Abstract
Objective
The aim of this study was to examine whether preterm very low birth weight (VLBW) or term born small for gestational age (SGA) adolescents have a reduced regional brain volume. Furthermore, we examined what perinatal factors are related to reduced brain volume in VLBW adolescents, and which regional brain volumes are associated with cognitive and perceptual functioning, and if these differ between the groups. Study design Fifty adolescent preterm VLBW (≤1500g) births and 49 term SGA births (birth weight < 10th percentile) were compared with 57 normal weight term births. An automated MRI segmentation technique was used. Cognitive and perceptual functions were evaluated by WISC-III and Visual Motor Integration (VMI) tests.
Results
The VLBW group had a reduced volume of thalamus and cerebellar white matter (P<0.002). The SGA group had a smaller total brain, and proportionally smaller regional brain volume. Cerebellar white matter in the VLBW, hippocampus in the SGA, and cerebral cortical in the control group were volumes which significantly predicted cognitive and perceptual functions.
Conclusions
We speculate that white matter injury may explain the impaired cognitive and perceptual functioning in the prematurely born, whereas hippocampal injury may be related to cognitive dysfunction in term SGA adolescents.
Keywords: VLBW, SGA, MRI brain volumes, cognitive and perceptual
INTRODUCTION
Being prematurely born and/or small for gestational age (SGA) are recognized risk factors for motor impairment as well as for cognitive and behavioral deficits later in life.1,2 Although it has been difficult to ascertain any specific pattern of brain abnormalities as the underlying cause of individual impairment, investigators have demonstrated that a reduction in regional brain volume near term in the preterm infant is a predictor of later cognitive outcome.3,4 Studies using an MRI scan suggest that abnormal cerebral findings in preterm infants persists into adolescence,5 especially as it pertains to white matter injury.6,7 Another group of infants believed to be susceptible to slight brain dysfunction are those born SGA at term.8 In previous studies, we did not find an increased rate of structural brain abnormalities9 or changes in white matter diffusion10 in the same cohort of SGA adolescents as was included in the current study. Their cognitive and perceptual scores were inferior to those of the control group so we therefore investigated whether their function could be reflected in a difference in regional brain volume.
The first aim of this study was to examine whether preterm VLBW and/or term SGA adolescents had reduced regional brain volumes when compared with the control group. Our hypothesis was that being born preterm affects specific regional brain volume, whereas being born term at a low birth weight results in a smaller overall brain, but with no reduction in specific regional brain volume. We further questioned whether brain development in prematurely born adolescents was related to known perinatal risk factors. Our third aim was to determine if a reduction in brain volume influences the association between brain volume and cognitive and perceptual function. We used an automated MRI segmentation technique specifically designed to classify the brain across many structures.11 The brain volumes we were most interested in were cerebral cortical and white matter, hippocampus, amygdale, thalamus, and cerebellar cortical and white matter.
MATERIAL AND METHODS
Study design
In a follow-up study of 15-year old children, we examined two groups with low birth weight births, i.e. prematurely born infants with very low birth weight (VLBW) and term SGA births which were compared with a group of term normal weight births. The VLBW children were admitted to the neonatal intensive care unit at the University Hospital in Trondheim (the referral hospital) from 1986 to 1988. The SGA and control group children were the second or third birth of mothers living in the Trondheim area. They were enrolled before gestational week 20 in a multicenter study conducted between January 1986 and March 1988 and followed prospectively through pregnancy. Details of the study design and population have been previously published.12
Study population
VLBW was defined as birth weight ≤ 1500 g. Due to imaging artifacts from dental braces, the MRI results of five adolescents were excluded, leaving 50 VLBW adolescents (26 males and 24 females) for the morphometric brain analyses. Cerebral palsy was diagnosed in six of the 50 adolescents, i.e. five cases of diplegia and one of hemiplegia, of whom all but one could walk. There were no major visual or hearing impairments in any of the adolescents who were examined using MRI.
SGA was defined as birth weight < 10th percentile adjusted for gestational age, gender and parity. Fifty SGA adolescents had MRI examinations. Due to imaging artifacts, one subject was excluded, leaving 49 MRI investigations (20 males and 29 females) for the morphometric analysis of the brain. Cerebral palsy (diplegia) was diagnosed in one SGA adolescent who underwent an MRI scan.
In the control group, 65 adolescents had an MRI examination. Due to imaging artifacts, eight MRI scans were excluded, leaving 57 MRI investigations (22 males and 35 females) for the morphometric studies.
Perinatal factors in the VLBW group
The perinatal factors investigated were intrauterine growth and the use of antenatal steroids, days on the ventilator, and days after birth used to regain the actual birth weight. Prior to delivery, 25 mothers in the VLBW group received steroids to enhance fetal lung maturation. To explore the influence of intrauterine growth on brain development, we calculated individual standard deviation scores (Z scores) for birth weight. This score represents the departure from the mean weight for gender, gestational age, and singleton13 or multiple births.14 Twenty-six infants had mechanical ventilation with a mean duration on the ventilator of seven days. The VLBW neonates regained their birth weight after 3 to 39 days. Other perinatal risk factors such as premature rupture of the membrane and intraventricular hemorrhage were not investigated since there were too few infants with these characteristics (e.g. four infants or less).
MRI Imaging
MRI studies were performed using a 1.5 Tesla Siemens Symphony Sonata (Siemens AG, Erlangen, Germany). The imaging for the morphometric analysis was a 3D inversion recovery prepared at a fast low flip angle gradient echo sequence (MP-RAGE) with 128 sagittal partitions, 1.33 mm slice thickness, TR between inversion pulses of 2730 ms, TR / TE / flip angle / TI: 7.1 ms / 3.45 ms / 7°/ 1000 ms, an acquisition matrix of 256×192×128, square FOV of 256 mm, NEX 1, and an acquisition duration of 8.5 minutes.
MRI volumetric analysis
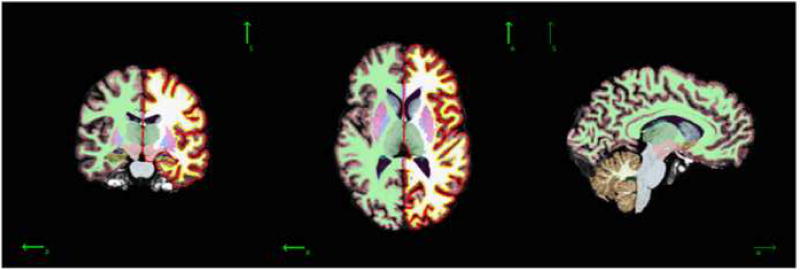
We employed the technique for the automated labeling of human brain structures as developed by Fischl et al.11 The volume for total brain and white matter and a series of gray matter structures were obtained. This technique uses whole brain segmentation and the automated labeling of neuroanatomical structures, with the correction for the partial volume effect performed by estimating the percentage of each voxel occupied by each tissue class that borders it, based on image intensity and class means. The technique is based on a set of manually labeled brains as a training set in order to compute prior probabilities and class statistics. A sample of the segmentation is shown in Figure 1.
Figure 1.
A sample of the automated segmentation of the brain volumes on one of the subjects. Left and middle: segmentation and surface representation left side, right: segmentation.
Clinical tests
Wechsler Intelligence Scales(WISC-III)
Total intelligence quotient (IQ) was estimated using two subtests of the Wechsler Intelligence Scales (WISC-III): Vocabulary and Block design tests.15 To estimate verbal and performance IQ, we administered the Arithmetic and Vocabulary subtests and the Block design and Picture Arrangement subtests, respectively.16
The Developmental Test of Visual-Motor Integration IV (VMI)
The Developmental Test of Visual-Motor Integration comprises 27 geometric designs with an increasing order of difficulty which must be copied, matched (visual perception test; VMIv) and traced (motor coordination test; VMIm), respectively. Scores were given according to the manual17 and raw scores were used.
Statistical analysis
Descriptive analyses included bivariate plots and calculation of percentiles, means and standard deviations. First, we studied the association of brain volume with the independent variables of interest. We employed multivariate linear regression with the following seven volumes as the dependent variables: cerebral cortical volume and white matter volume, hippocampal volume, amygdale volume, thalamus volume, cerebellar cortical volume and white matter volume. The predictor variables were gender, age at scan, and study group (SGA, VLBW, or control group). We also studied the relationship of proportional volume with respect to gender, age and study group. Thus, the proportional volume for each subject was calculated as the volume of a specific area, divided by the total intracranial volume. As shown in the results, analyses that compared study groups were controlled for gender and age at the time of the scan. Second, we sought to identify the strongest perinatal predictors of brain volume among VLBW births. To address this goal, we analyzed the VLBW group separately, employed stepwise linear regression, and controlled for gestational age, gender, age at scan, and intracranial volume. We used p-values of 0.05 and 0.10 to enter or remove, respectively. In these analyses, we considered the amount of days on a ventilator, days used to regain birth weight, use of antenatal steroids, and the Z score of birth weight as potential predictors. Finally, to identify the relationship between cognitive and perceptual test scores and brain regions, we conducted separate analyses within subject groups using stepwise linear regression, and again controlled for gender, age at scan, and intracranial volume. We used the same p-values to enter and remove as in the above analyses. For all regression analyses, diagnostics included the investigation of potential co-linearity, influential points, and normality and residual plots.
Ethics
The regional committee for medical research ethics approved the study protocol and written, informed consent was obtained from both the adolescents and parents.
RESULTS
Group characteristics
Table 1 summarizes the baseline characteristics of our study groups. The VLBW subjects had significantly lower gestational age than the control subjects. Birth weight, Z score of birth weight, and head circumference were significantly reduced in the VLBW and SGA groups. The VLBW adolescents were approximately four to five months younger than the control adolescents when they were scanned.
Table 1.
Group characteristics
| n | VLBW | n | SGA | n | Control | |
|---|---|---|---|---|---|---|
| Gender(boys/girls) | 50 | 26/24 | 49 | 20/29 | 57 | 22/35 |
| Gestational age in weeks | 50 | 29.1(2.7) * | 49 | 39.5 (1.1) | 57 | 39.6 (1.1) |
| Birth weight in grams | 50 | 1205 (233) * | 49 | 2915 (216)* | 57 | 3714 (486) |
| Z score of birth weight | 50 | −0.57 (1.44) * | 49 | −0.42 (0.30)* | 57 | 0.34 (0.96) |
| Head circumference at birth in cm | 49 | 27.1 (2.2) * | 48 | 33.8 (1.2)* | 57 | 35.4 (1.2) |
| MRI age in years | 50 | 15.2 (0.6) * | 49 | 15.6 (0.6) | 57 | 15.5 (0.5) |
Z score = (actual value – mean value)/SD, Mean (SD),
P<0.01 compared to control group
Brain volumes
The brain volume of the three study groups are presented in Table 2 (0nline). The differences in brain structures were explored in relation to the total intracranial volume, with proportions of brain volume still associated with age. As a result, after making the correction for gender and group, the cerebral cortical volume was negatively associated (F=10.31, P<0.01), and the cerebral white matter positively associated (F=14.21, P<0.001) with age at scan. Further, females had relatively larger hippocampus and cerebellum white matter than males (F=15.04, P<0.001, F=12.46, P<0.005, respectively when corrected for age and group). When adjusted for age at scan and gender, the VLBW group had a smaller volume of thalamus and cerebellar white matter in relation to the total volume than did the control group (thalamus: P<0.001, cerebellar white matter: P<0.002). For the SGA group, the average proportional volume for each cerebral region was similar to that found in the control groups, but in general the volumes were smaller.
Brain volumes in the VLBW group and perinatal factors
Additional analyses were performed to determine whether certain perinatal factors predicted definitive brain volume in the VLBW adolescents. To start, we assessed the association between gestational age and brain structure using linear regression after controlling for gender and age at scan. The thalamus volume of the VLBW group was the only part of the brain significantly associated with gestational age (P=0.04). Next, we assessed the potential association between brain volume and the antenatal administration of steroids, the Z score of birth weight, days needed to regain birth weight, and days on a ventilator. We controlled for gestational age, gender and age at scan and found no significant association between antenatal steroids and brain volume. We did find an association between the amount of days on the ventilator and the amount of days used to regain birth weight, i.e. 10 of the 11 individuals who took more than three weeks to regain their birth weight were on a ventilator. As a consequence, the amount of days on the ventilator was not included in subsequent analyses. The Z score of birth weight was positively associated with the volumes of cerebral white matter (P=0.003), thalamus (P=0.005), and cerebellar cortical (P=0.02) and white matter (P=0.002). Moreover, the amount of days needed to regain birth weight was negatively associated with cerebellar white matter volume. This was the difference in cerebellum white matter volume among those who took longer than three weeks to regain birth weight as compared to those who took less than two weeks: (P<0.05) After control for the additional effect of intracranial volume, no significant associations were found between the perinatal variables and regional brain volume.
Cognitive and perceptual results
Cognitive and perceptual scores are reported in Table 3. The IQ scores were significantly lower in the VLBW group than in the control group: total IQ was lower by approximately 11 points, verbal IQ by 11 points, and performance IQ by 20 points. The VMI, motor and visual perception scores were also lower in the VLBW group than in the control group. No significant differences were observed between the SGA and control adolescents.
Table 3.
Cognitive and perceptual scores; Mean (SD) and range.
| VLBW (n=50) |
SGA (n=49) |
control (n=57) |
||||
|---|---|---|---|---|---|---|
| IQ total | 87 (20) | (48–126)* | 95 (15) | (54–126) | 98 (15) | (59–129) |
| IQ verbal | 82 (19) | (42–117)* | 92 (17) | (65–126) | 93 (16) | (68–143) |
| IQ performance | 81 (28) | (32–138)* | 95 (20) | (44–135) | 101 (19) | (52–130) |
| VMI | 20 (4) | (10–26)* | 22 (3) | (14–26) | 23 (3) | (16–27) |
| VMIv | 23 (4) | (13–27)* | 24 (3) | (14–27) | 25 (3) | (14–27) |
| VMIm | 21 (3) | (14–27)* | 24 (2) | (16–27) | 24 (2) | (17–27) |
P< 0.05 vs. controls.
Abbreviations: SD=standard deviation, VMI=Visual-motor integration test, VMIv=visual perception test, VMIm=motor coordination test.
Associations between regional brain volumes and perceptual and cognitive scores
Each group was analyzed separately, with an adjustment for gender, age at scan and total intracranial volume (Table 4). In the VLBW group, cerebellar white matter and hippocampus volumes predicted total and performance IQ. Cerebellar white matter volume predicted VMI scores and VMI visual perception test scores, whereas thalamus volume predicted VMI scores. In the SGA group, hippocampus volume predicted total and verbal IQ scores, whereas cerebral white matter and cerebral cortex volume predicted verbal IQ and VMI motor scores, respectively. In the control group, the cerebral cortical volume predicted all IQ measures. Hippocampus volume predicted both IQ and VMI scores, whereas thalamus volume predicted VMI visual perception scores.
Table 4.
Stepwise regression analyzes. Results for functional outcomes. Controlling for gender, age at scan, and total intracranial volume.
| Outcome VLBW |
Volume (Predictor) |
Variances entered(no) |
Part R2 | F Value | P-value |
|---|---|---|---|---|---|
| IQ | |||||
| Step 1 | Cerebellar WM | 4 | 0.0588 | 5.03 | 0.0301 |
| Step 2 | Cerebellar WM | 7.24 | 0.0102 | ||
| Hippocampus | 5 | 0.0492 | 4.56 | 0.0386 | |
| IQ verbal | NS | ||||
| IQ performance | |||||
| Step 1 | Hippocampus | 4 | 0.0900 | 7.25 | 0.0101 |
| Step 2 | Hippocampus | 11.66 | 0.0101 | ||
| Cerebellar WM | 5 | 0.0906 | 8.59 | 0.0054 | |
| VMI | |||||
| Step 1 | Cerebellar WM | 4 | 0.1435 | 8.62 | 0.0053 |
| Step 2 | Cerebellar WM | 9.26 | 0.0040 | ||
| Thalamus | 5 | 0.0780 | 5.14 | 0.0286 | |
| VMI v | NS | ||||
| Step 1 | Cerebellar WM | 4 | 0.2076 | 13.65 | 0.0006 |
| VMI m | NS | ||||
| SGA | |||||
|
| |||||
| IQ | |||||
| Step 1 | Hippocampus | 4 | 0.1513 | 8.27 | 0.0062 |
| IQ verbal | |||||
| Step 1 | Cerebral WM | 4 | 0.1494 | 8.13 | 0.0066 |
| Step 2 | Cerebral WM | 7.92 | 0.0073 | ||
| Hippocampus | 5 | 0.1066 | 6.53 | 0.0142 | |
| Cerebral WM | 7.92 | 0.0073 | |||
| IQ performance | NS | ||||
| VMI | NS | ||||
| VMI v | NS | ||||
| VMI m | |||||
| Step 1 | Cerebral Cortex | 4 | 0.0637 | 4.24 | 0.0455 |
| Control | |||||
|
| |||||
| IQ | |||||
| Step 1 | Cerebral Cortex | 4 | 0.0841 | 5.32 | 0.0251 |
| Step 2 | Cerebral cortex | 0.0744 | 9.58 | 0.0032 | |
| Hippocampus | 5 | 5.08 | 0.0286 | ||
| IQ verbal | |||||
| Step 1 | Cerebral Cortex | 4 | 0.1147 | 7.39 | 0.0089 |
| IQ performance | |||||
| Step 1 | Cerebral Cortex | 4 | 0.1099 | 7.30 | 0.00 |
| VMI | |||||
| Step 1 | Hippocampus | 4 | 0.0765 | 4.53 | 4.53 |
| VMI v | NS | ||||
| VMI m | NS | ||||
| Step 1 | Thalamus | 4 | 0.0764 | 4.49 | 0.0390 |
VMI; The Developmental Test of Visual-Motor Integration, VMIv; visual perception test, VMIm;motor coordination test
DISCUSSION
We found that low birth weight was associated with reduced brain volume in adolescents. Whereas prematurity was associated with a reduction in specific brain regions, term low birth weight was associated with a lower scaling of the brain. The age at scan was an important determining factor in regional brain volume, and our findings, which showed a decrease in cortical gray matter and an increase in white matter volume by age, are consistent with other reports.18 The most common brain abnormality in premature infants is white matter injury, although this does not occur in isolation. Thus, in preterm infants a reduction in gray matter volume is reported in cortical gray matter19 thalami and basal ganglia20 as early as at term equivalent age. In these studies, the major predictors of volume reductions were white matter injury and gestational age at birth. Previous qualitative MRI studies of brain development of six-year old children in a subgroup of our adolescents indicated that a large proportion had periventricular leucomalacia.21 Furthermore, the VLBW group in the current study had lower fractional anisotropy values on diffusion tensor imaging in widespread white matter tracts, possibly as a result of disorganization of fibers or axonal loss.7 White matter injury impairs not only cerebral cortical development but also development of the remote cerebellum. Trophic interaction between the cerebrum and cerebellum is indicated by cerebellar growth failure with supratentorial white matter lesions.22, 23 While our adolescents had reduced thalamus and cerebellar white matter volume, they also tended to have reduced cerebral white matter volume. In preterm infants with white matter injury, neuropathological analyses reported the highest incidence of neuronal loss in the thalamus and, to a lesser extent, in the other gray matter regions.24 Similar to the findings of preterm infants at term age,20 we found a reduction in thalamus volume, and the lower the gestational age, the larger the reduction in volume. This volume reduction may be a consequence of an impaired interaction between the cerebrum and cerebellum, but may also represent a primary thalamic injury. Neuronal loss in the thalamus and negative retrograde effects may therefore have contributed to impaired cerebellar growth. The question of why the more common neuronal loss in the thalamus is related to the degree of immaturity and whether it is a primary or secondary injury is unclear. Our study suggests that the various volume reductions in premature infants as demonstrated at term equivalent age by others20 persist into adolescence. However, since we have no neonatal imaging data, we were unable to identify the effect of primary destructive events and/or maturational disturbances on brain volume.
Several potentially adverse factors can influence brain development, including exogenous and endogenous insults such as ischemia, inflammation exitotoxcicity, free-radical attacks, and under nutrition. After control for the effect of intracranial volume, the association between growth ( Z score of birth weight and days needed to regain birth weight) and regional brain volume in the preterm was no longer of significance to our study. It is possible however that such an influence would have been detected with larger numbers, and others have demonstrated the significant effect of nutrition on brain development.25 In the growth-restricted preterm infant, a decrease in brain volume and cerebral cortical26 and hippocampus volume was reported.27 We found an association between the amount of days on a ventilator and the amount days used to regain birth weight. The negative impact of poor weight gain on brain volume may be a marker of neonatal disease, as a reduction in brain volume has also been reported with prolonged oxygen requirements.28
Cognitive development is associated with the severity of white matter injury, which is the major mediator in altered cerebellar development in preterm infants.29 A reduction in cerebellar white matter volume predicted impaired cognitive and perceptual functioning in our prematurely born infants. However, injury may damage both the cerebral and cerebellar white matter, and both regions may be co-markers of the insult. Therefore, studies to investigate the association between cerebellar white matter volume and cognitive outcome, with control for white matter injury in VLBW adolescents, is needed. Hippocampal volume was not reduced in the VLBW group. A previous report showed that only in premature infants who were exposed to white matter injury, to postnatal steroids and to indomethacin treatment, was the hippocampal volume reduced at term equivalent age.4 This same study further reported a positive correlation between hippocampal volume and outcome for two-year old children. We also found an association between hippocampal volume and functional outcome, but no reduction in hippocampal volume. We were unable to identify any adverse factors which were an influence on hippocampus volume. Again, it is possible that such an influence may have been detected in a larger study.
We found no reduction in hippocampus volume in the term SGA group. Animal studies have demonstrated that prenatal stress and fetal growth restriction result in fewer neurons in the hippocampus and cerebellum, and30 higher cortisol concentrations have been reported in growth restricted infants.31 The vulnerability of the hippocampus may be more pronounced in early gestation, as premature intrauterine growth restricted infants who had a reduced hippocampus volume at term equivalent age, which in turn was associated with behavioral differences.27 As a possible effect of growth restriction, our study has pointed out the importance of the hippocampus in predicting the cognitive outcome for term born adolescents.
According to previous studies, which reported that variations in intelligence are primarily due to differences in gray matter volume,32 the volume of gray matter, particularly cerebral cortical, and to a lesser degree thalamus and hippocampus volume, was associated with cognitive outcome in our control group. There are certainly a number of brain characteristics other than volume that influence cognition and perception. Injury may damage both structure and functional processes, but not necessarily in association with volume. Therefore, the associations we have reported between volume and functional outcome may only be co-markers of cerebral injury, although it was beyond the aim of our study to investigate whether significant perinatal factors could mediate the association between outcome and volumes.
This study was limited by the paucity of information pertaining to regional brain volumes in the VLBW group. Our subjects did not undergo MRI examinations during their newborn period, and as a result the influence of white matter injury on regional brain structures could not be reported. We are also aware that SGA is a surrogate concept of intrauterine growth restriction. However, intrauterine growth is often inferred from size at birth, taking gestation into account. In our study, newborns with low birth weight that were not growth restricted may have possibly been included, thus diluting any significant differences between the SGA and the control group.
We are aware that the correlation between brain regions and functions is not usually strong, and the differences in volume, although statistically significant, are still small. The rationale for not using multivariate analyses, but instead to analyze each IQ volume separately was that there was already an á priori interest in some of these associations. We therefore performed separate stepwise analyses which were adjusted for gender, age at scan, and intracranial volume, and found it inappropriate to adjust for multiple comparisons.
In summary, our study has demonstrated that premature birth has a deleterious effect on total brain size and on the increase of specific brain volumes, such as the thalamic and cerebellar white matter. The degree of immaturity seems to influence structural brain development in the prematurely born. No specific regional volume reduction was seen in the SGA term adolescents, although their total brain volume was smaller than in controls. We speculate that white matter injury may explain the impaired cognitive and perceptual functioning in the prematurely born, whereas hippocampal injury may be related to cognitive dysfunction in term SGA adolescents.
Supplementary Material
Acknowledgments
The study was supported by the Department of Laboratory Medicine, Children’s and Women’s Health, Faculty of Medicine, Norwegian University of Science and Technology, and by St Olavs University Hospital, Trondheim, Norway. The study was also supported by the US National Institutes of Health (NICHD contract No. 1-HD-4-2803 and No. 1-HD-1-3127; R01-NS39581, R01-RR16594, P41-RR14075, and R01-RR13609), the Mental Illness and Neuroscience Discovery (MIND) Institute, and in part by the Biomedical Informatics Research Network Project (BIRN, http://www.nbirn.net), which is funded by the National Center for Research Resources at the National Institutes of Health, USA (NCRR BIRN Morphometric Project BIRN002).
We wish to thank neuropsychologist Siri Kulseng of St. Olavs University Hospital in Trondheim, Norway, for taking part in the clinical assessments of the study of adolescents.
Abbreviations
- VLBW
very low birth weight
- SGA
small for gestational age
- IQ
intelligence quotient
- VMI
the developmental test of visual-motor integration
- ICV
intracranial volume
Footnotes
Financial disclosure and conflict of interest: Dale, A.M. and Fischl, B. is funded by CorTechs Labs, Inc. Dale, A.M. has equity in CorTechs Labs, Inc.
References
- 1.Foulder-Hughes LA, Cooke RWI. Motor, cognitive, and behavioural disorders in children born very preterm. Dev Med & Child Neurol. 2003;45:97–103. [PubMed] [Google Scholar]
- 2.Larroque B, Bertrais S, Czernichow P, Léger J. School difficulties in 20-year-olds who were born small for gestational age at term in a regional cohort study. Pediatrics. 2001;108:111–5. doi: 10.1542/peds.108.1.111. [DOI] [PubMed] [Google Scholar]
- 3.Peterson BS, Anderson AW, Ehrenkranz R, Staib LH, Tageldin M, Colson E, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111:939–48. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- 4.Thompson DK, Wood SJ, Doyle LW, Warfield SK, Lodygensky GA, Anderson PJ, et al. Neonate hippocampal volumes: prematurity, perinatal predictors, and 2-year outcome. Ann Neurol. 2008;63:642–51. doi: 10.1002/ana.21367. [DOI] [PubMed] [Google Scholar]
- 5.Nosatri C, Al-Asady MHS, Frangou S, Stewart AL, Rifkin L, Murray RM. Adolescents who were born very preterm have decreased brain volumes. Brain. 2002;125:1616–23. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- 6.Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics; 2008;121:306–16. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- 7.Skranes J, Vangberg TR, Kulseng S, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130(3):654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- 8.Heinonen K, Räikkönen K, Pesonen A-K, Kajantie E, Andersson S, Eriksson JG, et al. Prenatal and postnatal growth and cognitive abilities at 56 months of age: a longitudinal study of infants born at term. Pediatrics. 2008;121:e1325–1333. doi: 10.1542/peds.2007-1172. [DOI] [PubMed] [Google Scholar]
- 9.Skranes JS, Martinussen M, Smevik O, Myhr G, Indredavik M, Vik T, et al. Cerebral MRI findings in very-low-birth-weight and small-for-gestational-age children at 15 years of age. Pediatr Radiol. 2005;35:758–65. doi: 10.1007/s00247-005-1446-2. [DOI] [PubMed] [Google Scholar]
- 10.Vangberg TR, Skranes J, Dale AM, Martinussen M, Brubakk A-M, Haraldseth O. Changes in white matter diffusion anisotropy in adolescents born prematurely. Neuroimage. 2006;32:1538–48. doi: 10.1016/j.neuroimage.2006.04.230. [DOI] [PubMed] [Google Scholar]
- 11.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 12.Martinussen M, Fischl B, Larsson HB, Skranes J, Kulseng S, Vangberg TR, et al. Cerebral cortex thickness in 15-year-old adolescents with low birth weight measured by an automated MRI-based method. Brain. 2005;128:2588–96. doi: 10.1093/brain/awh610. [DOI] [PubMed] [Google Scholar]
- 13.Skjæreven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand. 2000;79:440–9. [PubMed] [Google Scholar]
- 14.Glinianaia SV, Skjærven R, Magnus P. Birthweight percentiles by gestational age in multiple births A population-based study of Norwegian twins and triplets. Acta Obstet Gynecol Scand. 2000;79:450–8. [PubMed] [Google Scholar]
- 15.Wechsler Intelligence Scale for Children. Swedish version. 3. Psykologiförlaget AB: Stockholm; 1999. 1991. [Google Scholar]
- 16.Spreen OE, Strauss E . Administration, Norms, and Commentary. 2. Oxford University Press; New York: 1998. A Compendium of Neuropsychological tests. [Google Scholar]
- 17.Beery KE. Administration, scoring and teaching manual. 4. Modern Curriculum Press; 1997. The Beery-Buktencia Developmental test of visual-motor integration. [Google Scholar]
- 18.Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- 19.Inder TE, Warfield SK, Wang H, Hüppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–94. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 20.Boardman JP, Counsell SJ, Rueckert D, Kapellou O, Bhatia KK, Aljabar P, et al. Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. Neuroimage. 2006;32:70–8. doi: 10.1016/j.neuroimage.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Skranes J, Nilsen G, Smevik O, Vik T, Brubakk AM. Cerebral MRI of very low birth weight children at 6 years of age compared with the findings at 1 year. Pediatr Radiol. 1998;28:471–5. doi: 10.1007/s002470050387. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan L, Allsop J, Councell SJ, Boardman JP, Edwards JP, Rutherford M. Smaller cerebellar volumes in very preterm infants at term-equivalent are associated with the presence of supratentorial lesions. Am J Neuroradiol. 2006;27:573–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Limeropoulos C C, Soul JS, Haider H, Huppi PS, Bassan H, Warfield SK, et al. Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics. 2005;116:844–50. doi: 10.1542/peds.2004-2282. [DOI] [PubMed] [Google Scholar]
- 24.Pierson CR, Folkerth RD, Billiards SS, Trachtenberg FL, Drinkwater ME, Volpe JJ, et al. Gray matter injury associated with periventricular leucomalacia in the premature infant. Acta Neuropathol. 2007;114:619–31. doi: 10.1007/s00401-007-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isaacs EB, Gadian DG, Sabatini S, Chong WK, Quinn BT, Fischl BR, et al. The effect of early human diet on caudate volumes and IQ. Pediatr Res. 2008;63:308–14. doi: 10.1203/PDR.0b013e318163a271. [DOI] [PubMed] [Google Scholar]
- 26.Tolsa CB, Zimine S, Warfield SK, Freschi M, Rossignol AS, Lazeyras F, et al. Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res. 2004;56:132–8. doi: 10.1203/01.PDR.0000128983.54614.7E. [DOI] [PubMed] [Google Scholar]
- 27.Lodygensky GA, Seghier ML, Warfield SK, Tolsa CB, Sizonenko S, Lazeyras F, et al. Intrauterine growth restriction affects the preterm Infant`s hippocampus. Pediatr Res. 2008;63:438–43. doi: 10.1203/PDR.0b013e318165c005. [DOI] [PubMed] [Google Scholar]
- 28.Boardman JP, Counsell SJ, Rueckert D, Hajanal JV, Bhatia KK, Srinivasan L, et al. Early growth in brain volume is preserved in the majority of preterm infants. Ann Neurol. 2007;62:185–92. doi: 10.1002/ana.21171. [DOI] [PubMed] [Google Scholar]
- 29.Shah DK, Anderson PJ, Carlin JB, Pavlovic M, Howard K, Thompson DK, et al. Reduction in cerebellar volumes in preterm infants: relationship to white matter injury and neurodevelopment at two years of age. Pediatr Res. 2006;60:97–102. doi: 10.1203/01.pdr.0000220324.27597.f0. [DOI] [PubMed] [Google Scholar]
- 30.Mallard C, Loeliger M, Copolov D, Rees S. Reduced number of neurons in the hippocampus and the cerebellum in the postnatal guinea-pig following intrauterine growth-restriction. Neuroscience. 2000;100:327–33. doi: 10.1016/s0306-4522(00)00271-2. [DOI] [PubMed] [Google Scholar]
- 31.Seckl JR, Cleasby M, Nyirenda MJ. Glucocorticoids, 11β-hydroxysteroid dehydrogenase, and fetal programming. Kidney International. 57:1412–7. doi: 10.1046/j.1523-1755.2000.00984.x. 222. [DOI] [PubMed] [Google Scholar]
- 32.Frangou S, Chitins X, Williams SCR. Mapping IQ and gray matter density in healthy young people. Neuroimage. 2004;23:800–5. doi: 10.1016/j.neuroimage.2004.05.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.