Abstract
Cholecystokinin (CCK)-expressing neurons within the nucleus of the solitary tract (CCKNTS) are responsive to satiety signals and their chemogenetic activation suppresses appetite. Optogenetic activation of CCKNTS axon terminals within either the parabrachial nucleus (PBN) or the paraventricular nucleus of the hypothalamus (PVH) is sufficient to suppress feeding. An interesting dichotomy has been revealed when assessing the motivational valence of these two circuits. Activating CCKNTS cell bodies is aversive as demonstrated by conditioned taste aversion and place-preference assays. Activation of the CCKNTS→PBN pathway is also aversive; however, stimulating the CCKNTS→PVH pathway is appetitive when assayed using a real-time, place-preference task. Thus, these two projections from CCKNTS neurons reduce food intake through opposite motivational states; one pathway signals positive valence (CCKNTS→PVH) and the other signals negative valence (CCKNTS→PBN).
Graphical Abstract
INTRODUCTION
The advent of genetic and viral tools has led to an exponential increase in our understanding of neuronal circuits mediating food intake. By manipulating the activity of genetically identified cells within a specific region of the brain in vivo, researchers have uncovered several pathways that are directly involved in an animal’s motivation to eat or not to eat [for reviews see: (Graebner et al., 2015; Krashes and Kravitz, 2014; Sternson and Roth, 2014)]. Recently, we and others have elucidated a role for CCK-expressing neurons in the NTS in the cessation of feeding (D'Agostino et al., 2016; Roman et al., 2016). CCK is a well-studied peptide known for its role in suppressing feeding behavior both peripherally and centrally (Della Fera and Baile, 1979; Moran and Schwartz, 1994; Schick et al., 1990); however, the CCK-producing cells of the NTS have only recently received attention for their role in appetite suppression. Following a meal, CCKNTS neurons become activated and relay visceral input from the vagus to brain regions known to mediate changes in feeding (Rinaman, 2010). Interestingly, the same brain regions that are important for feeding behavior have also been shown to influence behavioral responses to emotionally relevant stimuli associated with fear, stress, and pain (Bernard and Besson, 1990; Bester et al., 2000; Casada and Dafny, 1992; Chagra et al., 2011; Han et al., 2015; LeDoux, 2000). Two such regions receiving especially dense innervation from CCKNTS neurons are the PBN and PVH. When CCKNTS neurons or their axon terminals in either of these two regions are stimulated, mice eat less, even in energy-depleted states (D'Agostino et al., 2016; Roman et al., 2016).
Appetite suppression may be the result of feeling pleasantly full (sated) or unpleasantly full after eating too much food too fast. Appetite suppression also occurs in response to visceral malaise induced by food poisoning, which can be mimicked by injecting rodents with lithium chloride (LiCl) or lipopolysaccharide (LPS), compounds that induce nausea and illness, respectively. When exposure to a novel food is followed by injection with LiCl or LPS, rodents will avoid that food in the future, a phenomenon known as conditioned taste aversion (CTA) (Cross-Mellor et al., 2004; Ingram, 1982).
Because obesity has become a major health problem and therapeutic interventions are being sought, it is important to know whether the neural circuits that are targeted relay aversive or appetitive feelings. Therefore, we aimed to investigate whether activation of the appetite-suppressing CCKNTS neurons and their axonal projections is associated with a pleasant state such as normal satiety or with an aversive state such as visceral malaise. Our experiments reveal that CCKNTS neurons can mediate either appetitive or aversive states, depending on the brain regions that are examined.
EXPERIMENTAL PROCEDURES
Mice
All animals were male CckIRES-Cre/+ (abbreviated to CckCre/+; Jackson Laboratory, CckTM1.1(cre)Zjh/J) mice backcrossed to C57Bl/6 mice for >6 generations (Taniguchi et al., 2011). All mice were group housed before stereotaxic surgery and maintained on a 12-h light/dark cycle with ad libitum access to rodent diet (Picolab, #5053 during CTA experiments and Research Diets, Inc. D12450B for all other experiments) unless otherwise stated. Mice (2.5 to 5 months old) were individually housed at least two weeks before behavioral testing. The total number of mice used across all experiments listed below was 55. All animal experiments were approved by the University of Washington Institutional Animal Care and Use Committee (IACUC).
Virus production
Cre-dependent, AAV-Ef1α-DIO-mCherry, and AAV-Ef1α-DIO-ChR2:mCherry plasmids were provided by K. Deisseroth. B. Roth provided Cre-dependent pAAV-hSyn-DIO-hM3Dq:mCherry plasmid. Viruses were prepared in our laboratory as described (Carter et al., 2013) using AAV serotype 1 helper plasmid (pDG1) and were suspended in buffer at ~2 × 109 particles/µl.
Stereotaxic surgery
Mice were anesthetized with isoflurane vapor and secured in a stereotaxic frame (David Kopf Instruments). After incision, the skull was exposed and two drill holes were created over the NTS at the following coordinates from bregma (AP: −6.8 mm, ML: ±0.38 mm). A 2-µl Hamilton syringe with a 32-gauge needle was positioned at a 10 degree angle coronally towards the back of the animal and was then inserted into each drill hole and lowered −5.2 mm from bregma. Virus (400 nl) was injected at a rate of 200 nl/min. Coordinate mapping and injection was performed using a robotic StereoDrive system (Neurostar). After 6 min, the needle was retracted and the incision was closed with a sterile suture. In mice used for in vivo optogenetic experiments, two fiber optic cannulae (Doric Lenses) were implanted above the lateral PBN (AP: −5.1 mm, ML: ±1.7 mm, DV: 3.0 mm) or a single fiber optic cannula was implanted above the PVH (AP: −0.8 mm, ML: 0 mm, DV: −4.3 mm) and affixed to the skull using Metabond (Parkell) and dental cement. All 55 mice used across subsequent experiments described below underwent stereotaxic surgery.
Food intake
Food-restriction assays in animals expressing hM3Dq were performed as previously described (Roman et al., 2016). For optogenetic experiments, mice were injected with both ChR2- and hM3Dq-containing viruses and prescreened for the ability of CNO (0.8 mg/kg body weight, i.p., Tocris) to decrease baseline food intake by > 40% at 2 and 4 h after lights out. Prescreening was performed to insure correct viral targeting to the NTS before optogenetic experiments were performed; approximately 55% of mice passed this screen. A total of 19 animals were acclimated to the fiber optic cables and feeding paradigm on the night prior to testing. On subsequent nights, mice were attached to fiber optic cables and all food (Research Diets, Inc. D12450B) was removed from the cage 2 h before lights out. At lights out, a measured amount of food was returned and the amount remaining was measured 2 h later. For baseline feeding (2 days) no stimulation occurred. On the third day, blue light (473 nm) was delivered in 10-ms pulses at 30 Hz for 1 s every 4 s starting 30 min before lights out and ending when food consumption was measured 2 h after lights out.
Conditioned Place Aversion (CPA)
Animals were kept at 85–90% body weight during the entire experiment by restricting the amount of food provided each day. CPA testing was performed on 11 animals using a counterbalanced design in a chamber (47 × 20 × 20 cm) consisting of two outer compartments and a center compartment. One outer compartment had black walls 20 cm in height while the other chamber had grey and black vertical striped-walls and a textured floor. The center compartment was white. Mice were habituated to saline injections for 3 days prior to testing. Animals were exposed to a 10-min pre-conditioning trial in which they were allowed to move freely in the CPA chamber to assess initial preference. Three animals were removed from further analysis because during this pre-conditioning trial, they each had a strong bias toward one compartment and spent over 71% of their time in one side of the chamber over the other. Three of the remaining animals had been previously injected with the control, mCherry virus and the small number in this group precluded further analysis. On conditioning trials, mice were injected daily with either saline or CNO (0.8 mg/kg; body weight, i.p., Tocris) dissolved in saline, alternating on consecutive days, and 45 min later they were placed into one side of the CPA chamber and restricted to that side for 60 min before being returned to the home cage. Conditioning trials occurred for 10 consecutive days. The following day (preference test), the mice were given access to the entire CPA chamber for 10 min. A second preference test was performed after animals had ad libitum access to food for 2 days to determine if their preference was altered by energy state or hunger; it was unaffected (data not shown).
Conditioned Taste Aversion (CTA)
Mice were exposed to conditioning sessions during which, after an overnight fast, mice were given access to a novel, high-fat diet (Research Diets, Inc. D12451) for 30 min prior to stimulation via either chemogenetic or optogenetic methods (9 animals each). For the chemogenetic approach, mice were injected with CNO (0.8 mg/kg) and allowed access to the novel food for another hour. These mice had been exposed to CNO in the CPA task 3 weeks earlier. For the optogenetic approach, mice received blue-light pulses at 30 Hz, 1 sec on 4 sec off, for 2 h via bilateral fiber-optic cannulae implanted over the PBN, during which mice had access to the novel food. Mice in this group had not been exposed to any prior photostimulation. Normal chow was returned and mice had ad libitum access to food for one night. The next night, mice were fasted again and a second conditioning session occurred. Mice were tested for food preference two days later, again after an overnight fast, by measuring their intake of normal chow and the high-fat diet during a 1-h session.
Real-Time, Place-Preference (RTPP)
Mice that had been food deprived 18–20 h were tested for RTPP using a counterbalanced design in a 47 × 20 × 20 cm chamber separated by a partition into two sides, one side having black walls and the other having black and grey vertical-striped walls and a textured floor. Mice were habituated to patch cords for 1 h on the day prior to testing and again 5 min prior to the testing session. Their preference for the two sides of the box was tested for 10 min without photostimulation. During the next 20 min, mice received photo stimulation (30 Hz, 1.5 sec on, 0.5 sec off) on only one side of the box. Photostimulation stopped when animals entered the non-paired side. A total of 24 animals were assayed in this single-trial RTPP test. One control animal was removed from analysis due to technical issues with the fiber-optic patch cord. For the repeated RTPP assay with stimulation over the PVH (n = 7), mice were tested every other day after overnight food deprivation.
Immunohistochemistry
Three hours after CNO injection (0.8 mg/kg) or 90 min after 30-Hz optogenetic stimulation, mice were anesthetized with pentobarbital and perfused transcardially with phosphate buffered saline (PBS) followed by 4% paraformaldehyde in PBS. Brains were extracted, post-fixed overnight, and protected in 30% sucrose dissolved in PBS for freezing and cryosectioning (30-µm sections). Sections were collected in cold PBS.
Brain sections were permeabilized with 0.2% Triton X-100 in PBS (PBST) for 30 min and treated with a blocking solution containing PBST and 3% normal donkey serum (Jackson Immunoresearch) for 45 min at room temperature. Sections were incubated overnight at 4°C in block solution containing goat polyclonal Fos antibody (1:500; Santa Cruz Biotech, c-Fos, 4:sc-52), and rabbit polyclonal DsRed antibody (1:1000; Clontech, #632496). After primary antibody incubation and washes in PBS, sections were incubated in Cy5-cojugated, donkey anti-goat IgG and Alexa Fluor 594 donkey anti-rabbit IgG (1:500; Jackson Immunoresearch) in block solution for 2 h at room temperature. Finally, sections were washed in PBS, mounted onto glass slides, and coverslipped with DAPI Fluromount-G (Southern Biotech, 0100-290) before image acquisition. Cannula placement and Fos expression were verified in tissues from all experimental animals. Images were collected on either a Nikon upright epifluorescent microscope with a QImaging Camera or a laser-scanning Olympus FV1200 confocal microscope.
Statistics
Data were analyzed using Prism 5.0 (GraphPad Software). All data analyzed using parametric tests passed either the Brown-Forsythe test to compare variances between more than two groups or the F-test to compare variances between two groups. All group data followed a Gaussian distribution as shown by Shapiro-Wilk normality tests, unless otherwise noted in the text. When data included non-normal distributions, non-parametric Kruskal-Walllis tests were used to compare means. Data are expressed as mean ± SEM. Statistical significance was considered with P < 0.05. Figures were prepared using Photoshop and Illustrator CS5 (Adobe Systems).
RESULTS
Stimulating anorexigenic CCKNTS neurons induces conditioned place aversion
To determine the motivational effects of CCKNTS neuron activation, we employed a conditioned place-aversion/preference (CPA or CPP) task, which uses Pavlovian conditioning to pair one distinct side of a chamber with an unconditioned stimulus (US). The US investigated here was stimulation of CCKNTS neurons using designer receptors exclusively activated by designer drugs (DREADDs). Before performing CPA experiments, we first validated that activation of CCKNTS neurons was sufficient to decrease feeding in our experimental animals.
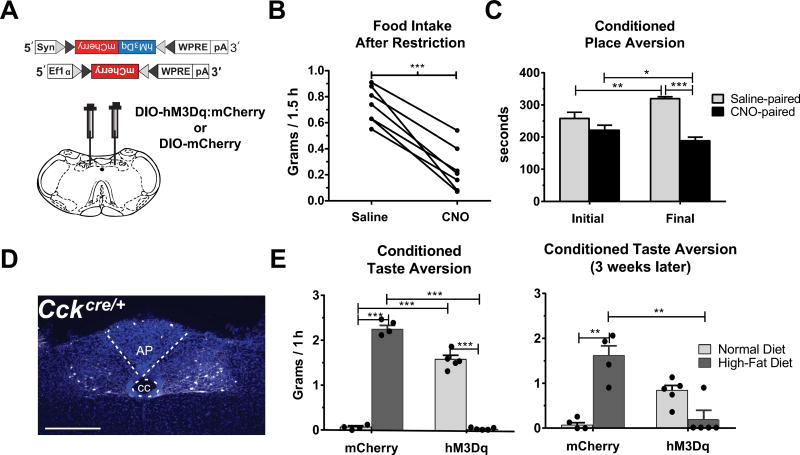
Mice expressing Cre-recombinase from the Cck locus (CckCre/+ mice) were injected bilaterally into the NTS with adeno-associated virus (AAV) encoding a Cre-dependent Gαq-coupled DREADD fused to a fluorescent protein (mCherry) for visualization (AAV-DIO-hM3Dq:mCherry) (Fig. 1A,D). Injection of the otherwise inert ligand, clozapine-N-oxide (CNO), was used to activate neurons expressing the DREADD receptor in vivo. Two independent studies have confirmed the ability of CNO to increase the firing rate in CCKNTS neurons expressing hM3Dq using ex vivo electrophysiology (D'Agostino et al., 2016; Roman et al., 2016). After allowing 2 weeks for viral expression of the receptor, animals were food-restricted and injected (i.p) with either saline or CNO (0.8 mg/kg) during the light cycle. Food consumption was measured for 1.5 h. In agreement with previous reports, activation of CCKNTS neurons with CNO decreased food intake when compared to intake following saline injection [Fig. 1B; (t(6)=8.52, p = 0.0001)].
Fig. 1.
CCKNTS neurons suppress appetite and encode negative valence. (A) Depiction of viral targeting site for the excitatory DIO-hM3Dq:mCherry or DIO-mCherry virus in a coronal section through the medial NTS within the mouse brainstem. (B) In food-restricted animals expressing DIO-hM3Dq:mCherry, activation of CCKNTS neurons with CNO decreased food intake at 2 h compared to intake after saline injection (n = 7). (C) Food-restricted mice expressing hM3Dq in CCKNTS neurons display decreased preference for the CNO-paired side of a box during the final test after conditioning (n = 5). (D) Representative coronal section of the NTS in a CckCre/+ mouse showing viral expression of the hM3Dq receptor in white and DAPI in blue. Scale bar, 100 µm. (E) Mice expressing DIO-hM3Dq:mCherry display CTA and avoided consumption of a high-fat diet that was previously paired with CNO in a choice test two days (left panel) and 3 weeks (right panel) after high-fat diet/CNO pairings (mCherry: n = 4, hM3Dq n = 5). All data represent means ± s.e.m., * P < 0.05, ** P < 0.01, *** P < 0.001. AP, area postrema; cc, central canal.
To assess whether this type of neuronal activation signals positive or negative valence, we performed a CPA task in food-restricted animals. On Day 1, the baseline preference of each mouse for two distinct sides of a rectangular chamber was measured during a 10-min session. For the following 10 days, each mouse received an injection of either saline or CNO (0.8 mg/kg) before being restricted to one side of the box or the other for 1 h. On Day 12, the mice were given access to both chambers of the box and the time spent in each chamber was recorded for 10 min. There was no significant preference for either of the two sides of the chamber before conditioning. However, after conditioning, all animals decreased their preference for the CNO-paired side of the box, indicating that it had become associated with a negative physiological or psychological state (Fig. 1C). A two-way repeated measures ANOVA detected a significant interaction of test day and side (F(1,8) = 33.52, p = 0.0004) and significant main effect of side (F(1,8) = 29.83, p = 0.0006). It is unlikely that the change in preference was due to a positive association with the saline-paired side, as saline injections are not considered to be appetitive and are often used as a control (Gauvin and Holloway, 1992).
Activation of CCKNTS neurons induces condition taste aversion to palatable food
Conditioned taste aversion (CTA) occurs when animals associate a novel food with visceral malaise and avoid that food in the future. We wanted to investigate whether activation of CCKNTS neurons was sufficient to induce CTA. For this test, CckCre/+ mice that had been injected in the NTS with a Cre-dependent virus encoding either the excitatory DREADD, hM3Dq:mCherry, or just mCherry (as a control), were exposed to a novel, high-fat diet for 30 min, injected with CNO (0.8 mg/kg i.p.), and allowed access to the food for an additional hour. This high-fat diet/CNO pairing was performed twice within one week in food-deprived mice. Mice were tested for CTA expression by giving them access to both the CNO-paired, high-fat diet and their normal diet for 1 hour. While control, mCherry-only mice consumed the high-fat diet almost exclusively (96.9% of their 1-h intake), mice expressing hM3Dq:mCherry in CCKNTS neurons avoided the high-fat diet, displaying a robust CTA (Fig. 1E, left panel). A two-way ANOVA detected a significant interaction of group and diet (F(1,14) = 1008, p < 0.0001), as well as significant main effects of group: (F(1,14) = 37.45, p < 0.001), and diet (F(1,14) = 27.32, p = 0.001). Mice were given the food-choice test again one, two, and three weeks later with no further injections of CNO or access to high-fat diet besides the 1-h tests. Even after 3 weeks, 4 out of 5 mice expressing hM3Dq:mCherry continued to avoid the high-fat diet while the mCherry controls retained a significant preference for the high-fat food (Fig. 1E, right panel). As one animal in the hM3Dq group no longer avoided the high-fat diet at the 3-week CTA test, and all other animals consumed no high-fat food, data for this group was no longer normally distributed. Therefore, a Kruskal-Wallis test was performed [H(3) = 12.88, p = .0004] and post-hoc tests revealed a significant mean rank difference (10.98) between the amount of high-fat diet consumed by the control, mCherry group compared to the group expressing hM3Dq (p = .0054). The ability to produce a robust CTA supports the hypothesis that CCKNTS stimulation encodes negative valence and suggests the activation of these neurons is perceived as an unpleasant visceral experience.
Because CCKNTS activation in these paradigms was performed using DREADDs and i.p injections of CNO, we were unable to pinpoint which specific downstream areas mediate the avoidance behaviors that were observed. Activating CCKNTS neurons using CNO activates neurons in many brain regions (assessed by Fos immunoreactivity) including the PVH, PBN, oval nucleus of the bed nucleus of the stria terminalis (BNSTov), central nucleus of the amygdala (CeA), and periaqueductal grey (PAG) (Roman et al., 2016). Activation of any one or a combination of these nuclei may be sufficient to induce avoidance behavior similar to that observed following activation of the entire population of CCKNTS neurons. Therefore, to localize the specific effects of particular downstream targets we had two main options: either locally infuse smaller doses of CNO into downstream nuclei through intracerebral cannulae, or locally stimulate axon terminals using a genetically-targeted channelrhodopsin and fiber optic cannula. We chose the latter option, as it provides more precise temporal and spatial control, and performed optogenetic experiments stimulating CCKNTS axon terminals in the PBN or the PVH.
CCKNTS axon stimulation in the PBN decreases food intake and elicits avoidance in a real-time, place-preference test
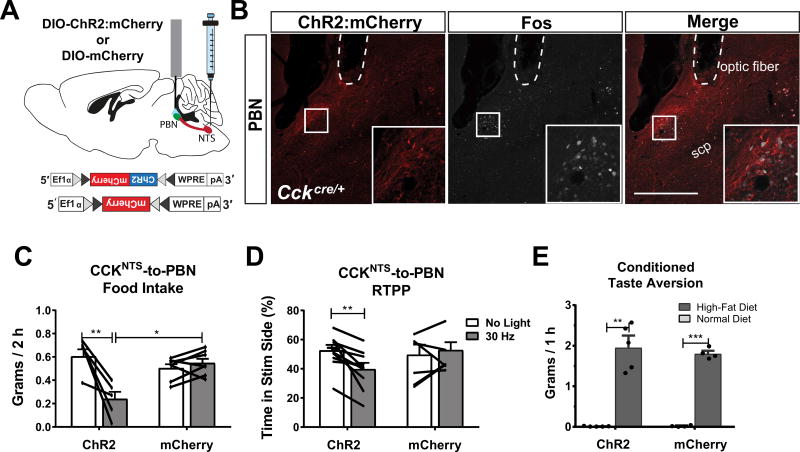
Both the PBN and the PVH receive dense innervation from excitatory CCKNTS neurons and express Fos immunoreactivity following either their chemogenetic activation (Roman et al., 2016) or optogenetic activation of their axon terminals (Fig. 2B, Fig. 3B). In addition, a number of genetically distinct subsets of cells within these areas involved in regulating energy balance and influencing appetite have been identified (Morton et al., 2014). Previous work shows that optogenetic stimulation of either the CCKNTS→PBN or the CCKNTS→PVH circuit alone is sufficient to suppress appetite (D'Agostino et al., 2016; Roman et al., 2016). We replicated these findings by assessing 2-h food intake during photostimulation of CCKNTS axon terminals in the PBN. CckCre/+ mice were injected with Cre-dependent AAV expressing channelrhodopsin (ChR2:mCherry) or mCherry alone (control) and fiber optic cannulae were implanted bilaterally over the PBN (Fig. 2A). After allowing several weeks for viral expression, 30-Hz photostimulation (blue light) decreased baseline feeding in mice expressing ChR2:mCherry when compared to non-stimulated (no light) intake or intake of mCherry-expressing controls (Fig. 2C). Baseline food-intake data for animals implanted with PBN fiber-optic cannula and expressing ChR2 (n = 5) were not normally distributed. Therefore, a Kruskal-Wallis test was performed [H(3) = 12.54, p = .0058] and Dunn’s post-hoc tests revealed that photostimulation of axon terminals in the PBN significantly decreased food intake in ChR2-expressing mice when compared to either baseline intake (mean rank difference = 15.1, p = 0.0015) or intake of mCherry-expressing control mice (mean rank difference = −9.171, p = 0.0267) (Fig. 2C).
Fig. 2.
A CCKNTS→PBN circuit suppresses appetite and elicits avoidance in a RTPP test. (A) Diagram of a sagittal mouse brain depicting site of viral injections and placement of fiber optic cannulae for photostimulation of axon terminals in the PBN. (B) Representative immunohistological section demonstrating expression of ChR2:mCherry within fibers and Fos within nuclei in the lateral PBN after 30-Hz photoactivation. Scale bar, 100 µm. (C) 30-Hz photoactivation of CCKNTS axon terminals in the PBN decreased food intake in ChR2-expressing mice (ChR2 n = 5, mCherry n = 7). (D) ChR2-expressing mice display a decreased preference for the 30-Hz, photostimulation-paired side in a RTPP assay, as compared to control, mCherry-expressing mice (ChR2 n = 10, mCherry n = 6). (E) ChR2-expressing mice did not display CTA and, similar to mCherry controls, chose to consume a high-fat diet that was previously paired with 30-Hz photoactivation in a choice test. All data represent means ± s.e.m., * P < 0.05, ** P < 0.01, *** P < 0.001. scp, superior cerebellar peduncle.
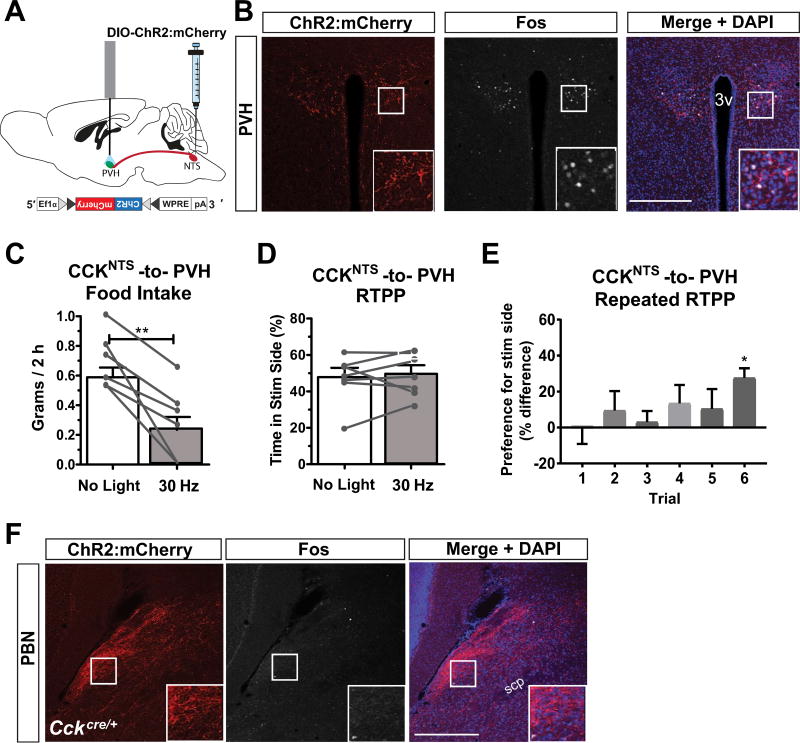
Fig. 3.
A CCKNTS→PVH circuit suppresses appetite and signals positive valence in a repeated RTPP test. (A) Diagram of a sagittal mouse brain depicting site of viral injections and placement of fiber optic cannula for photostimulation of axon terminals in the PVH. (B) Representative immunohistological section demonstrating expression of ChR2:mCherry within fibers and Fos within nuclei in the PVH after 30-Hz photoactivation. (C) 30-Hz photoactivation of CCKNTS axon terminals in the PVH decreased food intake in ChR2:mCherry-expressing mice when measured 2 h after lights out compared to non-stimulated (no light) intake (n = 7). (D) ChR2:mCherry-expressing mice did not display a preference for either side in a single RTPP assay when one side was paired with 30-Hz photostimulation of CCKNTS axon terminals in the PVH (n = 7). (E) After repeated RTPP sessions, animals increased their preference for the side of the chamber paired with photostimulation in the PVH (n = 7). (F) Immunohistological section demonstrating expression of ChR2:mCherry within fibers and absence of Fos immunoreactivity within nuclei in the lateral PBN after 30-Hz photoactivation of PVH afferents. All data represent means ± s.e.m., * P < 0.05, ** P < 0.01, *** P < 0.001. 3v, 3rd ventricle. Scale bars, 100 µm.
To investigate the motivational valence of optogenetically simulating the CCKNTS efferent projections to the PBN we used a real-time, place-preference (RTPP) test. CckCre/+ mice implanted with fiber optic cannulae over the PBN and virally expressing either ChR2:mCherry or mCherry within the NTS were food deprived overnight and allowed to explore the RTPP chamber for 10 min to assess baseline preference for either side of the chamber. Then, 30-Hz blue-light stimulation over the PBN was paired with one of the two distinct sides of the chamber during a 20-min trial. The ChR2:mCherry-expressing animals displayed a significant decrease in preference for the side of the chamber paired with photostimulation over the PBN (Fig. 2D), suggesting that it is aversive. A two-way ANOVA detected a significant interaction of group and treatment (F(1,14) = 8.077, p = 0.0131). This result is consistent with the direct, excitatory connection between CCKNTS neurons and CGRP neurons within the external lateral PBN found in previous work (Roman et al. 2016), because photostimulating CGRP neurons has been shown to induce aversive behaviors such as freezing (Han et al., 2015) and CTA (Carter et al., 2015). Although an aversion was elicited in the RTPP test and Fos was induced in the PBN (Fig. 2B,D), 30-Hz photostimulation over the PBN did not induce conditioned taste aversion (CTA) when it was paired on two separate days with high-fat food; both control and ChR2-expressing mice exclusively ate the high-fat diet during the test [Fig. 2E; significant main effect of diet (F(1,14) = 112.1, p < 0.0001)].
Stimulating the CCKNTS→PVH pathway elicits appetitive behavior after repeated trials in a real-time, place-preference test
Previous work investigating the CCKNTS→PVH circuit revealed that stimulation of this pathway encodes positive valence in food-deprived animals and increases preference for the photostimulation-paired side of a RTPP chamber or a Y-maze (D'Agostino et al., 2016). Those authors concluded that the appetitive nature of photostimulation is due to the relief from hunger that the animals experience during stimulation. We attempted to replicate these findings using a RTPP task in CckCre/+ mice injected with Cre-dependent ChR2:mCherry in the NTS and implanted with a fiber optic cannula over the PVH (Fig. 3A). Although photostimulation induced Fos immunoreactivity in the PVH (Fig. 3B) and decreased food intake [Fig. 3C; (t(6) = 4.977, p = 0.0025)], we did not observe a significant change in preference for one side of the chamber over the other with a single-trial RTPP task [Fig. 3D; (t(6) = 0.63, p = 0.55)]. However, when the stimulation was paired with one side of the chamber over several days in food-deprived mice, a significant preference for the photostimulation-paired side emerged (Fig. 3E). A one-way ANOVA approached significance (F(5,30) = 2.31, p = 0.0687) and Bonferroni post-hoc tests revealed that the mean for trial 1 (M = −0.0397, SEM = 8.8) was significantly different from the mean for trial 6 (M = 27.63, SEM 5.35). In addition, a significant preference for one side was observed for trial 6, as the percent time spent on the photostimulation-paired side was significantly greater than 50% using a one-sample t-test (t(6) = 5.162, p = 0.0021) data not shown. Following 30-Hz stimulation of terminals in the PVH, there was a substantial amount of Fos immunoreactivity in the PVH (Fig 3B), yet there was an absence of Fos immunoreactivity observed in the PBN [Fig 3F, (compare to Fig 2B; Fos expression after PBN terminal stimulation)]. These results show two separate projections from CCKNTS neurons are able to suppress appetite, yet do so by encoding opposite motivational valence.
DISCUSSION
CCKNTS neurons are activated following food intake and their chemogenetic activation reduces food intake and body weight (D'Agostino et al., 2016; Roman et al., 2016). It was hypothesized that CCKNTS neurons relay satiety signals from the periphery to forebrain regions to influence feeding behavior. Our previous study focused on the axonal projections to the PBN (Roman et al., 2016), while another laboratory’s work focused on the projections to the PVH (D'Agostino et al., 2016). The latter study included an assessment of motivational valence and concluded that activating the CCKNTS→PVH pathway “provided relief from the unpleasantness of energy deficit” because food deprived mice preferred the side of the apparatus where they were stimulated.
We investigated the motivational aspects of stimulating CCKNTS neurons as an entire population or just their afferents to either the PBN or PVH. Activating the entire population of CCKNTS neurons inhibited food intake and was aversive. Using the chemogenetic approach, activating CCKNTS neurons with CNO produced both a CPA and a robust, long-lasting CTA towards a highly palatable food. Induction of CTA not only indicates an aversive quality but also that it is perceived as a visceral stimulus because it is difficult to induce CTA if the unconditioned stimulus does not induce nausea or gastric upset (Garcia and Koelling, 1966).
It was previously established that stimulation of CCKNTS axon terminals in either the PBN or the PVH decreases appetite in hungry mice and we repeated those observations here. However, we found that activating these two independent pathways from CCKNTS neurons produced opposite results in a RTPP task. Activation of the CCKNTS→PBN pathway transmits negative motivational valence in that mice avoided the side in which they received ChR2 photostimulation. In contrast, activation of the CCKNTS→PVH circuit encodes positive valence and was preferred to no stimulation in hungry mice, in agreement with previous results (D'Agostino et al., 2016). Although the RTPP test utilized here relies on instrumental learning, active operant procedures could provide further information regarding the extent of the positive or negative valence associated with each circuit. For example, one could test whether or not an animal will nose poke or lever press to receive, or to turn off, photostimulation of a particular neural circuit. However, a major advantage of using either the CPP or the RTPP paradigm over an active, operant task is that the researcher need not design the experiment based on a hypothesis of whether the stimulation will be rewarding or aversive.
Mice avoided a distinct context when it was paired with CCKNTS→PBN circuit stimulation, suggesting negative valence, yet we did not observe CTA as a result of this stimulation. One explanation is that stimulation of the CCKNTS→PBN circuit is simply not aversive enough to produce CTA. We used a two-choice CTA test and two high-fat diet/photostimulation pairings; perhaps a CTA would be revealed with more pairings or using a less tempting food. Directly activating CGRP neurons in the PBN, one of the post-synaptic targets of CCKNTS neurons, is sufficient to induce a CTA (Carter et al., 2015), however, only a minority of CGRP neurons are activated by CCKNTS→PBN circuit stimulation (Roman et al. 2016). Multiple inputs to the CGRPPBN population, including noradrenergic neurons in the NTS that are activated following CCKNTS stimulation (Roman et al. 2016), may be necessary to generate a CTA. There also may be additional post-synaptic targets of the CCKNTS neurons in the PBN that modulate the effects of activating CGRPPBN neurons.
It is also likely that another projection site, such as the central nucleus of the amygdala (CeA) mediates the ability for CCKNTS neuronal stimulation to induce CTA. In addition to several studies implicating the CeA in taste aversion learning (Bahar et al., 2003; Lamprecht et al., 1997; Ma et al., 2011), Kinzig et al. (2002) have provided evidence that GLP-1 receptor activation in the CeA is sufficient to induce CTA, and receptor antagonism significantly attenuated LiCl-induced taste aversion learning. The GLP-1 neuronal population is found exclusively in the NTS and nearby area postrema and overlaps with CCKNTS neurons (Garfield et al., 2012; Merchenthaler et al., 1999; Zheng et al., 2015). Therefore, as some CCKNTS neurons release GLP-1, GLP-1 receptor activation within the CeA is a possible mechanism for CTA induction following CCKNTS cell-body stimulation and would be absent following CCKNTS→PBN circuit stimulation alone. Similar to CCKNTS neurons, stimulation of GLP-1 neurons via the DREADD system decreases food intake and body weight (Gaykema et al., 2017).
The CCKNTS→PVH circuitry appears to encode positive valence. Previous research demonstrated that mice prefer stimulation of CCKNTS axon terminals in the PVH in a RTPP and a Y-maze assay in an energy-dependent manner (i.e., only when they are hungry) (D'Agostino et al., 2016). In our experiments, the appetitive effect only materialized after repeated sessions and was not as robust as previously reported. This may be explained by differences in the experimental paradigms. The original study used 30-Hz stimulation interrupted every second by a 0.5-sec pause during the RTPP test. For technical reasons, the stimulus used here consisted of 30-Hz stimulation interrupted every 1.5 sec by a 0.5 second pause. In addition, unlike the original study, we first examined baseline preference in the absence of stimulation at the beginning of the RTPP task and used a distinguishing floor and wall pattern for each of the two sides of the RTPP chamber. Nevertheless, with repeated RTPP trials we observed a preference. We conclude that stimulation of the CCKNTS→PVH circuit signals positive valence and induces appetitive behavior, opposite to that of stimulating the CCKNTS→PBN pathway. The MC4R-expressing neurons that are the target of the CCKNTS→PVH circuit project their axons to the PBN and photoactivation of those terminals in the PBN has a positive valence (Garfield et al., 2015). Consequently, activation of that combined circuit has a pleasurable effect, perhaps reflecting satiety or absence of hunger.
Anatomical evidence shows that NTS neurons projecting to either the PBN or PVH have very few, if any, collaterals (Hermes et al., 2006; Kawai and Senba, 1996). Thus, any single CCKNTS neuron is unlikely to target both of these areas. Consistent with the lack of collaterals, we also did not observe Fos expression in the PBN after stimulating axon terminals in the PVH, which might occur via back-propagation of action potentials. It is not known how these two populations of CCKNTS neurons are activated in response to a meal. It is conceivable that during a meal the CCKNTS→PVH circuit is activated first, to stimulate satiety. As the meal progresses, mechano- and chemoreceptors in the stomach further activate the vagus and the CCKNTS→PBN circuit is activated as a secondary system, inciting CGRPPBN neurons to curtail the meal (Campos et al., 2016).
Acknowledgments
We thank Drs. B. Roth and K. Deisseroth for plasmids, M. Chiang for mouse husbandry, Drs. M. Carter, S. Han, and C. Campos for suggestions and technical advice as well as colleagues from the Palmiter laboratory for feedback and advice. This work was supported by the National Institutes of Health grants T32DK007247 (C.W.R.) and R01-DA24908 (R.D.P.).
Footnotes
Author Contributions
R.D.P, C.W.R, and S.R.S. conceived and designed the study. S.R.S. performed all experiments in Figure 1. C.W.R. performed all optogenetic behavioral experiments and immunohistology. C.W.R. and R.D.P wrote the manuscript.
References
- Bahar A, Samuel A, Hazvi S, Dudai Y. The amygdalar circuit that acquires taste aversion memory differs from the circuit that extinguishes it. Eur J Neurosci. 2003;17:1527–1530. doi: 10.1046/j.1460-9568.2003.02551.x. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1990;63:473–490. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- Bester H, Chapman V, Besson JM, Bernard JF. Physiological properties of the lamina I spinoparabrachial neurons in the rat. J Neurophysiol. 2000;83:2239–2259. doi: 10.1152/jn.2000.83.4.2239. [DOI] [PubMed] [Google Scholar]
- Campos CA, Bowen AJ, Schwartz MW, Palmiter RD. Parabrachial CGRP Neurons Control Meal Termination. Cell Metab. 2016;23:811–820. doi: 10.1016/j.cmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Han S, Palmiter RD. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J Neurosci. 2015;35:4582–4586. doi: 10.1523/JNEUROSCI.3729-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Evidence for two different afferent pathways carrying stress-related information (noxious and amygdala stimulation) to the bed nucleus of the stria terminalis. Brain Res. 1992;579:93–98. doi: 10.1016/0006-8993(92)90746-v. [DOI] [PubMed] [Google Scholar]
- Chagra SL, Zavala JK, Hall MV, Gosselink KL. Acute and repeated restraint differentially activate orexigenic pathways in the rat hypothalamus. Regul Pept. 2011;167:70–78. doi: 10.1016/j.regpep.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Mellor SK, Kavaliers M, Ossenkopp KP. Comparing immune activation (lipopolysaccharide) and toxin (lithium chloride)-induced gustatory conditioning: lipopolysaccharide produces conditioned taste avoidance but not aversion. Behav Brain Res. 2004;148:11–19. doi: 10.1016/s0166-4328(03)00181-5. [DOI] [PubMed] [Google Scholar]
- D'Agostino G, Lyons DJ, Cristiano C, Burke LK, Madara JC, Campbell JN, Garcia AP, Land BB, Lowell BB, Dileone RJ, et al. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. Elife. 2016;5 doi: 10.7554/eLife.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Fera MA, Baile CA. CCK-octapeptide injected in CSF causes satiety in sheep. Ann Rech Vet. 1979;10:234–236. [PubMed] [Google Scholar]
- Garcia J, Koelling RA. Relation of cue to consequence in avoidance learning. Psychonomic Science. 1966;4:123–124. [Google Scholar]
- Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci. 2015;18:863–871. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield AS, Patterson C, Skora S, Gribble FM, Reimann F, Evans ML, Myers MG, Jr, Heisler LK. Neurochemical characterization of body weight-regulating leptin receptor neurons in the nucleus of the solitary tract. Endocrinology. 2012;153:4600–4607. doi: 10.1210/en.2012-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin DV, Holloway FA. Historical factors in the development of ETOH-conditioned place preference. Alcohol. 1992;9:1–7. doi: 10.1016/0741-8329(92)90002-r. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Newmyer BA, Ottolini M, Raje V, Warthen DM, Lambeth PS, Niccum M, Yao T, Huang Y, Schulman IG, et al. Activation of murine pre-proglucagon-producing neurons reduces food intake and body weight. J Clin Invest. 2017;127:1031–1045. doi: 10.1172/JCI81335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graebner AK, Iyer M, Carter ME. Understanding how discrete populations of hypothalamic neurons orchestrate complicated behavioral states. Front Syst Neurosci. 2015;9:111. doi: 10.3389/fnsys.2015.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD. Elucidating an Affective Pain Circuit that Creates a Threat Memory. Cell. 2015;162:363–374. doi: 10.1016/j.cell.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes SM, Mitchell JL, Aicher SA. Most neurons in the nucleus tractus solitarii do not send collateral projections to multiple autonomic targets in the rat brain. Exp Neurol. 2006;198:539–551. doi: 10.1016/j.expneurol.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Lithium chloride-induced taste aversion in C57BL/6J and DBA/2J mice. J Gen Psychol. 1982;106:233–249. [PubMed] [Google Scholar]
- Kawai Y, Senba E. Organization of excitatory and inhibitory local networks in the caudal nucleus of tractus solitarius of rats revealed in in vitro slice preparation. J Comp Neurol. 1996;373:309–321. doi: 10.1002/(SICI)1096-9861(19960923)373:3<309::AID-CNE1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Kravitz AV. Optogenetic and chemogenetic insights into the food addiction hypothesis. Front Behav Neurosci. 2014;8:57. doi: 10.3389/fnbeh.2014.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, Hazvi S, Dudai Y. cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. J Neurosci. 1997;17:8443–8450. doi: 10.1523/JNEUROSCI.17-21-08443.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Ma L, Wang DD, Zhang TY, Yu H, Wang Y, Huang SH, Lee FS, Chen ZY. Region-specific involvement of BDNF secretion and synthesis in conditioned taste aversion memory formation. J Neurosci. 2011;31:2079–2090. doi: 10.1523/JNEUROSCI.5348-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Moran TH, Schwartz GJ. Neurobiology of cholecystokinin. Crit Rev Neurobiol. 1994;9:1–28. [PubMed] [Google Scholar]
- Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15:367–378. doi: 10.1038/nrn3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman CW, Derkach VA, Palmiter RD. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat Commun. 2016;7:11905. doi: 10.1038/ncomms11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick RR, Harty GJ, Yaksh TL, Go VL. Sites in the brain at which cholecystokinin octapeptide (CCK-8) acts to suppress feeding in rats: a mapping study. Neuropharmacology. 1990;29:109–118. doi: 10.1016/0028-3908(90)90050-2. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci. 2014;37:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Cai L, Rinaman L. Distribution of glucagon-like peptide 1-immunopositive neurons in human caudal medulla. Brain Struct Funct. 2015;220:1213–1219. doi: 10.1007/s00429-014-0714-z. [DOI] [PMC free article] [PubMed] [Google Scholar]