Abstract
Objectives
Current strategies to prevent adult pneumococcal disease have been recently reviewed in Italy. We did a postlicensure study to estimate the direct vaccine effectiveness (VE) of the 13-valent pneumococcal conjugate vaccine (PCV13) against adult pneumococcal community-acquired pneumonia (pCAP).
Study design
Between 2013 and 2015, a 2-year prospective cohort study of adults with CAP was conducted in the Apulia region of Italy where the average vaccine uptake of PCV13 was 32% among adults ≥65 years. The test-negative design was used to estimate VE against all episodes of confirmed pCAP and vaccine-type (VT)-CAP. VE in a subgroup of patients managed in the community was also estimated using a matched case–control design. VE was calculated as one minus the OR times 100%.
Results
The overall VE of PCV13 was 33.2% (95% CI −106.6% to 82%) against pCAP irrespective of serotype and 38.1% (95% CI −131.9% to 89%) against VT-CAP in the cohort of adults ≥65 years. The VE was 42.3% (95% CI −244.1% to 94.7%) against VT-CAP in the age group at higher vaccine uptake. For the subgroup of cases managed in the community, the overall VE against disease due to any pneumococcal strain was 88.1% (95% CI 4.2% to 98.5%) and 91.7% (95% CI 13.1% to 99.2%) when we controlled for underlying conditions.
Conclusions
Although our results are non-significant, PCV13 promises to be effective against all confirmed pCAP already with modest levels of uptake in the population of adults ≥65 years of age. Larger studies are needed to confirm the direct vaccine benefits.
Keywords: pcv13, pneumococcal conjugate vaccine, vaccine effectiveness, adult, community-acquired pneumonia
Strengths and limitations of this study.
The test-negative method used in this study has reduced the risk for selection bias since both cases and controls were recruited in one process and arose from the whole population when the same enrolment criteria were met.
The study was designed to capture community-acquired pneumonia (CAP) cases managed in the community, reducing the underestimate of the true impact of pneumococcal disease.
The active surveillance of CAP was performed in a single-regional setting leading to a small study size reducing the power to detect statistically significant effects.
The test-negative method may have overestimated 13-valent pneumococcal conjugate vaccine vaccine effectiveness if, by reducing the risk of acquiring vaccine-type serotypes, vaccination increases the risk of acquiring non-vaccine-type serotypes as is likely to be the case if there is serotype replacement.
Introduction
Routine administration of the 7-valent pneumococcal conjugate vaccine (PCV7) since 2000 and of the second-generation conjugate vaccines (PCV10 and PCV13) since 2010 has resulted in an overall reduction in the rates of pneumococcal disease in both vaccinated and unvaccinated children, and indirectly among adults in several countries, owing to herd immunity.1 2 However, the most recent available data suggest that significant burden still results from pneumococcal infection in older adults.
In the USA, the annual incidence of community-acquired pneumonia (CAP) requiring hospitalisation in 2010–2012 was 24.8 cases (95% CI 23.5 to 26.1) per 10 000 adults aged 18 years or older, with a prevalence of pneumococcal disease of 5% and an incidence that was almost five times as high among adults aged 65 years or older as among younger adults.3 In 2013, an estimated 10% of CAP cases in adults aged ≥65 years were caused by Streptococcus pneumoniae serotypes potentially preventable with the use of PCV13 in this population.4
In the UK, incidence of adult pneumococcal pneumonia declined over the 2008–2013 period, with serotypes included in PCV13 declining post-PCV13 introduction, suggesting an early herd protection effect from infant PCV13 on adult bacteraemic and non-bacteraemic disease. However, the most recent available data from 2012 to 2013 showed an incidence of 20.6 per 100 000 population for hospitalised adult pneumococcal CAP (pCAP) and 8.6 per 100 000 population for PCV13 serotype CAP.5
In Italy, high infant PCV13 coverage has been achieved since 2011 (vaccine uptake rate 90%–95%).6 For adults, only 23-valent pneumococcal polysaccharide vaccine (PPSV23) was recommended for routine immunisation of those aged ≥65 years and at-risk individuals, but the vaccine uptake rates have been low to date. In recent years, some Italian regions have recommended PCV13 to adults with underlying diseases and to the elderly.7
Impact of PCV infant vaccination on adult pneumococcal pneumonia has not been well established in Italy. The hospitalisation rates for pneumococcal pneumonia in the elderly population have remained relatively stable over the past decade, indicating a lack of herd protection among older age groups.8
In 2014, the Community-Acquired Pneumonia Immunization Trial in Adults (CAPITA) trial conducted in the Netherlands demonstrated the efficacy of PCV13 for the prevention, in those aged ≥65, of vaccine-type (VT) pCAP.9 Evidence from the CAPITA trial led to new Advisory Committee on Immunization Practices (ACIP) PCV13 recommendations,4 10 11 of which a review is planned for 2018 owing to potential changes in the epidemiological situation. In particular, studies evaluating the postlicensure effectiveness of PCV13 for prevention of invasive and non-bacteraemic pneumococcal pneumonia among adults ≥65 years old using a case–control design are needed.11 This study attempts to address this unmet need.
We report findings of the direct impact of PCV13 from a 2-year prospective study of a cohort of pCAP adults.
Methods
From January 2013 to January 2015, a prospective, multicentre, population-based, active surveillance study of adults with CAP was conducted over 2 years in Apulia, a large Italian region of approximately 4 000 000 inhabitants. PPSV23 was introduced in Apulia in 2000 for use in adults aged ≥65 years and was replaced by PCV13 in November 2011 for adults aged 65, 70 and ≥75 years.12 In 2015, the average vaccine uptake of PCV13 was 32% among adults aged 65–75 years (11 cohorts) and 10% in the overall population >75 years.13
According to 2013 census figures, the designated surveillance area included about 788 000 adults aged 65 years or older.14 Patients were enrolled in two different study settings from a network of 31 sentinel physicians. Surveillance for suspected CAP was conducted by 16 treating physicians among patients presenting at 13 hospitals located in the region (a total of 13 841 patients aged ≥65 years admitted to Departments of Respiratory Medicine in 2013–2014) and by 15 general practitioners (GPs) providing primary care for a total of 5010 persons aged ≥65 years throughout the region.
Adults ≥65 years with symptoms suggestive of lower respiratory tract infection were eligible for enrolment if they presented to a study hospital or to their GP for a clinical assessment; resided in the study region; had at least 2 of the 11 clinical criteria listed in box 1 and had evidence of new infiltrates on chest radiography consistent with pneumonia.15 Patients were excluded if they had been hospitalised recently (<10 days) or were functionally dependent nursing home, long-term care facility or other institution residents (healthcare-associated pneumonia cases).
Box 1. Clinical criteria for definition of community-acquired pneumonia.
New cough or sputum production.
Fever >38.0°C or hypothermia <36.1°C.
Chest pain.
Dyspnoea.
Tachypnoea.
New altered mental status.
Abnormal lung examination.
Respiratory failure.
Leucocytosis (white cell count >10×109/L or >15% bands) or leucopenia (white cell count <4.5×109/L).
C reactive protein value >3 times the upper limit of normal.
Hypoxaemia with a partial oxygen pressure <60 mm Hg while the patient was breathing room air.
Written informed consent was obtained from all the patients or their caregivers before enrolment. Study sentinel physicians used a standardised electronic case report form to collect information regarding patient demographics, clinical information, microbiological investigations and status regarding receipt of pneumococcal vaccination; pneumonia severity was assessed using the CURB-65 score (confusion, urea >7 mmol/L, respiratory rate ≥30 breaths/min, low systolic <90 mm Hg or diastolic ≤60 mm Hg blood pressure, age ≥65 years). Patients were contacted 30 days after enrolment for outcome measures collection (30-day mortality, recovery with sequelae).
The study was conducted according to the principles expressed in the Declaration of Helsinki.
Blood samples and nasopharyngeal swabs were obtained from the patients who presented to sentinel centres/GPs with symptoms of lower respiratory tract infection within 24 hours after presentation. In the case of patients with a productive cough, sputum was obtained. Bronchoalveolar-lavage (BAL) samples, blood culture and sputum specimens that had been obtained for clinical care were sent to the Regional Reference Laboratory for Invasive Bacterial Diseases and analysed for the study.
S. pneumoniae was isolated by PCR and multiplex sequential PCR. Bacterial genomic DNA was extracted from 200 µL of biological samples using the QIAamp DNeasy Blood and Tissue kit (Qiagen), according to the manufacturer’s instructions. Detection of S. pneumoniae was performed using a commercial multiplex assay (Pneumobacter ACE Detection for blood and Meningitis ACE Detection for Cerebro Spinal Fluid, Seegene; Sensitivity: detection limit of the Seeplex Pneumobacter ACE Detection=10 copy/reaction—10 copy/3 µL DNA). S. pneumoniae serotyping was performed on PCR positive samples through a sequential multiplex PCR.16 Twenty-nine primer pairs were designed to target serotypes 1, 3, 4, 5, 6 A/B, 7F, 7C, 8, 9V, 10A, 11A, 12F, 14, 15A, 15 B/C, 16F, 17F, 18, 19A, 19F, 20, 22F, 23F, 31, 33F, 34, 35B, 35F and 38. A primer pair (primers cpsA-f and cpsA-r) was also included as an internal control targeting the cpsA (pneumococcal capsular polysaccharide synthesis gene) locus found in all pneumococci.17 The amplified products, ranging from 250 bp to 988 bp, were analysed by means of electrophoresis on a 2% agarose gel (Life Technologies) and visualisation underultraviolet light.
Patients with a positive PCR result for S. pneumoniae on blood/sputum/BAL were deemed to have pCAP. Nasopharyngeal swab samples were not used for the diagnosis of pCAP, due to the poor sensitivity and specificity previously reported.3
Vaccine effectiveness analysis
To estimate the vaccine effectiveness (VE) of PCV13 in the prevention of pCAP, a test-negative design was performed on cases enrolled during the study surveillance period. The analysis included two primary end points:
VE in preventing confirmed pCAP irrespective of serotype, where cases were patients who had an episode of invasive or non-invasive pCAP due to any pneumococcal strain and controls were participants with an episode of non-pneumococcal pneumonia.
VE in preventing confirmed VT CAP, where cases were patients with an episode of invasive or non-invasive pCAP due to VT strains and controls were patients with non-VT (NVT) pCAP, non-typeable isolates or non-pneumococcal pneumonia.
These two end points were further assessed among patients who had underlying conditions.
The exposure of interest was vaccination with PCV13. The exposure to PPSV23 given <5 years prior to study enrolment was also assessed. Data were based on verified vaccine (both PCV13 and PPSV23) information and not self-report.
To further study the effectiveness of PCV13 in the prevention of episodes of confirmed pCAP managed in the community, post hoc analysis of a subgroup of cases reported by GPs network was performed. This analysis was performed according to a 1:3 matched case–control design. For every enrolled patient, a list of potential asymptomatic controls was generated from GPs subjects’ medical records. Three controls, matched by GP, age and gender, were selected at random for each case. Controls were enrolled if they provided written informed consent to their GP. Study personnel contacted these GPs to obtain a medical and vaccination history for every control. Immunisation information system was also used to verify vaccination histories.
Statistical analysis
Statistical analyses were performed in STATA (V.14; StataCorp).
χ2 analysis or Fisher’s exact test, two sided were used to calculate the p value for the difference between study groups in percentages of subjects reporting a pCAP or non-pneumococcal pneumonia.
Exact logistic regression was used to calculate the unadjusted ORs of vaccination together with 95% CIs. In the post hoc subgroup analysis, the matched ORs for vaccination in cases and controls were calculated using conditional logistic regression, controlling for the presence of underlying conditions. VE was calculated as one minus the OR times 100%.
Results
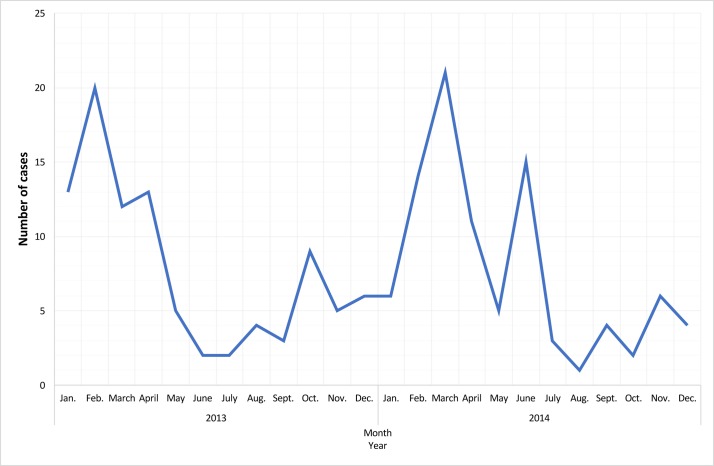
From the 1867 eligible adults identified over the 2-year period, 226 consented to the study. Main reasons why patients (or a relative) declined to participate were old age, difficulty in reading and/or understanding the invite letter, acute confusion or cognitive impairment, or a desire not to have medication altered. Of 226 patients recruited, 176 (77.9%) were admitted to Departments of Respiratory Medicine and 50 (22.1%) were registered with a GP. Pneumonia severity was low, moderate and high in 47 (20.8%), 167 (73.9%) and 12 (5.3%) adults, respectively. Forty patients were excluded as they were unable to provide a blood or a sputum/BAL sample, leaving 186 in the cohort for analyses. The median age of the cohort was 79 years (IQR, 73–85) and 65 (34.9%) were female. Twenty (10.8%) had received PCV13 and 60 (32.3%) had received PPSV23 <5 years prior to enrolment (table 1). The seasonal distribution of CAP cases followed a pattern similar to that of many other respiratory diseases, with similar peaks during the winter months of the 2-year period (figure 1).
Table 1.
Characteristics of adults with CAP requiring hospitalisation or managed in the community
| Pneumococcal group (n=59) | Non-pneumococcal group (n=127) | CAP cohort (n=186) | |
| Demographics | |||
| Age years | 79 (71 to 83) | 79 (73 to 85) | 79 (73 to 85) |
| Male | 42 (71.2) | 79 (62.2) | 121 (65.1) |
| Reporting | |||
| Hospital physicians | 38 (64.4) | 105 (82.7) | 143 (76.9) |
| GPs | 21 (35.6) | 22 (17.3) | 43 (23.1) |
| Any underlying comorbidity* | |||
| Chronic heart disease | 28 (47.5) | 70 (55.1) | 98 (52.7) |
| Chronic respiratory disease | 29 (49.2) | 52 (40.9) | 81 (43.6) |
| Diabetes | 14 (23.7) | 32 (25.2) | 46 (24.7) |
| Chronic kidney disease | 2 (3.4) | 9 (7.1) | 11 (5.9) |
| Chronic liver disease | 2 (3.4) | 3 (2.4) | 5 (2.6) |
| Malignancy | 0 | 4 (3.1) | 4 (2.1) |
| Asplenia | 1 (1.7) | 1 (0.8) | 2 (1.1) |
| Status regarding receipt of pneumococcal vaccination† | |||
| PCV13 | 5 (8.5) | 15 (11.8) | 20 (10.8) |
| PPSV23 given <5 years prior to study enrolment | 20 (33.9) | 40 (31.5) | 60 (32.3) |
| Outcomes‡ | |||
| 30-day mortality | 2 (3.4) | 2 (1.6) | 4 (2.2) |
| Recovery with sequelae | 5 (8.5) | 20 (15.7) | 25 (13.4) |
Data are number, median (IQR) or number (%).
*The groups were not mutually exclusive and therefore do not sum to 100%.
†For both vaccines, patients were considered to be vaccinated if they had received the vaccine at least 2 weeks before enrolment. Data were missing for four patients in the non-pneumococcal group. One patient had received a dose of PCV13 ≥1 year after receipt of a PPSV23 dose given <5 years prior to study enrolment.
‡Data were missing for 20 patients in the pneumococcal group and 40 patients in the non-pneumococcal group.
CAP, community-acquired pneumonia; GPs, general practitioners; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
Figure 1.
Seasonal distribution of cases in the community-acquired pneumonia cohort (n=186).
A nasopharyngeal swab was obtained from 171 of the 186 (91.9%) participants, a blood sample from 152 (81.7%), a sputum specimen from 139 (74.7%) and a BAL specimen from 3 (1.6%). S. pneumoniae was detected in 71 (41.5%) nasopharyngeal swab, 2 (1.3%) blood, 55 (39.6%) sputum and 2 (66.7%) BAL.
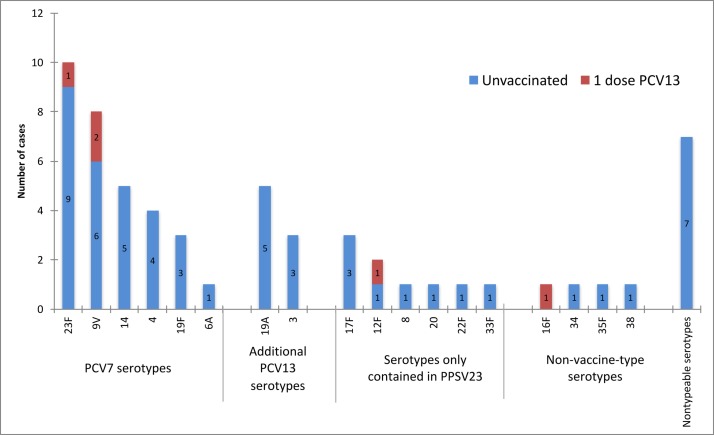
Of 186 in the CAP cohort, 59 (31.7%, 95% CI 25.7% to 38.9%) adults were identified as pCAP. More than half (31, 52.5%) had disease caused by one of the PCV7 serotypes, of which 23F, 9V, 14, 4 and 19F were the most common; 8 (13.6%) had CAP due to additional PCV13 serotypes, of which 19A and 3 were the most common; 9 (15.2%) had CAP due to serotypes only contained in PPSV23; four (6.8%) had NVT disease; 7 (11.9%) had non-typeable pCAP (figure 1). Five had received one dose of PCV13 and 20 one dose of PPSV23 <5 years prior to enrolment. Of 39 patients infected with serotypes contained in PCV13, three had received this vaccine (disease caused by 9V in two cases and 23F in one) (figure 2).
Figure 2.
Number of cases of pneumococcal community-acquired pneumonia by serotype and vaccination status (n=59). PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
Baseline characteristics and outcomes were balanced between pneumococcal and non-pneumococcal groups (table 1).
Vaccines effectiveness
PCV13 VE estimate was 33.2% (95% CI −106.6% to 82%) against pCAP irrespective of serotype and 38.1% (95% CI −131.9% to 89%) against VT CAP in the cohort of adults ≥65 years. The VE was 42.3% (95% CI −244.1% to 94.7%) with respect to VT-CAP in the age group at higher vaccine uptake (65–75 years).
The VE was 34.6% (95% CI −104.6% to 82.5%) against CAP due to any pneumococcal strain and 40.1% (95% CI −127.5% to 89.4%) against CAP due to VT strains for adults with underlying conditions.
PCV13 VE against the two primary end points in patients naïve to PPSV23 or vaccinated with PPSV23 ≥5 years prior to enrolment was 27.35% (95% CI −136.5% to 81.5%) and 17% (95% CI −234.7% to 85.9%), respectively, lower than VE estimates in subgroups defined irrespective of PPSV23 immune status (table 2).
Table 2.
PCV13 effectiveness estimates against all episodes of confirmed pCAP and CAP due to vaccine serotypes in adults by vaccination status and the presence of underlying conditions
| Cases vaccinated/ unvaccinated |
Controls vaccinated/ unvaccinated* |
Vaccine effectiveness (%) | 95% CI | |
| pCAP (any strain) | 5/54 | 15/108† | 33.2 | −106.6% to 82% |
| VT-CAP | 3/36 | 17/126‡ | 38.1 | −131.9% to 89% |
| VT-CAP in the age group at higher vaccine uptake (65–75 years) | 2/14 | 8/32‡ | 42.3 | −244.1% to 94.7% |
| pCAP in patients with ≥1 comorbid disorder | 5/46 | 15/90† | 34.6 | −104.6% to 82.5% |
| VT-CAP in patients with ≥1 comorbid disorder | 3/31 | 17/105‡ | 40.1 | −127.5% to 89.4% |
| pCAP in patients naïve to PPSV23 or vaccinated with PPSV23 ≥5 years prior to enrolment | 5/34 | 14/69† | 27.3 | −136.5% to 81.5% |
| VT-CAP in patients naïve to PPSV23 or vaccinated with PPSV23 ≥5 years prior to enrolment | 3/19 | 16/84‡ | 17 | −234.7% to 85.9% |
*Vaccine data were missing for four controls.
†Controls were patients with an episode of non- pneumococcal pneumonia.
‡Controls were patients with NVT pneumococcal CAP, non-typeable isolates or non- pneumococcal pneumonia.
CAP, community-acquired pneumonia; NVT, non-vaccine-type; pCAP, pneumococcal community-acquired pneumonia; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine; VT, vaccine-type.
PPSV23 was not shown to have effectiveness against pCAP in either all adults (VE −4%, 95% CI −115.4% to 50.4%) or those with comorbidities (VE −5.5%, 95% CI −130.5% to 52.2%) naïve to PCV13.
Post hoc subgroup analysis of 21 confirmed cases reported by GPs network (table 1) provided estimates of PCV13 effectiveness in preventing pCAP managed in the community.
We identified 4965 asymptomatic adults as potential controls, of whom 129 (2.6%) died and 95 (1.9%) were excluded because they left the GP’s practice during the study period. Among the remaining 4741 controls, 63 (three per case) were selected for the analysis. Nine (14.2%) controls were replaced for difficulty in obtaining consent.
Review of GP records showed that the controls were of similar age and gender to cases, but differed in vaccination and clinical history: one case (4.8%) and one control (1.6%) had received PCV13 at least 2 weeks before enrolment, whereas 18 controls (28.6%) were vaccinated during the study period; 16 cases (76.2%) and 36 controls (57.1%) had at least one comorbid disorder.
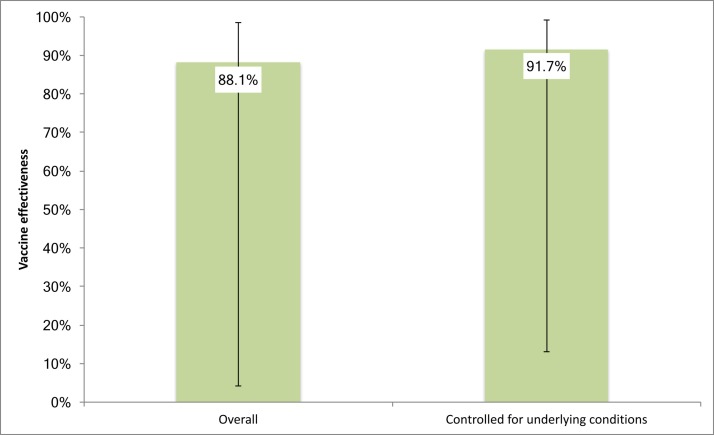
The overall effectiveness of PCV13 against disease due to any pneumococcal strain was 88.1% (95% CI 4.2% to 98.5%) and 91.7% (95% CI 13.1% to 99.2%) when we controlled for underlying conditions (figure 3).
Figure 3.
PCV13 effectiveness (%) estimates against CAP due to any pneumococcal strain managed in the community. Fourteen cases (66.7%) had CAP caused by one of the PCV7 serotypes, of which 14 and 9V were the most common; three cases (14.3%) were due to additional PCV13 serotype 19A; three cases (14.3%) had CAP due to other serotypes; one (4.7%) had non-typeable pneumococcal CAP. One case vaccinated with PCV13 was caused by serotype 12F. CAP, community-acquired pneumonia; PCV13, 13-valent pneumococcal conjugate vaccine.
Discussion
To our knowledge, this is the first study to investigate the effectiveness of PCV13 for prevention of invasive and non-invasive pneumococcal pneumonia among adults ≥65 years. The overall VE was 33.2% against pCAP irrespective of serotype and 38.1% against VT-CAP. The VE was 42.3% against VT-CAP in the age group at higher vaccine uptake. Moreover, we estimated a VE of 88.1% against confirmed pCAP managed in the community, where most patients are treated as outpatients. Given that a substantial proportion of studies are based on hospitalised patients, the true burden of disease is not known in Europe, where only Finland, Spain and the UK have precise epidemiological data on CAP.18 19
In this study, PCV13 serotypes accounted for 66% of all confirmed pCAP. National invasive bacterial diseases surveillance data from Italy showed that, despite an uncertain reduction in the proportion of PCV13 serotypes in the period 2010–2012, these were still responsible for about 56% of cases among over 65s.20 These data suggest that PCV13 VT pneumococcal disease continues to have a high burden in adults in Italy despite childhood PCV13 vaccination and would indicate a lack of herd protection effects in older age groups, in comparison to the vaccinated paediatric population.8 20 21 The most recent data in the UK suggest that, despite an ongoing trend of reduced incidence of PCV13 serotype CAP from paediatric conjugate vaccines,5 22 PCV13 serotypes currently account for 12.6% of all cases of CAP and 41% of pCAP in adults.18 In Ireland, over 5 years following PCV13 introduction to routine childhood vaccination, the number of invasive pneumococcal disease (IPD) associated with additional PCV13 serotypes in adults ≥65 years of age has remained relatively unchanged due to the persistence in serotypes 3 and 19A in this age group.23 In Spain, 13 years after introduction of PCV7/PCV13 for children, a significant proportion of adults continue to develop vaccine serotype CAP, suggesting an insufficient indirect protection.24 Because it cannot be assumed that a decline in pneumococcal disease incidence observed in some countries will always be mirrored elsewhere in the same time, adults aged ≥65 years may have a great potential for disease reduction from PCV13 and may be a primary target of vaccination programmes.9 This is particularly noteworthy for Italian population because the burden of CAP and pneumococcal disease in general is expected to increase with the ageing society, even with the impact of childhood and adult vaccine programmes.18 25
In our study, most of CAP was caused by PCV13 serotypes 23F, 9V, 14, 19A, 4, 19F and 3 (figure 2) that are among the less susceptible to antibiotics.26 Recent findings for Switzerland showed that, while non-susceptible serotypes 19A, 9V, 6B, 23F and 14 among invasive and non-invasive S. pneumoniae decreased over time in patients up to age 64 years due to PCVs infant vaccination,27–29 in patients older than 64 years with invasive S. pneumoniae resistance rates remained unchanged.30 By preventing disease caused by resistant strains, adult PCV13 vaccination provides a robust strategy for combating antimicrobial resistance that is a growing problem in Europe.31
On 19 January 2017, PCV13 has been introduced into the routine vaccination schedule in Italy for all adults aged 65 years followed by a dose of PPV23.32 The decision-making regarding its introduction was based on the CAPITA trial results and the ACIP recommendations, but also on a long history of experience of adult pneumococcal conjugate vaccination in some Italian regions including Apulia.7 12
In this prospective cohort study of adults with CAP, we found that PCV13 promise to be protective against all episodes of confirmed pCAP (VE 33.2%) and against disease caused by serotypes contained in the vaccine (VE 38.1%). Recently published findings of the exploratory efficacy endpoint analysis of the CAPITA trial showed VE of 29% for all episodes of confirmed pCAP and 43% for all non-bacteraemic and non-invasive episodes of VT pCAP,33 findings consistent with the primary efficacy analysis.9 On an ecological level, a preliminary analysis of hospitalisation rates for adult pCAP in Apulia region showed early PCV13 impact after the implementation of an adult vaccine programme (from 180.5 per 100 000 during 2006–2011 to 162.4 per 100 000 during 2012–2016; hospitalisation risk ratio: 0.9, 95% CI 0.83 to 0.97) (unpublished observations).
Moreover, our results showed that PCV13 promise to be effective for prevention of VT CAP already with modest levels of uptake in the target population (VE 42.3%). These data would suggest that rapid uptake and improved coverage of PCV13 among adults in the short term could maximise its impact.11 34
The incidence of pCAP is greatly increased in many individual clinical risk groups.35 Since when the ACIP recommended PCV13 for immunocompromised adults in 2012, there remains little evidence regarding the efficacy of the vaccine in at risk populations.9 36 37 Our findings would suggest that vaccination with PCV13 may be effective in preventing pneumococcal disease in adults ≥65 years with comorbid disorders. This observation, taken together with no effectiveness showed by PPV23 in our cohort, will require further studies to verify how adults with chronic diseases may fully benefit of the ACIP and the new Italian recommendations for the use of both PCV13 and PPSV23 in series. A recent systematic review and meta-analysis designed to estimate the efficacy of PPV23 in the prevention of pCAP, particularly in patients above 60 years of age and adults with underlying diseases showed that PPV23 vaccination ‘alone’ does not demonstrate clear efficacy, supporting the administration of a dose of PCV13 first followed by a dose of PPV23 at least 8 weeks later.38
Another recent systematic review of the burden of vaccine preventable pneumococcal disease in UK adults did not identify studies that were conducted in the community, where the majority of pCAP is managed.18 In our study, as it was designed to capture both invasive and non-invasive pCAP, approximately 36% of cases had been reported by GPs, suggesting that hospital-based studies may underestimate the true impact of pneumococcal disease. Post hoc subgroup analysis of cases managed in the community provided estimates of PCV13 effectiveness with respect to CAP from any pneumococcal serotype and this value did not change when we controlled for the presence of underlying disorders.
This study has several limitations. First, it was performed in a single region with a long-lasting history of experience of adult pneumococcal conjugate vaccination. Therefore, data from our setting may not be representative of the entire Italian adult population or generalisable to other settings. Moreover, data regarding the epidemiology of pCAP in adults in Italy are very limited and a large variability among published studies exists.20
Second, the recruitment rate was very low affecting the representativeness of the sample. The prestudy sample size calculation was 750 CAP cases aged over 64 years in the selected study area over a 3-year period of observation foreseen. We had estimated this sample size in the absence of a VE estimate as that provided by the CAPITA trial in 2014. As a reflection of the impact of stopping the study early (for administrative reasons), we had a much smaller recruitment than originally planned. However, there was not a risk for selection bias since both cases and controls were recruited in one process and arose from the whole population when the same enrolment criteria were met.
Third, 40 out of 226 enrolled patients were unable to provide a blood or a sputum/BAL sample, which could have led to underestimation or overestimation of pneumococcal aetiology rates. Owing to ethical and feasibility considerations, specimens were obtained only for clinical care and no invasive procedures were performed for this study, which may have reduced the microbiological yield. However, 82% of the adults had at least one specimen type available for S. pneumoniae detection.
The study size was small reducing the power to detect statistically significant effects, although pneumococcal aetiology was identified in 31.7% CAP adults. Recent corresponding figure from Rodrigo et al for UK was 29.3%.5 The main limitation, however, pertains to the small numbers of PCV13 vaccinated cases and controls underlying the estimation of the VE accounting for wide 95% CIs including zero. Although VE estimates should be interpreted with caution, our calculation reflected the still low PCV13 coverage achieved in the vaccinated cohorts. It was, therefore, too early to narrow the confidence limits around the point-estimate of effectiveness.
Fourth, the test-negative method may overestimate VE if, by reducing the risk of acquiring VT serotypes, vaccination increases the risk of acquiring NVT as is likely to be the case if there is serotype replacement.
Fifth, we were not able to assess the effectiveness of individual vaccine serotypes, as there were too few cases to allow statistical comparison between study groups.
Nonetheless, our study pointed out important gaps regarding the burden of pCAP in Italy where there are limited data outside of national surveillance of IPD and the only available data for non-invasive disease are limited to hospitalisation for pneumococcal pneumonia that, however, lack of serotyping information.20 The active surveillance of adult-confirmed CAP, drawn from a relative stable population over a 2-year period, was a strength of our study and allowed us to estimate the effectiveness of the PCV13 against all confirmed CAP, without regard to serotype, the presence of underlying conditions and the site of patient management (hospital/community).
This study has clinical practice implications for pneumococcal vaccination policies in adults in countries where PCV13 is becoming part of routine immunisation of the elderly. Although our results are non-significant, they can stimulate to perform larger studies that would probably confirm the VE but probably with narrower CIs than those presented here.
Supplementary Material
Acknowledgments
The authors thank the patients who consented to participate in this study; the sentinel physicians for their contributions to the enrolment of patients and collection of data (Maria Aliani, Mario Bisconti, Donato Vincenzo Catamerò, Vittoria Rosaria Costa, Elio Costantino, Gaetano D’Ambrosio, Francesco Dadduzio, Antonio De Maria, Mario Domenico Dell’Orco, Mario Lucio Dell’Orco, Pier Luigi Di Napoli, Oronzo Filieri, Maria Pia Foschino Barbaro, Vincenzo Frappampina, Donato Lacedonia, Giuseppe Vincenzo Laera, Luciana Labate, Paolo Lombardi, Michele Maiellari, Orazio Lippolis, Francesco Mariani, Antonio Metrucci, Gabriele Miolli, Onofrio Resta, Luigi Santoiemma, Teresa Scarabaggio, Salvatore Talamo); Anna Lisa De Robertis for her valuable laboratory work.
Footnotes
Contributors: RP and DM conceptualised and designed the work, analysed and interpreted data, and writing the manuscript. FF and MGC contributed to the data collection, managed the database and provided statistical support. MC performed the laboratory work. All authors have read and approved the final manuscript.
Funding: This work was in part supported by Pfizer unrestricted Investigator-Initiated Research Grant IRR WS 2164183.
Competing interests: RP reports grants from Pfizer, during the conduct of the study; grants, personal fees and non-financial support from Pfizer, personal fees and non-financial support from SanofiPasteurMSD, personal fees and non-financial support from GSK, outside the submitted work. DM reports grants and non-financial support from GSK, non-financial support from SanofiPasteurMSD, non-financial support from Pfizer, outside the submitted work.
Patient consent: Obtained.
Ethics approval: The study protocol was approved by the Institutional Review Board at the Apulian Regional Observatory for Epidemiology (PROT:18/OER/2012, 20 February 2012).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no unpublished data available.
References
- 1. Weil-Olivier C, Gaillat J. Can the success of pneumococcal conjugate vaccines for the prevention of pneumococcal diseases in children be extrapolated to adults? Vaccine 2014;32:2022–6. 10.1016/j.vaccine.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 2. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013;369:155–63. 10.1056/NEJMoa1209165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. Adults. N Engl J Med 2015;373:415–27. 10.1056/NEJMoa1500245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014;63:822–5. [PMC free article] [PubMed] [Google Scholar]
- 5. Rodrigo C, Bewick T, Sheppard C, et al. Impact of infant 13-valent pneumococcal conjugate vaccine on serotypes in adult pneumonia. Eur Respir J 2015;45:1632–41. 10.1183/09031936.00183614 [DOI] [PubMed] [Google Scholar]
- 6. Centro nazionale per la prevenzione delle malattie e la promozione della salute dell’Istituto superiore di sanità. Le vaccinazioni in Italia. 2016. http://www.epicentro.iss.it/temi/vaccinazioni/dati_Ita.asp (accessed 4 Aug 2017).
- 7. Castiglia P. Recommendations for pneumococcal immunization outside routine childhood immunization programs in Western Europe. Adv Ther 2014;31:1011–44. 10.1007/s12325-014-0157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prato R, Fortunato F, Martinelli D. Pneumococcal pneumonia prevention among adults: is the herd effect of pneumococcal conjugate vaccination in children as good a way as the active immunization of the elderly? Curr Med Res Opin 2016;32:543–5. 10.1185/03007995.2015.1131150 [DOI] [PubMed] [Google Scholar]
- 9. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015;372:1114–25. 10.1056/NEJMoa1408544 [DOI] [PubMed] [Google Scholar]
- 10. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015;64:944–7. 10.15585/mmwr.mm6434a4 [DOI] [PubMed] [Google Scholar]
- 11. Pilishvili T, Bennett NM. Pneumococcal disease prevention among adults: Strategies for the use of pneumococcal vaccines. Vaccine 2015;33 Suppl 4(Suppl 4):D60–D65. 10.1016/j.vaccine.2015.05.102 [DOI] [PubMed] [Google Scholar]
- 12. Bollettino Ufficiale Della Regione Puglia. Il Bollettino ufficiale della regione puglia si pubblica con frequenza infrasettimanale ed è diviso in due parti. 2014. http://beta.regione.puglia.it/documents/10180/4782589/N74_11_06_14.pdf (accessed 4 Aug 2017).
- 13. Osservatorio Epidemiologico Regione Puglia. Bollettino delle coperture vaccinali-resoconto sul monitoraggio delle attività vaccinali regionali condotte negli anni 2007-2015. 2016. https://www.sanita.puglia.it/documents/36126/269121/Bollettino+delle+Coperture+Vaccinali+-+Anni+2007+-+2015/f078e7d1-a19c-40de-8246-a2ae36278024?version=1.0&t=1491399965137 (accessed 4 Aug 2017).
- 14. Istituto Nazionale di Statistica. Popolazione Residente per età, sesso e stato civile al 1° gennaio 2015. 2015. http://www.demoistat.it.
- 15. Chow AW, Hall CB, Klein JO, et al. Evaluation of new anti-infective drugs for the treatment of respiratory tract infections. Infectious diseases society of America and the food and drug administration. Clin Infect Dis 1992;15(Suppl 1):S62–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J Clin Microbiol 2006;44:124–31. 10.1128/JCM.44.1.124-131.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mavroidi A, Godoy D, Aanensen DM, et al. Evolutionary genetics of the capsular locus of serogroup 6 pneumococci. J Bacteriol 2004;186:8181–92. 10.1128/JB.186.24.8181-8192.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chalmers JD, Campling J, Dicker A, et al. A systematic review of the burden of vaccine preventable pneumococcal disease in UK adults. BMC Pulm Med 2016;16:77 10.1186/s12890-016-0242-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012;67:71–9. 10.1136/thx.2009.129502 [DOI] [PubMed] [Google Scholar]
- 20. Rota MC, Bella A, D’Ancona F, et al. Vaccini anti-pneumococcici: dati ed evidenze per l’utilizzo nei soggetti a rischio di qualsiasi età e per l’eventuale ampliamento dell’offerta ai soggetti anziani (dicembre 2013). Roma: Istituto Superiore di Sanità, 2015. http://www.iss.it/binary/publ/cont/15_13.pdf (accessed 4 Aug 2017). [Google Scholar]
- 21. Fortunato F, Martinelli D, Cappelli MG, et al. Impact of pneumococcal conjugate universal routine vaccination on pneumococcal disease in italian children. J Immunol Res 2015;2015:1–6. 10.1155/2015/206757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Waight PA, Andrews NJ, Ladhani SN, et al. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis 2015;15:535–43. 10.1016/S1473-3099(15)70044-7 [DOI] [PubMed] [Google Scholar]
- 23. Corcoran M, Vickers I, Mereckiene J, et al. The epidemiology of invasive pneumococcal disease in older adults in the post-PCV era. Has there been a herd effect? Epidemiol Infect 2017;145:2390–9. 10.1017/S0950268817001194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Menéndez R, España PP, Pérez-Trallero E, et al. The burden of PCV13 serotypes in hospitalized pneumococcal pneumonia in Spain using a novel urinary antigen detection test. CAPA study. Vaccine 2017;35:5264–70. 10.1016/j.vaccine.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 25. Institute for Health Metrics and Evaluation (IHME). Life expectancy & probability of death. Seattle, WA: IHME, University of Washington, 2017. http://vizhub.healthdata.org/le/ (accessed 4 Aug 2017). [Google Scholar]
- 26. Song JH, Dagan R, Klugman KP, et al. The relationship between pneumococcal serotypes and antibiotic resistance. Vaccine 2012;30:2728–37. 10.1016/j.vaccine.2012.01.091 [DOI] [PubMed] [Google Scholar]
- 27. Hauser C, Kronenberg A, Allemann A, et al. Serotype/serogroup-specific antibiotic non-susceptibility of invasive and non-invasive Streptococcus pneumoniae, Switzerland, 2004 to 2014. Euro Surveill 2016;21:30239 10.2807/1560-7917.ES.2016.21.21.30239 [DOI] [PubMed] [Google Scholar]
- 28. Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae . N Engl J Med 2006;354:1455–63. 10.1056/NEJMoa051642 [DOI] [PubMed] [Google Scholar]
- 29. Dagan R, Juergens C, Trammel J, et al. Efficacy of 13-valent pneumococcal conjugate vaccine (PCV13) versus that of 7-valent PCV (PCV7) against nasopharyngeal colonization of antibiotic-nonsusceptible Streptococcus pneumoniae . J Infect Dis 2015;211:1144–53. 10.1093/infdis/jiu576 [DOI] [PubMed] [Google Scholar]
- 30. Meichtry J, Born R, Küffer M, et al. Serotype epidemiology of invasive pneumococcal disease in Swiss adults: a nationwide population-based study. Vaccine 2014;32:5185–91. 10.1016/j.vaccine.2014.07.060 [DOI] [PubMed] [Google Scholar]
- 31. European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe annual report of the European antimicrobial resistance surveillance network (EARS-Net) 2015. 2017. http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-europe-2015.pdf (accessed 4 Aug 2017).
- 32. Conferenza Permanente Per I Rapporti Tra Lo Stato Le Regioni E Le Province Autonome Di Trento E Bolzano. Intesa, ai sensi dell’articolo 8, comma 6, della legge 5 giugno 2003, n. 131, tra il Governo, le regioni e le province autonome di Trento e Bolzano sul documento recante "Piano nazionale prevenzione vaccinale 2017-2019. 2017. http://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=58185&completo=true (accessed 4 Aug 2017).
- 33. Webber C, Patton M, Patterson S, et al. Exploratory efficacy endpoints in the Community-Acquired Pneumonia Immunization Trial in Adults (CAPiTA). Vaccine 2017;35:1266–72. 10.1016/j.vaccine.2017.01.032 [DOI] [PubMed] [Google Scholar]
- 34. Mendes RE, Hollingsworth RC, Costello A, et al. Noninvasive streptococcus pneumoniae serotypes recovered from hospitalized adult patients in the united states in 2009 to 2012. Antimicrob Agents Chemother 2015;59:5595–601. 10.1128/AAC.00182-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Torres A, Blasi F, Dartois N, et al. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax 2015;70:984–9. 10.1136/thoraxjnl-2015-206780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Joint Committee on Vaccination and Immunisation, Department of Health, UK. Interim JCVI statement on adult pneumococcal vaccination in the UK - November 2015. 2015. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/477966/JCVI_pnemococcal.pdf (accessed 4 Aug 2017).
- 37. Baldo V, Cocchio S, Gallo T, et al. Pneumococcal conjugated vaccine reduces the high mortality for community-acquired pneumonia in the elderly: an italian regional experience. PLoS One 2016;11:e0166637 10.1371/journal.pone.0166637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schiffner-Rohe J, Witt A, Hemmerling J, et al. Efficacy of PPV23 in preventing pneumococcal pneumonia in adults at increased risk--a systematic review and meta-analysis. PLoS One 2016;11:e0146338 10.1371/journal.pone.0146338 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.