Abstract
Encapsulation of primary bovine adrenocortical cells in alginate is an efficacious model of a bioartificial adrenal cortex. Such a bioartificial adrenal cortex can be used for the restoration of lost adrenal function in vivo as well as for in vitro modeling of the adrenal microenvironment and for investigation of cell–cell interactions in the adrenals. The aim of this work was the optimization of a bioartificial adrenal cortex, that is the generation of a highly productive, self-regenerating, long-term functioning and immune tolerant bioartificial organ. To achieve this, it is necessary that adrenocortical stem and progenitor cells are present in the bioartificial gland, as these undifferentiated cells play important roles in the function of the mature gland. Here, we verified the presence of adrenocortical progenitors in cultures of bovine adrenocortical cells, studied the dynamics of their appearance and growth and determined the optimal time point for cell encapsulation. These procedures increased the functional life span and reduced the immunogenicity of the bioartificial adrenal cortex. This model allows the use of the luteinizing hormone-releasing hormone (LHRH) agonist triptorelin, the neuropeptide bombesin, and retinoic acid to alter cell number and the release of cortisol over long periods of time.
Introduction
Adrenal insufficiency is a life-threatening disorder that requires a complex and permanent hormone replacement. Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency is the most common form of adrenal insufficiency. Current treatment options with glucocorticoid substitution can only partially reverse the symptoms and exhibit unpleasant side effects [1]. Despite different treatment regimens, CAH remains a major therapeutic challenge sometimes requiring drastic therapeutic procedures such as bilateral adrenalectomy [2]. Transplantation of adrenal cells may be a feasible therapeutic alternative for those patients. However, this strategy is critically limited by a persistent lack of human donor organs and the requirement of chronic immune suppression.
Those problems could be solved by the transplantation of immunoisolated xenogeneic adrenal cells. Recently, we succeeded in creating a bioartificial adrenal cortex—a transplant, developed on the base of 3D xenogeneic primary Bovine Adrenocortical Cell (BAC) cultures, embedded in alginate [3]. This improved both the capacity of the adrenal cells for stable, long-term basal cortisol release as well as the response to stimulation with pituitary adrenocorticotropic hormone (ACTH). In addition, we successfully transplanted these bioartificial adrenal cortices intraperitoneally and subcutaneously into rats for a short period of time (approx. 3 weeks) within an implantable medical device without the use of immunosuppressive drugs [3].
Here, we improved this bioartificial adrenal cortex system by generating a long-term functioning, highly productive bioartificial organ that aims to induce minimal immunological responses. In this system, BACs were expanded in vitro in monolayer cultures for 1–7 days and then embedded in an alginate matrix for 3D growth for additional 114 days.
Because most BACs are mature cells with limited life span, it is essential that the cells in the bioartificial adrenal cortex include a population of adrenocortical progenitors. Previous reports showed the presence of adrenocortical progenitor cells in the adult rat adrenal cortex [4], suggesting that appropriate modeling of this organ should include immature cell populations. The presence of a side population of cells with stem and progenitor properties has also been demonstrated in mice [5, 6]. Experiments with transplantation of primary adrenocortical cells indirectly support the existence of adrenocortical progenitors in primary cultures of adrenocortical cells [5]. However, to our knowledge, no direct evidence of the presence of adrenocortical progenitor cells in primary cultures of bovine adrenocortical cells has been demonstrated.
Recently, the Sonic Hedgehog (Shh) signaling pathway was demonstrated to play an important role in the development of the adrenal cortex and to represent a population of undifferentiated cells [4, 7]. Shh-expressing cells are long-lived nonsteroidogenic progenitors of all steroidogenic cell types [8]. Additionally, these cells display a relatively high level of proliferation [4] and could replace apoptotic mature steroidogenic cells, thereby increasing the functional life span of the bioartificial adrenal cortex. Therefore, we investigated the expression of a transcription factor induced by Hh signaling, GLI1 and its receptor Patched1 in our BAC cultures.
DAX1 is critical for maintenance of adrenocortical progenitor cells, as previously reported by other labs [9]. Therefore we employed DAX1 as another marker of adrenocortical progenitors.
Nestin was originally identified as a marker of neural progenitor cells [10, 11]. However, later it was also shown to identify stem/progenitor cells in various tissues, including muscle, umbilical cord blood, testis, odontoblasts, hair follicle sheath, liver, and kidney [12]. Nestin-positive cells are also present in the human and murine adrenal cortex [13–15]. We have previously shown that Nestin-positive progenitor cells reside in the murine adult adrenal medulla and respond to stress by differentiation [16]. Another study revealed that Nestin-positive cells isolated from pancreatic islets are multipotent andable to differentiate into endocrine, exocrine and hepatic phenotypes [17]. In this research we reveal that Nestin-expressing cells originating from the adrenal cortex are sensitive to stimulation with ACTH. These observations suggest Nestin as an alternative progenitor marker.
Our concept is to identify adrenocortical stem and progenitor cells in the BAC culture, to determine the optimal time point for the onset of the 3D culture phase, when the cell culture has the maximal amount of progenitors, and to balance the volume of progenitors with the functionality of the bioartificial adrenal with the help of pharmacological agents. All these measures were designed to improve the outcome of adrenal replacement by transplantation of a bioartificial adrenal cortex.
Materials and methods
Cell preparation and culture
Adrenocortical cells were isolated from bovine adrenal glands shortly after slaughtering of 1–3 years old cattle as previously described [3]. Briefly, adrenal glands were transported to the laboratory in ice-cold Euro Collins Solution supplemented with 1% (vol/vol) penicillin-streptomycin solution (Thermo Fisher Scientific). The glands were then liberated from fat and connective tissue and rinsed several times with PBS through the central vein to remove remaining blood. Afterwards a longitudinal incision was made to cut the adrenals in halves, the medulla was removed and the cortex was scraped off the capsule and cut in small pieces. Adrenal capsule was discarded. Adrenal cortex was digested for 50 min in Dulbecco’s modified Eagle’s /Ham’s F12 (DMEM/F12) medium (Thermo Fisher Scientific), containing 2 mg/ml collagenase and 0.1 mg/ml DNase (both from Sigma-Aldrich) at 37°C while shaking. Obtained cells were washed with cultivation medium, pelleted by centrifugation (8 min, 300 x g) and filtered through 100-μm cell strainers (Becton Dickinson). After that, primary adrenocortical cells were placed in cell culture flasks (Thermo Fisher Scientific) and cultivated at 37°C in a humidified atmosphere (95% air, 5% CO2) in DMEM/F12 medium with 10% (vol/vol) FBS, 10% (vol/vol) horse serum (both from Thermo Fisher Scientific), 0.1 ng/ml recombinant FGF-2 (PromoCell GmbH) and 1% (vol/vol) penicillin-streptomycin solution. Medium change was performed every 2–3 days. Those cells that were cultivated for 7 days were stimulated with 3 ng/ml ACTH1-24 (Synacthen, Sigma-tau Arzneimittel GmbH) for 24 h on the 5-6th day of cultivation.
Cell encapsulation
Encapsulation of bovine adrenocortical cells in alginate was performed as previously described [3]. Briefly, the cells were dissociated from the culture flasks by trypsinization (TripLE, Thermo Fisher Scientific), pelleted by centrifugation and gently mixed with 3.5% (wt/vol) sterile alginate (UP-MVG, Novamatrix) dissolved in Custodiol-HTK solution (H.S. Pharma). Then 30 μl of the mixture of cells and alginate was placed on a glass plate and cross-linked with 70 mM strontium chloride containing 20 mM Hepes. Each bioartificial adrenal cortex contained 2 x 105 cells. During the cultivation period, the bioartificial adrenal cortices were stimulated with 3 ng/ml ACTH once a week for 24 h. Medium change was performed every 2–3 days.
Steroid release and measurement
To reveal how ACTH stimulation affects relative gene expressions, isolated BACs were seeded in 6 well plates. For stimulation of cortisol production one half of the cells was incubated in cultivation medium containing 3 ng/ml ACTH1-24 for 24 h on day 1, 7 and 10 following the isolation procedure. The remaining cells were cultivated in standard cell culture medium. RNA was collected from both groups of cells at the indicated time points, and the influence of ACTH stimulation on the expression of progenitor markers was measured on day 1, 7 and 10 after cell isolation.
Basal cortisol and aldosterone was measured in the supernatants of cell culture after 24 h of cultivation. For stimulation of cortisol production the cells were incubated in cultivation medium containing 3 ng/ml ACTH1-24 for 24 h. The concentration of cortisol or aldosterone in cell culture supernatants was detected by cortisol ELISA (IBL). Stimulation index was calculated by division of ACTH stimulated cortisol by basal cortisol.
Reverse-transcription and real-time PCR
Total RNA from bovine adrenocortical cells was isolated using the RNeasy Mini or Micro kit (Qiagen) where appropriate. DNA was eliminated using gDNA eliminator columns during RNA preparation according to the manufacturer’s protocol. For reverse transcription, up to 1μg of total RNA was converted to first-strand cDNA using M-MLV reverse transcriptase, reaction buffer, RNase inhibitor, dNTP mix and oligo(dT) 15/random hexamer primer according to the manufacturer’s instructions (Promega). Primers were designed by Primer-BLAST–NCBI software to span at least one intron to prevent nonspecific amplification of DNA remnants.
Real-time PCR was performed using SYBR green (Qiagen) and a Roche Light Cycler 1.5 (Roche). To normalize data, the RPS9 gene was used as an internal control gene. Evaluation of different housekeeping genes in our laboratory (GAPDH, β-actin, TBP) revealed that RPS9 is the most stable gene in our system. Typical genes used as internal controls (GAPDH and β-actin) increased their expression in cultured cells in response to traumatization compared to freshly isolated cells. These data corresponds with previously published results [18].Primers used are presented in Table 1.
Table 1. List of primers and amplification conditions for RT-PCR.
| Gene | Primer sequence | Annealing temperature, °C | Product size (base pairs) |
|---|---|---|---|
| RPS9 | F: CGGAACAAACGTGAGGTCT | 60–65 | 126 |
| R: CGCAACAGGGCATTACCTTC | |||
| Patched1 | F: GCTGCGAGCGAAGTTTCAAA | 60 | 174 |
| R: ACTCGTCCACCAACTTCCAC | |||
| GLI1 | F: AGACTCCAGCTCTGGACCG | 63 | 188 |
| R: GACCTGGCAGTCCTTCTGTC | |||
| Nestin | F: CCCTGGAGCAGGAGAAACAA | 60 | 128 |
| R: AGCCTCTAGGAGGGTCCTGT | |||
| IL1β | F: CTGCAGCTGGAGGAAGTAGAC | 65 | 277 |
| R: GCCAGTCCTCGGGGTTATTC | |||
| IL6 | F: ACGAAAGAGAGCTCCATCTGC | 63 | 71 |
| R: AATGGAGTGAAGGCGCTTGT | |||
| SF1 | F: GCTACGCCGCTGGACTTC | 60 | 388 |
| R: CACGTGTTGCTGGAGGTTTG | |||
| CYP17A1 | F: GGGGACATCTTCGGGGCTGG | 60 | 497 |
| R: CTCTGCAGCAGCCGGGACAT | |||
| DAX1 | F: TACTCTTCAACCCGGACCTGC | 60 | 190 |
| R: AACAGTTCAGCCAGGGTGTT |
F: forward, R: reverse
Immunofluorescence staining and microscopy
FDA/PI staining
Dual fluorescein diacetate and propidium iodide (FDA/PI) staining was applied to define the viability of the BACs. Stained cells were examined under the microscope (Axioplan, Carl Zeiss). Analysis was performed using Axiovision Software (Carl Zeiss). Viability was defined by the quantification of live (green) and dead (red) cells on microphotographs. To quantify cell number in bioartificial adrenals, the cells from six micrographs for each condition were counted using ImageJ 1.44 software. All images have been treated equally.
CYP11B1 staining
BACs were washed with PBS and fixed in 4% PFA in PBS for 30 min. After additional washing with PBS the cells were incubated in blocking solution (3% BSA, 0.1% Triton X-100 in PBS for 30 min at room temperature). Then the cells were washed with PBS and incubated with the primary antibody (CYP11B1, Santa Cruz Biotechnology Inc) overnight at 4°C, washed with PBS and incubated for 1 h in secondary antibody (donkey anti-goat IgG-FITC, Santa Cruz Biotechnology Inc) (both antibodies were diluted 1:100).
Experiments with triptorelin, bombesin and retinoic acid
The luteinizing hormone-releasing hormone (LHRH) agonist triptorelin (decapeptyl, [3]LHRH) was provided by one of us (AVS). Triptorelin was dissolved in DMSO and then diluted in culture medium to a concentration of 10μM. Cell culture medium, containing DMSO in a concentration 0.1% (vol/vol) was used as a control. Synthetic bombesin (Bachem) was dissolved in 0.9% NaCl and then diluted in culture medium to a concentration 0.5 μg/ml. Retinoic acid (Sigma-Aldrich) was dissolved in absolute ethanol (VWR International S.A.S) and then diluted in culture medium to a final concentration of 5 μM. Cell culture medium containing 5x10-4% (vol/vol) ethanol was used as a control.
Bioartificial adrenal cortices were cultivated with triptorelin, bombesin, retinoic acid and their respective controls for 6 days starting the day after cell encapsulation in alginate. During this period bioartificial adrenal cortices received freshly prepared compound-containing medium every day. After withdrawal of the compounds the bioartificial adrenal cortices were cultivated in standard medium for three months.
Statistical analyses
Quantitative data is represented as mean ± SEM. Statistical significance was determined by a two-tailed Student’s t-test or one- or two-way Analysis of Variance (ANOVA) with the post hoc Bonferroni’s multiple comparison test where appropriate by using GraphPad Prism 5 software. A value of p≤0.05 was considered as significant in all tests.
Results
Characterization of BAC growth during the monolayer expansion phase
Adrenocortical stem/progenitor cells are present in the primary culture of BACs
To demonstrate the presence of adrenocortical progenitor cell markers and the Shh pathway components GLI1 and Patched1 we used established PCR and Real Time (RT)-PCR methods. Additionally, we also detected the expression of DAX1 as well as expression of the neural and adrenomedullary progenitor marker nestin (S1 Fig) in the monolayer cell culture of BACs. Western blot confirmed the protein expression of the markers in BAC cell cultures (S2 Fig).
We performed time-course experiments to select the time point with maximal gene expression of these markers. The results showed a progressive upregulation of gene expression for Patched1 (2.3 fold, p = 0.06), GLI1 (90.2 fold, p<0.05) and nestin (3 fold, p<0.01) from day 1 to 7 after cell isolation. Gene expression was downregulated from day 7 to 10 (Fig 1). DAX1 followed a different course showing maximal expression at day 1.
Fig 1. Dynamics of the relative gene expression of progenitor markers in primary cultures of bovine adrenocortical cells.
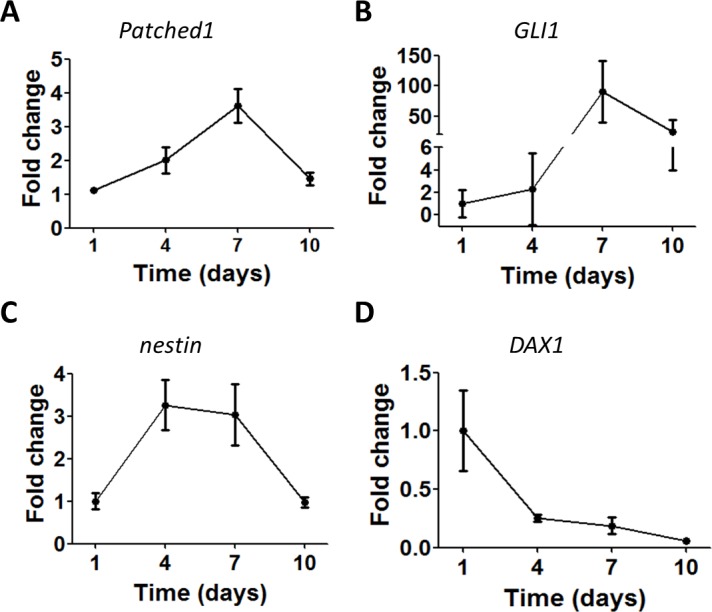
RT-PCR analysis of the expression of Patched1 (A), GLI1 (B), nestin (C) and DAX1 (D) in in BAC cultures for a period of 10 days after cell isolation. All data presented as mean ± SEM, n≥3 for each sample. Reference gene RPS9.
Stimulation with ACTH increases gene expression of steroid hydroxylases and promotes differentiation of progenitor cells
Having established cultures with a relatively high level of progenitor biomarker expression, we next used pharmacological treatments to fine-tune the balance between progenitor markers and activity of mature BACs.
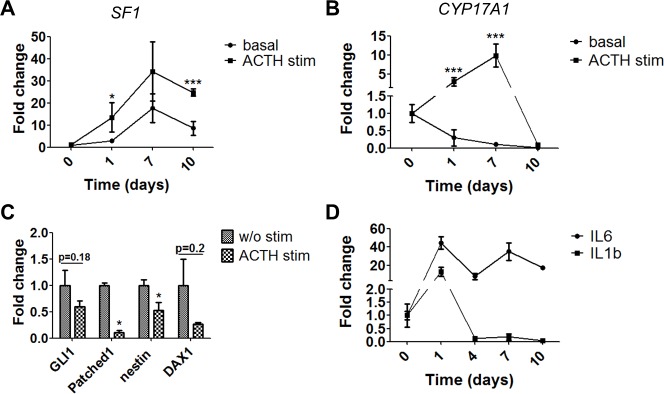
ACTH helps to maintain the function of mature BACs. Specifically, ACTH stimulates the expression of cytochrome P450 (CYP) genes [19, 20], opposing the progressive reduction and eventual loss of steroid hydroxylases in BACs [21]. Therefore, we stimulated the BAC cultures with ACTH for 24 h. ACTH increased the expression of SF1 (a proliferation marker of both progenitors and mature cells) and CYP17A1 (a marker of mature cells) (Figs 2A and 2B). Specifically, the expression of SF1 increased 4.8 fold for day 1 and 1.9 fold for day 7, and the expression of CYP17A1 increased 10.5 fold for day 1 and 90.5 fold for day 7 compared to unstimulated cells. The influence of ACTH stimulation and cultivation length were analyzed by two-way ANOVA, and a significant effect of ACTH stimulation on both gene expressions was found (F = 6.86, p = 0.0124 for SF1 and F = 15.77, p = 0.0003 for CYP17A1). The effect of cultivation length was also significant in both groups (F = 6.54, p = 0.001 for SF1 and F = 7.3, p = 0.0005 for CYP17A1 respectively). The stimulation has the same effect at all time points of cultivation for CYP17A1 (F = 8.32, p = 0.0002). For SF1 this effect was not significant (F = 0.83, p = 0.486).
Fig 2. Dynamics of the relative mRNA gene expression of SF1, CYP17A1 and interleukins 6 and 1β during cultivation of BAC.
A+B–Dynamics of SF1 (A) and CYP17A1 (B) expression with and without ACTH-stimulation for a period of 10 days after cell isolation. C–Downregulation of progenitor markers after ACTH-stimulation on day 7 after cell isolation. D–Dynamics of the gene expression of IL-6 and IL-1β 10 days after cell isolation. All data presented as mean ± SEM, n≥6 for each sample, *p≤0.05, ***p≤0.001. Reference gene RPS9.
ACTH treatment reduced the expression of the progenitor markers Patched1 and nestin. A similar trend was observed with GLI1 and DAX1 but those data were not significant (Fig 2C). ACTH affects differentiation and steroidogenesis partly through SF1-induced transcription of transcription of steroidogenic enzymes [22], but DAX1, on the opposite, represses SF1-mediated transcription [23].
The dynamics of pro-inflammatory cytokines in BAC cultures
A bioartificial adrenal cortex should optimally not produce pro-inflammatory cytokines. We therefore investigated the expression of such cytokines in our BAC cultures. ELISA analysis of the supernatant did not detect the presence of IL-1β and TNFα (S1 Methods). RT-PCR analysis revealed that 24 h after cell isolation the expression of IL-1β was upregulated 12.9 fold and IL-6 in 44.3 fold compared to freshly isolated cells. After 7 days in culture, the expression of these cytokines was reduced. The relative expression of IL-1β was 0.014 (p<0.01) and IL-6 0.78 (p>0.05 –not significant) on day 7 compared to day 1 after cell isolation (Fig 2D).
Optimal time point for the onset of the 3D bioartificial adrenal culture
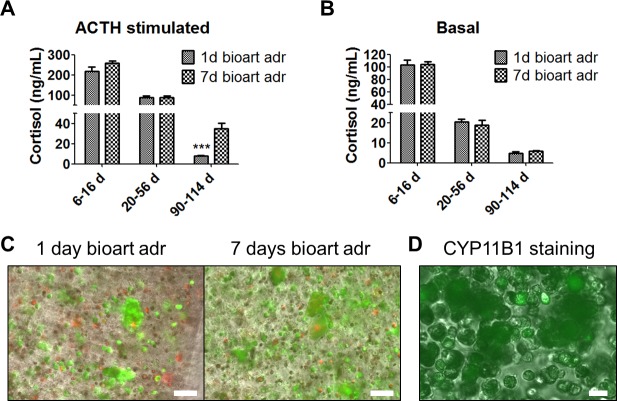
Given the differences in the BAC cultures between day 1 and 7 after cell isolation, we compared bioartificial adrenal cortices created at these two time points. Within the first two weeks after alginate encapsulation the average cortisol level was 103 ± 8 ng/ml for basal and 217±22 ng/ml for ACTH-stimulated day 1 bioartificial organs and 105±4 ng/ml and 258±10 ng/ml for day 7 bioartificial adrenal cortices, respectively. After 1–2 months of cultivation, both groups of bioartificial adrenal cortices produced similar amounts of cortisol (20.4±1.5 ng/ml for basal and 87.3±7.8 ng/ml for ACTH-stimulated day 1 and 18.7±2.4 ng/ml for basal and 89±7.7 ng/ml for ACTH-stimulated day 7 bioartificial adrenal cortices). After three months of cultivation, we detected a significantly higher ACTH-stimulated cortisol level in day 7 bioartificial adrenal cortices compared to day 1 (34.7±5.7 ng/ml vs. 8.1±0.4 ng/ml, respectively) (Fig 3A). Interestingly, we found no difference between basal cortisol productions in the two groups (Fig 3B). The stimulation index for day 7 bioartificial adrenal cortices was 5.9 and for day 1 it was 1.7. Basal aldosterone release of bioartificial adrenal cortices was determined two weeks after BAC encapsulation. Here, we found no difference in aldosterone production between the two groups (416±40 pg/ml for day 1 cortices and 391±37 pg/ml for day 7 cortices (p>0.1), respectively). The dynamics of aldosterone production was not evaluated. Moreover, microscopy of the bioartificial adrenal cortices revealed an increased cell cluster formation in day 7 bioartificial organs (Fig 3C and S4 Fig). The cell yield was assessed by the amount of cells. Cell counting at the end point of the experiment showed 3 times higher amount of cells (299±17.2% of day 1 bioartificial adrenal cortex, p<0.001) in bioartificial adrenal cortices that were created from 7 days old cell culture. CYP11B1 staining showed that cell clusters were formed from steroid producing cells (Fig 3D and S4 Fig), suggesting that the source for these cell clusters might be adrenocortical progenitor cells as well as transiently expressing cells.
Fig 3. Comparative analysis of bioartificial adrenal cortices, created from BAC cultures, cultivated for 1 or 7 days after isolation before encapsulation in alginate.
A–Dynamics of ACTH-stimulated cortisol production of bioartificial adrenal cortices during a cultivation period of 114 days (All data presented as mean ± SEM; n≥5 for each time point; ***p≤0.001). B—Dynamics of the basal cortisol release of bioartificial adrenal cortices during a cultivation period of 114 days (n≥5 for each time point). C–Cell cluster formation in bioartificial adrenal cortices formed from BACs 1 or 7 days after cell isolation (FDA/PI staining plus light microscopy). Scale bars = 100 μm. Uncropped images are seen in S4 Fig. D–Positive CYP11B1 staining (green) of cell clusters (Immunofluorescence plus light microscopy). Scale bar = 20 μm.
Interestingly, we found that adrenocortical progenitor cells maintained their presence in alginate matrix. Seven day cell cultures expressed GLI1 and bioartificial adrenals created from those cells also expressed GLI1 at the end point of the experiment. On the contrary, 1 day old cell cultures (which did not express GLI1 before encapsulation in alginate) failed to show GLI1 at the end of the experiment (114 days in alginate). The viability of encapsulated cells in bioartificial adrenal cortices was 77±3% for day 1 and 87±1.3% for day 7, respectively (p<0.01).
Altering the properties of the bioartificial adrenal cortex
We tested a range of pharmacological agents for their ability to influence the properties of the bioartificial adrenal cortex (S3 Fig). Many of these, including agonists of growth-hormone-releasing hormone (GHRH) (MR409, MR403, JI36 etc.) had no effect (S3 Fig). However, two compounds, triptorelin (LHRH agonist that in addition to stimulation of the secretion of luteinizing hormone and follicle-stimulating hormone, regulates the release of cortisol from adrenocortical cells [3, 24]), and bombesin (neuropeptide and growth factor that activates GLI1 and regulates proliferation in many progenitor cell types [25]) were shown to increase cell functionality and proliferative potential when applied on BACs encapsulated in alginate. Retinoic acid (metabolite of vitamin A that decreases basal cortisol in BACs [3] and promotes differentiation of progenitor cells [26]) in this improved bioartificial system also reduced the basal level of cortisol.
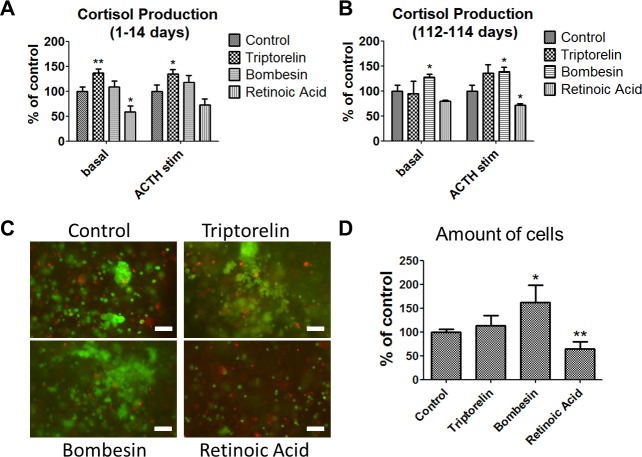
More specifically, during the first two weeks of treatment with triptorelin, a significant increase in basal and ACTH-stimulated cortisol production was observed. In the triptorelin-treated group the basal cortisol reached 136.7±8.1% compared to control, and for ACTH-stimulated BACs it was 134.5±9.2%. Treatment with bombesin showed a tendency towards cortisol increase, which, however, did not reach significance. Retinoic acid reduced cortisol production (58.2±12.1% for basal, p<0.05, and 72.2±12.9% p>0.1 for ACTH-stimulated BACs) (Fig 4A).
Fig 4. Short- and long-term effects of triptorelin, bombesin and retinoic acid on bioartificial adrenal cortices.
A–Short-term effect of the compounds on both basal and ACTH-stimulated cortisol release within the first two weeks of treatment (all data presented as mean ± SEM, n≥18 for each group, *p≤0.05, **p≤0.01). B–Long-term effect of the compounds on basal and ACTH-stimulated cortisol levels at the end point of the experiment (112–114 days of cultivation). All data presented as mean ± SEM, n = 5 for each group, *p≤0.05. C–Cell cluster formation in different groups of bioartificial adrenal cortices after cultivation for three months (FDA/PI staining). Scale bars = 100 μm. Uncropped images are seen in S4 Fig. D—Amount of cells, counted in bioartificial adrenal cortices after cultivation for 114 days (n = 6 for each group).
We also tested for long-term effects in an experimental paradigm where the bioartificial adrenal cortices were treated for 6 days and then maintained untreated for more than three months. In this system the most pronounced effects were induced by bombesin. Here, bombesin increased basal (127.2±6.3% of control) and ACTH-stimulated (138.7±9% of control) cortisol levels. In contrast, retinoic acid showed a reduction of both basal and ACTH-stimulated cortisol to 79.4±18.4% of control, (p≥0.05) and to 71.2±11.3% of control, (p≤0.05), respectively). Cortisol production in the triptorelin-treated group showed a tendency to increase, but did not reach significance (Fig 4B). Moreover, bioartificial adrenal cortices treated with this compound, showed a different morphology of cell cluster formation (Fig 4C and S4 Fig).
The amount of cells was 113±22% of control (p>0.1) for the triptorelin-treated group, 162±36% (p<0.05) of control for bombesin and 64±16% (p<0.01) of control for bioartificial adrenal cortices treated with retinoic acid (Fig 4D). RT-PCR performed at the end point of the experiment showed no effects of the compounds on the expression of SF1,CYP17A1 and the progenitor markers Patched1, GLI1, DAX1 and nestin compared to their controls.
Discussion
Here, we present an improved in vitro system that acts as a bioartificial adrenal cortex, based on cultures of BACs in an alginate matrix. Previously, we have shown that the application of alginate extracellular matrix on primary BAC cultures mimics several properties of the adrenal cortex [3]. Here, to generate an improved model, we optimized the conditions to maintain a progenitor cell population in the bioartificial adrenal cortex. This may lead to eventual application of this model in xenogeneic transplantation for the restoration of adrenal function in patients suffering from adrenal insufficiency or adrenal hyperplasia due to 21-hydroxylase deficiency and Addison’s disease.
Adrenocortical progenitor cells in cell culture might derive from the subcapsular region [27] during cell isolation. Our approach in modeling the composition of the adrenal cortex that contains both mature and progenitor cells was to isolate BACs, expand them in culture until the point (7 days), where the cultures contain the highest levels of progenitor markers, and then to use pharmacological treatments during the alginate culture phase to balance the content of progenitor biomarkers with the function of mature cells.
While working with primary cultures of adrenocortical cells, we have to take into consideration a progressive loss of steroid hydroxylases [21]. Stimulation with ACTH can significantly postpone this process [19, 20]. However, ACTH stimulation seems to downregulate the expression of adrenocortical progenitor markers, promoting differentiation of progenitor cells [28], which our data corroborate. Therefore, on one hand to prevent the loss of steroidogenic enzymes, adrenocortical cells need to be stimulated with ACTH. However, on the other hand, ACTH stimulation reduces the expression of adrenocortical stem/progenitor biomarkers in the culture, which in the long run might shorten the functional life-span of the created bioartificial organ. Precise refinements of this balance using pharmacological treatments may further improve this aspect of the method.
Production of pro-inflammatory cytokines by the graft is a limiting factor in transplantation approaches. Activation of cytokine signaling networks with a high production of proinflammatory interleukins is a universal response to any tissue damage occurring during primary cell isolation. Transplanted organs releasing cytokines would definitely cause a local host immune response. Cytokines could be produced not only by fibroblastic and macrophage elements, but also by the BACs themselves [29, 30]. However, as it has been already shown, proliferation and steroidogenesis of adrenocortical cells were closely linked to the level of IL-1β in BAC culture. Blocking the production of IL-1β significantly reduced or even abolished the elevation of proliferative activity and cortisol production by adrenocortical cells [31]. Similar effects were obtained in other studies. [32]. IL-6 also has a pronounced ability to stimulate proliferation and steroidogenesis [32–34]. Previous studies have demonstrated that inflammatory cytokines promote retrodifferentiation of mature cells to progenitor cells [35]. Overall, cytokines do not only play a pathogenic role, but also have an important physiological function in the activation of proliferative processes and steroidogenesis in adrenocortical cell cultures. In this study we show that, especially when cells that have been cultured for 7 days are selected to establish the bioartificial adrenal cortex, the levels of inflammatory cytokines are low.
Beyond differences in inflammatory cytokine production, the present study shows that BACs on day 1 and day 7 exhibit different biomarker expression profiles. The expression of CYP17A1 and DAX1 was highest on day 1 after cell isolation, whereas the expression of GLI1, Patched1, nestin and SF1 reached their maximum on day 7. The next step was to compare bioartificial adrenal cortices generated from BACs on day 1 and 7 after the isolation procedure. During the cultivation period we did not find any significant difference in cortisol production between the two groups of bioartificial organs, but at later time points, we noticed the formation of cell clusters in the bioartificial adrenal cortices created from 7 days old cell cultures but considerably less from the 1 day old cell cultures. By this time we detected significantly higher ACTH-stimulated cortisol release in this group. The productivity of bioartificial adrenal cortices correlated with the amount of newly formed cell clusters. Cell count at the end point of the experiment confirmed an increased cell yield in the day 7 bioartificial adrenal cortices. Moreover, 7 days bioartificial adrenal cortices showed significantly higher viability at the end point of the experiment. The elevated amount of viable cells might be responsible for the higher ACTH stimulated cortisol production in this group, compared to 1 day bioartificial adrenal cortices.
Another gene that was highly expressed in 7 days old cell culture was SF1. The role of SF1 in the homeostatic proliferation of the adult gland has already been demonstrated [9]. Additionally, SF1 acts as the obligate activator of most steroidogenic enzymes in the adrenal cortex and plays an essential part in both proliferation and differentiation (steroidogenesis) of the adult gland [9]. It is important to note that BACs, cultivated for 7 days before encapsulation in alginate and showing maximal gene expression of Patched1, GLI1 and nestin, exhibited a better outcome in ACTH stimulated cortisol production when cultured for more than 90 days.
Even though encapsulation of 7 days old cell cultures in the long run significantly improved the functionality of bioartifical adrenal cortices, still long-term cultivation unfortunately led to a major decrease in cortisol levels. One of the reasons for this loss could be that bioartifical adrenal cortices were mostly generated from mature adrenocortical cells with limited life span. Finding a way to additionally enrich the source cell culture with adrenocortical stem and progenitor cells could solve this problem.
The bioartificial adrenal cortices are amenable to pharmacological manipulation. Therefore, we tested a range of pharmacological agents for their ability to influence the properties of the bioartificial adrenal cortex. These agents were relevant for adrenal function [24, 36–38], and furthermore, they were known to have the ability to stimulate self-renewal and promote survival of for example cardiac stem cells [39]. In addition, we have previously shown that some of the GHRH agonists can stimulate growth, function, and engraftment of pancreatic islets after transplantation [40, 41]. During the cultivation of bioartificial adrenal cortices in triptorelin-containing medium and 8 days after withdrawal of triptorelin, the LHRH agonist treated group showed an increased basal and ACTH-stimulated cortisol production. These data corroborate previously published results, showing a surge of steroid hormone release after administration of triptorelin [42]. Other studies demonstrated an effect of bombesin on steroid production [43], which we were not able to confirm. On the other hand, the bombesin results support its proliferative potential [25] as assessed by the amount of cells at the end point of the experiment. Retinoic acid produced both short-term (2 weeks) and long-term (~3–4 months) effects. Short-term effects were manifested as a significant reduction of cortisol production. Long-term effects were assessed at the later stages of the experiment, where we detected a reduced cell yield and ACTH-stimulated cortisol release.
Our work extends our studies and improves our previous efforts to develop bioartificial adrenal cortices with long-term survival capacity providing the ability to respond to pharmacological treatments. Such efforts may form the basis for eventual transplantation strategies for patients with severe adrenal disorders.
Supporting information
(TIF)
The samples were loaded from the left to the right as following: adrenocortical cells from adrenals 1, 2, 3 and 4 cultivated for 1 day after cell isolation, for 4 days and then for 7 days.
(TIF)
All data presented as mean ± SEM, n≥3 for each sample, *p≤0.05, **p≤0.01.
(TIF)
Acknowledgments
We are thankful to Linda Friedrich and Uta Lehnert for expert technical assistance.
This work was in part supported by the International Fund Congenital Adrenal Hyperplasia (IFCAH) (to SRB), by the Deutsche Forschungsgemeinschaft (CRC-TRR 127 (to SRB and BL), CRC-TRR 205 (to SRB, BL and CS), IRTG 2251 (to SRB, BL and CS), and CRU 252 (to SRB and BL)), the German Academic Exchange Service (DAAD) (to GRB) and The TransCampus initiative between TU Dresden and King´s College London (to SRB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was in part supported by the International Fund Congenital Adrenal Hyperplasia (IFCAH) (to SRB), by the Deutsche Forschungsgemeinschaft (CRC-TRR 127 (to SRB and BL), CRC-TRR 205 (to SRB, BL and CS), IRTG 2251 (to SRB, BL and CS), and CRU 252 (to SRB and BL)), the German Academic Exchange Service (DAAD) (to GRB) and The TransCampus initiative between TU Dresden and King's College London (to SRB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365(9477):2125–36. doi: 10.1016/S0140-6736(05)66736-0 [DOI] [PubMed] [Google Scholar]
- 2.Merke DP, Bornstein SR, Braddock D, Chrousos GP. Adrenal lymphocytic infiltration and adrenocortical tumors in a patient with 21-hydroxylase deficiency. N Engl J Med. 1999;340(14):1121–2. doi: 10.1056/NEJM199904083401416 [DOI] [PubMed] [Google Scholar]
- 3.Balyura M, Gelfgat E, Ehrhart-Bornstein M, Ludwig B, Gendler Z, Barkai U, et al. Transplantation of bovine adrenocortical cells encapsulated in alginate. Proc Natl Acad Sci U S A. 2015;112(8):2527–32. doi: 10.1073/pnas.1500242112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guasti L, Paul A, Laufer E, King P. Localization of Sonic hedgehog secreting and receiving cells in the developing and adult rat adrenal cortex. Mol Cell Endocrinol. 2011;336(1–2):117–22. doi: 10.1016/j.mce.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn JC, Chu Y, Qin HH, Zupekan T. Transplantation of adrenal cortical progenitor cells enriched by Nile red. J Surg Res. 2009;156(2):317–24. doi: 10.1016/j.jss.2009.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang SP, Morrison HD, Nilsson F, Kenyon CJ, West JD, Morley SD. Cell proliferation, movement and differentiation during maintenance of the adult mouse adrenal cortex. PLoS One. 2013;8(12):e81865 doi: 10.1371/journal.pone.0081865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finco I, LaPensee CR, Krill KT, Hammer GD. Hedgehog signaling and steroidogenesis. Annu Rev Physiol. 2015;77:105–29. doi: 10.1146/annurev-physiol-061214-111754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci U S A. 2009;106(50):21185–90. doi: 10.1073/pnas.0909471106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walczak EM, Hammer GD. Regulation of the adrenocortical stem cell niche: implications for disease. Nat Rev Endocrinol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990;347(6295):762–5. doi: 10.1038/347762a0 [DOI] [PubMed] [Google Scholar]
- 11.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4):585–95. [DOI] [PubMed] [Google Scholar]
- 12.Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, et al. Nestin expression—a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61(19–20):2510–22. doi: 10.1007/s00018-004-4144-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toti P, Regoli M, Nesi G, Occhini R, Bartolommei S, Fonzi L, et al. Nestin expression in normal adrenal gland and adrenocortical tumors. Histol Histopathol. 2005;20(4):1115–20. doi: 10.14670/HH-20.1115 [DOI] [PubMed] [Google Scholar]
- 14.Bertelli E, Regoli M, Lucattelli M, Bastianini A, Fonzi L. Nestin expression in rat adrenal gland. Histochem Cell Biol. 2002;117(4):371–7. doi: 10.1007/s00418-002-0386-2 [DOI] [PubMed] [Google Scholar]
- 15.Steenblock C, Rubin de Celis MF, Androutsellis-Theotokis A, Sue M, Delgadillo Silva LF, Eisenhofer G, et al. Adrenal cortical and chromaffin stem cells: Is there a common progeny related to stress adaptation? Mol Cell Endocrinol. 2016. [DOI] [PubMed] [Google Scholar]
- 16.de Celis MFR, Garcia-Martin R, Wittig D, Valencia GD, Enikolopov G, Funk RH, et al. Multipotent Glia-Like Stem Cells Mediate Stress Adaptation. Stem Cells. 2015;33(6):2037–2051. doi: 10.1002/stem.2002 [DOI] [PubMed] [Google Scholar]
- 17.Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Muller B, et al. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50(3):521–33. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum. 2005;52(8):2386–95. doi: 10.1002/art.21215 [DOI] [PubMed] [Google Scholar]
- 19.Mukai K, Mitani F, Agake R, Ishimura Y. Adrenocorticotropic hormone stimulates CYP11B1 gene transcription through a mechanism involving AP-1 factors. Eur J Biochem. 1998;256(1):190–200. [DOI] [PubMed] [Google Scholar]
- 20.Hornsby PJ. Regulation of cytochrome P-450-supported 11 beta-hydroxylation of deoxycortisol by steroids, oxygen, and antioxidants in adrenocortical cell cultures. J Biol Chem. 1980;255(9):4020–7. [PubMed] [Google Scholar]
- 21.Hornsby PJ, Cheng CY, Lala DS, Maghsoudlou SS, Raju SG, Yang L. Changes in gene expression during senescence of adrenocortical cells in culture. J Steroid Biochem Mol Biol. 1992;43(8):951–60. doi: 10.1016/0960-0760(92)90323-B [DOI] [PubMed] [Google Scholar]
- 22.Sewer MB, Nguyen VQ, Huang CJ, Tucker PW, Kagawa N, Waterman MR. Transcriptional activation of human CYP17 in H295R adrenocortical cells depends on complex formation among p54(nrb)/NonO, protein-associated splicing factor, and SF-1, a complex that also participates in repression of transcription. Endocrinology. 2002;143(4):1280–90. doi: 10.1210/endo.143.4.8748 [DOI] [PubMed] [Google Scholar]
- 23.Babu PS, Bavers DL, Beuschlein F, Shah S, Jeffs B, Jameson JL, et al. Interaction between Dax-1 and steroidogenic factor-1 in vivo: Increased adrenal responsiveness to ACTH in the absence of Dax-1. Endocrinology. 2002;143(2):665–673. doi: 10.1210/endo.143.2.8658 [DOI] [PubMed] [Google Scholar]
- 24.Nekola MV, Pedroza E, Schally AV. Paradoxical effects of D-Trp6-LHRH in immature female rats correlated with changes in ACTH, prolactin, and corticosterone levels. Biol Reprod. 1981;24(3):505–11. [DOI] [PubMed] [Google Scholar]
- 25.Castellone MD, Laukkanen MO, Teramoto H, Bellelli R, Ali G, Fontanini G, et al. Cross talk between the bombesin neuropeptide receptor and Sonic hedgehog pathways in small cell lung carcinoma. Oncogene. 2015;34(13):1679–1687. doi: 10.1038/onc.2014.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung KF, Qin N, Androutsellis-Theotokis A, Bornstein SR, Ehrhart-Bornstein M. Effects of dehydroepiandrosterone on proliferation and differentiation of chromaffin progenitor cells. Mol Cell Endocrinol. 2011;336(1–2):141–8. doi: 10.1016/j.mce.2010.11.028 [DOI] [PubMed] [Google Scholar]
- 27.Kim AC, Barlaskar FM, Heaton JH, Else T, Kelly VR, Krill KT, et al. In search of adrenocortical stem and progenitor cells. Endocr Rev. 2009;30(3):241–63. doi: 10.1210/er.2008-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamins JA, Nedelkoska L, Lisak RP. Adrenocorticotropin hormone 1–39 promotes proliferation and differentiation of oligodendroglial progenitor cells and protects from excitotoxic and inflammation-related damage. J Neurosci Res. 2014;92(10):1243–51. doi: 10.1002/jnr.23416 [DOI] [PubMed] [Google Scholar]
- 29.Guzmán C H-BR, Morales-Montor J. Regulation of steroidogenesis in reproductive, adrenal and neural tissues by cytokines The Open Neuroendocrinology Journal. 2010(3):161–169. [Google Scholar]
- 30.Call GB, Husein OF, McIlmoil CJ, Adams A, Heckmann RA, Judd AM. Bovine adrenal cells secrete interleukin-6 and tumor necrosis factor in vitro. Gen Comp Endocrinol. 2000;118(2):249–61. doi: 10.1006/gcen.2000.7458 [DOI] [PubMed] [Google Scholar]
- 31.Haidan A, Bornstein SR, Glasow A, Uhlmann K, Lubke C, Ehrhart-Bornstein M. Basal steroidogenic activity of adrenocortical cells is increased 10-fold by coculture with chromaffin cells. Endocrinology. 1998;139(2):772–780. doi: 10.1210/endo.139.2.5740 [DOI] [PubMed] [Google Scholar]
- 32.Hanley N, Williams BC, Nicol M, Bird IM, Walker SW. Interleukin-1 beta stimulates growth of adrenocortical cells in primary culture. J Mol Endocrinol. 1992;8(2):131–6. [DOI] [PubMed] [Google Scholar]
- 33.Barney M, Call GB, McIlmoil CJ, Husein OF, Adams A, Balls AG, et al. Stimulation by interleukin-6 and inhibition by tumor necrosis factor of cortisol release from bovine adrenal zona fasciculata cells through their receptors. Endocrine. 2000;13(3):369–77. doi: 10.1385/ENDO:13:3:369 [DOI] [PubMed] [Google Scholar]
- 34.Mikhaylova IV, Kuulasmaa T, Jaaskelainen J, Voutilainen R. Tumor necrosis factor-alpha regulates steroidogenesis, apoptosis, and cell viability in the human adrenocortical cell line NCI-H295R. Endocrinology. 2007;148(1):386–92. doi: 10.1210/en.2006-0726 [DOI] [PubMed] [Google Scholar]
- 35.Dubois-Pot-Schneider H, Fekir K, Coulouarn C, Glaise D, Aninat C, Jarnouen K, et al. Inflammatory cytokines promote the retrodifferentiation of tumor-derived hepatocyte-like cells to progenitor cells. Hepatology. 2014;60(6):2077–90. doi: 10.1002/hep.27353 [DOI] [PubMed] [Google Scholar]
- 36.Freddi S, Arnaldi G, Fazioli F, Scarpelli M, Appolloni G, Mancini T, et al. Expression of growth hormone-releasing hormone receptor splicing variants in human primary adrenocortical tumours. Clin Endocrinol (Oxf). 2005;62(5):533–8. [DOI] [PubMed] [Google Scholar]
- 37.Doga M, Bonadonna S, Burattin A, Giustina A. Ectopic secretion of growth hormone-releasing hormone (GHRH) in neuroendocrine tumors: relevant clinical aspects. Ann Oncol. 2001;12 Suppl 2:S89–94. [DOI] [PubMed] [Google Scholar]
- 38.Ziegler CG, Brown JW, Schally AV, Erler A, Gebauer L, Treszl A, et al. Expression of neuropeptide hormone receptors in human adrenal tumors and cell lines: Antiproliferative effects of peptide analogues. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(37):15879–15884. doi: 10.1073/pnas.0907843106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Florea V, Majid SS, Kanashiro-Takeuchi RM, Cai RZ, Block NL, Schally AV, et al. Agonists of growth hormone-releasing hormone stimulate self-renewal of cardiac stem cells and promote their survival. Proc Natl Acad Sci U S A. 2014;111(48):17260–5. doi: 10.1073/pnas.1420375111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ludwig B, Ziegler C, Schally AV, Richter C, Steffen A, Block NL, et al. Agonist of Growth Hormone Releasing Hormone as a Potential Effector for Survival and Proliferation of Pancreatic Islets. Transplant International. 2010;23:61–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ludwig B, Rotem A, Schmid J, Weir GC, Colton CK, Brendel MD, et al. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(13):5022–5027. doi: 10.1073/pnas.1201868109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero E, Velez de Mendizabal N, Cendros JM, Peraire C, Bascompta E, Obach R, et al. Pharmacokinetic/pharmacodynamic model of the testosterone effects of triptorelin administered in sustained release formulations in patients with prostate cancer. J Pharmacol Exp Ther. 2012;342(3):788–98. doi: 10.1124/jpet.112.195560 [DOI] [PubMed] [Google Scholar]
- 43.Malendowicz LK, Lesniewska B, Baranowska B, Nowak M, Majchrzak M. Effect of bombesin on the structure and function of the rat adrenal cortex. Res Exp Med (Berl). 1991;191(2):121–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
The samples were loaded from the left to the right as following: adrenocortical cells from adrenals 1, 2, 3 and 4 cultivated for 1 day after cell isolation, for 4 days and then for 7 days.
(TIF)
All data presented as mean ± SEM, n≥3 for each sample, *p≤0.05, **p≤0.01.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.