Abstract
Aim
Because no approved medications exist for eosinophilic esophagitis (EoE), patients must use off-label drugs or create their own formulations. We assessed the efficacy of a standardized compounded budesonide suspension for treatment of EoE.
Materials and methods
We conducted a retrospective cohort study of EoE patients at the University of North Carolina treated with compounded budesonide dispensed by a specialty compounding pharmacy. Outcomes (symptomatic global response [yes/no], endoscopic response [% with individual findings], and histologic response [absolute eosinophil count; % with <15 eos/hpf])were assessed after the initial and last treatment in our system.
Results
We identified 48 patients treated with compounded budesonide (mean age 33.6; 69% male; 96% white; 2.4 mg mean initial dose). After a mean length of follow-up of 17.0 months (range: 4.2 - 56.3), there was a significant decrease in symptoms of dysphagia (95% vs. 32%, p < 0.001), improvements in heartburn (37% vs. 11%, p=0.06) and global symptom response (81%). The median of the peak eosinophil counts decreased from 55 to 20 eos/hpf (p<0.001) with 42% achieving a response of <15 eos/hpf. Esophageal candidiasis was rare (6%). In the 18 patients with prior non-response to corticosteroids or dietary elimination, 83% had symptomatic and 38% had histologic response.
Conclusion
Compounded budesonide suspension produced a durable symptomatic, endoscopic, and histologic response in a cohort followed for more than a year. Many patients previously refractory to prior therapy responded to compounded budesonide. This formulation can be used clinically until there are approved drugs with esophageal formulations for EoE.
Keywords: deglutition disorders, eosinophilia, endoscopy
Introduction
Eosinophilic esophagitis (EoE) is a chronic immune/antigen-mediated clinicopathologic condition characterized by eosinophilic-predominant inflammation and esophageal dysfunction. A diagnosis of EoE requires the presence of at least 15 eosinophils per high-power field in esophageal biopsies and the exclusion of alternative etiologies of esophageal eosinophilia.[1,2] In adolescents and adults, typical symptoms include dysphagia and food impaction while vomiting, regurgitation and feeding intolerance predominate in children.[3] EoE represents a major cause of esophageal morbidity and is now the leading cause of food bolus impaction and the second leading cause of esophagitis.[4,5]
Corticosteroids improve the clinical symptoms and histologic features of EoE[6] and represent the first-line pharmacologic therapy for this condition after non-response to proton pump inhibitors (PPIs).[1,2,7] As no FDA approved medications or esophageal-specific corticosteroid preparations are currently clinically available for EoE,[8] patients are forced to swallow asthma-specific preparations in an attempt to coat the esophagus. This is not ideal. Metered-dose inhalers produce sub-optimal esophageal delivery and unintentional pulmonary deposition, and the off-label use of budesonide slurries mixed by patients results in variable drug concentrations and mucosal contact times.[9–16] As such, a budesonide formulation compounded by a specialty pharmacy may represent a preferable alternative until drugs with esophageal-specific formulations are approved.[17]
In this study, we aimed to assess the efficacy of a standardized pharmacy compounded budesonide suspension for treatment of EoE in patients who were steroid naïve as well as previously refractory to other steroid or dietary treatments.
Methods
We conducted a retrospective cohort study at the University of North Carolina (UNC) at Chapel Hill utilizing the UNC EoE Clinicopathologic database. The UNC EoE Clinicopathologic database has previously been described.[18–21] Cases of EoE were diagnosed per consensus guidelines including the exclusion of PPI-responsive esophageal eosinophilia.[1] The University of North Carolina Institutional Review Board approved this study.
Using the UNC EoE Clinicopathologic Database, we identified all EoE patients treated with compounded budesonide suspension between 2010 and 2017. To help isolate the effect of compounded budesonide, data were abstracted from treatment periods when patients received compounded budesonide without systemic corticosteroids, additional topical corticosteroids, or other second-line EoE pharmacologic therapies, but patients who previously received these modalities were not excluded from the analysis. Patients maintained on a stable food elimination diet were included in this patient cohort. Patient inclusion required repeat endoscopy with biopsy subsequent to initiating compounded budesonide, in order to have both baseline and follow-up data.
After starting compounded budesonide, most commonly at a total daily dose of 2mg, or following a dose change, the typical clinical protocol was to repeat an upper endoscopy to assess for endoscopic and histologic response following approximately 8 to 12 weeks. For patients with a histologic response to compounded budesonide, defined as esophageal biopsies with < 15 eosinophils/high-powered field (eos/hpf),[22,23] compounded budesonide dosing was subsequently modified at the discretion of the provider. In some cases of complete response, the approach was to decrease the dose and to subsequently assess if response was maintained on the lower dose.
Compounded budesonide suspension was prescribed at a concentration of 1 mg/ 8 mL during the course of routine clinical care and both prepared and dispensed by a single specialty outpatient compounding pharmacy (Chapel Hill Compounding Pharmacy). The medication was formulated as a viscous suspension comprised of a micronized powder form of API Budesonide BP/EP (Medisca, Plattsburg, NY), Methocel E4M Premium hydroxypropyl methycellulose USP, and a sugar free sweetener and flavoring agent (Letco Medical, Decatur, AL). The suspension was prepared using strict quality control guidelines. Budesonide powder was first weighed on an analytical balance and validated by compounding software. The powder was then wet with a small amount of preserved water and incorporated into the methycellulose vehicle using geometric dilution. Each bottle was subsequently shaken thoroughly for 5 minutes to assure even dispersion. The final preparation was dispensed into an oval plastic amber bottle to protect the contents from light. Patients were instructed to store compounded budesonide suspension at room temperature and to discard the medication after 30 days per USP guidelines. In addition, patients were instructed to take compound budesonide 30 minutes prior to or after eating.
Following cohort assembly, data were extracted from medical records using a standardized data collection form. Abstracted data included demographics, symptoms, previous treatment, endoscopic findings, and outcomes (symptomatic global response [yes/no], endoscopic response [% with individual findings], and histologic response [absolute eosinophil count; % with < 15 eos/hpf]). As this was a retrospective study and validated patient reported outcome measures were not uniformly applied in clinical settings, symptoms were coded using dichotomous variables signifying their presence or absence [yes/no] and as a global symptomatic response [yes/no per patient perception of disease activity at follow-up appointments].[19,22] Additionally, given that the initiation of compounded budesonide preceded widespread adoption of the EFEFS scoring system,[24] endoscopic findings were coded by the presence or absence of individual findings [% with individual findings]. Data were collected for three time points: at baseline, after the initial compounded budesonide course, and following the last budesonide treatment in our system.
For analysis, descriptive statistics including the mean, standard deviation, and the shape of the distribution were calculated for all continuous variables. Frequencies were tabulated for categorical variables. Bivariable statistics analyzed the relationship between baseline and post-treatment symptomatic, endoscopic and histologic outcomes. As repeat measures were analyzed, McNemar's chi-squared test was used for dichotomous variables [symptomatic and endoscopic response] and paired Wilcoxon Signed-Rank for continuous variables [peak eosinophil counts]. Pearson's chi-squared test was otherwise used when comparing non-paired categorical variables. The Wilcoxon Rank Sum test was used for non-parametric and non-paired continuous variables. All analyses were performed using Stata 14.2 (StataCorp, College Station, TX).
Results
Baseline characteristics and prior treatments
A total of 48 patients with EoE met eligibility criteria for the study. The mean age was 33.6 years old, 69% were male, and 96% were white (Table 1). There were 9 (19%) patients younger then 18 years old at the time of compounded budesonide initiation. An associated atopic condition was reported in 56% of patients. The mean length of symptoms prior to diagnosis was 10.8 years. Patients were followed for a mean length of 13.2 months (range: 1.8 - 56.3 months) while treated with compounded budesonide suspension. There were 8 (17%) patients maintained on a stable food elimination diet at the time of compounded budesonide initiation. In 4 (50%) of these patients, a portion of the eliminated foods consisted of triggers for immediate-allergic food reactions, and all of the 8 still had active inflammation (>15 eos/hpf) at the start of the compounded budesonide treatment despite the dietary elimination.
Table 1. Patient demographics (n = 48).
| Patient descriptor | N ± SD, N (%) or range |
|---|---|
| Age at diagnosis† | 33.6 ± 16.1 |
| Symptom length in years before diagnosis | 10.8 ± 9.3 |
| Male | 33 (69%) |
| White | 44 (96%) |
| Private insurance | 37, (82%) |
| Atopic disease diagnosis | 27, (56%) |
| Food allergy | 16, (33%) |
| Length of follow-up in patient months | |
| All patients | 13.2 ± 11.8; 1.8-56.3 |
| Time to first follow-up biopsy | 6.2 ± 8.1; 1.4-42.0 |
| Time to final available biopsy | 17.0 ± 12.3; 4.2-56.3 |
| Initial compounded budesonide dose in milligrams | 2.4 ± 0.9; 1 - 6 |
| Final compounded budesonide dose | 2.2 ± 1.3; 0.5 - 4 |
| Concurrent food elimination diet‡ | 8 (17%) |
SD = standard deviation;
Compounded budesonide was added to a targeted food elimination diet in 8 patients. The targeted elimination diets varied from a single food removed (i.e. low-heat eggs) to 8 separate foods removed from a patient's diet (i.e. nuts, chocolate, soy, oats, barley, sesame, and shellfish, coconut, peaches, and apples).In 4 of these patients, a portion of the eliminated foods was a trigger of immediate-allergic food allergies.
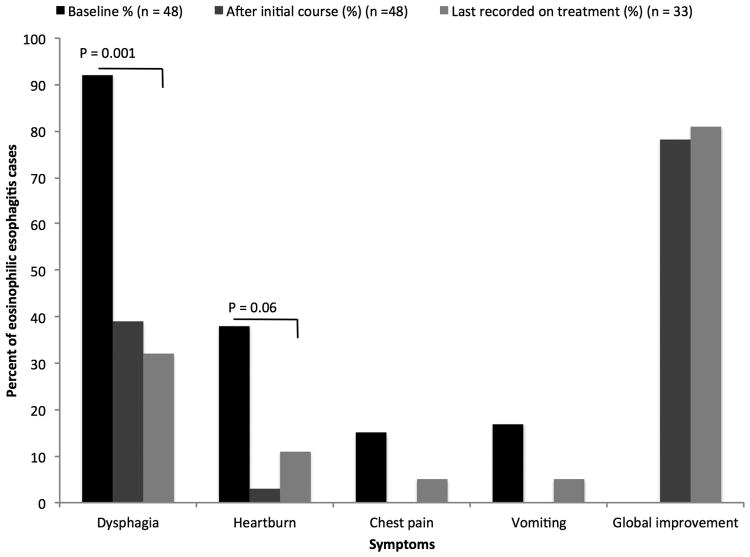
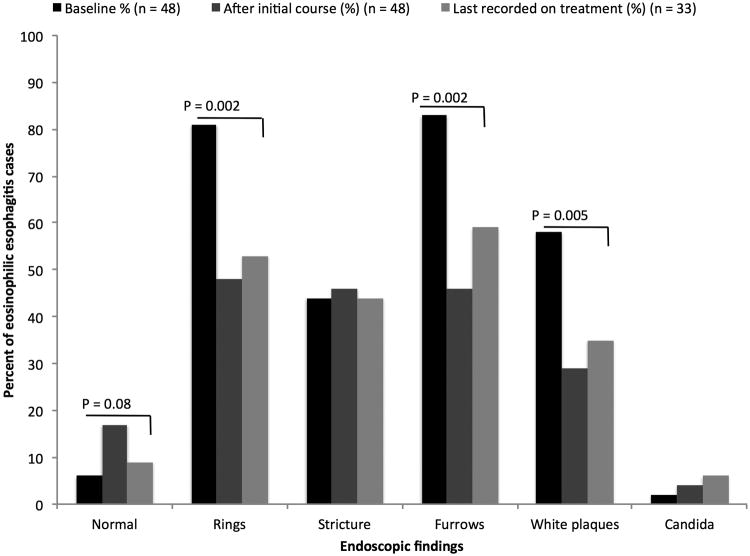
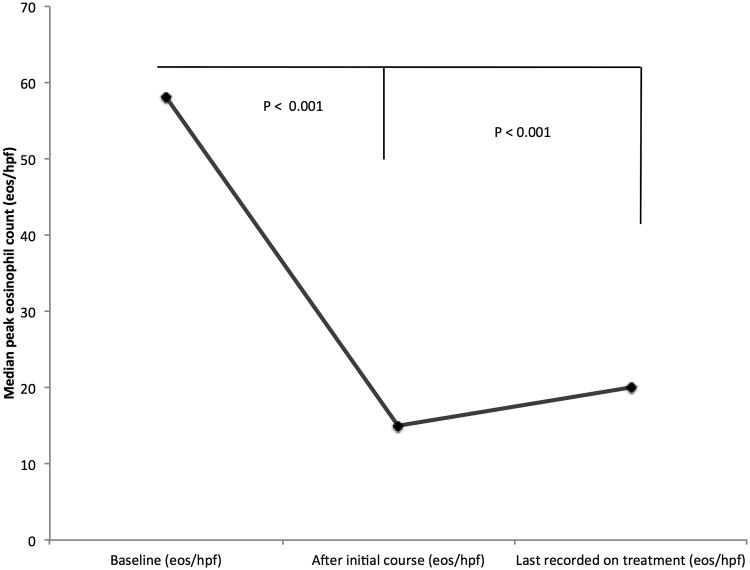
Prior to therapy, the most commonly reported symptom was dysphagia (92%) followed by heartburn (38%) (Figure 1A). Typical endoscopic findings of EoE were prevalent at baseline including rings (81%), furrows (83%), and edema/decreased vascularity (67%) (Figure 1B). The median eosinophil count prior to treatment was 58 eos/hpf (IQR: 39 - 80) (Figure 1C). Most patients (67%) had previously been treated with a topical corticosteroid or food elimination diet before compounded budesonide suspension (Table 2). More specifically, 27% and 60% of all patients had been prescribed a food elimination diet or topical corticosteroid before compounded budesonide suspension was started, respectively. Non-response and/or loss of response to topical corticosteroids or food elimination diet had been recorded in 18 of 32 (56%) patients.
Figure 1.
(A) Symptom reporting across the study period and statistical comparison between baseline and last available findings; (B) Endoscopic features across the treatment period and statistical comparison between baseline and last available findings; (C) Peak median eosinophil count across the treatment period and statistical comparison between baseline and initial findings as well as baseline and last available findings
Table 2. Prior treatment history (n, %).
| Treatment history | N (%) |
|---|---|
| Fluticasone (multi-dose inhaler) | 19 (40) |
| Budesonide (mixed into a slurry from the respule formulation) | 20 (42) |
| Systemic steroids | 3 (6) |
| Leukotriene receptor antagonist | 6 (13) |
| Dilation | 19 (40) |
| Patients receiving FED† or tCS‡ before compounded budesonide | 32 (67) |
| Food elimination diet | 13 (27) |
| Targeted elimination | 10 (21) |
| Six food elimination diet | 3 (6) |
| Patients receiving tCS before compounded budesonide | 29 (60) |
| No response to or loss of response to tCS or FED | 18 (56) |
FED: food elimination diet;
tCS: topical corticosteroids
Initial Response to Compounded Budesonide
Each included patient underwent at least one follow-up endoscopy with biopsies. There were 33 patients who had two or more follow-up endoscopies with biopsies while being treated with compounded budesonide suspension. The median interval between compounded budesonide initiation and initial follow-up examination was 3.1 months (IQR: 2.3 - 5.0).
For the 48 patients in this study, the mean initial daily dose of compounded budesonide was 2.4 mg (range 1 - 6 mg/day). The average initial monthly dose was 72 mg (range 30 - 180 mg). There were 26 patients (54%) who initiated compounded budesonide at once daily dosing. The remainder of patients started the medication at twice daily dosing (Table 1). A significant decrease in symptoms of dysphagia (90% vs. 39%, p < 0.001), heartburn (33% vs. 3%, p = 0.001), chest pain (18% vs. 0%, p = 0.008), abdominal pain (10% vs. 0%, p = 0.05), and vomiting (21% vs. 0%, p = 0.005) was documented after initiating compounded budesonide. An improvement in nausea trended toward statistical significance (11% vs. 3%, p = 0.18). Global symptomatic improvement was recorded in 78% of treated patients (Figure 1A). Statistically significant improvements in endoscopic features of EoE were also recorded after the initiation of treatment for the cohort. These included improvement in rings (81% vs. 48%, p = 0.002), furrows (83% vs. 46%, p < 0.001), white plaques (58% vs. 29%, p = 0.006), and decreased vascularity (67% vs. 29%, p < 0.001). Narrowing also significantly improved (29% vs. 17%, p = 0.03), but strictures did not. Overall, the incidence of esophageal candidiasis was rare after the initiation of therapy and documented in 4% of the cohort; this was typically asymptomatic and noted incidentally on follow-up endoscopy. Esophageal dilation was performed in a similar number of patients at baseline and after compounded budesonide suspension was started (46% vs. 50%, p = 0.56) (Figure 1B).
After initial treatment with compounded budesonide, the median of the peak eosinophil count decreased from 58 to 15 eos/hpf (IQR: 0 - 95) (p = < 0.001), and 48% achieved a post-treatment eosinophil count of < 15 eos/hpf (Figure 1C). The mean initial steroid dose did not differ between patients who did and did not have a histologic response to compounded budesonide (2.4 vs. 2.4 mg; p = 0.85).
Sub-group analysis
A sub-group analysis was conducted comparing the initial outcomes between patients without and with at least a second follow-up endoscopy while receiving compounded budesonide. For the 15 patients who did not complete an additional follow-up endoscopy, the median eosinophil count was 3 eos/hpf (60 vs. 3, p = 0.005) with 80% achieving a response of <15 eos/hpf after starting compounded budesonide. Additionally, 11 (73%) of these patients had an initial global symptomatic response. The 33 patients in whom there were additional follow-up endoscopies had a median eosinophil count of 30 (55 vs. 30, p=0.003) following compounded budesonide initiation. Of these 33 patients, 39% achieved a histologic response of < 15 eos/hpf and 81% reported global improvement in symptoms. These two groups of patients did not differ by initial post-treatment median eosinophil count (3 vs. 30, p =0.08), or by the proportion of patients with global symptomatic improvement (73% vs. 81%, p= 0.58).
Final Response to Compounded Budesonide
There were 33 patients included in the analysis of treatment outcomes at the end of the follow-up period. The average dose at the end of the available follow-up was 2.2 mg (Table 1). The mean number of follow-up endoscopies after initiating compounded budesonide was 2.8. Among these 33 patients, 19 (59%) were on a different dose of compounded budesonide at the end of follow-up. There were 13 patients whose dose was decreased after histologic improvement, and 1 patient in whom the dose was decreased for esophageal candidiasis. The dose was increased in 5 patients after an inadequate initial histologic response. After a mean 17.0-month follow-up interval, durable responses were seen in symptomatic, endoscopic, and histologic outcomes. When compared to the pre-treatment findings of patients with final follow-up data, 81% of patients reported a global improvement in symptoms. There remained a significant decrease in symptoms of dysphagia (95% vs. 32%, p < 0.001), while heartburn (37% vs. 11%, p=0.06) and chest pain trended toward significant improvements (10% vs. 5%, p=0.56) (Figure 1A). Similarly, the previously documented statistical improvements in endoscopic features persisted: rings (85% vs. 53%, p = 0.002), furrows (88% vs. 59%, p < 0.002), white plaques (68% vs. 35%, p = 0.005), and decreased vascularity (74% vs. 35%, p = 0.005) (Figure 1B). Esophageal candidiasis was more prevalent at this point in treatment (3% vs. 6%, p = 0.56) but did not statistically differ from baseline findings.
No differences in esophageal narrowing (29% vs. 32%, p = 0.71) or stricturing (56% vs. 44%; p = 0.16)were found compared to baseline. Furthermore, the same proportion of patients was dilated at baseline and at the end of follow-up (56% vs. 56%, p = 1.00).
At the end of the mean 17.0-month follow-up period, esophageal eosinophil counts remained significantly lower that at baseline. The median eosinophil count was 20 eos/hpf (55 vs. 20, p < 0.001) with 42% achieving a response of <15 eos/hpf (Figure 1C). Of the 48% who achieved an initial histologic response, 69% maintained this response at the end of the follow-up period.
Additional sub-group analyses
In a sub-group analysis of the 18 patients who previously did not respond to or lost response to a topical corticosteroid or a food elimination diet, compounded budesonide suspension was variably efficacious. Among these 18 patients, 83% had a global symptomatic response, but only 38% had a histologic response of < 15 eos/hpf at the time of their final available evaluation while on compounded budesonide suspension. In a second subgroup analysis, among the 8 patients who were concurrently treated with a food elimination diet and compounded budesonide suspension, 50% achieved a final post-treatment histologic response of < 15 eos/hpf. The median peak eosinophil count for these 8 patients (14 eos/hpf) did not differ from the rest of the cohort (20 eos/hpf) at the end of follow-up (p = 0.38). Finally, assessing differences by patient age, the mean peak histologic counts (8 vs. 23, p = 0.12) did not differ between children and adults at the end of treatment, but a greater proportion of adults reported a global symptom response (50% vs. 87%, p = 0.02).
For the 23 patients with an initial histologic response to compounded budesonide, final eosinophil counts remains suppressed but slightly attenuated by the end of the study. Here, the median eosinophil counts after the initial therapy and last available therapy were 0 and 9 eos/hpf, respectively (p = 0.002). Approximately two-thirds of initial responders maintained histologic response at the available follow-up time. However, the final daily compounded budesonide dose in the initial responders had decreased by 1.0 mg (p = 0.005). Lastly, we separately examined patients with 2 or more follow-up endoscopies that did not have an initial histologic response to compounded budesonide. Among these 20 patients, only 5 (25%) attained a histologic response of < 15 eos/hpf at the end of follow-up, even with a mean compounded budesonide dose increase of 0.3 mg (p = 0.35).
Discussion
Topical corticosteroids improve the clinical, histologic, and endoscopic features of EoE and are the first line medical therapy for the management of this disease after PPI non-response.[6] At present, however, no FDA approved steroid preparations are available for EoE. This predicament forces patients to utilize off-label steroid inhalers or liquid corticosteroids, such as budesonide respules, which they use to produce their own budesonide formulations. In clinical practice, a simpler means of supplying topical steroid suspensions may be preferable for patient care.[1–3,7] For this study, we evaluated the efficacy of a pharmacy-dispensed compounded budesonide suspension in a cohort of adults and children with EoE. We found that a compounded budesonide suspension produced statistically significant and durable symptomatic, endoscopic, and histologic responses in a cohort followed for a mean length of 17.0 months. Furthermore, after restricting the analysis to those patients who previously had no response to or loss of response to a topical corticosteroid and/or a food elimination diet, we found that 38% of the cohort exhibited a histologic response of < 15 eos/hpf. Given the notable clinical response to a compounded budesonide suspension, this formulation may represent a viable option in the primary or secondary treatment of EoE patients in routine clinical practice.
Topical corticosteroids were developed to mitigate the associated risk of systemic corticosteroids. Among the topical formulations, fluticasone, dispensed from an MDI, and budesonide, dispensed as viscous slurry or a swallowed nebulized vapor, are best described in the medical literature.[25–29] The evidence for their efficacy, largely assessed by histologic improvement but also with clinical improvement, is robust and has been synthesized within 4 meta-analyses of 7 randomized controlled trials[25–28] as well as a meta-regression.[29] More recent studies have suggested that the formulation and/or preparation is of paramount importance for the efficacy of the corticosteroid.[9,17,30,31] One study showed that an effervescent budesonide tablet is effective as a delivery vehicle in a phase 2 placebo-controlled RCT,[30] and this medication also proved highly efficacious in a phase 3 trial.[31] An additional phase 2 RCT found a muco-adherent viscous budesonide formulation to be effective in improving the clinical, endoscopic and histologic features of the disease. This medication is now being studied in a phase 3 placebo-controlled RCT.[32] Improvement in clinical symptoms, remission of histolopathological features, and the avoidance of long-term complications embody the goals of EoE treatment.[17] Our study shows that compounded viscous budesonide is effective in all of these domains but also over an extended period of follow-up time. For patients with an initial histologic response to compounded budesonide, over two-thirds continued to demonstrate a histologic response to the medication despite a 1 mg reduction from the initial dose. These results are similar though more favorable than findings from previous long-term studies of tCS in the management of EoE,[20,33] and they lend credence to some patients losing disease control with time. Our results also support previous work suggesting that steroid doses should be carefully reduced to avoid loss of histologic response.[34] Lastly, we also demonstrated that continued steroid treatment at a fixed dose does not significantly increase the proportion of patients responding to therapy after initial non-response.[35]
One issue to consider with using a compounded medication is cost. Both food elimination strategies and tCS (e.g. oral viscous budesonide slurries and fluticasone delivered via multi-dose inhaler) are expensive and potentially burdensome for patients and payers alike.[36,37] Compounded budesonide may be an exception to this paradigm. In an analysis of several pharmacies in Minnesota,[36] a formulation of budesonide respules at 1 mg twice daily cost $1,613 for 6 weeks of treatment.[38] This compared to compounded budesonide at a dose of 3 mg twice daily that cost $141 for 6 weeks of therapy,[38] which is similar in cost to the product used in this study ($80/mo). However, though often much more expensive, insurance companies may reimburse commercially available products but may not pay for compounded formulations.
There are some limitations to this study. First, it has a retrospective design, so symptoms were assessed subjectively, and symptom response data must be interpreted with caution. However, we were able to use endoscopic findings clearly documented at the time of the procedure, as well as histologic findings, which are relatively objective. Second, the study was from a single center, so results may not be generalizable. This cohort, though, had symptomatic and endoscopic findings similar to a non-referral population.[39] Third, a small proportion of the cohort received a concomitant food elimination diet. This may conceivably confound the measured treatment effect. However, in these cases, budesonide was an add-on therapy to a food elimination diet because there was incomplete response to the dietary treatment. Finally, this study did not include a placebo arm, so it would not be possible to conclude how the medication would fare in an intervention study. This is salient, as multiple prior trials in EoE showed an improvement in symptoms in both the treatment and placebo arms when non-validated outcome measures were applied.[9,13,40–43] Multiple strengths also characterize this study. Data extraction was rigorous, the cohort was homogenous and included patients only meeting a consensus diagnosis of EoE, and the compounded budesonide, while provided clinically, was compounded in a standard fashion ensuring all patients received the stated doses and concentrations.
In conclusion, compounded budesonide suspension produced a durable symptomatic, endoscopic, and histologic response in a cohort of patients followed for more than a year. Just under a half of patients who were previously refractory to a prior therapy, including topical corticosteroids, responded to compounded budesonide. This formulation can be used clinically until there are approved drugs with esophageal formulations for EoE.
Acknowledgments
Grant support: This research was funded by NIH Awards T32 DK007634 (CCR), K24 DK100548, and R01 DK101856 (ESD).
Potential competing interests: Dr. Dellon is a consultant for Adare, Alivio, Allakos, Banner, Enumeral, GSK, Receptos/Celegene, Regeneron, and Shire, receives research funding from Adare, Meritage, Miraca, Nutricia, Receptos/Celgene, Regeneron, and Shire, and has received an educational grant from Banner.
Footnotes
None of the other authors report and potential conflicts of interest with this study.
No additional potential conflicts of interest exist for the authors of this paper.
All authors made substantial contributions to the study conception and design, data acquisition, or analysis and interpretation of data. All authors were involved in the drafting of the article or critical revision. Each author approved the final version of this manuscript.
References
- 1.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) Am J Gastroenterol. 2013;108(5):679–92. doi: 10.1038/ajg.2013.71. [DOI] [PubMed] [Google Scholar]
- 2.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES, Dohil R, Falk GW, Gonsalves N, Gupta SK, Katzka DA, Lucendo AJ, Markowitz JE, Noel RJ, Odze RD, Putnam PE, Richter JE, Romero Y, Ruchelli E, Sampson HA, Schoepfer A, Shaheen NJ, Sicherer SH, Spechler S, Spergel JM, Straumann A, Wershil BK, Rothenberg ME, Aceves SS. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 3.Furuta GT, Liacouras Ca, Collins MH, Gupta SK, Justinich C, Putnam PE, Bonis P, Hassall E, Straumann A, Rothenberg ME. First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133(4):1342–1363. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Desai TK, Stecevic V, Chang CH, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest Endosc. 2005;61(7):795–801. doi: 10.1016/s0016-5107(05)00313-5. [DOI] [PubMed] [Google Scholar]
- 5.Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43(2):201–218. doi: 10.1016/j.gtc.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aceves SS, Newbury RO, Chen D, Mueller J, Dohil R, Hoffman H, Bastian JF, Broide DH. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy Eur J Allergy Clin Immunol. 2010;65(1):109–116. doi: 10.1111/j.1398-9995.2009.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dellon ES, Liacouras CA. Advances in clinical management of eosinophilic esophagitis. Gastroenterology. 2014;147(6):1238–1254. doi: 10.1053/j.gastro.2014.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothenberg ME, Aceves S, Bonis PA, Collins MH, Gonsalves N, Gupta SK, Hirano I, Liacouras CA, Putnam PE, Spergel JM, Straumann A, Wershil BK, Furuta GT. Working with the US Food and Drug Administration: Progress and timelines in understanding and treating patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2012;130(3):617–619. doi: 10.1016/j.jaci.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellon ES, Sheikh A, Speck O, Woodward K, Whitlow AB, Hores JM, Ivanovic M, Chau A, Woosley JT, Madanick RD, Orlando RC, Shaheen NJ. Viscous Topical is More Effective than Nebulized Steroid Therapy for Patients with Eosinophilic Esophagitis. Gastroenterology. 2012;143(2):321–324. doi: 10.1053/j.gastro.2012.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noel RJ, Putnam PE, Collins MH, Assa'ad AH, Guajardo JR, Jameson SC, Rothenberg ME. Clinical and immunopathologic effects of swallowed fluticasone for eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2004;2(7):568–575. doi: 10.1016/s1542-3565(04)00240-x. [DOI] [PubMed] [Google Scholar]
- 11.Konikoff MR, Noel RJ, Blanchard C, Kirby C, Jameson SC, Buckmeier BK, Akers R, Cohen MB, Collins MH, Assa'ad AH, Aceves SS, Putnam PE, Rothenberg ME. A Randomized, Double-Blind, Placebo-Controlled Trial of Fluticasone Propionate for Pediatric Eosinophilic Esophagitis. Gastroenterology. 2006;131(5):1381–1391. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 12.Schaefer ET, Fitzgerald JF, Molleston JP, Croffie JM, Pfefferkorn MD, Corkins MR, Lim JD, Steiner SJ, Gupta SK. Comparison of Oral Prednisone and Topical Fluticasone in the Treatment of Eosinophilic Esophagitis: A Randomized Trial in Children. Clin Gastroenterol Hepatol. 2008;6(2):165–173. doi: 10.1016/j.cgh.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Alexander JA, Jung KW, Arora AS, Enders F, Katzka DA, Kephardt GM, Kita H, Kryzer LA, Romero Y, Smyrk TC, Talley NJ. Swallowed Fluticasone Improves Histologic but Not Symptomatic Response of Adults With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2012;10(7):742–749. doi: 10.1016/j.cgh.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Aceves SS, Bastian JF, Newbury RO, Dohil R. Oral viscous budesonide: A potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol. 2007;102(10):2271–2279. doi: 10.1111/j.1572-0241.2007.01379.x. [DOI] [PubMed] [Google Scholar]
- 15.Aceves SS, Dohil R, Newbury RO, Bastian JF. Topical viscous budesonide suspension for treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2005;116(3):705–706. doi: 10.1016/j.jaci.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139(2):418–429.e1. doi: 10.1053/j.gastro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Dellon ES, Katzka DA, Collins MH, Hamdani M, Gupta SK, Hirano I MP-101-06 Investigators. Budesonide Oral Suspension Improves Symptomatic, Endoscopic, and Histologic Parameters Compared With Placebo in Patients With Eosinophilic Esophagitis. Gastroenterology. 2017;152(4):776–786. doi: 10.1053/j.gastro.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Dellon ES, Gibbs WB, Fritchie KJ, Rubinas TC, Wilson LA, Woosley JT, Shaheen NJ. Clinical, Endoscopic, and Histologic Findings Distinguish Eosinophilic Esophagitis From Gastroesophageal Reflux Disease. Clin Gastroenterol Hepatol. 2009;7(12):1305–1313. doi: 10.1016/j.cgh.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed CC, Fan C, Koutlas N, Shaheen NJ, Dellon ES. Food elimination diets are effective for long-term treatment of adults with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2017 Nov;46(9):836–844. doi: 10.1111/apt.14290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eluri S, Runge TM, Hansen J, Kochar B, Reed CC, Robey BS, Woosley JT, Shaheen NJ, Dellon ES. Diminishing Effectiveness of Long-Term Maintenance Topical Steroid Therapy in PPI Non-Responsive Eosinophilic Esophagitis. Clin Transl Gastroenterol. 2017;8(6):e97. doi: 10.1038/ctg.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Runge TM, Eluri S, Cotton CC, Burk CM, Woosley JT, Shaheen NJ, Dellon ES. Causes and Outcomes of Esophageal Perforation in Eosinophilic Esophagitis. J Clin Gastroenterol. 2016;51(9):1. doi: 10.1097/MCG.0000000000000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf WA, Cotton CC, Green DJ, Hughes JT, Woosley JT, Shaheen NJ, Dellon ES. Evaluation of Histologic Cutpoints for Treatment Response in Eosinophilic Esophagitis. J Gastroenterol Hepatol Res. 2015;4(10):1780–1787. doi: 10.17554/j.issn.2224-3992.2015.04.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed CC, Wolf W, Cotton CC, Rusin S, Perjar I, Hollyfield J, Woosley JT, Shaheen NJ, Dellon ES. Optimal Histologic Cutpoints for Treatment Response in Patients With Eosinophilic Esophagitis: Analysis of Data From a Prospective Cohort Study. Clin Gastroenterol Hepatol. 2017 Oct;4(17):S1542–3565. 31188–6. doi: 10.1016/j.cgh.2017.09.046. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62(4):489–495. doi: 10.1136/gutjnl-2011-301817. [DOI] [PubMed] [Google Scholar]
- 25.Sawas T, Dhalla S, Sayyar M, Pasricha PJ, Hernaez R. Systematic review with meta-analysis: pharmacological interventions for eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015 May;41(9):797–806. doi: 10.1111/apt.13147. [DOI] [PubMed] [Google Scholar]
- 26.Tan ND, Xiao YLCM. Steroid therapy for eosinophilic esophagitis: Systematic review and meta-analysis. J Dig Dis. 2015;16:431–432. doi: 10.1111/1751-2980.12265. [DOI] [PubMed] [Google Scholar]
- 27.Chuang MY, Chinnaratha MA, Hancock DG, Woodman R, Wong GR, Cock C, Fraser RJ. Topical Steroid Therapy for the Treatment of Eosinophilic Esophagitis (EoE): A Systematic Review and Meta-Analysis. Clin Transl Gastroenterol. 2015 Mar 26;6:e82. doi: 10.1038/ctg.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murali AR, Gupta A, Attar BM, Ravi V, Koduru P. Topical steroids in Eosinophilic Esophagitis: Systematic Review and Meta-analysis of Placebo Controlled Randomized Clinical Trials. J Gastroenterol Hepatol. 2015 doi: 10.1111/jgh.13281. [DOI] [PubMed] [Google Scholar]
- 29.Cotton CC, Eluri S, Wolf WA, Dellon ES. Six-Food Elimination Diet and Topical Steroids are Effective for Eosinophilic Esophagitis: A Meta-Regression. Dig Dis Sci. 2017;62(9):2408–2420. doi: 10.1007/s10620-017-4642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miehlke S, Hruz P, Von Arnim U, Madisch A, Vieth M, Bussmann C, Bajbouj M, Schlag C, Fibbe C, Wittenburg H, Allescher H, Reinshagen M, Schubert S, Tack JF, Mueller R, Dilger K, Greinwald R, Straumann A. Two new budesonide formulations are highly efficient for treatment of active eosinophilic esophagitis: Results from a randomized, double-blind, double-dummy, placebo-controlled multicenter trial. Gastroenterology. 2014;146(5):S–16. [Google Scholar]
- 31.Lucendo AJ, Miehlke S, Vieth M, Schlag C, Von Arnim U, Molina-Infante J, Hartmann D, Bredenoord AJ, De Los Rios C, Schubert S, Bruckner S, Madisch A, Hayat JO, Tack JF, Attwood SE, Mueller R, Greinwald R, Schoepfer AM, Straumann A. Budesonide orodispersible tablets are highly effective for treatment of active eosinophilic esophagitis: results from a randomized, double-blind, placebo- controlled, pivotal multicenter trial. Gastroenterology. 2017;152(5):S207. [Google Scholar]
- 32.An Extension Study to Evaluate Maintenance of Efficacy and Long-Term Treatment Effect of Oral Budesonide Suspension (OBS) in Adults and Adolescents With Eosinophilic Esophagitis (EoE) (ORBIT2) (SHP621-302) Report No.: NCT02736409. Available from: URL: https://clinicaltrials.gov/ct2/show/NCT02736409.
- 33.Rajan J, Newbury RO, Anilkumar A, Dohil R, Broide DH, Aceves SS. Long-term assessment of esophageal remodeling in patients with pediatric eosinophilic esophagitis treated with topical corticosteroids. J Allergy Clin Immunol. 2016;137(1):147–156.e8. doi: 10.1016/j.jaci.2015.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreae DA, Hanna MG, Magid MS, Malerba S, Andreae MH, Bariella E, Chehade M. Swallowed Fluticasone Propionate Is an Effective Long-Term Maintenance Therapy for Children With Eosinophilic Esophagitis. Am J Gastroenterol. 2016;111(8):1187–1197. doi: 10.1038/ajg.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dellon ES, Katzka DA, Collins MH, Hamdani M, Gupta SK, Hirano I. Safety and Efficacy of Oral Budesonide Suspension for Maintenance Therapy in Eosinophilic Esophagitis: Results from a Prospective Open-Label Study of Adolescents and Adults. Gastroenterology. 2016;150(4):S188. [Google Scholar]
- 36.Cotton CC, Erim D, Eluri S, Palmer SH, Green DJ, Wolf WA, Runge TM, Wheeler S, Shaheen NJ, Dellon ES. Cost Utility Analysis of Topical Steroids Compared With Dietary Elimination for Treatment of Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2017;15(6):841–849.e1. doi: 10.1016/j.cgh.2016.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf WA, Huang KZ, Durban R, Iqbal ZJ, Robey BS, Khalid FJ, Dellon ES. The Six-Food Elimination Diet for Eosinophilic Esophagitis Increases Grocery Shopping Cost and Complexity. Dysphagia. 2016;31(6):765–770. doi: 10.1007/s00455-016-9739-1. [DOI] [PubMed] [Google Scholar]
- 38.Alexander JA. Topical steroid therapy for eosinophilic esophagitis. Gastroenterol Hepatol. 2014;10(5):327–329. [PMC free article] [PubMed] [Google Scholar]
- 39.Dellon ES. Diagnosis and management of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2012;10(10):1066–1078. doi: 10.1016/j.cgh.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miehlke S, Hruz P, Vieth M, Bussman C, von Arnim U, Bajbouj M, Schlag C, Madisch A, Fibbe C, Wittenberg H, Allescher HD, Reinshagen M, Schubert S, Tack J, Muller M, Krummeneril P, Arts J, Mueller R, Dilger K, Greinwald R, Straumann A. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut. 2015;65(3):390–399. doi: 10.1136/gutjnl-2014-308815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katzka DA, Geno DM, Ravi A, Smyrk TC, Lao-Sirieix P, Miremadi A, Debiram I, O'Donovan M, Kita H, Kephart GM, Kryzer LA, Camilleri M, Alexander JA, Fitzgerald RC. Accuracy, safety, and tolerability of tissue collection by cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2015;13(1):77–83. doi: 10.1016/j.cgh.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 42.Assa'ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, Perschy TL, Jurgensen CH, Ortega HG, Aceves SS. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141(5):1593–1604. doi: 10.1053/j.gastro.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 43.Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G, O'Gorman MA, Abonia JP, Young J, Henkel T, Wilkins HJ, Liacouras CA. Reslizumab in children and adolescents with eosinophilic esophagitis: Results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129(2):456–463. doi: 10.1016/j.jaci.2011.11.044. e3. [DOI] [PubMed] [Google Scholar]