Abstract
Despite the widespread use of bacillus Calmette–Guérin vaccination, Mycobacterium tuberculosis infection remains globally the leading cause of death from a single infectious disease. The complicated and often protracted dynamics of infection and disease make clinical trials to test new tuberculosis vaccines extremely complex. Preclinical selection of only the most promising candidates is therefore essential. Because macaque monkeys develop a disease very similar to humans, they have potential to provide important information in addition to small animal models. To assess the relative merits of rhesus and cynomolgus monkeys as screens for tuberculosis vaccines, we compared the efficacy of bacillus Calmette–Guérin vaccination and the course of infection in both species. Unvaccinated rhesus and cynomolgus monkeys both developed progressive disease with high levels of C-reactive protein, M. tuberculosis-specific IgG, and extensive pathology including cavitation and caseous necrosis. Bacillus Calmette–Guérin vaccination protected cynomolgus almost completely toward the development of pathology, reflected in a striking 2-log reduction in viable bacteria in the lungs compared with nonvaccinated animals. Rhesus, on the other hand, were not protected efficiently by the bacillus Calmette–Guérin. The vaccinated animals developed substantial pathology and had negligible reductions of colony-forming units in the lungs. Comparative studies in these closely related species are likely to provide insight into mechanisms involved in protection against tuberculosis.
Tuberculosis (TB) is the leading global cause of death from a single infectious disease (1, 2), accelerated by the HIV epidemic and appearance of multidrug-resistant Mycobacterium tuberculosis (3). The efficiency of bacillus Calmette–Guérin, the only TB vaccine available for humans, ranges between 0 and 85% (4). Improved vaccines are needed urgently, and a large number of new TB vaccine candidates and delivery systems is being tested in small animal models (5–7). Human trials to evaluate new TB vaccines will be very complex, will often occur in bacillus Calmette–Guérin-immunized populations, will be of long duration, and require large cohorts (8). Effective screens to select only the most promising candidates, delivery systems, and formulations for clinical testing will be extremely valuable. Up to now, almost all efficacy testing of TB vaccine candidates has been in the mouse and/or guinea pig (9). A more human-like response to TB has been described in the closely related rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) macaques (reviewed in ref. 10). This similarity in response may be because several host molecules implicated in TB infections are present in man and nonhuman primates but are not found, or differ fundamentally, in mice and guinea pigs. For example, many primate species, including human, share the presence of functional Mhc-DR, -DQ, and -DP regions (11). The relevance of this structural similarity is reflected in the observation that specific mycobacterial peptides reactive with HLA-DR17 also bind to, and can be presented by, related MHC molecules from rhesus macaques (12). Group 1 CD1 molecules, present in both human and nonhuman primates, present several nonpeptide mycobacterial products to T cells (13, 14), whereas CD1d, the only CD1 molecule in the mouse, is not involved in resistance to M. tuberculosis (15). Finally, although small animals and humans show fundamental differences in T cell antigen receptor repertoire, including γδ T cells, which recognize several mycobacterial ligands (16, 17), human and macaque repertoires are comparable (18, 19). Taken together, macaques provide attractive models for the final selection of new TB vaccines.
Previous studies have suggested that rhesus are highly susceptible to TB (20, 21), and the closely related cynomolgus macaque is somewhat more resistant (22). However, up to now all information on the course of infection after vaccination is from studies in rhesus, and no direct comparison of TB infection in these species has been reported. Because bacillus Calmette–Guérin is the standard by which new vaccines will be judged, here the effect of bacillus Calmette–Guérin vaccination on TB is evaluated and compared in these species for the first time. Data from this study show important differences with respect to the efficacy of bacillus Calmette–Guérin vaccination in these species and provide valuable information for the rational choice of models for the evaluation of future vaccine candidates.
Materials and Methods
Monkeys.
Six rhesus monkeys (M. mulatta) and six cynomolgus monkeys (M. fascicularis), all adult males (5–10 kg, 5–10 years of age) born and raised in captivity, were housed at the Biomedical Primate Research Centre facilities. Before the study all animals were examined clinically and radiologically and tuberculin skin-tested. For all handling, animals were sedated with ketamine. Experiments were reviewed and approved by the institutional ethical committee according to Dutch law.
Bacteria.
M. tuberculosis Erdman strain was grown in modified Sauton medium enriched with 0.5% sodium pyruvate and 0.5% glucose (9 days, shaking platform, 35.5°C) and centrifuged (2,500 × g, 30 min). The pellet was resuspended in 10 ml of Sauton medium and glass beads and shaken (350 rpm, 15 min) to obtain a single cell suspension (concentration ≈5 × 108 bacteria per ml), diluted 1:1 in 3% (wt/vol) Trypton Soya Broth medium (Oxoid, Hampshire, UK) containing 17% (wt/vol) glycerol, and aliquoted (storage at −80°C). Colony-forming units (cfu) were determined on 7H11 agar plates. For challenge, a stock culture was diluted in PBS to 1,000 cfu/ml. Samples were plated and showed no loss of viability on thawing.
Bacillus Calmette–Guérin Vaccination and Chemotherapeutic Treatment of Monkeys.
Animals were assigned randomly to groups. Three rhesus and three cynomolgus monkeys were vaccinated intradermally with 1–4 × 105 cfu bacillus Calmette–Guérin Copenhagen (strain 1331; Statens Serum Institute, Copenhagen, Denmark) freshly prepared from a lyophilized stock. After 8 weeks, daily oral treatment (20 mg/kg isoniazid, 25 mg/kg rifadin) was initiated and continued for 3 weeks. After drug treatment, the animals were rested for 6 weeks before challenge with M. tuberculosis.
Challenge of Monkeys.
On the same day and with the same preparation, all animals (sedated with 7 mg/kg tilatamine/zolazepam and 1 ml/kg Atropine) received 3,000 cfu M. tuberculosis by intratracheal installation of 3 ml of the bacterial suspension. By using a laryngoscope, a catheter was installed in the trachea to the depth of the carina, instilled with 0.2 ml of 2% lidocaine without epinephrine followed by saline. The bacterial suspension was instilled slowly followed by sufficient saline to flush the catheter. The head and body were held up at a slight angle before animals were placed in right lateral recumbency. Animals were housed in biosafety level-three facilities.
Clinical Assessment.
After infection the animals were observed daily for behavior, eating, and coughing. Weight, temperature, and radiologic outcome (on masked radiographs) of anterior, posterior, and lateral views of the chest (22) were recorded.
Immunological Assays.
Skin tests were recorded 72 h after the administration of 0.1 ml (2,500 international units) of Old Tuberculin (OT, Statens Serum Institute) to the abdomen (23). Heparinized blood was used to isolate peripheral blood mononuclear cells (PBMCs) using Ficoll-Hypaque density gradient centrifugation. PBMCs were washed, counted, and resuspended to 2 × 106 cells per ml in RPMI 1640 medium containing gentamycin and 10% heat-inactivated FCS. In triplicate wells (final volume 100 μl), PBMCs were incubated with 10 μg/ml of tuberculin purified protein derivative (PPD, RT49, Statens Serum Institute), 5 μg/ml Con A, or medium only in 96-well round-bottom microtiter plates. After incubation (37°C, 5% CO2, 72 h), 2.5 μCi (1 Ci = 37 GBq) of [3H]thymidine was added for another 18 h. Cells were harvested, and emission was determined on a Helium counter (Packard Matrix Counter). Proliferation is expressed as the relative increase in 3H incorporation in stimulated cells vs. nonstimulated cells (mean cpm with antigen) − (mean cpm-negative control). In a separate assay, we harvested supernatants of PBMCs cultured for 72 h with or without antigen to measure IFN-γ levels (U-Cytech, Utrecht, The Netherlands).
Serum was stored at −20°C. Antibody determinations were done in 1:300 diluted serum samples by using an M. tuberculosis-specific IgG ELISA (Kreatech Diagnostics, Amsterdam, The Netherlands; ref. 24). The data show the percentage increase in OD compared with prevaccination serum. C-reactive protein (CRP) serum levels were measured by the regional hospital.
Fluorescence-Activated Cell Sorter Analysis.
Phenotypic characterization was done by incubating 100 μl of heparinized blood with the following mAbs: CD8-PE (Dako), CD4-FITC, CD64-FITC, and γδ TCR-PE (PharMingen) (30 min, room temperature). After washing, red blood cells were lysed with fluorescence-activated cell sorter lysing solution (Becton Dickinson) and washed, and the cells were fixed with 1% formaldehyde. Cells were analyzed within a lymphocyte or monocyte gate by flow cytometry by using Cell Quest software (FACScan, Becton Dickinson). Isotype-matched control mAbs were used in all experiments. The results are expressed as a percentage of positive cells.
Pathologic Examination.
Between 59 and 65 days after infection animals were killed, and necropsies were undertaken. After a longitudinal incision, the body cavity was opened to examine the organs. Samples from lungs and other organs when affected were fixed (4% buffered formalin). Paraffin sections (5 μm) were prepared and stained (hematoxylin/eosin or Ziehl-Neelsen). Scoring criteria for the amount of gross pathology, lymphadenopathy, and hematogenous spreading (Table 1) were based on the judgment of an experienced pathologist. Histopathological slides were evaluated by a pathologist with extensive experience with murine and human TB. For both pathologists the experimental groups were masked throughout all procedures.
Table 1.
Pathologic characteristics in nonvaccinated and BCG-vaccinated rhesus and cynomolgus monkeys
| Treatment | Animal* | Lung disease† | Lymphadenopathy‡ | Hematogenous spread |
|---|---|---|---|---|
| None | R1 | 5 | 2 | Yes |
| R2 | 4 | 1 | Yes | |
| R3 | 3 | 3 | No | |
| BCG | R4 | 3 | 2 | No |
| R5 | 3 | 3 | No | |
| R6 | 4 | 2 | No | |
| None | C1 | 2 | 2 | No |
| C2 | 3 | 2 | No | |
| C3 | 3 | 3 | No | |
| BCG | C4 | 1§ | 0 | No |
| C5 | 1§ | 0 | No | |
| C6 | 1§ | 0 | No |
R rhesus and C cynomolgus monkeys.
Right lungs were scored according to proportion affected by disease. 1, <10%; 2, 10–30%; 3, 30–50%; 4, 50–70%; 5, 70–90%. Only in C3 was the left lung affected (2).
Enlargement of tracheobronchial lymph nodes; 0, none; 1, mild; 2, moderate; 3, severe.
C4 had 10 granulomas, C5 had approximately 20 granulomas, and C6 had 1 granuloma.
Determination of Bacterial Counts.
At necropsy, three parts (0.5–1 cm3) of the affected lungs were taken by the pathologist from the basal part of the apical, middle, and lower lobes. The experimental groups were masked to the pathologist. No cavitary lesions were included. Mycobacteria were quantified by crushing known weights of the lung tissue with sea sand in saline. Three parts of H2SO4 [6% (wt/vol)] were added to two parts of suspended tissue (room temperature, 10 min). The pH was neutralized with buffered caustic phosphate solution with a pH indicator. Duplicate 10-fold dilutions were incubated (7H10 agar plates, 35.5°C, 4 weeks), and the number of colonies was counted.
Results
Immune Responses to Bacillus Calmette–Guérin Vaccination.
All animals received a skin test with OT 13 weeks after bacillus Calmette–Guérin vaccination. Nonvaccinated animals showed a slight response to OT (mean induration 5 mm in nonvaccinated rhesus and 5.7 mm in nonvaccinated cynomolgus monkeys). Bacillus Calmette–Guérin-vaccinated animals had slightly larger indurations, but no differences were observed between rhesus and cynomolgus monkeys (mean diameter 8.7 and 8.3 mm, respectively).
We also tested the lymphoproliferative responses of PBMCs to PPD 13 weeks after bacillus Calmette–Guérin vaccination. PBMCs from vaccinated rhesus and cynomolgus monkeys showed antigen-specific proliferation to PPD, with rhesus responding more strongly than cynomolgus (Fig. 1A).
Figure 1.
PPD-induced lymphoproliferative responses (A) and production of IFN-γ (B) by PBMCs from bacillus Calmette–Guérin (BCG)-vaccinated and nonvaccinated rhesus and cynomolgus monkeys. The results are the means (SEM) of each group. For each species, significant differences were evident for both the lymphoproliferative responses and the IFN-γ responses between nonvaccinated and bacillus Calmette–Guérin-vaccinated groups (unpaired t test, P = <0.05)
The control of mycobacterial infections in mice and humans involves IFN-γ-mediated immune responses (25, 26). PPD-induced IFN-γ production by PBMCs was assessed after bacillus Calmette–Guérin vaccination. PBMCs from all vaccinated animals, but not of nonvaccinated animals, produced IFN-γ in response to PPD, but PBMCs from rhesus produced more than twice the amount than PBMCs from cynomolgus monkeys (Fig. 1B). Mitogen-induced IFN-γ production by PBMCs did not differ between rhesus (2855 ± 46 pg ml−1) and cynomolgus (2916 ± 73 pg ml−1).
Clinical Parameters During Infection.
During the infection period, no coughing was observed in either group of cynomolgus or in the bacillus Calmette–Guérin-vaccinated rhesus group. In the nonvaccinated rhesus group, one animal started to cough in week 6, and by week 8 all were coughing. None of the animals showed a decrease in weight during the observation period. In cynomolgus, no increase in body temperature was observed, but an increase of ≈1°C was evident in all rhesus monkeys from week 4 of infection to the end of the study.
At week 7 of infection, the animals were examined radiographically. All rhesus showed fibronodular infiltrates, mainly in the lower and middle lobes of the right lung. Evidence for cavitation was present in all nonvaccinated rhesus and in one of the three vaccinated rhesus. In nonvaccinated cynomolgus, fibronodular infiltrates were present, mainly in the right lungs. One animal showed cavitation. In contrast, the radiographs of bacillus Calmette–Guérin-vaccinated cynomolgus showed no lung abnormalities.
Immunological Parameters During Infection.
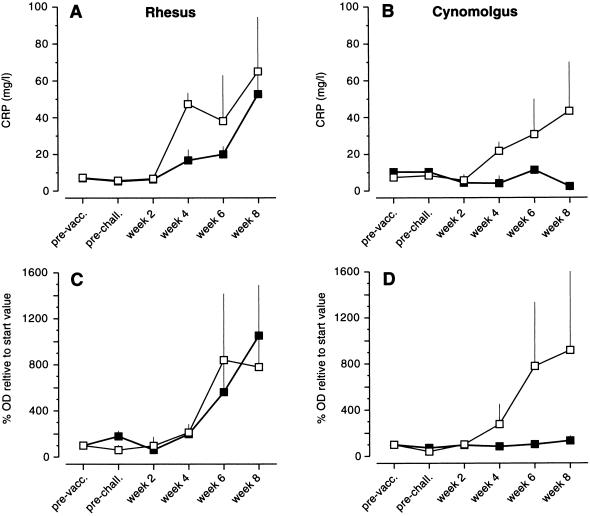
CRP levels were determined before and during TB infection. CRP levels in nonvaccinated, noninfected animals were 7.2 ± 1.0 (rhesus) and 8.8 ± 3.8 mg/liter (cynomolgus). Bacillus Calmette–Guérin vaccination did not result in increased CRP levels. At week 2 of infection, CRP levels were normal, but in nonvaccinated rhesus and cynomolgus CRP levels gradually increased thereafter. There was a tendency for a sharper increase in rhesus compared with cynomolgus. Although somewhat slower than in nonvaccinated rhesus, CRP levels also increased in vaccinated rhesus (Fig. 2A). In marked contrast, vaccinated cynomolgus showed no significant elevation of CRP during the observation period (Fig. 2B).
Figure 2.
Mean levels (SEM) of CRP (A and B) and M. tuberculosis-specific IgG (C and D) during M. tuberculosis infection of nonvaccinated (□) and bacillus Calmette–Guérin (BCG)-vaccinated (■) rhesus (A and C) and cynomolgus (B and D) monkeys.
At week 8 of infection, skin test reactivity to OT was variable. Two of the nonvaccinated rhesus showed indurations of 3 and 4 cm, and the third had an induration of 7 mm. Vaccinated rhesus had smaller indurations (mean 10.7 ± 6.4 mm). One nonvaccinated cynomolgus showed an induration of 1 cm, and the other two animals had indurations of 7 mm. Vaccinated cynomolgus monkeys had a mean induration of 7.3 ± 1.2 mm. The induration size did not correlate with disease progression. This variability in skin-test reactivity by using OT is consistent with earlier reports using PPD (22).
Antibody responses were determined by using a diagnostic kit developed for the detection of M. tuberculosis IgG responses. After bacillus Calmette–Guérin vaccination, a weak response was observed in rhesus but not in cynomolgus. In both rhesus groups and the nonvaccinated cynomolgus group, an increase in M. tuberculosis-specific IgG was detected from week 4 of infection to the end of the study (Fig. 2, C and D). In bacillus Calmette–Guérin-vaccinated cynomolgus monkeys, we found only a very low increase in specific serum IgG levels (Fig. 2D).
In all groups, the number of CD4+ and CD8+ T lymphocytes was similar and did not change during the infection. No increase of γδ T cells was observed at week 4, followed by a slight nonsignificant increase in control (6.6 ± 3.1 to 8.5 ± 1.8%) and bacillus Calmette–Guérin-vaccinated rhesus (3.4 ± 1.1 to 13.8 ± 7%) and control (3.4 ± 1.8 to 6.3 ± 2.4%) and bacillus Calmette–Guérin-vaccinated cynomolgus (3.8 ± 1.2 to 6.1 ± 1.6%) (weeks 2 and 6, respectively). The percentage of CD64-expressing cells was increased significantly at week 4 of infection followed by a slight decline thereafter in all groups (Table 2).
Table 2.
Expression of CD64 during M. tuberculosis infection in nonvaccinated and BCG-vaccinated rhesus and cynomolgus macaques
| Week of
infection
|
|||
|---|---|---|---|
| 2 | 4 | 6 | |
| NVR | 43.4 (4.6)* | 76.4 (3.4) | 53.9 (8.0) |
| VR | 35.1 (12.1) | 65.2 (1.9) | 53.9 (13.8) |
| NVC | 32.5 (1.0) | 66.4 (1.4) | 36.2 (8.5) |
| VC | 32.3 (3.0) | 54.9 (9.6) | 49.9 (5.5) |
NV, nonvaccinated; V, BCG-vaccinated; R, rhesus monkey; C, cynomolgus monkey.
Results are expressed as percentage positive cells (SEM) in the monocyte gate.
Gross Pathology.
At autopsy, nonvaccinated rhesus showed granulomas and large necrotic abscesses in the right lower and apical lung lobes and moderately to severely enlarged tracheobronchial lymph nodes. The left lungs were hardly affected, although in one animal hyperemic foci were present near the bifurcation. In two nonvaccinated rhesus, miliary dissemination was present in the pericardium, spleen, and/or liver. In bacillus Calmette–Guérin-vaccinated rhesus monkeys, gross pathology in the lung was comparable to the nonvaccinated rhesus monkeys, but no dissemination to other organs was found. Approximately 50–80% of the right lung was involved in the nonvaccinated group, and 40–60% of the right lung was involved in the bacillus Calmette–Guérin-vaccinated group (Table 1). Liquefaction and cavitation formation was observed in both groups. In cynomolgus monkeys, for comparison, a large difference was found between vaccinated and nonvaccinated animals. In nonvaccinated animals, granulomas with caseous necrosis were present throughout the right lower and apical lung lobes (Table 1). In one animal, both lungs were affected heavily. As in rhesus, nonvaccinated cynomolgus presented severely enlarged tracheobronchial lymph nodes with necrotic granulomas, although no evidence for miliary dissemination was observed. In contrast to the extensive pathology found in the nonvaccinated animals, less than 5% of the lungs were affected in the lungs of bacillus Calmette–Guérin-vaccinated cynomolgus, and no necrosis was found (Table 1). The tracheobronchial lymph nodes were macroscopically normal, and there was no evidence for hematogenous spread of the infection.
Histopathology.
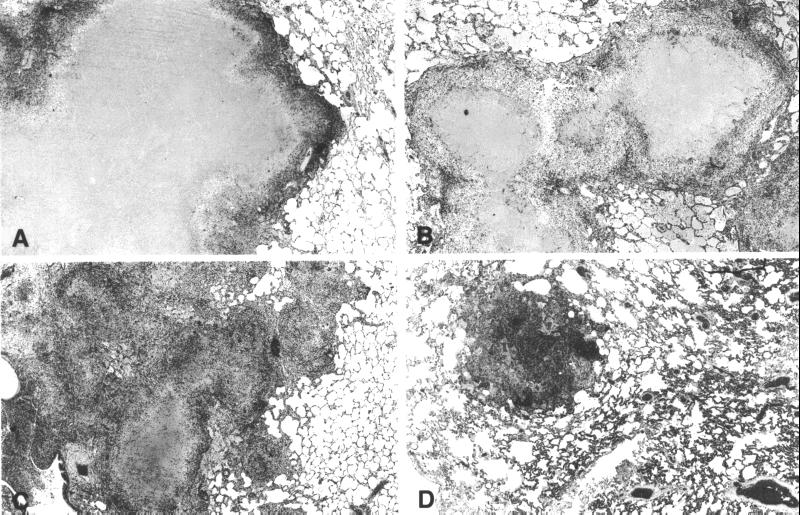
On microscopic examination, nonvaccinated rhesus showed large lung granulomas consisting of central caseation necrosis with epithelioid cells and numerous Langhans' giant cells. A similar pattern of inflammation was observed in bacillus Calmette–Guérin-vaccinated rhesus (Fig. 3, A and B). Nonvaccinated cynomolgus also developed typical granulomas with central caseation necrosis, although fewer Langhans' giant cells were present (Fig. 3C). Lung sections of bacillus Calmette–Guérin-vaccinated cynomolgus monkeys showed significantly less inflammation compared with the nonvaccinated controls. Moreover, the small granulomas encountered were largely fibrotic without caseation necrosis and Langhans' cells, and mycobacteria could not be found (Fig. 3D). In the other groups, limited numbers of bacteria were present in necrotic areas and occasionally in the cytoplasm of macrophages. In general, the number of bacteria paralleled the amount of necrosis.
Figure 3.
Histopathology of lungs at week 9 of TB infection in nonvaccinated (A) and bacillus Calmette–Guérin-vaccinated (B) rhesus and nonvaccinated (C) cynomolgus monkeys showing large granulomas with caseous necrosis (A, B, and C). In vaccinated cynomolgus monkeys (D), only a few largely fibrotic granulomas are evident (hematoxylin/eosin staining, original magnification ×6.6).
Bacterial Counts.
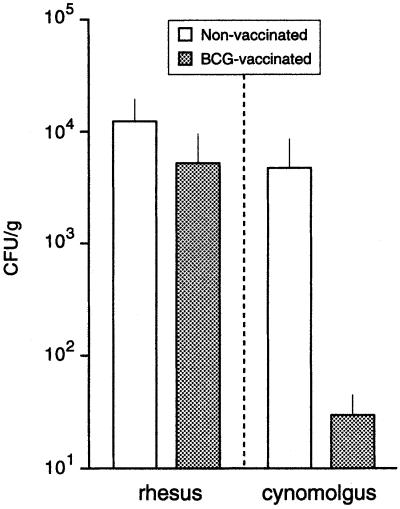
At autopsy, parts of the lung tissue were taken for determination of cfu. The lung tissue of bacillus Calmette–Guérin-vaccinated rhesus monkeys contained only two to three times less cfu than that of nonvaccinated rhesus, and this difference was not statistically significant. The number of cfu in lung tissue of nonvaccinated cynomolgus was similar to that of rhesus monkeys, but in marked contrast to rhesus monkeys, bacillus Calmette–Guérin vaccination efficiently protected cynomolgus and resulted in more than a 100-fold lower cfu than in nonvaccinated controls (Fig. 4).
Figure 4.
Mean levels (SEM) of M. tuberculosis cfu/g of lung at week 9 of infection. The effect of vaccination in cynomolgus monkeys was significant (unpaired t test, P = <0.01). BCG, bacillus Calmette–Guérin.
Discussion
Both rhesus and cynomolgus monkeys are susceptible to M. tuberculosis, although to different degrees. Bacillus Calmette–Guérin-induced protection in these animals is markedly different. Bacillus Calmette–Guérin vaccination protects cynomolgus monkeys from a challenge with 3,000 cfu M. tuberculosis, resulting in >2-log reduction of the bacterial load and diminished pathology, whereas only a minimal effect of bacillus Calmette–Guérin was observed in rhesus. These two closely related species represent the extremes of bacillus Calmette–Guérin-induced protection seen in humans. Such differences in bacillus Calmette–Guérin-induced protection and susceptibility to infection suggest that further studies will provide insight relevant to an understanding of the human response to vaccination and infection.
Both species showed specific cell-mediated immune responses after bacillus Calmette–Guérin vaccination as observed by skin test, lymphoproliferation, and IFN-γ production. Although PPD-specific IFN-γ responses after bacillus Calmette–Guérin vaccination were higher in vaccinated rhesus than in vaccinated cynomolgus, in rhesus bacillus Calmette–Guérin vaccination reduced pathology and bacterial load in the lungs after challenge only marginally. This result suggests that although IFN-γ is essential to control mycobacterial infection (26), the amount of IFN-γ induced in lymphocytes of vaccinated individuals will not be a reliable indicator for the protective capacity of a vaccine. We have found similar differences in IFN-γ response in an ongoing study comparing rhesus and cynomolgus monkeys immunized with a TB subunit vaccine and subsequently challenged. Notably, after challenge early secretory antigenic target 6-induced release of IFN-γ by PBMCs from these animals was more than 5-fold lower in protected animals than in nonprotected animals (unpublished data). Together, these data indicate that the amount of IFN-γ produced before and during the infection is not very predictive with respect to protection.
Several cell types including γδ T lymphocytes have been implicated in the control of mycobacterial infections (17). All animals showed a small, nonsignificant increase in γδ T cells during the infection, but it is possible that the activation of these T lymphocytes differs between the groups (27). An increase in CD64-expressing cells, indirectly indicating production of IFN-γ (28), was also observed in all animals. The various cell populations will be the subject of additional studies.
Granulomas were markedly different in the bacillus Calmette–Guérin-immunized cynomolgus monkeys when compared with other groups, the most notable difference being that they were fibrotic rather than necrotic, indicating healing processes. Overall, we observed in both species well defined granulomas with a core of caseation necrosis surrounded by histiocytes, Langhans' giant cells, and various lymphocytic infiltrates. This closely matches the human picture, as does the inverse correlation between the density of the infiltrate and the extent of necrosis. Both humans and monkeys display caseous necrotic areas containing only few mycobacteria. In contrast, immunologically intact M. tuberculosis-infected mice show differences in the pulmonary granulomatous response such as a lack of caseating necrosis and the absence of Langhans' giant cells (29). Mice deficient in IFN-γ form numerous Langhans' giant cells (30), whereas in nonhuman primates we observed numerous Langhans' giant cells in rhesus, the animals that produced most IFN-γ. An essential role for IFN-γ in the formation of Langhans' giant cells from human monocytic cells has been reported (31), and although it is possible that the observed differences in numbers of Langhans' cells are caused by differences in IFN-γ production between the two species, it is not likely. Patients with diminished IFN-γ production do not show a diminished appearance of Langhans' cells in granulomas (32). Although Langhans' cells have been implicated in the limitation of bacterial spreading (33), we found numerous Langhans' cells in the most susceptible animals. Interestingly, we did not find them in protected bacillus Calmette–Guérin-vaccinated cynomolgus monkeys, in which the occurrence of fibrosis in the granulomas indicates clearance or containment of the bacteria. One of the dangerous processes in human TB is the liquefaction of solid caseous lesions and cavity formation (34). Apart from macaques, rabbits are the only animal model showing this clinically important process in TB (35). Rhesus and cynomolgus are the only animal models that display the whole range of clinical and pathological changes induced by TB in humans. Moreover, most immunological reagents for humans can be used in macaques, which makes evaluation of a wide range of immune responses possible.
Our observation that bacillus Calmette–Guérin does not substantially protect rhesus monkeys from pulmonary M. tuberculosis infection contradicts earlier studies (20, 36–38). This might be due in part to the higher M. tuberculosis challenge dose that we used to allow a direct comparison between cynomolgus and rhesus (26). In earlier studies, animals were challenged within 2 months of bacillus Calmette–Guérin vaccination, and it is likely that some or all of the observed protection was because of bystander effects caused by an inflammatory response to bacillus Calmette–Guérin. We avoided this by removing bacillus Calmette–Guérin with chemotherapy followed by a rest period. In a subsequent study, we have confirmed the lack of bacillus Calmette–Guérin-induced protection in rhesus (data not shown).
Recent advances in TB genomics (39) will result in the identification of many potential vaccine candidates. Because of the complexity of human trials (8), only the most promising candidates should be advanced. New attenuated, live vaccines are likely to require thorough safety testing in models that are as close to human as possible before going into clinical trials. To justify human testing, new attenuated vaccine approaches must offer the prospect of significantly improved performance over bacillus Calmette–Guérin. Because of concerns over the safety of bacillus Calmette–Guérin especially in immuno-compromised individuals, subunit vaccines offering similar performance to bacillus Calmette–Guérin might be attractive replacements. Because of the need to compare performance with bacillus Calmette–Guérin, the selection of appropriate macaque models will be critical for both vaccine approaches. Our results suggest that cynomolgus monkeys offer a good experimental model for the evaluation of new TB subunit vaccines, whereas efficacy studies on improved live, attenuated vaccines may show clear improvement over bacillus Calmette–Guérin in the rhesus model.
The comparative failure of bacillus Calmette–Guérin in the outbred rhesus is unusual in that bacillus Calmette–Guérin immunization has been protective in nearly all animal species studied thus far. Further comparative studies between these species, which diverged phylogenetically only 1 million years ago, may provide critical insight into mechanisms involved in the induction of protection by bacillus Calmette–Guérin and in other host resistance factors against TB.
Acknowledgments
We thank Dr. Ronald Bontrop for critical reading of the manuscript and Con Regeer and the excellent animal care staff. This work was supported by ZorgOnderzoek Nederland Grant 2100.0017 and the EC DGXII program INCO-DEV Grants CT97-9104 and KA2 CT99-1903.
Abbreviations
- TB
tuberculosis
- cfu
colony-forming unit(s)
- PBMC
peripheral blood mononuclear cell
- PPD
purified protein derivative
- CRP
C-reactive protein
- OT
Old Tuberculin
References
- 1.Enarson D A, Chretien J. Curr Opin Pulm Med. 1999;5:128–135. doi: 10.1097/00063198-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Control. Switzerland: World Health Organization Geneva; 2001. [Google Scholar]
- 3.Murray C J, Salomon J A. Proc Natl Acad Sci USA. 1998;95:13881–13886. doi: 10.1073/pnas.95.23.13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colditz G A, Brewer T F, Berkey C S, Wilson M E, Burdick E, Fineberg H V, Mosteller F. J Am Med Assoc. 1994;271:698–702. [PubMed] [Google Scholar]
- 5.Berthet F X, Lagranderie M, Gounon P, Laurent-Winter C, Ensergueix D, Chavarot P, Thouron F, Maranghi E, Pelicic V, Portnoi D, Marchal G, Gicquel B. Science. 1998;282:759–762. doi: 10.1126/science.282.5389.759. [DOI] [PubMed] [Google Scholar]
- 6.Guleria I, Teitelbaum R, McAdam R A, Kalpana G, Jacobs W R, Jr, Bloom B R. Nat Med. 1996;2:334–337. doi: 10.1038/nm0396-334. [DOI] [PubMed] [Google Scholar]
- 7.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D'Souza C, et al. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 8.Ellner J J. J Infect Dis. 1997;176:1351–1359. doi: 10.1086/514132. [DOI] [PubMed] [Google Scholar]
- 9.Baldwin S L, D'Souza C, Roberts A D, Kelly B P, Frank A A, Lui M A, Ulmer J B, Huygen K, McMurray D M, Orme I M. Infect Immun. 1998;66:2951–2959. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMurray D N. Clin Infect Dis. 2000;30, Suppl. 3:S210–S212. doi: 10.1086/313885. [DOI] [PubMed] [Google Scholar]
- 11.Bontrop R E, Otting N, de Groot N G, Doxiadis G G. Immunol Rev. 1999;167:339–350. doi: 10.1111/j.1600-065x.1999.tb01403.x. [DOI] [PubMed] [Google Scholar]
- 12.Geluk A, Elferink D G, Slierendregt B L, van Meijgaarden K E, de Vries R R, Ottenhoff T H, Bontrop R E. J Exp Med. 1993;177:979–987. doi: 10.1084/jem.177.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porcelli S, Morita C T, Brenner M B. Nature (London) 1992;360:593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 14.Shinkai K, Locksley R M. J Exp Med. 2000;191:907–914. doi: 10.1084/jem.191.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behar S M, Dascher C C, Grusby M J, Wang C R, Brenner M B. J Exp Med. 1999;189:1973–1980. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Constant P, Davodeau F, Peyrat M A, Poquet Y, Puzo G, Bonneville M, Fournie J J. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 17.Carding S R, Egan P J. Immunol Rev. 2000;173:98–108. doi: 10.1034/j.1600-065x.2000.917302.x. [DOI] [PubMed] [Google Scholar]
- 18.Poccia F, Malkovsky M, Pollak A, Colizzi V, Sireci G, Salerno A, Dieli F. Mol Med. 1999;5:471–476. [PMC free article] [PubMed] [Google Scholar]
- 19.Bontrop R E, Otting N, Slierendregt B L, Lanchbury J S. Immunol Rev. 1995;143:33–62. doi: 10.1111/j.1600-065x.1995.tb00669.x. [DOI] [PubMed] [Google Scholar]
- 20.Ribi E, Anacker R L, Barclay W R, Brehmer W, Harris S C, Leif W R, Simmons J. J Infect Dis. 1971;123:527–538. doi: 10.1093/infdis/123.5.527. [DOI] [PubMed] [Google Scholar]
- 21.Good R C. Ann NY Acad Sci. 1968;154:200–213. doi: 10.1111/j.1749-6632.1968.tb16710.x. [DOI] [PubMed] [Google Scholar]
- 22.Walsh G P, Tan E V, dela Cruz E C, Abalos R M, Villahermosa L G, Young L J, Cellona R V, Nazareno J B, Horwitz M A. Nat Med. 1996;2:430–436. doi: 10.1038/nm0496-430. [DOI] [PubMed] [Google Scholar]
- 23.Stunkard J A, Szatalowicz F T, Sudduth H C. Am J Vet Res. 1971;32:1873–1878. [PubMed] [Google Scholar]
- 24.Julian E, Matas L, Hernandez A, Alcaide J, Luquin M. Int J Tuberc Lung Dis. 2000;4:1082–1085. [PubMed] [Google Scholar]
- 25.Jouanguy E, Lamhamedi-Cherradi S, Lammas D, Dorman S E, Fondaneche M C, Dupuis S, Doffinger R, Altare F, Girdlestone J, Emile J F, et al. Nat Genet. 1999;21:370–378. doi: 10.1038/7701. [DOI] [PubMed] [Google Scholar]
- 26.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behr-Perst S I, Munk M E, Schaberg T, Ulrichs T, Schulz R J, Kaufmann S H. J Infect Dis. 1999;180:141–149. doi: 10.1086/314844. [DOI] [PubMed] [Google Scholar]
- 28.Perussia B, Dayton E T, Lazarus R, Fanning V, Trinchieri G. J Exp Med. 1983;158:1092–1113. doi: 10.1084/jem.158.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhoades E R, Frank A A, Orme I M. Tuber Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 30.Sugawara I, Yamada H, Kazumi Y, Doi N, Otomo K, Aoki T, Mizuno S, Udagawa T, Tagawa Y, Iwakura Y. J Med Microbiol. 1998;47:871–877. doi: 10.1099/00222615-47-10-871. [DOI] [PubMed] [Google Scholar]
- 31.Anderson J M. Curr Opin Hematol. 2000;7:40–47. doi: 10.1097/00062752-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Altare F, Durandy A, Lammas D, Emile J F, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Doffinger R, Bernaudin F, et al. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 33.Byrd T F. Cell Immunol. 1998;188:89–96. doi: 10.1006/cimm.1998.1352. [DOI] [PubMed] [Google Scholar]
- 34.Dannenberg A M, Rook G A W. In: Tuberculosis: Pathogenesis, Protection, and Control. Bloom B R, editor. Washington, DC: Am. Soc. Microbiol.; 1994. pp. 459–483. [Google Scholar]
- 35.Converse P J, Dannenberg A M, Estep J E, Sugisaki K, Abe Y, Schofield B H, Pitt M L. Infect Immun. 1996;64:4776–4787. doi: 10.1128/iai.64.11.4776-4787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barclay W R, Anacker R L, Brehmer W, Leif W, Ribi E. Infect Immun. 1970;2:574–582. doi: 10.1128/iai.2.5.574-582.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barclay W R, Busey W M, Dalgard D W, Good R C, Janicki B W, Kasik J E, Ribi E, Ulrich C E, Wolinsky E. Am Rev Respir Dis. 1973;107:351–358. doi: 10.1164/arrd.1973.107.3.351. [DOI] [PubMed] [Google Scholar]
- 38.Janicki B W, Good R C, Minden P, Affronti L F, Hymes W F. Am Rev Respir Dis. 1973;107:359–366. doi: 10.1164/arrd.1973.107.3.359. [DOI] [PubMed] [Google Scholar]
- 39.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry C E, 3rd, et al. Nature (London) 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]