Abstract
Background & Aims
The severity of sepsis can be linked to excessive inflammatory responses resulting in hepatic injury. P2X7 receptor activation by extracellular ATP (eATP) exacerbates inflammation by augmenting cytokine production; while CD39 (ENTPD1) scavenges eATP to generate adenosine, thereby limiting P2X7 activation and resulting in A2A receptor stimulation. We aim to determine the functional interaction of P2X7 and A2A receptors on controlling macrophage response, consequently impacting the outcome of sepsis and liver injury.
Methods
Sepsis was induced by cecal ligation and puncture in C57BL/6 wild-type (WT) and CD39−/− mice. Several in vitro assays were performed using peritoneal or bone marrow derived macrophages to determine CD39 ectonucleotidase activity and its role in sepsis-induced liver injury.
Results
CD39 expression in macrophages limits ATP-P2X7 receptor pro-inflammatory signaling. P2X7 receptor paradoxically boosts CD39 activity. Inhibition and/or deletion of P2X7 receptor in LPS-primed macrophages attenuates cytokine production and inflammatory signaling as well as preventing ATP-induced increases in CD39 activity. Septic CD39−/− mice exhibit higher levels of inflammatory cytokines and show more pronounced liver injury than WT mice. Pharmacological P2X7 blockade largely prevents tissue damage, cell apoptosis, cytokine production, and the activation of inflammatory signaling pathways in the liver from septic WT, while only attenuating these outcomes in CD39−/− mice. Furthermore, the combination of P2X7 blockade with adenosine A2A receptor stimulation completely inhibits cytokine production, the activation of inflammatory signaling pathways, and protects septic CD39−/− mice against liver injury.
Conclusions
CD39 attenuates sepsis-associated liver injury by scavenging eATP and ultimately generating adenosine. We propose boosting of CD39 would suppress P2X7 responses and trigger adenosinergic signaling to limit systemic inflammation and restore liver homeostasis during the acute phase of sepsis.
Keywords: Extracellular ATP, Ectonucleotidases, Adenosine, Kupffer cells, Systemic inflammation
Graphical abstract
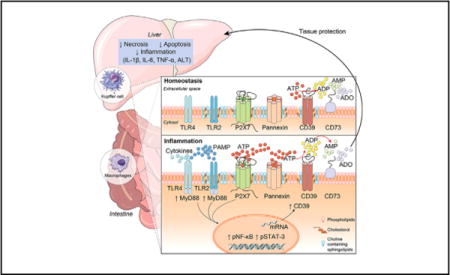
Introduction
Sepsis is a leading cause of death in intensive care units worldwide1 and represents a major public health issue due to high numbers of deaths of economically productive people and major morbidity in survivors. Aspects of the associated morbidity and mortality are related to onset of shock and hemodynamic compromise with multiple organ system dysfunction and failure due to the excessive, unfettered inflammation.1,2
The liver plays a central role in metabolic homeostasis and in the host-defense against pathogens; albeit liver function can be acutely compromised by shock as in severe sepsis.3 Sepsis causes acute liver damage because of hemodynamic compromise but direct hepatocyte and canalicular injury is also feasible. The development of liver failure in sepsis is recognized as a major complication, which contributes to severity of the sepsis and limits positive outcomes.
The inflammatory response in sepsis is dependent upon activation of pathogen recognition receptors (i.e. Toll-like receptors [TLRs]) in monocyte/macrophages (Kupffer cells in the liver), which are crucial to the pathogenesis of sepsis and sepsis-associated liver injury.4,5 The TLR-mediated activation of the MyD88/NF-κB pathway in these cells induces a hyper-inflammatory state mediated by the production of large amounts of tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and IL-1β.4,6 In turn, TNF-α and IL-1β stimulate hepatocytes and Kupffer cells to produce IL-6, which induces acute-phase protein production via STAT3 activation.3,7 Therefore, crosstalk between activated macrophages and hepatocytes may be a central component of the pathogenesis of sepsis-associated liver injury.4
Purinergic signaling modulates inflammation, coagulation and immune responses.8,9 Adenosine triphosphate (ATP) may be secreted into the extracellular space as a result of cellular damage or TLR activation, as part of different pathogen-associated molecular patterns (PAMPs) that induce leukocyte activation and migration to the site of infection.10,11 Through the activation of P2 receptors, extracellular ATP (eATP) can initiate inflammation by acting as a ‘danger’ signal in the extracellular medium.9,12 The P2 receptor family comprises of P2Y G-protein coupled receptors and P2X (P2X1-7) ligand-gated ion channels.13,14 The P2X7 receptor has been the most extensively studied,9 as activation of this receptor induces maturation and release of pro-inflammatory cytokines, such as IL-1β,15 together with activation of acid sphingomyelinase16 and the production of reactive nitrogen and oxygen species.17 Furthermore, P2X7 receptor signaling is involved in host resistance against a wide variety of microbial pathogens.9,18,19
The ATP-dependent pro-inflammatory signaling mediated by the P2X7 receptor is modulated by cell surface ectonucleotidases, such as CD39 also known as ecto-nucleoside triphosphate diphosphohydrolase-1 (ENTPD1) and CD73/ecto-5′-nucleotidase, the major ectonucleotidases expressed in immune and vascular cells.20 CD39 hydrolyzes extracellular tri- and diphosphonucleo-sides to monophosphonucleosides, whereas the ubiquitously expressed CD73 is responsible for AMP hydrolysis, and ultimately the generation of adenosine, which has anti-inflammatory properties.21 Interestingly, Cohen et al.11 have reported that ectonucleotidases exert important monocyte-macrophage autoregulatory functions in limiting cellular activation by catalyzing the rapid conversion of ATP, ultimately into adenosine. In addition, the CD39/CD73-axis has been found to decrease mortality and organ damage in sepsis.21,22 However, the exact cellular mechanisms and signaling pathways underling these observations remains unclear.
We note that: (i) the liver plays a central role in host-defense against pathogens and that hepatic dysfunction contributes to severity and outcome of sepsis;3 (ii) monocyte-macrophage responses are crucial to the pathogenesis of abdominal inflammation and liver failure;4,5 and (iii) P2X7 receptor activation, CD39/CD73-axis, as well as, adenosinergic signaling modulate macrophage responses and plasticity,11 as eATP-P2X7 receptor mediated immune responses in activated macrophages are counterbalanced by ectonucleotidases, such as CD39, which scavenge ATP. We hypothesized that CD39 ectonucleotidase activity would restore liver homeostasis during sepsis and could be hepatoprotective in such experimental settings.
We found that CD39 expression in macrophages limits ATP-P2X7 mediated pro-inflammatory responses. Moreover, CD39 deletion exacerbates sepsis-induced liver dysfunction. The combination of P2X7 blockade with an adenosine A2A receptor agonist (ATL146e) completely protects the liver during sepsis, improving experimental outcomes. Therefore, these data indicate that CD39 attenuates sepsis-associated liver injury by both scavenging eATP and ultimately generating adenosine, suggesting innovative therapeutic avenues to explore the possible benefits in clinical sepsis.
Material and methods
Mice and experimental procedures
Male, 8–10-week old wild-type (WT), P2X7 receptor deficient (P2X7−/−) (originally from the Jackson Laboratory, USA) or CD39 deficient mice (CD39−/−) C57BL/6 were used. All experiments were approved by the Commission for the Ethical Use of Research Animals (CEUA) from the Federal University of Rio de Janeiro (UFRJ) (approved protocol number: IBCCF138) and by the Institutional Animal Care and Use Committees (IACUC) of Beth Israel Deaconess Medical Center (approved protocol number: 019-2015). Sepsis was induced by cecal ligation and puncture (CLP) as previously described.23
Statistical analysis
Results are expressed as means ± standard error of mean (SEM). Statistical analysis was performed by one-way analysis of variance (ANOVA), followed by a Turkey multiple range tests. Differences between groups were considered statistically significant when p <0.05. The statistical significance of lethality was analyzed using the Kaplan-Meier method and log-rank test.
For further details regarding the materials used, please refer to the Supplementary data and CTAT table.
Results
P2X7 receptor boosts CD39 activity and contributes to NF-κB and STAT3 activation in LPS-primed macrophages
To investigate whether the sepsis-like stimulus (LPS) and P2X7 receptor are involved in the modulation of CD39 activity and inflammatory signaling pathways (MyD88/NF-κB and STAT3), we primed murine peritoneal resident macrophages and BMDM with LPS in vitro and treated these cells with P2X7 agonists ATP (500 μM), BzATP (100 μM; a potent P2X7 agonist) or ATPγs (100 μM; a non-hydrolysable ATP analog; data not shown) for 3 h, in the presence or absence of the specific P2X7 antagonist A740003 (Fig. 1A and B). We noted that P2X7 agonists potentiated ATP hydrolysis in LPS-primed macrophages (*p <0.05), albeit this effect was reversed by pharmacological blockade of the P2X7 receptor (p >0.05) (Fig. 1A and B). A similar pattern of response was observed in THP-1 derived human macrophages (data not shown). In agreement with the observed changes in biochemical ecto-enzymatic activity, we also noted an associated increase in CD39 mRNA levels (Fig. 1C), suggesting transcriptional control of CD39 by TLR and P2X7 receptor activation. Indeed, macrophages derived from P2X7−/− mice were less able to scavenge ATP after LPS and ATP stimulation (Fig. 1D).
Fig. 1. P2X7 receptor boosts CD39 activity and contributes to NF-κB and STAT3 activation in LPS-primed macrophages.
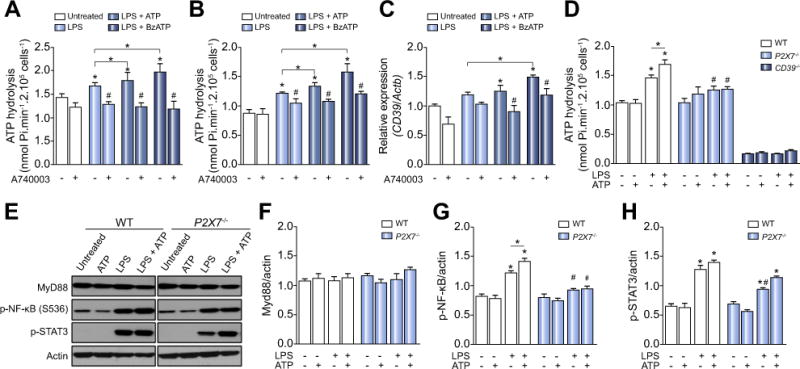
(A) ATP hydrolysis by LPS-primed peritoneal macrophages and (B) BMDM stimulated with P2X7 receptor agonists pretreated or not with A740003. (C) CD39 gene expression in LPS-primed BMDM. (D) ATP hydrolysis by P2X7 and CD39 deficient LPS-primed macrophages stimulated with ATP. (E) Representative Western blot membranes with densitometric analysis of (F) MyD88, (G) p-NF-κB, and (H) p-STAT3 protein expression in LPS-primed macrophages derived from WT or P2X7−/− mice stimulated with 500 μM ATP. Data are expressed as mean ± SEM of three independent experiments performed in triplicates analyzed by one-way ANOVA, Turkey tests. Asterisks represent statistically significant differences (p <0.05; *) compared to unstimulated control group. The number signs (#) represent statistically significant difference (p <0.05) when comparing groups of animals and WT vs. P2X7−/−.
Regarding the possibility that P2X7 triggers intracellular inflammatory pathways, we next observed significant increases in NF-κB and STAT3 activation when ATP stimulation of LPS-primed macrophages was studied (Fig. 1E). Again, these ATP effects on NF-κB activation were not observed in LPS-primed macrophages derived from P2X7−/− mice (Fig. 1E). Indeed, LPS-primed P2X7−/− macrophages exhibited significantly lower levels of STAT3 activation, when compared to the WT counterparts (Fig. 1E). These data suggest functional links between P2X7 activation and intracellular responses to LPS.
CD39−/− macrophages stimulated with LPS and ATP exhibit an increased NF-κB activation and IL-1β production
In order to test whether CD39 enzyme activity is important to limit P2X7 receptor mediated NF-κB activation in LPS-primed macrophages, we next compared MyD88 and p-NF-κB expression in WT and CD39−/− macrophages. These cells were pretreated with P2X7 receptor antagonists or imipramine (operational as an acid sphingomyelinase inhibitor); and then stimulated with LPS and P2X7 agonists. It is already known that P2X7 stimulates acid sphingomyelinase to boost cytokine release;16 hence inhibition of acid sphingomyelinase might inhibit, at least in part, inflammatory signaling post activation of P2X7 receptor.
The results confirmed that both BzATP and ATP are able to enhance the NF-κB activation induced by LPS per se. The pretreatment with P2X7 receptor antagonists (A740003 and oATP); or imipramine attenuate this effect (Fig. 2A). In comparison to the WT group, CD39−/− macrophages show increased p-NF-κB activation, both at baseline and after stimulation with LPS and/or with P2X7 agonists (Fig. 2B). Likewise, P2X7 receptor antagonists or imipramine were able to attenuate NF-κB activation. These findings support the notion that CD39 limits P2X7 receptor response. Moreover, CD39 expression is also crucial for controlling P2X7 induced cytokine release. As shown in Fig. 2C and E, CD39−/− macrophages showed more IL-1β and generated less IL-10 expression after stimulation with LPS and ATP; in comparison to WT macrophages. Furthermore, both P2X7 receptor antagonists (A740003 and oATP) decreased the production of IL-1β (Fig. 2C), while oATP alone induced a significant decrease in IL-6 and IL-10 levels (Fig. 2D and E) in both groups. Importantly, the same profile of cytokine production was observed after stimulation with LPS + BzATP (data not shown).
Fig. 2. P2X7 receptor blockade decreases LPS-mediated NF-κB activation and cytokine production in WT and CD39−/− BMDM.
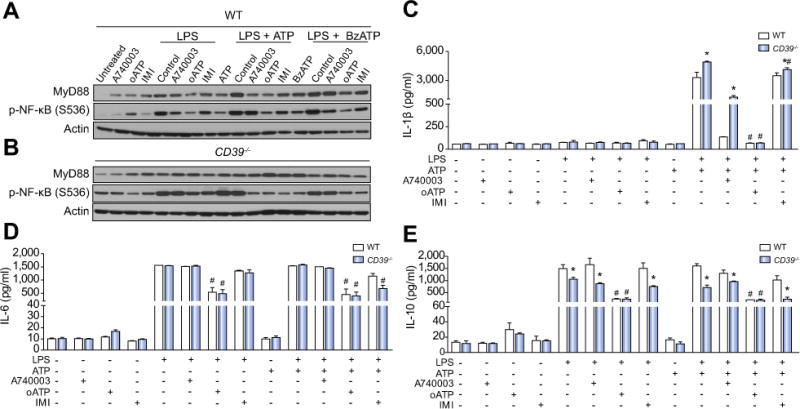
(A) MyD88 and p-NF-κB protein expression (B) IL-1β, (C) IL-6, and (D) IL-10 production in WT and CD39−/− LPS-primed BMDM stimulated with ATP (500 μM) or BzATP (100 μM). For P2X7 receptor inhibition, samples were pretreated with the P2X7 receptor antagonists oATP (300 μM, for 2 h) or A740003 (0.1 μM, for 30 min) or with imipramine (IMI, 30 μM for 30 min) before priming. Data are expressed as mean ± SEM of three independent experiments analyzed by one-way ANOVA, Turkey tests. Asterisks represent statistically significant differences (p <0.05; *) when comparing WT to CD39−/− or groups. Number signs (#) represent statistically significant differences (p <0.05) when the group pretreated with antagonists were compared to the same stimulated group non-pretreated.
Extracellular ATP acting via P2X7 receptor contributes to inflammatory exacerbation in sepsis
Extracellular ATP has been considered an immune adjuvant that can initiate inflammation at sites of infection and/or damage. We studied the CLP model of polymicrobial sepsis in mice to explore the role of ATP-P2X7 receptor signaling in the modulation of immune responses during early phase of abdominal sepsis.
Since neutrophils are rapidly mobilized from the circulation to sites of inflammation during acute infection, we first investigated whether engagement of P2X7 receptor modulates neutrophil infiltration into the peritoneal cavity during sepsis. We observed a significant increase in the number of neutrophils (CD11b+Ly6G+ cells) in the peritoneal cavity 3 h after sepsis induction (Fig. 3A). P2X7 receptor pharmacological inhibition using the antagonist brilliant blue G (BBG) (Fig. 3A) or with genetic deletion of P2X7 (Fig. 3A) significantly (p <0.05) decreased CLP-induced neutrophil recruitment to the peritoneal cavity.
Fig. 3. P2X7 receptor blockade attenuates systemic inflammatory responses in septic WT and CD39−/− mice.
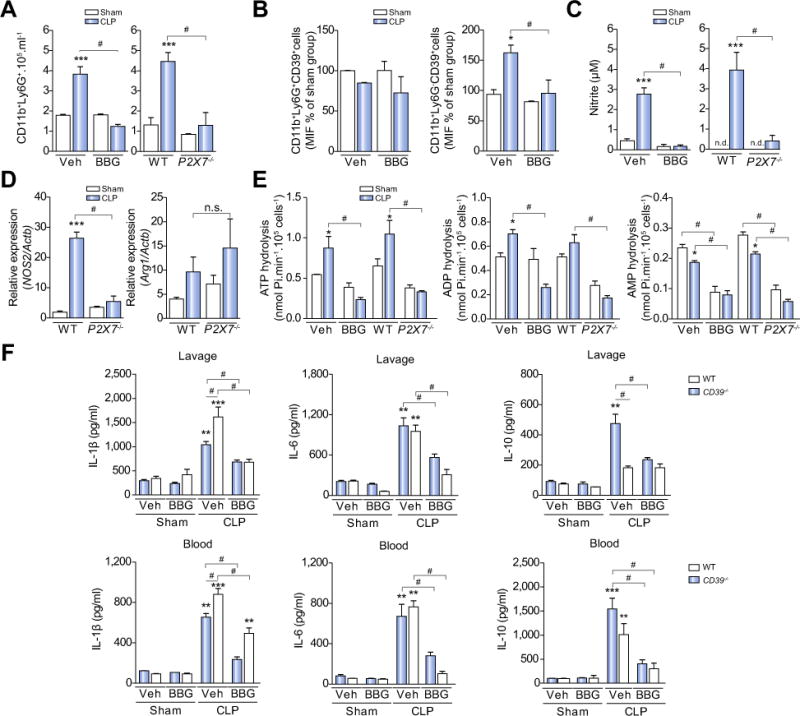
Peritoneal lavage fluid was collected 3 h after surgery and the (A) number of neutrophils (CD11b+Ly6G+) and monocyte/macrophages (CD11b+Ly6G−) was quantified by flow cytometry, as well as, (B) CD39 expression in these cells. (C) Nitric oxide production and (D) Nos2 expression in peritoneal cavity cells from WT or P2X7−/− or BBG-pretreated septic mice. (E) ATP, ADP, and AMP hydrolysis by peritoneal cavity cells from WT or P2X7−/− or BBG-pretreated septic mice. (F) Cytokine production in peritoneal lavage fluid and blood from WT or CD39−/− mice. Data are expressed as mean ± SEM of four independent experiments (min. n = 3 mice/experiment) analyzed by one-way ANOVA, Turkey tests. Statistically significant differences between Sham and CLP, and between CLP groups (CLP vehicle vs. BBG, CLP WT vs. P2X7−/− or CD39−/−) are indicated by asterisks (*, p <0.05; **, p <0.01; ***, p <0.001) and by the number sign (#, p <0.05), respectively.
We further observed a significant increase in nitric oxide (NO) production in the peritoneal cavity of septic mice (p <0.001). Genetic deletion or pharmacological inhibition of the P2X7 receptor was able to significantly decrease NO levels in the peritoneum in this setting (Fig. 3C; p <0.001). In accordance with these results, we also found an increased expression of Nos2 in peritoneal cells obtained from WT mice, but not in cells from the null P2X7−/− (Fig. 3D; p <0.001). Conversely, arginase1 gene expression did not differ among the studied groups at this time point (Fig. 3D; p >0.05). Collectively, these results suggest a role for the ATP-P2X7 signaling in the activation and migration of inflammatory cells to the peritoneal cavity during sepsis.
CD39 activity is increased and modulated by P2X7 receptor in peritoneal cells from septic mice
Extracellular ATP metabolism was evaluated through the activity of CD39 and CD73 enzymes in peritoneal cells at 3 h after CLP-induced sepsis. We observed a significant increase in ATP and ADP hydrolysis – suggestive of an increase in CD39 activity – by peritoneal cells from septic mice (CLP, vehicle-treated or WT) when compared to sham-operated mice (p <0.05) (Fig. 3E, left and middle), whereas the AMP hydrolysis was decreased – suggestive of diminished CD73 activity (Fig. 3E, right). Pharmacological inhibition or genetic deletion of the P2X7 receptor abrogated these sepsis-induced effects (p <0.05).
Moreover, a subpopulation of monocytes/macrophages expressing CD39 (CD11b+Ly6G−CD39+), but not neutrophils (CD11b+Ly6G+CD39+) in the peritoneal cavity appeared responsible for the enhanced extracellular nucleotide phosphohydrolysis, associated with sepsis (Fig. 3B, p >0.05). Indeed, BBG pretreatment prevented sepsis-induced increase in a number of these CD39+ monocyte/macrophage (Fig. 3B), further supporting a role for P2X7 in controlling CD39 activity in this setting.
CD39 deletion contributes to excessive cytokine production after CLP-induced sepsis
Levels of IL1-β, IL-6, and IL-10 were significantly increased in both serum and peritoneal fluid from WT and CD39−/− mice after 3 h of CLP model induction (p <0.05; Fig. 3F). Notably, IL-10 levels in peritoneal lavage fluid obtained from CD39−/− mice did not change despite sepsis induction (p >0.05; Fig. 3F). Moreover, septic CD39−/− mice had significantly higher levels of IL-1β, and lower levels of IL-10 when compared to septic WT mice (p <0.05; Fig. 3F). Furthermore, the inhibition of P2X7 receptor with BBG attenuated cytokine production in all septic mice samples (p <0.05; Fig. 3F). Altogether, these results implicate a role for CD39 in limiting the eATP-P2X7 pro-inflammatory effects and immune responses during sepsis and peritoneal inflammation. Of note, imipramine treatment (20 mg/kg) did not show any significant protective effects in septic WT or CD39−/− mice (data not shown).
Simultaneous P2X7 receptor blockade and adenosine A2A receptor activation completely protects from liver injury during sepsis
While exploring the role of purinergic signaling in sepsis progression and acute liver injury, histological analysis indicated foci of liver necrosis in tissues obtained from WT and CD39−/− mice 24 h after sepsis induction (Fig. 4A and B). Moreover, CD39−/− animals had enlarged pathological areas of cell death, in comparison to WT mice (Fig. 4A and B). Pre-treating these animals with BBG completely protected the liver in WT mice, but only attenuated the liver injury in CD39−/− animals (Fig. 4A and B).
Fig. 4. P2X7 receptor pharmacological inhibition and adenosine A2A receptor activation completely protects the liver from WT and CD39−/− mice during sepsis.
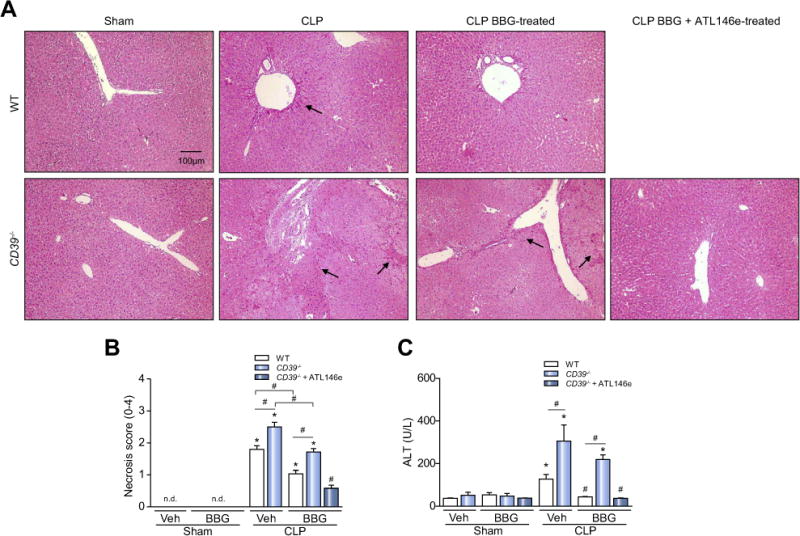
The liver was harvested 24 h after surgery for the evaluation of histopathological changes. Representative photomicrographs of hepatic parenchyma in (A) WT and CD39−/− mice are shown. Arrows show the necrotic areas. (B) Necrosis score; values represent mean ± SEM of four animals per group, considering 10 random fields per mice. (C) Alanine aminotransferase (ALT) levels in septic mice. Data are expressed as mean ± SEM of two independent experiments (n = 6) analyzed by one-way ANOVA, Turkey tests. Statistically significant differences between Sham and CLP, and between CLP groups (CLP vehicle vs. CLP BBG, CLP vehicle vs. CLP BBG + ATL146e) are indicated by asterisks (*, p <0.05) and by number sign (#, p <0.05), respectively.
Given these data, we therefore hypothesized that CD39 is important not only for the removal of extracellular ATP, but also for providing substrate (AMP) for ultimately generating adenosine by the enzyme CD73. We, therefore, tested this assumption by pre-treating CD39−/− mice with BBG, inducing the CLP model of polymicrobial sepsis and injecting an A2A adenosine receptor agonist right after the surgery.
The association of P2X7 receptor inhibition with adenosine A2A receptor activation was able to completely protect CD39−/− animals from liver injury during sepsis. In agreement with these findings, serum levels of alanine aminotransferase (ALT) were increased in septic WT and CD39−/− mice, suggestive of liver injury, and these levels were significantly higher in septic CD39−/− mice (Fig. 4C). Pretreatment with BBG could prevent ALT increases in WT mice, but not in septic CD39−/− mice (Fig. 4C). However, the combination of BBG (P2X7 blockade) and ATL146e (adenosine A2A receptor agonist) abrogated the serum ALT enzyme increase in septic CD39−/− animals (Fig. 4C), suggesting that both P2X7 receptor blockade and adenosine generation are pivotal to maintain liver integrity during sepsis. Of note, the administration of A2A agonist alone did not confer any protective effect in WT or CD39−/− animals (data not shown).
P2X7 receptor inhibition and adenosine A2A receptor activation decreased the number of apoptotic cells in the liver from septic mice
Tissues from both WT and CD39−/− mice presented a significant increase in the number of TUNEL-positive cells, with morphology indicative of sinusoidal cells, 24 h after sepsis induction (Fig. 5A and B; p <0.05). Once more, pretreatment with BBG significantly decreased the number of apoptotic cells in the liver of septic WT and CD39−/− mice (Fig. 5A and B; p <0.05), but a complete rescue was obtained only in the latter when animals were treated with BBG + ATL146e.
Fig. 5. P2X7 receptor inhibition decreases the number of apoptotic cells in the liver from septic WT or CD39−/− mice.
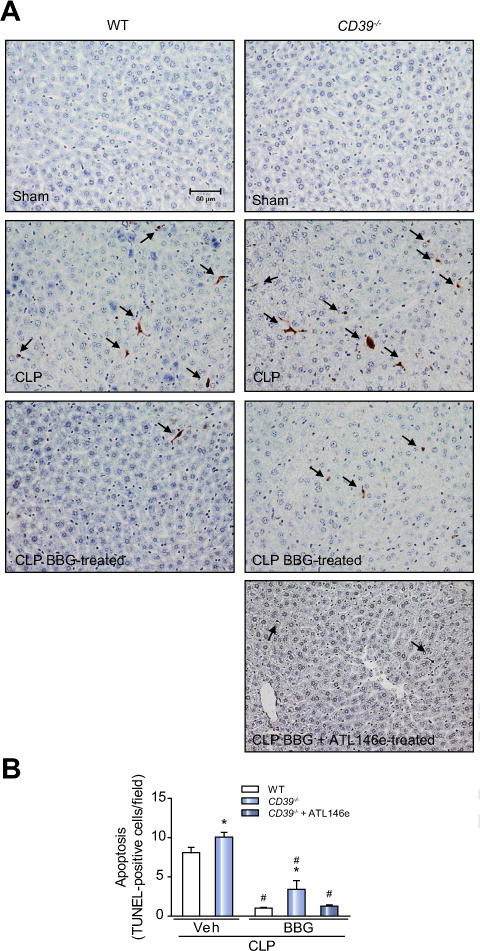
The liver was harvested 24 h after surgery. (A) Photomicrographs show the hepatic TUNEL-positive cells (arrows) in septic WT and CD39−/− mice. (B) Number of TUNEL-positive cells per field. Values represent mean ± SEM of four animals per group, considering five random fields per mice. Data are expressed as mean ± SEM of two independent experiments (n = 6) analyzed by one-way ANOVA, Turkey tests. Statistically significant differences between Sham and CLP, and between CLP groups (CLP vehicle vs. CLP BBG, CLP vehicle vs. CLP BBG + ATL146e) are indicated by asterisks (*, p <0.05) and by the number sign (#, p <0.05), respectively.
P2X7 receptor blockade and adenosine A2A receptor activation reduces the cytokine production and NF-κB and STAT3 activation in the liver of septic mice
Increased levels of IL-1β, IL-6, TNF-α, and IL-10 were found in liver samples from both septic WT and CD39−/− mice (Fig. 6A respectively; p <0.05). IL-1β production was significantly higher in the liver from septic CD39−/− mice when compared to the septic WT mice (Fig. 6A, p <0.05). Pretreatment with BBG attenuated the production of all cytokines in septic WT mice (Fig. 6A; p <0.05), while this effect was less pronounced in the CD39−/− group (note the elevated production of IL-1β and IL-6 in relation to the corresponding sham group) (Fig. 6A). However, associating P2X7 receptor blockade with adenosine A2A receptor activation completely inhibited sepsis-induced cytokine production in the liver from these CD39−/− animals (Fig. 6A). Of note, the same cytokine pattern was observed in blood and peritoneal lavage fluid from WT and CD39−/− mice fluid 24 h after sepsis induction and pharmacological treatments (Fig. S1).
Fig. 6. P2X7 receptor blockade and concurrent adenosine A2A receptor activation inhibit inflammatory cytokine production and NF-κB/STAT3 activation in the liver from septic WT or CD39−/− mice.
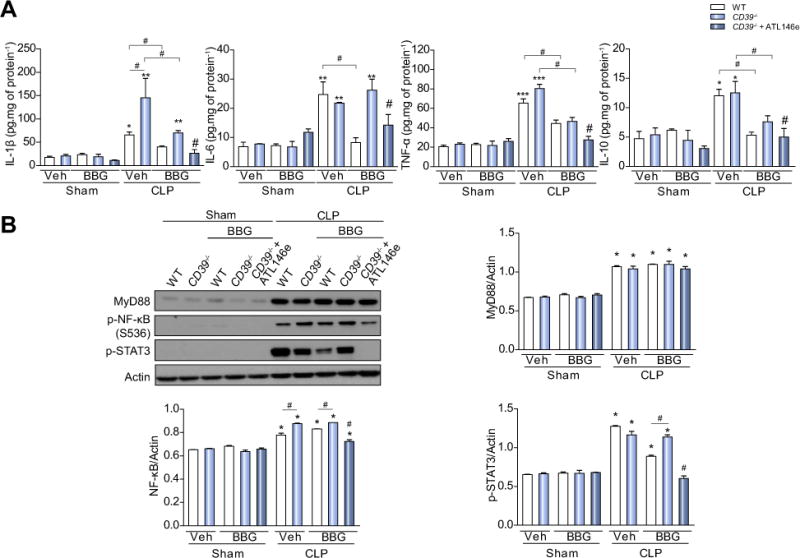
The liver was harvested 24 h after surgery and (A) cytokine (IL-1β, IL-6, TNF-α, and IL-10) production were determined by ELISA. (B) MyD88, p-NF-κB and p-STAT3 protein levels in the liver from septic mice treated or not treated with BBG or BBG + ATL146e. Data are presented as mean ± SEM from three independent experiments (n = 6) analyzed by one-way ANOVA, Turkey tests. Statistically significant differences between Sham and CLP, and between CLP groups (CLP vehicle vs. CLP BBG, CLP vehicle vs. CLP BBG + ATL146e) are indicated by asterisks (*, p <0.05; **, p <0.01; ***, p <0.001) and by the number sign (#, p <0.05), respectively.
Further, we also detected an increase in NF-κB and STAT3 activation in the liver from septic WT and CD39−/− mice. Indeed, the p-NF-κB increase was higher in CD39−/− compared to septic WT mice (Fig. 6B). Again, pretreatment with BBG attenuated the increase in the levels of p-STAT3 in WT, but not in septic CD39−/− animals. Combined treatment with BBG and adenosine A2A receptor agonist significantly decreased NF-κB and STAT3 activation in mouse liver from septic CD39−/− mice. The expression of MyD88 was increased in both septic WT and CD39−/− mice, but no level reversion was seen despite the pharmacological treatments (Fig. 6B).
Finally, we observed that BBG and BBG + ATL146e treatments prolonged the survival time of the septic WT mice by approximately 40% (p = 0.08), and 55% (p = 0.02), respectively (Fig. 7).
Fig. 7. P2X7 receptor blockade and adenosine A2A receptor activation prolong time to euthanasia and death of septic mice.
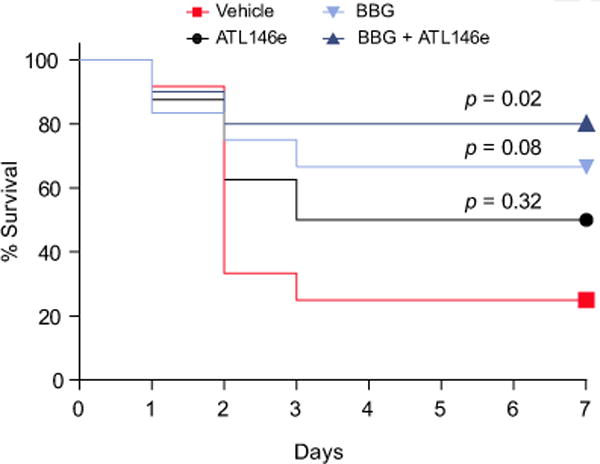
Survival curves of septic WT mice after pharmacological treatments (ATL146e, BBG or BBG + ATL146e) showing time to euthanasia or death. The statistical significances were analyzed using the Kaplan-Meier method and Log-rank test (n = 8–12).
Discussion
Severe sepsis induces hepatic dysfunction and acute liver injury and indicates a poor prognosis with deleterious outcomes in clinically ill patients. Monocyte/macrophages are key players in the pathogenesis of acute liver injury in sepsis as well as in other liver related illnesses.4,5 TLR-mediated NF-κB activation in monocyte-macrophages induces the production of inflammatory cytokines, such as TNF-α and IL-1β.4,6 In turn, TNF-α and IL-1β stimulate hepatocytes and Kupffer cells to produce IL-6, which induces acute-phase protein production via STAT3 activation, further contributing to systemic activation of immune responses.3,7 In addition, NF-κB and STAT3 activation may also increase CD39 expression.24
Here we show that NF-κB and STAT3 phosphorylation is potentiated by P2X7 receptor activation in LPS primed-macrophages, suggesting a contribution for this receptor in a model of sepsis related inflammation and liver injury. TLR4 and P2X7 receptor activation both induce increases in the activity and gene expression of CD39 in macrophages, in a putative negative feed-back loop, to scavenge extracellular ATP and thereby limit inflammation and sepsis-associated liver damage.11
CD39 is the rate-limiting enzyme in the extracellular nucleotide to adenosine phosphohydrolytic cascade, thereby modulating the activation of purinergic receptors and inflammation in the liver.20,25 CD39 per se is able to reduce cellular responses triggered by P2X7, such as apoptosis and release of pro-inflammatory cytokines in macrophages.26 Our data show that CD39 limits the P2X7-mediated increased p-NF-κB levels and controls cytokines released by macrophages. Further, the lack of CD39 boosts IL-1β levels, probably as a consequence of increased levels of extracellular ATP (second signal to the inflammasome activation).15
We also note that IL-10 production is decreased in CD39−/− macrophages, which can be explained by the fact that absence of CD39 would compromise adenosine generation and might limit A2B receptor activation, which otherwise would lead to IL-10 production.27 Moreover, CD39−/− macrophages treated with P2X7 antagonists decrease IL-1β release, reinforcing a link between CD39 and P2X7 receptor in controlling cell signaling transcription and translation of inflammatory cytokines. Considering monocyte-macrophage responses dictate the severity of liver injury, it is feasible that CD39 could be a potential therapeutic target to limit P2X7-dependent inflammatory responses, thereby protecting the liver and improving outcomes in sepsis.
CD39-P2X7 receptor modulatory effects have also been demonstrated in vivo during hepatic ischemia reperfusion injury,28 acetaminophen acute liver failure,29 and in polymicrobial sepsis.18,22 P2X7 receptor activation is important in providing a protective mechanism by enhancing macrophage bacterial killing ability,19 and increasing secretion of IgM by B1 cells in sepsis.30 However, P2X7 receptor activation might contribute to the production of NO, inflammatory cytokines and exacerbate inflammatory response in sepsis.18,31 Similarly, CD39 genetic deletion increases the mortality rate and the production of cytokines in septic mice, operational through purinergic mechanisms.22
Here, we show that P2X7 receptor pharmacological inhibition with BBG decreases the production of inflammatory cytokines (IL1-β, IL-6, and IL-10), the NO production, and the neutrophil recruitment to peritoneal cavity 3 h after induction of sepsis. NO may enhance bacterial killing by inducing neutrophil activation and migration to the sites of infection.32 On the other hand, NO induces vasodilatation, activation of inflammatory pathways, and reduction of cardiac function, which is likely to increase sepsis severity.18,32 In addition to NO, excessive cytokine production is related to poor outcomes in sepsis.
Therefore, our data reinforce the idea that eATP acting through P2X7 receptor contributes to inflammatory response in early phases of sepsis. In addition, the inhibition of this receptor with BBG could be a therapeutic strategy to limit sepsis progression and treat established inflammatory disease.
BBG has already been tested in clinical trials and no toxicity or adverse effects were reported in humans.33,34 In addition, BBG treatment has protective effects in different animal models of inflammatory and liver disease.31,35 The AZD9056 – a selective orally active inhibitor of the purinergic receptor P2X7 – has shown beneficial effects in patients with moderate-to-severe Crohn’s Disease.36 We also show that pharmacological inhibition of P2X7 has a protective effect, decreasing liver damage, the number of apoptotic cells, the cytokine production, and the activation of inflammation signaling pathways (NF-κB and STAT3) in the liver of septic WT mice, while minimally attenuated those effects in the CD39−/− group. This result might be explained by the fact that septic CD39−/− mice present with exacerbated inflammatory processes, generated by the lack of ectonucleotidases. We propose that this lack of CD39 results in ATP/adenosine imbalance and favors the pro-inflammatory response over the anti-inflammatory one. This assumption was confirmed by our data showing that a combination of P2X7 blockade with the specific A2A receptor agonist ATL146e was able to completely protect CD39−/− mice from harmful effects of sepsis, such as liver injury and progression of the inflammatory process. In addition, BBG + ATL146e treatment was able to improve the survival of septic WT mice by approximately 55%. Our investigations support previous studies showing that stimulation of A2A receptor by specific agonists (ALT146e or ATL313) significantly decreases animal mortality in sepsis37,38 and attenuates hepatic ischemia reperfusion injury.39 Although animal models do not completely reflect the complexity and heterogeneity of sepsis in humans, these data have clinical relevance since they suggest that targeting this system by removing the extracellular ATP and increasing adenosine levels may be a new strategy to prevent sepsis progression, hepatotoxicity, and death.
In summary, we show that P2X7 receptor may modulate the functionality of CD39, increasing the associated ecto-enzymatic activity. CD39 functions as an extracellular ATP scavenger, thus limiting P2X7 activation and inhibiting pro-inflammatory macrophage responses. Moreover, during the progression of sepsis, the functionality of CD39 is simultaneously crucial for the generation of adenosine, which has anti-inflammatory, immunosuppressive, and protective effects in sepsis-associated liver injury (Fig. 8). Thus, these results support the involvement of the purinergic signaling in macrophage activation and sepsis-induced liver injury. Furthermore, these data suggest possible therapeutic approaches based on the administration of soluble apyrases or truncated forms of CD39, which mimic ectonucleotidase activity. These approaches might be combined with the administration of P2X7 receptor blockers to limit monocyte-macrophage responses and liver injury during the acute phase of systemic and abdominal sepsis.
Fig. 8. Schematic representation of P2X7 receptor and CD39 interactions on monocyte-macrophages that modulate liver injury during inflammation.
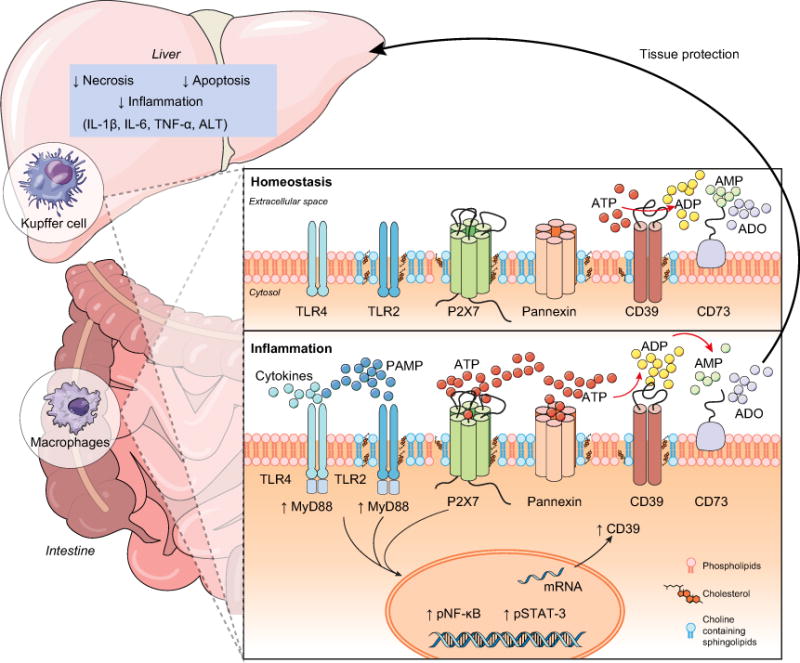
The recognition of PAMPs – such as bacterial LPS – by PRRs can induce ATP release activating the P2X7 receptor. P2X7 receptor activation induces large-scale ATP release that can occur mainly via pannexin hemichannels boosting purinergic signaling. CD39 functions to generate AMP from extracellular ATP to limit P2X7 activation and inhibit pro-inflammatory responses. Then, CD73 hydrolyzes AMP to adenosine (ADO) providing for anti-inflammatory effects and tissue protection. This purinergic regulatory mechanism is functional in limiting liver injury and restoring homeostasis during sepsis.
Supplementary Material
Lay summary.
CD39 expression in macrophages limits P2X7-mediated pro-inflammatory responses, scavenging extracellular ATP and ultimately generating adenosine. CD39 genetic deletion exacerbates sepsis-induced experimental liver injury. Combinations of a P2X7 antagonist and adenosine A2A receptor agonist are hepatoprotective abdominal sepsis.
Highlights.
CD39 expression by monocyte-macrophages limits P2X7-mediated inflammatory responses.
CD39 genetic deletion exacerbates sepsis-induced liver injury and systemic outcomes.
P2X7 blockade and concurrent adenosine A2A receptor activation in sepsis provide substantive hepatoprotective effects.
Simultaneous P2X7 blockade and adenosine A2A receptor activation improve survival in sepsis.
Acknowledgments
Financial support
This work was supported by funds from the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico do Brasil (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Programa de Núcleos de Excelência (PRONEX), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ – Pós-doutorado Nota 10 Edital 05/2016), the Instituto Nacional de Ciência e Tecnologia para Pesquisa Translacional em Saúde e Ambiente na Região Amazônica (INPeTAm/UFRJ), Fundação Lemann, and the National Institutes of Health (NIH) P01 HL107152 and R21 CA164970; Ben and Rose Cole Charitable PRIA Foundation grants to SCR as well as the USA Department of Defence GRANT12027970 (PR151953).
Footnotes
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
Luiz Eduardo B. Savio has made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, and wrote the manuscript. Paola de Andrade Mello; Vanessa R. Figliuolo, Thiago F. A. Almeida, Patrícia T. Santana, Suellen D’arc S. Oliveira, Eva Csizmadia, and Linda Feldbrügge participates in acquisition of data, and/or analysis and interpretation of data. Claudia L. M. Silva, Richard D. Minshall, Maria Serena Longhi, Yan Wu, Robson Coutinho-Silva and Simon C. Robson all contributed to conception, design and interpretation of data, and manuscript preparation. All authors reviewed critically the article content and gave final approval of the version to be submitted.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jhep.2017.05.021.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nesseler N, Launey Y, Aninat C, Morel F, Malledant Y, Seguin P. Clinical review: The liver in sepsis. Crit Care. 2012;16:235. doi: 10.1186/cc11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Possamai LA, Thursz MR, Wendon JA, Antoniades CG. Modulation of monocyte/macrophage function: a therapeutic strategy in the treatment of acute liver failure. J Hepatol. 2014;61:439–445. doi: 10.1016/j.jhep.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 5.Antoniades CG, Khamri W, Abeles RD, Taams LS, Triantafyllou E, Possamai LA, et al. Secretory leukocyte protease inhibitor: a pivotal mediator of anti-inflammatory responses in acetaminophen-induced acute liver failure. Hepatology. 2014;59:1564–1576. doi: 10.1002/hep.26933. [DOI] [PubMed] [Google Scholar]
- 6.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 7.Bode JG, Albrecht U, Haussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins–regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kappaB-dependent signaling. Eur J Cell Biol. 2012;91:496–505. doi: 10.1016/j.ejcb.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Bours MJ, Swennen EL, Di VF, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Morandini AC, Savio LE, Coutinho-Silva R. The role of P2X7 receptor in infectious inflammatory diseases and the influence of ectonucleotidases. Biomed J. 2014;37:169–177. doi: 10.4103/2319-4170.127803. [DOI] [PubMed] [Google Scholar]
- 10.Kawamura H, Kawamura T, Kanda Y, Kobayashi T, Abo T. Extracellular ATP-stimulated macrophages produce macrophage inflammatory protein-2 which is important for neutrophil migration. Immunology. 2012;136:448–458. doi: 10.1111/j.1365-2567.2012.03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen HB, Briggs KT, Marino JP, Ravid K, Robson SC, Mosser DM. TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood. 2013;122:1935–1945. doi: 10.1182/blood-2013-04-496216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di VF. Purinergic signalling in the immune system. A brief update. Purinergic Signal. 2007;3:1–3. doi: 10.1007/s11302-006-9048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 14.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 16.Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009;28:1043–1054. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewinson J, Mackenzie AB. P2X(7) receptor-mediated reactive oxygen and nitrogen species formation: from receptor to generators. Biochem Soc Trans. 2007;35:1168–1170. doi: 10.1042/BST0351168. [DOI] [PubMed] [Google Scholar]
- 18.Santana PT, Benjamim CF, Martinez CG, Kurtenbach E, Takiya CM, Coutinho-Silva R. The P2X7 Receptor Contributes to the Development of the Exacerbated Inflammatory Response Associated with Sepsis. J Innate Immun. 2015;7:417–427. doi: 10.1159/000371388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csoka B, Nemeth ZH, Toro G, Idzko M, Zech A, Koscso B, et al. Extracellular ATP protects against sepsis through macrophage P2X7 purinergic receptors by enhancing intracellular bacterial killing. FASEB J. 2015;29:3626–3637. doi: 10.1096/fj.15-272450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasko G, Csoka B, Koscso B, Chandra R, Pacher P, Thompson LF, et al. Ecto-5′-nucleotidase (CD73) decreases mortality and organ injury in sepsis. J Immunol. 2011;187:4256–4267. doi: 10.4049/jimmunol.1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Csoka B, Nemeth ZH, Toro G, Koscso B, Kokai E, Robson SC, et al. CD39 improves survival in microbial sepsis by attenuating systemic inflammation. FASEB J. 2015;29:25–36. doi: 10.1096/fj.14-253567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalmin F, Mignot G, Bruchard M, Chevriaux A, Vegran F, Hichami A, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36:362–373. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Vaughn BP, Robson SC, Burnstock G. Pathological roles of purinergic signaling in the liver. J Hepatol. 2012;57:916–920. doi: 10.1016/j.jhep.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levesque SA, Kukulski F, Enjyoji K, Robson SC, Sevigny J. NTPDase1 governs P2X7-dependent functions in murine macrophages. Eur J Immunol. 2010;40:1473–1485. doi: 10.1002/eji.200939741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemeth ZH, Lutz CS, Csoka B, Deitch EA, Leibovich SJ, Gause WC, et al. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beldi G, Banz Y, Kroemer A, Sun X, Wu Y, Graubardt N, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51:1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoque R, Sohail MA, Salhanick S, Malik AF, Ghani A, Robson SC, et al. P2X7 receptor-mediated purinergic signaling promotes liver injury in acetaminophen hepatotoxicity in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1171–G1179. doi: 10.1152/ajpgi.00352.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proietti M, Cornacchione V, Rezzonico JT, Romagnani A, Faliti CE, Perruzza L, et al. ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer’s patches to promote host-microbiota mutualism. Immunity. 2014;41:789–801. doi: 10.1016/j.immuni.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Savio LE, Andrade MG, de Andrade MP, Santana PT, Moreira-Souza AC, Kolling J, et al. P2X7 receptor signaling contributes to sepsis-associated brain dysfunction. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0168-9. http://dx.doi.org/10.1007/s12035-016-0168-9. [DOI] [PubMed]
- 32.Fortin CF, McDonald PP, Fulop T, Lesur O. Sepsis, leukocytes, and nitric oxide (NO): an intricate affair. Shock. 2010;33:344–352. doi: 10.1097/SHK.0b013e3181c0f068. [DOI] [PubMed] [Google Scholar]
- 33.Remy M, Thaler S, Schumann RG, May CA, Fiedorowicz M, Schuettauf F, et al. An in vivo evaluation of Brilliant Blue G in animals and humans. Br J Ophthalmol. 2008;92:1142–1147. doi: 10.1136/bjo.2008.138164. [DOI] [PubMed] [Google Scholar]
- 34.Totan Y, Guler E, Guragac FB, Uzun E, Dogdu E. Brilliant blue G assisted macular surgery: the effect of air infusion on contrast recognisability in internal limiting membrane peeling. Br J Ophthalmol. 2015;99:75–80. doi: 10.1136/bjophthalmol-2014-305077. [DOI] [PubMed] [Google Scholar]
- 35.Tung HC, Lee FY, Wang SS, Tsai MH, Lee JY, Huo TI, et al. The beneficial effects of P2X7 antagonism in rats with bile duct ligation-induced cirrhosis. PLoS One. 2015;10:e0124654. doi: 10.1371/journal.pone.0124654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eser A, Colombel JF, Rutgeerts P, Vermeire S, Vogelsang H, Braddock M, et al. Safety and efficacy of an oral inhibitor of the purinergic receptor P2X7 in adult patients with moderately to severely active crohn’s disease: a randomized placebo-controlled, double-blind, phase IIa study. Inflamm Bowel Dis. 2015;21:2247–2253. doi: 10.1097/MIB.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan GW, Fang G, Linden J, Scheld WM. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J Infect Dis. 2004;189:1897–1904. doi: 10.1086/386311. [DOI] [PubMed] [Google Scholar]
- 38.Moore CC, Martin EN, Lee GH, Obrig T, Linden J, Scheld WM. An A2A adenosine receptor agonist, ATL313, reduces inflammation and improves survival in murine sepsis models. BMC Infect Dis. 2008;8:141. doi: 10.1186/1471-2334-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


