Abstract
Background
In walking rehabilitation trials, self-selected walking speed has emerged as the dominant outcome measure to assess walking ability. However, this measure cannot differentiate between recovery of impaired movement and compensation strategies. Spatiotemporal variables and asymmetry ratios are frequently used to quantify gait deviations and are hypothesized markers of recovery.
Objectives
The purpose of this review is to investigate spatiotemporal variables and asymmetry ratios as mechanistic recovery measures in physical therapy intervention studies post-stroke.
Methods
A systematic literature search was performed to identify physical therapy intervention studies with a statistically significant change in self-selected walking speed post intervention and concurrently collected spatiotemporal variables. Methodological quality was assessed using the Cochrane Collaboration’s tool. Walking speed, spatiotemporal, and intervention data were extracted.
Results
46 studies met the inclusion criteria, 41 of which reported raw spatiotemporal measures and 19 reported asymmetry ratio calculations. Study interventions included: aerobic training (n=2), functional electrical stimulation (n=5), hippotherapy (n=2), motor dual task training (n=2), multidimensional rehabilitation (n=4), robotics (n=4), sensory stimulation training (n=8), strength/resistance training (n=4), task specific locomotor rehabilitation (n=9), and visually guided training (n=6).
Conclusions
Spatiotemporal variables help describe gait deviations, but scale to speed, so consequently, may not be an independent factor in describing functional recovery and gains. Therefore, these variables are limited in explaining mechanistic changes involved in improving gait speed. Use of asymmetry measures provides additional information regarding the coordinative requirements for gait and can potentially indicate recovery. Additional laboratory-based mechanistic measures may be required to truly understand how walking speed improves.
Keywords: stroke, walking speed, rehabilitation, spatio-temporal analysis
Introduction
Stroke is the leading cause of disability in the United States, and the majority of those who survive stroke and achieve independent ambulation will demonstrate limitations in walking ability.1,2 Regaining the ability to ambulate and maximizing independence are two main goals of stroke rehabilitation, and these may be accomplished by compensating for remedial deficits, by promoting recovery of impaired movements, or by a combination thereof.3 Walking rehabilitation post-stroke has been historically dominated by strategies designed to compensate for impaired motor control, balance, and stabilization. However, improved understanding of neuroplasticity as a foundation for rehabilitation has led to a paradigm shift in which therapeutic interventions now target the nervous system’s ability to recover normalized movement patterns.4 While “recovery” is often used to describe functional gains, it will be operationally defined within this manuscript as a return to pre-morbid patterns of movement associated with independent walking.5 Traditional outcome measurements, such as walking speed, are focused on performance of functional tasks, but these measures do not identify the mechanisms by which an individual recovers.
Self-selected walking speed (SSWS) is the most commonly used outcome measure of walking ability in locomotor rehabilitation,6 likely because it is simple,7 cost effective, reliable,8 valid,9,10 sensitive,11 and specific.12 Gait speed has been described as the sixth vital sign13 and has been shown to be a predictor of independence, functional level at home and within the community,14 hospital length of stay, discharge disposition,15,16 mortality,17 health status,18 and quality of life.19 However, simply because a patient post-stroke has learned to walk at an increased speed, this does not necessarily indicate that the patient walks without impairments or that they have recovered normalized and/or premorbid movements. Even with a normal walking speed, significant deficits in the gait pattern may occur.20 These deficits potentially decrease patient safety and increase energy expenditure,21 leading to increased falls risk,22 increased walking-related fatigue,23 and reduced ambulatory efficiency.24 Regaining a normal, functional walking speed cannot distinguish between motor compensation (with an abnormal gait pattern) and motor recovery (with a normal gait pattern). While many interventions can lead to an increased walking speed, it is not clear which interventions promote acquisition and reinforcement of compensatory walking skills and/or which interventions promote recovery via changes in underlying mechanisms.
While SSWS has emerged as the dominant outcome measure for walking rehabilitation clinical trials, there remains inconsistent measurement of the mechanisms that may contribute to recovery. Spatiotemporal variables, including cadence, step length/width parameters, and support time parameters, are frequently used to quantify gait deviations,21,25 calculate asymmetries,26,27 determine appropriate therapy,9,28 and track patient progress.29–31 Since these variables reflect alterations in movement patterns and degrees of impairment, using spatiotemporal asymmetry ratio measures may be effective as markers of recovery.32 Many therapy trials collect data that allude to recovery potential, but 1) there is no systematic and consistent use of outcome measures to determine the contributing mechanisms of speed change; and 2) there is no systematic pooling and assimilation of data to infer collective knowledge about mechanisms.
The purposes of this systematic review are to: 1) examine changes in spatiotemporal variables and asymmetry measures in intervention studies associated with significant changes in SSWS; and 2) differentiate between spatiotemporal raw variables and measures of asymmetry in their potential relationship with motor recovery.
Methods
Identification and selection of studies
Literature searches were performed in three databases: PubMed, Ovid, and CINAHL, on January 10, 2016. Search terms in PubMed and Ovid included the following medical subject headings: “Stroke” OR “Stroke, Lacunar” OR “Brain Infarction” OR “Cerebral Infarction” OR “Subarachnoid Hemorrhage” OR “Intracranial Hemorrhages” OR “Intracranial Aneurysm” AND “Gait” OR “Walking”. In CINAHL, identical search terms were used as CINAHL headings, except when unavailable. Consequently, key word searching was used for the terms “Brain Infarction” and “Cerebral Infarction”, and the medical subject heading “Intracranial Aneurysm” was substituted with the equivalent CINAHL heading “Cerebral Aneurysm”. Search filters consisted of English language and human subjects.
One reviewer (ECW) screened all titles and abstracts to identify relevant studies and delete duplicates. A second reviewer (MGB) screened 10% of the titles and abstracts for reliability. Full-text articles were then retrieved and assessed for eligibility by both reviewers. Studies selected for inclusion in the systematic review met the following inclusion criteria: (a) Adult participants, defined as >18 years of age; (b) All study participants are clinically diagnosed with stroke, regardless of time since diagnosis and lesion site; (c) Studies utilized any clinical physical therapy intervention to effect gait; (d) Studies included both a functional outcome measure of self-selected gait speed and a mechanistic outcome measure of spatiotemporal gait analysis; (e) Intervention group and/or comparison group yielded a statistically significant change in SSWS pre- to post-intervention.
Data extraction and analysis
One reviewer (ECW) extracted significant data elements from the included studies, and a second reviewer (MGB) verified the information. Extracted data included: sample demographics, design characteristics, intervention type and details, functional and mechanistic outcomes measured and method of measurement, gait speed data pre- and post-intervention, and statistically significant differences observed.
Effect sizes for change in gait speed were calculated, using Cohen’s d (mean difference/SD), for all intervention and control groups in order to standardize the difference between means and increase ease of comparison between studies. Cohen’s d effect size is interpreted as small (0.2), medium (0.5), or large (0.8).33 In addition, each study was evaluated for changes in spatiotemporal variables, and when applicable, changes in spatiotemporal asymmetry ratios.
Assessment of study quality
Levels of evidence were applied using a Hierarchy of Evidence diagram adapted from Melnyk and Fineout-Overholt34 accessed on the Medical University of South Carolina’s Library website.35 The methodological quality and bias was assessed using the Cochrane Collaboration’s tool for assessing risk of bias.36 The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement was used throughout this review.37
Results
Flow of studies through the review
The outline of the search for relevant studies is shown in Figure 1. The initial search yielded 3,530 articles. After removing duplicates, screening records’ titles and abstracts, and reviewing reference lists, 232 full text articles were retrieved. 186 articles failed to meet the inclusion criteria, so 46 studies were included for qualitative synthesis. Reviewers’ agreement rate was 93.2%. Disagreements were resolved through discussion.
Figure 1.
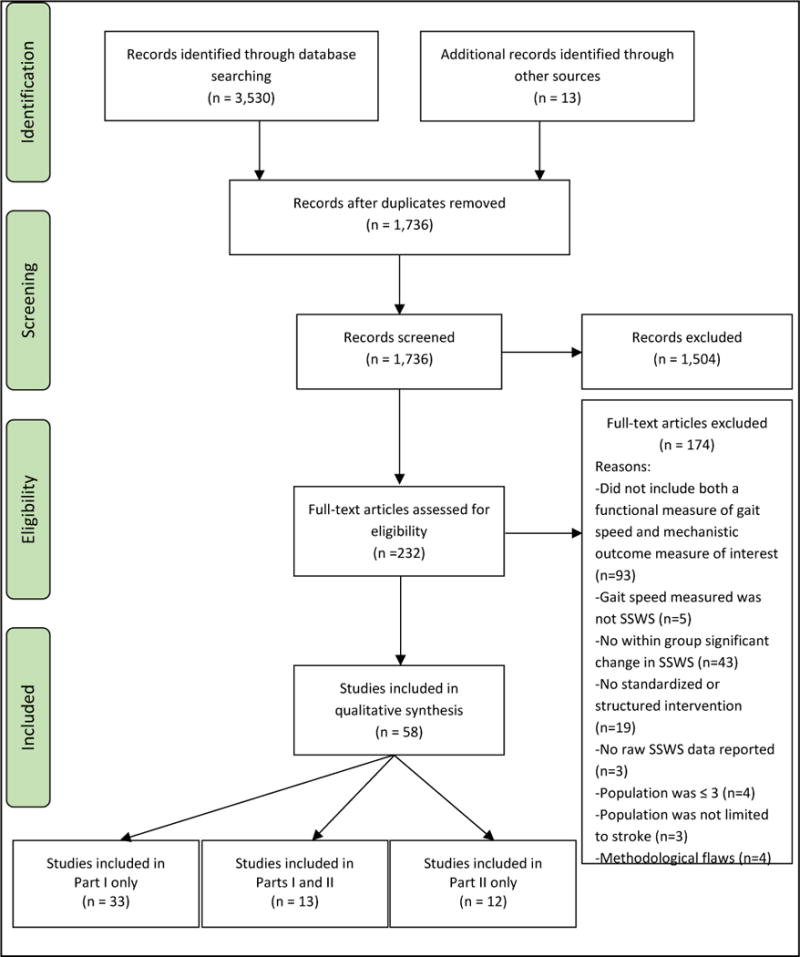
Flow of studies through the review.
Description of studies
Of the included studies, one was published in the 1990’s, eight in the 2000’s, and 37 between 2010 and January 10, 2016 (Figure 2). One study design was classified as a case series, 11 as quasi-experimental, and 34 as randomized-controlled trials. The quality of the studies, including design, level of evidence, and assessment of risk of bias, is presented in Table 1.
Figure 2.
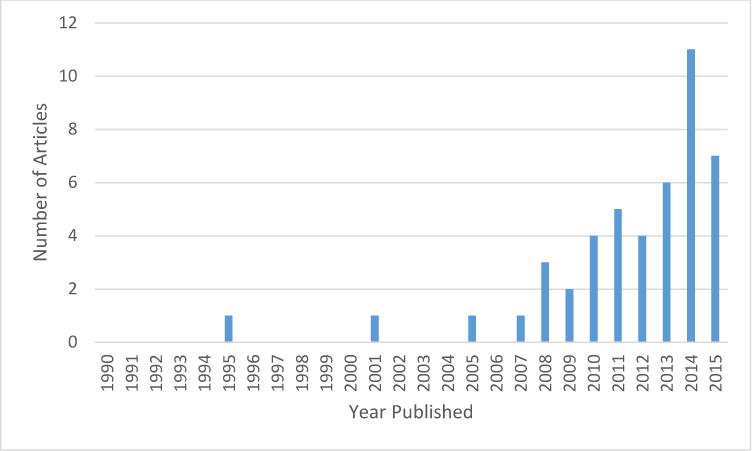
Growth of the literature including both functional and mechanistic measures.
Table 1.
Quality of included studies
| Assessing Risk of Bias36 | |||||||
|---|---|---|---|---|---|---|---|
| Study, Year | Design/Level of evidence 34 | Sequence Generation | Allocation conceal-ment | Blinding | Incomplete data | Selective outcome reporting | Other sources of bias |
| Alon, 2011 | Case Series/VI | No | No | No | Yes | No | No |
| Bowden, 2013 | Quasi-experimental design/III | No | No | No | Yes | Yes | Yes |
| Cha, 2014 | RCT/II | Unclear | Yes | Unclear | Yes | Yes | Yes |
| Chae, 2011 | RCT/II | Unclear | Unclear | Unclear | Yes | Yes | No |
| Chen, 2014 | RCT/II | Yes | Yes | Yes | Yes | Yes | Yes |
| Cheng, 2010 | RCT/II | Yes | Yes | Yes | Yes | Yes | Yes |
| Combs-Miller, 2014 | RCT/II | Unclear | Yes | Yes | Yes | Yes | Yes |
| Dunsky, 2008 | Quasi-experimental design/III | No | No | No | Yes | No | Yes |
| Engardt, 1995 | Quasi-experimental design/III | No | Unclear | Unclear | Yes | Yes | Yes |
| Forrester, 2011 | Quasi-experimental design/III | No | No | No | Yes | Yes | Yes |
| Forrester, 2014 | RCT/II | Unclear | No | No | Yes | No | Yes |
| Furnari, 2014 | RCT/II | Unclear | Unclear | Yes | Yes | Yes | Yes |
| Gama, 2015 | RCT/II | Unclear | Unclear | Yes | Yes | Yes | No |
| Holleran, 2014 | RCT/II | No | No | No | Yes | Yes | Yes |
| Hornby, 2008 | RCT/II | Yes | Yes | Yes | Yes | Yes | Yes |
| Ji, 2014 | RCT/II | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Ji, 2015 | RCT/II | Yes | Yes | Yes | Yes | Yes | Yes |
| Jonsdottir, 2010 | RCT/II | Yes | Unclear | Yes | Yes | Yes | Yes |
| Jung, 2015 | RCT/II | Yes | Yes | Yes | Yes | Yes | Yes |
| Kim, 2009 | RCT/II | Unclear | Unclear | Yes | Yes | Yes | Yes |
| Kim, 2012 | RCT/II | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Kim, 2013 | RCT/II | Unclear | Yes | Unclear | Yes | Yes | Yes |
| Kim, 2014 | Quasi-experimental design/III | No | No | No | Yes | Yes | Yes |
| Kim, 2015 | RCT/II | Yes | Yes | Yes | Unclear | Yes | Yes |
| Lee, C-W, 2014 | Quasi-experimental design/III | Unclear | Unclear | Unclear | Unclear | Yes | No |
| Lee, C-H, 2014 | RCT/II | Yes | Yes | Yes | Yes | Yes | Yes |
| Lee, H-J, 2013 | RCT/II | Yes | Unclear | Yes | Yes | Yes | Yes |
| Lee, I-H, 2015 | RCT/II | Unclear | Unclear | Yes | Yes | Yes | Yes |
| Lee, N-K, 2013 | RCT/II | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Lee, S-W, 2013 | RCT/II | Yes | Yes | Yes | Yes | Yes | Yes |
| Morgan, 2015 | Quasi-experimental design/III | No | No | No | Yes | No | Yes |
| Paoloni, 2010 | RCT/II | Yes | Yes | Yes | Yes | Yes | Yes |
| Park, 2013 | Quasi-experimental design/III | No | No | No | Yes | Yes | Yes |
| Park, 2014 | RCT/II | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Patterson, 2008 | Quasi-experimental design/III | No | No | No | Yes | Yes | Yes |
| Sabut, 2010 | RCT/II | Unclear | Unclear | Yes | Yes | Yes | Yes |
| Seo, 2012 | RCT/II | Unclear | Unclear | Unclear | Unclear | Yes | Yes |
| Shim, 2012 | RCT/II | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Sousa, 2011 | Quasi-experimental design/III | No | No | No | Yes | Yes | Yes |
| Teixeira-Salmela, 2001 | Quasi-experimental design/III | No | No | No | Yes | Yes | Yes |
| Verma, 2011 | RCT/II | Yes | Yes | Yes | Yes | Yes | Yes |
| Westlake, 2009 | RCT/II | Yes | Unclear | Unclear | Unclear | Yes | Yes |
| Yang, 2005 | RCT/II | Yes | Yes | No | Yes | Yes | Yes |
| Yang, 2007 | RCT/II | Yes | Yes | Yes | Unclear | Yes | Yes |
| Yom, 2015 | RCT/II | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| You, 2012 | RCT/II | Unclear | Unclear | Unclear | Yes | Yes | Yes |
RCT: Randomized Controlled Trial
Participant characteristics
Number of participants included in each study ranged from 8 to 61. All studies were conducted on chronic stroke survivors, except three that were done with early post-acute stroke patients.38–40 Also, one study examined and compared both the sub-acute and chronic populations.41 Participant demographics are presented in Table 2.
Table 2.
Overview of participant characteristics, intervention categories, gait effect sizes, and spatiotemporal parameters
| Study, Year | Participant Demographics | Intervention | Outcome Measures | ||||
|---|---|---|---|---|---|---|---|
| n | Age (yrs) | Time Since Stroke | Category | Grp | ES | Spatiotemporal Parameters | |
| Chronic Stroke | |||||||
| Alon, 2011 | 10 | 59 ± 13.25 | 7.7 ± 10.56 (y) | FES | I | 0.33 | Cadence (steps/min), P Single Limb Stance (s) |
| Bowden, 2013 | 27 | 58.74 ± 12.97 | 22.70 ± 16.38 (m) | TSLR | I | 1.11 | P Step Ratio |
| Cha, 2014 | 10 | 59.8 ±11.7 | 14.5 ±5.5 (m) | SST | I | 1.18 | Cadence (steps/min)*, P Stride Length (cm)*, P Double Support Period (%)*, NP Stride Length (cm)*, NP Double Support Period (%)* |
| 10 | 63.0 ±14.1 | 14.7±5.4 (m) | C | 0.22 | |||
| Chae, 2011 | 9 | 56.33 | 21.44 ± 6.85 (m) | SST | I | 0.06 | Cadence (steps/min)*, Step length (cm)*, Step Length Asymmetry Ratio, Single Support Time Asymmetry Ratio |
| Chen, 2013 | 15 | 54.8 ± 8.1 | 2.2 ± 2.0 (y) | TSLR | I | 0.61 | Stride Length (m), Cadence (steps/min), Temporal Asymmetry Ratio*, Spatial Asymmetry Ratio |
| Cheng, 2010 | 8 | 52.87 ± 8.74 | 33.6 ± 37.9 (m) | FES | I | 0.71 | Spatial Asymmetry Ratio*, Temporal Asymmetry Ratio |
| Combs-Miller, 2014 | 10 | 65.50 ± 6.17 | 60.00 ± 51.68 (m) | TSLR | C | 0.45 | Step Length Symmetry Ratio, Swing Time Symmetry Ratio*, Stance Time Symmetry Ratio |
| Dunsky, 2008 | 17 | 57.47 ± 9.25 | 45.94 ± 27.14 (m) | V-G T | I | 0.88 | Cadence (steps/min)*, Stride length (cm)*, P Step Length (cm)*, NP Step Length (cm)*, P Single Limb Support (%)*, NP Single Limb Support (%), Double Support (%)*, Gait Symmetry Index (%)* |
| Engardt, 1995 | 10 | 64.6 ± 6.2 | 27.8 ± 12.0 (m) | S/R T | Ib | 0.40 | Swing Phase Duration (%)* |
| Forrester, 2011 | 8 | 62.38 ± 10.45 | 72.5 ± 36.7 (m) | R | I | 0.93 | Stride Length (cm)*, Cadence (steps/min)*, P Single Limb Support (%)*, Double Support (%)* |
| Furnari, 2014 | 20 | 68 ± 3 | 7 ± 1.6 (m) | M-DR | I | 1.20 | Semi-Step Length (cm), Cadence (steps/min)*, Stance Phase (%)*, Swing Phase (%)*, Double Support Phase (%)* |
| 20 | 72 ± 5 | 6 ± 1.4 (m) | C | 0.50 | |||
| Gama, 2015 | 14 | 52.92 ±9.51 | 35.36 ±26.87 (m) | TSLR | I | 0.50 | Cadence (steps/min), Stride Length (m)*, P Step Length (m)*, NP Step Length (m)*, P Stance Time (%), NP Stance Time (%), Double Stance Time (s), P Swing Time (%), NP Swing Time (%), Symmetry Ratio |
| Hornby, 2008 | 24 | 57 ± 10 | 50 ± 51 (m) | R | I | 0.37 | Single Limb Stance (%), Step Asymmetry |
| 24 | 57 ± 11 | 73 ± 87 (m) | C | 0.59 | |||
| Ji, 2014 | 10 | 52.9 ± 9.9 | 7.1 ± 3.4 (m) | FES | Ia | 3.54 | Cadence (steps/min)*, Step Length (cm)*, Stride Length (cm)* |
| 10 | 48.6 ± 8.5 | 7.3 ± 2.9 (m) | Ib | 2.45 | |||
| 10 | 54.6 ± 9.2 | 6.7 ± 2.3 (m) | C | 1.28 | |||
| Ji, 2015 | 16 | 55.2 ±7.5 | 4.3 ± 1.5 (m) | V-G T | I | 0.79 | Single Limb Stance (%)*, Stance Phase (%), Step Length (cm)*, Stride Length (cm)*, Swing Phase (%), Cadence (step/min), Step Width (cm)* |
| 15 | 54.3 ±8.7 | 4.5 ± 1.3 (m) | C | 0.49 | |||
| Jonsdottir, 2010 | 10 | 61.6 ± 13.1 | 5.9 ± 10.5 (y) | SST | I | 1.01 | Stride Length (%h)* |
| Jung, 2015 | 11 | 56.4 ±11.1 | 6.2 ±2.5 (m) | TSLR | I | 0.94 | P Single Limb Support Phase (%)* |
| Kim, 2009 | 12 | 52.42 ± 10.09 | 25.91 ± 9.96 (m) | V-G T | I | 0.75 | Cadence (steps/min)*, Step time (s)*, Swing Time (s), Stance Time (s), Single Limb Support Time (s), Double Limb Support Time (s), Step length (cm)*, Stride length (cm)* |
| Kim, 2012 | 10 | 65.2 ± 6.8 | 15.8 ± 2.3 (m) | SST | I | 2.80 | P Stride Length (cm)*, NP Stride Length (cm)*, Stride Length Ratio*, P Single Limb Support Time (ms)*, NP Single Limb Support Time (ms)*, Single Support Time Ratio* |
| 10 | 64.5 ± 8.1 | 15.3 ± 3.0 (m) | C | 1.75 | |||
| Kim, 2013 | 9 | 55.3 ± 12.1 | 8.3 ± 3.3 (m) | V-G T | Ia | 1.16 | Cadence (steps/min)*, P Step Length (cm)*, P Stride Length (cm)*, P Single Limb Support (%)*, P Double Limb Support (%)* |
| 9 | 54.8 ± 8.8 | 7.3 ± 0.7 (m) | Ib | 0.57 | |||
| 9 | 59.8 ± 8.9 | 8.5 ± 3.6 (m) | C | 0.45 | |||
| Kim, 2014 | 17 | 63.9 ± 8.7 | 23.6 ± 2.8 (m) | H | I | 0.67 | Cadence (steps/min)*, P Stride Length (cm)*, NP Stride Length (cm)*, P Double Limb Support (%)*, NP Double Limb Support (%)* |
| Kim, 2015 | 10 | 59.20 ± 7.72 | 8.12 ± 4.95 (m) | M-DR | Ia | 0.60 | Cadence (step/min)*, P Stride Length (cm)* Gait Symmetry Ratio*, Double Support Period (%)* |
| 10 | 58.53 ± 11.83 | 7.99 ± 3.85 (m) | Ib | 0.68 | |||
| Lee, C-W, 2014 | 15 | 63.8 ± 6.2 | H | I | 3.25 | Step Length Asymmetry Ratio (%)* | |
| Lee, C-H, 2014 | 10 | 47.9 ± 12.0 | 11.7 ± 4.5 (m) | V-G T | I | 0.95 | Cadence (steps/min)*, P Step Length (cm)*, NP Step Length (cm)*, P Stride Length (cm)*, NP Stride Length (cm)* |
| Lee, H-J, 2013 | 15 | 52.47 ± 9.41 | 4.0 ± 0.41 (m) | FES | I | 2.00 | Cadence (steps/min)*, P Step Length (cm)*, P Stride Length (cm)* |
| 15 | 56.73 ± 7.24 | 4.07 ± 1.03 (m) | C | 0.95 | |||
| Lee, N-K, 2013 | 14 | 60.3 ± 7.5 | 19.2 ± 5.2 (m) | S/R T | I | 2.43 | Step Length (cm)*, Stride Length (cm)*, Heel-to-heel Base of Support (%)*, Step Time (s)*, Double Support (%)* |
| Lee, S-W, 2013 | 16 | 53.31 ± 8.37 | 56.94 ± 25.73 (m) | SST | I | 0.58 | Cadence (steps/min)*, P Step Length (cm)*, P Single Limb Support (s)* |
| 15 | 55.73 ± 8.27 | 49.93 ± 29.97 (m) | C | 0.16 | |||
| Morgan, 2015 | 12 | 56.0 ± 16.8 | 29.3 ± 19.7 (m) | S/R T | I | 0.54 | Cadence*, P Step Length*, NP Step Length* |
| Paoloni, 2010 | 22 | 59.5 ± 13.3 | 1.85 ± 0.59 (y) | SST | I | 0.69 | Cadence (step/min), P Toe-off (%)*, NP Toe-off (%), P Step Length (m), NP Step Length (m), P Stride Length (m)*, NP Stride Length (m)*, P Step Width (m), NP Step Width (m), P Swing Velocity (m/s), NP Swing Velocity (m/s)* |
| Park, 2013 | 13 | 58.46 ± 8.53 | 53.15 ± 7.28 (m) | SST | I | 0.75 | Cadence (steps/min)*, P Step Length (cm)*, NP Step Length (cm)*, P Stride Length (cm)*, NP Stride Length (cm)* |
| Park, 2014 | 15 | 71.2 ±3.46 | 18.66 ±2.46 (m) | SST | I | 0.47 | Cadence (steps/min)*, P Step Length (cm)*, P Stride Length (cm)* |
| Patterson, 2008 | 39 | 64 ± 8 | 20.55 ± 64 (m) | AT | I | 0.34 | Cadence (steps/min)*, Stride length (cm)*, P Step Length (cm)*, NP Step Length (cm)*, P Step Time (s)*, NP Step Time (s)*, P Stance (%)*, NP Stance (%), Double Limb Support (%)*, P Swing (%)*, NP Swing (%), Asymmetry Ratios |
| Sabut, 2010 | 16 | 49.5 ± 8.9 | 20 (m) | FES | I | 0.46 | Cadence (steps/min)*, Step Length (cm)*, Stride Length (cm)* |
| 14 | 47.1 ± 12.4 | 15 (m) | C | 0.26 | |||
| Seo, 2012 | 20 | 61.5 ± 2.8 | 5.1 ± 4.8 | TSLR | I | 3.00 | Step Time (s)*, Double Limb Support (%)*, Stance Phase (%)*, Step Length (cm)*, Heel-to-heel Base of Support (%)*, Step/Extremity Ratio (%)* |
| Shim, 2012 | 17 | 65.59 ± 5.81 | 16.29 ± 2.62 (m) | MDTT | I | 0.97 | Cadence (steps/min)*, P Step Length (cm)*, NP Step Length (cm)*, P Stride Length (cm)*, NP Stride Length (cm)*, P Single Limb Support (%)*, NP Single Limb Support (%)*, P Double Limb Support (%)*, NP Double Limb Support (%)* |
| 16 | 61.56 ± 6.17 | 17.44 ± 3.67 (m) | C | 0.31 | |||
| Sousa, 2011 | 12 | 53.2 ± 7.5 | 4.6 ± 3.0 (y) | TSLR | I | 0.57 | P Step Length (m)*, NP Step Length (m), P Stride Length (m)*, NP Stride Length (m)*, P Stride Speed (m/s)*, NP Stride Speed (m/s)*, Double Limb Stance (%), P Single Limb Support (%), NP Single Limb Support (%) |
| Teixeira-Salmela, 2001 | 13 | 67.7 ± 9.2 | 7.7 ± 9.4 (y) | M-DR | I | 0.41 | Cadence (steps/min)*, Stride Length (m)*, Double Support (%), Stance (%), Symmetry Ratio |
| Westlake, 2009 | 8 | 58.6 ± 16.9 | 43.8 ± 26.8 (m) | R | I | 0.32 | P Step Length Ratio* |
| Yang, 2005 | 13 | 63.38 ± 7.7 | 5.45 ± 3.03 (m) | TSLR | I | 1.29 | Cadence (steps/min)*, Stride Length (m)*, Gait Cycle (s)*, Temporal Symmetry Index* |
| 12 | 63.42 ± 11.06 | 7.33 ± 2.42 (m) | C | 0.25 | |||
| Yang, 2007 | 13 | 59.46 ± 11.83 | 4.08 ± 3.13 (y) | MDTT | I | 1.50 | Cadence (steps/min)*, Stride Time (s)*, Stride Length (cm)*, Temporal Symmetry Index |
| Yom, 2015 | 10 | 64.6 | 11.14 (m) | V-G T | I | 0.70 | Cadence (step/sec)*, Step Length (cm)*, Stride Length (cm)*, Stance Time (%)*, Swing Time (%)*, Double Limb Support (%)* |
| 10 | 78.1 | 11.63 (m) | C | 0.20 | |||
| You, 2012 | 13 | 61.46 ± 5.12 | 13.30 ± 3.35 (m) | S/R T | I | 1.81 | P Step Time (s)*, Cycle Time (s)*, P Step Length (cm)*, Stride length (cm)*, P Swing (%)*, P Stance (%)* |
| 14 | 59.07 ± 4.66 | 11.35 ± 2.84 (m) | C | 1.26 | |||
| Chronic and Sub-Acute Stroke | |||||||
| Holleran, 2014 | 10 | 55 ± 8.8 | 42 ± 58 (m) | AT | Ia | 0.88 | Single Limb Stance (%)*, Step Length Symmetry (%) |
| 12 | 52 ± 13 | 3.2 ± 1.8 (m) | Ib | 1.22 | |||
| Sub-Acute Stroke | |||||||
| Forrester, 2014 | 18 | 63.3 ± 2.3 | 11.9 ± 1.5 (d) | R | I | 6.03 | P Step Time (s)*, NP Step Time (s)*, Step-time Symmetry* P Step Length (cm)*, NP Step Length (cm)*, Step-length Symmetry |
| 16 | 60.0 ± 3.1 | 10.8 ± 1.2 (d) | C | 4.47 | |||
| Lee, I-H, 2015 | 31 | 65.45 ± 4.37 | 40.93 ± 8.67 (d) | TSLR | Ia | 2.00 | P Step Length (m)*, NP Step Length (m)*
Step Width (m), Cadence (steps/min)* |
| 30 | 63.16 ± 8.22 | 34.77 ± 4.48 (d) | Ib | 2.17 | |||
| Verma, 2011 | 15 | 53.27 ± 8.53 | 6.07 ± 3.30 (w) | M-DR | I | 2.13 | Cadence*, Step Length Asymmetry (cm), Stride Length Asymmetry (cm) |
| 15 | 55.07 ± 6.80 | 6.60 ± 3.20 (w) | C | 0.57 | |||
denotes statistically significant change in mechanistic outcome measure
Mean ± SD
Time Since Stroke: d: days; w: weeks; m: months; y: years; Intervention Categories: AT: Aerobic Training; FES: Functional Electrical Stimulation; H: Hippotherapy; MDTT: Motor Dual Task Training; M-DR: Multi-dimensional Rehabilitation; R: Robotics; SST: Sensory Stimulation Training; S/R T: Strength/Resistance Training; TSLR: Task Specific Locomotor Rehabilitation; V-G T: Visually-Guided Training. Intervention Groups: I: Intervention; C: Control; Outcome Measures: ES: Effect Size; Spatiotemporal Parameters: P: Paretic; NP: Non-Paretic.
Functional gait outcome – gait speed
All included studies utilized gait speed as an outcome measure and showed a statistically significant increase in SSWS post-intervention. Gait speed was measured by utilizing an instrumented walkway,29–31,38,41–69 a motion analysis or instrumented gait analysis system,70–74 or by timing ambulation along the following distances: 9 meters;75 10 meters;39,40,76–78 or 30 meters.79
Interventions that elicited functional improvements
The physical therapy interventions used within the included studies are variable. In order to structure the large number and variety of interventions, each study is assigned to one of 10 categories.
Aerobic training
Two included studies utilized an aerobic training intervention. Aerobic treadmill59 training produced an effect size of 0.34, while aerobic over-ground, reciprocal stepping training41 had effect sizes of 0.88 and 1.22 for change in gait speed.
Functional electrical stimulation
Five included studies utilized interventions involving functional electrical stimulation in combination with cycling,42 body weight supported treadmill training (BWSTT),54 mirror therapy,70 conventional therapy,77 or electrical stimulation with active ankle dorsi-flexion on a rocker board.45 Change in gait speed effect sizes range dramatically from 0.26 to 3.54.
Hippotherapy
Two included studies involved hippotherapy, or horse-back riding therapy, interventions. The study utilizing hippotherapy53 elicited an effect size of 3.25, and another involving a hippotherapy simulation49 elicited effect size of 0.67.
Motor dual task training
Of the included studies, two investigated the effects of dual task training/exercise.58,62 The change in gait speed effect sizes range from 0.31 to 1.50.
Multidimensional rehabilitation
Four of the included studies have multidimensional exercise programs that included strengthening and aerobic exercise,60 circuit class training,40 circuit tilt table,66 or hydro-kinesiotherapy.47 Change in gait speed effect sizes range from 0.41 to 2.13.
Robotics
Of the included studies, four involved robotic interventions, specifically: robotic gait training with body weight support,48,61 or ankle training with a robotic device.38,46 The change in gait speed effect sizes that resulted range from 0.32 to 6.03.
Sensory stimulation training
Eight of the included studies investigated interventions involving sensory stimulation training, including rhythmic auditory stimulation,64 spinal stabilization with visual feedback,43 auditory stimulation training,51 exercise with TENS,68 ankle proprioceptive control training,30 local vibration stimulus training program,56 segmental muscle vibration,74 and biofeedback.63 Change in gait speed effect sizes following sensory stimulation training range from 0.06 to 2.80.
Strength/Resistance training
Four of the included studies utilized a strength training intervention through progressive resistance training,55 power training,67 exercise standing on one leg,31 or by comparing eccentric to concentric resistance training.79 The change in gait speed effect sizes range from 0.40 to 2.43.
Task specific locomotor rehabilitation
Nine of the included studies investigated the effects of locomotor training and variations thereof. Study interventions included: BWSTT,73,78,80 treadmill training without body weight support,39 body weight support over ground walking,71 turning-based treadmill training,44 backward walking training,75 walking exercise on a ramp,57 and gait training with a cane.65 Change in gait speed effect sizes that resulted range from 0.25 to 3.00.
Visually-guided training
Six of the included studies investigated the effects of visually guided interventions such as action observation training,50 motor imagery,29 mirror therapy,72 or virtual or augmented reality interventions.52,69,76 The resulting effect sizes range from 0.20 to 1.16.
Potential mechanisms of change - spatiotemporal parameters and asymmetry ratios
Spatiotemporal variables are variables concerning placement of the feet and time aspects of events during the gait cycle, and include step length, stride length, cadence, single limb support time, double limb support time, stance time, and swing time measurements. The 46 included studies analyzed gait using a variety of spatiotemporal variables and/or asymmetry ratio calculations, recorded for either or both the paretic and non-paretic limbs. Refer to Table 2 for details regarding inclusion of spatiotemporal parameters and asymmetry ratios and significant changes observed post-intervention. Statistically significant improvements reported were observed in the hypothesized direction of change based on each variable (i.e. cadence and step length increased, while double limb support decreased). The most commonly used data collection method for spatiotemporal variables is through the use of an instrumented walkway or motion analysis system. All included studies utilized these methods expect two: one study used inked footprints on paper for quantitative gait analysis40 and one used footswitches on the paretic side.79
Step length
Of the included studies, 24 recorded paretic step length; of those, 23 found statistically significant changes in the paretic step length following the physical therapy intervention.29–31,38,39,43,50,52,54–59,67–73,76,77 Non-paretic step length was less commonly reported (n=11). Nine of those studies found statistically significant changes in non-paretic step length.29,30,38,39,52,58,59,67,73
Stride length
28 studies recorded paretic stride length, and of those, 27 found statistically significant changes in this spatial measure following physical therapy intervention.29–31,46,49–52,54,55,58–60,62–64,66,68–77 Non-paretic stride length was less commonly reported (n=8). However, non-paretic stride length was found to be statistically significant in all eight of those studies.30,49,51,52,58,64,71,74
Cadence
Cadence was the most commonly reported of all the spatial and temporal parameters, as 30 studies included this measure. Of those 30 studies, cadence was found to significantly increase in 25 of them.29,30,39,40,43,46,47,49,50,52,54,56,58–60,62,64,66–70,75–77
Single limb support
Paretic single limb support was reported in 13 studies and was found to be statistically significant in nine.29,41,46,50,51,56,58,65,72 Non-paretic single limb support was reported in 4 studies and found to be statistically significant in half of them.51,58
Double limb support
Double limb support was reported, as a period of time or as a phase of gait cycle, in 11 of the included studies. Seven of those studies found a statistically significant change in this variable.29,46,47,55,59,66,69 More specifically, paretic double limb support was reported and statistically significant in four other included studies.49,50,58,64 Additionally, three of those four studies reported non-paretic double limb support, all exhibiting statistically significant changes.49,58,64
Stance time
Stance time/phase was reported in six studies and found to be statistically significant in only two of them.47,69 Three studies reported paretic stance time, two of which found statistically significant changes.31,59 Lastly, non-paretic stance time was reported in two studies and was not found to have a statistically significant change in either.
Swing time
Paretic limb swing time was reported in eight of the included studies, and it was found to be statistically significant in five of those.31,47,59,69,79 Non-paretic limb swing time was reported in only two studies59,73 and was not statistically significant in either.
Asymmetry ratios
Additionally, 19 studies quantified the spatiotemporal variables by calculating measures of asymmetry.29,38,40,41,43–45,48,51,53,59–62,66,73,75,78,80 These methods varied and are listed in Table 3.
Table 3.
Overview of spatiotemporal asymmetry ratio calculations
| Spatial | Calculation | Study, Year | |
|---|---|---|---|
| Step Length Asymmetry Ratios |
|
Combs-Miller, 201478 Patterson, 200859 Forrester, 201438 |
|
|
|
* Cheng, 201045 Verma, 201140 |
||
|
|
Chen, 201344 * Westlake, 200961 |
||
|
|
* Holleran, 201441 | ||
|
|
Hornby, 200848 | ||
| Stride Length Asymmetry Ratio |
|
* Kim, 201251 Verma, 201140 |
|
| Paretic Step Ratio (aka Distance Phasing) |
|
Bowden, 201380 Patterson, 200859 |
|
| Temporal | |||
| Single Support Time Asymmetry Ratios |
|
* Dunsky, 200829 | |
|
|
Cheng, 201045 * Kim, 201251 |
||
|
|
* Chen, 201344 | ||
|
|
* Yang, 200575 | ||
|
|
Yang, 200762 | ||
| Stance Time Symmetry Ratio |
|
Combs-Miller, 201478 Patterson, 200859 |
|
|
|
Patterson, 200859 | ||
| Swing Symmetry Ratio |
|
* Combs-Miller, 201478 Gama, 201473 Teixeira-Salmela, 200160 |
|
|
|
Patterson, 200859 | ||
| Step Time Symmetry Ratio |
|
* Forrester, 201438 | |
| Gait Symmetry Ratio |
|
* Kim, 201566 |
denotes statistically significant change in mechanistic outcome measure
NOTE: Spatiotemporal asymmetry ratio equations not available for Chae, 2011 and Lee, 2014
Discussion
This systematic review sought to identify mechanistic variables that potentially detect motor recovery and explain improvements in gait speed following physical therapy interventions in the stroke population. Measuring mechanistic factors, such as spatiotemporal parameters and asymmetries, may assist in the understanding of how walking speed improves, and will enable researchers and therapists to better focus rehabilitation of gait post-stroke. Spatiotemporal parameters and asymmetry measures are commonly used outcomes, likely due to the decreased cost, increased ease, and time effectiveness of collecting this type of data. Although closely related, as raw spatiotemporal parameters are used to calculate asymmetry ratios, asymmetry improvements may reflect whole body gains in coordination and movement patterning, and likely provide differing insight into gait events and mechanisms.
Of the 41 studies that report raw spatiotemporal measures, 38 found at least one parameter to be significantly changed, indicating that these measures are highly responsive to physical therapy interventions, but may not differentiate the mechanisms by which individuals alter walking speed. Within the three studies where a spatiotemporal parameter was not significantly altered, the change in walking speed was minimal, with small to moderate effect sizes ranging from 0.33 to 0.61.42,44,48 While significantly improved from baseline, these increases were perhaps not large enough to generate concomitant changes in spatiotemporal factors. Also, within these three studies, they reported only one or two raw spatiotemporal variables each, and it is possible recovery may have been observed elsewhere. These studies investigated the interventions functional electrical stimulation,42 robotic gait training,48 and turning-based treadmill training,44 and the results imply that perhaps they were not robust enough to promote spatiotemporal improvements. There does not appear to be any commonality within the interventions that resulted in specific spatiotemporal changes, and thus, we cannot interpret the relationships between spatiotemporal parameters and these varying interventions. The high number of significant spatiotemporal measurement changes observed may be due to the number of parameters, such as step length, stride length, and cadence that scale to gait speed.81 Consequently, these measures are not representative of factors that are independent of the changes in walking speed. Due to this dependent relationship, spatiotemporal measures may not give insight into the true mechanism of change. For example, do increases in walking speed drive increases in step/stride length or do step/stride length increases drive the speed change? In actuality, all are possibly a product of a separate mechanism, such as improved propulsive force production, that results from the intervention. Similarly, cadence and speed increases can both be a product of improved motor control, thus improved capacity to increase the frequency of the reciprocal gait pattern. One must apply restraint in considering these variables as the mechanism that drives recovery, as they may similarly be a product of that recovery.
Spatiotemporal asymmetry measures describe the body’s ability to produce normalized walking patterns and evaluate interlimb coordination and, therefore, may represent the potential for a clinical measurement to indicate motor recovery. Asymmetry measures were calculated in 19 of the included studies, with only 10 studies yielding statistically significant changes following the intervention. All but two of those studies reported changes in raw spatiotemporal variables.44,48 Thus, it is probable that gait asymmetry ratios are more sensitive measures and better reflect recovery as compared to raw spatiotemporal measures. However, as with the raw spatiotemporal parameters, there does not appear to be any consistency within the interventions that did and did not elicit specific spatiotemporal asymmetry changes. Detecting a pattern regarding what therapies improve asymmetries became even more difficult due to the variability of the calculations (see Table 3) used within the included studies. Likewise, no conclusions were definitively drawn regarding asymmetry measures and their relationship to walking changes due to the calculation variability of ratio measurements. This lack of standardization of gait asymmetry measures in the stroke population has been addressed by Patterson et al. with recommendations made to use the symmetry ratio (paretic variable/non-paretic variable) for ease of interpretation.27 Even so, the current data do not conclusively demonstrate that asymmetry measures reflect recovery, and one must continue to apply restraint in considering asymmetry measures to be the mechanism of change. Although there is potential here to assess recovery in the future, it would require a more standardized battery of assessments of true mechanisms, that reflect motor control and force production, to understand the improvements in interlimb asymmetry and coordination.
This review of the literature identifies the need for more direct measures to quantify the mechanisms by which human walking is recovered after stroke. While spatiotemporal parameters provide therapists with the ability to better track patient progress regarding gait deviations, these measures are intermediary, since they closely relate to walking speed. True mechanistic measures are required to provide a greater understanding of how physical therapy interventions improve gait speed. This will likely require more laboratory-based measures of motor recovery, including measures of exercise capacity, muscle activation, movement analysis, and force production that will all be examined in Part II of this review.
Limitations
There were a number of limitations to this systematic review. First, there was a high risk of bias within many of the included studies. This is primarily due to study design, level of evidence, lack of control group, and decreased use of randomization. Also, several experiments included small sample sizes. Another limitation is the high variability of the included studies due to the variation in type, duration, and intensity of the interventions used, as well as the methods in which spatiotemporal parameters were collected and spatiotemporal asymmetry ratios calculated. Additionally, the gait speeds reported in this systematic review were self-selected. Although SSWS appears to be more common, it has been suggested that assessment at one’s self selected speed alone may not be sufficient to identify underlying impairments in hemiparetic walking.82 Furthermore, raw spatiotemporal variables are likely not sensitive in detecting true mechanistic changes, as they are a product of the functional changes following the intervention. Lastly, although it is likely that neurological recovery is greater in the early post-acute stroke population, because there were so few of the included studies examining patients during the sub-acute phase, no definitive assessment could be made regarding a differential level of recovery.
Clinical and research implications
This systematic review highlights specific physical therapy interventions that resulted in gait speed improvements with concurrent measurement of spatiotemporal variables. Spatiotemporal measurements are clinically feasible and provide more quantifiable gait information than gait speed alone. As the causal links are still missing with this method of gait measurement, researchers have begun to connect asymmetry ratios and measured patterns of ground reaction force development.26 Due to these missing links, there is great potential and opportunity for researchers to link laboratory-based measurements to clinical improvements.28,83
Conclusions
Our aim was to review the available literature in order to identify potential mechanisms associated with speed changes, as they relate to physical therapy interventions and outcomes after stroke. The methods in which gait is analyzed have evolved dramatically, and there has been a shift to not only understand, but also to quantify the underlying mechanistic changes that contribute to the functional gains. The growth of literature observed in this systematic review demonstrates the sharp increase in studies including both functional and mechanistic measures beginning in the late 2000s (Figure 2). The recent evolution of the gait literature and the increased ease of collecting spatiotemporal parameters in the clinic has led to an increase in spatiotemporal data, which helps to better describe gait deviations but may not be able to explain mechanistic changes. Spatiotemporal variables are often utilized as a surrogate for recovery, however, these variables may be a by-product of the speed at which someone walks and thus not independent in describing functional recovery and gains. The use of asymmetry measures may provide additional information regarding the coordinative requirements for gait and can potentially indicate recovery. Additional mechanistic measures may be required to gain a greater understanding of how walking speed improves. Part II of this review will examine more laboratory-based measures of motor recovery including measures of exercise capacity, muscle activation, movement analysis, and force production and will similarly discuss them within the context of increased walking speed.
Acknowledgments
This work was supported by a VA Career Development Award-2 RR&D N0787-W (MGB) and Institutional Development Award from the National Institute of General Medical Sciences of the NIH under grant number P20-GM109040 (MGB).
The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
The authors would like to thank Heather Shaw Bonilha, PhD, CCC-SLP for her guidance in the early stages of this manuscript idea.
We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated and, if applicable, we certify that all financial and material support for this research and work are clearly identified in the title page of the manuscript.
Abbreviations
- SSWS
self-selected walking speed
- BWSTT
body weight supported treadmill training
References
- 1.Ada L, Dean CM, Vargas J, Ennis S. Mechanically assisted walking with body weight support results in more independent walking than assisted overground walking in non-ambulatory patients early after stroke: a systematic review. Journal of physiotherapy. 2010;56(3):153–61. doi: 10.1016/s1836-9553(10)70020-5. [DOI] [PubMed] [Google Scholar]
- 2.Polese JC, Ada L, Dean CM, Nascimento LR, Teixeira-Salmela LF. Treadmill training is effective for ambulatory adults with stroke: a systematic review. Journal of physiotherapy. 2013;59(2):73–80. doi: 10.1016/S1836-9553(13)70159-0. [DOI] [PubMed] [Google Scholar]
- 3.Buurke JH, Nene AV, Kwakkel G, Erren-Wolters V, Ijzerman MJ, Hermens HJ. Recovery of gait after stroke: what changes? Neurorehabilitation and neural repair. 2008;22(6):676–83. doi: 10.1177/1545968308317972. [DOI] [PubMed] [Google Scholar]
- 4.Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Physical therapy. 2006;86(10):1406–25. doi: 10.2522/ptj.20050212. [DOI] [PubMed] [Google Scholar]
- 5.Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabilitation and neural repair. 2009;23(4):313–9. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- 6.Eng JJ, Tang PF. Gait training strategies to optimize walking ability in people with stroke: a synthesis of the evidence. Expert review of neurotherapeutics. 2007;7(10):1417–36. doi: 10.1586/14737175.7.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. The journals of gerontology Series A, Biological sciences and medical sciences. 2000;55(4):M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. Journal of rehabilitation medicine. 2005;37(2):75–82. doi: 10.1080/16501970410017215. [DOI] [PubMed] [Google Scholar]
- 9.Richards CL, Olney SJ. Hemiparetic gait following stroke. Part II: Recovery and physical therapy. Gait & posture. 1996:149–62. [Google Scholar]
- 10.Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Physical therapy. 2002;82(2):128–37. doi: 10.1093/ptj/82.2.128. [DOI] [PubMed] [Google Scholar]
- 11.van Iersel MB, Munneke M, Esselink RA, Benraad CE, Olde Rikkert MG. Gait velocity and the Timed-Up-and-Go test were sensitive to changes in mobility in frail elderly patients. Journal of clinical epidemiology. 2008;61(2):186–91. doi: 10.1016/j.jclinepi.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Harada N, Chiu V, Damron-Rodriguez J, Fowler E, Siu A, Reuben DB. Screening for balance and mobility impairment in elderly individuals living in residential care facilities. Physical therapy. 1995;75(6):462–9. doi: 10.1093/ptj/75.6.462. [DOI] [PubMed] [Google Scholar]
- 13.Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. Journal of geriatric physical therapy (2001) 2009;32(2):46–9. [PubMed] [Google Scholar]
- 14.Bowden MG, Balasubramanian CK, Behrman AL, Kautz SA. Validation of a speed-based classification system using quantitative measures of walking performance poststroke. Neurorehabilitation and neural repair. 2008;22(6):672–5. doi: 10.1177/1545968308318837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabadi MH, Blau A. Admission ambulation velocity predicts length of stay and discharge disposition following stroke in an acute rehabilitation hospital. Neurorehabilitation & Neural Repair. 2005;19(1):20–6. doi: 10.1177/1545968304272762. [DOI] [PubMed] [Google Scholar]
- 16.Salbach NM, Mayo NE, Higgins J, Ahmed S, Finch LE, Richards CL. Responsiveness and predictability of gait speed and other disability measures in acute stroke. Archives of physical medicine and rehabilitation. 2001;82(9):1204–12. doi: 10.1053/apmr.2001.24907. [DOI] [PubMed] [Google Scholar]
- 17.Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182–9. doi: 10.1016/j.physio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Studenski S, Wallace D, Chandler J, et al. Gait speed as a clinical vital sign in the care of older adults. J Am Geriatr Soc. 2002 [Google Scholar]
- 19.Schmid A, Duncan PW, Studenski S, et al. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38(7):2096–100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 20.Nadeau S, Betschart M, Bethoux F. Gait analysis for poststroke rehabilitation: the relevance of biomechanical analysis and the impact of gait speed. Physical medicine and rehabilitation clinics of North America. 2013;24(2):265–76. doi: 10.1016/j.pmr.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Olney SJ, Richards C. Hemiparetic gait following stroke. Part I: Characteristics. Gait & posture. 1996:136–48. [Google Scholar]
- 22.Weerdesteyn V, de Niet M, van Duijnhoven HJ, Geurts AC. Falls in individuals with stroke. Journal of rehabilitation research and development. 2008;45(8):1195–213. [PubMed] [Google Scholar]
- 23.Rose DK, Behrman AL, Nadeau SE, et al. Does exercise tolerance testing at 60 days poststroke predict rehabilitation performance? Archives of physical medicine and rehabilitation. 2013;94(7):1223–9. doi: 10.1016/j.apmr.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 24.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Archives of physical medicine and rehabilitation. 2005;86(8):1552–6. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait & posture. 2005;22(1):51–6. doi: 10.1016/j.gaitpost.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Archives of physical medicine and rehabilitation. 2007;88(1):43–9. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait & posture. 2010;31(2):241–6. doi: 10.1016/j.gaitpost.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Bowden MG, Behrman AL, Woodbury M, Gregory CM, Velozo CA, Kautz SA. Advancing measurement of locomotor rehabilitation outcomes to optimize interventions and differentiate between recovery versus compensation. Journal of neurologic physical therapy : JNPT. 2012;36(1):38–44. doi: 10.1097/NPT.0b013e3182472cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunsky A, Dickstein R, Marcovitz E, Levy S, Deutsch J. Home-based motor imagery training for gait rehabilitation of people with chronic poststroke hemiparesis. Archives of Physical Medicine & Rehabilitation. 2008;89(8):1580–8. doi: 10.1016/j.apmr.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 30.Park Y-H, Kim Y-M, Lee B-H. An Ankle Proprioceptive Control Program Improves Balance, Gait Ability of Chronic Stroke Patients. Journal of Physical Therapy Science. 2013;25(10):1321–4. doi: 10.1589/jpts.25.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.You YY, Her JG, Ko T, Chung SH, Kim H. Effects of Standing on One Leg Exercise on Gait and Balance of Hemiplegia Patients. Journal of Physical Therapy Science. 2012;24(7):571–5. [Google Scholar]
- 32.Allen JL, Kautz SA, Neptune RR. Step length asymmetry is representative of compensatory mechanisms used in post-stroke hemiparetic walking. Gait & posture. 2011;33(4):538–43. doi: 10.1016/j.gaitpost.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 3rd. Philadelphia, PA: F.A. Davis Company; 2015. [Google Scholar]
- 34.Melnyk B, Finout-Overholt E. Evidence-Based Practice in Nursing and Healthcare: A Guide to Best Practice. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 35.Medical Students: Study Design. 2014 Dec 6; http://musc.libguides.com/c.php?g=107902&p=699682.
- 36.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011 Mar; www.cochrane-handbook.org.
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International journal of surgery (London, England) 2010;8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Forrester LW, Roy A, Krywonis A, Kehs G, Krebs HI, Macko RF. Modular ankle robotics training in early subacute stroke: a randomized controlled pilot study. Neurorehabilitation and neural repair. 2014;28(7):678–87. doi: 10.1177/1545968314521004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee IH. Does the speed of the treadmill influence the training effect in people learning to walk after stroke? A double-blind randomized controlled trial. Clinical rehabilitation. 2015;29(3):269–76. doi: 10.1177/0269215514542637. [DOI] [PubMed] [Google Scholar]
- 40.Verma R, Arya KN, Garg RK, Singh T. Task-oriented circuit class training program with motor imagery for gait rehabilitation in poststroke patients: a randomized controlled trial. Topics in stroke rehabilitation. 2011;18(Suppl 1):620–32. doi: 10.1310/tsr18s01-620. [DOI] [PubMed] [Google Scholar]
- 41.Holleran CL, Straube DD, Kinnaird CR, Leddy AL, Hornby TG. Feasibility and potential efficacy of high-intensity stepping training in variable contexts in subacute and chronic stroke. Neurorehabilitation and neural repair. 2014;28(7):643–51. doi: 10.1177/1545968314521001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alon G, Conroy VM, Donner TW. Intensive Training of Subjects with Chronic Hemiparesis on a Motorized Cycle Combined with Functional Electrical Stimulation (FES): A Feasibility and Safety Study. Physiotherapy Research International. 2011;16(2):81–91. doi: 10.1002/pri.475. [DOI] [PubMed] [Google Scholar]
- 43.Chae JB, Lee MH, Lee SY. Post-Stroke Rehabilitation Intervention: Effect of Spinal Stabilization with Visual Feedback on the Mobility of Stroke Survivors. Journal of Physical Therapy Science. 2011;23(2):225–8. [Google Scholar]
- 44.Chen IH, Yang YR, Chan RC, Wang RY. Turning-based treadmill training improves turning performance and gait symmetry after stroke. Neurorehabilitation and neural repair. 2014;28(1):45–55. doi: 10.1177/1545968313497102. [DOI] [PubMed] [Google Scholar]
- 45.Cheng JS, Yang YR, Cheng SJ, Lin PY, Wang RY. Effects of combining electric stimulation with active ankle dorsiflexion while standing on a rocker board: a pilot study for subjects with spastic foot after stroke. Archives of physical medicine and rehabilitation. 2010;91(4):505–12. doi: 10.1016/j.apmr.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 46.Forrester LW, Roy A, Krebs HI, Macko RF. Ankle training with a robotic device improves hemiparetic gait after a stroke. Neurorehabilitation and neural repair. 2011;25(4):369–77. doi: 10.1177/1545968310388291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furnari A, Calabr RS, Gervasi G, et al. Is hydrokinesitherapy effective on gait and balance in patients with stroke? A clinical and baropodometric investigation. Brain injury. 2014;28(8):1109–14. doi: 10.3109/02699052.2014.910700. [DOI] [PubMed] [Google Scholar]
- 48.Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke; a journal of cerebral circulation. 2008;39(6):1786–92. doi: 10.1161/STROKEAHA.107.504779. [DOI] [PubMed] [Google Scholar]
- 49.Kim H, Her JG, Ko J. Effect of Horseback Riding Simulation Machine Training on Trunk Balance and Gait of Chronic Stroke Patients. Journal of Physical Therapy Science. 2014;26(1):29–32. doi: 10.1589/jpts.26.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JH, Lee BH. Action observation training for functional activities after stroke: a pilot randomized controlled trial. NeuroRehabilitation. 2013;33(4):565–74. doi: 10.3233/NRE-130991. [DOI] [PubMed] [Google Scholar]
- 51.Kim J-S, Oh D-W. Home-Based Auditory Stimulation Training for Gait Rehabilitation of Chronic Stroke Patients. Journal of Physical Therapy Science. 2012;24(8):775–7. [Google Scholar]
- 52.Lee C-H, Kim Y, Lee B-H. Augmented reality-based postural control training improves gait function in patients with stroke: Randomized controlled trial. Hong Kong Physiotherapy Journal. 2014;32(2):51–7. [Google Scholar]
- 53.Lee C-W, Kim SG, Yong MS. Effects of Hippotherapy on Recovery of Gait and Balance Ability in Patients with Stroke. Journal of Physical Therapy Science. 2014;26(2):309–11. doi: 10.1589/jpts.26.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee HJ, Cho KH, Lee WH. The effects of body weight support treadmill training with power-assisted functional electrical stimulation on functional movement and gait in stroke patients. American journal of physical medicine & rehabilitation/Association of Academic Physiatrists. 2013;92(12):1051–9. doi: 10.1097/PHM.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 55.Lee NK, Son SM, Nam SH, Kwon JW, Kang KW, Kim K. Effects of Progressive Resistance Training Integrated with Foot and Ankle Compression on Spatiotemporal Gait Parameters of Individuals with Stroke. Journal of Physical Therapy Science. 2013;25(10):1235–7. doi: 10.1589/jpts.25.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S-W, Cho K-H, Lee W-H. Effect of a local vibration stimulus training programme on postural sway and gait in chronic stroke patients: a randomized controlled trial. Clinical rehabilitation. 2013;27(10):921–31. doi: 10.1177/0269215513485100. [DOI] [PubMed] [Google Scholar]
- 57.Seo K, Lee J, Lee S. Impact of PNF-based Walking Exercise on a Ramp on Gait Performance of Stroke Patients. Journal of Physical Therapy Science. 2012;24(12):1243–6. [Google Scholar]
- 58.Shim S, Yu J, Jung J, Kang H, Cho K. Effects of Motor Dual Task Training on Spatiotemporal Gait Parameters of Post-stroke Patients. Journal of Physical Therapy Science. 2012;24(9):845–8. [Google Scholar]
- 59.Patterson SL, Rodgers MM, Macko RF, Forrester LW. Effect of treadmill exercise training on spatial and temporal gait parameters in subjects with chronic stroke: a preliminary report. Journal of rehabilitation research and development. 2008;45(2):221–8. doi: 10.1682/jrrd.2007.02.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teixeira-Salmela LF, Nadeau S, McBride I, Olney SJ. Effects of muscle strengthening and physical conditioning training on temporal, kinematic and kinetic variables during gait in chronic stroke survivors. Journal of rehabilitation medicine. 2001;33(2):53–60. doi: 10.1080/165019701750098867. [DOI] [PubMed] [Google Scholar]
- 61.Westlake KP, Patten C. Pilot study of Lokomat versus manual-assisted treadmill training for locomotor recovery post-stroke. Journal of neuroengineering and rehabilitation. 2009;6:18. doi: 10.1186/1743-0003-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang YR, Wang RY, Chen YC, Kao MJ. Dual-task exercise improves walking ability in chronic stroke: a randomized controlled trial. Archives of physical medicine and rehabilitation. 2007;88(10):1236–40. doi: 10.1016/j.apmr.2007.06.762. [DOI] [PubMed] [Google Scholar]
- 63.Jonsdottir J, Cattaneo D, Recalcati M, et al. Task-oriented biofeedback to improve gait in individuals with chronic stroke: motor learning approach. Neurorehabilitation and neural repair. 2010;24(5):478–85. doi: 10.1177/1545968309355986. [DOI] [PubMed] [Google Scholar]
- 64.Cha Y, Kim Y, Hwang S, Chung Y. Intensive gait training with rhythmic auditory stimulation in individuals with chronic hemiparetic stroke: a pilot randomized controlled study. Neuro Rehabilitation. 2014;35(4):681–8. doi: 10.3233/NRE-141182. [DOI] [PubMed] [Google Scholar]
- 65.Jung K, Kim Y, Cha Y, In TS, Hur YG, Chung Y. Effects of gait training with a cane and an augmented pressure sensor for enhancement of weight bearing over the affected lower limb in patients with stroke: a randomized controlled pilot study. Clinical rehabilitation. 2015;29(2):135–42. doi: 10.1177/0269215514540923. [DOI] [PubMed] [Google Scholar]
- 66.Kim CY, Lee JS, Kim HD, Kim JS. The effect of progressive task-oriented training on a supplementary tilt table on lower extremity muscle strength and gait recovery in patients with hemiplegic stroke. Gait & posture. 2015;41(2):425–30. doi: 10.1016/j.gaitpost.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 67.Morgan P, Embry A, Perry L, Holthaus K, Gregory CM. Feasibility of lower-limb muscle power training to enhance locomotor function poststroke. Journal of Rehabilitation Research & Development. 2015;52(1):77–84 8. doi: 10.1682/JRRD.2014.04.0109. [DOI] [PubMed] [Google Scholar]
- 68.Park J, Seo D, Choi W, Lee S. The effects of exercise with TENS on spasticity, balance, and gait in patients with chronic stroke: a randomized controlled trial. Medical science monitor : international medical journal of experimental and clinical research. 2014;20:1890–6. doi: 10.12659/MSM.890926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yom C, Cho H-Y, Lee B. Effects of virtual reality-based ankle exercise on the dynamic balance, muscle tone, and gait of stroke patients. Journal of Physical Therapy Science. 2015;27(3):845–9 5. doi: 10.1589/jpts.27.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ji S-G, Cha H-G, Kim M-K, Lee C-R. The Effect of Mirror Therapy Integrating Functional Electrical Stimulation on the Gait of Stroke Patients. Journal of Physical Therapy Science. 2014;26(4):497–9. doi: 10.1589/jpts.26.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sousa CO, Barela JA, Prado-Medeiros CL, Salvini TF, Barela AM. Gait training with partial body weight support during overground walking for individuals with chronic stroke: a pilot study. Journal of neuroengineering and rehabilitation. 2011;8:48. doi: 10.1186/1743-0003-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ji SG, Kim MK. The effects of mirror therapy on the gait of subacute stroke patients: a randomized controlled trial. Clinical rehabilitation. 2015;29(4):348–54. doi: 10.1177/0269215514542356. [DOI] [PubMed] [Google Scholar]
- 73.Gama GL, de Lucena Trigueiro LC, Simao CR, et al. Effects of treadmill inclination on hemiparetic gait: controlled and randomized clinical trial. American journal of physical medicine & rehabilitation/Association of Academic Physiatrists. 2015;94(9):718–27. doi: 10.1097/PHM.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 74.Paoloni M, Mangone M, Scettri P, Procaccianti R, Cometa A, Santilli V. Segmental muscle vibration improves walking in chronic stroke patients with foot drop: a randomized controlled trial. Neurorehabilitation and neural repair. 2010;24(3):254–62. doi: 10.1177/1545968309349940. [DOI] [PubMed] [Google Scholar]
- 75.Yang YR, Yen JG, Wang RY, Yen LL, Lieu FK. Gait outcomes after additional backward walking training in patients with stroke: a randomized controlled trial. Clinical rehabilitation. 2005;19(3):264–73. doi: 10.1191/0269215505cr860oa. [DOI] [PubMed] [Google Scholar]
- 76.Kim JH, Jang SH, Kim CS, Jung JH, You JH. Use of virtual reality to enhance balance and ambulation in chronic stroke: a double-blind, randomized controlled study. American Journal of Physical Medicine & Rehabilitation. 2009;88(9):693–701. doi: 10.1097/PHM.0b013e3181b33350. [DOI] [PubMed] [Google Scholar]
- 77.Sabut SK, Sikdar C, Mondal R, Kumar R, Mahadevappa M. Restoration of gait and motor recovery by functional electrical stimulation therapy in persons with stroke. Disability and rehabilitation. 2010;32(19):1594–603. doi: 10.3109/09638281003599596. [DOI] [PubMed] [Google Scholar]
- 78.Combs-Miller SA, Kalpathi Parameswaran A, Colburn D, et al. Body weight-supported treadmill training vs. overground walking training for persons with chronic stroke: a pilot randomized controlled trial. Clinical rehabilitation. 2014;28(9):873–84. doi: 10.1177/0269215514520773. [DOI] [PubMed] [Google Scholar]
- 79.Engardt M, Knutsson E, Jonsson M, Sternhag M. Dynamic muscle strength training in stroke patients: effects on knee extension torque, electromyographic activity, and motor function… this paper was presented in part at the International Congress on Stroke Rehabilitation, Berlin, Germany, November 21–24, 1993. Archives of Physical Medicine & Rehabilitation. 1995;76(5):419–25. doi: 10.1016/s0003-9993(95)80570-2. [DOI] [PubMed] [Google Scholar]
- 80.Bowden MG, Behrman AL, Neptune RR, Gregory CM, Kautz SA. Locomotor rehabilitation of individuals with chronic stroke: difference between responders and nonresponders. Archives of physical medicine and rehabilitation. 2013;94(5):856–62. doi: 10.1016/j.apmr.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 81.Perry J, Burnfield J. Gait Analysis: Normal and Pathological function. 2. Slack Incorporated; 2010. [Google Scholar]
- 82.Beaman CB, Peterson CL, Neptune RR, Kautz SA. Differences in self-selected and fastest-comfortable walking in post-stroke hemiparetic persons. Gait & posture. 2010;31(3):311–6. doi: 10.1016/j.gaitpost.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gregory CM, Embry A, Perry L, Bowden MG. Quantifying human movement across the continuum of care: From lab to clinic to community. Journal of neuroscience methods. 2014;231:18–21. doi: 10.1016/j.jneumeth.2014.04.029. [DOI] [PubMed] [Google Scholar]


