Abstract
The lack of capacity to provide laboratory confirmation of a diagnosis of tuberculosis disease (TB) is contributing to enormous gaps in the ability to find, treat and follow TB patients. WHO estimates that globally only about 57% of the notified new cases of pulmonary TB in 2012 and about 19% of rifampicin-resistant TB cases were laboratory confirmed. The Cepheid Xpert® MTB/RIF assay has been credited with revolutionizing laboratory testing to aid in the diagnosis of TB and rifampicin-resistant TB. This semi-automated test can detect both the causative agent of TB and mutations that confer rifampicin resistance from clinical specimens within 2 h after starting the test. In this article, we review the performance of the test, its pathway to regulatory approval and endorsement, guidelines for its use and lessons learned from the implementation of the test in low-burden, high-resource countries and in high-burden, low-resource countries.
Keywords: automated nucleic acid amplification, molecular detection of resistance, rifampicin resistance, tuberculosis
Background & need
In humans, tuberculosis (TB) disease most commonly affects the lungs, yet extrapulmonary TB disease can affect any organ of the body [1]. Although TB patients often are diagnosed clinically, based on symptoms and chest x-rays, definitive diagnosis requires laboratory detection of Mycobacterium tuberculosis complex bacteria (MTBC) in clinical specimens. Bacteriologic confirmation of TB relies on acid-fast bacilli (AFB) smear microscopy, culture isolation or molecular detection of MTBC (reviewed in [1]). Historically, challenges to providing rapid, reliable diagnostic testing have included the limited sensitivity and specificity of widely used tests; slow bacterial growth (18–24 h generation time) and requirements for sophisticated laboratory containment or biosafety equipment and facilities to work safely with materials with a high potential for generating infectious aerosols.
Because MTBC is transmitted from person-to-person by airborne droplet nuclei, the global strategy to control TB has prioritized the detection and treatment of persons with pulmonary TB disease [2]. Consequently, most countries in the world have relied on diagnosis of the most infectious TB patients through broad-scale implementation of microscopic examination of smears made from expectorated sputum and other respiratory specimens to identify AFB in persons with clinical signs and symptoms of pulmonary TB disease. However, AFB microscopy is limited by its ability to detect only about 50–70% of culture-positive pulmonary TB cases, its inability to distinguish MTBC from other mycobacteria and its reduced sensitivity for detecting TB disease in persons with pauci-bacillary disease, such as in people living with HIV (PLHIV), in children and in individuals with extrapulmonary TB [3,4]. The inadequacy of AFB smear microscopy as a diagnostic tool has hampered efforts to detect, treat and control TB in high-burden, vulnerable populations. It is estimated that a more sensitive test capable of replacing AFB smear microscopy as the initial diagnostic test for TB could improve TB case detection rates significantly and has a potential global market size of 30.8 million tests per year [5].
The isolation of mycobacteria from sputum and other clinical specimens by culture is currently the most sensitive laboratory method for the diagnosis of TB, but it can take 4–6 weeks to obtain results by solid media. Although the use of liquid media can reduce this time to detection by half, the cost of the test and facilities and the need for highly trained laboratory workers have limited attempts to build culture capacity in many resource-limited settings [6,7]. Even in the USA, TB laboratory capacity was a challenge until the unexpected increase in TB incidence during the mid-1980s and early 1990s in association with several outbreaks of rapidly progressive and fatal disease due to multidrug-resistant MTBC (MDR TB; caused by MTBC resistant to at least isoniazid and rifampicin), mostly in persons with HIV infection. This public health crisis prompted the development of national recommendations and investment in laboratories focusing on improving the reliability of results and reducing diagnostic delays [8]. Remarkable advances in laboratory services contributed to subsequent decreases in the national incidence of TB and MDR TB in the USA [8]. In Europe and Canada, standards for TB laboratory services have also been published in recent years [9,10].
Globally, the lack of readily accessible, rapid and reliable laboratory services to establish the diagnosis of TB and monitor response to therapy continues to hamper efforts to combat TB [11]. In 2012, only about 66% of the estimated 8.6 million new cases of TB were reported to WHO [11]. Furthermore, only about 57% of the 4.57 million notified cases of pulmonary TB were laboratory confirmed, demonstrating enormous gaps in our ability to find, treat and follow TB patients. Furthermore, the emergence and spread of drug-resistant strains of MTBC in several countries are revealing even larger gaps in laboratory services. An estimated 450,000 new cases of MDR TB, some of which also include extensively drug-resistant TB, occurred globally in 2012 [11]. Only about 84,000 (19%) of these were laboratory confirmed, in part, because of the lack of culture capacity in many resource-limited settings. Conventional drug susceptibility testing (DST) involves culturing the bacteria from a clinical specimen and determining if the bacteria can grow in media containing an anti-TB drug. The indirect proportion DST method (isolation by culture then DST) using solid or liquid media is most commonly used by laboratories [12]. Because of the slow growth of MTBC, the indirect proportion method typically requires 6–8 weeks on solid media and 3–4 weeks in liquid media after the receipt of the specimen to provide results, leading to substantial delays in the diagnosis and initiation of optimal treatment for drug-resistant TB [8–10].
Molecular methods can reduce the time required for detection of drug resistance to as few as 2 h after receipt of the sample in the laboratory. When coupled with earlier initiation of effective treatment, the rapid detection of rifampicin resistance by molecular methods holds promise to reduce periods of infectiousness of MDR TB cases by as much as 6 weeks, to limit the further spread of MDR TB and to improve treatment outcomes [13]. Molecular methods for the detection of MTBC have been available since the mid-1990s (e.g., Amplified M. tuberculosis Direct Test, Amplicor M. tuberculosis Test, laboratory developed tests) (reviewed in [14,15]). However, the use of these early molecular tests for detecting MTBC has been limited, largely because of the complexities of DNA extraction, amplification and detection, the cost of the tests and the need for sophisticated laboratory infrastructure and trained personnel. In addition, the early commercially available molecular tests were less sensitive than culture for detecting pulmonary TB disease, especially for smear-negative TB disease [14,15]. The early commercially available tests did not detect resistance, so culture was the only method available for DST.
Although there were no FDA-approved molecular tests to detect drug-resistant TB disease in the USA prior to the approval of the Xpert® MTB/RIF assay (Xpert MTB/RIF) in 2013, several validated molecular tests (line-probe assays [LPAs], molecular beacons and DNA sequencing) based on analyte-specific reagents, often called ‘home-brew’ or ‘in-house’ tests, have been used in the USA (reviewed in [13–15]). In general, the availability of such laboratory-developed tests was quite limited, the performance of the laboratory-developed tests were variable and little information was available on the programmatic impact of such tests [13–15], in part because the tests were not available as quality controlled, commercial products.
LPAs were among the first commercially available molecular tests developed in the late 1990s to aid in the diagnosis of TB, rifampicin-resistant TB and MDR TB disease. LPAs were endorsed by WHO in 2008 for use with smear-positive sputum specimens or culture isolates [16]. In many settings, particularly where fixed-dose combination first-line anti-TB drugs are used, more than 85% of rifampicin-resistant strains are MDR and rifampicin resistance is considered a proxy for MDR TB [17,18]. The LPAs held promise for shortening the time to diagnosis of drug-resistant TB, improving testing throughput and expanding access by having fewer biosafety requirements when performed directly from positive sputum specimens. However, the uptake of the tests in resource-limited settings was inhibited by the same factors that limited the uptake of molecular tests to detect MTBC, in particular the need for separate rooms for unidirectional workflow, labor-intensive specimen processing and well-trained personnel. Nonetheless, LPA implementation was a critical component of the EXPAND-TB project and their use aided in the detection of more than 72,000 MDR TB case between 2009 and 2013 [19]. A discussion of these methods is outside the scope of this review and the reader is referred to the WHO policy statements on LPAs for additional details [16].
A breakthrough in molecular testing for TB came with the development of Xpert MTB/RIF, an automated PCR test, run on the GeneXpert platform (Cepheid, Sunnyvale, CA, USA) [20]. The assay is based on real-time PCR that amplifies a region of the rpoB gene known as the rifampicin resistance determining region (RRDR). Mutations in this region are associated with resistance to rifampicin. Five overlapping molecular beacons (combinations of hybridization probes, fluorophors and quenchers) are used to detect the amplified portion of the rpoB gene. Hybridization indicates the presence of the wild-type sequence, which predicts rifampicin susceptibility. Lack of hybridization indicates the presence of a mutated sequence, which predicts rifampicin resistance. Details of the development of the assay and technical details are described in [20].
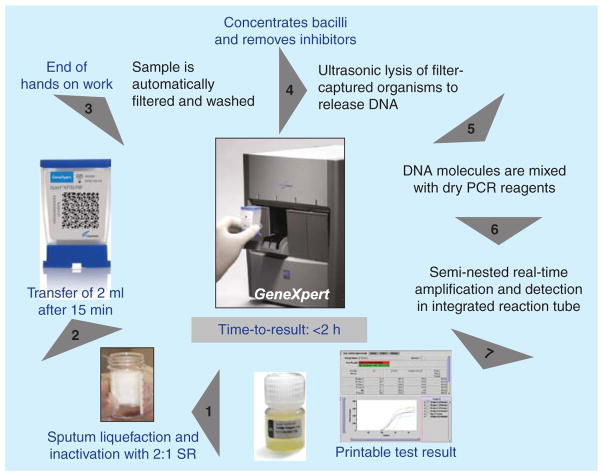
Xpert MTB/RIF can detect both MTBC and rifampicin resistance from clinical specimens within 2 h after starting the test, with minimal hands-on time and minimal biosafety infrastructure requirements for facilities. There are several important, game-changing aspects to this test (Figure 1). Sputum sample processing is simple and only requires adding the provided sample reagent to the specimen, transferring 2 ml of liquefied sputum into the Xpert MTB/RIF cartridge and loading the cartridge into the instrument. All further steps (recovery of the bacteria, lysis, DNA extraction, PCR amplification and detection) are automated. In addition, because the sample reagent kills mycobacteria, the test requires the same biosafety considerations as direct sputum smear microscopy. Thus, theoretically, the test can be conducted in peripheral laboratories, which should make it easier for patients to access the services. Practical limitations, however, such as the need for a reliable supply of electricity, air conditioning and security for the external computer, have led to the test being placed at the district level rather than in peripheral microscopy centers in many settings.
Figure 1. Assay procedure for Xpert MTB/RIF.
Diagram supplied by C Boehme, Foundation for Innovative New Diagnostics with permission [22].
The remainder of this article focuses on the Xpert MTB/RIF test and the reader is referred to recent reviews for detailed discussions of the currently approved conventional and other molecular laboratory methods for TB [21].
Regulatory approval or endorsement
Much of the early data on the performance of the Xpert MTB/RIF test came from evaluation and demonstration projects led by the Foundation for Innovative New Diagnostics (FIND) [22]. In these studies, which enrolled 6648 participants, a single Xpert MTB/RIF test detected 90.3% (95% CI: 88.4–92.0) of the culture-confirmed TB cases. The test was 99.0% specific (95% CI: 98.5–99.3) for the detection of TB. With respect to the detection of rifampicin resistance, the sensitivity of the Xpert MTB/RIF test compared with conventional DST was 94.4% (95% CI: 90.8–96.6) and its specificity was 98.3% (95% CI: 97.1–99.0). These data formed much of the basis of the applications to regulatory agencies including those in the EU and the USA.
In April 2009, Xpert MTB/RIF received Conformité Européene marking as an in vitro diagnostic medical device. In the USA, in vitro diagnostic devices for the detection of MTBC by nucleic acid amplification (NAA) directly from clinical specimens (i.e., Amplified M. tuberculosis Direct Test and Amplicor M. tuberculosis Test) have been classified by the FDA into the highest risk category, class III, based on precedents. However, as a new device that detects both MTBC and mutations associated with rifampicin resistance, Xpert MTB/RIF was eligible for consideration for the de novo classification process in which a device without a precedent (or ‘predicate’) could receive consideration for lower risk classification with the establishment of special controls, which together with general controls, provide reasonable assurance of safety and effectiveness [23].
The FDA granted market authorization of Xpert MTB/RIF via the de novo classification process in the USA in July 2013 [24]. Although the test cartridges and procedures are the same as those used globally, the FDA has authorized Xpert MTB/RIF as a qualitative test and does not provide a semi-quantitative value [23]. The intended specimen types for use of Xpert MTB/RIF are unprocessed sputum (induced or expectorated) and concentrated sputum sediments from patients with suspected TB disease. The test is not intended for use in patients who have received treatment for more than 3 days. When compared with culture, clinical performance, as described in the US Xpert MTB/RIF package insert [25], revealed a sensitivity of 99.7% (95% CI: 98.4–99.9%) and specificity of 98.8% (95% CI: 91.9–99.7%) for 417 patients with smear-positive TB and a sensitivity of 76.1% (95% CI: 67.6–82.9%) and specificity of 98.8% (95% CI: 97.5–99.4%) in 679 patients with smear-negative TB. In comparison to phenotypic DST, Xpert MTB/RIF had a sensitivity of 94.7% (95% CI: 75.4–99.1%) and specificity of 99% (95% CI: 97.5–99.6%) for detection of rifampicin resistance [25]. Because of the low prevalence of rifampicin resistance in the USA, an Xpert MTB/RIF result indicating the detection of rifampicin resistance must have results confirmed by a reference laboratory.
Many high-burden, resource-limited countries do not have established regulatory authorities for diagnostics and in specific instances view endorsement of a test by WHO as equivalent to regulatory approval. To evaluate new diagnostic tests and develop policy recommendations, WHO uses the GRADE system (Grades of Recommendations, Assessment, Development and Evaluation) to provide a consistent, structured framework for evaluating the accuracy and the impact of new interventions on patients and public health [26–28].
In September 2010, WHO convened an Expert Group [28,29] to assess the available data on the performance of the Xpert MTB/RIF test using the GRADE system. Based on the evaluation of the evidence, the Expert Group recommended that:
Xpert MTB/RIF should be used as the initial diagnostic test for patients with suspected pulmonary MDR TB or HIV-associated TB (strong recommendation, moderate quality evidence).
Xpert MTB/RIF may be used as a follow-on test to microscopy in settings where MDR TB or HIV is of lesser concern, especially for smear-negative specimens (conditional recommendation recognizing major resource implications, moderate quality evidence).
The WHO Strategic and Technical Advisory Group for TB (STAG-TB) supported the Expert Group recommendations [30] and Xpert MTB/RIF was endorsed by WHO early in December 2010 [31].
Programmatic implementation began immediately after WHO endorsement and has continued to scale up rapidly, largely because of substantial infusions of support from donors such as the US President’s Emergency Plan for AIDS Relief (PEPFAR), US Agency for International Development, UNITAID, Global Fund to Fight AIDS, TB and Malaria and the Canadian International Development Agency (CIDA). Key among this support was negotiations among the manufacturer, to the list of participants in the negotiation which are US President’s Emergency Plan for AIDS Relief, US Agency for International Development, UNITAID and the Bill and Melinda Gates Foundation that led to a concessionary price of US$9.98 per test cartridge [28]. By the end of June 2014, more than 3000 GeneXpert instruments and more than 7.5 million Xpert MTB/RIF test cartridges were procured in the public sector in 108 countries [32].
Along with this rapid deployment came many new, independent studies of the performance of the test, as well as information from post-market surveillance. The WHO endorsement process includes re-review of polices and recommendations as new information becomes available. Thus, in 2013, WHO commissioned systematic reviews and convened an Expert Group to evaluate the new data (summarized in Table 1) and consider the utility of Xpert MTB/RIF for the detection of MTBC and rifampicin resistance in pulmonary, extrapulmonary and pediatric TB [4,33–35]. The Expert Group concluded that for detection of pulmonary TB, rifampicin-resistant TB and HIV-associated TB, there was high-quality evidence for adults and very low-quality evidence for children; for the detection of extrapulmonary TB there was very low-quality evidence both in adults and children. The Expert Group recommended that:
Table 1.
Performance of Xpert in adults and children.
| Detect | Specimen | Population | Reference method | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|
| TB | Sputum | Adults | Culture | 88% (84–92%) | 99% (98–99%) |
| TB | Sputum | Adults, smear+ | Culture | 98% (97–99%) | N/A |
| TB | Sputum | Adults; smear− | Culture | 68% (61–74%) | 99% (98–99%) |
| TB | Sputum | HIV+ adults | Culture | 79% (70–86%) | 98% (96–99%) |
| TB | Sputum | HIV–adults | Culture | 86% (76–92%) | 99% (98–100%) |
| TB | Sputum | Children | Culture | 66% (52–77%) | 98% (96–100%) |
| TB | Gastric aspirate | Children | Culture | 66% (51–81%) | 98% (96–100%) |
| TB | Lymph node tissue, aspirate | Adults and children | Culture | 85% (72–92%) | 93% (80–97%) |
| TB | Other tissue type | Adults and children | Culture | 81% (68–90%) | 98% (87–100%) |
| TB | Cerebral spinal fluid | Adults and children | Culture | 80% (62–90%) | 99% (96–100%) |
| RIF-R | Adults | Culture DST | 95% (90–97%) | 98% (97–99%) | |
| RIF-R | Children | Culture DST | 86% (53–98%) | 98% (94–100%) |
Xpert MTB/RIF should be used rather than conventional microscopy, culture and DST as the initial diagnostic test in adults and children presumed to have pulmonary MDR TB or HIV-associated TB.
Xpert MTB/RIF may be used rather than conventional microscopy and culture as the initial diagnostic test in all adults and children presumed to have pulmonary TB.
Xpert MTB/RIF should be used in preference to conventional microscopy and culture as the initial diagnostic test in testing cerebrospinal fluid specimens from patients presumed to have TB meningitis.
Xpert MTB/RIF may be used as a replacement test for usual practice for testing of specific non-respiratory specimens (lymph nodes and other tissues) from patients presumed to have extrapulmonary TB.
STAG-TB supported the Expert Group recommendations and policy recommendations on the Xpert MTB/RIF test were issued by WHO in early 2014 [34].
Evidence-based guidelines for laboratorians, clinicians & program managers
Regulatory approval or endorsement opens the market for a test. However, uptake and acceptance of the test often depends on clear guidance on how to implement the test; which testing algorithms to use; laboratory controls, quality assurance and follow-up testing and interpretation of and acting on test results. The Association of Public Health Laboratories (APHL) and the US CDC described considerations for use of Xpert MTB/RIF in the USA. APHL provided guidance to laboratories for incorporation of the assay into an existing test algorithm dependent on the level of service performed [36]. CDC provided information [37] that was based on previous recommendations for NAA testing as a standard of practice [38] and guidelines for infection control [39]. Considerations included the continued requirement for mycobacterial culture and conventional DST because culture remains the most sensitive method for detection of MTBC. Because of the low prevalence of rifampicin resistance in the USA [40], an Xpert MTB/RIF result indicating the detection of rifampicin resistance should be confirmed by DNA sequencing of rpoB. Additionally, consideration should be given for DNA sequencing of at least inhA and katG to test for mutations associated with isoniazid resistance when confirming the rifampicin result. Minimal language for reporting of laboratory results was suggested pending confirmation of results (Table 2). When rifampicin resistance is confirmed, examination of other loci associated with resistance to first and second-line anti-TB drugs is warranted pending results from conventional DST. NAA results from Xpert MTB/RIF may be considered as part of infection control practice to rule out contagious TB because of its increased sensitivity and specificity compared with AFB smear microscopy.
Table 2.
Suggested reporting language.
| Result generated by GeneXpert System | Minimum laboratory report language |
|---|---|
| MTB detected, RIF resistance detected | MTBC detected. A mutation in rpoB gene has been detected, indicating possible rifampicin resistance. Confirmatory testing should follow |
| MTB detected, RIF resistance not detected | MTBC detected. No rpoB gene mutations detected; probably rifampicin susceptible |
| MTB detected, RIF resistance indeterminate | MTBC detected; presence of rpoB gene mutations cannot be accurately determined |
| MTB not detected | MTBC not detected |
MTBC: Mycobacterium tuberculosis complex bacteria.
The European Center for Disease Prevention and Control and the European Reference Laboratory Network for TB reviewed existing data (systematic review performed 2011) and developed considerations to guide the implementation and use of rapid molecular assays like Xpert MTB/RIF for the detection of MTBC and mutations associated with drug resistance for EU member countries [41]. Expert opinion included the following considerations concerning the use of assays like Xpert:
Assays with the ability for direct detection of MTBC and mutations associated with resistance should not replace standard diagnostic methods including phenotypic DST.
Molecular assays can serve as a supplement to standard diagnostic methods and conventional DST in smear-positive pulmonary TB patients, especially to rule out MDR TB. However, the evidence does not yet support routine use of Xpert MTB/RIF for smear-negative pulmonary specimens.
Molecular assays can serve as an adjunct to standard diagnostic methods for non-respiratory specimens, although the evidence is limited for cerebrospinal fluid.
Some evidence supports the use of molecular methods for detection of MTBC and drug resistance in HIV/MTBC co-infected patients with smear-positive TB in the context of standard diagnostic methods including phenotypic DST.
Although evidence is lacking, use of molecular methods is recommended for children with suspected pulmonary TB when combined with standard methods.
For the use of Xpert MTB/RIF in resource-limited settings, WHO issued guidance for laboratorians, clinicians and programs as part of its policy statements and implementation manuals [31,34,42].
If Xpert MTB/RIF detects MTBC without rifampicin resistance, a WHO-recommended first-line treatment regimen should be used.
-
If Xpert MTB/RIF detects MTBC with rifampicin resistance, the patient should be evaluated for risk factors associated with MDR TB [43,44].
In patients at high risk of MDR TB, a WHO-recommended regimen for MDR TB with the addition of isoniazid should be initiated, and another sputum sample should be sent for DST to at least isoniazid, fluoroquinolones and second-line injectable anti-TB drugs. When results of the DST are available, treatment can be adjusted accordingly.
-
In patients at low risk of MDR TB, a fresh sample should be collected and tested by Xpert MTB/RIF or LPA to exclude possible errors in the pre- and post-analytic processes.
If the second Xpert MTB/RIF (or LPA) detects MTBC but not rifampicin resistance, a WHO-recommended first-line regimen should be used.
If the second Xpert MTB/RIF (or LPA) detects MTBC and rifampicin resistance, a WHO-recommended regimen for MDR TB with the addition of isoniazid may be started.
In either situation, an additional sample should be used for follow-up DST to assess susceptibility to rifampicin, and as needed, to isoniazid, fluoroquinolones and second-line injectable anti-TB drugs. When DST results are available, treatment should be adjusted if appropriate.
In the event that Xpert MTB/RIF results are discordant with phenotypic DST or LPA results, the culture isolate should be sent to a reference laboratory for DNA sequencing.
The addition of isoniazid to the WHO-recommended MDR TB regimen for patients for whom Xpert MTB/RIF detects rifampicin-resistant MTBC addresses concerns about regional variations in the utility of rifampicin resistance as a proxy for MDR TB. Data from 14 Supranational TB Reference Laboratories indicated that 0.5–11.6% of isolates with phenotypic rifampicin resistance did not have associated isoniazid resistance [17]. A retrospective analysis of aggregate data from >81 countries and subnational settings reported by the WHO/International Union Against TB and Lung Diseases (The Union) Global Project on Anti-TB Drug Resistance Surveillance [45] from 1994 to 2007 revealed that >40% of rifampicin-resistant isolates from new TB cases did not display resistance to isoniazid in settings with relatively low MDR TB prevalence [18].
The recommendation to initiate therapy based on the Xpert MTB/RIF result is bolstered by a recent study indicating that when clinical decisions are based on a rapid test that detects only rifampicin resistance; additional testing for isoniazid resistance has minimal impact on treatment outcomes or transmission of TB, MDR TB or isoniazid-monoresistant TB [46].
The decision algorithm for persons with suspected TB who are at low risk of having MDR TB reflects a concern that the positive predictive value of Xpert MTB/RIF is low for the detection of rifampicin resistance in a setting with a low frequency of MDR TB [28] (the predictive value of a test is dependent on the prevalence of the condition in the population being tested). For example, with a specificity of 98% and MDR TB prevalence of 5% relative to all TB, the anticipated positive predictive value would be about 70%. In some countries with a low incidence of TB, MDR TB is less than 2% of incident TB.
Lessons from early implementers
Although TB remains a public health threat in the USA, the incidence rate has declined and was reported as 3.2 cases per 100,000 persons in 2012 [40]. As with other low-burden settings, the performance of Xpert MTB/RIF may vary depending on the test algorithm and patient population. A cross-sectional study of Xpert MTB/RIF compared with culture in 217 respiratory specimens (sputum and bronchial) from patients in the western USA revealed an overall sensitivity of 89% and specificity of 95%. The sensitivity among smear-positive, culture-positive specimens was 98% and 72% among smear-negative, culture-positive specimens. No cross-reactivity was detected in 41 specimens that were culture positive for 7 different non-tuberculous mycobacteria. Each of the three specimens found to be rifampicin resistant by Xpert MTB/RIF were found to be susceptible by repeat testing of the isolate or DNA sequencing [47]. Similar sensitivities and specificities have been reported for detection of MTBC in respiratory specimens in other low-burden settings [48,49]. However, a prospective study of 502 patients at a university hospital TB clinic in Canada [50] found an overall sensitivity of 46% and specificity of 100% when compared with culture for induced sputum specimens. The overall sensitivity was primarily influenced by a low sensitivity of 28% among patients with smear-negative, culture-positive TB. The authors concluded that Xpert MTB/RIF has limited use in decreasing time to diagnosis in a setting where patients may be evaluated early in the disease process and the diagnostic algorithm is sound. However, in an environment without ready access to rapid laboratory testing or healthcare providers experienced in diagnosing TB, Xpert MTB/RIF may have value even in a high-resource, low-burden setting, especially for communities that may experience a higher incidence of disease.
A crucial lesson gained by early implementers is the need to consider all costs associated with the introduction of Xpert MTB/RIF testing. For the laboratory, this includes the cost of the instrument, computer (desktop or laptop), supplies (cartridges, pipettes, etc.), back-up power supply, facilities, training and external quality assessment [42]. Other important country-specific costs to consider include shipping and transportation and custom and import fees. An important, ongoing cost is the cost of annual calibration, which is US$1800 for the 4 modules when done at the company facilities or US$450 using an on-site calibration kit [42,51]. All 4-module instruments shipped after 24 April 2012 include a 2-year warranty. Validity of the second year of the warranty, however, is dependent on calibration of all modules at the end of the first year. Calibration costs are not included in initial 2-year warranty [51]. Recently, extended warranty options have become available to include maintenance and calibration (US$2900 annually or US$7900 for a 3-year extension) [51,52].
Costs are much lower in the public sector in the 145 high-burden or low-resource countries eligible for concessionary pricing [52,53]. For example, the ex works price of the 4-module GX4 with computer is US$17,000–17,500 in eligible countries and US$78,200 in non-eligible countries (e.g., USA and European Community) and cartridges are US$9.98 in eligible countries and approximately US$71.00 (50 euros) in non-eligible countries. In addition to the difference in commodity prices, there are differences in labor and infrastructure costs. Analyses that take into account all costs (supplies, labor, equipment, infrastructure, etc.) revealed that the average cost of an Xpert MTB/RIF test ranged from US$17 to 25 in high-burden countries and was an estimated average of US$98 in the USA [54,55]. Interestingly, in Brazil, the cost of an Xpert MTB/RIF test in the public sector which received the concessionary price was calculated to be US$17.80, while in the private sector, patients were charged US$254 for an Xpert MTB/RIF test [56].
Despite the higher costs in high-resource, low-burden countries, rapid NAA tests, such as Xpert MTB/RIF, have the potential to reduce healthcare-associated costs for respiratory isolation because of their increased sensitivity over AFB smear microscopy and their ability to distinguish MTBC from other mycobacteria. A cost–benefit analysis of the use of a single Xpert MTB/RIF to rule out infectious TB versus the recommended two negative smears from early morning specimens collected on different days revealed the potential for a savings of US$533,520 per year in a public US hospital and an average reduction in isolation time from 2.7 to 1.4 days per patient [54]. In a US hospital setting, an observational cohort study of the routine use of Xpert MTB/RIF as part of the diagnostic algorithm shortened duration of respiratory isolation for individuals without active TB and successfully identified all active cases [57].
The use of Xpert MTB/RIF may also reduce time to initiation of therapy in some settings, thus reducing associated healthcare costs. A retrospective analysis in Spain, a low-burden country, examined time to initiation of treatment and cost per patient by comparing AFB smear-negative to AFB smear-positive cases. AFB smear-negative cases were found to have delays in treatment initiation, more frequent and longer hospitalizations and more tests (e.g., bronchoscopy) prior to diagnosis than AFB smear-positive cases. With a sensitivity of 68% for Xpert MTB/RIF in smear-negative cases in that study, the authors concluded that 86% of all culture-confirmed TB cases over a 3-year period could have been rapidly detected by Xpert, which would thereby reduce the time to diagnosis and associated healthcare costs, especially for smear-negative cases [58]. A US economic evaluation compared standard diagnostic algorithms (i.e., AFB smear microscopy, with or without NAA test other than Xpert MTB/RIF, and culture) with an algorithm incorporating testing one sputum sample by Xpert MTB/RIF from patients with suspected TB [59]. The results indicated that incorporation of Xpert MTB/RIF into an algorithm that tested only one sputum specimen from all patients with suspected TB would be highly cost-effective when considering all costs to the health system despite the higher laboratory costs.
The WHO guidelines for Xpert MTB/RIF also address cost–effectiveness, testing algorithms, strengthening the entire diagnostic cascade, training, quality assurance and monitoring and evaluating performance and impact. Models have indicated that, compared with smear microscopy-based approaches, Xpert MTB/RIF can be cost-effective for the detection of TB in low-and middle-income settings, among PLHIV initiating antiretro-viral therapy (ART) irrespective of symptoms [55,60], and for the reduction of mortality among PLHIV within the first 6 months of ART [61], Although Xpert MTB/RIF testing overall is projected to be less costly than conventional diagnostics for TB and MDR TB [62], the cost per test in clinics as opposed to laboratories in South Africa is 50% higher [63] and once fully implemented, will yield a 35% increase in overall cost of diagnosis and treatment in South Africa [64]. Compared with smear microscopy, introduction of Xpert MTB/RIF in two large cities in Brazil led to 46% lower costs to patients [65]. However, it must be noted that these estimates are likely to be highly setting-, population- and algorithm-dependent and must be confirmed with additional data obtained from routine programmatic implementation settings.
Although data from many large-scale, multi-country programmatic implementation projects will not be available until late 2014, data from early implementers and pragmatic studies are highlighting the importance of selecting patients to test who are at high risk of having TB or MDR TB and strengthening systems to maximize the outcomes and impact associated with Xpert MTB/RIF introduction. Although Xpert MTB/RIF detects more cases than sputum smear microscopy and increases bacteriological confirmation of TB disease [66–69], sensitivity of the assay depends on degree of immunosuppression and TB disease severity [68,70]. For example, in a study using Xpert MTB/RIF to screen HIV-infected persons enrolling in ART centers in South Africa, sensitivity of Xpert MTB/RIF for detecting smear-negative, culture-positive TB was only 43.4% (61) compared with the 68% pooled sensitivity observed in the evaluation studies (Table 1). Also, the potential impact of Xpert MTB/RIF with respect to case finding and treatment outcomes may be substantially decreased in settings in which clinicians initiate empiric TB treatment in the absence of bacteriological confirmation [69,71,72], thereby diminishing the apparent improvements in patient outcomes such as decreased mortality. Initial loss to follow-up and time to treatment initiation also have proven to be highly context specific and are likely to be influenced by sample referral systems versus point-of-care testing and delays in result reporting. Early implementation of Xpert MTB/RIF across nine countries demonstrated that differences in testing algorithms and proximity of testing to the patient yielded times from initial symptom screen to Xpert MTB/RIF result ranging from 0 to 10 days [73]. In a multicenter randomized controlled trial in four countries in Africa, Theron et al. found that Xpert MTB/RIF conducted at the point of care led to an increase in the proportion of cases initiating treatment on the same day as diagnosis [69]. In a community-based active case finding study in Cambodia, specimens were transported to the testing sites on a routine basis and results were transmitted to clinicians via SMS as soon as they were available; greater than 94% of cases initiated treatment and the median time to treatment initiation from symptom screening was 8 days [74]. Among MDR TB cases, time to second-line treatment initiation was decreased by 25 days with an Xpert MTB/RIF-containing algorithm compared with an LPA-based algorithm [75].
To maximize the short- and long-term utility and outcomes associated with any diagnostic test, comprehensive quality assurance (QA) programs must be introduced and maintained. Various specific approaches to instrument verification and proficiency testing are currently under evaluation and global consensus is expected in the near term [76–78]. However, irrespective of the specific details, any Xpert MTB/RIF QA program should assess pre-analytic (e.g., collection of good-quality specimens), analytic and post-analytic (e.g., prompt reporting) phases of testing through prospective monitoring (e.g., analysis of standardized performance indicators) as well as cross-sectional evaluations (e.g., proficiency testing) and should focus on the entire quality cycle, including feedback, corrective action and continuous quality improvement.
To measure programmatically important impact, a robust monitoring and evaluation system needs to be put in place, including indicators and support for data collection and analysis. It is especially important to monitor the positive effect that Xpert MTB/RIF can have on detection and improved diagnosis of smear-negative and drug-resistant TB, treatment initiation rates and reduced delays in TB diagnosis and initiation of a treatment regimen matched to the susceptibility pattern. Currently recommended outcome indicators include:
Number of pulmonary TB cases detected.
Proportion of TB cases that are bacteriologically confirmed.
Proportion of all persons with suspected TB tested with Xpert MTB/RIF.
Proportion of TB cases detected with Xpert MTB/RIF among all persons with suspected TB and tested with Xpert MTB/RIF.
Proportion of all TB cases that are treated with a regimen matched to the susceptibility.
Proportion of TB cases detected using Xpert MTB/RIF that are treated with a regimen matched to the susceptibility.
Average number of days between sputum collection and initiation of treatment among confirmed TB cases.
Assessment of these indicators will require a system that can efficiently link diagnostic and clinical information, which is not yet in place in most high-burden countries.
Lastly, to take full advantage of the promise of Xpert MTB/RIF to increase the number of TB and MDR TB cases that are detected, the implementation of Xpert MTB/RIF must be matched with increased treatment capacity to meet the demand, and healthcare facilities will need to enhance infection control procedures to prevent nosocomial transmission. Countries may need to make significant investments in human resources and TB control programs to ensure that all diagnosed patients are placed on therapy. Of particular concern is a growing gap between the number of MDR TB or rifampicin-resistant TB patients diagnosed and the number placed on therapy [11]. Adequate human and clinical resources and management of second-line drug inventories will become critical as more MDR TB cases are detected [11,43].
Expert commentary
A laboratory test is just one part of the diagnostic process, which starts with a clinician evaluating a patient and ordering of a test and continues through the receipt and interpretation of the results and initiation of appropriate TB treatment. Delays in any of these steps can reduce the clinical and public health impact of a laboratory test. To strengthen all steps in the cascade, a systems approach should be used which emphasizes access to services and uses quality management principles to ensure prompt and reliable flow of specimens and information [8]. This can dramatically reduce the time from the ordering a test to making a treatment decision as well as increase access to laboratory services for all patients. For example, by combining an efficient specimen transport system, Xpert MTB/RIF and an efficient reporting system, Boehme et al. [22] showed that use of Xpert MTB/RIF reduced the median time from the date of first sputum collection to the date of treatment initiation of culture-diagnosed TB cases from 56 days to 5 days. Similarly, Kwak et al. [79] in South Korea demonstrated that for patients diagnosed with pulmonary TB based on Xpert MTB/RIF had a median time to anti-TB treatment initiation to 7 days, compared with a median time of 21 days for starting anti-TB treatment in patients diagnosed with pulmonary TB and in whom Xpert MTB/RIF was not the basis of this diagnosis.
The clinical and public health impact of Xpert MTB/RIF will vary according to the epidemiologic setting, target population (e.g., all patients with TB disease, patients with HIV-associated TB or patients with MDR TB), current testing and treatment algorithms and Xpert MTB/RIF testing algorithms. In settings where laboratory confirmation relies on direct AFB smear microscopy, Xpert MTB/RIF may increase TB case detection by 30–40%. In contrast, in settings where laboratory confirmation relies on culture, Xpert MTB/RIF may not increase TB case detection. Potential decreases in the time to initiation of therapy will also depend on current clinical practices. For example, Xpert MTB/RIF may not decrease time to initiation of treatment for smear-negative TB patients if the current algorithm is to initiate treatment based on x-ray findings or on clinical signs and symptoms. Similarly, the detection of MDR TB may not increase in settings where conventional culture and DST of all TB patients is routine, such as it is in the USA.
Although recommendations indicate that Xpert MTB/RIF can replace conventional microscopy, culture and DST as the initial diagnostic test for patients with presumed TB, the test does have limitations. Xpert MTB/RIF is not as sensitive as culture for detecting MTBC in specimens from smear-negative TB patients, is not as sensitive as culture-based proportion tests for detecting resistant bacteria in a mixture of resistant and susceptible bacteria, does not provide information on susceptibility to anti-TB drugs other than rifampicin and cannot be used for monitoring the response to therapy. As such, high-quality conventional microscopy, culture and DST are still needed for monitoring therapy, for surveillance, for having an isolate for molecular epidemiologic investigations and for selecting treatment regimens for drug-resistant TB cases. Interestingly, although the introduction of Xpert MTB/RIF into a national testing algorithm may decrease the need for laboratory capacity that uses culture as an initial diagnostic test to detect MTBC, the anticipated increase in the detection of MDR TB may increase the need for culture to monitor response to therapy and for DST of other anti-TB drugs to guide the design of treatment regimens. Overall, national public health authorities should take advantage of the introduction of Xpert MTB/RIF to update their TB laboratory strategy, strengthen the entire TB laboratory network, improve the capacity of the laboratory system for conventional culture and DST for other first- and second-line anti TB drugs and other molecular methods and improve systems for specimen referral and reporting results.
As with any laboratory test, Xpert MTB/RIF can generate false-positive and false-negative results compared with the reference standard (culture or conventional DST). Suspected false-positive results were described in case reports of culture-negative patients previously treated for TB [80]. These patients improved without anti-TB treatment indicating that positive Xpert MTB/RIF results were possibly due to the detection of nucleic acid from dead bacilli. Because we are still learning about the frequency and mechanisms of false-positive and false-negative results for Xpert MTB/RIF as well as for the reference standard, it is recommended that in settings with a low prevalence of MDR TB or for patients without a known risk factor for MDR TB that the detection of rifampicin resistance trigger additional testing by conventional and molecular methods. In the case of discrepancies between conventional and molecular test results for rifampicin, clinicians should use judgment for case management decisions until the discrepancy is resolved. It is essential that healthcare providers understand the benefits and limitations of molecular testing.
The accuracy of the calculated positive predictive value lies in the accuracy of the specificity of the diagnostic test. However, determining the specificity of a test entails difficulties when the reference test has its own limitations. For example, in the studies used to calculate specificity, samples that were rifampicin resistant by Xpert MTB/RIF but susceptible by conventional DST were considered false-positive results. However, recent data indicate that some of the isolates actually represented false-susceptible results of the conventional DST because of mutations resulting in low-level or borderline rifampicin resistance [81–83]. Sequencing of the isolates revealed non-synonymous mutations in the RRDR and follow-up of these patients revealed a greater than expected rate of treatment failure with a rifampicin-based regimen. Strains with these types of mutations (e.g., 511Pro, 516Tyr, 526Asn, 526Leu and 533Pro) and certain mutations outside the RRDR (e.g., 572Phe) can exhibit highly discordant results among different conventional methods used for DST. On the other hand, silent mutations (i.e., mutations that do not change the amino acid sequence such as 514Phe (TTC/TTT)) in the RRDR have been found that generated false-positive results with Xpert MTB/RIF [84–86]. Early reports of false-positive rifampicin resistance in strains later found to be wild-type for rpoB led to modifications of the assay to improve performance [87–89]. False-positive results for rifampicin resistance could lead to an incorrect diagnosis of MDR TB and subject patients to longer and less-effective treatment with second-line drugs.
Conversely, false-negative results for rifampicin resistance have been reported for strains containing the Leu533Pro mutation in rpoB covered by probe E in the assay, possibly caused by variability in hybridization [90]. Additionally, false-negative Xpert MTB/RIF results could be due in part to the presence of simultaneous infection with rifampicin-susceptible and -resistant strains where the proportion of wild-type sequences for rpoB is predominant, thereby allowing all probes to hybridize. In at least one study, false-negative rifampicin resistance results were associated with poor clinical outcome [91].
Nucleic acid sequencing of the RRDR of the rpoB gene has been a powerful tool to investigate discordant results, but it has also revealed complexities in the association of specific mutations with changes in rifampicin susceptibility [81–96]. Some mutations are associated with large increases in the rifampicin MIC compared with strains with wild-type rpoB, while other mutations are associated with smaller increases in MIC, and some only confer increases in MIC in certain genetic backgrounds or in the presence of compensatory changes in other genes such as rpoC. While research is needed to define the association of mutations and clinical resistance, the current consensus is that, in the case of discordant results between phenotypic DST and sequencing, the detection of a change in the amino acid sequence of the RRDR should be considered confirmation of clinically significant rifampicin resistance [34].
A limitation of Xpert MTB/RIF is that it detects only rifampicin resistance. As the frequency of resistance to other drugs (e.g., fluoroquinolones, injectable second-line drugs) and incidence of extensively drug-resistant TB increase, diagnostic tests will be needed that can detect resistance to all clinically significant drugs. Nucleic acid sequencing of genes associated with drug resistance can be used to detect mutations associated with resistance to other drugs. In the USA, CDC offers a service that determines the sequence of nine genetic loci associated with resistance to eight anti-TB drugs: rifampicin, isoniazid, ethambutol, pyrazinamide, fluoroquinolones, amikacin, kana-mycin and capreomycin [97]. The service can provide results within 2 days of receiving the sample. Nucleic acid sequencing methods are amenable to high-throughput approaches. This sort of centralized service using high-throughput sequencing approaches coupled with an efficient specimen transport system and a rapid reporting system might enable one laboratory to provide services to a large population.
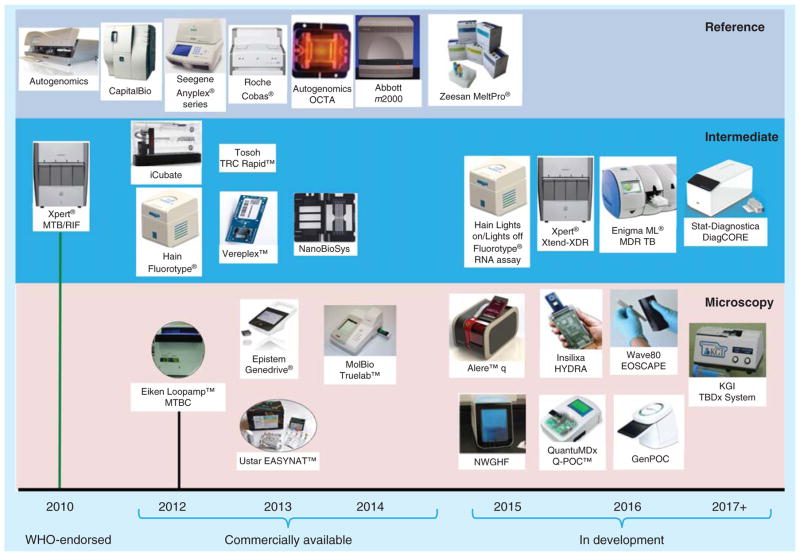
Xpert MTB/RIF is currently the only automated system for the detection of TB and rifampicin-resistant TB that has been endorsed by WHO or approved by the FDA. However, a number of ‘fast-followers’ and competing technologies are nearing the marketplace (Figure 2), and the diagnostic landscape could change dramatically in the next few years [98]. Sequencing of the entire genome has also been used to discover mutations associated with drug resistance and understand development and spread of drug-resistant TB [92–95], and as these technologies improve, bioinformatic analyses become more automated, costs are reduced and instruments suitable for use in peripheral laboratories are developed, sequencing may become the preferred test for detecting drug resistance [99].
Figure 2. Commercial tuberculosis products and development pipeline.
Reproduced with permission from UNITAID [98].
Five-year view
For the immediate future, Xpert MTB/RIF and LPAs are the only WHO-approved assays for the detection of TB and rifampicin resistance. Similar products are approaching the marketplace, but their penetration into the large untapped market (Xpert MTB/RIF has only ~3% of the anticipated market) in high-burden countries may be somewhat limited outside few countries with national regulatory processes because of the long, costly process to get globally recognized regulatory approval or endorsement. As such, Xpert MTB/RIF is likely to dominate molecular testing for TB at the district-level for the next few years, while LPAs with their potential higher throughput still have a role to play at higher levels in the laboratory system. It is important to note that a laboratory test such as Xpert MTB/RIF is but one part of the system that is needed to provide reliable laboratory services. The improvements in laboratory networks and systems catalyzed by the implementation of Xpert MTB/RIF will facilitate the implementation of any new molecular test that becomes available.
The diagnostic test in greatest need in high-burden countries is a rapid, affordable, robust point-of-care test that can provide results in less than an hour, while the patient waits, such that the healthcare worker can initiate optimal therapy on the same day. Xpert MTB/RIF gets us partway to this goal. The next-generation tests will need to operate under the conditions typically found in peripheral settings (clinics or laboratories), minimize infrastructure and human resource needs and provide information on the susceptibility to the important first- and second-line anti-TB drugs.
Molecular detection of mutations associated with drug resistance holds great promise for improving access to drug susceptibility information, improving the predictive value of the information and shortening the time to obtaining the information. As whole genome sequencing of microbial pathogens becomes affordable and accessible, molecular information may be available to design individualized treatment regimens within hours of obtaining a specimen. A critical need for these tests will be information that correlates clinical outcomes with the detection of mutations.
Key issues.
The definitive diagnosis of tuberculosis (TB) relies on laboratory testing to detect the presence of the causative agent (Mycobacterium tuberculosis complex bacteria, MTBC).
Only about 57% of the estimated 4.57 million notified cases of pulmonary TB in 2012 and about 19% of rifampicin-resistant TB cases were laboratory confirmed, which indicate enormous gaps in the ability to find, treat and follow TB patients.
The Cepheid Xpert® MTB/RIF assay (Xpert MTB/RIF) has the potential to revolutionize laboratory testing to aid in the diagnosis of TB and rifampicin-resistant TB.
Xpert MTB/RIF can detect both MTBC and rifampicin resistance from clinical specimens within 2 h after starting the test, with minimal hands-on time and minimal requirements for facilities.
A single Xpert MTB/RIF has sensitivity and specificity equivalent to a single culture on solid media for detecting MTBC, which may increase TB case detection by 30–40% in settings where laboratory confirmation relies on direct acid-fast bacilli smear microscopy.
However, conventional culture and drug-susceptibility test is still needed to detect MTBC in acid-fast bacilli-negative samples, confirm rifampicin resistance in persons at low risk of having MDR TB and determining susceptibilities to other drugs.
As is true for all tests, clinicians should interpret any laboratory result on the basis of the clinical situation.
-
The impact of Xpert MTB/RIF on clinical care and public health will vary according to the epidemiologic setting, target population (e.g., all patients with TB disease, patients with HIV-associated TB or patients with MDR TB), current testing and treatment algorithms and Xpert MTB/RIF testing algorithms.
In settings that rely on AFB microscopy, the use of Xpert MTB/RIF can increase case detection and reduce time to initiation of appropriate therapy, particularly for patients with MDR TB.
In settings that rely on clinical findings to initiate therapy, the use of Xpert MTB/RIF is likely to have much less effect on case finding and time to initiation of therapy.
Xpert MTB/RIF has been approved by the FDA for use in the USA, received Conformité Européene marking and endorsed by WHO for use in TB-endemic countries.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US CDC.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.ATS/CDC/IDSA. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161(4):1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. The global plan to stop TB 2011–2015: transforming the fight towards elimination of tuberculosis. World Health Organization; Geneva: 2010. WHO/HTM/STB/2010.2. Available from: www.stoptb.org/global/plan/ [Google Scholar]
- 3.Gomez-Pastrana D. Diagnosis of pulmonary tuberculosis in children. J Infect Dis Therap. 2013;1:17–24. [Google Scholar]
- 4.Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593. doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Kik SV, Denkinger CM, Chedore P, Pai M. Replacing smear microscopy for the diagnosis of tuberculosis: what is the market potential? Eur Respir J. 2014;43(6):1793–6. doi: 10.1183/09031936.00217313. Describes potential market for a rapid tuberculosis (TB) test such as Xpert. [DOI] [PubMed] [Google Scholar]
- 6.Global Laboratory Initiative. A roadmap for ensuring quality tuberculosis laboratory services within national laboratory strategic plans. World Health Organization; Geneva: 2010. Available from: www.stoptb.org/wg/gli/assets/documents/GLI_Roadmap_First_Issue_2010110.pdf. [Google Scholar]
- 7.World Health Organization. Framework for implementing TB diagnostics. 2011 Available from: www.who.int/tb/laboratory/whopolicyframework_rev_june2011.pdf.
- 8.CDC. National plan for reliable tuberculosis laboratory services using a systems approach: recommendations from CDC and the Association of Public Health Laboratories Task Force on Tuberculosis Laboratory Services. MMWR Morb Mortal Wkly Rep. 2005;54(RR-6):1–12. [PubMed] [Google Scholar]
- 9.Drobniewski FA, Hoffner S, Rusch-Gerdes S WHO European Laboratory Strengthening Task Force. Recommended standards for modern tuberculosis laboratory services in Europe. Eur Respir J. 2006;28(5):903–9. doi: 10.1183/09031936.06.00084906. [DOI] [PubMed] [Google Scholar]
- 10.Canadian Thoracic Society. Canadian Tuberculosis Standards, 7th Edition. Canadian Respir J. 2013;20(Suppl A) Available on-line only from www.respiratoryguidelines.ca/tb-standards-2013. [Google Scholar]
- 11.World Health Organization. Global tuberculosis report 2013. World Health Organization; Geneva: 2013. WHO/HTM/TB/2013.11. Available from: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. [Google Scholar]
- 12.World Health Organization. World Health Organization; Geneva: 2008. Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosis drugs. WHO/HTM/TB/2008.392. Available from: http://whqlibdoc.who.int/hq/2008/WHO_HTM_TB_2008.392_eng.pdf. [PubMed] [Google Scholar]
- 13.Wells WA, Boehme CC, Cobelens FG, et al. Alignment of new tuberculosis drug regimens and drug susceptibility testing: a framework for action. Lancet Infect Dis. 2013;13(5):449–58. doi: 10.1016/S1473-3099(13)70025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinnes J, Deeks J, Kunst H, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess. 2007;11:1–196. doi: 10.3310/hta11030. [DOI] [PubMed] [Google Scholar]
- 15.Flores LL, Pai M, Colford JM, Jr, Riley LW. In-house nucleic acid amplification tests for the detection of Mycobacterium tuberculosis in sputum specimens: meta-analysis and meta-regression. BMC Microbiol. 2005;5:55. doi: 10.1186/1471-2180-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis (MDR TB) Policy Statement. World Health Organization; Geneva: 2008. Available from: http://who.int/tb/features_archive/policy_statement.pdf. [Google Scholar]
- 17.Kurbatova EV, Cavanaugh JS, Shah NS, et al. Rifampicin-resistant Mycobacterium tuberculosis: susceptibility to isoniazid and other anti-tuberculosis drugs. Int J Tuberc Lung Dis. 2012;16(3):355–7. doi: 10.5588/ijtld.11.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SE, Kurbatova EV, Cavanaugh JS, Cegielski JP. Global isoniazid resistance patterns in rifampin-resistant and rifampin-susceptible tuberculosis. Int J Tuberc Lung Dis. 2012;16(2):203–5. doi: 10.5588/ijtld.11.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foundation for Innovative New Diagnostics. The EXPAND-TB Project. Available from: www.finddiagnostics.org/programs/scaling_up/unitaid_expand_tb/
- 20.Helb D, Jones M, Story E, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near patient technology. J Clin Microbiol. 2010;48(1):229–37. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stop TB partnership global laboratory initiative. Resources for WHO policy recommendations and supporting documents. Available from: www.stoptb.org/wg/gli/documents.asp?xpand=1.
- 22•.Boehme C, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377(9776):1495–505. doi: 10.1016/S0140-6736(11)60438-8. Established performance of the test in resource-limited settings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Food and Drug Administration, Medical Devices Advisory Committee. Discussion and recommendations regarding the classification of NAAT-based rapid M. tuberculosis diagnostics and the classification of interferon Gamma release assays. 2011 Available from: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/MicrobiologyDevicesPanel/UCM260828.pdf.
- 24.Food and Drug Administration. 501(k) decision summary for de novo request for evaluation of automatic class III designation for the Xpert® MTB/RIF Assay for use with the GeneXpert® Instrument Systems, including the GeneXpert® Diagnostic (Dx) Systems and the GeneXpert® Infinity Systems. 2013 Available from: http://www.accessdata.fda.gov/cdrh_docs/reviews/K131706.pdf.
- 25.package insert. Sunnyvale; CA: Cepheid; 2013. Xpert MTB/RIF assay. [Google Scholar]
- 26.World Health Organization. Handbook for guideline development. World Health Organization; Geneva: 2012. Available from: http://apps.who.int/iris/bitstream/10665/75146/1/9789241548441_eng.pdf. [Google Scholar]
- 27.Schünemann J, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–10. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Weyer K, Mirzayev F, Migliori GB, et al. Rapid molecular TB diagnosis: evidence, policy making and global implementation of Xpert MTB/RIF. Eur Respir J. 2013;42(1):252–71. doi: 10.1183/09031936.00157212. Overview of Xpert performance and standard setting processes at WHO. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Xpert MTB/RIF: expert Group Meeting Report. World Health Organization; Geneva: 2010. Available from: www.who.int/tb/laboratory/en/EGM_report. [Google Scholar]
- 30.World Health organization. Strategic and Technical Advisory Group for Tuberculosis (STAG-TB). Report of the 10th meeting; Geneva: World Health Organization; 2010. WHO/HTM/TB/2010.18. Available from: www.who.int/entity/tb/advisory_bodies/STAG_report2013.pdf. [Google Scholar]
- 31.World Health Organization. Policy statement. World Health Organization; Geneva: 2011. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. WHO/HTM/TB/2011.4. Available from: http://whqlibdoc.who.int/publications/2011/9789241501545_eng.pdf. [PubMed] [Google Scholar]
- 32.World Health Organization. WHO monitoring of Xpert MTB/RIF roll-out. Available from: www.who.int/tb/laboratory/mtbrifrollout/en/
- 33.Denkinger CM, Schumacher SG, Boehme CC, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2014 doi: 10.1183/09031936.00007814. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34••.World Health Organization. World Health Organization; Geneva: 2013. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system for the diagnosis of pulmonary and extrapulmonary TB in adults and children: policy update. WHO/HTM/TB/2013.14. Available from: http://apps.who.int/iris/bitstream/10665/112472/1/9789241506335_eng.pdf?ua=1Most recent guidance on the use of Xpert in resource-limited and high-burden settings. [PubMed] [Google Scholar]
- 35.World Health Organization. Expert Group Meeting Report. World Health Organization; Geneva: 2013. Using the Xpert MTB/RIF assay to detect pulmonary and extrapulmonary tuberculosis and rifampicin resistance in adults and children. WHO/HTM/TB/2013.14. Available from: www.who.int/tb/laboratory/expert_group_report.pdf?ua=1. [Google Scholar]
- 36.Association of Public Health Laboratories. Laboratory considerations for use of Cepheid Xpert® MTB/RIF Assay. 2013 Available from: www.aphl.org/AboutAPHL/publications/Documents/ID_2013Nov_Cepheid-Xpert-Fact-Sheet.pdf.
- 37.Centers for Disease Control and Prevention (CDC) Availability of an assay for detecting Mycobacterium tuberculosis, Including rifampin-resistant strains, and considerations for its use - United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;62(41):821–4. [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC) Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009;58:7–10. [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention (CDC) Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Morb Mortal Wkly Rep. 2005;54(RR-17):1. [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention (CDC) Reported tuberculosis in the United States, 2012. Department of Health and Human Services, CDC; Atlanta: 2013. Available from: www.cdc.gov/tb/statistics/reports/2012/pdf/report2012.pdf. [Google Scholar]
- 41.European Centre for Disease Prevention and Control. ERLN-TB expert opinion on the use of the rapid molecular assays for the diagnosis of tuberculosis and detection of drug-resistance. Stockholm: ECDC; 2013. Catalogue number TQ-02-13-155-EN-C. Available from: www.ecdc.europa.eu/en/publications/Publications/ERLN-TB-use-rapid-molecular-assays-diagnosis-tuberculosis-detection-drug-resistance.pdf. [DOI] [Google Scholar]
- 42.World Health Organization. Technical and operational ‘How-to’ practical considerations. World Health Organization; Geneva: 2014. Rapid implementation of the Xpert MTB/RIF diagnostic test. WHO/HTM/TB/2014.1. Available from: http://apps.who.int/iris/bitstream/10665/112469/1/9789241506700_eng.pdf?ua=1. [Google Scholar]
- 43.World Health Organization. World Health Organization; Geneva: 2011. Guidelines for the programmatic management of drug-resistant tuberculosis – 2011 update. WHO/HTM/TB/2011.6. Available from: http://whqlibdoc.who.int/publications/2011/9789241501583_eng.pdf. [PubMed] [Google Scholar]
- 44.World Health Organization. World Health Organization; Geneva: 2008. Guidelines for the programmatic management of drug-resistant tuberculosis: emergency update 2008. WHO/HTM/TB/2008.402. Available from: http://whqlibdoc.who.int/publications/2008/9789241547581_eng.pdf. [Google Scholar]
- 45.World Health Organization. World Health Organization; Geneva: 2008. Anti-tuberculosis drug resistance in the world: fourth global report. WHO/HTM/TB/2008.394. Available from: www.who.int/tb/publications/2008/drs_report4_26feb08.pdf. [Google Scholar]
- 46.Denkinger CM, Pai M, Dowdy DW. Do we need to detect isoniazid resistance in addition to rifampicin resistance in diagnostic tests for tuberculosis? PLoS One. 2014;9(1):e84197. doi: 10.1371/journal.pone.0084197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marlowe EM, Novak-Weekley SM, Cumpio J, et al. Evaluation of the Cepheid Xpert MTB/RIF assay for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol. 2011;49(4):1621–3. doi: 10.1128/JCM.02214-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bunsow E, Ruiz-Serrano MJ, López Roa P, et al. Evaluation of GeneXpert MTB/RIF for the detection of Mycobacterium tuberculosis and resistance to rifampin in clinical specimens. J Infect. 2014;68(4):338–43. doi: 10.1016/j.jinf.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Moure R, Muñoz L, Torres M, et al. Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. J Clin Microbiol. 2011;49(3):1137–9. doi: 10.1128/JCM.01831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sohn H, Aero AD, Menzies D, et al. Xpert MTB/RIF testing in a low tuberculosis incidence, high-resource setting: limitations in accuracy and clinical impact. Clin Infect Dis. 2014;58(7):970–6. doi: 10.1093/cid/ciu022. [DOI] [PubMed] [Google Scholar]
- 51.Cepheid. Available from: www.cepheidcares.com/tb/cepheid-warranty.html.
- 52.Foundation for Innovative New Diagnostics. Available from: www.finddiagnostics.org/about/what_we_do/successes/find-negotiated-prices/xpert_mtb_rif.html.
- 53.Cepheid. Available from: www.cepheidcares.com/tb/cepheid-vision.html.
- 54.Millman AJ, Dowdy DW, Miller CR, et al. Rapid molecular testing for TB to guide respiratory isolation in the U.S: A cost-benefit analysis. PLoS One. 2013;8(11):e79669. doi: 10.1371/journal.pone.0079669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vassall A, van Kampen S, Sohn H, et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med. 2011;8(11):e1001120. doi: 10.1371/journal.pmed.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.TB diagnostics market analysis consortium. Market assessment of tuberculosis diagnostics in Brazil in 2012. PloS One. 2014;9(8):e104105. doi: 10.1371/journal.pone.0104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lippincott CK, Miller MB, Popowitch EB, et al. Xpert MTB/RIF assay shortens airborne isolation for hospitalized patients with presumptive tuberculosis in the United States. Clin Infect Dis. 2014;59(2):186–92. doi: 10.1093/cid/ciu212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muñoz L, Moure R, Porta N, et al. GeneXpert® for smear-negative pulmonary tuberculosis: does it play a role in low-burden countries? Diagn Microbiol Infect Dis. 2013;75(3):325–6. doi: 10.1016/j.diagmicrobio.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 59.Choi HW, Miele K, Dowdy D, Shah M. Cost-effectiveness of Xpert® MTB/RIF for diagnosing pulmonary tuberculosis in the United States. Int J Tuberc Lung Dis. 2013;17(10):1328–35. doi: 10.5588/ijtld.13.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andrews JR, Lawn SD, Rusu C, et al. The cost-effectiveness of routine tuberculosis screening with Xpert MTB/RIF prior to initiation of antiretroviral therapy: a model-based analysis. AIDS. 2012;26(8):987–95. doi: 10.1097/QAD.0b013e3283522d47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abimbola TO, Marston BJ, Date AA, et al. Cost-effectiveness of tuberculosis diagnostic strategies to reduce early mortality among persons with advanced HIV infection initiating antiretroviral therapy. AIDS. 2012;60(1):e1–7. doi: 10.1097/QAI.0b013e318246538f. [DOI] [PubMed] [Google Scholar]
- 62•.Pantoja A, Fitzpatrick C, Vassall A, et al. Xpert MTB/RIF for diagnosis of tuberculosis and drug-resistant tuberculosis: a cost and affordability analysis. Eur Respir J. 2013;42(3):708–20. doi: 10.1183/09031936.00147912. Xpert testing overall is projected to be less costly than conventional diagnostics for TB and multidrug-resistant TB in resource-limited, high-burden countries. [DOI] [PubMed] [Google Scholar]
- 63.Schnippel K, Meyer-Rath G, Long L, et al. Diagnosing Xpert MTB/RIF negative TB: impact and cost of alternative algorithms for South Africa. S Afr Med J. 2013;103(2):101–6. doi: 10.7196/samj.6182. [DOI] [PubMed] [Google Scholar]
- 64.Meyer-Rath G, Schnippel K, Long L, et al. The impact and cost of scaling up GeneXpert MTB/RIF in South Africa. PLoS One. 2012;7(5):e36966. doi: 10.1371/journal.pone.0036966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.da Silva Antunes R, Pinto M, Trajman A. Patient costs for the diagnosis of tuberculosis in Brazil: comparison of Xpert MTB/RIF and smear microscopy. Int J Tuberc Lung Dis. 2014;18:547–51. doi: 10.5588/ijtld.13.0637. [DOI] [PubMed] [Google Scholar]
- 66.Hanrahan CF, Selibas K, Deery CB, et al. Time to treatment and patient outcomes among TB suspects screened by a single point-of-care Xpert MTB/RIF at a primary care clinic in Johannesburg, South Africa. PLoS One. 2013;8(6):e65421. doi: 10.1371/journal.pone.0065421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lawn SD, Brooks SV, Kranzer K, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med. 2011;8(7):e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lawn SD, Kerkhoff AD, Vogt M, Wood R. HIV-associated tuberculosis: relationship between disease severity and the sensitivity of new sputum-based and urine-based diagnostic assays. BMC Med. 2013;11:231. doi: 10.1186/1741-7015-11-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69••.Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383(9915):424–35. doi: 10.1016/S0140-6736(13)62073-5. Xpert may have limited impact and cost–effectiveness in settings where treatment is initiated in the absence of bacteriologic confirmation. [DOI] [PubMed] [Google Scholar]
- 70.Lawn SD, Kerkhoff AD, Vogt M, et al. Characteristics and early outcomes of patients with Xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin Infect Dis. 2012;54(8):1071–9. doi: 10.1093/cid/cir1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Theron G, Peter J, Meldau R, et al. Accuracy and impact of Xpert MTB/RIF for the diagnosis of smear-negative or sputum-scarce tuberculosis using bronchoalveolar lavage fluid. Thorax. 2013;68(11):1043–51. doi: 10.1136/thoraxjnl-2013-203485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Theron G, Peter J, Dowdy D, et al. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis. 2014;14(6):527–32. doi: 10.1016/S1473-3099(13)70360-8. [DOI] [PubMed] [Google Scholar]
- 73.Creswell J, Codlin AJ, Andre E, et al. Results from early programmatic implementation of Xpert MTB/RIF testing in nine countries. BMC Infect Dis. 2014;14:2. doi: 10.1186/1471-2334-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lorent N, Choun K, Thai S, et al. Community-based active tuberculosis case finding in poor urban settlements of Phnom Penh, Cambodia: a feasible and effective strategy. PLoS ONE. 2014;9(3):e92754. doi: 10.1371/journal.pone.0092754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naidoo P, du Toit E, Dunbar R, et al. A comparison of multidrug-resistant tuberculosis treatment commencement times in MDRTBPlus line probe assay and Xpert MTB/RIF-based algorithms in a routine operational setting in Cape Town. PLoS One. 2014;9:e103328. doi: 10.1371/journal.pone.0103328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scott LE, Gous N, Cunningham BE, et al. Dried culture spots for Xpert MTB/RIF external quality assessment: results of a phase 1 pilot study in South Africa. J Clin Microbiol. 2011;49:4356–60. doi: 10.1128/JCM.05167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gous N, Cunningham B, Kana B, et al. Performance monitoring of Mycobacterium tuberculosis dried culture spots for use with the GeneXpert system within a national program in South Africa. J Clin Microbiol. 2013;51:4018–21. doi: 10.1128/JCM.01715-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scott L, Albert H, Gilpin C, et al. Multicenter feasibility study to assess external quality assessment panels for Xpert MTB/RIF assay in South Africa. J Clin Microbiol. 2014;52:2493–9. doi: 10.1128/JCM.03533-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kwak N, Choi SM, Lee J, et al. Diagnostic accuracy and turnaround time of the Xpert MTB/RIF assay in routine clinical practice. PLoS One. 2013;8(10):e77456. doi: 10.1371/journal.pone.0077456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boyles TH, Hughes J, Cox V, et al. False-positive Xpert MTB/RIF assays in previously treated patients: need for caution in interpreting results. Int J Tuberc Lung Dis. 2014;18(7):876–8. doi: 10.5588/ijtld.13.0853. [DOI] [PubMed] [Google Scholar]
- 81••.Van Deun A, Maug AKJ, Bola V, et al. Rifampicin drug resistance tests for tuberculosis: challenging the gold standard. J Clin Microbiol. 2013;51(8):2633–40. doi: 10.1128/JCM.00553-13. Reports evidence that molecular detection of rifampicin resistance may be a better indicator than phenotypic drug susceptibility testing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rigouts L, Gumusboga M, Bram de Rijka W, et al. Rifampin resistance missed in automated liquid culture system for Mycobacterium tuberculosis isolates with specific rpoB mutations. J Clin Microbiol. 2013;51(8):2641–5. doi: 10.1128/JCM.02741-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ocheretina O, Escuyer VE, Mabou M, et al. Correlation between genotypic and phenotypic testing for resistance to rifampin in Mycobacterium tuberculosis clinical isolates in Haiti: investigation of cases with discrepant susceptibility results. PLoS One. 2014;9(3):e90569. doi: 10.1371/journal.pone.0090569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williamson DA, Basu I, Bower J, et al. An evaluation of the Xpert MTB/RIF assay and detection of false-positive rifampicin resistance in Mycobacterium tuberculosis. Diagn Microbiol Infect Dis. 2012;74:207–9. doi: 10.1016/j.diagmicrobio.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 85.Alonso M, Palacios JJ, Herranz M, et al. Isolation of Mycobacterium tuberculosis strains with a silent mutation in rpoB leading to potential misassignment of resistance category. J Clin Microbiol. 2011;49(7):2688–90. doi: 10.1128/JCM.00659-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moure R, Martin R, Alcaide F. Silent mutation in rpoB detected from clinical samples with rifampin-susceptible Mycobacterium tuberculosis. J Clin Microbiol. 2011;49(10):3722. doi: 10.1128/JCM.05314-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Rie A, Mellet K, John MA, et al. False-positive rifampicin resistance on Xpert® MTB/RIF: case report and clinical implications. Int J Tuberc Lung Dis. 2012;16(2):206–8. doi: 10.5588/ijtld.11.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Foundation for Innovative New Diagnostics. Performance of Xpert MTB/RIF Version G4 assay. Available from: www.stoptb.org/wg/gli/assets/documents/map/findg4cartridge.pdf.
- 89.Deggim V, Somoskovi A, Voit A, et al. Integrating the Xpert MTB/RIF assay into a diagnostic workflow for rapid detection of Mycobacterium tuberculosis in a low-prevalence area. J Clin Microbiol. 2013;51(7):2396–9. doi: 10.1128/JCM.00151-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Somoskovi A, Deggim V, Ciardo D, Bloemberg GV. Diagnostic implications of inconsistent results obtained with the Xpert MTB/Rif assay in detection of Mycobacterium tuberculosis isolates with an rpoB mutation associated with low-level rifampin resistance. J Clin Microbiol. 2013;51(9):3127–9. doi: 10.1128/JCM.01377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zetola NM, Shin SS, Tumedi KA, et al. Mixed Mycobacterium tuberculosis complex infections and false-negative results for rifampin resistance by GeneXpert MTB/RIF are associated with poor clinical outcomes. J Clin Microbiol. 2014;52(7):2422–9. doi: 10.1128/JCM.02489-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Comas I, Borrell S, Roetzer A, et al. Whole-genome sequencing of rifampicin-resistant Mycobacterium tuberculosis strains identifies compensatory mutations in RNA polymerase genes. Nat Genet. 2011;44(1):106–10. doi: 10.1038/ng.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Casali N, Nikolayevsky V, Balabanova Y, et al. Evolution and transmission of drug-resistant tuberculosis in a Russian population. Nat Genet. 2014;46:279–86. doi: 10.1038/ng.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Merker M, Kohl TA, Roetzer A, et al. Whole genome sequencing reveals complex evolution patterns of multidrug-resistant Mycobacterium tuberculosis Beijing strains in patients. PLoS One. 2013;8(12):e82551. doi: 10.1371/journal.pone.0082551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Farhat MR, Shapiro BJ, Kieser KJ, et al. Genomic analysis identifies targets of convergent positive selection in drug-resistant Mycobacterium tuberculosis. Nat Genet. 2013;45:1183–9. doi: 10.1038/ng.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Springer B, Lucke K, Calligaris-Maibach R, et al. Quantitative drug susceptibility testing of Mycobacterium tuberculosis by use of MGIT 960 and EpiCenter instrumentation. J Clin Microbiol. 2009;47:1773–80. doi: 10.1128/JCM.02501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Centers for Disease Control and Prevention (CDC) CDC; Atlanta: 2012. Laboratory user guide for U.S. Public Health Laboratories: molecular detection of drug resistance (MDDR) in Mycobacterium tuberculosis complex by DNA sequencing. Available from: www.cdc.gov/tb/topic/laboratory/MDDRUsersGuide.pdf. [Google Scholar]
- 98.UNITAID. Tuberculosis diagnostic technology and market landscape. 3. World Health Organization; Geneva: 2014. Available from: http://unitaid.org/images/marketdynamics/publications/UNITAID_TB_Diagnostics_Landscape_3rd-edition.pdf. [Google Scholar]
- 99.Dunne WM, Jr, Westblade LF, Ford B. Next-generation and whole-genome sequencing in the diagnostic clinical microbiology laboratory. Eur J Clin Microbiol Infect Dis. 2012;31(8):1719–26. doi: 10.1007/s10096-012-1641-7. [DOI] [PubMed] [Google Scholar]