Abstract
Background
Orbital decompression is an important surgical procedure for treatment of Graves’ ophthalmopathy (GO), especially in women. It is reasonable for balanced orbital decompression of the lateral and medial wall. Various surgical approaches, including endoscopic transnasal surgery for medial wall and eye-side skin incision surgery for lateral wall, are being used nowadays, but many of them lack the validity, safety, or cosmetic effect.
Patients and methods
Endoscopic orbital decompression of lateral wall through hairline approach and decompression of medial wall via endoscopic transnasal surgery was done to achieve a balanced orbital decompression, aiming to improve the appearance of proptosis and create conditions for possible strabismus and eyelid surgery afterward. From January 29, 2016 to February 14, 2017, this surgery was performed on 41 orbits in 38 patients with GO, all of which were at inactive stage of disease. Just before surgery and at least 3 months after surgery, Hertel’s ophthalmostatometer and computed tomography (CT) were used to check proptosis and questionnaires of GO quality of life (QOL) were completed.
Findings
The postoperative retroversion of eyeball was 4.18±1.11 mm (Hertel’s ophthalmostatometer) and 4.17±1.14 mm (CT method). The patients’ QOL was significantly improved, especially the change in appearance without facial scar. The only postoperative complication was local soft tissue depression at temporal region. Obvious depression occurred in four cases (9.76%), which can be repaired by autologous fat filling.
Interpretation
This surgery is effective, safe, and cosmetic. Effective balanced orbital decompression can be achieved by using this original and innovative surgery method. The whole manipulation is safe and controllable under endoscope. The postoperative scar of endoscopic surgery through hairline approach is covered by hair and the anatomic structure of anterior orbit is not impacted.
Keywords: orbital decompression, Graves’ ophthalmopathy, hairline approach, endoscopic surgery, lateral wall
Introduction
Graves’ ophthalmopathy (GO) is an organ-specific autoimmune disease. The most common orbital disease in adults has two stages: active and inactive stages.1 For patients in the active stage, it is not effective for sight-threatening and the glucocorticoid shock therapy, and orbital decompression has to be performed to preserve the visual function.2 For patients in the inactive stage with an appearance of proptosis, the quality of life (QOL) can be improved only by surgical treatment.3 Orbital decompression has become the premise and foundation of strabismus and eyelid surgery afterward.
Balanced orbital decompression involves lateral and medial wall decompressions simultaneously and is thought to reduce the risk of postoperative diplopia by “balancing” the amount of decompression on both sides of the orbit, thus reducing horizontal globe shifts.4–7 The most commonly used method for the decompression of the medial wall is endoscopic transnasal surgery, which is effective, safe, and cosmetic.8,9 For lateral wall, the methods include lateral incision, lateral canthotomy incision, eyelid crease incision with or without extension, fornical conjunctiva incision, and scalp coronal incision.10–15 However, there are still limitations with lateral wall surgery. The incidence of GO is higher in women, who need to pay more attention to the appearance;16 however, the incision of eye-side skin causes visible scars after surgery, leading to women being unwilling to accept it. Although there is no visible scar after operation through approach of fornical conjunctiva incision, the normal anatomic structure of the orbit is influenced. In addition, previous studies reported the endoscopic orbital surgery through skin of lid, but adipose in the orbit impeded the reveal and operation space.17,18 Due to the urgent need to improve the QOL, an effective, safe, and cosmetic surgery method of balanced orbital decompression is necessary.
So far, orbital decompression of lateral bone wall under direct vision out of orbit with endoscope has not been reported. This study is designed to explore and verify an endoscopic orbital decompression of lateral wall through hairline approach for the first time, observing the alleviation of proptosis and the improvement of QOL.
Patients and methods
Study design and patients
Based on practice using computer 3D design software and simulant operation of corpse head, this innovative endoscopic balanced orbital decompression of lateral wall through hairline approach and medial wall through transnasal approach was designed to achieve the effect of no visible scar after surgery and to avoid the destruction of normal structure in orbit, aiming to alleviate the proptosis and retraction of the eyelid. Intraoperative and postoperative complications were to be found and treated. All aspects of this research were approved by the Ethics Committee of The Second Xiangya Hospital, Central South University. All patients provided written informed consent and the patient agreed to the use of the photos in this paper.
A total of 38 patients were included from January 29, 2016 to February 14, 2017. Patients’ inclusion criteria were age not less than 20 years; thyroid function returning to normal; proptosis lasting for >2 years and affecting the appearance, maintaining stable condition for >6 months; clinical activity score not being >3 points (7-points method).
The patients at the grade of visual impairment, including compressive optic neuropathy and exposed corneal ulcer, were not chosen and morbidity of this grade was only about 5% in GO epidemiologically.19–23
At least 3 months after the operation, the examinations, including visual acuity (VA), intraocular pressure (IOP), retraction of upper eyelid (RUE), proptosis, diplopia, and score of quality of life (sQOL), were reviewed.
Surgical technique
Decompression of lateral wall
The procedures are as follows (shown in Figure 1):
Position: set patient in lateral position (shown in Figure 1-2a).
“Three point” incisions (Figure 1-1A, B, 1-2b): Make the first cut along the superficial temporal artery in front of crus of Helix and then free and ligate the superficial temporal artery (pay attention to protection of the concomitant vein and the auriculotemporal nerve). Make the second cut at 5 cm above the peak of helix and the third cut at 4 cm in front of the second cut. All these cuts locate in the posterior area of the hairline and each cut is no longer than 1 cm.
Surgical field building (Figure 1-1C, 1-2c): The puncture needle enters from the second cut along the loose subcutaneous tissue and the needle tip arrives at the subcutaneous area of temporal fossa. About 15 mL expanding liquid (epinephrine saline) is injected to the temporal fossa, and dilator is used to expand the space below to the superficial fascia. Ten millimeter trocar is placed into this area. The endoscope enters the area through the trocar. Under the endoscopic vision we place 5 or 10 mm trocar through the first cut and 10 mm trocar through the third cut.
The lateral orbit wall exposure: At the beginning, ultrasonic scalpel is used to cut proper volume of temporalis away behind the zygomatic process, and thus the lateral orbit wall is exposed (Figure 1–1D, –2d, 1–3e, f). This area has four boundaries: the frontier is the external orbit rim, the superior is parallel to the geisoma, the inferior is parallel to the zygomatic arch, and the posterior is near the zygomatic crest of greater wing of the sphenoid bone. Later, we just cut through (not cut away) the temporalis attached to the temporal fossa to avoid soft tissue depression after the operation.
Windowing: The electric grinding head enters the surgical field through the trocar from the third cut, and the head is used to wear through the exposed lateral orbit wall. In this window, the orbital fat can be revealed sufficiently (Figure 1–1E, 1–3g).
Drainage: Check the incision and find no active bleeding. Put the rubber membrane for draining at the orbit window. Then close each cut (Figure 1–1F, 1–3h).
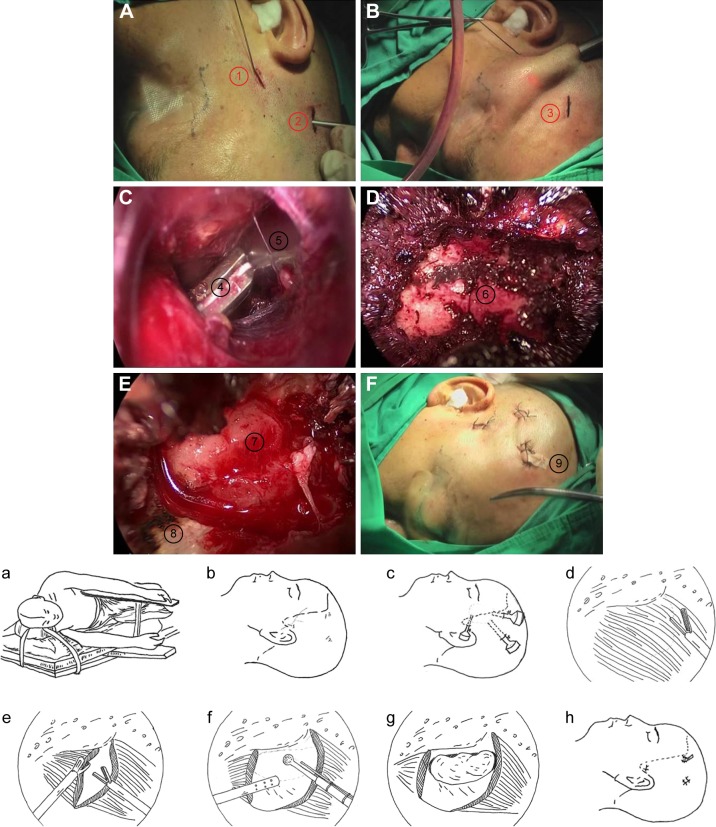
Figure 1.
Decompression of lateral wall.
Notes: (A, B) “Three-point” incisions. ①=First cut; ②=second cut; ③=third cut. (a, b) Diagrammatic sketch of the “three-point” incisions. (C) The surgical field building. In the endoscopic vision through the second cut, ultrasonic scalpel and other auxiliary instruments were placed through the first cut and the third cut; ④,⑤=Surgical instruments. (c) Diagrammatic sketch of the surgical field building. (D) The lateral orbit wall exposure. Ultrasonic scalpel was used to cut about 3–4 cm2 temporalis away behind the zygomatic process. The temporalis was moved away to expose the lateral orbit wall. The temporalis attached to the temporal fossa was cut through to avoid soft tissue depression after operation. ⑥=Bone of the lateral orbit wall in the fossae termporalis. (d, e, f) Diagrammatic sketch of the lateral orbit wall exposure. (E) Windowing of lateral wall of bone and fat. The electric grinding head was used to wear through the exposed lateral orbit bone wall and the orbital fat protruded sufficiently. ⑦=Orbital fat; ⑧=lateral orbit bone wall. (g) Diagrammatic sketch of the windowing of lateral wall of bone and fat. (F) Drainaging. Check the wound and find no active bleeding. Put the rubber membrane for drainaging at the orbit window and then close each cut. ⑨=Drainage strip. (h) Diagrammatic sketch of drainaging.
Decompression of medial wall
The procedures are as follows:
The uncinate process is incised and excised.
Ethmoid sinus is opened fully and lamina papyracea is exposed synchronously.
Apertures of sphenoid sinus and maxillary sinus are opened adequately to prevent inflammation of the sinus cavity.
Lamina papyracea is excised as far as possible.
Periosteum and orbital fascia are cut open to bring about bulge of orbital fat.
Exophthalmometry
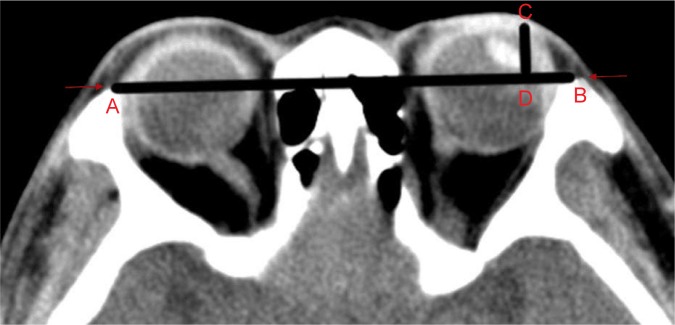
We first assessed the degree of proptosis using Hertel’s exophthalmometry. To minimize bias, we also employed the computed tomography (CT) examination to assess the degree of proptosis. CT examination was performed axially with 2 mm thickness, parallel to the infraorbitomeatalline with a 128-Slice Multi-Detector CT Scanner (Definition AS; Siemens Health care, Forchheim, Germany). All scanning were performed at a constant window level and width settings of 50 and 250 HU, respectively. Patient was asked to maintain forward gaze and gentle eye closure during scanning process to avoid asymmetric extraocular muscle contraction.23 The degree of exophthalmus was measured at the midglobe section on axial scans. The interzygomatic line (a line drawn at the anterior portions of the zygomatic arches) at the midglobe section was used as a reference line. The degree of exophthalmus is given as the perpendicular distance between the interzygomatic line and the anterior margin of the globe (Figure 2).
Figure 2.
CT method used to assess the degree of proptosis.
Notes: “A” point to “B” point shows the interzygomatic line. “C” point to “D” point is the distance of protrusion. The arrows present the anterior margins of lateral bony orbital rim.
Abbreviation: CT, computed tomography.
Assessment of QOL
Information about the QOL were collected through two questionnaires of GO-QOL24,25 that measure visual function and appearance, each of which was measured by eight three-category questions and was administered preoperatively and at least 3 months postoperatively. Score = (original score − minimum score)/(maximum score − minimum score) × 100. The maximum score is 24, the minimum score is 8, and the original score is the actual score of the patient.
Statistical analysis
Statistical analysis was performed using paired-samples independent t-test to evaluate preoperative and postoperative data such as protrusion of eyeball, sQOL, VA, and IOP. For data analysis the statistical program SPSS 14.0 was used. Independent-sample t-test was used for normally distributed data sets. Chi-square test was used for nonparametric statistical analysis. Significance was assumed if P<0.05.
Results
Total 41 endoscopic balanced orbital decompressions were performed on 38 patients with GO (Table 1), and the effect of balanced orbital decompression was achieved because the bone of lateral and medial wall was abraded moderately (Figure 3). There were 18 men and 20 women and a mean age of 41.95±12.65 (range 20–71) years. Three patients underwent bilateral procedure. Because most complications and operative effects became apparent within the first few months after surgery, we chose to evaluate them and operate on the other orbit on a minimum of 3 months after the surgery.26 Data of patients and comparison of preoperative and postoperative results were listed in Table 1.
Table 1.
The clinical data of surgery patients
| No of patients | Age (years) |
Sex | Side | Exophthalmometry (mm) (Hertel) |
Visual acuity
|
IOP (mmHg) |
sQOL-appearance
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||||
| 1 | 20 | F | L | 19.50 | 17.00 | 0.6 | 0.6 | 21 | 19 | 37.50 | 43.75 |
| 2 | 34 | F | L | 23.50 | 20.00 | 0.8 | 0.8 | 22 | 22 | 62.50 | 75.00 |
| 3 | 53 | M | R | 20.50 | 16.00 | 0.4 | 0.6 | 24 | 15 | 50.00 | 68.75 |
| 4 | 71 | F | L | 20.00 | 16.50 | 0.8 | 0.8 | 20 | 16 | 37.50 | 62.50 |
| 5 | 23 | M | R | 24.00 | 20.00 | 1.0 | 1.0 | 17 | 14 | 50.00 | 68.75 |
| 6 | 52 | F | L | 20.50 | 16.00 | 1.0 | 1.0 | 20 | 17 | 43.75 | 75.00 |
| 7 | 51 | M | R | 19.00 | 17.00 | 0.25 | 0.3 | 20 | 21 | 50.00 | 68.75 |
| 8 | 51 | M | L | 22.00 | 17.00 | 0.5 | 0.6 | 23 | 21 | 25.00 | 31.25 |
| 9 | 51 | M | L | 18.00 | 16.00 | 0.6 | 0.6 | 18 | 18 | 50.00 | 56.25 |
| 10 | 44 | M | L | 26.00 | 22.50 | 0.1 | 0.1 | 26 | 18 | 50.00 | 62.50 |
| 11 | 49 | F | R | 16.50 | 13.00 | 0.8 | 0.8 | 21 | 22 | 62.50 | 62.50 |
| 12 | 42 | M | L | 18.00 | 13.00 | 1.0 | 1.0 | 20 | 18 | 31.25 | 43.75 |
| 13 | 28 | M | L | 21.50 | 17.00 | 0.6 | 0.7 | 20 | 18 | 37.50 | 68.75 |
| 14 | 40 | F | L | 21.00 | 17.00 | 1.0 | 1.0 | 18 | 16 | 68.75 | 87.50 |
| 15 | 20 | F | R | 18.50 | 15.50 | 0.25 | 0.5 | 24 | 16 | 43.75 | 62.50 |
| 16 | 43 | M | L | 23.00 | 18.50 | 1.0 | 1.0 | 18 | 19 | 50.00 | 75.00 |
| 17 | 61 | F | R | 18.00 | 14.50 | CF/BE | 0.1 | 25 | 17 | 56.25 | 81.25 |
| 18 | 30 | M | R | 17.50 | 14.00 | 1.0 | 1.0 | 18 | 18 | 50.00 | 75.00 |
| 19 | 61 | F | L | 25.00 | 19.00 | 0.6 | 0.8 | 24 | 18 | 56.25 | 75.00 |
| 20 | 51 | M | R | 22.00 | 17.00 | 0.3 | 0.5 | 15 | 16 | 31.25 | 62.50 |
| 21 | 47 | F | L | 19.50 | 15.50 | 0.8 | 0.8 | 14 | 13 | 56.25 | 68.75 |
| 22 | 58 | F | L | 22.00 | 18.00 | HM/20 cm | 0.3 | 26 | 21 | 43.75 | 75.00 |
| 23 | 33 | M | R | 17.00 | 13.50 | 1.0 | 1.0 | 19 | 20 | 68.75 | 87.50 |
| 24 | 53 | F | L | 22.50 | 18.00 | 1.0 | 1.0 | 19 | 18 | 50.00 | 81.25 |
| 25 | 35 | M | R | 22.00 | 17.00 | 1.0 | 1.0 | 21 | 19 | 37.50 | 68.75 |
| 26 | 50 | M | L | 27.00 | 20.00 | 1.0 | 1.0 | 23 | 21 | 31.25 | 50.00 |
| 27 | 28 | M | L | 22.50 | 18.00 | 0.2 | 0.4 | 18 | 18 | 37.50 | 62.50 |
| 28 | 37 | F | R | 23.50 | 18.50 | 0.1 | 0.1 | 16 | 17 | 43.75 | 62.50 |
| 29 | 44 | M | R | 26.00 | 22.00 | 0.1 | 0.1 | 18 | 17 | 37.50 | 75.00 |
| 30 | 37 | F | R | 25.50 | 18.00 | 0.5 | 0.6 | 20 | 19 | 18.75 | 62.50 |
| 31 | 58 | M | L | 15.00 | 10.50 | 0.5 | 0.5 | 21 | 17 | 62.50 | 87.50 |
| 32 | 50 | F | L | 15.50 | 12.00 | 0.6 | 0.8 | 22 | 14 | 62.50 | 81.25 |
| 33 | 37 | F | L | 21.00 | 17.00 | 0.8 | 0.8 | 19 | 17 | 50.00 | 75.00 |
| 34 | 24 | F | L | 21.50 | 17.50 | HM/BE | 0.1 | 17 | 14 | 6.25 | 50.00 |
| 35 | 30 | M | R | 18.00 | 14.50 | 0.6 | 0.6 | 18 | 18 | 50.00 | 75.00 |
| 36 | 30 | F | L | 19.00 | 14.00 | 0.8 | 0.8 | 15 | 15 | 56.25 | 93.75 |
| 37 | 26 | F | L | 20.00 | 17.00 | 1.0 | 1.0 | 18 | 17 | 56.25 | 68.75 |
| 38 | 52 | F | L | 19.50 | 14.50 | 0.25 | 0.3 | 14 | 14 | 43.75 | 87.50 |
| 39 | 49 | F | L | 19.00 | 14.00 | 0.8 | 0.8 | 21 | 18 | 50.00 | 68.75 |
| 40 | 25 | F | R | 18.00 | 13.50 | 0.5 | 0.5 | 17 | 17 | 50.00 | 75.00 |
| 41 | 42 | F | R | 19.00 | 16.00 | HM/BE | HM/BE | 20 | 15 | 37.50 | 87.50 |
Abbreviations: BE, blind eye; CF, counting fingers; F, female; HM, hand movement; IOP, intraocular pressure; L, left; M, male; R, right; sQOL, score of quality of life.
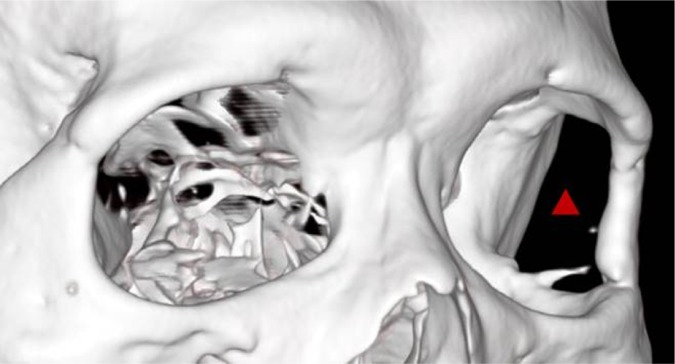
Figure 3.
Postoperative stereogram of bone.
Note: The red triangle represents decompressed lateral bone wall.
The surgery was effective and precise under endoscopy: the reduction of proptosis and RUE was successfully achieved (Table 1). The VA was slightly improved postoperatively, especially in patients 15, 17, 22, and 34, but there was no statistical difference compared with preoperative data. There was no significant difference in IOP before and after operation. The RUE was slightly improved from 0 to 3 mm postoperatively. Increased strabismus with aggravation of diplopia after surgery was not developed in each patient (Table 1). Limitation of eyelid closure and disorder of occlusal movement were not supervened postoperatively.
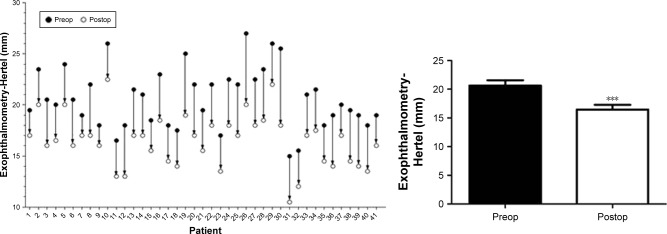
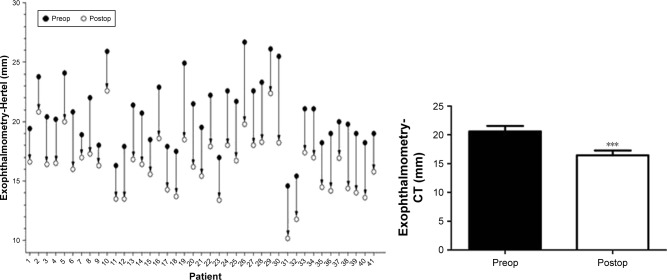
Obviously, the surgery also had the following advantages: no damage of orbital structures (eg, Whitnall tubercle), and no exposed surgical scars (Figure 4). The postoperative proptosis was significantly reduced an average of 4.18±1.11 mm (range 2.0–7.5 mm) with a follow-up of at least 3 months to each patient with Hertel’s exophthalmometry (Figure 5), compared to the preoperative proptosis (P<0.0001). The postoperative mean proptosis was also significantly reduced compared to the preoperative mean proptosis by CT-method exophthalmometry with an average reduction of 4.17±1.14 mm (range 1.70–7.30 mm; Figure 6, P<0.0001).
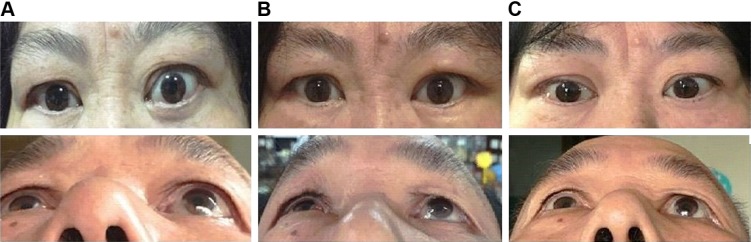
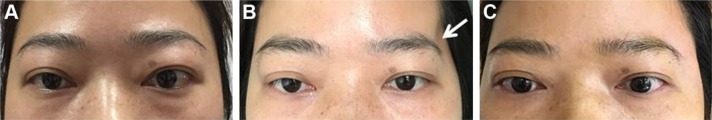
Figure 4.
Postoperative appearance of patients.
Notes: (A) Appearance of preoperative; (B) appearance on first day after surgery; (C) appearance at 3 months after surgery. Left orbits of them were under operation. The above was anterior view, and below was head up 45° view.
Figure 5.
Reduction of proptosis obtained in 41 operations.
Notes: Proptosis was measured with Hertel’s exophthalmometry. ***P<0.0001.
Figure 6.
Reduction of proptosis obtained in 41 operations.
Notes: Proptosis was measured with CT method. ***P<0.0001.
Abbreviation: CT, computed tomography.
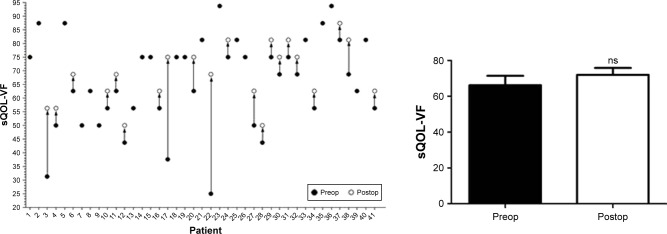
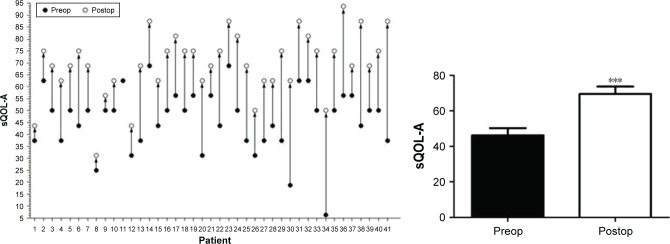
This new type of surgery successfully improved the appearance of patients. Furthermore, the enhancement of the QOL was also evaluated. As reflected by questionnaires of GO-QOL (visual function questionnaire and appearance questionnaire), the postoperative visual function was only slightly improved (Figure 7), while the postoperative appearance was obviously improved (Figure 8, P<0.0001).
Figure 7.
sQOL of visual function questionnaire.
Note: There is no significant difference.
Abbreviations: sQOL -VF, score of quality of life of visual function; ns, not significant.
Figure 8.
sQOL of appearance questionnaire.
Note: ***P<0.0001.
Abbreviation: sQOL-A, score of quality of life of appearance.
There were some intraoperative complications observed, which included bleeding, nerve injury, and depression of soft tissue at regions temporalis. After improved surgical operation, these complications could avoid and handle well. The postoperative complication included surgical reactivity edema of orbital tissue mid miopragia of temporal muscle and soft tissue depression in the temporal region. For orbital decompression of lateral wall, the only complication was depression of soft tissue at regions temporalis, just as that glabellar dimpling or depression was the postoperative complication of endoscopic forehead rejuvenation.27 Four cases (9.75%) suffered this complication with overt depression of soft tissue at regions temporalis within 3 months after surgery and were effectively rectified by autologous fat filling (Figure 9). This complication existed only at the early stage of development of this innovative surgery, due to too much resection of temporalis to expose the temporal fossa. After improvement (eg, cutting through instead of cutting away the temporalis attached to the temporal fossa), no overt depression of soft tissue arose anymore.
Figure 9.
Overt depression of soft tissue at regions temporalis was rectified with autologous fat filling.
Notes: (A) The appearance at preoperation. (B) Overt depression of soft tissue at regions temporalis at 2 months after surgery (arrow); (C) repaired soft tissue depression.
Soft tissue hemorrhage or bone bleeding was the only difficult point during the surgery of decompression of lateral wall. To avoid the hemorrhage, the following procedures were conducted: ultrasonic scalpel was used to stop the emergent hemorrhage of soft tissue and a mixture of bone wax and yarn was used to stop the bone bleeding through oppressing.
Discussion
In the present design, we pioneered an endoscopic surgery with a hairline-through path to operate on the temporal fossa. Innovative endoscopic balanced orbital decompression of lateral wall through hairline approach and medial wall through transnasal approach was feasible, effective, safe, and cosmetic. Lateral orbital wall was reached and exposed at fossae temporalis and was abraded effectively and safely under endoscope. Combined with endoscopic transnasal surgery, effective balanced orbital decompression and cosmetic results could be achieved. Furthermore, normal structures of orbit including conjunctiva, lateral central angle, and lateral orbital tubercle (Whitnall tubercle) were not destroyed. The main purpose of the surgery, retroversion of eyeball with improvement of appearance, could be achieved by this innovative balanced orbital decompression. The effect of two wall decompressions could also be achieved from a quantitative point of view.14,28 In consideration of individual difference of measurement with Hertel’s exophthalmometry, more objective measurement of exophthalmos with CT was applied in this study.
Balanced orbital decompression, involving medial and lateral wall decompressions simultaneously, is the optimal choice to remedy the exophthalmos of GO. The medial wall of the orbit can be decompressed through the nasal cavity under endoscopy with a valid, safe, and cosmetic effect. However, the deep operation with eye-side incision under indirect vision commonly adopted by traditional surgery of lateral wall can cause visible scar after surgery, as well as direct damage to the orbital structure. We adopted the hairline-through path, which could avoid the facial surgical scar, thus to improve the appearance of patients. Two questionnaires of GO-QOL, visual function questionnaire and appearance questionnaire, were used in this study. The visual function was slightly improved after operation with obvious improvement in individual patients along with an obviously improved appearance of patient. This improvement of appearance achieved the goal of the innovation approach.
The concept of surgical procedures has been completely changed by the use of endoscopy under direct vision, which makes the surgery trauma minimal and incision hidden. This technology also enables the oculist major to perform operations on the bony orbital wall and the orbital structure through the nasal cavity and sinus cavity. Nowadays, trans-nasal endoscopic orbital decompression is frequently used for bony decompression of medial orbital wall and medial part of inferior orbital wall. In the present study, endoscopic surgery caused no external incision and no damage to the internal structure of the orbit, and enabled operation under direct vision.
The postoperative adverse reaction would be slight if the bleeding was stopped thoroughly during operation and the effect of eyeball retroversion could be achieved without exerted surgical trauma. Among the included subjects, a long-term depression of temporal soft tissue arose postoperatively in four patients and simple orthomorphic resection was needed to rectify the depression, most possibly because the muscular temporalis was resected too much to expose the lateral orbital bone wall at fossae temporalis. This complication was reduced and/or avoided later by just cutting through (not cutting away) the temporalis attached to the temporal fossa. The complication could also be corrected by filling of autologous fat.
We observed no statistical difference in IOP before and after operation, probably because all the patients included were at inactive stage with mild or moderate grade. The RUE was slightly decreased from 0 to 3 mm postoperatively, attributed to reduction of exophthalmos, which was a factor of RUE. A significant advantage of balanced orbital decompression was the stability of eyeball position after operation because of the simultaneous decompression of lateral and medial walls. In addition, aggravation of diplopia did not arise in any patient after operation.
Altogether, this innovative, effective surgery is easily accepted by the majority of patients because of safe and operative procedures being performed under direct vision, no breached orbital structures, and no exerted scar post operatively. In addition, the surgery can be performed safely with few complications that can be prevented and/or dispelled easily. It would be a new method of balanced orbital decompression in pace with the popularization. However, this is a new surgical technique and the results obtained are preliminary and, therefore, large case series will be necessary in order to validate the findings.
More importantly, this development was inspired by a safe and feasible endoscopic thyroidectomy via the areola of breast approach, which has been widely used in clinic for several years with a great amount of successful operation leaving no visible scars.29–31 Based on these, we apply this idea to orbital decompression surgery; our innovation also provided clinical and theoretical basis for expanding this type of surgery approach.
Acknowledgments
We thank all the nursing teams for their involvement in patient care. We thank Li-hong Cai at the Third Hospital of Changsha for simulating the operation using computer 3D design software. We thank Yan Cai at the anatomy department of Xiangya Medical College of Central South University for the simulant operation of corpse head at endoscopic animal operation room. We thank Lei Wang at Department of Respiratory Medicine, Xuanwu Hospital, Capital Medical University for revising and proofreading the paper. We thank Li-fang Guo, Hong-yu Li, Yang-ping Liu, Luo-sheng Tang, and Jiang-sheng Huang for their assistance in manuscript preparation. Working team including all the authors has received the award of new medical technology in the Second Xiangya Hospital of Central South University, Changsha, Hunan, China. Wei Xiong has been invited to give lecture about this innovative surgery and has received lecture fee at ophthalmological conference of Chinese Medical Association.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Stan MN, Garrity JA, Bahn RS. The evaluation and treatment of graves ophthalmopathy. Med Clin North Am. 2012;96(2):311–328. doi: 10.1016/j.mcna.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goh MS, McNab AA. Orbital decompression in Graves’ orbitopathy: efficacy and safety. Intern Med J. 2005;35(10):586–591. doi: 10.1111/j.1445-5994.2005.00933.x. [DOI] [PubMed] [Google Scholar]
- 3.Wickwar S, Mcbain H, Ezra DG, Hirani SP, Rose GE, Newman SP. The psychosocial and clinical outcomes of orbital decompression surgery for thyroid eye disease and predictors of change in quality of life. Ophthalmology. 2015;122(12):2568. doi: 10.1016/j.ophtha.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Baril C, Pouliot D, Molgat Y. Optic neuropathy in thyroid eye disease: results of the balanced decompression technique. Can J Ophthalmol. 2014;49(2):162–166. doi: 10.1016/j.jcjo.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Alsuhaibani AH, Carter KD, Policeni B, Nerad JA. Orbital volume and eye position changes after balanced orbital decompression. Ophthal Plast Reconstr Surg. 2011;27(3):158–163. doi: 10.1097/IOP.0b013e3181ef72b3. [DOI] [PubMed] [Google Scholar]
- 6.Yao WC, Sedaghat AR, Yadav P, Fay A, Metson R. Orbital decompression in the endoscopic age: the modified inferomedial orbital strut. Otolaryngol Head Neck Surg. 2016;154(5):963–969. doi: 10.1177/0194599816630722. [DOI] [PubMed] [Google Scholar]
- 7.Boboridis KG, Bunce C. Surgical orbital decompression for thyroid eye disease. Cochrane Database Syst Rev. 2011;(12):CD007630. doi: 10.1002/14651858.CD007630.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Wu W, Selva D, Bian Y, et al. Endoscopic medial orbital fat decompression for proptosis in type 1 graves orbitopathy. Am J Ophthalmol. 2015;159(2):277–284. doi: 10.1016/j.ajo.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Castelnuovo P, Turri-Zanoni M, Battaglia P, Locatelli D, Dallan I. Endoscopic endonasal management of orbital pathologies. Neurosurg Clin N Am. 2015;26(3):463–472. doi: 10.1016/j.nec.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Cansiz H, Yilmaz S, Karaman E, et al. Three-wall orbital decompression superiority to 2-wall orbital decompression in thyroid-associated ophthalmopathy. J Oral Maxillofac Surg. 2006;64(5):763–769. doi: 10.1016/j.joms.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Graham SM, Brown CL, Carter KD, Song A, Nerad JA. Medial and lateral orbital wall surgery for balanced decompression in thyroid eye disease. Laryngoscope. 2003;113(7):1206–1209. doi: 10.1097/00005537-200307000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Pelton RW. The anterior eyelid crease approach to the orbit. Curr Opin Ophthalmol. 2009;20(5):401–405. doi: 10.1097/ICU.0b013e32832ec3f7. [DOI] [PubMed] [Google Scholar]
- 13.Ben Simon GJ, Wang L, Mccann JD, Goldberg RA. Primary-gaze diplopia in patients with thyroid-related orbitopathy undergoing deep lateral orbital decompression with intraconal fat debulking: a retrospective analysis of treatment outcome. Thyroid. 2004;14(5):379. doi: 10.1089/105072504774193221. [DOI] [PubMed] [Google Scholar]
- 14.Kingdom TT, Davies BW, Durairaj VD. Orbital decompression for the management of thyroid eye disease: an analysis of outcomes and complications. Laryngoscope. 2015;125(9):2034–2040. doi: 10.1002/lary.25320. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg RA, Weinberg DA, Shorr N, Wirta D. Maximal, three-wall, orbital decompression through a coronal approach. Ophthalmic Surg Lasers. 1997;28(10):832. [PubMed] [Google Scholar]
- 16.Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves’ ophthalmopathy. Thyroid. 2002;12(10):855–860. doi: 10.1089/105072502761016476. [DOI] [PubMed] [Google Scholar]
- 17.Prabhakaran VC, Selva D. Orbital endoscopic surgery. Indian J Ophthalmol. 2008;56(1):5–8. doi: 10.4103/0301-4738.37587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasperbauer JL, Hinkley L. Endoscopic orbital decompression for Graves’ ophthalmopathy. Am J Rhinol. 2005;19(6):603–606. [PubMed] [Google Scholar]
- 19.Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev. 2000;21(2):168. doi: 10.1210/edrv.21.2.0393. [DOI] [PubMed] [Google Scholar]
- 20.Bartalena L, Tanda ML. Clinical practice. Graves’ ophthalmopathy. N Engl J Med. 2009;360(10):994–1001. doi: 10.1056/NEJMcp0806317. [DOI] [PubMed] [Google Scholar]
- 21.Ponto KA, Binder H, Diana T, et al. Prevalence, phenotype, and psychosocial well-being in euthyroid/hypothyroid thyroid-associated orbitopathy. Thyroid. 2015;25(8):942–948. doi: 10.1089/thy.2015.0031. [DOI] [PubMed] [Google Scholar]
- 22.Tanda ML, Piantanida E, Liparulo L, et al. Prevalence and natural history of Graves’ orbitopathy in a large series of patients with newly diagnosed graves’ hyperthyroidism seen at a single center. J Clin Endocrinol Metab. 2013;98(4):1443–1449. doi: 10.1210/jc.2012-3873. [DOI] [PubMed] [Google Scholar]
- 23.Chen YL, Chang TC, Huang KM, Tzeng SS, Kao SC. Relationship of eye movement to computed tomographic findings in patients with Graves’ ophthalmopathy. Acta Ophthalmol (Copenh) 1994;72(4):472–477. doi: 10.1111/j.1755-3768.1994.tb02800.x. [DOI] [PubMed] [Google Scholar]
- 24.Wiersinga WM. Quality of life in Graves’ ophthalmopathy. Best Pract Res Clin Endocrinol Metab. 2012;26(3):359–370. doi: 10.1016/j.beem.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Terwee CB, Gerding MN, Dekker FW, Prummel MF, Pol JPVD, Wiersinga WM. Test-retest reliability of the GO-QOL: a disease-specific quality of life questionnaire for patients with Graves’ ophthalmopathy. J Clin Epidemiol. 1999;52(9):875–884. doi: 10.1016/s0895-4356(99)00069-4. [DOI] [PubMed] [Google Scholar]
- 26.Orbitopathy EGOG, Mourits MP, Bijl H, et al. Outcome of orbital decompression for disfiguring proptosis in patients with Graves’ orbitopathy using various surgical procedures. Br J Ophthalmol. 2009;93(11):1518. doi: 10.1136/bjo.2008.149302. [DOI] [PubMed] [Google Scholar]
- 27.Baker CDC. Endoscopic forehead rejuvenation: I. limitations, flaws, and rewards: reply. Plast Reconstr Surg. 2007;119(3):1116–1119. doi: 10.1097/01.prs.0000253466.90945.71. [DOI] [PubMed] [Google Scholar]
- 28.Kacker A, Kazim M, Murphy M, Trokel S, Close LG. “Balanced” orbital decompression for severe Graves’ orbitopathy: technique with treatment algorithm. Otolaryngol Head Neck Surg. 2003;128(2):228–235. doi: 10.1067/mhn.2003.61. [DOI] [PubMed] [Google Scholar]
- 29.Wang CC, Hu YZ, Lai ZW, et al. Endoscopic thyroidectomy via the areola of breast approach. Zhonghua Wai Ke Za Zhi. 2009;47(14):1067–1069. [PubMed] [Google Scholar]
- 30.Zheng BX, Shi TX, Deng JW. Clinical application of modified endoscopic thyroidectomy. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;48(10):818–822. [PubMed] [Google Scholar]
- 31.Xia LY, He C, Huang XW, Xi X, Liu XK. The operation experience of endoscopic thyroidectomy by areola and axilla approach. Eur Arch Otorhinolaryngol. 2016;273(3):555–558. doi: 10.1007/s00405-014-3424-5. [DOI] [PubMed] [Google Scholar]