Abstract
Background
Tumor differentiation is an important process in the development of cancer. It is valuable to identify key differentiation related genes in the prognosis and therapy of pancreatic adenocarcinoma.
Methods
The mRNA expression data were downloaded from the Cancer Genome Atlas database. Then, differentially expressed tumor differentiation related genes were identified. Additionally, Gene Ontology functional categories and Kyoto Encyclopedia of Genes and Genomes biochemical pathway was used to explore the function. In addition, receiver operating characteristic and survival analysis were carried out to assess the diagnosis and prognosis value. Finally, the electronic validation of selected tumor differentiation related genes was performed.
Results
A total of 932 genes were identified. Among which, 8 genes including JUB, ERLIN1, HMGA2, FAM110B, EGFR, MCM2, TCTA and SSTR1 were differentially expressed in all different tumor differentiation grades. Functional analysis revealed those genes between highly differentiated and other differentiation were remarkably enriched in pancreatic adenocarcinoma and cell cycle pathway. Finally, ERLIN1, HMGA2, FAM110B, EGFR, MCM2, BCL2L1, E2F1 and RAC1 were associated with the survival time of pancreatic adenocarcinoma patient. Among these genes, JUB, ERLIN1, FAM110B, MCM2 and BCL2L1 also had a diagnosis value for pancreatic adenocarcinoma. Additionally, the expression trend of JUB, HMGA2 and MCM2 was increased along with the tumor differentiation grades. And the expression trend of FAM110B was decreased along with the tumor differentiation grades. The electronic validation result was consistent with the bioinformatics analysis.
Conclusions
12 tumor differentiation related genes including JUB, ERLIN1, HMGA2, FAM110B, EGFR, MCM2, TCTA, SSTR1, BCL2L1, E2F1, RAC1 and STAT1 played crucial roles in the differentiation of pancreatic adenocarcinoma.
Introduction
Pancreatic adenocarcinoma is an important leading cause of cancer-related mortality in the Western world [1]. Generally, the vast majority of pancreatic adenocarcinoma patients present with no specific symptoms until appear jaundice and weight loss, signs of advanced stage of pancreatic adenocarcinoma [2]. The lethal characteristic of pancreatic adenocarcinoma is the high metastatic potential to distant organs and the lymphatic system [3]. Additionally, lack of effective chemotherapy also contributes to the high mortality of pancreatic adenocarcinoma patients [4].
UP to now, the therapy of pancreatic adenocarcinoma remains a great challenge in clinical oncology on account of the similar rate between incidence and mortality [5]. According to the report of cancer statistics in 2015, in contrast to the steady increase in survival for most cancers, advance has been slow for pancreatic adenocarcinoma, for which the 5-year relative survival is recent 7% [6]. Therefore, advances in understanding the molecular biology and discovery of novel therapeutic targets will contribute to clinical management of pancreatic adenocarcinoma.
Based on the degree of tumor differentiation, pancreatic adenocarcinoma is divided into four grades including highly differentiated (G1), moderately differentiated (G2), poorly differentiated (G3) and un-differentiation (G4). Moreover, patients with poorly differentiated tumors have a worse prognosis than those with well differentiated tumors [7]. It is noted that The Cancer Genome Atlas (TCGA, https://tcga-data.nci.nih.gov/tcga/) database is a publicly funded project that is consisted of multidimensional data of different cancers in patients at DNA, RNA and protein levels. Therefore, the objective of our study is to identify the tumor differentiation related genes based on the TCGA dataset, shedding light on molecule mechanism and identifying new therapy targets for the treatment of pancreatic adenocarcinoma.
Material and methods
The Cancer Genome Atlas dataset
The Cancer Genome Atlas (TCGA, https://tcga-data.nci.nih.gov/tcga/) is a publicly funded project that is consisted of multidimensional data of different cancers in patients at DNA, RNA and protein levels. In this study, TCGA dataset was used to retrieve the gene expression data in pancreatic adenocarcinoma.
Identification of tumor differentiation related differentially expressed genes
In order to find potential genes in the process of tumor differentiation, differentiation related transcription sequencing data were analyzed by linear by linear association test in 176 pancreatic adenocarcinoma patients. The lbl.Test function analysis in the cion package of R software was used to identify differentiation related genes. The detailed process was as follows: Firstly, the sample was divided into four express quantity interval according to the quartiles of each gene expression quantity. Secondly, the correlation between express quantity interval and tumor grade was tested. Thirdly, tumor differentiation related genes were selected according to the threshold of p < 0.01. Finally, the expression of each gene in different tumor differentiation grades was further analyzed through tukey's honest, and p < 0.05 was considered the statistical significant difference.
Functional annotation of tumor differentiation related differentially expressed genes
The Gene Ontology (GO) function was used to enrich the biological function of genes in biological progress, cellular component and molecular function [8]. The Kyoto Encyclopedia of Genes and Genomes (KEGG) dataset is applied to analyze gene function and genome information systematically, which is helpful in studying gene expression in a network. It is consisted of six subdata including PATHWAY, BRITE, MODULE, DISEASE, GENES and GENOME. Among which, PATHWAY dataset contains the most advanced functional information such as metabolism, membrane transport, signal transmission and cell cycle. To further understand the biological function of tumor differentiation related genes in pancreatic adenocarcinoma, we performed the GO functional categories and the KEGG biochemical pathway using GeneCodis3 (http://genecodis.cnb.csic.es/analysis). False discovery rate < 0.05 was considered to be statistically significant.
Receiver operating characteristic (ROC) analyses
In order to assess the diagnostic value of tumor differentiation related differentially expressed genes in pancreatic adenocarcinoma, we performed the receiver operating characteristic (ROC) analyses through the pROC package in R language. The area under the curve (AUC) under binomial exact confidence interval was calculated and the ROC curve was generated.
Survival analysis of tumor differentiation related differentially expressed genes
The R package (3.4.0 version, 2017) was used to explore the prognostic ability to predict patient survivability of identified tumor differentiation related differentially expressed genes in the TCGA database.
Electronic validation of tumor differentiation related genes in GEO database
We employed the Gene Expression Omnibus (GEO) database to validate the expression of selected tumor differentiation related genes. We compared the expression levels of these genes in different tumor differentiation grades including G1, G2, G3 and G4. The difference of expression levels was displayed by box-plots.
Results
Clinical information
In this study, the mRNA expression data of patients with pancreatic adenocarcinoma were downloaded from the platform of UNC_IlluminaHiSeq_RNASeqV2 in TCGA data portal. Downloaded mRNA expression data were standardized. Those patients who had a tumor grade of GX were ruled out for this study. Finally, 176 patients with clinical tumor grade information and mRNA expression data were included in this study. Among which, 31 were highly differentiated (G1), 95 were moderately differentiated (G2), 48 were poorly differentiated (G3) and 2 were un-differentiated (G4). G3 and G4 were combined together (G3/G4). Detailed information of pancreatic adenocarcinoma patients was presented in Table 1.
Table 1. Information of patients with pancreatic adenocarcinoma.
| Parameter | Patients (N = 176) | |
|---|---|---|
| Gender | Male | 97 |
| Female | 79 | |
| Age | >60 (Mean ± SD) | 70.72±6.43 |
| ≤60 (Mean ± SD) | 51.97±6.08 | |
| Race | White | 156 |
| Black or African American | 6 | |
| Asian | 10 | |
| Vital status | Unknown | 3 |
| Alive | 117 | |
| Dead | 59 | |
| Follow-up(days) | >5 years (Mean ± SD) | 5.73±0.68 |
| <5 years(Mean ± SD) | 0.90±0.89 | |
| Tumor differentiation | G1 | 31 |
| G2 | 95 | |
| G3/G4 | 50 | |
| Radiation therapy | Yes | 31 |
| No | 102 | |
| Unknown | 42 | |
| Not Available | 1 | |
| Yes | 1 | |
| History of neoadjuvant treatment | No | 175 |
| Yes | 89 | |
| No | 30 | |
| Targeted molecular therapy | Not applicable | 57 |
G1: highly tumor differentiated; G2: moderately tumor differentiated; G3/G4: poorly tumor differentiation and un-differentiated.
Tumor differentiation related differentially expressed genes
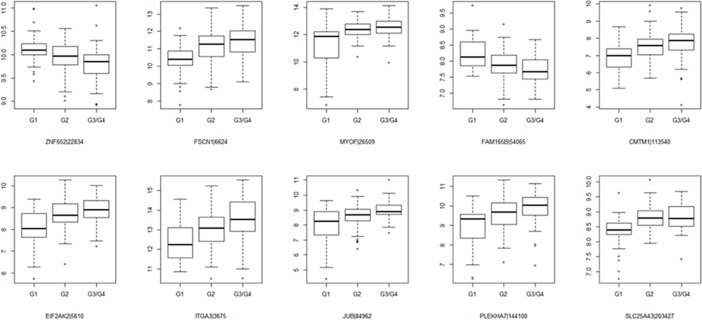
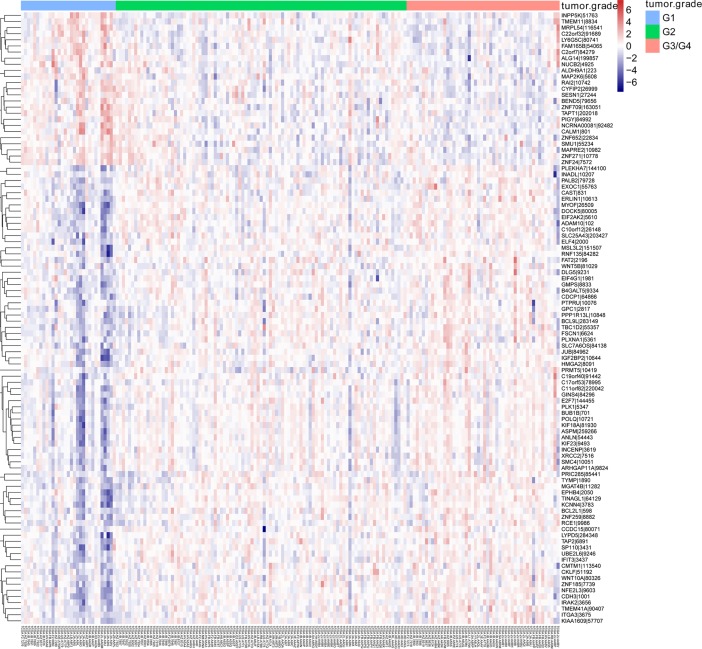
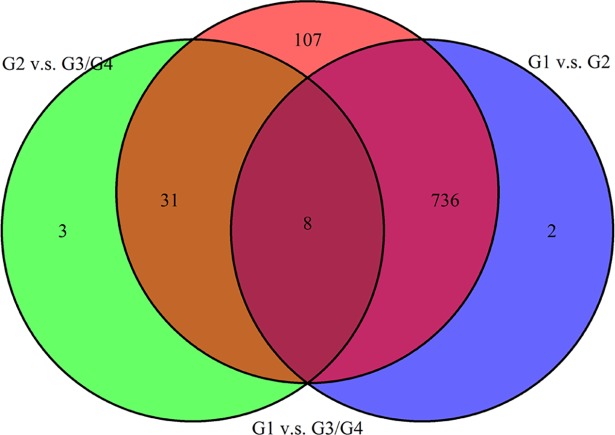
Based on transcriptome sequencing data analysis in the context of tumor differentiation grade, 932 (614 positively associated and 318 negatively associated) related genes were identified in pancreatic adenocarcinoma. The top 10 tumor differentiation related genes were shown in Table 2. The Box-plot of top 10 tumor differentiation related genes was also shown in Fig 1. In addition, the heat map of top 100 tumor differentiation related genes was presented in Fig 2. Additionally, tukey's honest significant difference was used to further investigate the different expression of each gene in different tumor differentiation grades. The analysis results revealed that there were 746 differentially expressed genes between G1 and G2, 882 differentially expressed genes between G1 and G3/G4 and 42 differentially expressed genes between G2 and G3/G4. The Venn of genes expression overlaps in diverse tumor differentiation grades was shown in Fig 3. It is worth mentioning that 8 genes including JUB, ERLIN1, HMGA2, FAM110B, EGFR, MCM2, TCTA and SSTR1 were differentially expressed in distinct tumor differentiation grades.
Table 2. The top 10 tumor differentiation-related genes.
| Gene ID | Gene symbol | Mean G1 | Mean G2 | Mean G3/G4 | P Value | Association |
|---|---|---|---|---|---|---|
| 22834 | ZNF652 | 10.1460 | 9.9536 | 9.8123 | 7.94E-06 | Negative |
| 6624 | FSCN1 | 10.3484 | 11.1331 | 11.4693 | 1.26E-05 | Positive |
| 26509 | MYOF | 11.0974 | 12.3869 | 12.5488 | 1.26E-05 | Positive |
| 54065 | FAM165B | 8.2168 | 7.8843 | 7.7171 | 1.26E-05 | Negative |
| 113540 | CMTM1 | 6.9000 | 7.5645 | 7.7247 | 1.99E-05 | Positive |
| 5610 | EIF2AK2 | 8.0822 | 8.7083 | 8.8838 | 1.99E-05 | Positive |
| 3675 | ITGA3 | 12.3691 | 13.0239 | 13.4195 | 1.99E-05 | Positive |
| 84962 | JUB | 7.9763 | 8.6089 | 8.9946 | 1.99E-05 | Positive |
| 144100 | PLEKHA7 | 8.9381 | 9.6629 | 9.9410 | 1.99E-05 | Positive |
| 203427 | SLC25A43 | 8.3147 | 8.8164 | 8.8370 | 1.99E-05 | Positive |
Fig 1. The Box-plot of top 10 tumor differentiation related genes in pancreatic adenocarcinoma.
The x-axis shows the tumor differentiation grades and y-axis shows expression reads counts.
Fig 2. The heat map of top 100 tumor differentiation related genes in pancreatic adenocarcinoma.
Diagram presents the result of a two-way hierarchical clustering of top 100 tumor differentiation-related genes and samples. The clustering is constructed using the complete-linkage method together with the Euclidean distance. Each row represents a gene and each column, a sample. The gene clustering tree is shown on the right. The colour scale illustrates the relative level of gene expression: red, below the reference channel; blue, higher than the reference. G1: highly tumor differentiated; G2: moderately tumor differentiated; G3/G4: poorly tumor differentiation and un-differentiated.
Fig 3. The Venn of genes expression overlaps in different tumor differentiation grades in pancreatic adenocarcinoma.
G1: highly tumor differentiated; G2: moderately tumor differentiated; G3/G4: poorly tumor differentiation and un-differentiated.
Biological function of tumor differentiation related differentially expressed genes
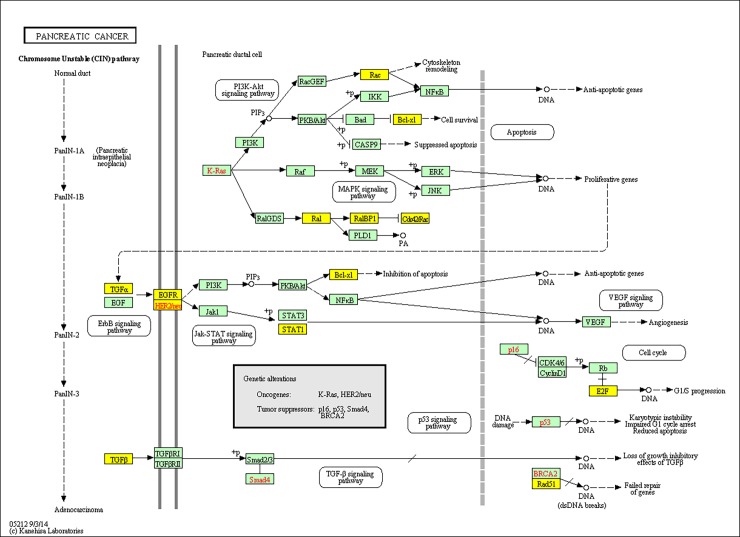
To investigate the gene function, GO and KEGG were applied to perform function annotation in 744 differentially expressed genes (the intersection genes between G1 vs G2 and G1 vs G3/G4) and 705 genes were recognized. GO enrichment analysis revealed that these genes were significantly involved in cell division (false discovery rate = 2.15E–17), cell cycle (false discovery rate = 2.19E–17) and cell proliferation (false discovery rate = 1.17E–07). Additionally, KEGG pathway enrichment analysis showed that these genes were remarkably enriched in pathways in cancer (false discovery rate = 8.56E–07), cell cycle (false discovery rate = 3.48E–06) and pancreatic adenocarcinoma (false discovery rate = 1.10E–05). Top 15 Go terms and top 3 KEGG pathways for differentially expressed genes were listed in Table 3 and Table 4, respectively. Remarkably, 11 genes including STAT1, BCL2L1, TGFA, ERBB2, E2F1, RAD51, RALB, RALBP1, TGFB2, EGFR and RAC1 were involved in the pathway of pancreatic adenocarcinoma. 15 genes including MCM4, CCNB2, CDC20, CCNA2, CCND2, E2F1, CCNB1, TFDP2, TGFB2, PLK1, BUB1B, PKMYT1, TTK, BUB1 and MCM2 were involved in the cell cycle. The KEGG map of pancreatic adenocarcinoma was showed in Fig 4.
Table 3. Top 15 Go terms for differentially expressed genes in pancreatic adenocarcinoma.
| GO ID | GO Term | Count | False discovery rate |
|---|---|---|---|
| Biological process | |||
| GO:0051301 | cell division (BP) | 39 | 2.15E-17 |
| GO:0007049 | cell cycle (BP) | 47 | 2.19E-17 |
| GO:0000278 | mitotic cell cycle (BP) | 35 | 1.56E-13 |
| GO:0000087 | M phase of mitotic cell cycle (BP) | 21 | 2.11E-13 |
| GO:0007067 | mitosis (BP) | 26 | 7.60E-12 |
| GO:0000236 | mitotic prometaphase (BP) | 18 | 3.95E-11 |
| GO:0001525 | angiogenesis (BP) | 21 | 5.42E-09 |
| GO:0008283 | cell proliferation (BP) | 27 | 1.17E-07 |
| GO:0007165 | signal transduction (BP) | 55 | 4.06E-06 |
| GO:0006468 | protein phosphorylation (BP) | 28 | 4.39E-06 |
| GO:0006915 | apoptotic process (BP) | 35 | 6.57E-06 |
| GO:0031581 | hemidesmosome assembly (BP) | 6 | 1.02E-05 |
| GO:0006355 | regulation of transcription, DNA-dependent (BP) | 66 | 1.42E-05 |
| GO:0045892 | negative regulation of transcription, DNA-dependent (BP) | 27 | 1.47E-05 |
| GO:0006260 | DNA replication (BP) | 16 | 1.50E-05 |
| Molecular function | |||
| GO:0005515 | protein binding (MF) | 230 | 2.76E-39 |
| GO:0005524 | ATP binding (MF) | 97 | 2.78E-21 |
| GO:0000166 | nucleotide binding (MF) | 116 | 8.85E-20 |
| GO:0046872 | metal ion binding (MF) | 108 | 1.38E-07 |
| GO:0003677 | DNA binding (MF) | 76 | 2.57E-07 |
| GO:0016787 | hydrolase activity (MF) | 49 | 1.16E-06 |
| GO:0042803 | protein homodimerization activity (MF) | 32 | 3.63E-06 |
| GO:0008270 | zinc ion binding (MF) | 76 | 5.26E-06 |
| GO:0019901 | protein kinase binding (MF) | 20 | 8.90E-06 |
| GO:0004674 | protein serine/threonine kinase activity (MF) | 24 | 8.46E-05 |
| GO:0042802 | identical protein binding (MF) | 20 | 2.76E-04 |
| GO:0008134 | transcription factor binding (MF) | 18 | 4.85E-04 |
| GO:0005509 | calcium ion binding (MF) | 32 | 5.00E-04 |
| GO:0005488 | binding (MF) | 34 | 5.10E-04 |
| GO:0004386 | helicase activity (MF) | 12 | 5.47E-04 |
Table 4. Top 3 KEGG pathways for differentially expressed genes in pancreatic adenocarcinoma.
| KEGG ID | KEGG term | Count | False discovery rate | Genes |
|---|---|---|---|---|
| hsa05200 | Pathways in cancer | 26 | 8.56E-07 | WNT10A, MET, STAT1, LAMA3, BCL2L1, CTNNA1, FZD6, TGFA, ERBB2, ITGA3, CASP8, E2F1, RAD51, RALB, PIAS3, FADD, RALBP1, LAMB3, TGFB2, PML, EGFR, CKS1B, LAMC2, RAC1, SLC2A1, CTNNB1 |
| hsa04110 | Cell cycle | 15 | 3.48E-06 | MCM4, CCNB2, CDC20, CCNA2, CCND2, E2F1, CCNB1, TFDP2, TGFB2, PLK1, BUB1B, PKMYT1, TTK, BUB1, MCM2 |
| hsa05212 | Pancreatic cancer | 11 | 1.10E-05 | STAT1, BCL2L1, TGFA, ERBB2, E2F1, RAD51, RALB, RALBP1, TGFB2, EGFR, RAC1 |
Fig 4. The tumor differentiation related gene enriched KEGG map of pancreatic adenocarcinoma.
The yellow colours represents the enriched genes in the pathway of pancreatic adenocarcinoma.
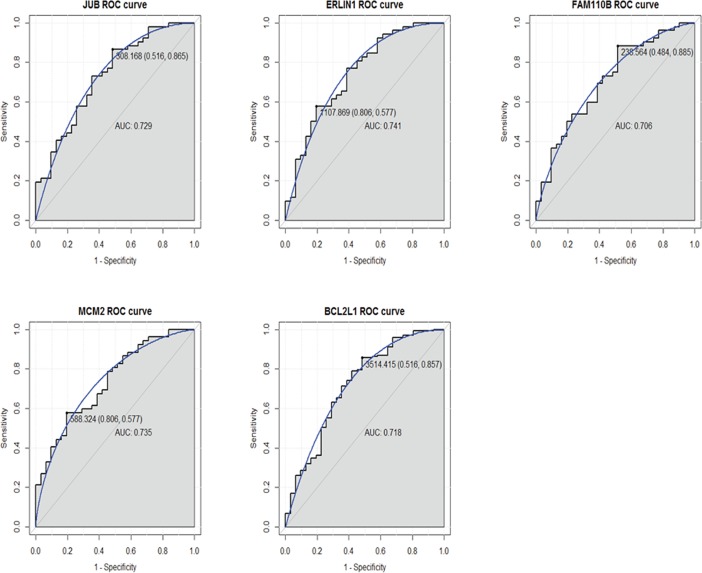
ROC curve analysis
We performed ROC curve analyses and calculated the AUC to assess the discriminatory ability of five tumor differentiation related differentially expressed genes in the TCGA dataset. The AUC of these genes including JUB (0.729), ERLIN1 (0.741), FAM110B (0.706), MCM2 (0.735) and BCL2L1 (0.718) was more than 0.7 (Fig 5). For pancreatic adenocarcinoma diagnosis, the 1-specificity (proportion of false positive) and sensitivity (proportion of true positive) of JUB was 51.6% and 86.5%, respectively; the 1- specificity and sensitivity and of ERLIN1 was 80.6% and 57.7%, respectively; the 1- specificity and sensitivity and of FAM110B was 48.4% and 88.5%, respectively; the 1- specificity and sensitivity and of MCM2 was 80.6% and 57.7%, respectively; the 1- specificity and sensitivity and of BCL2L1 was 51.6% and 85.7%, respectively.
Fig 5. ROC curves of selected differentially expressed genes between pancreatic adenocarcinoma and healthy controls.
The ROC curves were used to show the diagnostic ability of these selected differentially expressed genes with 1-specificity (the proportion of false positive) and sensitivity (the proportion of true positive). The x-axis shows 1-specificity and y-axis shows sensitivity.
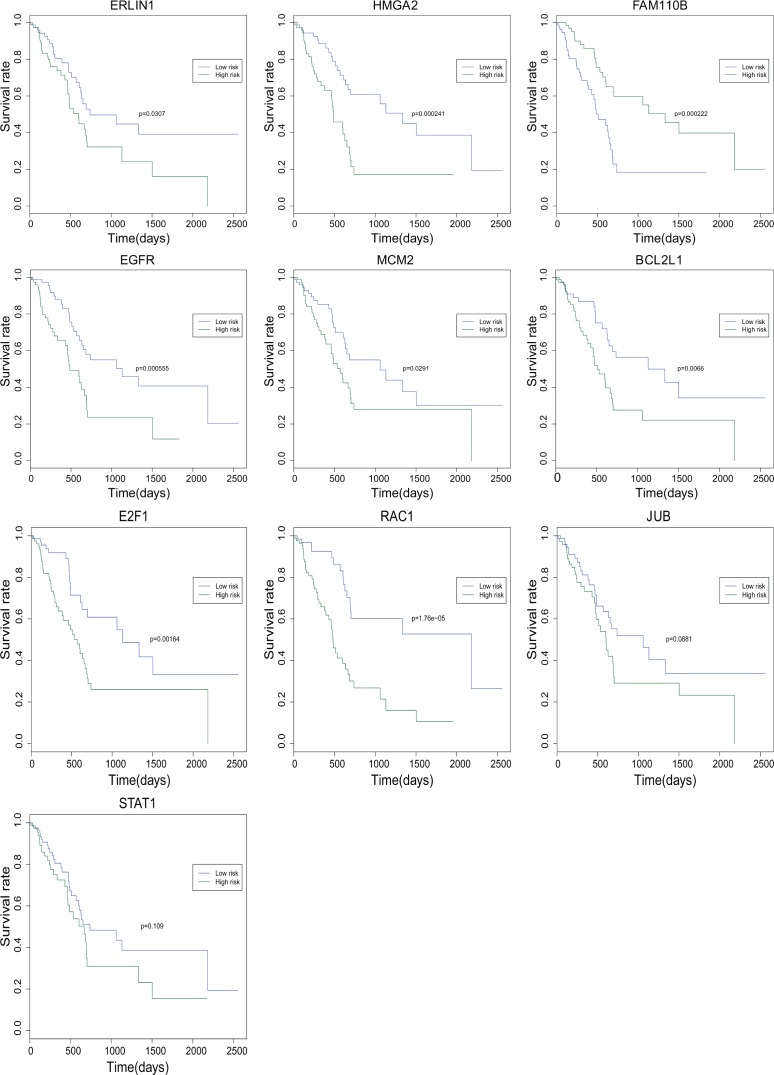
Survival prediction of tumor differentiation related differentially expressed genes
To analyze the potential prognostic characteristics of tumor differentiation related genes in pancreatic adenocarcinoma, 6 differentially expressed genes in all different tumor differentiation grades (JUB, ERLIN1, HMGA2, FAM110B, EGFR and MCM2) and four differentially expressed genes that enriched in pancreatic adenocarcinoma signaling pathway (BCL2L1, E2F1, RAC1 and STAT1) were analyzed using the R package (3.4.0 version, 2017). Finally, 8 differentially expressed genes (ERLIN1, HMGA2, FAM110B, EGFR, MCM2, BCL2L1, E2F1 and RAC1) were considered to be significantly negatively associated with survival (P < 0.05) time of pancreatic adenocarcinoma patients. However, JUB and STAT1 were not remarkably related to the survival time of pancreatic adenocarcinoma patients. The survival curves of above 10 genes were illustrated in Fig 6.
Fig 6. The survival curves of ten tumor differentiation-related genes in pancreatic adenocarcinoma.
The x-axis shows the survive time (days) and y-axis shows survival rate.
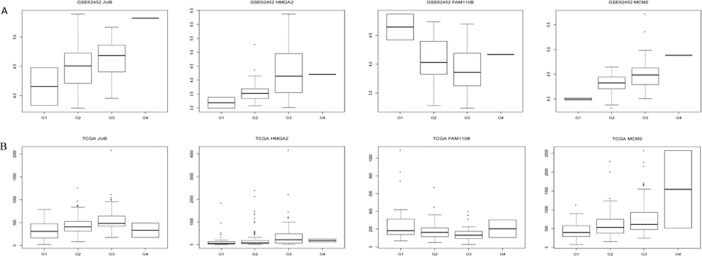
Electronic validation of tumor differentiation related genes in GEO database
Based on the sequencing data, four tumor differentiation related genes (JUB, HMGA2, FAM110B and MCM2) were selected to perform the expression validation by GEO database (Fig 7A). Different expression levels of these genes in G1, G2, G3 and G4 were analyzed and depicted through box-plots. The box-plots were displayed by median and inter-quartile range visually. The expression levels of JUB, HMGA2 and MCM2 were increased gradually along with the tumor differentiation grades. However, the expression level of FAM110B was decreased gradually along with the tumor differentiation grades. The expression trend of these genes in different tumor differentiation grades was consistent with the TCGA sequencing data (Fig 7B).
Fig 7. The validation of the expression levels of JUB, HMGA2, FAM110B and MCM2 in pancreatic adenocarcinoma based on GEO database.
The x-axis shows the case and normal groups and y-axis shows expression reads counts. (A) The expression validation in the GEO database (B): The expression in the TCGA sequencing data.
Discussion
Pancreatic adenocarcinoma is the fourth most common cause of cancer-related mortality [9]. It is worth mentioning that tumor differentiation is a universal process in the development of different cancers. Therefore, the objective of this study was to identify tumor differentiation related genes in pancreatic adenocarcinoma. In this study, we found 932 tumor differentiation related genes. After different expression analysis of each gene in diverse tumor differentiation grades, 8 genes were differentially expressed in all grades. GO and KEGG annotation in 744 differentially expressed genes (the intersection genes between G1 vs G2 and G1 vs G3/G4) revealed that pancreatic adenocarcinoma and cell cycle were significantly enriched pathways. ROC and survival prediction analysis identified several genes that had a diagnosis and prognosis value for pancreatic adenocarcinoma. In conclusion, 8 tumor differentiation related genes (JUB, ERLIN1, HMGA2, FAM110B, EGFR, MCM2, TCTA and SSTR1), pancreatic adenocarcinoma pathway related genes (BCL2L1, E2F1, RAC1 and STAT1) and cell cycle pathway related genes (BUB1, BUB1B, CCNA2, CCNB2, CCND2, CDC20, PLK1, TGFB2 and TTK) played fatal roles in the development of pancreatic adenocarcinoma.
Ajuba LIM protein (JUB, also called AJUBA) is a well-known cancer associated protein, which is involved in tumor invasion and migration [10]. It has been reported that the inactivation of JUB is implicated in deregulation of cell differentiation in head and neck squamous cell carcinomas [11]. Significantly, we found that JUB was differentially expressed in all tumor differentiation grades and the expression level increased gradually along with the tumor differentiation grades, which suggested the positive association with tumor differentiation of pancreatic adenocarcinoma. Furthermore, ROC analysis indicated that JUB had a diagnostic value for pancreatic adenocarcinoma patients.
ER lipid raft associated 1 (ERLIN1) encodes members of the prohibitin that defines lipid-raft-like domains of the endoplasmic reticulum [12]. It is reported that the expression of ERLIN1 is decreased in laser capture microdissection gastric carcinomas compared to histologic macrodissection [13]. Herein, we first found that the expression of ERLIN1 was positively related to tumor differentiation of pancreatic adenocarcinoma. In addition, ERLIN1 had a diagnosis value and associated with the survival time of pancreatic adenocarcinoma patients. Therefore, we concluded that ERLIN1 played crucial roles in tumor differentiation and could be a diagnosis and prognosis marker for pancreatic adenocarcinoma.
High mobility group AT-hook 2 (HMGA2) is a transcription factor primarily expressed in the mesenchyme and regulates mesenchymal proliferation and differentiation [14, 15]. It is demonstrated that immunoreactivity of HMGA2 is correlated to poor differentiation in pancreatic ductal adenocarcinoma [16]. Our study first found the different expression of HMGA2 in tumor differentiation of pancreatic adenocarcinoma. The expression of HMGA2 was increased along with the tumor differentiation grades. In addition, we also found that HMGA2 was significantly related to the survivability of pancreatic adenocarcinoma. Thus, we supposed that HMGA2 was a promising molecular target for pancreatic adenocarcinoma therapy and prognosis.
Cell mitosis is an important process in the cell cycle, which contributes to the biological development of cell differentiation. Family with sequence similarity 110 member B (FAM110B, also called C8orf72) accumulates at the spindle poles and centrosomes in mitosis and aberrant expression will influence cell cycle progression in G1 phase [17]. It is stated that FAM110B is a survival predictor of breast cancer stem cells [18]. Herein, we found that the expression of FAM110B was decreased along with the tumor differentiation grades, which showed that FAM110B was negatively associated with tumor differentiation and could be served as diagnosis and survival marker of pancreatic adenocarcinoma.
It is suggested that the activation of epidermal growth factor receptor (EGFR) affects cellular growth, proliferation and differentiation [19]. By contrast, the blockade of EGFR expression reduces the growth and metastatic potential of pancreatic tumor [20]. It is suggested that the major role of EGFR in pancreatic tumorigenesis is controlling the differentiation of neoplastic precursors [21]. Additionally, high expression of EGFR has been related to shorter survival of pancreatic adenocarcinoma [22]. Herein, we found that the expression of EGFR was associated with tumor differentiation and survivability of pancreatic adenocarcinoma, which was in line with previous reports.
Some studies have demonstrated that the minichromosome maintenance complex component 2 (MCM2) is not only the marker of cellular proliferation also required for cell cycle [23]. Moreover, it is reported that withdrawal of cells from the cell cycle into the differentiated state is accompanied by down-regulated expression of MCM2 [24, 25]. It is worth mentioning that it is up-regulation in primary pancreatic tumors compared to normal pancreas [26]. In this study, we found that MCM2 was increased along with the tumor differentiation grades, which further demonstrated the crucial role of MCM2 in cell differentiation of pancreatic adenocarcinoma. Significantly, we also found the diagnosis and prognosis value of MCM2 in pancreatic adenocarcinoma.
Generally, T-cell leukemia translocation altered (TCTA) mRNA is expressed in normal tissues, with high-level of expression in the kidney [27]. Of note, the expression of TCTA is reduced in three of four small cell lung cancer cell lines [28]. Meaningfully, we first found expression of TCTA in different tumor differentiation grades of pancreatic adenocarcinoma, and the expression was negatively related to tumor differentiation. Therefore, our data indicated that TCTA could be considered as the therapy target of pancreatic adenocarcinoma.
Somatostatin (SST), a small cyclic neurpeptide, has been applied for treating pancreatic adenocarcinoma in pre-clinical trials as adjuvants on account of their inhibitory effects on cell proliferation and growth hormone release [29, 30]. It is reported that gene transfer using somatostatin receptor 1 (SSTR1) inhibits the growth of pancreatic adenocarcinoma through cell cycle arrest in vivo and in vitro [2]. Our study found that SSTR1 was expressed in all grades of tumor differentiation of pancreatic adenocarcinoma, which further demonstrated the crucial role of SSTR1 in the development of pancreatic adenocarcinoma.
According to the KEGG pathway analysis, we found that several tumor differentiation related genes (such as BCL2L1, E2F1, RAC1 and STAT1) were remarkably enriched in pancreatic adenocarcinoma signaling pathway. Bcl-xL, BCL2 like 1 (BCL2L1) encoded protein expressed in various malignant tumors and involved in facilitating resistance to chemotherapy [31–33]. Additionally, it is over-expression in pancreatic tumor cells [34, 35]. It is suggested that the E2F transcription factor 1 (E2F1) is both an oncogenic inducer and a tumor suppressor [36, 37]. Moreover, a direct correlation between E2F1 and cell proliferation, as well as an inverse association between E2F1 and disease-associated survival has been observed in pancreatic adenocarcinoma [38]. In this study, we found the role of BCL2L1 and E2F1 in the process of pancreatic adenocarcinoma, which was in line with previous researches. Significantly, we also found that BCL2L1 had a diagnosis value for pancreatic adenocarcinoma.
Several studies report that ras-related C3 botulinum toxin substrate (RAC1) is involved in controlling cell cycle, growth and survival [39, 40]. It is worth mentioning that increased expression of RAC1 has been found in pancreatic adenocarcinoma and is correlated with patient prognosis [41, 42]. It is noted that signal transducer activator of transcription 1 (STAT1) is expressed in 88% of pancreatic adenocarcinoma tissue specimens, and the expression is inversely related to tumor differentiation of pancreatic adenocarcinoma [43]. Additionally, patients with high STAT1 have a better prognosis compared to those with low expression [44]. Herein, we found that BCL2L1, E2F1, RAC1 and STAT1 were expressed in pancreatic adenocarcinoma, which was consistent with previous reports. Significantly, we also found the roles of BCL2L1, E2F1 and RAC1 in tumor differentiation and survival prediction of pancreatic adenocarcinoma.
Alteration in cell cycle regulatory mechanisms plays a vital role in the tumor development. Besides pancreatic cancer, cell cycle was the common biological function of differentially expressed genes in GO and KEGG, which involved several genes including BUB1, BUB1B, CCNA2, CCNB2, CCND2, CDC20, PLK1, TGFB2 and TTK. It is found that BUB1 is a remarkably altered gene in CD4+ peripheral blood cells of pancreatic adenocarcinoma patients compared with healthy volunteers [45]. BUB1B is associated with DNA repair and known to drive the development of cancer [46, 47]. It is reported that deleterious variant in BUB1B gene is more frequent in patients with familial pancreatic adenocarcinoma [48]. CCNA2 has been demonstrated up-regulated in pancreatic adenocarcinoma tissue samples and significantly involved in the cell cycle pathway [49]. Nakamura T and Sato N et al found that CCNB2 was also up-regulated in human pancreatic adenocarcinoma [50, 51]. CCND2 plays an important role in the proliferation of pancreatic islet b-cell and the mRNA expression of CCND2 is rarely detected in pancreatic adenocarcinoma cell lines [52–54]. It has been found that CCND2 is hypermethylated in the progression of pancreatic adenocarcinoma [54]. It is reported that CDC20 is up-regulated in pancreatic adenocarcinoma cells [55]. Deregulation of PLK1 occurred early in carcinogenesis and over-expression in pancreatic intraepithelial neoplasia III lesions of pancreatic adenocarcinoma patients [56]. It is noted that PLK1 pathway is a potential therapeutic target of pancreatic adenocarcinoma [57]. TGFB2 is found to be over-expressed in pancreatic adenocarcinoma [58, 59]. TTK is also over-expressed in pancreatic adenocarcinoma and plays a crucial role in maintaining the viability and proliferative potential of pancreatic adenocarcinoma cells [60]. Thus it can be seen that these genes were associated with the development of pancreatic adenocarcinoma and may play an important role in the cell cycle of pancreatic adenocarcinoma differentiation.
Conclusions
Our study provided the molecular clues in understanding the pathological mechanism of pancreatic adenocarcinoma, especially in tumor differentiation of pancreatic adenocarcinoma. Additionally, we identified several diagnosis and survivability-related differentially expressed genes, which may be regarded as diagnosis and prognosis markers in the development of pancreatic adenocarcinoma. Of course, there were limitations to our study. Firstly, we aimed to study the biological function of differentially expressed genes between highly differentiated group (G1) and other differentiation groups (G2 and G3/G4). Anyway, it is necessary to study the biological function of genes in non-intersection set (G2 vs G3/G4). Therefore, the biological function of those genes in non-intersection set is needed in our further research. Secondly, some in vivo and in vitro experiments are essential for elucidation of the biological roles of tumor differentiation related differentially expressed genes in pancreatic adenocarcinoma in the future work.
Data Availability
All the data are available to other researchers and have been presented in the reported study.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Prasad S. DeVita, Hellman, and Rosenberg's Cancer: Principles & Practice of Oncology: Tenth Edition. 2015. [Google Scholar]
- 2.Li M, Wang X, Li W, Li F, Yang H, Wang H, et al. Somatostatin receptor-1 induces cell cycle arrest and inhibits tumor growth in pancreatic cancer. Cancer science. 2008;99(11):2218–23. Epub 2008/10/01. doi: 10.1111/j.1349-7006.2008.00940.x ; PubMed Central PMCID: PMCPmc2930023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan JJ, Yang M. The role of epithelial-mesenchymal transition in pancreatic cancer. Journal of Gastrointestinal Oncology. 2011;2(3):151–6. doi: 10.3978/j.issn.2078-6891.2011.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar FH, Li Y, Wang Z, Kong D. Pancreatic cancer stem cells and EMT in drug resistance and metastasis. Minerva chirurgica. 2009;64(5):489–500. Epub 2009/10/28. ; PubMed Central PMCID: PMCPmc2878773. [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63(1):11–30. Epub 2013/01/22. doi: 10.3322/caac.21166 . [DOI] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65(1):5–29. Epub 2015/01/07. doi: 10.3322/caac.21254 . [DOI] [PubMed] [Google Scholar]
- 7.Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP: Journal of the pancreas. 2008;9(2):99–132. Epub 2008/03/11. . [PubMed] [Google Scholar]
- 8.Ochs C, Perl Y, Halper M, Geller J, Lomax J. Quality assurance of the gene ontology using abstraction networks. Journal of bioinformatics and computational biology. 2016;14(3):1642001 Epub 2016/06/16. doi: 10.1142/S0219720016420014 . [DOI] [PubMed] [Google Scholar]
- 9.Oncology TL. Pancreatic cancer in the spotlight. Lancet Oncology. 2014;15(3):586–600. [DOI] [PubMed] [Google Scholar]
- 10.Nola S, Daigaku R, Smolarczyk K, Carstens M, Martin-Martin B, Longmore G, et al. Ajuba is required for Rac activation and maintenance of E-cadherin adhesion. The Journal of cell biology. 2011;195(5):855–71. Epub 2011/11/23. doi: 10.1083/jcb.201107162 ; PubMed Central PMCID: PMCPmc3257575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrence MS, Sougnez C, Lichtenstein L, Cibulskis K, Lander E, Gabriel SB, et al. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–82. Epub 2015/01/30. doi: 10.1038/nature14129 ; PubMed Central PMCID: PMCPmc4311405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browman DT, Resek ME, Zajchowski LD, Robbins SM. Erlin-1 and erlin-2 are novel members of the prohibitin family of proteins that define lipid-raft-like domains of the ER. Journal of cell science. 2006;119(Pt 15):3149–60. Epub 2006/07/13. doi: 10.1242/jcs.03060 . [DOI] [PubMed] [Google Scholar]
- 13.Kim HK, Kim J, Korolevich S, Choi IJ, Kim CH, Munroe DJ, et al. Distinctions in gastric cancer gene expression signatures derived from laser capture microdissection versus histologic macrodissection. BMC medical genomics. 2011;4:48 Epub 2011/06/04. doi: 10.1186/1755-8794-4-48 ; PubMed Central PMCID: PMCPmc3141377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashar HR, Fejzo MS, Tkachenko A, Zhou X, Fletcher JA, Weremowicz S, et al. Disruption of the architectural factor HMGI-C: DNA-binding AT hook motifs fused in lipomas to distinct transcriptional regulatory domains. Cell. 1995;82(1):57–65. Epub 1995/07/14. . [DOI] [PubMed] [Google Scholar]
- 15.Anand A, Chada K. In vivo modulation of Hmgic reduces obesity. Nature genetics. 2000;24(4):377–80. Epub 2000/03/31. doi: 10.1038/74207 . [DOI] [PubMed] [Google Scholar]
- 16.Hristov AC, Cope L, Reyes MD, Singh M, Iacobuzio-Donahue C, Maitra A, et al. HMGA2 protein expression correlates with lymph node metastasis and increased tumor grade in pancreatic ductal adenocarcinoma. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2009;22(1):43–9. Epub 2008/10/10. doi: 10.1038/modpathol.2008.140 ; PubMed Central PMCID: PMCPmc2769577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauge H, Patzke S, Aasheim HC. Characterization of the FAM110 gene family. Genomics. 2007;90(1):14–27. Epub 2007/05/15. doi: 10.1016/j.ygeno.2007.03.002 . [DOI] [PubMed] [Google Scholar]
- 18.Yin ZQ, Liu JJ, Xu YC, Yu J, Ding GH, Yang F, et al. A 41-gene signature derived from breast cancer stem cells as a predictor of survival. Journal of experimental & clinical cancer research: CR. 2014;33:49 Epub 2014/06/08. doi: 10.1186/1756-9966-33-49 ; PubMed Central PMCID: PMCPmc4229870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SJ, Gu MJ, Lee DS, Yun SS, Kim HJ, Choi JH. EGFR expression in pancreatic intraepithelial neoplasia and ductal adenocarcinoma. International journal of clinical and experimental pathology. 2015;8(7):8298–304. Epub 2015/09/05. ; PubMed Central PMCID: PMCPmc4555728. [PMC free article] [PubMed] [Google Scholar]
- 20.Bruns CJ, Harbison MT, Davis DW, Portera CA, Tsan R, McConkey DJ, et al. Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clinical cancer research: an official journal of the American Association for Cancer Research. 2000;6(5):1936–48. Epub 2000/05/18. . [PubMed] [Google Scholar]
- 21.Ardito CM, Gruner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer cell. 2012;22(3):304–17. Epub 2012/09/15. doi: 10.1016/j.ccr.2012.07.024 ; PubMed Central PMCID: PMCPmc3443395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamanaka Y, Friess H, Kobrin MS, Buchler M, Beger HG, Korc M. Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer research. 1993;13(3):565–9. Epub 1993/05/01. . [PubMed] [Google Scholar]
- 23.Lei M, Tye BK. Initiating DNA synthesis: from recruiting to activating the MCM complex. Journal of cell science. 2001;114(Pt 8):1447–54. Epub 2001/04/03. . [DOI] [PubMed] [Google Scholar]
- 24.Shetty A, Loddo M, Fanshawe T, Prevost AT, Sainsbury R, Williams GH, et al. DNA replication licensing and cell cycle kinetics of normal and neoplastic breast. British journal of cancer. 2005;93(11):1295–300. Epub 2005/11/10. doi: 10.1038/sj.bjc.6602829 ; PubMed Central PMCID: PMCPmc2361513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoeber K, Tlsty TD, Happerfield L, Thomas GA, Romanov S, Bobrow L, et al. DNA replication licensing and human cell proliferation. Journal of cell science. 2001;114(Pt 11):2027–41. Epub 2001/08/09. . [DOI] [PubMed] [Google Scholar]
- 26.Thakur A, Bollig A, Wu J, Liao DJ. Gene expression profiles in primary pancreatic tumors and metastatic lesions of Ela-c-myc transgenic mice. Molecular cancer. 2008;7:11 Epub 2008/01/26. doi: 10.1186/1476-4598-7-11 ; PubMed Central PMCID: PMCPmc2259361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanke Y, Yago T, Kobashigawa T, Kawamoto M, Yamanaka H, Kotake S. A novel peptide from TCTA protein inhibits proliferation of fibroblast-like synoviocytes of rheumatoid arthritis patients. Central-European journal of immunology. 2014;39(4):468–70. Epub 2014/01/01. doi: 10.5114/ceji.2014.47730 ; PubMed Central PMCID: PMCPmc4439957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aplan PD, Johnson BE, Russell E, Chervinsky DS, Kirsch IR. Cloning and characterization of TCTA, a gene located at the site of a t(1;3) translocation. Cancer research. 1995;55(9):1917–21. Epub 1995/05/01. . [PubMed] [Google Scholar]
- 29.Bousquet C, Puente E, Buscail L, Vaysse N, Susini C. Antiproliferative effect of somatostatin and analogs. Chemotherapy. 2001;47 Suppl 2:30–9. Epub 2001/03/29. 49159. doi: 10.1159/000049159 . [DOI] [PubMed] [Google Scholar]
- 30.Hejna M, Schmidinger M, Raderer M. The clinical role of somatostatin analogues as antineoplastic agents: much ado about nothing? Annals of oncology: official journal of the European Society for Medical Oncology. 2002;13(5):653–68. Epub 2002/06/22. . [DOI] [PubMed] [Google Scholar]
- 31.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science (New York, NY). 1998;281(5381):1322–6. Epub 1998/09/12. . [DOI] [PubMed] [Google Scholar]
- 32.Walczak H, Bouchon A, Stahl H, Krammer PH. Tumor necrosis factor-related apoptosis-inducing ligand retains its apoptosis-inducing capacity on Bcl-2- or Bcl-xL-overexpressing chemotherapy-resistant tumor cells. Cancer research. 2000;60(11):3051–7. Epub 2000/06/13. . [PubMed] [Google Scholar]
- 33.Liu R, Page C, Beidler DR, Wicha MS, Nunez G. Overexpression of Bcl-x(L) promotes chemotherapy resistance of mammary tumors in a syngeneic mouse model. The American journal of pathology. 1999;155(6):1861–7. Epub 1999/12/14. doi: 10.1016/S0002-9440(10)65505-8 ; PubMed Central PMCID: PMCPmc1866947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trauzold A, Roder C, Sipos B, Karsten K, Arlt A, Jiang P, et al. CD95 and TRAF2 promote invasiveness of pancreatic cancer cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19(6):620–2. Epub 2005/01/27. doi: 10.1096/fj.04-2984fje . [DOI] [PubMed] [Google Scholar]
- 35.Hinz S, Trauzold A, Boenicke L, Sandberg C, Beckmann S, Bayer E, et al. Bcl-XL protects pancreatic adenocarcinoma cells against CD95- and TRAIL-receptor-mediated apoptosis. Oncogene. 2000;19(48):5477–86. Epub 2000/12/15. doi: 10.1038/sj.onc.1203936 . [DOI] [PubMed] [Google Scholar]
- 36.Johnson DG. The paradox of E2F1: oncogene and tumor suppressor gene. Molecular carcinogenesis. 2000;27(3):151–7. Epub 2000/03/09. . [DOI] [PubMed] [Google Scholar]
- 37.La Thangue NB. The yin and yang of E2F-1: balancing life and death. Nature cell biology. 2003;5(7):587–9. Epub 2003/07/02. doi: 10.1038/ncb0703-587 . [DOI] [PubMed] [Google Scholar]
- 38.Yamazaki K, Yajima T, Nagao T, Shinkawa H, Kondo F, Hanami K, et al. Expression of transcription factor E2F-1 in pancreatic ductal carcinoma: an immunohistochemical study. Pathology, research and practice. 2003;199(1):23–8. Epub 2003/03/26. doi: 10.1078/0344-0338-00348 . [DOI] [PubMed] [Google Scholar]
- 39.Chiariello M, Marinissen MJ, Gutkind JS. Regulation of c-myc expression by PDGF through Rho GTPases. Nature cell biology. 2001;3(6):580–6. Epub 2001/06/05. doi: 10.1038/35078555 . [DOI] [PubMed] [Google Scholar]
- 40.Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science (New York, NY). 1995;269(5228):1270–2. Epub 1995/09/01. . [DOI] [PubMed] [Google Scholar]
- 41.Baron A. Gene expression profiles of pancreatic cancer and stromal desmoplasia. Oncogene. 2001;20(50):7437–46. doi: 10.1038/sj.onc.1204935 [DOI] [PubMed] [Google Scholar]
- 42.Wang P, Chen L, Zhang J, Chen H, Fan J, Wang K, et al. Methylation-mediated silencing of the miR-124 genes facilitates pancreatic cancer progression and metastasis by targeting Rac1. Oncogene. 2014;33(4):514–24. Epub 2013/01/22. doi: 10.1038/onc.2012.598 . [DOI] [PubMed] [Google Scholar]
- 43.Sun Y, Yang S, Sun N, Chen J. Differential expression of STAT1 and p21 proteins predicts pancreatic cancer progression and prognosis. Pancreas. 2014;43(4):619–23. Epub 2014/03/25. doi: 10.1097/MPA.0000000000000074 . [DOI] [PubMed] [Google Scholar]
- 44.Widschwendter A, Tonko-Geymayer S, Welte T, Daxenbichler G, Marth C, Doppler W. Prognostic significance of signal transducer and activator of transcription 1 activation in breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2002;8(10):3065–74. Epub 2002/10/11. . [PubMed] [Google Scholar]
- 45.Komura T, Sakai Y, Harada K, Kawaguchi K, Takabatake H, Kitagawa H, et al. Inflammatory features of pancreatic cancer highlighted by monocytes/macrophages and CD4+ T cells with clinical impact. Cancer science. 2015;106(6):672–86. Epub 2015/04/02. doi: 10.1111/cas.12663 ; PubMed Central PMCID: PMCPmc4471781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruark E, Snape K, Humburg P, Loveday C, Bajrami I, Brough R, et al. Mosaic PPM1D mutations are associated with predisposition to breast and ovarian cancer. Nature. 2013;493(7432):406–10. Epub 2012/12/18. doi: 10.1038/nature11725 ; PubMed Central PMCID: PMCPmc3759028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., Kinzler KW. Cancer genome landscapes. Science (New York, NY). 2013;339(6127):1546–58. Epub 2013/03/30. doi: 10.1126/science.1235122 ; PubMed Central PMCID: PMCPmc3749880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts NJ, Norris AL, Petersen GM, Bondy ML, Brand R, Gallinger S, et al. Whole Genome Sequencing Defines the Genetic Heterogeneity of Familial Pancreatic Cancer. Cancer discovery. 2016;6(2):166–75. Epub 2015/12/15. doi: 10.1158/2159-8290.CD-15-0402 ; PubMed Central PMCID: PMCPmc4744563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long J, Liu Z, Wu X, Xu Y, Ge C. Screening for genes and subnetworks associated with pancreatic cancer based on the gene expression profile. Molecular medicine reports. 2016;13(5):3779–86. Epub 2016/04/02. doi: 10.3892/mmr.2016.5007 ; PubMed Central PMCID: PMCPmc4838159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura T, Fidler IJ, Coombes KR. Gene expression profile of metastatic human pancreatic cancer cells depends on the organ microenvironment. Cancer research. 2007;67(1):139–48. Epub 2007/01/11. doi: 10.1158/0008-5472.CAN-06-2563 . [DOI] [PubMed] [Google Scholar]
- 51.Sato N, Fukushima N, Maitra A, Iacobuzio-Donahue CA, van Heek NT, Cameron JL, et al. Gene expression profiling identifies genes associated with invasive intraductal papillary mucinous neoplasms of the pancreas. The American journal of pathology. 2004;164(3):903–14. Epub 2004/02/26. doi: 10.1016/S0002-9440(10)63178-1 ; PubMed Central PMCID: PMCPmc1613263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. The Journal of clinical investigation. 2004;114(7):963–8. Epub 2004/10/07. doi: 10.1172/JCI22098 ; PubMed Central PMCID: PMCPmc518666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ebert MP, Hernberg S, Fei G, Sokolowski A, Schulz HU, Lippert H, et al. Induction and expression of cyclin D3 in human pancreatic cancer. Journal of cancer research and clinical oncology. 2001;127(7):449–54. Epub 2001/07/27. . [DOI] [PubMed] [Google Scholar]
- 54.Matsubayashi H, Sato N, Fukushima N, Yeo CJ, Walter KM, Brune K, et al. Methylation of cyclin D2 is observed frequently in pancreatic cancer but is also an age-related phenomenon in gastrointestinal tissues. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9(4):1446–52. Epub 2003/04/10. . [PubMed] [Google Scholar]
- 55.Ouellet V, Guyot MC, Le Page C, Filali-Mouhim A, Lussier C, Tonin PN, et al. Tissue array analysis of expression microarray candidates identifies markers associated with tumor grade and outcome in serous epithelial ovarian cancer. International journal of cancer. 2006;119(3):599–607. Epub 2006/03/31. doi: 10.1002/ijc.21902 . [DOI] [PubMed] [Google Scholar]
- 56.Weichert W, Schmidt M, Jacob J, Gekeler V, Langrehr J, Neuhaus P, et al. Overexpression of Polo-like kinase 1 is a common and early event in pancreatic cancer. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al. ]. 2005;5(2–3):259–65. Epub 2005/04/28. doi: 10.1159/000085280 . [DOI] [PubMed] [Google Scholar]
- 57.Gray PJ Jr., Bearss DJ, Nagle R, Tsao MS, Dean N, et al. Identification of human polo-like kinase 1 as a potential therapeutic target in pancreatic cancer. Molecular cancer therapeutics. 2004;3(5):641–6. Epub 2004/05/14. . [PubMed] [Google Scholar]
- 58.Friess H, Yamanaka Y, Büchler M, Ebert M, Beger HG, Gold LI, et al. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993;105(6):1846–56. [DOI] [PubMed] [Google Scholar]
- 59.Satoh K, Shimosegawa T, Hirota M, Koizumi M, Toyota T. Expression of transforming growth factor beta1 (TGFbeta1) and its receptors in pancreatic duct cell carcinoma and in chronic pancreatitis. Pancreas. 1998;16(4):468 [DOI] [PubMed] [Google Scholar]
- 60.Kaistha BP, Honstein T, Muller V, Bielak S, Sauer M, Kreider R, et al. Key role of dual specificity kinase TTK in proliferation and survival of pancreatic cancer cells. British journal of cancer. 2014;111(9):1780–7. Epub 2014/08/20. doi: 10.1038/bjc.2014.460 ; PubMed Central PMCID: PMCPmc4453723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are available to other researchers and have been presented in the reported study.