Abstract
Purpose
To examine if the role of obesity in the risk of gestational diabetes differs between immigrant and US-born women.
Methods
We used New York City linked 2010–2014 birth certificate and hospital data. We created four racial/ethnic groups (Non-Hispanic Black, Hispanic, Non-Hispanic White, and Asian) and three subgroups (Mexican, Indian, and Chinese). Gestational diabetes mellitus (GDM) was ascertained by the birth certificate checkbox and discharge ICD-9 codes. We calculated relative risks for immigrant status and BMI with GDM using covariate-adjusted log-binomial regression. We calculated multivariable population attributable risk (PAR) to estimate the proportion of GDM that could be eliminated if overweight/obesity were eliminated, by immigrant status.
Results
Immigrant women had higher risk of GDM than US-born women, with adjusted relative risks ranging from 1.2 among Non-Hispanic Black women (95%Confidence Interval (CI)=1.2, 1.3) to 1.6 among Hispanic women (95%CI=1.4, 1.8). Increasing BMI was associated with GDM risk in all groups, but relative risks were weaker among immigrants (p-value for interaction<0.05). The PAR for overweight/obesity was lower in immigrant women than in US-born women in all racial/ethnic groups.
Conclusions
The lower proportion of GDM attributable to overweight/obesity among immigrant women may point to early life and migration influences on risk of GDM.
Keywords: gestational diabetes, obesity, immigrant, ethnicity, disparities, lifecourse
Introduction
Immigrant women are at a decreased risk of many adverse birth outcomes, despite greater socioeconomic risk – a phenomenon known as the healthy migrant effect or Hispanic paradox1. Contrary to this paradox, evidence is substantial that immigrant women are at an increased risk of some maternal adverse outcomes, in particular, gestational diabetes mellitus (GDM).2–6 GDM is glucose intolerance occurring or first recognized during the 2nd or 3rd trimesters of pregnancy.7,8 GDM is thought to affect about 6–8% of pregnancies in the US9, and has serious life course health consequences both for the mother and her child.9,10 Women with gestational diabetes are at an increased risk of later developing Type 2 diabetes and possibly hypertension11, and their children are at an increased risk of obesity.12 An understanding of how etiology of GDM differs between immigrant and US-born women can inform the development of maternal interventions targeting immigrant groups as well as hypotheses of the etiology of GDM across the life course.
Obesity is an established risk factor for GDM, with risks two, four, and eight times the risk for overweight, obese, and severely obese women relative to normal weight women, respectively.13 Previous research has shown that the role obesity plays in gestational diabetes differs by race/ethnicity, with the percent of GDM cases that could be prevented if overweight/obesity were eliminated (population attributable risk) varying from 50–65% among African American women to only 15–23% among Asian women.14–16 Evidence also exists that the role of obesity in GDM varies by nativity, with risk ratios for overweight/obese in association with GDM of larger magnitude in most foreign-born compared to US-born groups.17 Despite a lower prevalence of obesity among immigrant women, they have a greater risk of developing gestational diabetes compared to non-immigrant women, suggesting fewer cases of GDM can be attributed to obesity in immigrant compared to US-born women.18 However, the partial population attributable risk (PAR) of overweight/obesity on GDM by immigrant status has not been investigated. The PAR is important because it clearly communicates the potential impact of gestational diabetes prevention strategies on different population groups, holding other risk factors constant. We hypothesized that obesity would be a cause of a lower proportion of GDM cases in immigrant women. Our hypothesis is framed by the lifecourse theory of cardiometabolic disease in low- and middle-income countries, which posits that high rates of cardiometabolic disease even at low levels of obesity are a result of maternal undernutrition during fetal development followed by exposure to an increasingly obesogenic environment.19 We analyzed linked birth certificate – hospital discharge data from the years 2010–2014 with the following objectives: 1) Confirm in recent data an increased risk of GDM among foreign-born women, 2) Test if BMI shows a similar pattern of increased risk of GDM by nativity, and 3) Calculate the population attributable risk for obesity in association with GDM for each racial/ethnic/immigrant group.
Materials and Methods
Data source
The New York State Department of Health linked New York City birth certificate data to New York State Hospitalization data (SPARCS) for the years 2010–2014. Each birth record was linked to the hospital discharge record for the corresponding delivery, representing 591,454 deliveries. We excluded women with pre-gestational diabetes (n=5405) and women with multiple gestations (n=18,574). We excluded records with missing values because of the relatively low proportion of missing and overall large sample size: BMI (0.75%), parity (0.07%), and maternal education (0.28%). We also excluded women with race-ethnicity categorized as “other” for ease of interpretation (n=9028). The final dataset for analysis included 565,839 women. We obtained approvals from the Institutional Review Board of the New York City Department of Health and Mental Hygiene, the New York State Department of Health, and the Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai.
Gestational diabetes
We classified women as gestational diabetes cases if gestational diabetes was indicated either on the birth certificate or hospital discharge record.5 This approach produces high sensitivity with few false positives.20,21. Cases were identified from hospital discharge diagnosis ICD-9 codes 648.8–648.84, and from the birth certificate check box indicating the presence of gestational diabetes during pregnancy. Pre-gestational diabetes cases were identified with the same “either/or” approach, using the birth certificate check box and hospital discharge diagnosis ICD-9 codes 250.00-250.93, 357.2, 362.01, 362.02, 363.03–363.07, 366.41, and 648.01–648.04.
Obesity and other covariates
Maternal height and pre-pregnancy weight were obtained from the birth certificate and used to calculate body mass index (BMI) in kg/m2. We categorized BMI using the World Health Organization (WHO) categorization22. Validity studies in Florida and Pennsylvania have shown that obesity ascertained from the birth certificate has good validity.23,24 We obtained place of birth (US vs. foreign-born) and race/ethnicity from the birth certificate. We used several steps to create our US-born and immigrant categories for analysis. First, we created four categories of race/ethnicity from mother’s self-reported race and Hispanic ancestry: Non-Hispanic Black (Black), Hispanic, non-Hispanic White (White), Asian, excluding those of other race/ethnicity. We next created additional country-specific categories that were available in the data and had sufficient sample size for analysis: Mexican, Indian, and Chinese. The category All Hispanic includes Mexicans, and All Asian includes Indians and Chinese – we chose this approach rather than excluding the ethnic subgroups to increase generalizability and facilitate comparisons with previous research. We then divided each category into US-born and immigrant, based on the variable “country of birth”. We used payment source (Medicaid vs. private insurance/self/other) and maternal education (Less than high school/high school/greater than high school) from the birth certificate as measures of socioeconomic status.
Statistical analysis
We examined descriptive statistics for the study sample by race/ethnicity and nativity, testing differences with Chi-square tests for categorical variables and t-test for continuous variables. We used log binomial regression to estimate associations between obesity categories and gestational diabetes, with normal weight as the reference category. We chose covariates that have been cited in the literature as risk factors for GDM and may be associated with nativity status. We examined the covariates in a directed acyclic graph to determine there would be no induced bias by the relationships between the variables, and then included them in a covariate-adjusted model. Covariates included maternal age, parity, infant sex, maternal education, Medicaid status, and maternal height. We compared nested models (covariate-adjusted models with and without interaction terms) using the log likelihood Chi-square difference test to evaluate if the association between BMI and GDM varied by immigrant status.
We estimated partial population attributable risk (PAR) for pre-pregnancy overweight/obesity (BMI≥25 kg/m2) and gestational diabetes by race/ethnicity/nativity using a SAS macro published by Spiegelman et al.25 The partial PAR measures the proportion of gestational diabetes cases that could be prevented if overweight/obese women were to achieve a normal BMI, while adjusting for other covariates considered “non-modifiable”. The partial PAR is appropriate when the disease of interest is multifactorial, and other risk factors are not expected to change as a result of the hypothetical intervention.26 In contrast, the full PAR is appropriate when the disease of interest depends on a single binary risk factor.27 Our New York City study population was used to estimate the prevalence of overweight/obesity for the partial PAR calculation. Covariates included in the multivariable model were the same as listed above.
Because alternative cut points to define overweight/obesity are recommended for Asian populations,28,29 in a sensitivity analysis we recalculated the partial PAR for All Asian, Indian, and Chinese women using the cut point of 23 kg/m2 to define overweight/obesity.
Results
Study characteristics are shown in Table 1. All tests of differences had p<0.05 due to the large sample size. Among Blacks, All Hispanics, and Mexicans, immigrant women were older and more likely to be multiparous than US-born. Asian immigrant women were also more likely to be multiparous but slightly younger than US-born women. All Hispanic, Mexican, All Asian, Indian, and Chinese immigrant women were less educated and more likely to be insured by Medicaid than their US-born counterparts, but the opposite was true among Black women. Immigrant and US-born White women had similar characteristics except immigrant women were more likely to be insured by Medicaid.
Table 1.
Study characteristics by general racial/ethnic categories, racial/ethnic subgroups, and nativity, New York City, New York City, 2010–2014
| General racial/ethnic categories | ||||||||
|---|---|---|---|---|---|---|---|---|
| Non-Hispanic Black | All Hispanic | All Asian | Non-Hispanic White | |||||
|
| ||||||||
| US-born | Foreign-born | US-born | Foreign-born | US-born | Foreign-born | US-born | Foreign-born | |
|
|
||||||||
| Total | n=67,215 | n=49,321 n=78,170 | n=93,194 | n=22,490 | n=72,461 | n=129,279 | n=49,072 | |
| Mother’s age | ||||||||
| mean (std) | 27.1 (6.3) | 30.8 (6.0) | 26.6 (6.3) | 28.6 (6.0) | 31.9 (4.9) |
30.2 (5.2) |
31.3 (5.7) | 31.1 (5.5) |
| Mother’s education (%) | ||||||||
| Less than high school | 23.3 | 16 | 27.1 | 44.7 | 4.8 | 28.0 | 7.4 | 9.3 |
| High school | 25.3 | 29.2 | 22.8 | 25.2 | 7.6 | 22.0 | 20.5 | 15.3 |
| Greater than high school | 51.4 | 54.8 | 50.1 | 30.1 | 87.6 | 50.0 | 72.1 | 75.3 |
| Mother’s insurance (%) | ||||||||
| Medicaid | 70.4 | 69.7 | 69.5 | 86.0 | 30.0 | 70.2 | 30.3 | 46.4 |
| Private | 26.2 | 24.1 | 28.2 | 11.6 | 68.8 | 28.3 | 68.8 | 51.3 |
| Uninsured | 1.9 | 3.9 | 1.3 | 1.5 | 0.5 | 0.9 | 0.3 | 1.4 |
| Other | 1.5 | 2.3 | 1.1 | 0.9 | 0.8 | 0.5 | 0.7 | 0.9 |
| Pre-pregnancy BMI (%) | ||||||||
| Underweight (<18.5) | 3.8 | 3.4 | 3.5 | 2.8 | 8.4 | 13.0 | 5.5 | 7.2 |
| Normal weight (18.5 – 24.9) | 37.5 | 39.7 | 42.7 | 48.0 | 68.9 | 67.1 | 66.7 | 65.3 |
| Overweight (25.0 – 29.9) | 27.6 | 32.2 | 28.1 | 31.6 | 16.9 | 15.3 | 18 | 19.5 |
| Obese Class I (30.0–34.9) | 16.8 | 16.4 | 15.2 | 12.5 | 4.5 | 3.8 | 6.3 | 5.9 |
| Obese Class II (35.0 – 39.9) | 8.3 | 5.8 | 6.6 | 3.6 | 1.0 | 0.7 | 2.2 | 1.6 |
| Obese Class III (≥40) | 6.1 | 2.5 | 3.9 | 1.4 | 0.3 | 0.2 | 1.2 | 0.5 |
| Height | ||||||||
| mean (std) | 64.7 (2.9) | 64.8 (2.8) | 63.5 (2.8) | 62.2 (3.1) | 63.5 (2.4) |
62.9 (2.3) |
64.5 (2.7) | 64.8 (2.8) |
| Parity (%) | ||||||||
| Nulliparous | 47.9 | 39.8 | 49.4 | 50.6 | 60.1 | 47.9 | 47.4 | 47.1 |
| Multiparous | 52.1 | 60.2 | 50.6 | 64.9 | 39.9 | 52.1 | 52.6 | 52.9 |
| Racial/ethnic Subgroups | ||||||
|---|---|---|---|---|---|---|
| Mexican | Indian | Chinese | ||||
|
| ||||||
| US-born | Foreign-born | US-born | Foreign-born | US-born | Foreign-born | |
|
|
||||||
| Total | n=5283 | n=28,863 | n=9892 | n=17,766 | n=4212 | n=40,817 |
| Mother’s age | ||||||
| mean (std) | 23.3 (6.1) | 28.3 (5.8) | 30.7 (4.8) |
29.3 (5.3) | 32.6 (4.7) |
30.1 (5.0) |
| Mother’s education (%) | ||||||
| Less than high school | 41.1 | 64.2 | 7.6 | 23.5 | 1.9 | 34.6 |
| High school | 22.2 | 26.9 | 9.3 | 28.0 | 4.1 | 22.3 |
| Greater than high school | 36.7 | 8.9 | 83.0 | 48.5 | 94 | 43.2 |
| Mother’s insurance (%) | ||||||
| Medicaid | 78.8 | 94.5 | 35.2 | 76.7 | 15.1 | 74.3 |
| Private | 18.9 | 3.7 | 63.5 | 20.9 | 84.4 | 24.8 |
| Uninsured | 1 | 1.1 | 0.6 | 1.6 | 0.1 | 0.7 |
| Other | 1.3 | 0.8 | 0.8 | 0.8 | 0.4 | 0.2 |
| Pre-pregnancy BMI (%) | ||||||
| Underweight (<18.5) | 4 | 2.3 | 7.6 | 6.2 | 7.4 | 17.2 |
| Normal weight (18.5–24.9) | 47 | 43.9 | 62.9 | 54.1 | 74.6 | 73.7 |
| Overweight (25.0–29.9) | 27.8 | 34.5 | 22.0 | 28.6 | 13.9 | 7.9 |
| Obese Class I (30.0–34.9) | 13.7 | 14.1 | 6.0 | 8.8 | 3.0 | 1.0 |
| Obese Class II (35.0–39.9) | 5 | 3.9 | 1.2 | 1.8 | 0.7 | 0.2 |
| Obese Class III (≥40) | 2.4 | 1.3 | 0.3 | 0.6 | 0.3 | 0.0 |
| Height | ||||||
| mean (std) | 62.4 (2.6) | 60.8 (3.0) | 63.4 (2.3) |
62.6 (2.5) | 63.6 (2.3) |
63.1 (2.1) |
| Parity (%) | ||||||
| Nulliparous | 58.7 | 26.1 | 57.1 | 40.2 | 63.3 | 50.1 |
| Multiparous | 41.3 | 73.9 | 42.9 | 59.8 | 36.7 | 49.9 |
The overall risk of GDM was 7.6%, and was highest in immigrant Indian women (22.9%) and lowest in US-born White women (4.2%) (Table 2). Immigrant women were at a greater risk of GDM than US-born women for all racial/ethnic groups, with adjusted risk ratios of 1.4 (95%Confidence Interval (CI)=1.4, 1.5) among Black women, 1.2 (1.2, 1.3) among All Hispanic women, 1.6 (95%CI=1.4, 1.8) among Mexican women, 1.5 (95%CI=1.4, 1.6) among White women, 1.4 (95%CI=1.4, 1.5) among All Asian women, 1.5 (95%CI=1.4, 1.6) among Indian women, and 1.4 (95%CI=1.3, 1.6) among Chinese women. Adjusted risk ratios were similar or slightly attenuated compared to unadjusted risk ratios for all groups except Mexicans, which was attenuated from 2.8 to 1.6 after adjusting for covariates, in particular, maternal age.
Table 2.
Unadjusted and adjusted risk ratios for foreign-born status and gestational diabetes
| Race/ethnicity | Percent GDM | Risk ratios for GDM Foreign-born vs. US-born | Adjusted* RRs for GDM foreign-born vs. US-born | |
|---|---|---|---|---|
| US-born | Foreign-born | |||
| General racial/ethnic categories | ||||
| Black | 5.7 | 9.9 | 1.7 (1.7, 1.8) | 1.4 (1.4, 1.5) |
| All Hispanic | 6 | 8.5 | 1.4 (1.4, 1.5) | 1.2 (1.2, 1.3) |
| White | 4.2 | 6.1 | 1.5 (1.4, 1.5) | 1.5 (1.4, 1.6) |
| All Asian | 10.2 | 15.1 | 1.5 (1.4, 1.5) | 1.4 (1.4, 1.5) |
| Racial/ethnic subgroups | ||||
| Mexican | 4.2 | 11.9 | 2.8 (2.5, 3.2) | 1.6 (1.4, 1.8) |
| Indian | 12.8 | 22.9 | 1.8 (1.7, 1.9) | 1.5 (1.4, 1.6) |
| Chinese | 9.2 | 11.6 | 1.5 (1.4, 1.6) | 1.4 (1.3, 1.6) |
Adjusted for maternal age, maternal education, BMI, height, parity, and infant sex
Unadjusted and adjusted risk ratios for BMI categories in association with GDM showed similar trends of increasing risk of GDM with increasing obesity within all racial/ethnic/immigrant groups (Table 3). The effect of overweight/obesity on GDM was of greater magnitude in US-born women than in immigrant women. Tests for interaction were statistically significant in all racial/ethnic groups except All Asians (Indians and Chinese were not tested due to insufficient cell sizes). For example, among US-born Black women, the adjusted risk ratios for overweight and obese class 1, obese class 2, and obese class 3 were 1.7 (95%CI=1.5, 1.8), 2.4 (95%CI=2.2, 2.7), 2.9 (95%CI=2.6, 3.2), and 3.8 (95%CI=3.4, 4.2), respectively, whereas for immigrant Black women they were 1.5 (95%CI=1.4, 1.7), 2.0 (95%CI=1.9, 2.2), 2.3 (95%CI=2.1, 2.6) and 2.8 (95%CI=2.5, 3.1), respectively.
Table 3.
WHO classification of BMI and nativity in association with gestational diabetes, New York City, 2010–2014
| BMI in kg/m2 | %GDM US-born | aRR (95%CI) |
%GDM Foreign-born | aRR (95%CI) |
p-value | |
|---|---|---|---|---|---|---|
| Racial/ethnic category or subgroup | ||||||
| Black | Underweight (<18.5) | 2.4 | 0.8 (0.6, 1.1) | 4.7 | 0.7 (0.6, 0.9) | p=0.02 |
| Normal weight (18.5–24.9) |
2.9 | ref | 6.2 | ref | ||
| Overweight (25.0–29.9) | 5.4 | 1.7 (1.5, 1.8) | 10.6 | 1.5 (1.4, 1.7) | ||
| Obese Class I (30.0–34.9) | 8.3 | 2.4 (2.2, 2.7) | 14.4 | 2.0 (1.9, 2.2) | ||
| Obese Class II (35.0–39.9) | 9.9 | 2.9 (2.6, 3.2) | 16.8 | 2.3 (2.1, 2.6) | ||
| Obese Class III (≥40) | 12.7 | 3.8 (3.4, 4.2) | 18.3 | 2.8 (2.5, 3.1) | ||
| All Hispanic | Underweight (<18.5) | 2 | 0.7 (0.6, 1.0) | 4 | 0.7 (0.6, 0.9) | p<0.001 |
| Normal weight (18.5–24.9) |
3 | ref | 5.5 | ref | ||
| Overweight (25.0–29.9) | 6 | 1.9 (1.8, 2.1) | 9.8 | 1.6 (1.5, 1.6) | ||
| Obese Class I (30.0–34.9) | 9.6 | 3.0 (2.8, 3.3) | 14.3 | 2.1 (2.0, 2.3) | ||
| Obese Class II (35.0–39.9) | 12.8 | 4.0 (3.6, 4.4) | 17.2 | 2.6 (2.4, 2.8) | ||
| Obese Class III (≥40) | 14.4 | 4.5 (4.1, 5.0) | 18.8 | 3.3 (3.0, 3.6) | ||
| Mexican | Underweight (<18.5) | 1.3 | 0.6 (0.1, 2.5) | 6.1 | 0.7 (0.5, 1.0) | p=0.001 |
| Normal weight (18.5–24.9) |
1.9 | ref | 7.6 | ref | ||
| Overweight (25.0–29.9) | 5.2 | 2.9 (2.0, 4.2) | 13.4 | 1.5 (1.4, 1.6) | ||
| Obese Class I (30.0–34.9) | 8 | 4.1 (2.8, 6.0) | 18.4 | 2.0 (1.8, 2.4) | ||
| Obese Class II (35.0–39.9) | 9.4 | 5.0 (3.1, 7.9) | 22.4 | 2.3 (2.0, 3.6) | ||
| Obese Class III (≥40) | 10.6 | 5.8 (3.9, 5.7) | 21 | 2.5 (2.2, 2.9) | ||
| White | Underweight (<18.5) | 2.3 | 0.9 (0.8, 1.1) | 3.7 | 0.8 (0.7, 1.1) | p<0.001 |
| Normal weight (18.5–24.9) |
2.9 | ref | 4.5 | ref | ||
| Overweight (25.0–29.9) | 5.9 | 2.1 (1.9, 2.2) | 9 | 2.0 (1.8, 2.2) | ||
| Obese Class I (30.0–34.9) | 10.1 | 3.5 (3.2, 3.7) | 13.8 | 3.1 (2.8, 3.4) | ||
| Obese Class II (35.0–39.9) | 13 | 4.5 (4.0, 5.9) | 16.6 | 3.7 (3.1, 4.3) | ||
| Obese Class III (≥40) | 14.3 | 5.3 (4.7, 6.0) | 15.8 | 4.8 (3.8, 6.1) | ||
| All Asian | Underweight (<18.5) | 5.1 | 0.7 (0.6, 0.8) | 8.3 | 0.7 (0.6, 0.7) | p=0.24 |
| Normal weight (18.5–24.9) |
8 | ref | 13 | ref | ||
| Overweight (25.0–29.9) | 16.5 | 2.0 (1.8, 2.2) | 25.1 | 1.8 (1.7, 1.9) | ||
| Obese Class I (30.0–34.9) | 24.5 | 2.9 (2.6, 3.3) | 29.8 | 2.1 (1.9, 2.2) | ||
| Obese Class II (35.0–39.9) | 25.1 | 3.0 (2.4, 3.7) | 33.3 | 2.1 (1.9, 2.4) | ||
| Obese Class III (≥40) | 15.8 | 2.2 (1.4, 3.4) | 30.6 | 2.3 (2.0, 2.7) | ||
| Indian | Underweight (<18.5) | 4 | 0.4 (0.3, 0.6) | 9.1 | 0.5 (0.5, 0.7) | * |
| Normal weight (18.5–24.9) |
9.8 | ref | 18.8 | ref | ||
| Overweight (25.0–29.9) | 19.1 | 1.8 (1.6, 2.0) | 29.2 | 1.4 (1.4, 1.5) | ||
| Obese Class I (30.0–34.9) | 27.9 | 2.5 (2.2, 2.9) | 33.4 | 1.6 (1.4, 1.7) | ||
| Obese Class II (35.0–39.9) | 28.7 | 2.6 (2.0, 3.5) | 36.2 | 1.5 (1.3, 1.7) | ||
| Obese Class III (≥40) | * | * | 41.8 | 1.9 (1.6, 2.3) | ||
| Chinese | Underweight (<18.5) | 5.7 | 0.8 (0.5, 1.3) | 8.5 | 0.8 (0.8, 0.9) | * |
| Normal weight (18.5–24.9) |
8 | ref | 11.3 | ref | ||
| Overweight (25.0–29.9) | 13.6 | 1.6 (1.3, 2.1) | 20 | 1.6 (1.5, 1.7) | ||
| Obese Class I (30.0–34.9) | 21.9 | 3.1 (2.2, 4.4) | 21.4 | 1.7 (1.4, 2.0) | ||
| Obese Class II (35.0–39.9) | * | * | 23.3 | 1.9 (1.2, 2.9) | ||
| Obese Class III (≥40) | * | * | ? | 1.9 (0.6, 6.2) |
The partial PAR for overweight/obesity in association with GDM ranged from 17.5% in immigrant Chinese women (95%CI=10.5, 24.3) to 60.0% in US-born Mexicans (95%CI=47.1, 70.4) (Figure 1). The partial PAR was lower in immigrant women than in US-born women in all racial/ethnic groups except All Asians and Indians. Groups in which the PARs were significantly different by nativity included Blacks (US-born: 47.4%, 95%CI=43.9, 50.7 vs. immigrant: 37.5%, 95%CI=34.2, 40.7), All Hispanics (US-born: 50.0%, 95%CI=47.1, 52.7 vs. immigrant: 35.8%, 95%CI=33.4, 38.2), Mexicans (US-born: 60.0%, 95%CI=47.1, 70.4 vs. immigrant: 34.8%, 95%CI=30.8, 38.7), Whites (US-born: 35.4%, 33.4, 37.3 vs. immigrant: 28.5%, 95%CI=25.4, 31.6). Partial PARs did not significantly differ in Asian groups, but were of greater magnitude in US-born groups (Figure 1). In the sensitivity analysis using the alternative cut point for overweight/obesity in Asian groups, the magnitude of the partial PAR increased for all groups, but US-born vs. immigrant differences were similar (Figure 1).
Figure 1.
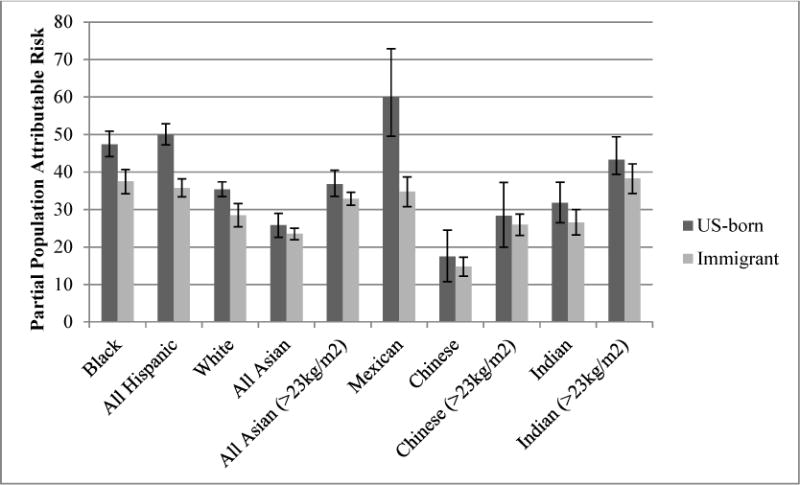
Partial population attributable risks for overweight/obesity in association with gestational diabetes, by general racial/ethnic category, racial/ethnic subgroup and nativity, New York City 2010–2014
Discussion
In a population-based study of 565,839 women, we found that immigrant women are at increased risk of GDM in all racial/ethnic groups, but that overweight/obesity plays a weaker role in GDM in immigrant groups than US-born women of the same race/ethnicity. Associations between BMI and GDM were of a smaller magnitude within immigrant groups, and the proportion of GDM that could be eliminated with the elimination of overweight and obesity was smaller. An exception was All Asian and Indian women, for whom overweight/obesity seemed to play a more similar role among immigrant and US-born women. This is the first study to present partial PARs for US-born compared to immigrant women, and in doing so, clearly defined the public health importance of identifying and targeting other risk factors for gestational diabetes in immigrant women. Most importantly, our finding that immigrant women have an increased risk of GDM despite a weaker role of obesity provides insight into life course theories of cardiometabolic risk and specific GDM prevention needs in immigrant populations.
Previous research examining the US population as a whole has concluded that a large percentage of GDM could be prevented with the prevention of overweight and obesity.30 However, our findings suggest that among immigrant women this may not be the case, even among ethnic groups for which a high partial PAR has previously been reported. For example, Hedderson et al. reported a partial PAR of 54% among Hispanic women, which is similar to our finding of a partial PAR of 50% among US-born Hispanic women, but much higher than the partial PAR for immigrant Hispanic women we reported of 35.8%.14 Our findings that Asian groups have lower partial PARs than other ethnic groups is also consistent with earlier research14, including the finding that Asian subgroups different markedly in their risk profile.31 In New York City, immigrant Chinese women had a partial PAR of overweight/obesity on GDM of only 14.8%, while the partial PAR for immigrant Indian women was 26.6%; in the sensitivity analysis, the partial PAR for immigrant Chinese women was 26.6% and in immigrant Indian women it was 38.6%. Our finding that the partial PARs using modified cut points for Asian groups is consistent with the goal of the lowered cut point to identify a greater number of women at high risk of diabetes, but nevertheless, the Asian subgroups still were notably different. Our results regarding Indian women differed somewhat from previous research – we found similar partial PARs for overweight/obesity for immigrant and US-born Indian, whereas in Florida, BMI had a greater role in GDM in US-born vs. immigrant Indian women.17 One possible reason for this difference is that Indian immigrants to Florida compared to the Indian immigrant women we studied in New York City were more highly educated (86% >12 years in Florida compared to 50% in New York City) and less likely to have Medicaid (15% in Florida compared to 76% in New York City). Further,the larger prevalence of overweight/obesity we found in immigrant Indian women in NYC in 2010–2014 compared to in Florida from 2004–2007 (39.8% vs. 30.5%) may reflect a growing obesity epidemic in India.32 Such differences are a reminder to clinicians and researchers that GDM risk among immigrant groups may vary based on migration cohort, and prevention strategies should be developed with strong ties to community organizations supporting immigrant women.
The mechanism for the differing role of obesity in immigrant and US-born women is unclear, but may be related to differences in adiposity, nutritional influences, country of origin factors, and life course cardiometabolic trajectories. Visceral adipose tissue (VAT) depth as measured by ultrasound in the 1st trimester predicts GDM independently of BMI33, and South Asians and Chinese have been found to have greater VAT at similar BMI.34 Diet may differ based on immigrant status and acculturation, and to the extent that the diet from the sending country is higher in elements such as saturated fats that are known to increase the risk of GDM independent of BMI35, cultural norms around diet may result in a greater role of diet in GDM among immigrant women. In support of the hypothesis that less acculturated women may be at higher risk of GDM, a study of ethnic enclaves and gestational diabetes in New York City found that among South Central Asian and Mexican women, living in their ethnic enclave was associated with an increased risk of GDM.36 The life course perspective on cardiometabolic risk ties together potential explanations for difference in the role of obesity in GDM risk between immigrant and US-born women.37 The life course trajectory of immigrant women has its roots in their country of origin, where they may have a lower birthweight than their US-born counterparts, and low birth weight may be an independent risk factor for GDM.38 In fact, intergenerational undernutrition has been hypothesized as a factor in the obesity epidemics in India and Latin America.19,38 Subsequently, upon immigration, increasing time in the US is associated with increased BMI in Asian and Latina women suggesting that changes in lifestyle factors and the US environment further influences cardiometabolic risk.39,40 Thus the combination of lower weight at birth and obesogenic environment upon arrival may work across the life course to influence GDM risk, even among women with “normal” BMI. The perinatal period is an opportune time to intervene on this life course trajectory. However, culturally sensitive preconception, prenatal, and post-partum interventions tested in both obese and non-obese immigrant populations are needed to prevent the recurrence of gestational diabetes and development of later Type 2 diabetes and hypertension in vulnerable immigrant populations.41
Our study has several limitations to be considered. Measures of GDM by race/ethnicity/nativity may be influenced by screening practices. Universal screening for GDM at 24–28 weeks was recommended in 2013 by the American College of Gynecologists and Obstetricians and by the U.S. Preventive Services Taskforce, so it is likely that screening was universal in New York City for the study period (2010–2014).42,43 However, institutions may vary in their cut points for diagnosis and whether they use a one-step or two-step approach, although the two-stop is the norm in U.S. practice.44 Potential differences in 1st trimester screening for pre-gestational diabetes might also impact our estimates – in 2010 the International Association of Diabetes and Pregnancy Study Groups recommended that high-risk women be screened in the first trimester and glucose intolerance diagnosed as overt diabetes.45 If any of these screening practices differ by race/ethnicity or nativity and by obesity, they could influence our reported associations.
Another limitation of our data is that both GDM and obesity cases may be missed in our administrative data. The validity of both GDM and obesity may vary somewhat by race/ethnicity.21,23,24 If cases of obesity were misclassified mostly among immigrant women with gestational diabetes, it could make the reported partial PARs among those groups spuriously lower. However, given that our overall pattern of findings is similar to previous research, it is unlikely that misclassification alone could explain our findings.
Our study has several features that strengthen the validity and importance of our research. Our data is population-based and representative of all women delivering in New York City, regardless of insurance status or ability to speak English. This stands in contrast to clinical or insurance-based studies, which are prone to selection biases which limit generalizability, especially for research on immigrant groups. Because New York City is a large and diverse city in which half of births are foreign-born, we had sufficient sample size to analyze important immigrant subgroups.
Conclusion
Our study adds evidence that one paradox does not fit all when it comes to immigrant reproductive health.46 Immigrant women are at an increased risk of GDM yet obesity plays a weaker role in GDM in immigrant compared to US-born populations. Clinicians and public health practitioners should be aware that in immigrant women interventions targeting weight loss as a GDM prevention strategy would have a smaller impact on GDM risk than in the non-immigrant populations in which many intervention trials are conducted. The etiology of GDM should be explored in specific immigrant groups to identify other modifiable risk factors such as diet, physical activity, and psychosocial stress to be targeted in culturally sensitive interventions.
Acknowledgments
Funding
Research reported in this publication was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD007651. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fuentes-Afflick E, Lurie P. Low birth weight and Latino ethnicity: examining the epidemiologic paradox. Archives of pediatrics & adolescent medicine. 1997;151(7):665–674. doi: 10.1001/archpedi.1997.02170440027005. [DOI] [PubMed] [Google Scholar]
- 2.Gagnon AJ, McDermott S, Rigol-Chachamovich J, Bandyopadhyay M, Stray-Pedersen B, Stewart D. International migration and gestational diabetes mellitus: a systematic review of the literature and meta-analysis. Paediatric and perinatal epidemiology. 2011;25(6):575–592. doi: 10.1111/j.1365-3016.2011.01230.x. [DOI] [PubMed] [Google Scholar]
- 3.Hedderson MM, Darbinian JA, Ferrara A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatric and perinatal epidemiology. 2010;24(5):441–448. doi: 10.1111/j.1365-3016.2010.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kieffer EC, Martin JA, Herman WH. Impact of maternal nativity on the prevalence of diabetes during pregnancy among US ethnic groups. Diabetes Care. 1999;22(5):729–735. doi: 10.2337/diacare.22.5.729. [DOI] [PubMed] [Google Scholar]
- 5.Savitz D, Janevic T, Engel S, Kaufman J, Herring A. Ethnicity and gestational diabetes in New York City, 1995–2003. BJOG: An International Journal of Obstetrics & Gynaecology. 2008;115(8):969–978. doi: 10.1111/j.1471-0528.2008.01763.x. [DOI] [PubMed] [Google Scholar]
- 6.Urquia M, Glazier RH, Berger H, Ying I, De Souza L, Ray JG. Gestational diabetes among immigrant women. Epidemiology. 2011;22(6):879–880. doi: 10.1097/EDE.0b013e31823199ee. [DOI] [PubMed] [Google Scholar]
- 7.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes care. 2014;37(Supplement 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 8.Association AD. 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Supplement 1):S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Mayo R, Chatry A, Hu G. Gestational Diabetes Mellitus: Its Epidemiology and Implication beyond Pregnancy. Current Epidemiology Reports. 2016;3(1):1–11. [Google Scholar]
- 10.Wendland EM, Torloni MR, Falavigna M, et al. Gestational diabetes and pregnancy outcomes-a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC pregnancy and childbirth. 2012;12(1):23. doi: 10.1186/1471-2393-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pace R, Brazeau A-S, Meltzer S, Rahme E, Dasgupta K. Conjoint associations of gestational diabetes and hypertension with diabetes, hypertension, and cardiovascular disease in parents: A retrospective cohort study. American Journal of Epidemiology. 2017 doi: 10.1093/aje/kwx263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Y, Olsen SF, Mendola P, et al. Growth and obesity through the first 7 y of life in association with levels of maternal glycemia during pregnancy: a prospective cohort study. The American journal of clinical nutrition. 2016;103(3):794–800. doi: 10.3945/ajcn.115.121780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu SY, Callaghan WM, Kim SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes care. 2007;30(8):2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- 14.Hedderson M, Ehrlich S, Sridhar S, Darbinian J, Moore S, Ferrara A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes care. 2012;35(7):1492–1498. doi: 10.2337/dc11-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SY, England L, Sappenfield W, et al. Racial/ethnic differences in the percentage of gestational diabetes mellitus cases attributable to overweight and obesity, Florida, 2004–2007. Preventing chronic disease. 2012:9. doi: 10.5888/pcd9.110249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pu J, Zhao B, Wang EJ, et al. Racial/ethnic differences in gestational diabetes prevalence and contribution of common risk factors. Paediatric and perinatal epidemiology. 2015;29(5):436–443. doi: 10.1111/ppe.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SY, Sappenfield W, Sharma AJ, et al. Racial/ethnic differences in the prevalence of gestational diabetes mellitus and maternal overweight and obesity, by Nativity, Florida, 2004-2007. Obesity. 2013;21(1) doi: 10.1002/oby.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao SM, Wakeel F, Nazinyan Y, Sun S. Does Preconception Health Differ by Nativity?: Findings from the Los Angeles Mommy and Baby (LAMB) Study. Maternal and child health journal. 2016;20(4):769–777. doi: 10.1007/s10995-015-1907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Jaramillo P, Gomez-Arbelaez D, Sotomayor-Rubio A, Mantilla-Garcia D, Lopez-Lopez J. Maternal undernutrition and cardiometabolic disease: a Latin American perspective. BMC medicine. 2015;13(1):41. doi: 10.1186/s12916-015-0293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lain SJ, Hadfield RM, Raynes-Greenow CH, et al. Quality of data in perinatal population health databases: a systematic review. Medical care. 2012;50(4):e7–e20. doi: 10.1097/MLR.0b013e31821d2b1d. [DOI] [PubMed] [Google Scholar]
- 21.Robledo CA, Yeung EH, Mendola P, et al. Examining the Prevalence Rates of Preexisting Maternal Medical Conditions and Pregnancy Complications by Source: Evidence to Inform Maternal and Child Research. Maternal and child health journal. 2017;21(4):852–862. doi: 10.1007/s10995-016-2177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Organization WH. Obesity: preventing and managing the global epidemic. World Health Organization; 2000. [PubMed] [Google Scholar]
- 23.Bodnar LM, Abrams B, Bertolet M, et al. Validity of Birth Certificate-Derived Maternal Weight Data. Paediatric and perinatal epidemiology. 2014;28(3):203–212. doi: 10.1111/ppe.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S, Sappenfield WM, Bish C, Bensyl DM, Goodman D, Menges J. Reliability and validity of birth certificate prepregnancy weight and height among women enrolled in prenatal WIC program: Florida, 2005. Maternal and child health journal. 2011;15(7):851–859. doi: 10.1007/s10995-009-0544-4. [DOI] [PubMed] [Google Scholar]
- 25.Spiegelman D, Hertzmark E, Wand H. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes & Control. 2007;18(5):571–579. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 26.Wong BH, Peskoe SB, Spiegelman D. The effect of risk factor misclassification on the partial population attributable risk. Statistics in medicine. 2018 doi: 10.1002/sim.7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Philadelphia: Lippincott, Williams and Wilkins; 2008. [Google Scholar]
- 28.Hsu WC, Araneta MRG, Kanaya AM, Chiang JL, Fujimoto W. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes care. 2015;38(1):150–158. doi: 10.2337/dc14-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO EC. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London, England) 2004;363(9403):157. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 30.Kim SY, England L, Wilson HG, Bish C, Satten GA, Dietz P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. American Journal of Public Health. 2010;100(6):1047–1052. doi: 10.2105/AJPH.2009.172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karter AJ, Schillinger D, Adams AS, et al. Elevated rates of diabetes in Pacific Islanders and Asian subgroups. Diabetes care. 2013;36(3):574–579. doi: 10.2337/dc12-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Misra A, Khurana L. The metabolic syndrome in South Asians: epidemiology, determinants, and prevention. Metabolic syndrome and related disorders. 2009;7(6):497–514. doi: 10.1089/met.2009.0024. [DOI] [PubMed] [Google Scholar]
- 33.De Souza LR, Berger H, Retnakaran R, et al. First-trimester maternal abdominal adiposity predicts dysglycemia and gestational diabetes mellitus in midpregnancy. Diabetes Care. 2016;39(1):61–64. doi: 10.2337/dc15-2027. [DOI] [PubMed] [Google Scholar]
- 34.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT) The American journal of clinical nutrition. 2007;86(2):353–359. doi: 10.1093/ajcn/86.2.353. [DOI] [PubMed] [Google Scholar]
- 35.Schoenaker DA, Mishra GD, Callaway LK, Soedamah-Muthu SS. The role of energy, nutrients, foods, and dietary patterns in the development of gestational diabetes mellitus: a systematic review of observational studies. Diabetes Care. 2016;39(1):16–23. doi: 10.2337/dc15-0540. [DOI] [PubMed] [Google Scholar]
- 36.Janevic T, Borrell L, Savitz D, Echeverria S, Rundle A. Ethnic enclaves and gestational diabetes among immigrant women in New York City. Social Science & Medicine. 2014;120:180–189. doi: 10.1016/j.socscimed.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 37.Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends in Endocrinology & Metabolism. 2010;21(4):199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Yeung E, Hu F, Solomon C, et al. Life-course weight characteristics and the risk of gestational diabetes. Diabetologia. 2010;53(4):668–678. doi: 10.1007/s00125-009-1634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akresh IR. Overweight and obesity among foreign-born and US-born Hispanics. Biodemography and Social Biology. 2008;54(2):183–199. doi: 10.1080/19485565.2008.9989141. [DOI] [PubMed] [Google Scholar]
- 40.Singh GK, Siahpush M, Hiatt RA, Timsina LR. Dramatic increases in obesity and overweight prevalence and body mass index among ethnic-immigrant and social class groups in the United States, 1976–2008. Journal of community health. 2011;36(1):94–110. doi: 10.1007/s10900-010-9287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez NG, Niznik CM, Yee LM. Optimizing postpartum care for the patient with gestational diabetes mellitus. American Journal of Obstetrics and Gynecology. 2017 doi: 10.1016/j.ajog.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mellitus GD. ACOG Practice Bulletin. Obstetrics & Gynaecology. 2013:406. [Google Scholar]
- 43.Moyer VA. Screening for gestational diabetes mellitus: US Preventive Services Task Force recommendation statement. Annals of internal medicine. 2014;160(6):414–420. doi: 10.7326/M13-2905. [DOI] [PubMed] [Google Scholar]
- 44.ACOG Practice Bulletin: Gestational Diabetes Mellitus. Obstetrics and Gynecology. 2017;130(1) doi: 10.1097/AOG.0000000000002159. [DOI] [PubMed] [Google Scholar]
- 45.International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes care. 2010;33(3):676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urquia ML, O’Campo PJ, Heaman MI. Revisiting the immigrant paradox in reproductive health: the roles of duration of residence and ethnicity. Social science & medicine. 2012;74(10):1610–1621. doi: 10.1016/j.socscimed.2012.02.013. [DOI] [PubMed] [Google Scholar]


