Abstract
This study compares the time to transfusion in traumatically injured children receiving uncrossmatched whole blood with time to infusion in a historical cohort that received blood components.
Hemorrhagic shock is the most common cause of preventable death in pediatric civilian trauma.1 Balanced resuscitation with blood components has become the standard of care for initial hemostatic resuscitation in adults and children2,3,4 to mitigate the deleterious effects of trauma-induced coagulopathy.5 Whole blood (WB) contains plasma, red blood cells (RBCs), and platelets and requires less processing and dilution compared with reconstituting WB using components, making it an ideal resuscitative fluid. However, in non–group O recipients, there is a theoretical risk for hemolysis from anti-A and anti-B antibodies in group O WB units. Recently, the transfusion of WB in bleeding adult male trauma patients has been instituted in a large adult trauma center.6 Whole blood has never been transfused in a pediatric civilian trauma population; the novel use of transfusion of WB in injured children is herein described.
Methods
This study was conducted at Children’s Hospital of Pittsburgh of the University of Pittsburgh Medical Center. Group O WB units were collected from male donors with low titers of both anti-A and anti-B antibodies (<50). Whole blood units were stored between 1°C and 6°C; those not used by storage day 14 were processed into an RBC unit for use elsewhere. One WB unit was stored in the emergency department (ED) of a pediatric level 1 hospital and additional units were stored in the hospital blood bank. Patients eligible for WB included injured children 3 years and older weighing 15 kg or more. These factors mitigate the risk for hemolysis; by age 3 years, children have full expression of A/B antigens that can adsorb anti-A and/or anti-B antibodies in the group O WB unit. Furthermore, children weighing 15 kg or more have a sufficient blood volume to dilute these antibodies. A maximum volume of 20 mL/kg of WB was chosen because it is nearly equivalent to 2 units of WB in adults, which had been proven safe in that population.6 A hemolysis panel including haptoglobin, total bilirubin, reticulocyte count, and lactate dehydrogenase was performed at the time of WB transfusion and on posttrauma days 1 and 2. Transfusion-related complications were recorded. The University of Pittsburgh institutional review board approved this study, which was conducted with a waiver of informed consent.
The median (interquartile range [IQR]) time elapsed from ED admission to receipt of at least 1 RBC, plasma, and platelet unit (time ending at the start of the third product administered) in a historical (2013-2016) pre-WB cohort of 50 traumatically injured children was compared with the median (IQR) time elapsed from ED admission to the start of the first WB transfusion among the WB recipients.
Results
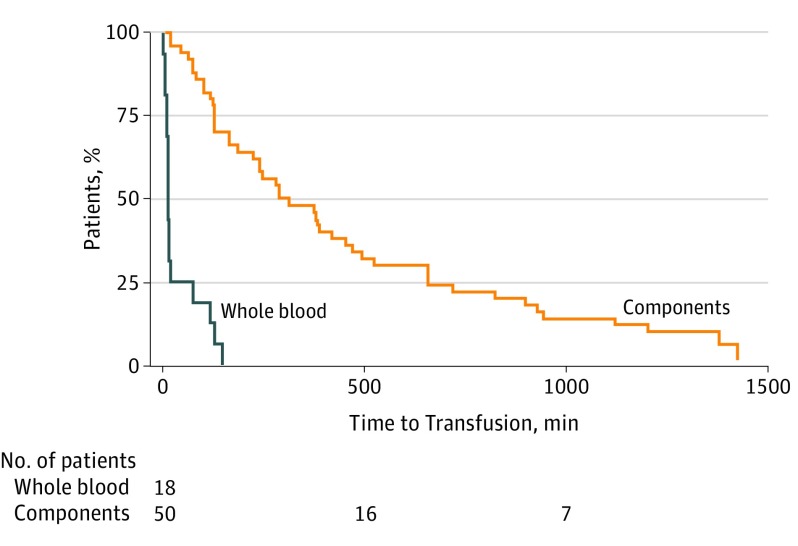
Between June 2016 and June 2017, 18 injured children received WB (Table).7,8 Eleven patients (61%) were male, 15 patients (83%) were of white race/ethnicity, and median (IQR) age was 11 (5-14) years. The median (IQR) injury severity score was 32 (25-42) and 8 patients (44%) died. Eight patients were blood group O, 10 patients were non–group O. Median (IQR) WB transfusion volume was 15 (9-23) mL/kg, roughly 1.5 (1-2) WB units. In 16 of these patients (88%), the first unit of WB was administered in the ED. The Wilcoxon rank sum test was used for comparison of nonparametric data; significance was set at P < .05. All P values are 2-tailed. There were no significant differences between group O and non–group O WB recipients at time of transfusion in biochemical hemolysis markers (1.25% vs 1.25%), in creatinine (0.009 mg/dL vs 0.007 mg/dL), and in potassium (4.05 mEq/L vs 3.70 mEq/L). There were no significant differences between the 2 groups on day 1 in biochemical hemolysis markers (1.40% vs 1.40%), in creatinine (0.005 mg/dL vs 0.005 mg/dL), and in potassium (3.80 mEq/L vs 3.80 mEq/L) (all P > .38). To convert reticulocyte count to proportion of red blood cells, multiply by 0.01; creatinine to μmol/L, multiply by 88.4; and potassium to μmol/L, multiply by 1.0. No transfusion reactions were reported. The median (IQR) time from ED admission to the start of the WB transfusion was 15 (14-77) minutes, compared with 303 (129-741) minutes (P < .001) for administration of at least 1 unit of RBCs, plasma, and platelets in the historical cohort (Figure).
Table. Demographic and Injury Characteristics of Children Receiving Whole-Blood Transfusiona.
| Variable | All Patients (N = 18) |
|---|---|
| Patient and injury | |
| Age, y, median (IQR) | 11 (5-14) |
| Sex, No. (%) | |
| Female | 7 (39) |
| Male | 11 (61) |
| Race, No. (%) | |
| White | 15 (83) |
| Black | 3 (17) |
| Mechanism of injury, % | |
| Blunt | 14 (78) |
| Penetrating | 2 (11) |
| Abusive | 2 (11) |
| Admission GCS7 | 3 (3-15) |
| Injury severity score8 | 34 (26-38) |
| Outcome | |
| Mortality, No. (%) | 8 (44) |
| Hospital length of stay, d, median (IQR) | |
| All patients | 7.5 (3-13) |
| Survivors | 13 (9-14) |
| ICU Length of stay, d, median (IQR) | 3.5 (2-6) |
| Time receiving mechanical ventilation, d, median (IQR) | 2 (1-5) |
Abbreviations: GCS, Glasgow coma scale; ICU, intensive care unit; IQR, interquartile range.
Use of whole blood is restricted to patients aged ≥3 years and weighing ≥15 kg.
Figure. Time From Emergency Department Admission to Receipt of Transfusion in Civilian Pediatric Trauma Patients.
Time from emergency department admission until whole blood administration in whole blood cohort or receipt of red blood cells, plasma, and platelets in the component cohort.
Discussion
To our knowledge, this is the first cohort of pediatric civilian trauma patients to receive WB during resuscitation. These preliminary data suggest that WB transfusion of up to 20 mL/kg is safe in children with severe injuries; there was no evidence of hemolysis in non–group O recipients, and no transfusion reactions were reported. Based on these data, the transfusion committee of Children’s Hospital of Pittsburgh of the University of Pittsburgh Medical Center approved an increase in maximum volume of WB transfusion to 30 mL/kg. Larger cohorts are necessary for further study to determine if WB administration will affect outcomes, including number of donor exposures, cost, total volume transfused, and mortality.
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6)(suppl):S3-S11. [DOI] [PubMed] [Google Scholar]
- 2.Dehmer JJ, Adamson WT. Massive transfusion and blood product use in the pediatric trauma patient. Semin Pediatr Surg. 2010;19(4):286-291. [DOI] [PubMed] [Google Scholar]
- 3.Hendrickson JE, Shaz BH, Pereira G, et al. Implementation of a pediatric trauma massive transfusion protocol: one institution’s experience. Transfusion. 2012;52(6):1228-1236. [DOI] [PubMed] [Google Scholar]
- 4.Chidester SJ, Williams N, Wang W, Groner JI. A pediatric massive transfusion protocol. J Trauma Acute Care Surg. 2012;73(5):1273-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leeper CM, Kutcher M, Nasr I, et al. Acute traumatic coagulopathy in a critically injured pediatric population: definition, trend over time, and outcomes. J Trauma Acute Care Surg. 2016;81(1):34-41. [DOI] [PubMed] [Google Scholar]
- 6.Yazer MH, Jackson B, Sperry JL, Alarcon L, Triulzi DJ, Murdock AD. Initial safety and feasibility of cold-stored uncrossmatched whole blood transfusion in civilian trauma patients. J Trauma Acute Care Surg. 2016;81(1):21-26. [DOI] [PubMed] [Google Scholar]
- 7.Baker SP, O’Neill B, Haddon W Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187-196. [PubMed] [Google Scholar]
- 8.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81-84. [DOI] [PubMed] [Google Scholar]