Key Points
Question
What is the pattern of alcoholism-associated cortical volume deficits, and are they accelerated with aging or augmented by drug dependence or hepatitis C virus infection comorbidity in alcohol-dependent men and women spanning adulthood?
Findings
This combined cross-sectional/longitudinal study evaluated magnetic resonance imaging data collected during 14 years in 199 control and 222 alcohol-dependent participants. Findings revealed frontally distributed cortical volume deficits in individuals with alcohol dependence, accelerated age-dependent decline, and compounded deficits with drug dependence or hepatitis C virus infection comorbidity.
Meaning
These findings raise concern for heightened risk of accelerated cortical aging with alcohol dependence even when alcohol misuse develops later in life.
Abstract
Importance
The prevalence of alcohol misuse increased substantially over a decade in adults, particularly in those aged 65 years or older. Ramifications for brain structural integrity are significant, especially in older adults.
Objectives
To combine cross-sectional, longitudinal data to test age-alcoholism interactions and examine the association between prevalent comorbidities (drug dependence and hepatitis C virus [HCV] infection) and cortical volume deficits in alcohol dependence.
Design, Setting, and Participants
During 14 years, 826 structural magnetic resonance images were acquired in 222 individuals with alcohol dependence and 199 age-matched control participants (aged 25-75 years at initial study), parcellated with a common atlas, and adjusted for brain volume. Longitudinal data were available on 116 participants with alcoholism and 96 control participants. DSM-IV criteria determined alcohol and drug diagnoses; serology testing determined HCV status. The study was conducted at SRI International and Stanford University School of Medicine from April 11, 2003, to March 3, 2017.
Main Outcomes and Measures
Magnetic resonance imaging–derived regional cortical volumes corrected for supratentorial volume and sex.
Results
Of the 222 participants with alcoholism, 156 (70.3%) were men; mean (SD) age was 48.0 (10.0) years; the mean age for the 199 control participants was 47.6 (14.0) years. Participants with alcohol dependence had volume deficits in frontal (t = −5.732, P < .001), temporal (t = −3.151, P = .002), parietal (t = −5.063, P < .001), cingulate (t = −3.170, P = .002), and insular (t = −4.920, P < .001) cortices; deficits were prominent in frontal subregions and were not sex dependent. Accelerated aging occurred in frontal cortex (t = −3.019, P < .02) and precentral (t = −2.691, P < .05) and superior gyri (t = −2.763, P < .05) and could not be attributed to the amount of alcohol consumed, which was greater in younger-onset than older-onset participants with alcoholism (t = 6.1191, P < .001). Given the high drug-dependence incidence (54.5%) in the alcoholism group, analysis examined drug subgroups (cocaine, cannabis, amphetamines, opiates) compared with drug-dependence–free alcoholism and control groups. Although the alcohol plus cocaine (t = −2.310, P = .04) and alcohol plus opiate (t = −2.424, P = .04) groups had smaller frontal volumes than the drug-dependence–free alcoholism group, deficits in precentral (t = −2.575, P = .01), supplementary motor (t = −2.532, P = .01), and medial (t = −2.800, P = .01) volumes endured in drug-dependence–free participants with alcoholism compared with control participants. Those with HCV infection had greater deficits than those without HCV infection in frontal (t = 3.468, P = .01), precentral (t = 2.513, P = .03), superior (t = 2.533, P = .03), and orbital (t = 2.506, P = .03) volumes, yet total frontal (t = 2.660, P = .02), insular (t = 3.526, P = .003), parietal (t = 2.414, P = .03), temporal (t = 3.221, P = .005), and precentral (t = 3.180, P = .01) volume deficits persisted in the uninfected participants with alcoholism compared with control participants with known HCV status.
Conclusions and Relevance
Drug dependence and HCV infection compounded deleterious effects of alcohol dependence on frontal cortical volumes but could not account for the frontally distributed volume deficits in the drug-free participants with alcoholism. We speculate that age-alcohol interactions notable in frontal cortex put older adults at heightened risk for age-associated neurocompromise even if alcohol misuse is initiated later in life.
This cross-sectional study examines the association between alcoholism and cortical volume deficits in alcohol-dependent individuals.
Introduction
It is well established through in vivo neuroimaging1,2,3 and postmortem4,5 studies that chronic, excessive alcohol consumption can result in regional brain shrinkage. This assertion is supported by longitudinal evidence indicating that at least partial reversal of tissue volume deficits and ventricular dilatation occur early in abstinence—over days to weeks6,7—and that further cortical tissue shrinkage ensues with resumption of drinking.1,8,9 The most commonly reported regions affected in vivo are frontally distributed, notably superior and middle lateral, orbital, and medial frontal gyri in individuals with treated10,11,12 and never-treated13 alcoholism. Prefrontal cortex is also a target of normal aging, showing pronounced volume decline from approximately age 50 years onward.14,15,16 The potential interaction of normal aging and alcoholism has been borne out in several cross-sectional studies in which accelerated volume declines appear by approximately age 45 years in alcohol-dependent groups.17,18 Assessing the association between age-alcoholism interactions and brain structure has new urgency considering current epidemiologic data indicating a major increase, measured throughout a decade, of 106.7% (from 1.5% to 3.1% of the US population) in the prevalence of alcohol use disorder (AUD) in individuals aged 65 years or older. This increasing prevalence of AUD is also occurring in younger adults, ranging from a 44.4% increase in individuals aged 18 to 29 years to 47.7% in those aged 30 to 44 years and 81.5% in adults aged 45 to 64 years.19 To date, the locus and extent of enduring regional cortical volume deficits, however, have not been examined longitudinally across the adult age range in large samples of participants with alcohol dependence.
Factors that contribute to persistent or accelerated brain volume abnormalities are still being identified but likely include alcohol consumption variables of frequency and amount drunk, age at onset of alcohol misuse, and conditions resulting from years of abusive drinking, such as withdrawal signs and symptoms20 and alcoholism-related nutritional deficiencies.21,22 Perhaps the most salient factor is age, which may render the older brain especially vulnerable to insult from other factors, including excessive alcohol consumption and attendant events. Among these events are accruing detoxification experiences; comorbid licit and illicit drug dependence, which is a frequent concomitant factor of alcohol misuse23; and acquired medical conditions, such as hepatitis C virus (HCV) infection, which is prevalent in individuals who misuse alcohol.24,25
Alcohol use disorder and drug use comorbidity rates are high, highlighting the relevance of potentially compounding effects of substance dependence on brain structural integrity. Estimates indicate that 4 of 5 adults with a substance use disorder also have AUD26 and that 15% of adults with AUD also have a substance use disorder.27 Commonly abused substances in the United States include cocaine, opiates, amphetamines, and cannabis. Several studies report enhancement of activation in reward networks of the striatum or enlargement in striatal structures compared with control participants with drug dependence meta-analyses,28,29 whereas studies of alcoholism report striatal volume deficits involving caudate nucleus, nucleus accumbens, or globus pallidus.10 Overall, evidence indicates that various constellations of drug and alcohol misuse confer some selective and other overlapping effects on brain structure.
Hepatitis C virus infection also occurs with high prevalence in individuals with AUD25 and is the most common blood-borne infection in the United States. Hepatitis C virus can infiltrate the brain30 with presence in the frontal but not occipital cortex.31 The prevalence of HCV is highest (approximately 75%) in those born between 1945 and 1965 who are now approximately aged 50 to 70 years, which is the time of notable senescent brain structural declines in unaffected persons14,15,16 and accelerates in individuals with alcoholism.17 Alcoholism and HCV infection comorbidity also increases the risk of chronic liver disease morbidity and mortality.25 Although intravenous drug use is the most recognized vehicle of infection, other non–drug-related causes exist and include male-to-male sex and body tattoos.32
Given the known independent contributions of age, alcoholism, drug, and HCV infection to frontal volume insult, we tested the hypotheses that alcohol-dependent adults (1) would exhibit significant cortical volume deficits and show accelerated aging selective to frontal cortical loci; (2) would have greater volume deficits, especially affecting frontal sites, than those without drug dependence; (3) would show compounded alcoholism-related volume deficits in frontal cortex when infected with HCV; and (4) who were drug-free and HCV-infection-free would have enduring volume deficits.
Methods
Participants
The participants were drawn from our ongoing longitudinal studies of brain magnetic resonance imaging (MRI) (control participants15 and those with alcoholism33). Clinical psychologists (including S.A.S.) or research nurses administered the Structured Clinical Interview for DSM-IV34 to all study participants.33 Only participants meeting DSM-IV criteria for alcohol dependence were included in the patient group. Prospective control participants did not meet DSM-IV criteria for any Axis I disorder. Quantity of lifetime alcohol consumption and date of last drink were obtained from all participants by interview.35,36,37 The study was conducted from April 11, 2003, to March 3, 2017. All study participants provided written informed consent, and the study was approved by the institutional review boards of Stanford University School of Medicine and SRI International. Participants received financial compensation.
Of the 222 participants with alcoholism, 123 (55.4%) also met historical DSM-IV criteria for substance dependence. Substances most commonly used to dependence were cocaine, cannabis, amphetamines, and opiates: 86 participants with alcoholism (38.7%) had a lifetime history of cocaine dependence, 50 (22.5%) had a history of cannabis dependence, 44 (19.8%) had a history of amphetamine dependence, and 30 (13.5%) had a history of opiate dependence. Approximate mean remission time for the most recent nonalcohol substance of abuse/dependence was 495.8 weeks (median, 294 weeks). One control participant developed cannabis dependence at a later MRI scan but had no drug diagnosis at her initial visit. Of the 222 participants with alcoholism, 107 (48.2%) had current nicotine dependence, 35 (15.8%) had a history of nicotine dependence, 60 (27.0%) never had nicotine dependence, and the status of 20 (9.0%) was unknown. Of the 199 control participants, 14 (7.0%) had current nicotine dependence, 6 (3.0%) had a history of nicotine dependence, 117 (58.8%) never had nicotine dependence, and the status of 62 (31.2%) was unknown.
MRI Acquisition and Analysis
Image Acquisition
Data obtained with MRI were acquired between April 11, 2003, and March 3, 2017 (3-T GE whole-body MR systems; General Electric Healthcare). An 8-channel phased-array head coil and the same axial acquisition protocol were used throughout (eAppendix 1 in the Supplement).
Statistical Analysis
All statistical analyses (eAppendix 2 in the Supplement) were performed with the R statistical language software.38 The magnitude of cortical gray matter volume is correlated with supratentorial volume (svol). To examine each gray matter volume region independent of svol, the regression of regional volume on svol was computed for control participants with a general linear model (lm in R); this function was then applied to the data of all participants at each scan. Only control participants were used in the fitting function to ensure that the estimate of association was not influenced by disease.35,39 This procedure also minimized sex effects given that women, in general, have smaller heads and svol than men (mean svol for control women, 1199.3 mL vs control men, 1360.5 mL; t = 11.397; P < 10−16 in the present sample).
Results
A control group was selected to match the 25- to 75-year age range of the alcoholism group at study entry. Mean (SD) age of the 222 alcohol-dependent participants was 48.0 (10.0) years; the mean age for the 199 control participants was 47.6 (14.0) years. A total of 826 MRIs were analyzed: 417 acquired in 199 control and 409 acquired in 222 alcohol-dependent participants. Of the 199 control and 222 alcohol-dependent participants scanned at entry, 96 control (48.2%) and 116 alcohol-dependent participants (52.3%) had 2 or more MRIs. Of these, 47 control (5.0%) and 71 alcohol-dependent participants (61.2%) had 2 MRIs, 21 control (2.1%) and 31 alcohol-dependent participants (26.7%) had 3 MRIs, 11 control (11.5%) and 9 alcohol-dependent participants (7.8%) had 4 MRIs, and 17 control (17.7%) and 5 alcohol-dependent participants (4.3%) had 5 or more MRIs (eFigure 1 in the Supplement). Consistent with epidemiologic studies of alcoholism,19 the groups comprised more men than women, but the control and alcoholism groups had similar sex representation and were of similar ages (Table; eTable 1 in the Supplement). All analyses were based on regional brain volumes adjusted for total brain volume (svol), which minimized differences attributable to sex.
Table. Demographic Data for the Control and Alcoholism Groups.
| Demographic Variablea | Control | Alcoholism |
|---|---|---|
| Sex, No. (%) | ||
| Men | 107 (53.8) | 156 (70.3) |
| Women | 92 (46.2) | 66 (29.7) |
| Self-defined race/ethnicity, No. (%) | ||
| Asian | 28 (14.1) | 3 (1.4) |
| African American | 28 (14.1) | 69 (31.1) |
| White | 106 (53.2) | 98 (44.1) |
| Other/unknown | 37 (18.6) | 52 (23.4) |
| Detoxifications or treatment, No. (%) | NA | |
| Yes | 83 (46.6) | |
| No | 95 (53.4) | |
| Drank to stop symptoms, No. (%) | NA | |
| Yes | 96 (48.7) | |
| No | 101 (51.3) | |
| Reported seizures | NA | |
| Yes | 14 (7.0) | |
| No | 186 (93.0) | |
| Age, y, mean (SD) | ||
| Men | 47.7 (13.7) | 48.5 (10.2) |
| Women | 45.9 (14.3) | 48.2 (9.4) |
| Education, mean (SD), y | 16.0 (2.4) | 13.3 (2.4) |
| Socioeconomic status, mean (SD)b | 25.4 (11.3) | 41.8 (14.4) |
| WTAR FSIQ estimate, mean (SD)c | 105.6 (9.3) | 98.3 (11.5) |
| Alcoholism onset age, mean (SD), y | NA | 25.5 (9.6) |
| Lifetime alcohol consumed at final MRI, mean (SD), kg | 34.0 (57.0) | 1202.0 (885.8) |
| Alcohol consumed in year before MRI, mean (SD), kg | NA | 35.3 (46.1) |
| Days since last drink, mean (SD) | NA | 290.5 (689.3) |
| Alcohol consumed in year before MRI, median, kg | NA | 20.2 |
| Days since last drink, median | NA | 92 |
Abbreviations: FSIQ, full-scale intelligence quotient; MRI, magnetic resonance imaging; NA, not applicable; WTAR, Wechsler Test of Adult Reading.
Data on some variables were not obtained for all patients.
Lower score indicates higher status.
Participants had either the National Adult Reading Test or the WTAR for IQ; 10 points were added to the National Adult Reading Test IQ to make it comparable to the WTAR Full Scale IQ.
Regional Volume Deficits in Participants With Alcoholism
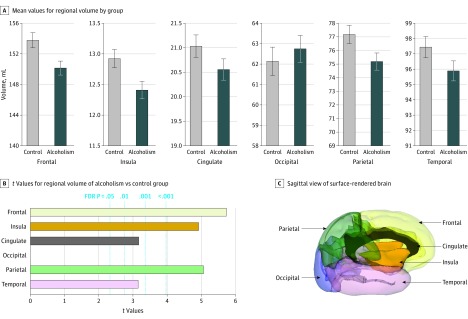
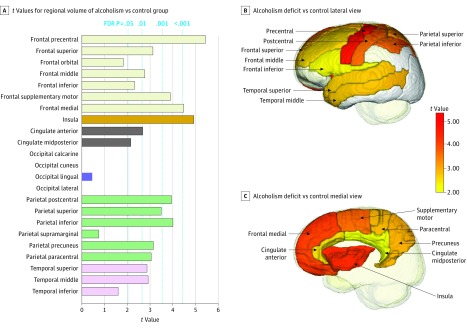
Examination of the 6 major cortical volumes identified 5 regions showing volume deficits in the alcoholism compared with control groups: frontal (t = −5.732, P < .001), temporal (t = −3.151, P = .002), parietal (t = −5.063, P < .001), cingulate (t = −3.170, P = .002), and insular (t = −4.920, P < .001) cortices (Figure 1; eTable 2A in the Supplement); the exception was the occipital lobe. Analysis of the 23 cortical subregions (eFigure 2 for frontal subregions in the Supplement) revealed gray matter volume deficits in the alcoholism compared with the control group in 16 regions (false discovery rate corrected; eTable 2B in the Supplement): precentral (t = −5.428, P < .001), superior (t = −3.131, P = .005), middle (t = −2.763, P = .01), inferior (t = −2.318, P = .03), supplementary motor (t = −3.891, P < .001), medial (t = −4.481, P < .001) frontal; insula (t = −4.920, P < .001); anterior (t = −2.681, P = .01) and midposterior (t = −2.156, P = .05) cingulate; postcentral (t = −3.946, P < .001), superior (t = −3.492, P = .002), inferior (t = −4.002, P < .001), precuneus (t = −3.148, P = .005), paracentral (t = −3.051, P = .006) parietal; and superior (t = −2.865, P = .01) and middle (t = −2.914, P = .01) temporal. Volume deficits were prominent in frontal, parietal, and insular cortices and were less so but still significant in temporal and cingulate regions (Figure 2). Testing for diagnosis-by-sex interactions yielded no significant effects in either the 6- or the 23-regional volume analyses.
Figure 1. Regional Cortical Volumes Showing Volume Deficits in 222 Alcohol-Dependent Participants.
A, Mean values for each volume by group; error bars indicate 95% CI. B, Values from t tests for regional volumes indicating group differences and false discovery rate (FDR)–corrected P values. In 5 of the 6 regions, the alcoholism group had smaller volumes than the control group. The t value for the occipital comparison indicated a nonsignificant higher value for the alcoholic than control group. C, Sagittal view of a surface-rendered brain indicating the 6 global cortical regions used for volumetric analysis.
Figure 2. Gray Matter Regions Showing Volume Deficits in 222 Alcohol-Dependent Participants.
Values from t tests for regional volumes indicating group differences and false discovery rate (FDR)–corrected P values. The t values for 3 occipital regional comparisons indicated a nonsignificant higher value for the alcoholic than control group. (A). In general, the alcohol-dependent group had smaller volumes than the control group. Lateral (B) and medial (C) sagittal views of the gray matter regions show volume deficits in alcohol-dependent participants.
Age-Alcoholism Interactions
The effect of age was examined independently for the control and alcoholism groups. The control group showed significant aging effects in 5 of the 6 cortical regions: frontal t = −11.672, P < .001; cingulate t = −4.471, P < .001; occipital t = −2.983, P < .001; parietal t = 11.660, P < .001; temporal t = −13.210, P < .001 (not insula). The alcoholism group showed aging effects in 5 of the 6 cortical regions: frontal t = −10.998, P < .001; insula t = −2.511, P = .01; cingulate t = −2.374, P = .02; parietal t = −6.195, P < .001; temporal t = −5.535, P < .0001 (not occipital). Age-alcohol interactions occurred in the alcoholism group over and above those measured in the control group for the frontal cortex only (t = −3.019, P = .02) (eTable 3A in the Supplement).
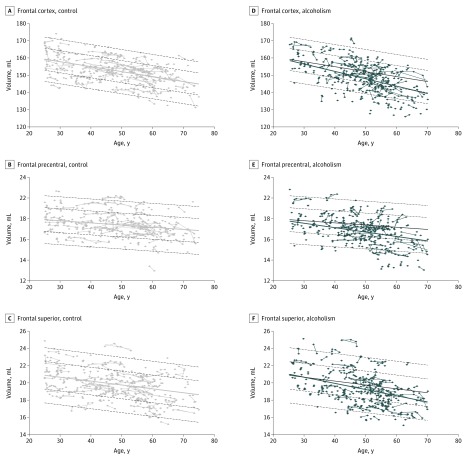
Age-associated declines were detected in all 7 frontal subregions of the alcoholism and control groups (eTable 3B in the Supplement). Furthermore, the alcoholism group showed age-alcoholism interactions in the precentral (t = −2.691, P = .04) and superior frontal (t = −2.763, P = .04) cortices that exceeded the age declines identified in the control participants (Figure 3; eFigure 3 in the Supplement).
Figure 3. Age-Alcoholism Interactions Shown at Each Magnetic Resonance Imaging (MRI) of the 199 Individual Control Participants vs 222 Alcohol-Dependent Participants.
A-C, Frontal regional volumes by age at each MRI in the control participants plotted on their mean (solid gray regression) ± 1 and 2 SDs (dashed gray lines). D-F, Frontal regional volumes by age at each MRI of the alcohol-dependent participants plotted on their mean regression (blue line) and overplotted on the control mean (solid gray regression) ± 1 and 2 SDs (dashed gray lines).
To identify factors that contributed to the age-alcoholism interactions, we examined drinking variables commonly associated with age. Total alcohol ingested in a lifetime correlated with mean age of alcohol-dependent individuals (r = 0.263; P < .001), and older age of alcoholism onset correlated with older age at examination (r = 0.367; P < .001) (eFigure 4 in the Supplement). Smaller, age-adjusted frontal cortical volumes showed a correlational trend with total lifetime alcohol consumption (r = −0.122; P = .07). Many participants had a relatively late onset of alcohol dependence. To test for regional volume differences in older participants (age ≥40 years), we divided that alcoholism group into those with early onset (by age 30 years, n = 117) and those with late-onset (age ≥40 years, n = 24). This comparison revealed smaller age-adjusted frontal cortical volumes in the late-onset relative to the early-onset group (t = −2.271; P = .03) even having controlled for normal aging effects. The late-onset group had lower lifetime alcohol consumption than the early-onset group (early mean, 1480.8 kg; late mean, 759.9 kg; t = 6.1191; P < .001), but these groups did not differ significantly in days since last drink (t = 1.4525; P = .15).
Drug and Alcohol Dependence Comorbidity
The first test of drug comorbidity examined volumes of the 5 alcoholism subgroups (101 alcohol only, 86 alcohol-cocaine, 30 alcohol-opiates, 44 alcohol-amphetamines, 50 alcohol-cannabis) against control volumes of the 6 lobar regions. Each of these 5 subgroups had volume deficits in frontal, insula, and parietal cortices relative to control participants (P < .004; eTable 4). Furthermore, alcohol-dependent participants without a drug history (t = −2.86, P = .02) and those with a cocaine history (t = −2.586, P = .03) also showed volume deficits in temporal cortices; in addition, the cocaine group had deficits in cingulate cortex (t = −2.717, P = .03). Those with alcohol dependence with amphetamine (t = 2.448, P = .04) or cannabis (t = 2.596, P = .04) histories had larger occipital volumes than control participants (eTable 4 in the Supplement). The second test examined volumes of the 4 alcoholism subgroups with histories of drug dependence against the alcoholism subgroup with no history of drug dependence. The alcohol-cocaine (t = −2.310, P = .04) and alcohol-opiate (t = −2.424, P = .04) groups had smaller frontal volumes than the alcoholism group without drug histories (eTable 4 and eFigure 5 in the Supplement), whereas the alcoholism subgroup with amphetamine (t = 2.591, P = .02) or cannabis (t = 2.722, P = .02) histories had larger occipital volumes than the non–drug-dependent alcoholism subgroup.
Given the observed frontal deficits, we tested for group differences in the 7 frontal volumes. Three regions showed significant volume deficits in the alcoholism group with no history of drug dependence and each of the 4 alcohol-drug groups relative to control participants: precentral (t = −2.575, P = .01), supplementary motor (t = −2.532, P = .01), and medial (t = −2.800, P = .01) cortices. Comparisons of the alcohol-drug groups with the nondrug alcoholism group did not yield significant differences for any of the 7 frontal subregions (eTable 4 in the Supplement).
HCV-Alcoholism Comorbidity
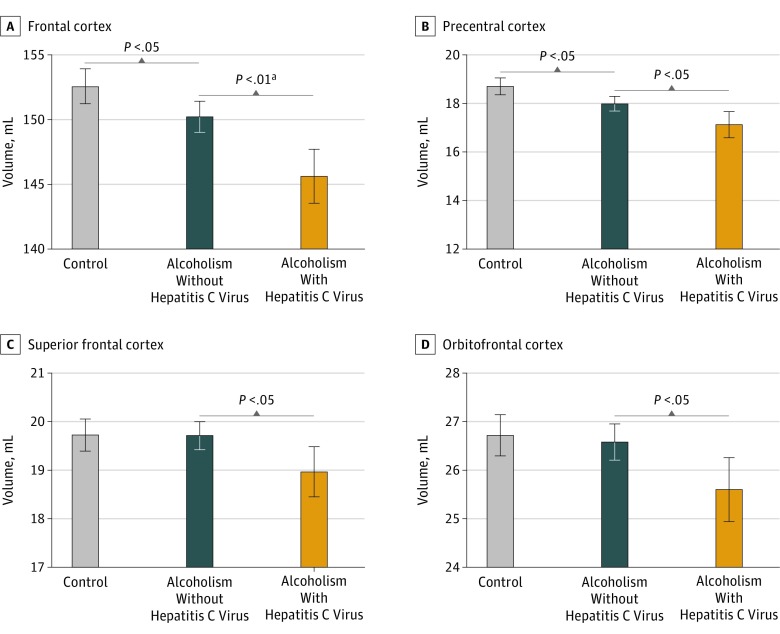
We compared the volumes of 6 lobar regions in alcohol-dependent participants with and without HCV infection comorbidity (eTable 1 in the Supplement). Results indicated smaller volumes in those with HCV infection than without HCV infection in the frontal volumes only (t = 3.468, P = .01) (Figure 4; eTable 5 in the Supplement). Analysis of the 7 frontal subregions revealed larger volumes for alcohol-dependent participants without HCV infection in the precentral (t = 2.513, P = .03), superior (t = 2.533, P = .03), and orbital (t = 2.506, P = .03) cortices (eTable 5A in the Supplement). Compared with the 89 control participants with known HCV status, the 115 alcohol-dependent participants free of HCV infection had significant volume deficits in frontal (t = 2.660, P = .02), insular (t = 3.526, P = .003), parietal (t = 2.414, P = .03), temporal (t = 3.221, P = .005), and precentral (t = 3.180, P = .01) cortical volumes (eTable 5B in the Supplement).
Figure 4. Alcoholism and Hepatitis C Virus (HCV) Infection in Alcohol-Dependent (Alc) and Control (Con) Participants.
Mean volumes in participants without HCV infection (Alc-HCV) vs those with HCV infection (Alc+HCV) in frontal cortex (A), precentral cortex (B), superior frontal cortex (C), and orbitofrontal cortex (D). Serologic HCV status known in all included participants. Error bars indicate 95% CI.
aP value false discovery rate corrected.
Group Differences in Alcohol-Dependent Participants Scanned Once vs Multiple Times
Questioning whether alcohol-dependent participants who received 1 MRI differed in demographic or volume data from those who returned for multiple examinations revealed significant differences in alcohol consumption variables between these subgroups (eTable 6 in the Supplement). The subgroup scanned once had drunk more alcohol in the year before scanning (t = 2.076, P = .04) and had a shorter interval between the last drink and the scan (t = −2.030, P = .04). Even greater differences were identified for the number of detoxifications (χ2 = 106.69. P < .001), history of drinking to stop symptoms (χ2 = 72.04. P < .001), and seizures (χ2 = 12.432. P < .001), all of which occurred with greater frequency in the single- than multiple-scan subgroup. Our reanalysis of the MRI data with respect to these subgroups revealed a similar pattern of regional volume deficits and frontal age interactions in both subgroups (single group t = −3.339; P < .001; multiple group t = −2.510; P = .01) (eFigure 6 in the Supplement), with the single MRI group having even greater volume deficits than the multiple MRI group in frontal, temporal, parietal, and occipital regions.
Discussion
Examination of cortical brain structure using atlas-based, quantitative MRI revealed regionally selective volume deficits in the 222 alcohol-dependent participants relative to a control group spanning the same 50-year adult age range. Regional volumes most extensively affected included lateral and medial frontal, parietal, and insular cortices with additional deficits in temporal and cingulate regions. These effects endured when examining alcohol-dependent participants without comorbidity of drug dependence or HCV infection, and there was evidence for compounded untoward effects of drug dependence and HCV infection with alcoholism. Although our cohort of nearly 200 control participants showed an expected age-related cortical volume decline salient in precentral and superior frontal regions, longitudinal analysis of the alcoholism group data identified age-alcoholism interactions beyond those observed in control participants. These findings in alcohol-dependent and control participants, examined 1 to 8 times or more during intervals of 1 week to 12.5 years, representing, to our knowledge, the largest and longest-studied group to date, support our study hypotheses regarding alcoholism-associated accelerated aging and cortical volume deficits independent of drug dependence or HCV infection comorbidity.
Pattern of Cortical Volume Deficits Associated With Alcohol Dependence and Aging
Courville's40 early claim of focal neuronal loss in alcoholism cases on postmortem study included the superior dorsal surfaces of the frontal cortex in addition to the precentral, postcentral, and superior parietal regions with sparing of temporal, inferior parietal, and occipital regions. This postmortem pattern of regional effects of alcoholism on cortex reflects the in vivo pattern observed herein and consistent with other in vivo studies2,10 of abstinent alcohol-dependent participants and reviews.12,13
A central aim of this longitudinal analysis was to test for age-alcoholism interactions. Accordingly, we observed a selectivity of frontal cortex to age-alcoholism interaction beyond normal aging effects and independent of deficits related to drug dependence. This interaction is consistent with that of a cross-sectional study, which reported an age-alcoholism interaction on nonspecific, total gray matter/white matter volume ratios in alcohol-dependent participants without drug dependence history, but not in those with a comorbid lifetime cocaine use disorder.41 Our age-alcoholism interaction identified longitudinally supports earlier cross-sectional findings showing that older alcohol-dependent participants had greater cortical volume deficits selective to prefrontal and frontal regions beyond those observed in normal aging.17,35 The accelerated volume deficits in the older alcohol-dependent participants could not readily be attributed to more years of heavy drinking, given that many had a late onset of their disorder and lower lifetime alcohol consumption estimates than their early-onset counterparts.
Alcohol and Drug Dependence Comorbidity
Given the high incidence (54.5%) of drug dependence in these alcohol-dependent participants, additional analysis examined subgroups according to the drugs most misused (ie, cocaine, cannabis, amphetamines, opiates) compared with participants with alcoholism free of drug dependence and control participants. Each of these 4 drugs is associated with cortical volume abnormalities, some unique and many overlapping with each other, notably in frontal regions.23,42
A few studies have considered alcohol-drug comorbidities. One study reported a similar level of prefrontal volume deficits in 6-week abstinent crack-cocaine dependents with or without alcohol dependence.43 Yet, a larger study failed to detect independent influence of comorbid cocaine dependence on gray matter volume deficits in alcohol-dependent men.41 Herein, although the alcohol-cocaine and alcohol-opiate groups had smaller frontal volumes than the drug-dependence–free alcohol-dependent participants, deficits in precentral, supplementary motor, and medial volumes endured when the analysis was limited to drug-dependence–free alcohol-dependent participants.
Alcohol and HCV Infection Comorbidity
Alcohol-dependent participants with HCV infection had greater deficits than those without HCV infection in precentral, superior, and orbital frontal volumes. Nonetheless, the total frontal, insular, and precentral volume deficits were significant in the uninfected alcoholism group compared with control participants with known HCV status. Thus, HCV infection, while having focal effects on frontal brain systems,30,31 targeted frontally based systems also vulnerable to chronic and extensive alcohol consumption. Whether the compounded untoward effects of alcoholism and HCV infection on brain structure can be ameliorated with successful treatment of the infection remains to be determined.
Limitations
One limitation of the study is that alcohol-dependent participants were recruited from community-based treatment centers, which, according to estimates, account for less than 25% of individuals needing treatment.44 Thus, our results cannot necessarily generalize to all adults with AUD.18 A further limitation is the absence of non–alcohol-dependent drug or HCV-infected comparison groups, which were unavailable. Although formal testing for diagnosis-by-sex interactions identified no sex effects, we are cautious to conclude that sex differences do not occur in alcohol dependence, especially given some evidence from cross-sectional studies reporting greater volume deficits in women than men,45 although others do not.46,47 Finally, although testing of functional correlates was beyond the scope of this analysis, ultimate consideration of neurocompromise in the context of the observed frontal distribution of the aging-alcoholism acceleration of volume shrinkage may identify substrates of cognitive, emotion, or motor compromise potentially ameliorated with adequate retraining efforts.
Conclusions
Alcoholism's target of prefrontal and frontal cortical tissue has been thematic for nearly a century of quantitative analysis. In vivo neuroimaging findings have continued this theme in demonstrating consistencies in compromise of frontal-fugal systems12 extending to insular and parietal sites, now associated with behaviors commonly observed in alcoholism, such as problems with inhibitory control, poor insight, visuospatial disabilities,48,49,50 and liability for relapse.11 Alcohol is a critical agent in understanding observed brain structural compromise given that neither drug dependence nor HCV infection comorbidities accounted for the frontally distributed volume deficits in the drug-free alcohol-dependent group. Finally, the presence of age-alcoholism interactions notable in frontal cortex puts older alcohol-dependent individuals at heightened risk for age-associated functional compromise,19 even if excessive drinking is initiated later in life.
eAppendix 1. Further Study Details
eAppendix 2. R Code
eFigure 1. Horizontal Lines Represent Individual Participants; Dots Represent Each MRI per Participant Over Time for the Alcoholic (Blue) and Control (Gray) Participants
eFigure 2. Color-Coded Atlas Used for Identifying the 23 Gray Matter Regional Volumes
eFigure 3. Mean ±95% Confidence Interval (CI) Volume Slopes, Expressed as Change per Year (cc), for the 199 Control (Gray Bars) and 222 Alcoholic (Blue Bars) Participants
eFigure 4. Age and Alcohol Variables and Frontal Cortical Volumes in the Alcoholic Participants Related To Total Lifetime Alcohol Consumption (Kg), Age of Alcoholism Onset, Age of Onset Divided by Early- and Late-Onset Subgroups
eFigure 5. Mean ±95% CI of the 199 Controls (Con), 101 Alcoholic Participants Only, and the Four Alcohol-Dependent With Drug-Dependent Comorbidity: Cocaine, Opiates, Amphetamines, and Cannabis
eFigure 6. Age-Alcohol Interactions for Alcohol-Dependent Subgroups Scanned Once and Multiple Times
eTable 1. Study Entry Demographics of the Study Groups
eTable 2A. 199 Controls vs 222 Alcoholics: Group Differences for 6 Cortical Volumes
eTable 2B. 199 Controls vs 222 Alcoholics: Group Differences for 23 Cortical Volumes
eTable 3A. Control and Alcoholic Aging Effects for 6 Cortical Volumes
eTable 3B. Control and Alcoholic Aging Effects in Frontal Cortical Volumes
eTable 4. Group Differences: Controls vs Alcoholics Without a Drug Dependence History vs Alcoholics With a Drug Dependence History
eTable 5A. Group Differences: 37 Alcoholics With HCV<115 Alcoholics Without HCV Infection
eTable 5B. Group Differences: 89 Controls With Known HCV Status >115 Alcoholics Without HCV Infection
eTable 6. Study Entry Demographics of Alcohol Dependent Participants With Single vs Multiple MRIs
References
- 1.Segobin SH, Chételat G, Le Berre AP, et al. Relationship between brain volumetric changes and interim drinking at six months in alcohol-dependent patients. Alcohol Clin Exp Res. 2014;38(3):739-748. [DOI] [PubMed] [Google Scholar]
- 2.Le Berre AP, Pitel AL, Chanraud S, et al. Chronic alcohol consumption and its effect on nodes of frontocerebellar and limbic circuitry: comparison of effects in France and the United States. Hum Brain Mapp. 2014;35(9):4635-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bühler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res. 2011;35(10):1771-1793. [DOI] [PubMed] [Google Scholar]
- 4.Harper CG, Kril JJ, Sheedy D, et al. Neuropathological studies: the relationship between alcohol and aging In: Gomberg ESL, Hegedus AM, Zucker RA, eds. Alcohol Problems and Aging. Vol 33 Bethesda: National Institute on Alcohol Abuse and Alcoholism; 1998:117-134. [Google Scholar]
- 5.Sutherland GT, Sheedy D, Kril JJ. Neuropathology of alcoholism. Handb Clin Neurol. 2014;125:603-615. [DOI] [PubMed] [Google Scholar]
- 6.van Eijk J, Demirakca T, Frischknecht U, Hermann D, Mann K, Ende G. Rapid partial regeneration of brain volume during the first 14 days of abstinence from alcohol. Alcohol Clin Exp Res. 2013;37(1):67-74. [DOI] [PubMed] [Google Scholar]
- 7.Wang GY, Demirakca T, van Eijk J, et al. Longitudinal mapping of gyral and sulcal patterns of cortical thickness and brain volume regain during early alcohol abstinence. Eur Addict Res. 2016;22(2):80-89. [DOI] [PubMed] [Google Scholar]
- 8.Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34(3):879-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55(10):905-912. [DOI] [PubMed] [Google Scholar]
- 10.Makris N, Oscar-Berman M, Jaffin SK, et al. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64(3):192-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rando K, Hong KI, Bhagwagar Z, et al. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am J Psychiatry. 2011;168(2):183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zahr NM, Pfefferbaum A, Sullivan EV. Perspectives on fronto-fugal circuitry from human imaging of alcohol use disorders. Neuropharmacology. 2017;122:189-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fein G, Shimotsu R, Barakos J. Age-related gray matter shrinkage in a treatment naïve actively drinking alcohol-dependent sample. Alcohol Clin Exp Res. 2010;34(1):175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fjell AM, Westlye LT, Amlien I, et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009;19(9):2001-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. Neuroimage. 2013;65:176-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockhart SN, DeCarli C. Structural imaging measures of brain aging. Neuropsychol Rev. 2014;24(3):271-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21(3):521-529. [DOI] [PubMed] [Google Scholar]
- 18.Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naïve alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26(4):558-564. [PMC free article] [PubMed] [Google Scholar]
- 19.Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(9):911-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duka T, Gentry J, Malcolm R, et al. Consequences of multiple withdrawals from alcohol. Alcohol Clin Exp Res. 2004;28(2):233-246. [DOI] [PubMed] [Google Scholar]
- 21.Pitel AL, Zahr NM, Jackson K, et al. Signs of preclinical Wernicke’s encephalopathy and thiamine levels as predictors of neuropsychological deficits in alcoholism without Korsakoff’s syndrome. Neuropsychopharmacology. 2011;36(3):580-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomson AD, Guerrini I, Bell D, et al. Alcohol-related brain damage: report from a Medical Council on Alcohol Symposium, June 2010. Alcohol Alcohol. 2012;47(2):84-91. [DOI] [PubMed] [Google Scholar]
- 23.Mackey S, Paulus M. Are there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants? Neurosci Biobehav Rev. 2013;37(3):300-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novo-Veleiro I, Calle CdeL, Domínguez-Quibén S, Pastor I, Marcos M, Laso FJ. Prevalence of hepatitis C virus infection in alcoholic patients: cohort study and systematic review. Alcohol Alcohol. 2013;48(5):564-569. [DOI] [PubMed] [Google Scholar]
- 25.Fuster D, Sanvisens A, Bolao F, et al. Impact of hepatitis C virus infection on the risk of death of alcohol-dependent patients. J Viral Hepat. 2015;22(1):18-24. [DOI] [PubMed] [Google Scholar]
- 26.Lipari RN, Van Horn SL The CBHSQ Report: Trends in Substance Use Disorders Among Adults Aged 18 or Older. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [PubMed] [Google Scholar]
- 27.National Institute on Alcohol Abuse and Alcoholism Other Substance Abuse. http://www.niaaa.nih.gov/alcohol-health/special-populations-co-occurring-disorders/other-substance-abuse. Accessed January 29, 2018.
- 28.Luijten M, Schellekens AF, Kühn S, Machielse MW, Sescousse G. Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry. 2017;74(4):387-398. [DOI] [PubMed] [Google Scholar]
- 29.Hall MG, Alhassoon OM, Stern MJ, et al. Gray matter abnormalities in cocaine versus methamphetamine-dependent patients: a neuroimaging meta-analysis. Am J Drug Alcohol Abuse. 2015;41(4):290-299. [DOI] [PubMed] [Google Scholar]
- 30.Silverstein PS, Kumar S, Kumar A. HIV-1, HCV and alcohol in the CNS: potential interactions and effects on neuroinflammation. Curr HIV Res. 2014;12(4):282-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Letendre S, Paulino AD, Rockenstein E, et al. ; HIV Neurobehavioral Research Center Group . Pathogenesis of hepatitis C virus coinfection in the brains of patients infected with HIV. J Infect Dis. 2007;196(3):361-370. [DOI] [PubMed] [Google Scholar]
- 32.National Institute on Drug Abuse Why are cocaine users at risk for contracting HIV/AIDS and hepatitis? http://www.drugabuse.gov/publications/research-reports/cocaine/are-cocaine-abusers-risk-contracting-hivaids-hepatitis-b-c. Accessed January 29, 2018.
- 33.Pfefferbaum A, Rosenbloom MJ, Chu W, et al. White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. Lancet Psychiatry. 2014;1(3):202-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 35.Pfefferbaum A, Lim KO, Zipursky RB, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16(6):1078-1089. [DOI] [PubMed] [Google Scholar]
- 36.Skinner HA. Development and Validation of a Lifetime Alcohol Consumption Assessment Procedure. Toronto, Canada: Addiction Research Foundation; 1982. [Google Scholar]
- 37.Skinner HA, Sheu WJ. Reliability of alcohol use indices. the lifetime drinking history and the MAST. J Stud Alcohol. 1982;43(11):1157-1170. [DOI] [PubMed] [Google Scholar]
- 38.R Team A language and environment for statistical computing. 2017. https://www.R-project.org/. Accessed July 4, 2017.
- 39.Mathalon DH, Sullivan EV, Rawles JM, Pfefferbaum A. Correction for head size in brain-imaging measurements. Psychiatry Res. 1993;50(2):121-139. [DOI] [PubMed] [Google Scholar]
- 40.Courville CB. Effects of Alcohol on the Nervous System of Man. Los Angeles: San Lucas Press; 1955. [Google Scholar]
- 41.Bjork JM, Grant SJ, Hommer DW. Cross-sectional volumetric analysis of brain atrophy in alcohol dependence: effects of drinking history and comorbid substance use disorder. Am J Psychiatry. 2003;160(11):2038-2045. [DOI] [PubMed] [Google Scholar]
- 42.Bjork JM, Momenan R, Smith AR, Hommer DW. Reduced posterior mesofrontal cortex activation by risky rewards in substance-dependent patients. Drug Alcohol Depend. 2008;95(1-2):115-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68(1):87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Institute on Alcohol Abuse and Alcoholism National Epidemiologic Survey on Alcohol and Related Conditions: Selected Findings. Vol. 29, No. 2, 2006. Washington, DC: National Institute on Alcohol Abuse and Alcoholism; 2006. [PMC free article] [PubMed] [Google Scholar]
- 45.Hommer D, Momenan R, Kaiser E, Rawlings R. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry. 2001;158(2):198-204. [DOI] [PubMed] [Google Scholar]
- 46.Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan E. Sex differences in the effects of alcohol on brain structure. Am J Psychiatry. 2001;158(2):188-197. [DOI] [PubMed] [Google Scholar]
- 47.Pfefferbaum A, Sullivan EV. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage. 2002;15(3):708-718. [DOI] [PubMed] [Google Scholar]
- 48.Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36(5):357-368. [DOI] [PubMed] [Google Scholar]
- 49.Oscar-Berman M, Valmas MM, Sawyer KS, Ruiz SM, Luhar RB, Gravitz ZR. Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. Handb Clin Neurol. 2014;125:183-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Berre AP, Fama R, Sullivan EV. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol Clin Exp Res. 2017;41(8):1432-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Further Study Details
eAppendix 2. R Code
eFigure 1. Horizontal Lines Represent Individual Participants; Dots Represent Each MRI per Participant Over Time for the Alcoholic (Blue) and Control (Gray) Participants
eFigure 2. Color-Coded Atlas Used for Identifying the 23 Gray Matter Regional Volumes
eFigure 3. Mean ±95% Confidence Interval (CI) Volume Slopes, Expressed as Change per Year (cc), for the 199 Control (Gray Bars) and 222 Alcoholic (Blue Bars) Participants
eFigure 4. Age and Alcohol Variables and Frontal Cortical Volumes in the Alcoholic Participants Related To Total Lifetime Alcohol Consumption (Kg), Age of Alcoholism Onset, Age of Onset Divided by Early- and Late-Onset Subgroups
eFigure 5. Mean ±95% CI of the 199 Controls (Con), 101 Alcoholic Participants Only, and the Four Alcohol-Dependent With Drug-Dependent Comorbidity: Cocaine, Opiates, Amphetamines, and Cannabis
eFigure 6. Age-Alcohol Interactions for Alcohol-Dependent Subgroups Scanned Once and Multiple Times
eTable 1. Study Entry Demographics of the Study Groups
eTable 2A. 199 Controls vs 222 Alcoholics: Group Differences for 6 Cortical Volumes
eTable 2B. 199 Controls vs 222 Alcoholics: Group Differences for 23 Cortical Volumes
eTable 3A. Control and Alcoholic Aging Effects for 6 Cortical Volumes
eTable 3B. Control and Alcoholic Aging Effects in Frontal Cortical Volumes
eTable 4. Group Differences: Controls vs Alcoholics Without a Drug Dependence History vs Alcoholics With a Drug Dependence History
eTable 5A. Group Differences: 37 Alcoholics With HCV<115 Alcoholics Without HCV Infection
eTable 5B. Group Differences: 89 Controls With Known HCV Status >115 Alcoholics Without HCV Infection
eTable 6. Study Entry Demographics of Alcohol Dependent Participants With Single vs Multiple MRIs