Abstract
The relationship between ABO blood groups (BG) and risk of incidence in cancers including gynecological cancers has been widely studied, showing increased incidence risk for BG A patients. As available data are inconsistent we investigated whether BG and their anti-glycan antibodies (anti-A and anti-B) have prognostic values in gynecological cancers. We retrospectively evaluated 974 patients with gynecological cancers in three cancer centers (Switzerland and Australia) between 1974 and 2014 regarding the relationships between clinico-pathological findings and the BG. Time to disease recurrence was significantly influenced by BG in patients with ovarian (n = 282) and vulvar (n = 67) cancer. BG O or B patients showed a significantly increased risk for ovarian cancer relapse compared to A, 59% and 82%, respectively (p = 0.045; HR O vs A = 1.59 (CI 1.01–2.51) and (p = 0.036; HR A vs B = 0.55 (CI 0.32–0.96). Median time to relapse for advanced stage (n = 126) ovarian cancer patients was 18.2 months for BG O and 32.2 for A (p = 0.031; HR O vs A = 2.07 (CI 1.07–4.02)). BG also significantly influenced relapse-free survival in patients with vulvar cancer (p = 0.002), with BG O tending to have increased relapse risk compared to A (p = 0.089). Blood groups hence associate with recurrence in ovarian and vulvar cancer: women with BG O seem to have a lower ovarian cancer incidence, however are more likely to relapse earlier. The significance of the BG status as a prognostic value is evident and may be helpful to oncologists in prognosticating disease outcome and selecting the appropriate therapy.
Introduction
The ABO blood group (BG) in humans is the most important BG system in transfusion and transplantation medicine. It is defined by two glycans, antigen A (GalNAcα1-3(Fucα1–2)Galβ1) and B (Galα1-3(Fucα1–2)Galβ1) and is determined by the ABO gene that encodes the A and B allele, resulting in two different glycosyltransferase activities. These activities add either N-acetylgalactosamine or galactose to the precursor H antigen to form A or B antigen, respectively. The absence of both antigens in BG O owes to a frameshift mutation at the N-terminus of the enzyme [1]. ABO BG antigens are commonly expressed on cell surface glycosphingolipids or glycoproteins present on erythrocytes and on a variety of other human cells and tissues (e.g. gastro-intestinal, bronchopulmonary, skin and urogenital epithelial cells [2]), and also occur in various body fluids and secretions [3].
ABO BG are involved in several benign and malignant diseases [4] and the relationship between human BG and cancer is well known [5]. Several studies have shown associations between ABO BG and incidence and risk for various cancers [5] including ovarian cancer[5–7] and several plausible explanations have been proposed explaining the observed associations of ABO BG and cancer: these include inflammation, immune surveillance for malignant cells, modified expression of ABO BG antigens on cancer cells as a consequence of altered glycosyltransferase activities [8], intercellular adhesion and membrane signaling [9], single nucleotide polymorphisms and epigenetics [10].
Ovarian cancer (OC), usually diagnosed at an advanced FIGO stage, is the fifth leading cause of cancer death for women and the most lethal gynecological cancer in women and despite improved surgical techniques and drug regimens, overall survival has not changed significantly for several decades [11]. In addition, currently used screening methods seem not accurate enough: the combination of tumor marker CA125 and transvaginal ultrasound allows measurement of triaging indices such as the Risk of Malignancy Index (RMI) and facilitates discrimination between benign and malignant ovarian masses [12], and screening trials (PLCO-, UKCTOCS-trials) have not shown benefit in terms of disease specific survival [13, 14].
The search for new and highly specific biomarkers for both early disease detection and disease prognosis is still needed and ongoing. We have previously shown that the level of plasma-derived anti-glycan antibodies to P1 trisaccharide significantly discriminates between OC patients and healthy women, suggesting P1 as an OC-associated carbohydrate antigen[15]. Interestingly, P1 carbohydrate antigen belongs to the human P BG system and shares oligosaccharide sequences with Pk and P antigens [16]. In analogy to this and as we know that the classical blood system, with his anti-glycan antibodies, is involved in the pathogenesis of several malignancies, we were interested in looking for survival association in gynecological cancer liable to identify a prognostic marker.
Only a small body of data has been reported regarding associations between ABO BG and survival [17–20] and even fewer and inconsistent data are available for gynecological cancers in general and OC in particular [21, 22]. We therefore retrospectively evaluated and compared the clinic-pathological findings including relapse-free survival (RFS), disease-specific survival (DSS), and overall survival (OS) of a large gynecological cancer patient cohort (n = 974) to the ABO BG status.
Materials and methods
Study cohort description
Clinicopathological databases between 1974 and 2014 from three gynecological cancer centers in Switzerland (Basel, Zurich) and Australia (Sydney) were reviewed. Patients’ eligibility criteria included at least a histologically confirmed gynecological cancer, a complete remission after primary treatment, and an available BG status. Patients who deceased from causes other than cancer or developed a second primary tumor which is different from the cancer they had initially were censored in this study. Disease recurrence was diagnosed on a clinical basis (symptoms) and/or increasing tumor marker followed by radiological confirmation. Histological grades were defined according to the World Health Organization, and extent of disease by FIGO stage. All patients underwent surgery with curative intent, adjuvant chemotherapy and/or radiotherapy unless refused, as recommended by the interdisciplinary tumor conferences. These recommendations were based on international data and guidelines and were individualized depending to the patient’s co-morbidities. BG status (ABO system) of all patients was determined serologically before their surgery. Patients were followed up every 3 months for the first two years and then every 6 months until 5 years after completion of primary treatment, and then then once yearly. In total 974 patients were analyzed, subdivided into 282 cases of ovarian, 56 peritoneal, 23 tubal, 377 endometrial, 149 cervical, 11 vaginal, 67 vulvar, and 9 synchronous ovarian/endometrial cancers. This study was approved by the Swiss Medical Ethical Committee, EKNZ 2015–436. Neither written nor oral consent was necessary for thir retrospective study and data accession was anonymous.
Statistical analysis
Descriptive statistics comparing the study groups are reported as counts and percentages or as mean and standard deviation (SD) as appropriate. Corresponding p-values were calculated using Fisher’s exact tests (counts) and t-Tests (ordinal data). Relationships between clinic-pathological findings, ABO BG, and outcome (RFS, DSS, and OS) were analyzed. RFS was defined as the period from the date of diagnosis to the date of disease recurrence (as described above). DSS was calculated from the date of diagnosis to death from disease. Deaths of unknown cause or other than disease were censored. Kaplan-Meier analysis was used to calculate the survival rate or time to event analysis (RFS, DSS, and OS) with a 95% confidence interval (CI). Data for 5-year OS were also reported. The Log-rank test was used to compare the survival curves. Additionally, Cox-Regression analysis including a center effect comparing each blood group was performed. Analyses were not adjusted for covariates. Results (median values) are reported with hazard ratios (HR), corresponding 95% CI’s and p-values. Reported p-values were two-sided and p < 0.05 was considered statistically significant. The statistical analyses were performed using R version 3.0.1.
Results
Clinico-pathological characteristics for all gynecological cancers patients sorted by blood groups
This study includes 974 patients with various gynecological cancers. The mean age was 62.8 ± 13.7 years and the mean follow-up 4.8 ± 33.7 years. The BG distribution was 471 patients with BG A (48.4%), 94 patients with B (9.6%), 375 patients with O (38.5%), 34 patients with AB (3.5%), and was similar to that of the general population in Europe. The cohort comprised ovarian (n = 282), peritoneal (n = 56), tubal (n = 23), cervical (n = 149), endometrial (n = 377), vaginal (n = 11), and vulvar cancer (n = 67) patients. This and additional information on the study cohort including tumor type, histology, stage, grade, residual disease, survival status, and recurrence status regarding the BG status is summarized in Table 1.
Table 1. Clinico-pathological data of gynecological cancer cohort sorted by blood group.
| ABO blood group | |||||||
|---|---|---|---|---|---|---|---|
| ALL | O | A | AB | B | p | n | |
| Characteristic | |||||||
| Number of women | 974 | 375 | 471 | 34 | 94 | ||
| Percentage of total (%) | 100.0 | 38.5 | 48.4 | 3.5 | 9.7 | ||
| Cancer Center | 0.038 | 974 | |||||
| Basel | 708 (72.7%) | 265 (70.7%) | 364 (77.3%) | 22 (64.7%) | 57 (60.6%) | ||
| Sydney | 210 (21.6%) | 89 (23.7%) | 83 (17.6%) | 9 (26.5%) | 29 (30.9%) | ||
| Zürich | 56 (5.75%) | 21 (5.60%) | 24 (5.10%) | 3 (8.82%) | 8 (8.51%) | ||
| Mean age (years) (±SD) | 62.8 (13.7) | 63.1 (13.8) | 62.9 (13.5) | 62.3 (12.2) | 61.5 (14.5) | 0.786 | 940 |
| Organ | 0.035 | 974 | |||||
| Cervix | 149 (15.3%) | 54 (14.4%) | 81 (17.2%) | 5 (14.7%) | 9 (9.57%) | ||
| Endometrium | 377 (38.7%) | 156 (41.6%) | 182 (38.6%) | 13 (38.2%) | 26 (27.7%) | ||
| Ovaries | 282 (29.0%) | 100 (26.7%) | 138 (29.3%) | 10 (29.4%) | 34 (36.2%) | ||
| Ovaries & Endometrium | 9 (0.92%) | 2 (0.53%) | 5 (1.06%) | 1 (2.94%) | 1 (1.06%) | ||
| Peritoneum | 56 (5.75%) | 22 (5.87%) | 18 (3.82%) | 3 (8.82%) | 13 (13.8%) | ||
| Fallopian tube | 23 (2.36%) | 9 (2.40%) | 9 (1.91%) | 1 (2.94%) | 4 (4.26%) | ||
| Vagina | 11 (1.13%) | 8 (2.13%) | 3 (0.64%) | 0 (0.00%) | 0 (0.00%) | ||
| Vulva | 67 (6.88%) | 24 (6.40%) | 35 (7.43%) | 1 (2.94%) | 7 (7.45%) | ||
| Tumour type | 0.183 | 974 | |||||
| Adenocarcinoma | 733 (75.3%) | 286 (76.3%) | 345 (73.2%) | 25 (73.5%) | 77 (81.9%) | ||
| Squamous cell carcinoma | 193 (19.8%) | 71 (18.9%) | 103 (21.9%) | 6 (17.6%) | 13 (13.8%) | ||
| Carcinosarcoma (MMMT) | 26 (2.67%) | 11 (2.93%) | 12 (2.55%) | 1 (2.94%) | 2 (2.13%) | ||
| Adenosarcoma | 1 (0.10%) | 1 (0.27%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||
| Adenosquamous carcinoma | 2 (0.21%) | 1 (0.27%) | 1 (0.21%) | 0 (0.00%) | 0 (0.00%) | ||
| Sarcoma | 7 (0.72%) | 4 (1.07%) | 0 (0.00%) | 2 (5.88%) | 1 (1.06%) | ||
| Carcinoid | 1 (0.10%) | 1 (0.27%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||
| Brenner tumor | 3 (0.31%) | 0 (0.00%) | 2 (0.42%) | 0 (0.00%) | 1 (1.06%) | ||
| Sertoli-Leydig tumor | 2 (0.21%) | 0 (0.00%) | 2 (0.42%) | 0 (0.00%) | 0 (0.00%) | ||
| Granulosacell tumor | 6 (0.62%) | 0 (0.00%) | 6 (1.27%) | 0 (0.00%) | 0 (0.00%) | ||
| Histology | 0.427 | 709 | |||||
| Serous | 251 (35.4%) | 92 (33.3%) | 115 (34.3%) | 9 (37.5%) | 35 (47.3%) | ||
| Endometrioid | 339 (47.8%) | 132 (47.8%) | 165 (49.3%) | 11 (45.8%) | 31 (41.9%) | ||
| Mucinous | 24 (3.39%) | 9 (3.26%) | 10 (2.99%) | 1 (4.17%) | 4 (5.41%) | ||
| Clear cell | 23 (3.24%) | 9 (3.26%) | 12 (3.58%) | 2 (8.33%) | 0 (0.00%) | ||
| Neuroendocrine | 3 (0.42%) | 1 (0.36%) | 1 (0.30%) | 0 (0.00%) | 1 (1.35%) | ||
| Mixed/unknown/other | 69 (9.73%) | 33 (4.65%) | 32 (4.51%) | 1 (0.14%) | 3 (0.42%) | ||
| FIGO Stage | 0.524 | 662 | |||||
| I | 259 (39.1%) | 105 (40.5%) | 111 (36.2%) | 11 (45.8%) | 32 (44.4%) | ||
| II | 85 (12.8%) | 31 (12.0%) | 45 (14.7%) | 4 (16.7%) | 5 (6.94%) | ||
| III | 242 (36.6%) | 95 (36.7%) | 110 (35.8%) | 7 (29.2%) | 30 (41.7%) | ||
| IV | 76 (11.5%) | 28 (10.8%) | 41 (13.4%) | 2 (8.33%) | 5 (6.94%) | ||
| Tumour grade | 0.510 | 762 | |||||
| G1 | 185 (24.3%) | 66 (22.2%) | 96 (26.2%) | 3 (12.5%) | 20 (26.7%) | ||
| G2 | 238 (31.2%) | 98 (33.0%) | 112 (30.6%) | 6 (25.0%) | 22 (29.3%) | ||
| G3 | 339 (44.5%) | 133 (44.8%) | 158 (43.2%) | 15 (62.5%) | 33 (44.0%) | ||
| Residual Disease | 0.571 | 441 | |||||
| optimal debulking | 303 (68.7%) | 118 (67.4%) | 144 (70.9%) | 10 (76.9%) | 31 (62.0%) | ||
| suboptimal debulking | 138 (31.3%) | 57 (32.6%) | 59 (29.1%) | 3 (23.1%) | 19 (38.0%) | ||
| Survival status | 0.962 | 933 | |||||
| alive | 829 (88.9%) | 317 (89.0%) | 401 (88.5%) | 29 (87.9%) | 82 (90.1%) | ||
| dead of disease | 104 (11.1%) | 39 (11.0%) | 52 (11.5%) | 4 (12.1%) | 9 (9.89%) | ||
| Recurrence | 0.009 | 974 | |||||
| no | 750 (77.0%) | 298 (79.5%) | 368 (78.1%) | 24 (70.6%) | 60 (63.8%) | ||
| yes | 108 (23.0%) | 77 (20.5%) | 103 (21.9%) | 10 (29.4%) | 34 (36.2%) | ||
Data from gynecological cancer centers (Basel and Zurich, Switzerland) and Sydney (Australia) collected between 1974 and 2014. P-values calculated by t-test or Fisher’s exact tests.
Effect of blood group status on RFS, DSS, and OS in all gynecological cancers patients
Time-to-event analysis was performed for all 7 gynecological cancer types. For DSS, no significant associations to BG were found for these cancer types: ovarian (p = 0.696), peritoneal (p = 0.28), tubal (p = 0.366), cervical (p = 0.723), endometrial (p = 0.39), vaginal (p = 0.26), and vulvar (VC, p = 0.29) cancer. No significant associations to BG were also found for RFS in peritoneal (p = 0.889), tubal (p = 0.814), cervical (p = 0.638), endometrial (p = 0.492) or vaginal (p = 0.480) cancer. In contrast, associations for RFS and BG were found for OC and VC. No significant associations for OS were found for the whole cohort (p = 0.287).
Effect of blood group status on RFS and OS in ovarian cancer patients
The OC group comprised 282 patients with a mean age at diagnosis of 60.7 ± 13.7 years and mean follow-up time of 3.26 ± 4.12 years. BG distribution was 100 (35.5%) with BG O, 138 (48.9%) with A, 10 (3.5%) with AB, and 34 (12.1%) with B. These and additional clinico-pathological data are given in Table 2.
Table 2. Clinico-pathological data of ovarian cancer cohort sorted by blood group.
| ABO blood group | |||||||
|---|---|---|---|---|---|---|---|
| ALL | O | A | AB | B | p | n | |
| Characteristic | |||||||
| Number of women | 282 | 100 | 138 | 10 | 34 | 282 | |
| Percentage of total (%) | 100 | 35.46 | 48.94 | 3.55 | 12.06 | ||
| Cancer Center | 0.16 | 282 | |||||
| Basel | 160 (56.7%) | 55 (55.0%) | 85 (61.6%) | 6 (60.0%) | 14 (41.2%) | ||
| Sydney | 87 (30.9%) | 36 (36.0%) | 33 (23.9%) | 3 (30.0%) | 15 (44.1%) | ||
| Zürich | 35 (12.4%) | 9 (9.00%) | 20 (14.5%) | 1 (10.0%) | 5 (14.7%) | ||
| Mean age (years) (±SD) | 60.7 (13.7) | 61.1(13.9) | 60.2 (13.3) | 57.4 (12.3) | 63.1 (15.1) | 0.599 | 276 |
| Tumour type | 0.397 | 282 | |||||
| Adenocarcinoma | 257 (91.1%) | 93 (93.0%) | 123 (89.1%) | 10 (100%) | 31 (91.2%) | ||
| Carcinosarcoma (MMMT) | 13 (4.61%) | 6 (6.00%) | 5 (3.62%) | 0 (0.00%) | 2 (5.88%) | ||
| Carcinoid | 1 (0.35%) | 1 (1.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||
| Brenner tumor | 3 (1.06%) | 0 (0.00%) | 2 (1.45%) | 0 (0.00%) | 1 (2.94%) | ||
| Sertoli-Leydig tumor | 2 (0.71%) | 0 (0.00%) | 2 (1.45%) | 0 (0.00%) | 0 (0.00%) | ||
| Granulosacell tumor | 6 (2.13%) | 0 (0.00%) | 6 (4.35%) | 0 (0.00%) | 0 (0.00%) | ||
| Histology | 0.645 | 261 | |||||
| Serous | 160 (61.3%) | 53 (56.4%) | 83 (65.9%) | 4 (40.0%) | 20 (64.5%) | ||
| Endometrioid | 42 (16.1%) | 16 (17.0%) | 18 (14.3%) | 2 (20.0%) | 6 (19.4%) | ||
| Mucinous | 21 (8.05%) | 8 (8.51%) | 8 (6.35%) | 1 (10.0%) | 4 (12.9%) | ||
| Clear cell | 11 (4.21%) | 5 (5.32%) | 4 (3.17%) | 2 (20.0%) | 0 (0.00%) | ||
| Neuroendocrine | 1 (0.38%) | 1 (1.06%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||
| Mixed/unknown/other | 26 (9.96%) | 11 (11.70%) | 13 (10.32%) | 1 (10.0%) | 1 (3.23%) | ||
| FIGO Stage | 0.59 | 247 | |||||
| I | 64 (25.9%) | 22 (23.9%) | 27 (23.7%) | 4 (40.0%) | 11 (35.5%) | ||
| II | 21 (8.50%) | 10 (10.9%) | 8 (7.02%) | 1 (10.0%) | 2 (6.45%) | ||
| III | 126 (51.0%) | 44 (47.8%) | 61 (53.5%) | 4 (40.0%) | 17 (54.8%) | ||
| IV | 36 (14.6%) | 16 (17.4%) | 18 (15.8%) | 1 (10.0%) | 1 (3.23%) | ||
| Tumour grade | 0.519 | 216 | |||||
| G1 | 34 (15.7%) | 14 (17.5%) | 14 (13.7%) | 1 (12.5%) | 5 (19.2%) | ||
| G2 | 35 (16.2%) | 17 (21.2%) | 12 (11.8%) | 1 (12.5%) | 5 (19.2%) | ||
| G3 | 147 (68.1%) | 49 (61.3%) | 76 (74.5%) | 6 (75.0%) | 16 (61.5%) | ||
| Residual Disease | 0.783 | 170 | |||||
| optimal debulking | 106 (62.4%) | 41 (61.2%) | 47 (62.7%) | 5 (83.3%) | 13 (59.1%) | ||
| suboptimal debulking | 64 (37.6%) | 26 (38.8%) | 28 (37.3%) | 1 (16.7%) | 9 (40.9%) | ||
| Survival status | 0.781 | 274 | |||||
| alive | 226 (82.5%) | 81 (82.7%) | 107 (80.5%) | 9 (90.0%) | 29 (87.9%) | ||
| dead of disease | 48 (17.5%) | 17 (17.3%) | 26 (19.5%) | 1 (10.0%) | 4 (12.1%) | ||
| Recurrence | 0.128 | 282 | |||||
| no | 174 (61.7%) | 67 (67.0%) | 86 (62.3%) | 6 (60.0%) | 15 (44.1%) | ||
| yes | 108 (38.3%) | 33 (33.0%) | 52 (37.7%) | 4 (40.0%) | 19 (55.9%) | ||
Data from gynecological cancer centers (Basel and Zürich, Switzerland) and Sydney (Australia) collected between 1974 and 2014. P-values calculated by T-Tests or Fisher’s exact Tests.
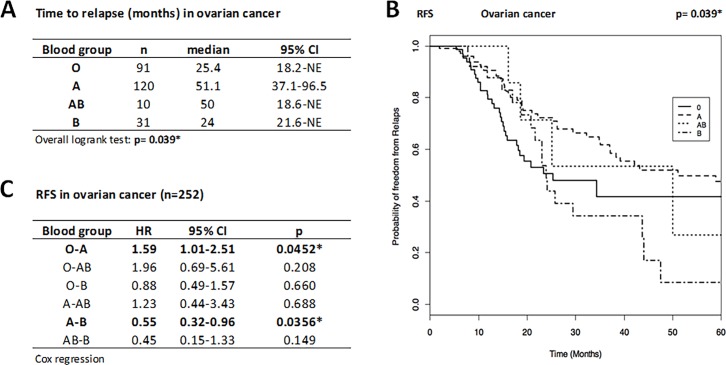
The median time until relapse for OC patients was 25.4 months for BG O, 51.1 months for A, 50.0 months for AB and 24.0 months for B (Fig 1A and 1B), indicating that patients with BG A relapsed about two years later than patients with BG O.
Fig 1.
Time to relapse (A), Kaplan-Meier curve for RFS (B), and HR for disease recurrence (C) in ovarian cancer patients (n = 252). BG O and B patients showed a significantly increased risk for relapse compared to A patients (59%, p = 0.045 and 82%, p = 0.036, respectively; Cox regression). Hence, BG A patients have better prognosis with a significant longer RFS than those with O and B. Time to relapse presented as median (months) and 95%CI and compared by overall logrank test and disease recurrence risk presented as HR and 95%CI. Statistical significance marked by asterisks (*) or highlighted. NE, not estimable. RFS given as probability of freedom from relapse as a function of time (months).
Statistical analysis showed a significant (p = 0.039) overall influence of the BG on the time to relapse in OC (Fig 1C): patients with BG O showed a statistically significant (p = 0.045) 59% (HR O vs A = 1.59) increased risk for OC relapse and patients with BG B a statistically significant (p = 0.036) 82% (HR B vs A = 1.82 = 1/0.55 given in Fig 1C) increased risk for OC relapse compared to those with BG A, indicating that patients with BG A have better prognosis with a significant longer relapse-free survival than those with BG O and BG B. The other comparisons did not reveal any significant differences for relapse risk. Likewise, no difference in OS was found among the blood groups (p = 0.665). The 5-year OS was 0.694 (95%CI: 0.531–0.907) for BG O and 0.734 (95%CI: 0.624–0.863) for BG A (values for BG AB and BG B not reported because of low number of cases and events).
Effect of blood group status on RFS and OS in vulvar cancer patients
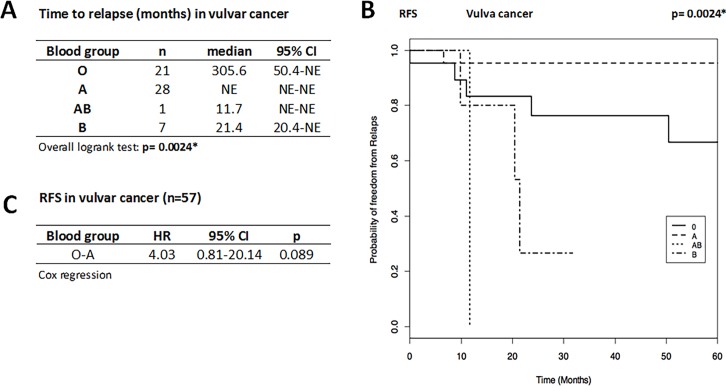
The group with VC comprised 67 patients with a mean age at diagnosis of 70.0 ± 13.8 years and mean follow-up time of 5.15 ± 5.5 years. BG distribution was 35.5% with BG O, 52.2% with A, 1.5% with AB, and 10.5% with B. The data showed (Fig 2A and 2B) that the median time until relapse was 305.6 months for BG O (n = 21), 21.4 months for BG B (n = 7), 11.7 months for the one patient with BG AB, and was not estimable for the 28 patients with BG A (none relapsed within 60 months). Despite the low number of cases and non-estimable data, a highly significant (p = 0.0024) relationship between the BG and the time to relapse for VC was found. Cox regression (Fig 2C) analysis for patients with BG O compared to A showed a trend for a 4-times longer time to relapse for BG A patients, whereas comparisons among the other groups were obsolete because of respectively low number of cases and non-estimable data. Data for OS are not reported due to low number of cases and events.
Fig 2.
Time to relapse (A), Kaplan-Meier curve for RFS (B), and HR for disease recurrence (C) in vulvar cancer patients (n = 57). BG O patients tend to have increased risk for relapse compared to A patients A (HR O vs A = 4.03, 95%CI: 0.81–20.14, p = 0.089), i.e. A patients have better prognosis with a trend to longer RFS than patients with BG O. Time to relapse presented as median (months) and 95%CI and compared by overall Logrank Test. Disease recurrence risk presented as HR and 95%CI by Cox regression (only possible for O vs A owing the small sample size for AB and B). Statistical significance marked by asterisks (*) or highlighted. NE, not estimable. RFS given as probability of freedom from relapse as a function of time (months).
Taken together, the data indicate that BG O is associated with higher risk for disease recurrence in OC and VC.
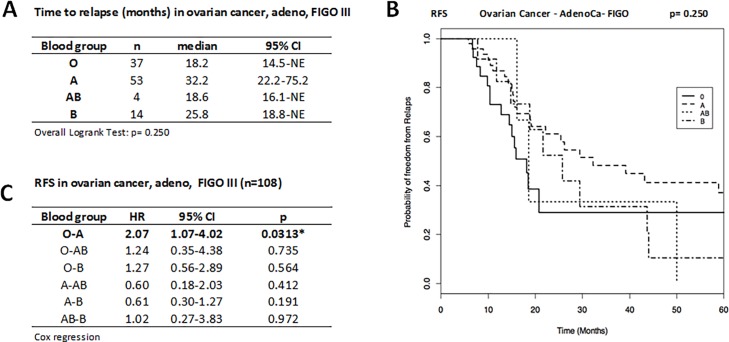
Effect of blood group status on FIGO III ovarian adenocarcinoma
In order to eliminate the contribution of the prognostic effect of the FIGO stage we determined the RFS and OS in FIGO stage III ovarian adenocarcinoma patients. In this more homogenous subgroup (n = 108), the median time until relapse was 18.2 months for BG O (n = 37), 32.2 months for A (n = 53), 18.6 months for AB (n = 4), and 14 months for B (n = 14), and hence were not significantly different among each other (Fig 3A and 3B). Cox regression analysis showed a statistically significant two time increased recurrence risk for FIGO III ovarian carcinoma patients with BG O compared to BG A (HR O vs A = 2.07; 95%CI: 1.07–4.02; p = 0.0313), while other comparisons did not reveal any significant differences (Fig 3B). The OS did not differ among the blood groups (p = 0.115). The 5-year OS was 0.714 (95%CI: 0.478–1.000) for BG O and 0.789 (95%CI: 0.619–1.000) for BG A (data for BG AB and BG B not reported due to low number of cases and events).
Fig 3.
Time to relapse (A), Kaplan-Meier curve for RFS (B), and HR for disease recurrence (C) in the FIGO III adenocarcinoma patient subgroup (n = 108). BG O patients have two time increased risk for relapse compared to A patients A, i.e. BG A patients have better prognosis with a significant longer RFS than patients with O. Time to relapse presented as median (months) and 95%CI and compared by overall Logrank test. Disease recurrence risk presented as HR and 95%CI by Cox regression. Statistical significance marked by asterisks (*) or highlighted. NE, not estimable. RFS given as probability of freedom from relapse as a function of time (months).
Discussion
This retrospective study with various gynecological malignancies showed that relapse-free survival (RFS) in OC and VC (but not on other gynecological cancers such as peritoneal, cervical, endometrial, tubal, and endometrial cancers) was significantly influenced the ABO BG status. Specifically, BG O and B ovarian cancer patients had a considerably increased risk for recurrence compared to BG A patients (i.e. BG A patients have a marked better prognosis with a longer time-to-relapse than BG O and B patients) and BG A vulvar cancer patients at least tend to have a longer time-to-relapse than patients with BG O. No influence was observed for DSS and OS for both cancers. Our data indicate that RFS in OC and VC patients is associated with the ABO blood type, suggesting that the ABO status is an important factor for RFS. The present study is the first to show the prognostic value of the ABO BG status for RFS, in particular BG A, in OC and VC and is also the largest data in the English literature addressing the prognostic value of ABO BG in regards to RFS and DSS in gynecological cancers.
Very little is known from the literature about the relationship between ABO BG and cancer prognosis (summarized in Table 3). One study on gynecological cancers published (in Italian) in 1995 [21] reported negative associations between overall survival (OS) and BG A in endometrial and OC when compared to BG O, and a positive association between BG A and OS in cervical cancer patients. Considering this it seems that OC patients with BG A have a lower risk for recurrence, no different DSS, but a worse OS than BG O patients, but opposed results were reported very recently in study with over 700 patients, demonstrating a significantly better OS in OC patients with BG A compared to BG O or non-A [22]. In our study, however, no difference in OS was found in OC patients for any BG.
Table 3. Studies on the prognostic value of ABO blood group by cancer type.
| Author | Year | n | Cancer | Blood group | Influence on prognostic data | Country | Publication | |
|---|---|---|---|---|---|---|---|---|
| negative/positive | Survival data | |||||||
| Kaffenberger | 2012 | 900 | RCC | non-O | negative | OS | USA | BJU international 2012;110: E641-6 |
| de Martino | 2014 | 556 | RCC | no association | Austria | BJU international 2014;113: E62-6 | ||
| Lee | 2015 | 3'172 | RCC | no association | Korea | J Cancer Res Clin Oncol | ||
| Unal | 2013 | 81 | NSCLC | no association | Turkey | APJCP 2013;14: 3945–8 | ||
| Fukumoto | 2015 | 333 | NSCLC | A, AB | negative | DFS, OS | Japan | Journal of epidemiology 2015;25: 110–6. |
| Yang | 2014 | 496 | ESCC | non-O | positive | OS | China | Int J Clin Exp Med 2014;7: 2214–8 |
| Qin | 2015 | 548 | ESCC > subgroup with negativ NL | non-AB | positive | OS | China | OncoTargets and therapy 2015;8: 947–53 |
| Xu | 2016 | 1'412 | Gastric | AB | positive | OS | China | J Surg Res 2016;201: 188–95. |
| > subgroup after Gastrectomy | A | negative | OS | |||||
| Dandona | 2010 | 417 | Pancreas | no association | USA | J Natl Cancer Inst 2010;102: 135–7 | ||
| Ben | 2011 | 1'431 | Pancreas | no association | China | Int J Cancer 011;128: 1179–86 | ||
| 316 | > subgroup with curative resection | O | positive | OS | ||||
| Rahbari | 2012 | 627 | Pancreas | O | positive | OS | Germany | BMC cancer 2012;12: 319 |
| Cao | 2014 | 1'555 | Colon | AB | positive | OS | China | British journal of cancer 2014;111: 174–80 |
| Holdsworth | 1985 | 1'001 | Breast | O | positive | DFS | GB | Br Med J 1985;290: 671–3 |
| Costantini | 1990 | 315 | Breast | O | positive | OS | Italy | Oncology 1990;47: 308–12 |
| Klimant | 2011 | 426 | Breast | no association | USA | Clinical medicine & research 2011;9: 111–8 | ||
| Gates | 2012 | 2'036 | Breast | no association | USA | Int J Cancer 2012;130: 2129–37 | ||
| Cihan | 2014 | 335 | Breast | A, O | positive | DFS, OS | Turkey | APJCP 2014;15: 4055–60 |
| Marinaccio | 1995 | 92 | Ovary | A | negative | OS | Italy | Minerva ginecologica 1995;47: 69–76 |
| 237 | Endometrium | A | negative | OS | ||||
| 639 | Cervix | no association | ||||||
| Cozzi | 2017 | 713 | Ovary | positive | OS | USA | PLoS One 2017; 30;12 (5):e0178965 | |
| Montavon | 2018 | 282 | Ovary | A | positive | RFS | Swiss, AUS | |
| no association | OS | |||||||
| 56 | Peritonum | no association | ||||||
| 23 | Fallopian tube | no association | ||||||
| 377 | Endometrium | no association | ||||||
| 149 | Cervix | no association | ||||||
| 67 | Vulva | A | positiv | RFS | ||||
| no association | OS | |||||||
| 11 | Vagina | no association | ||||||
RCC (renal cell carcinoma), NSCLC (Non-small Cell Lung Cancer), ESCC (oesophageal squamous cell carcinoma), OS (overall survival), DFS (Disease Free Survival), RFS (Relapse Free Survival). Current study in italic.
The largest body of data available however relates to the association of ABO BG with cancer risk and incidence. The first indication of a possible relationship between BG and cancer risk was published in 1953, reporting a 20% increased incidence of gastric cancer in BG A compared to O [23]. Since then an increasing numbers of often inconsistent data were published, suggesting that the biological role of ABO antigens may be disease-specific. Patients with BG A and AB have an increased risk of gallbladder [24], and nasopharyngeal carcinomas [25], whereas non-O female blood group carriers have been identified with a higher risk of developing renal cell cancer [26]. BG B has been significantly associated with cardiac and oesophageal carcinomas [27, 28]. BG O carriers have a reduced risk of developing basal cell carcinoma, squamous cell carcinoma of the skin [29], and a lower risk of pancreatic cancer [30]. Other associations have been reported, but the data are not reproducible for lung [31, 32], breast [33, 34] and colorectal cancers [35, 36]. A recent meta-analysis of 89 eligible studies with 100’554 cases from 30 cancer sites calculated a pooled OR for overall cancer risk of 1.12 for A vs non-A groups and 0.84 for O vs non-O groups [5]. Reports for gynecological cancers are also rare and mainly suggest increased OC risk for BG A compared to non-A and a decreased risk for BG O [5–7]. No significant association of blood groups with cervical cancer risk were reported in 8 studies [7] and two studies reported no significant associations in endometrial cancer [7]. Other gynecological cancers are underreported with only two studies without any significant difference in invasive squamous cell carcinoma of the vulva [37, 38].
All these studies underline the biological role of the BG regarding incidence risk, prognosis, and outcome in malignant diseases including ovarian, vulvar, and endometrial cancer. Associations between ABO blood type and modified immune response have been described [39, 40], but this may not fully explain how the ABO BG are linked to cancer risk incidence. Welshinger et al. have shown that, although the ovarian surface epithelium usually does not express BG antigens, ABO antigens were expressed in some areas of activated surface epithelium, in inclusion cysts and also by one-half of ovarian carcinomas [41], but it is still poorly understood how BG antigens or altered abundance of BG antigens influence carcinogenesis and whether BG antigen expression is a consequence of malignant transformation.
It is also poorly understood how BG antigens relate to disease outcome and prognosis. The expression of BG antigens on cancer cells can be subject to genetic and epigenetic modifications, e.g. ABO promotor methylation, which in turn might be related to tumor invasion and metastasis [10]. Certain tumor antigens may mimic the structure of antigens of the ABO system. Forssmann antigen, predominantly expressed in stomach and colon tumors, is structurally almost identical to A antigen determinant: BG A carriers may have a diminished tumor immune response owing the reduced ability in recognizing and attacking tumor cells expressing Forssmann antigen[5]. Abundance of A and B antigen in carcinomas derived from tissues normally not expressing these antigens may promote cancer aggressiveness by increasing cell motility, resistance to apoptosis, and immune escape [2].
The most important finding of the present study is that OC patients and to some extent vulvar cancer patients with BG A have substantially longer RFS compared to BG O (and B). This finding is in line with the recently reported longer survival for OC patients with BG A compared to BG O or non-A [22] and suggests that the lower recurrence risk accounts for this better survival. This finding presents a novelty regarding outcome and ABO BG in gynecological cancers. But open questions remain such as why BG A patients have a better RFS prognosis, why this advantage is not reflected for DSS, and why no association was found for other gynecological cancers. At least our data suggest that abundance of A antigen (or anti-B antibodies) delays recurrence or that B antigen (or anti-A antibodies) promotes recurrence in these cancers, but it is unknown by which mechanisms these immune molecules influence disease recurrence. Likewise intriguing is the observed reciprocal relationship between incidence risk and RFS in OC: women with BG O seem to have a lower incidence, however are more likely to relapse earlier.
Despite the strong indication of a positive role of BG A on prolonged RFS in OC, we recognizes that the occasionally small sample number (despite an almost 1000 patient mighty cohort) may limit the value of statistical analysis e.g. for AB patients (least frequent blood group), rare gynecological cancers (e.g. vulvar, vagina), and small subgroups (e.g. histology, stage, grade), warranting the conduction of studies with larger cohorts.
Abbreviations
- BG
blood group
- CA125
cancer antigen 125
- CI
confidence interval
- DSS
disease-free survival
- FIGO
International Federation of Gynecology and Obstetrics
- HR
Hazard ratio
- OC
ovarian cancer
- OS
overall survival
- RFS
relapse-free survival
- RMI
risk of malignancy index
- VC
vulvar cancer
Data Availability
All relevant data are within the paper.
Funding Statement
Funded by Swiss National Science Foundation (310030_156982, 310030_143619 and 310030_143632 to VHS); OncoSuisse Grant (KFS_3013-08-2012 to VHS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yamamoto F, Clausen H, White T, Marken J, Hakomori S. Molecular genetic basis of the histo-blood group ABO system. Nature. 1990;345(6272):229–33. doi: 10.1038/345229a0 . [DOI] [PubMed] [Google Scholar]
- 2.Le Pendu J, Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Clement M. ABH and Lewis histo-blood group antigens in cancer. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2001;109(1):9–31. Epub 2001/04/12. . [DOI] [PubMed] [Google Scholar]
- 3.Seltsam A, Hallensleben M, Kollmann A, Blasczyk R. The nature of diversity and diversification at the ABO locus. Blood. 2003;102(8):3035–42. doi: 10.1182/blood-2003-03-0955 . [DOI] [PubMed] [Google Scholar]
- 4.Liumbruno GM, Franchini M. Beyond immunohaematology: the role of the ABO blood group in human diseases. Blood Transfus. 2013;11(4):491–9. doi: 10.2450/2013.0152-13 ; PubMed Central PMCID: PMCPMC3827391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang B-L, He N, Huang Y-B, Song F-J, Chen K-X. ABO Blood Groups and Risk of Cancer: a Systematic Review and Meta-analysis. Asian Pacific Journal of Cancer Prevention. 2014;15(11):4643–50. doi: 10.7314/apjcp.2014.15.11.4643 [DOI] [PubMed] [Google Scholar]
- 6.Poole EM, Gates MA, High BA, Chanock SJ, Cramer DW, Cunningham JM, et al. ABO blood group and risk of epithelial ovarian cancer within the Ovarian Cancer Association Consortium. Cancer causes & control: CCC. 2012;23(11):1805–10. Epub 2012/09/11. doi: 10.1007/s10552-012-0059-y ; PubMed Central PMCID: PMC3474344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuzhalin AE, Kutikhin AG. ABO and Rh Blood Groups in Relation to Ovarian, Endometrial and Cervical Cancer Risk Among The Population of South-East Siberia. Asian Pacific Journal of Cancer Prevention. 2012;13(10):5091–6. doi: 10.7314/apjcp.2012.13.10.5091 [DOI] [PubMed] [Google Scholar]
- 8.Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochimica et biophysica acta. 1999;1473(1):247–66. . [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto H, Muramatsu H, Shimotakahara T, Yanagi M, Nishijima H, Mitani N, et al. Correlation of expression of ABH blood group carbohydrate antigens with metastatic potential in human lung carcinomas. Cancer. 1993;72(1):75–81. Epub 1993/07/01. . [DOI] [PubMed] [Google Scholar]
- 10.Gao S, Worm J, Guldberg P, Eiberg H, Krogdahl A, Liu CJ, et al. Genetic and epigenetic alterations of the blood group ABO gene in oral squamous cell carcinoma. International journal of cancer Journal international du cancer. 2004;109(2):230–7. Epub 2004/01/30. doi: 10.1002/ijc.11592 . [DOI] [PubMed] [Google Scholar]
- 11.Bowtell DD, Bohm S, Ahmed AA, Aspuria PJ, Bast RC Jr., Beral V, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. 2015;15(11):668–79. doi: 10.1038/nrc4019 ; PubMed Central PMCID: PMCPMC4892184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manegold-Brauer G, Buechel J, Knipprath-Meszaros A, Schoetzau A, Hacker NF, Tercanli S, et al. Improved Detection Rate of Ovarian Cancer Using a 2-Step Triage Model of the Risk of Malignancy Index and Expert Sonography in an Outpatient Screening Setting. Int J Gynecol Cancer. 2016. doi: 10.1097/IGC.0000000000000718 . [DOI] [PubMed] [Google Scholar]
- 13.Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945–56. doi: 10.1016/S0140-6736(15)01224-6 ; PubMed Central PMCID: PMCPMC4779792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305(22):2295–303. doi: 10.1001/jama.2011.766 . [DOI] [PubMed] [Google Scholar]
- 15.Jacob F, Anugraham M, Pochechueva T, Tse BW, Alam S, Guertler R, et al. The glycosphingolipid P(1) is an ovarian cancer-associated carbohydrate antigen involved in migration. British journal of cancer. 2014;111(8):1634–45. Epub 2014/08/29. doi: 10.1038/bjc.2014.455 ; PubMed Central PMCID: PMC4200095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaczmarek R, Buczkowska A, Mikolajewicz K, Krotkiewski H, Czerwinski M. P1PK, GLOB, and FORS blood group systems and GLOB collection: biochemical and clinical aspects. Do we understand it all yet? Transfusion medicine reviews. 2014;28(3):126–36. doi: 10.1016/j.tmrv.2014.04.007 . [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Huang Y, Feng JF. Is there an association between ABO blood group and overall survival in patients with esophageal squamous cell carcinoma? International journal of clinical and experimental medicine. 2014;7(8):2214–8. Epub 2014/09/19. ; PubMed Central PMCID: PMC4161570. [PMC free article] [PubMed] [Google Scholar]
- 18.Xu YQ, Jiang TW, Cui YH, Zhao YL, Qiu LQ. Prognostic value of ABO blood group in patients with gastric cancer. J Surg Res. 2016;201(1):188–95. doi: 10.1016/j.jss.2015.10.039 . [DOI] [PubMed] [Google Scholar]
- 19.Cao X, Wen ZS, Sun YJ, Li Y, Zhang L, Han YJ. Prognostic value of ABO blood group in patients with surgically resected colon cancer. British journal of cancer. 2014;111(1):174–80. Epub 2014/06/06. doi: 10.1038/bjc.2014.302 ; PubMed Central PMCID: PMC4090745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cihan YB. Significance of ABO-Rh Blood Groups in Response and Prognosis in Breast Cancer Patients Treated with Radiotherapy and Chemotherapy. Asian Pacific Journal of Cancer Prevention. 2014;15(9):4055–60. doi: 10.7314/apjcp.2014.15.9.4055 [DOI] [PubMed] [Google Scholar]
- 21.Marinaccio M, Traversa A, Carioggia E, Valentino L, Coviello M, Salamanna S, et al. [Blood groups of the ABO system and survival rate in gynecologic tumors]. Minerva ginecologica. 1995;47(3):69–76. Epub 1995/03/01. . [PubMed] [Google Scholar]
- 22.Cozzi GD, Levinson RT, Toole H, Snyder MR, Deng A, Crispens MA, et al. Blood type, ABO genetic variants, and ovarian cancer survival. PloS one. 2017;12(4):e0175119 doi: 10.1371/journal.pone.0175119 ; PubMed Central PMCID: PMCPMC5407760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aird I, Bentall HH, Roberts JA. A relationship between cancer of stomach and the ABO blood groups. British medical journal. 1953;1(4814):799–801. Epub 1953/04/11. ; PubMed Central PMCID: PMC2015995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandey M, Gautam A, Shukla VK. ABO and Rh blood groups in patients with cholelithiasis and carcinoma of the gall bladder. BMJ (Clinical research ed). 1995;310(6995):1639 Epub 1995/06/24. ; PubMed Central PMCID: PMC2550011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheng L, Sun X, Zhang L, Su D. ABO blood group and nasopharyngeal carcinoma risk in a population of Southeast China. International journal of cancer Journal international du cancer. 2013;133(4):893–7. Epub 2013/02/08. doi: 10.1002/ijc.28087 . [DOI] [PubMed] [Google Scholar]
- 26.Joh HK, Cho E, Choueiri TK. ABO blood group and risk of renal cell cancer. Cancer epidemiology. 2012;36(6):528–32. Epub 2012/07/31. doi: 10.1016/j.canep.2012.07.001 ; PubMed Central PMCID: PMC3717382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su M, Lu SM, Tian DP, Zhao H, Li XY, Li DR, et al. Relationship between ABO blood groups and carcinoma of esophagus and cardia in Chaoshan inhabitants of China. World journal of gastroenterology: WJG. 2001;7(5):657–61. Epub 2002/01/31. doi: 10.3748/wjg.v7.i5.657 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Liu L, Wang Z, Lu X, Wei M, Lin T, et al. ABO blood group and esophageal carcinoma risk: from a case-control study in Chinese population to meta-analysis. Cancer causes & control: CCC. 2014;25(10):1369–77. Epub 2014/07/30. doi: 10.1007/s10552-014-0442-y . [DOI] [PubMed] [Google Scholar]
- 29.Xie J, Qureshi AA, Li Y, Han J. ABO blood group and incidence of skin cancer. PloS one. 2010;5(8):e11972 Epub 2010/08/10. doi: 10.1371/journal.pone.0011972 ; PubMed Central PMCID: PMC2915921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nature genetics. 2009;41(9):986–90. Epub 2009/08/04. doi: 10.1038/ng.429 ; PubMed Central PMCID: PMC2839871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oguz A, Unal D, Tasdemir A, Karahan S, Aykas F, Mutlu H, et al. Lack of any association between blood groups and lung cancer, independent of histology. Asian Pacific journal of cancer prevention: APJCP. 2013;14(1):453–6. Epub 2013/03/29. . [DOI] [PubMed] [Google Scholar]
- 32.Urun Y, Utkan G, Cangir AK, Oksuzoglu OB, Ozdemir N, Oztuna DG, et al. Association of ABO blood group and risk of lung cancer in a multicenter study in Turkey. Asian Pacific journal of cancer prevention: APJCP. 2013;14(5):2801–3. Epub 2013/06/28. . [DOI] [PubMed] [Google Scholar]
- 33.Gates MA, Xu M, Chen WY, Kraft P, Hankinson SE, Wolpin BM. ABO blood group and breast cancer incidence and survival. International journal of cancer Journal international du cancer. 2012;130(9):2129–37. Epub 2011/06/03. doi: 10.1002/ijc.26220 ; PubMed Central PMCID: PMC3655700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miao SY, Zhou W, Chen L, Wang S, Liu XA. Influence of ABO blood group and Rhesus factor on breast cancer risk: a meta-analysis of 9665 breast cancer patients and 244,768 controls. Asia-Pacific journal of clinical oncology. 2014;10(2):101–8. Epub 2013/05/30. doi: 10.1111/ajco.12083 . [DOI] [PubMed] [Google Scholar]
- 35.Khalili H, Wolpin BM, Huang ES, Giovannucci EL, Kraft P, Fuchs CS, et al. ABO blood group and risk of colorectal cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(5):1017–20. Epub 2011/03/19. doi: 10.1158/1055-9965.epi-10-1250 ; PubMed Central PMCID: PMC3089692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urun Y, Ozdemir NY, Utkan G, Akbulut H, Savas B, Oksuzoglu B, et al. ABO and Rh blood groups and risk of colorectal adenocarcinoma. Asian Pacific journal of cancer prevention: APJCP. 2012;13(12):6097–100. Epub 2012/01/01. . [DOI] [PubMed] [Google Scholar]
- 37.Redman R, Massoll NA, Wilkinson EJ. Association between invasive squamous cell carcinoma of the vulva and ABO blood group. Journal of lower genital tract disease. 2005;9(2):89–92. Epub 2005/05/05. . [DOI] [PubMed] [Google Scholar]
- 38.Rolfe KJ, Nieto JJ, Reid WM, Perrett CW, MacLean AB. Is there a link between vulval cancer and blood group? European journal of gynaecological oncology. 2002;23(2):111–2. Epub 2002/05/16. . [PubMed] [Google Scholar]
- 39.Paterson AD, Lopes-Virella MF, Waggott D, Boright AP, Hosseini SM, Carter RE, et al. Genome-wide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Arteriosclerosis, thrombosis, and vascular biology. 2009;29(11):1958–67. Epub 2009/09/05. doi: 10.1161/ATVBAHA.109.192971 ; PubMed Central PMCID: PMC3147250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbalic M, Dupuis J, Dehghan A, Bis JC, Hoogeveen RC, Schnabel RB, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Human molecular genetics. 2010;19(9):1863–72. Epub 2010/02/20. doi: 10.1093/hmg/ddq061 ; PubMed Central PMCID: PMC2850624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welshinger M, Finstad CL, Venkatraman E, Federici MG, Rubin SC, Lewis JL Jr., et al. Expression of A, B, and H blood group antigens in epithelial ovarian cancer: relationship to tumor grade and patient survival. Gynecologic oncology. 1996;62(1):106–12. Epub 1996/07/01. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.