Abstract
Background
Recent research has shown that in schistosome-endemic areas preschool-aged children (PSAC), that is, ≤5 years, are at risk of infection. However, there exists a knowledge gap on the dynamics of infection and morbidity in this age group. In this study, we determined the incidence and dynamics of the first urogenital schistosome infections, morbidity and treatment in PSAC.
Methods
Children (6 months to 5 years) were recruited and followed up for 12 months. Baseline demographics, anthropometric and parasitology data were collected from 1502 children. Urinary morbidity was assessed by haematuria and growth-related morbidity was assessed using standard WHO anthropometric indices. Children negative for Schistosoma haematobium infection were followed up quarterly to determine infection and morbidity incidence.
Results
At baseline, the prevalence of S haematobium infection and microhaematuria was 8.5% and 8.6%, respectively. Based on different anthropometric indices, 2.2%–8.2% of children were malnourished, 10.1% underweight and 18.0% stunted. The fraction of morbidity attributable to schistosome infection was 92% for microhaematuria, 38% for stunting and malnutrition at 9%–34%, depending on indices used. S haematobium-positive children were at greater odds of presenting with microhaematuria (adjusted OR (AOR)=25.6; 95% CI 14.5 to 45.1) and stunting (AOR=1.7; 95% CI 1.1 to 2.7). Annual incidence of S haematobium infection and microhaematuria was 17.4% and 20.4%, respectively. Microhaematuria occurred within 3 months of first infection and resolved in a significant number of children, 12 weeks post-praziquantel treatment, from 42.3% to 10.3%; P<0.001.
Conclusion
We demonstrated for the first time the incidence of schistosome infection in PSAC, along with microhaematuria, which appears within 3 months of first infection and resolves after praziquantel treatment. A proportion of stunting and malnutrition is attributable to S haematobium infection. The study adds scientific evidence to the calls for inclusion of PSAC in schistosome control programmes.
Keywords: dynamics, incidence, morbidity, paediatric, praziquantel efficacy, prevalence, preschool, schistosomiasis, Zimbabwe
Key questions.
What is already known about this topic?
Epidemiological studies indicate that preschool-aged children (PSAC) aged ≤5 years are exposed to schistosome infection, the consequences of which manifest later in life.
Unlike school-aged children, there are no longitudinal studies tracking the dynamics of new infections, the development of morbidity and implications on current and future health in this age group.
What are the new findings?
We determined for the first time levels of schistosome morbidity in PSAC attributable to Schistosoma haematobium infection, that is, 92% of microhaematuria, 38% of stunting and depending on what index is used, 9%–34% of malnutrition. We recorded significant annual incidence of new schistosome infection (17.7%) and urinary morbidity (microhaematuria; 20.4%) with significant quarterly incidences.
We showed that microhaematuria occurred within 3 months of first infection and resolved after praziquantel (PZQ) treatment.
We indicated that a significant amount of morbidity, as measured by microhaematuria, resolved within 3 months of effective treatment with PZQ (significant reduction from 42.3% vs to 10.3% (P<0.0001)).
Recommendations for policy
The findings indicate that schistosome morbidity in PSAC can be reversed by PZQ treatment.
The findings contribute to the scientific evidence base for prioritising schistosome treatment in PSAC, to reduce infection and morbidity and promote child health and development.
Introduction
Of the 123 million children worldwide affected with schistosomiasis, about 50 million are preschool-aged children (PSAC), that is, ≤5 years.1 Nonetheless, schistosome infection and morbidity dynamics in this age group are less characterised compared with school-aged children (SAC), that is, ≥6 years. For example, there are several studies describing and quantifying schistosome-related morbidity including haematuria, nutritional deficiencies and delayed growth and cognition in SAC2 3 but no comparable comprehensive studies in PSAC.
Epidemiological studies in PSAC clearly indicate that infection occurs in early childhood,4–7 and if untreated, the infection can lead to health consequences later in life.8 Despite this importance of childhood infections, there is a paucity of longitudinal studies tracking the dynamics of new first infections, the development of morbidity and implications on current and future health in this age group. The definition of schistosome pathology and morbidity continues to be refined in attempts to better characterise clinical manifestations, for example, as with female genital schistosomiasis,9 and to identify applicable morbidity markers of disease, for example, urine albumin–creatinine ratio.10 Growth and nutrition-related morbidities associated with schistosomiasis have also only recently become more widely recognised.11 There is therefore a need to collate all of this new knowledge to better define schistosomiasis in PSAC, where manifestations of disease are poorly described.6
In this study, we aimed to describe the baseline dynamics of schistosome infection and morbidity in Zimbabwean PSAC exposed to Schistosoma haematobium. A cohort of schistosome-negative children was followed for a year to document infection and morbidity incidence, as well as the effects of treatment on infection and morbidity. This study investigates the ability of existing diagnostic and morbidity tools to quantify and monitor early infection and morbidity. It also contributes to disease burden estimates and the dynamics of infection and morbidity in PSAC. This knowledge will inform the design and implementation of interventions targeted at this age group.
Methods
Consent
Permission to conduct the study in the province was obtained from the Provincial Medical Director. Prior to enrolment, the study aims and procedures were explained to all participants and their parents/guardians in English or in the local language, Shona. Written informed consent was obtained from the participants’ parents/guardians as appropriate. Recruitment into the study was voluntary and parents/guardians were free to withdraw the participants at any time with no further obligation.
Study site and period
The study was conducted in 13 villages in the Shamva district, northeast of Zimbabwe (17°10′0″S 31°40′0″E) from February 2016 through to February 2017. This is one of seven districts in the Mashonaland Central province of Zimbabwe, whose people are primarily subsistence farmers. There is a cold dry (April–July), hot dry (August–October) and rainy season (November–March).12 13 The area was selected for this study on urogenital schistosomiasis because the prevalence of S haematobium is high (>50%), while the prevalence of Schistosoma mansoni and soil-transmitted helminths is low (<15%).14
Study design
This study was part of a larger longitudinal parasitological and immunological project, following the treatment-reinfection study design widely used in human helminth field studies. There was a cross-sectional study at baseline, followed by a 1-year longitudinal study. Recruited children were screened at baseline for schistosome infection and morbidity to describe the epidemiology of infection and morbidity in this population. The larger study is comparing the impact of regular quarterly screening and treatment (group 1) and biennial screening and treatment (group 2). This study reports on the findings in the children screened quarterly (group 1). Following the baseline recruitment, age and sex-matched S haematobium-negative children who fulfilled the inclusion criteria were randomly allocated into groups 1 and 2. A total of 1783 children were invited to participate, of which 1502 provided samples for parasitological diagnosis at baseline. After allocation to the two groups of the study, 525 children who were schistosome-negative by egg count, provided a blood sample for serological assays and consented to participate in the longitudinal follow-up, formed the group 1 cohort, which was followed up every 3 months to detect new schistosome infections by egg count, and morbidity by microhaematuria.
Children were recruited from crèches, early child development centres, and preschools. Parents/guardians of children not attending any of the educational programmes (eg, children <3 years) were invited through the community nurse and village health workers to report to the sampling centre; that is, the centre used by the community for the Expanded Program for Immunisation (eg, school or primary health centre) for enrolment into the project. A questionnaire designed in English and translated into the local dialect (Shona) was administered to gather demographic data and establish exposure behaviour.
Study inclusion criteria
At baseline, the study enrolled children aged 6 months to 5 years who met the following inclusion criteria. Participants had to (i) be lifelong residents of the study area, (ii) have no previous antihelminthic treatment, (iii) be negative for S mansoni and (iv) consent to participate. To be included in the longitudinal cohort, children who had fulfilled the inclusion criteria described above had to meet an additional criterion of being diagnosed negative for S haematobium by egg count at baseline.
Anthropometry
Weight (nearest 0.1 kg) and height (nearest 0.1 cm) without shoes and in light clothing was measured using an electronic scale and a stadiometer, respectively. For very young babies, height was measured with an infantometer baby board, and weight measured with a baby scale. The mid-upper arm circumference (MUAC) was measured (nearest 1 mm) using a child MUAC tape; on the left arm, midpoint between the shoulder and the tip of the elbow, with the arm relaxed and hanging down the body. Growth and nutritional status was assessed using the WHO Anthro software V.3.0.1 (http://www.who.int/childgrowth/en/).15 This generated Z-scores for specific measures of nutrition and growth, that is, stunting by height-for-age (HAZ), underweight by weight-for-age (WAZ) and body mass index-for-age (BAZ) and malnutrition by MUAC (MUAC and MUACZ) and weight-for-height (WHZ). Measures were considered abnormal when Z scores were <–2.16
Parasitological diagnosis
About 50 mL of urine sample was collected from each participant on three successive days and a stool specimen was collected on a single day from each participant. Samples were collected between 10:00 hours and 14:00 hours, and processed within 2 hours of collection. For very young children, urine bags (Hollister 7511 U-Bag Urine Specimen Collector, Hollister, Chicago, Illinois, USA) and disposable diapers were used to collect urine and stool samples respectively. Urine samples were examined microscopically for S haematobium infection following the standard urine filtration method17 and the number of eggs was reported per 10 mL of urine. Stool samples collected were processed using the Kato–Katz method18 and parasite eggs enumerated under a light microscope for S mansoni (in duplicates) per gramme of stool.
Children were diagnosed positive for helminth infection if at least one parasite egg was detected in their urine or stool samples. All children who were positive for S haematobium infection were treated with a single dose of praziquantel (PZQ) at the standard 40 mg/kg bodyweight at each visit. Tablets were crushed and administered with squash and sliced bread19 by local nurses. A post-treatment efficacy check (egg count) was carried out for all such participants at each subsequent follow-up (12 weeks post treatment).
Detection of urinary morbidity
Urine samples collected were examined for visible haematuria (macrohaematuria). Microhaematuria was determined by dipping the reagent end of Uristix reagent strips (Uripath, Plasmatec, UK) into fresh, well-mixed urine for 40 s and the test area compared with a standard colour chart as per manufacturer’s instructions. The strength of the colour change indicates varying concentrations of blood present in the sample, that is, negative, trace, positive (+), positive (++), positive (+++) and positive (++++). For analysis purposes, microhaematuria was classified as either negative or positive.
Sample size calculations
The sample size used for this study was based on the larger, longer-lasting study comparing reinfection rates following different treatment regimens, of which this is a subset. Calculations were based on previous studies showing that PZQ treatment reduces reinfection rates by at least 50%.5 14 Statistical power analysis was performed using Gpower V.3.1.5.20 Based on an expected prevalence of 6.7%, as derived from our previous study in children aged 1–5 years,21 a sample size of 214 in each group would provide α=0.05 and power=0.80. Allowing for a 20% dropout rate, the sample size required for each group during follow-up was 256. The sample size for the larger reinfection study, that is, 525 in a group, was therefore sufficient for this aspect of the study.
Statistical methods
Data analyses were performed using SPSS V.22 (IBM) and GraphPad Prism V.7.02 (GraphPad Software). The χ2 (or Fisher’s exact test for small sample sizes) and the Mann-Whitney was used to test for differences in categorical and numerical variables, respectively. Infection intensity for S haematobium was defined as the arithmetic mean egg count/10 mL of at least two urine samples collected on three consecutive days. The egg count data was further log-transformed (log10 [egg count+1]) to meet the normality assumption of parametric statistics. At baseline, infection intensity (log-transformed) and its relationship as a function of age was determined using a linear regression model. To determine the infection prevalence based on a binary response as a function of age, logistic regression modelling was used as previously described.22 The effect of different factors on the prevalence of schistosome infection and morbidity was determined using logistic regression and the results reported as adjusted ORs (AORs) and 95% CI, along with the test for significance.
For a given morbidity marker, the attributable fraction (AF) in the exposed population (S haematobium-positive) and in the total population was calculated using Miettinen’s formulae23:
In the formulae, RR is the risk ratio of morbidity associated with exposure, Pe is the prevalence of morbidity among the exposed (S haematobium-positive). Because the AFs in this case are from a helminth study and estimated at the cross-sectional level, we substituted the RR with prevalence ratios (PR) as recommended in helminth epidemiology.24 The PR was estimated as a ratio of the proportion of infected individuals with morbidity to the proportion of uninfected individuals with morbidity. AFs were calculated on morbidity markers with PR>1, suggesting an increased risk of morbidity from schistosome infection.24 Treatment efficacy was assessed by means of egg reduction rates (ERR) and cure rates (CR) as described previously.21
Approximate CIs were calculated using the modified Wald method25 and P<0.05 was considered significant.
Results
Demographics
Of the 1502 recruited into the study, 794 (52.9%) were male. Age range was between 0.5 and 5 years (median=3.5 years; IQR 2.5–4.3). The youngest participant in whom S haematobium infection was detected was a year old. Maximum loss to follow-up was recorded at first follow-up in May 2016 (64 participants; 12.2%). Overall follow-up rates in the longitudinal cohort, including participants for post-treatment efficacy check, were 87.8% in May 2016, 93.7% in August 2016, 95.1% in November 2016 and 88.8% in February 2017.
S haematobium epidemiology at baseline
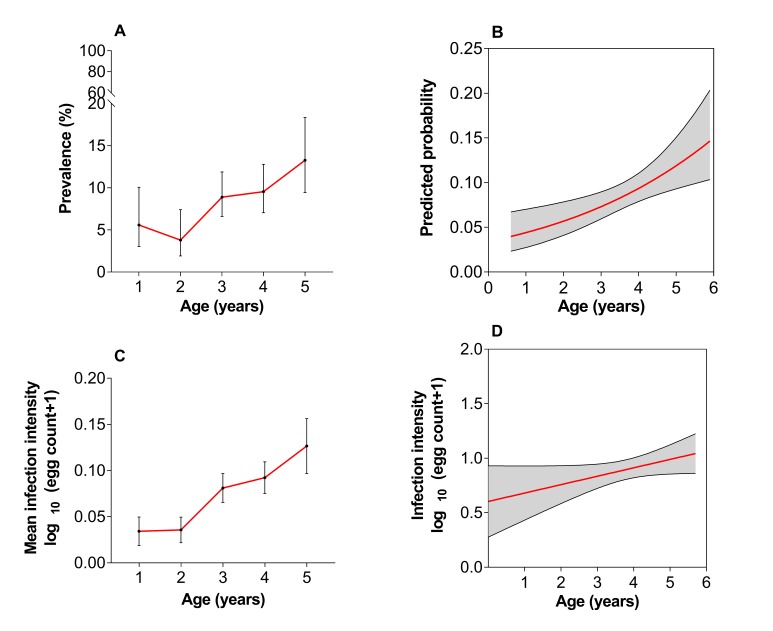
S haematobium infection prevalence at baseline in the 1502 participants was 8.5% (95% CI 7.2 to 10.0). The median age of children positive for infection was significantly higher compared with those negative for infection (4.0 vs 3.5 years; P=0.001). Infection prevalence increased with age as shown in figure 1A,B. The overall mean infection intensity was 7.9 eggs/10 mL urine (95% CI 6.4 to 9.7). The majority of children, 93.7% (95% CI 87.9 to 97.0) presented with light infections (<50 eggs/10 mL of urine) based on the WHO classification.19 Infection intensity increased with age as shown in figure 1C,D. There was no significant difference in infection prevalence between males and females; 8.9% (95% CI 7.1 to 11.1) and 8.1% (95% CI 6.3 to 10.4; P=0.067), respectively.
Figure 1.
(A) Schistosoma haematobium infection prevalence with age; prevalence varied with age (P<0.001) and (B) age-predicted probability of infection; prevalence increased as children grew older (P=0.002). (C) S haematobium infection intensity with age; intensity varied with age (P<0.001) and (D) age-predicted intensity of infection; infection intensity increased as children grew older. Error bars indicate 95% CI (A) or SEM (C), and shaded areas indicate 95% CI; (B, D).
Morbidity at baseline
Prevalence of urinary morbidity was 0.7% (95% CI 0.3 to 1.5) for macrohaematuria and 8.6% (95% CI 6.9 to 10.6) for microhaematuria. Malnutrition measured by different indices were as follows: MUAC, 2.2% (95% CI 1.4 to 3.2), MUACZ, 7.4% (95% CI 6.0 to 9.1) and WHZ, 8.2% (95% CI 6.8 to 9.9). Prevalence of underweight measured by WAZ was 10.1% (95% CI 8.5 to 11.9), and stunting by HAZ was 18.0% (95% CI 16.0 to 20.3). Comparing infected versus uninfected children, prevalence of microhaematuria (43.5%; 95% CI 34.8 to 52.6 vs 3.4%; 95% CI 2.4 to 5.0; P<0.001) and stunting (27.0%; 95% CI 19.9 to 35.6 vs 17.0%; 95% CI 14.9 to 19.4; P=0.009) was significantly higher among children with S haematobium infection.
Morbidity attributable to S haematobium infection
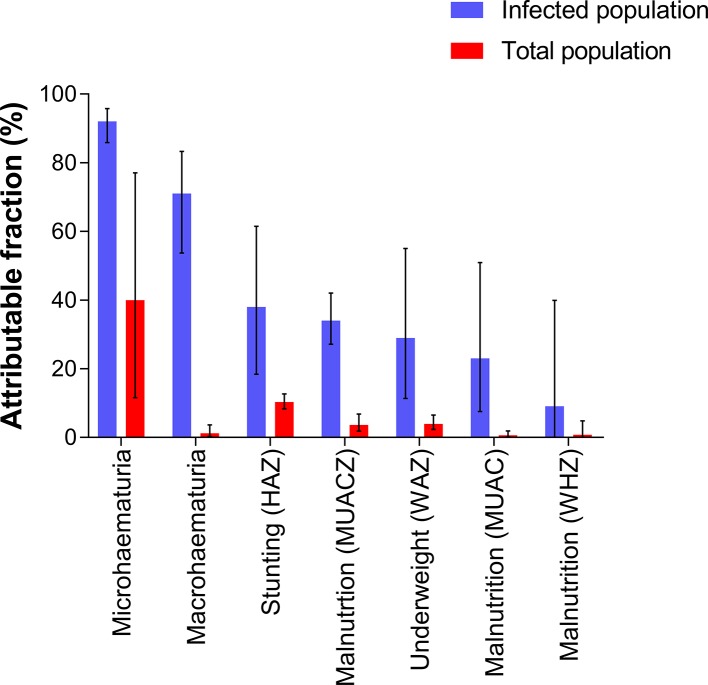
Morbidities from schistosome infection are not specific and may relate to different physiological, biochemical and immunological processes. We determined how much of the detected morbidity was attributable to schistosome infection by first determining PRs. All the morbidity markers considered had PR >1 (significant association with schistosome infection) except underweight by BAZ (table 1). Based on AFs, microhaematuria was the most dominant morbidity marker attributed to schistosome infection both in infected children and at the population level. Macrohaematuria, on the other hand, was highly attributable to schistosome infection in the infected population but this was not the case in the total population. Of the anthropometric markers, stunting was the most dominant marker attributed to schistosome infection both at the population level and among the infected children (figure 2).
Table 1.
Prevalence ratios (PRs) for detected schistosome-related morbidity
| Morbidity | Diagnostic tool | PR (95% CI) |
| Microhaematuria | Urine dipsticks | 12.6 (11.6 to 14.1) |
| Macrohaematuria | Visual inspection (colorimetry) | 3.4 (1.9 to 5.4) |
| Stunting | HAZ | 1.6 (1.05 to 2.31) |
| Malnutrition | WHZ | 1.1 (0.9 to 1.4) |
| MUACZ | 1.5 (1.3 to 1.9) | |
| MUAC | 1.3 (0.8 to 1.9) | |
| Underweight | WAZ | 1.4 (1.2 to 1.6) |
| BAZ | 1.0 (0.8 to 1.3) |
BAZ, body mass index for age Z scores; HAZ, height-for-age Z scores, MUAC, mid-upper arm circumference Z scores; WHZ, weight-for-height Z scores; WAZ, weight-for-age Z scores.
Figure 2.
Estimated proportion of morbidity attributable to Schistosoma haematobium infection in the infected population (blue; AFe) and in the total population (red; AFp). Error bars indicate 95% CIs. BAZ, body mass index-for-age Z scores; HAZ, height-for-age Z scores; MUAC, mid-upper arm circumference Z scores; WAZ, weight-for-age Z scores; WHZ, weight-for-height Z scores .
Likelihood of schistosome infection and morbidity
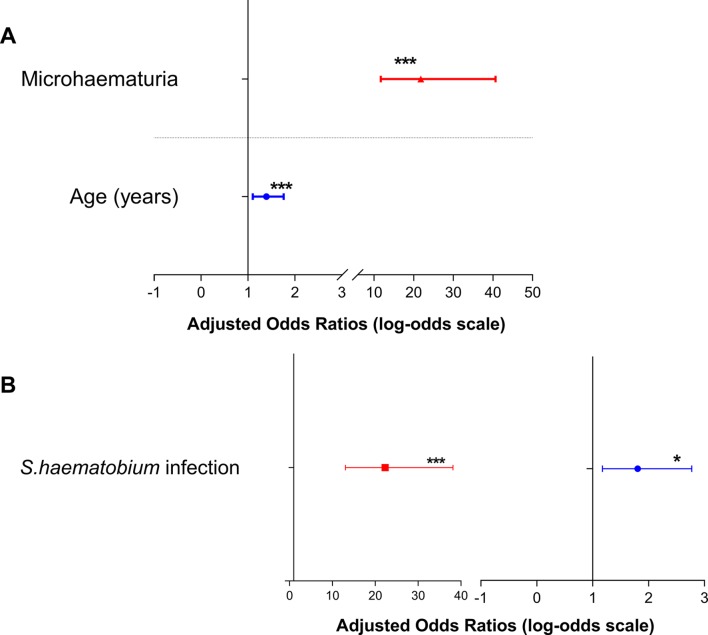
Multiple logistic regression analysis showed that with every unit increase in age, children were more likely to acquire S haematobium infection (AOR=1.4; 95% CI 1.1 to 1.8; P=0.005). Children who presented with microhaematuria were more likely to be positive for S haematobium infection (AOR=21.8; 95% CI 11.7 to 40.7; P<0.001) as shown in figure 3A. Similarly, children presenting with S haematobium infection were more likely to present with microhaematuria (AOR=25.6; 95% CI 14.5 to 45.1; P<0.001) and stunting (AOR=1.7; 95% CI 1.1 to 2.7; P=0.014) as shown in figure 3B.
Figure 3.
Forest plot showing (A) the odds of presenting with Schistosoma haematobium infection and (B) odds of presenting with microhaematuria (left) and stunting (right). Error bars indicate the 95% CIs. *P<0.05, ***P<0.001. Non-significant variables were excluded from the final logistic regression model.
Incidence of infection and morbidity
To determine infection and morbidity incidence, 525 schistosome-negative children were followed quarterly for 12 months to determine schistosome infection and morbidity acquired in the previous three months. Based on the longitudinal data, annual incidence of S haematobium infection was 17.4% (95% CI 13.7 to 21.8) and that of microhaematuria was 20.4% (95% CI 15.8 to 26.0). S haematobium incidence rates in the dry season was 4.9% in May (95% CI 3.1 to 7.8) and 6.5% in August (95% CI 4.1 to 9.9) while that in the rainy season was 3.8% in November (95% CI 2.1 to 6.6) and 3.7% in February (95% CI 2.1 to 6.5). Difference in overall incidence rates however was not significant between the dry (10.4%; 95% CI 7.6 to 14.1) and rainy seasons (7.4% total; 95% CI 5.0 to 10.9; P=0.175). The quarterly incidence of microhaematuria recorded was 2.0% in May (95% CI 0.4 to 5.9), 2.8% in August (95% CI 1.0 to 6.6), 13.3% in November (95% CI 9.6 to 18.3) and 4.3% in February (95% CI 2.2 to 8.1).
Treatment efficacy and effects on morbidity
The treatment efficacy was calculated from children positive for infection at baseline and those that became infected throughout the year. Thus, a total of 187 children were treated for infection (127 at baseline and 60 from the longitudinal cohort), of which post-treatment data were available for 156 (follow-up rate: 83.4%). PZQ was efficacious in reducing S haematobium infection, as indicated by the high CR (96.2%; 95% CI 91.7 to 98.4) and ERR (99.8%; 95% CI 99.2 to 100). In addition, the mean infection intensity pretreatment (7.1 eggs/10 mL urine; 95% CI 5.9 to 8.6) was significantly reduced at post-treatment follow-up (1.1 eggs/10 mL urine; 95% CI 1.0 to 1.2; P<0.001).
To determine the effects of treatment on morbidity identified at baseline, data for microhaematuria were available for 78 of the 127 S haematobium-positive cases identified. Within this cohort, 42.3% (95% CI 32.0 to 53.4) were positive for microhaematuria and this declined significantly post-treatment (10.3%; 95% CI 5.1 to 19.2; P<0.001).
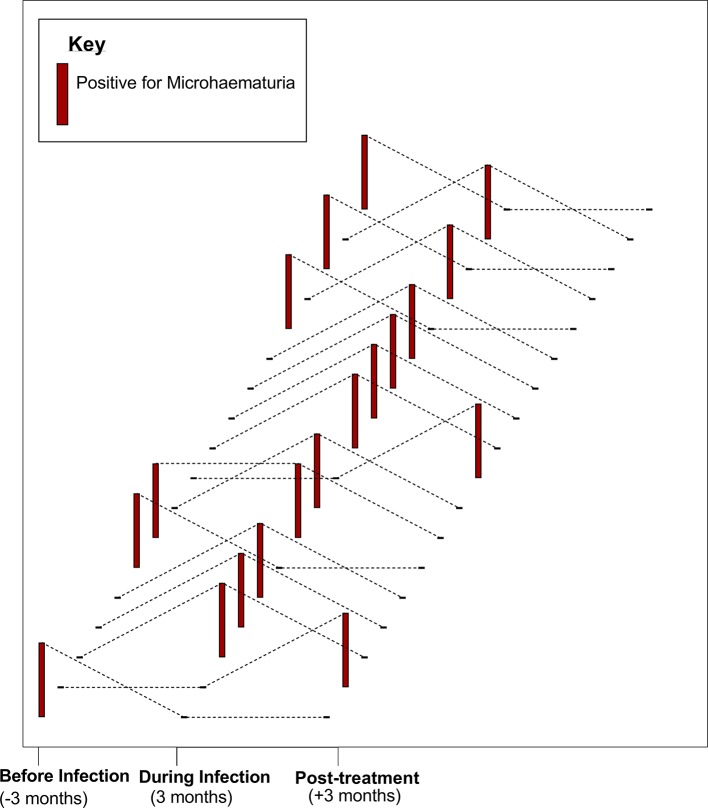
A pooled analysis of participants in whom new infections were detected throughout the follow-up period (group 1) was done to determine the dynamics of microhaematuria with infection, before, during and post infection. A total of 60 new infections were detected throughout the follow-up period. Of this, microhaematuria was detected among 18 individuals; 6 (33.3%) preinfection, 11 (61.1%) during infection and 2 (11.1%) post treatment. In 61.1% of these individuals, microhaematuria coincided with the detection of S haematobium infection (within 3 months) and had resolved by the next survey at 3 months post treatment of infection (figure 4).
Figure 4.
Impact of Schistosoma haematobium infection and praziquantel (PZQ) treatment on morbidity (microhaematuria). Microhaematuria status for 18 individual participants is shown at three time points: before, during and post infection (post treatment). Each data set (dotted line) represents one individual. Tall, red bars indicate positive microhaematuria, and a black dash indicates negative microhaematuria at specific time points.
Discussion
Contrary to the previously held assumption of low risk to schistosome infection in PSAC,26 the frequency of schistosome infections among infants and young children is being increasingly recognised.27 We conducted a longitudinal study in a cohort of Zimbabwean PSAC to determine the prevalence, dynamics and incidence of first urogenital schistosome infection and morbidity and its associated risks and health impacts. We showed that PSAC present with schistosome infection (estimated by egg count) and associated morbidity (determined by microhaematuria, growth and nutritional markers) from an early age. We also found that schistosome infection and morbidity can be detected early in PSAC using parasitology and microhaematuria within 3 months of infection and is resolved after treatment.
The observed baseline prevalence of S haematobium infection in PSAC (8.5%) is comparable to levels recorded in PSAC in Ghana (11.2%),4 Malawi 10.7%28 and in our recent studies in Zimbabwe, that is, 13.5%10 and 6.7%.21 PSAC however present with light infections22 27 29 and parasitological egg counts underestimate the prevalence of schistosome infection.4 22 We anticipate this prevalence to increase if the more sensitive serological diagnostic tools are used.22 In agreement with previous findings,7 22 30–32 infection prevalence and intensity increased as children grew older.
The majority of morbidity biomarkers associated with schistosomiasis are non-specific and relate to various physiological, biochemical and immunological processes.33 We determined the prevalence of morbidity and how much of this was attributable to S haematobium infection. Microhaematuria was the most dominant marker for schistosome-related morbidity, and children with S haematobium infection were more likely to present with microhaematuria and vice versa. This agrees with our previous findings in Zimbabwe10 and that by researchers in Nigeria34 on the significance of microhaematuria as a point of care field marker of morbidity in PSAC.
In addition to biomarkers, we also investigated the prevalence of stunting and malnutrition in the children. To the best of our knowledge, this is the first study to show the relationship between S haematobium infection and chronic growth failure (Stunting by HAZ) in PSAC, although studies on polyparasitism35 36 and few schistosome-specific studies37 38 have documented this effect in older children. In accordance with our findings, stunting as detected in older children and adolescents is believed to be the result of chronic antiparasite inflammation which persists during childhood.39 Causality is difficult to establish in this case due to the lag time between the initial infection and the time at which we measured growth failure, and the impact of confounding factors including diet and coinfections. However, there is the need for longer-term studies investigating the impact of treatment on growth and development measures in PSAC. Statistical modelling suggests that with early, repetitive treatment of infection before 6 years of age ‘catch-up growth’ can be effectively facilitated.40
While baseline prevalence and intensity of schistosome infection have been described in PSAC from several African countries, there has not been an incidence study published to date. Here, we document the incidence of urogenital schistosome infection and morbidity in PSAC. Our quarterly incidence is an indication of new schistosome infections in PSAC in endemic areas and the applicability of current tools (urine filtration and urine dipstick) to screen for early infection and morbidity. The incidence of microhaematuria and the AF analyses suggest that even in the very first episodes of infection PSAC suffer morbidity, reflected as microhaematuria; an indication of active bladder and ureteral lesions41 42 and blood loss even in mild schistosome infection.43
PZQ, the antihelminthic of choice for treatment of schistosomiasis, is safe and efficacious in PSAC.44 Our results 12 weeks post treatment showed that a single standard dose of PZQ was effective against S haematobium infection. This is consistent with reports on the efficacy of PZQ treatment for schistosomiasis in PSAC.5 Microhaematuria correlates with S haematobium infection,42 45 and treatment with PZQ reduces morbidity (microhaematuria) as used in large-scale chemotherapy for SAC.33 46 We observed that microhaematuria occurred rapidly within 3 months of exposure to infection and resolved within 3 months after treatment with PZQ. This is an important indicator for non-delayed schistosome screening and treatment in PSAC, to avert cumulative morbidity which can affect overall health.1 Observation from our field studies prove that suggestions to empower health workers to screen for infection, and making PZQ available in health centres for treatment on detection will be an important control strategy in this age group.47
The seasonal pattern of infection incidence detected is in agreement with the fact that during the dry seasons snail vectors and larval schistosomes become concentrated at permanent and slow-moving water sources, increasing the risk of infection.48 Our observation from fieldwork also indicates that during the rainy seasons, households are less reliant on water sources for chores and children are less likely to visit water bodies for recreational purposes.
The impact of schistosome infection on the health of children is likely to be greater than those explored here, for example, its impact on neurocognitive development. Mechanistic and epidemiological studies separating the effects of schistosome infections from other confounders would be informative in identifying and portioning causation in AFs. The present study did not measure the impact of existing feeding, nutrition habits and socioeconomic status on stunting and its relationship to schistosome infections. A limitation of the parasitological detection of infection is that some light infections may have been missed, resulting in underestimation of the infection rates observed. Nonetheless, the study allows comparison with other studies while parasitological methods remain the predominant schistosome diagnostic in PSAC.
Conclusions
We demonstrated for the first time the incidence of schistosome infection and morbidity in PSAC. We have also shown that a significant proportion of stunting and malnutrition is attributable to S haematobium infection. Morbidity assessed by microhaematuria occurs rapidly within 3 months of first infection and resolves post treatment. More importantly for childhood health and development, schistosome treatment leads to a significant decline in microhaematuria and this resolution occurs within 3 months of PZQ treatment. The study adds scientific evidence to the calls for inclusion of PSAC in schistosome control programmes. Non-delayed schistosome screening and treatment in PSAC is essential to avert accumulative morbidity which can affect overall health.
Acknowledgments
The authors thank the local nurses, health workers and community nurses for their help with the fieldwork. Special thanks are due the study participants and their parents/guardians. They also thank members of the Understanding Bilharzia project in Zimbabwe for their technical help and all the members of the Parasite Immuno-epidemiology Group at the University of Edinburgh for their useful comments in shaping this manuscript.
Footnotes
Handling editor: Alberto L Garcia-Basteiro
Contributors: DNMO, TM, NM, MEJW and FM were involved in conceptualisation. MEJW and FM designed the study. TM, NM, MEJW and FM were involved in project supervision DNMO, TM, NM, MJM-M, TC, EE, TM, LTP, WMW, SAA, JM, CT and FM conducted the field work. DNMO, TC, EE, LTP and WMW curated the data. DNMO, MCT, WMW, MEJW and FM analysed the data. DNMO and FM prepared the draft manuscript, and all authors were involved in review and editing of the manuscript. All authors read and approved the final manuscript.
Funding: Our paediatric schistosomiasis project is funded by the Thrasher Research Fund 12440 and the Wellcome Trust 108061/Z/15/Z. This research is also commissioned by the National Institute of Health Research, using Official Development Assistance (ODA) funding 16/136/33.
Disclaimer: The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute of Health Research or the Department of Health. The funders had no role in the conception, study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: DNMO is supported by the Darwin Trust of Edinburgh and MCT is supported by a Wellcome Trust Strategic Award WT095831.
Patient consent: Parental/guardian consent obtained.
Ethics approval: The study received institutional approval from the University of Edinburgh and ethical approval from the Medical Research Council of Zimbabwe.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.World Health Organization. Report of a meeting to review the results of studies on the treatment of schistosomiasis in preschool-age children. Geneva: World Health Organization, 2010. [Google Scholar]
- 2.Gryseels B, Polman K, Clerinx J, et al. Human schistosomiasis. Lancet 2006;368:1106–18. 10.1016/S0140-6736(06)69440-3 [DOI] [PubMed] [Google Scholar]
- 3.van der Werf MJ, de Vlas SJ, Brooker S, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop 2003;86:125–39. 10.1016/S0001-706X(03)00029-9 [DOI] [PubMed] [Google Scholar]
- 4.Bosompem KM, Bentum IA, Otchere J, et al. Infant schistosomiasis in Ghana: a survey in an irrigation community. Trop Med Int Health 2004;9:917–22. 10.1111/j.1365-3156.2004.01282.x [DOI] [PubMed] [Google Scholar]
- 5.Mutapi F, Rujeni N, Bourke C, et al. Schistosoma haematobium treatment in 1-5 year old children: safety and efficacy of the antihelminthic drug praziquantel. PLoS Negl Trop Dis 2011;5:e1143 10.1371/journal.pntd.0001143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odogwu SE, Ramamurthy NK, Kabatereine NB, et al. Schistosoma mansoni in infants (aged < 3 years) along the Ugandan shoreline of Lake Victoria. Ann Trop Med Parasitol 2006;100:315–26. 10.1179/136485906X105552 [DOI] [PubMed] [Google Scholar]
- 7.Woolhouse ME, Mutapi F, Ndhlovu PD, et al. Exposure, infection and immune responses to Schistosoma haematobium in young children. Parasitology 2000;120(Pt 1):37–44. [DOI] [PubMed] [Google Scholar]
- 8.Subramanian AK, Mungai P, Ouma JH, et al. Long-term suppression of adult bladder morbidity and severe hydronephrosis following selective population chemotherapy for Schistosoma haematobium. Am J Trop Med Hyg 1999;61:476–81. [DOI] [PubMed] [Google Scholar]
- 9.Kjetland EF, Leutscher PD, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol 2012;28:58–65. 10.1016/j.pt.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 10.Wami WM, Nausch N, Midzi N, et al. Identifying and evaluating field indicators of urogenital schistosomiasis-related morbidity in preschool-aged children. PLoS Negl Trop Dis 2015;9:e0003649 10.1371/journal.pntd.0003649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn 2008;4:65–79. 10.1177/1742395307084407 [DOI] [PubMed] [Google Scholar]
- 12.Ingham K, Bradley K, Sanger CW. Encyclopædia Britannica. Zimbabwe: Encyclopædia Britannica, Inc, 2017. [Google Scholar]
- 13.Chandiwana SK. Seasonal patterns in water contact and the influence of water availability on contact activities in two schistosomiasis-endemic areas in Zimbabwe. Cent Afr J Med 1987;33:8–15. [PubMed] [Google Scholar]
- 14.Midzi N, Mduluza T, Chimbari MJ, et al. Distribution of schistosomiasis and soil transmitted helminthiasis in Zimbabwe: towards a national plan of action for control and elimination. PLoS Negl Trop Dis 2014;8:e3014 10.1371/journal.pntd.0003014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mondal D, Minak J, Alam M, et al. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis 2012;54:185–92. 10.1093/cid/cir807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malawi MOH. Guidelines for Community-Based Management of Acute Malnutrition. 2nd edn Malawi: LilongweMinistry of Health, 2016. [Google Scholar]
- 17.Mott KE, Baltes R, Bambagha J, et al. Field studies of a reusable polyamide filter for detection of Schistosoma haematobium eggs by urine filtration. Tropenmed Parasitol 1982;33:227–8. [PubMed] [Google Scholar]
- 18.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 1972;14:397–400. [PubMed] [Google Scholar]
- 19.WHO Expert Committee. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rep Ser 2002;912:1–57. [PubMed] [Google Scholar]
- 20.Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 21.Wami WM, Nausch N, Midzi N, et al. Comparative assessment of health benefits of praziquantel treatment of urogenital schistosomiasis in preschool and primary school-aged children. Biomed Res Int 2016;2016:9162631 10.1155/2016/9162631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wami WM, Nausch N, Bauer K, et al. Comparing parasitological vs serological determination of Schistosoma haematobium infection prevalence in preschool and primary school-aged children: implications for control programmes. Parasitology 2014;141:1962–70. 10.1017/S0031182014000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol 1974;99:325–32. 10.1093/oxfordjournals.aje.a121617 [DOI] [PubMed] [Google Scholar]
- 24.Booth M. The application of attributable risk analysis in helminth epidemiology. Parasitol Today 1998;14:497–500. 10.1016/S0169-4758(98)01349-0 [DOI] [PubMed] [Google Scholar]
- 25.Agresti A, Coull BA. Approximate is Better than “Exact” for Interval Estimation of Binomial Proportions. J Am Stat Assoc 1998;52:119–26. 10.1080/00031305.1998.10480550 [DOI] [Google Scholar]
- 26.Jordan P, Webbe G. Human Schistosomiasis. Illinois: C. C. Thomas, 1969. [Google Scholar]
- 27.Stothard JR, Sousa-Figueiredo JC, Betson M, et al. Closing the praziquantel treatment gap: new steps in epidemiological monitoring and control of schistosomiasis in African infants and preschool-aged children. Parasitology 2011;138:1593–606. 10.1017/S0031182011001235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poole H, Terlouw DJ, Naunje A, et al. Schistosomiasis in pre-school-age children and their mothers in Chikhwawa district, Malawi with notes on characterization of schistosomes and snails. Parasit Vectors 2014;7:153 10.1186/1756-3305-7-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colley DG, Bustinduy AL, Secor WE, et al. Human schistosomiasis. Lancet 2014;383:2253–64. 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanamura HY, Silva RM, Chiodelli SG, et al. IgM-immunofluorescence test as a diagnostic tool for epidemiologic studies of Schistosomiasis in low endemic areas. Mem Inst Oswaldo Cruz 2002;97:485–9. [DOI] [PubMed] [Google Scholar]
- 31.Lengeler C, Utzinger J, Tanner M. Screening for schistosomiasis with questionnaires. Trends Parasitol 2002;18:375–7. [DOI] [PubMed] [Google Scholar]
- 32.van Dam GJ, Wichers JH, Ferreira TM, et al. Diagnosis of schistosomiasis by reagent strip test for detection of circulating cathodic antigen. J Clin Microbiol 2004;42:5458–61. 10.1128/JCM.42.12.5458-5461.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webster JP, Koukounari A, Lamberton PH, et al. Evaluation and application of potential schistosome-associated morbidity markers within large-scale mass chemotherapy programmes. Parasitology 2009;136:1789–99. 10.1017/S0031182009006350 [DOI] [PubMed] [Google Scholar]
- 34.Salawu OT, Odaibo AB. Urogenital schistosomiasis and urological assessment of hematuria in preschool-aged children in rural communities of Nigeria. J Pediatr Urol 2014;10:88–93. 10.1016/j.jpurol.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 35.Bustinduy AL, Parraga IM, Thomas CL, et al. Impact of polyparasitic infections on anemia and undernutrition among Kenyan children living in a Schistosoma haematobium-endemic area. Am J Trop Med Hyg 2013;88:433–40. 10.4269/ajtmh.12-0552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mupfasoni D, Karibushi B, Koukounari A, et al. Polyparasite helminth infections and their association to anaemia and undernutrition in Northern Rwanda. PLoS Negl Trop Dis 2009;3:e517 10.1371/journal.pntd.0000517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Assis AM, Barreto ML, Prado MS, et al. Schistosoma mansoni infection and nutritional status in schoolchildren: a randomized, double-blind trial in northeastern Brazil. Am J Clin Nutr 1998;68:1247–53. [DOI] [PubMed] [Google Scholar]
- 38.Coutinho HM, Acosta LP, McGarvey ST, et al. Nutritional status improves after treatment of schistosoma japonicum-infected children and adolescents. J Nutr 2006;136:183–8. [DOI] [PubMed] [Google Scholar]
- 39.Friedman JF, Kanzaria HK, Acosta LP, et al. Relationship between Schistosoma japonicum and nutritional status among children and young adults in Leyte, the Philippines. Am J Trop Med Hyg 2005;72:527–33. [PubMed] [Google Scholar]
- 40.Gurarie D, Wang X, Bustinduy AL, et al. Modeling the effect of chronic schistosomiasis on childhood development and the potential for catch-up growth with different drug treatment strategies promoted for control of endemic schistosomiasis. Am J Trop Med Hyg 2011;84:773–81. 10.4269/ajtmh.2011.10-0642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdel-Salam E, Ehsan A. Cystoscopic picture of Schistosoma haematobium in Egyptian children correlated to intensity of infection and morbidity. Am J Trop Med Hyg 1978;27:774–8. [DOI] [PubMed] [Google Scholar]
- 42.Mott KE, Dixon H, Osei-Tutu E, et al. Relation between intensity of Schistosoma haematobium infection and clinical haematuria and proteinuria. Lancet 1983;1:1005–8. [DOI] [PubMed] [Google Scholar]
- 43.Mahmood A. Blood loss caused by helminthic infections. Trans R Soc Trop Med Hyg 1966;60:766–9. [DOI] [PubMed] [Google Scholar]
- 44.Mutapi F, Rujeni N, Bourke C, et al. Schistosoma haematobium treatment in 1-5 year old children: safety and efficacy of the antihelminthic drug praziquantel. PLoS Negl Trop Dis 2011;5e1143 10.1371/journal.pntd.0001143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkins HA, Goll P, Marshall TF, et al. The significance of proteinuria and haematuria in Schistosoma haematobium infection. Trans R Soc Trop Med Hyg 1979;73:74–80. [DOI] [PubMed] [Google Scholar]
- 46.Koukounari A, Gabrielli AF, Toure S, et al. Schistosoma haematobium infection and morbidity before and after large-scale administration of praziquantel in Burkina Faso. J Infect Dis 2007;196:659–69. 10.1086/520515 [DOI] [PubMed] [Google Scholar]
- 47.Bustinduy AL, Friedman JF, Kjetland EF, et al. Expanding Praziquantel (PZQ) Access beyond Mass Drug Administration Programs: Paving a Way Forward for a Pediatric PZQ Formulation for Schistosomiasis. PLoS Negl Trop Dis 2016;10:e0004946 10.1371/journal.pntd.0004946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woolhouse ME, Chandiwana SK. Spatial and temporal heterogeneity in the population dynamics of Bulinus globosus and Biomphalaria pfeifferi and in the epidemiology of their infection with schistosomes. Parasitology 1989;98(Pt 1):21–34. 10.1017/S0031182000059655 [DOI] [PubMed] [Google Scholar]