Abstract
Background
Eosinophilic esophagitis (EoE) is an allergic inflammatory disease that is triggered by food allergens and characterized by progressive esophageal dysfunction. Esophageal biopsy specimens are characterized by eosinophilia and expression of TH2 cytokines.
Objective
To ascertain whether TH2 cells can exist in the peripheral blood in patients with milk-induced EoE.
Methods
Peripheral blood mononuclear cells from 20 children with milk-induced EoE were collected during active EoE (EoE-A) while consuming milk and inactive EoE (EoE-I) while not consuming milk, and 8 healthy patients without EoE were used as controls. The samples were analyzed for T-cell phenotype, including intracellular cytokines before and after incubation with milk antigens and assessed by flow cytometry.
Results
We found a significant increase in CD4+ TH2 cells in the peripheral blood of patients with EoE-A compared with the controls. Furthermore, we observed a significant mean (SD) increase in the activation marker of CD154+ T cells (0.17% [0.047%]) in patients with EoE-A compared with control patients (0.034% [0.007%]) and EoE-I (0.025% [0.008]). These CD4+ T cells expressed significantly increase levels of TH2 cytokines (interleukins 4, 5, and 13) compared with the EoE-I and control groups. CD3+CD4+CD154+IL-5+ cells were significantly increased by milk antigens in both milk-induced EoE-A (0.050% [0.008%] to 0.079% [0.017%]) and EoE-I (0.0045% [0.002%] to 0.014% [0.008%]) compared with the controls (0.008% [0.003%] to 0.003% [0.001%]).
Conclusion
Our findings indicate that in EoE peripheral T cells have specific activation to milk allergens.
Introduction
Eosinophilic esophagitis (EoE) is a chronic immune- and antigen-mediated clinicopathologic disease, which is characterized by eosinophil infiltration into the esophageal epithelium and results in esophageal fibrosis and dysfunction.1 EoE is emerging as an increasingly common cause of esophagitis in children and adults and requires intensive monitoring and treatment to prevent complications, including poor growth, nutritional deficiencies, food impaction, stricture formation, and spontaneous esophageal perforation. In EoE, there is an inflammation with eosinophils, mast cells, basophils, and typical TH2 cells limited to the esophagus. The inflammation is postulated to lead to esophageal dysfunction and fibrosis, which ultimately lead to the typical symptoms of esophageal dysfunction and limited elasticity.2,3 It is postulated that in genetically predisposed individuals, the damaged esophageal barrier secretes various alarmins, such as thymic stromal lymphopoietin (TSLP) and interleukin (IL) 25, triggering the TH2 phenotype. Furthermore, TH2 cytokines are also secreted by innate (eosinophils, basophils, mast cells, invariant natural killer T cells and adaptive (TH2 cells) immune cells.4–7 As in atopic dermatitis, these TH2 cytokines lead to downregulation of epithelial barrier and increased permeability, which in turn leads to the establishment of sensitization to food allergens that ultimately then represent the chronic trigger of the disease in most patients.8 These food allergens have been demonstrated to have a causative role in EoE, fulfilling the Koch postulate.9–11
In children and adults, milk is the most common trigger of EoE.9,12–15 However, the mechanism on how milk triggers EoE is unclear. It does not appear to be IgE mediated1 because IgE detected by skin prick test or specific IgE has not proven successful in the identification of causative foods in EoE,12,16,17 and the use of omalizumab has not been successful in the treatment of EoE.18,19 Furthermore, children reintroducing a food after outgrowing IgE-mediated food allergy or have successfully completing oral immunotherapy can develop EoE to the same food.20–22 An alternative, IgG4-mediated pathway has been proposed by Clayton and colleagues.19 It is also known that TH2 cells cytokines can increase IgG4, providing a link between the 2 mechanisms.23
We postulate that T cells indeed play a central role in EoE because mice that lack T cells, but not B cells, do not develop EoE.23 In this article, we hypothesized that T cells are activated by milk in EoE, and this activation is not limited to the esophagus but can be observed in the peripheral circulating T-cell repertoire. Therefore, we hypothesize that food allergy in EoE, albeit a local inflammatory process, may have systemic manifestation, such as peripheral T-cell activation.
To test our hypothesis, we studied patients who were being evaluated for EoE because of milk allergy and obtained peripheral lymphocytes during a milk elimination diet and on reintroduction of the milk. Lymphocytes from milk sensitive patients were evaluated for markers of activation and cytokine production ex vivo and after culture with milk antigen.
Methods
EoE
All studies were conducted with approval from the Children’s Hospital of Philadelphia Institutional Review Board. All participants meet the clinical criteria of EoE1 with abnormal esophagogastroduodenoscopy results with a biopsy specimen with more than 15 eosinophils per high-power field (eos/hpf) after 2-month treatment with a high-dose proton pump inhibitor.
Milk-Induced EoE
Milk-induced EoE was defined as having active EoE (EoE-A) with greater than 15 eos/hpf isolated to the esophagus when the individuals were following a milk-containing diet. The same participants had to have inactive EoE (EoE-I) (<10 eos/hpf in the esophagus on biopsy) with only removing milk from their diet. No other alterations in their medications or diet were permitted. All patients were treated with a proton pump inhibitor before the endoscopy and had not been taking swallowed corticosteroids for at least 1 year. In addition, all patients had EoE symptoms while on a milk diet and no symptoms while off a milk diet. These participants were recruited as part of a clinical trial examining milk desensitization by an epicutaneous method (ClinicalTrials.gov Identifier: NCT02579876). Other foods triggering EoE were defined in a similar fashion. All participants had negative specific IgE test results to milk.
Atopic Phenotypes
Asthma, allergic rhinitis, and atopic dermatitis were determined by physician diagnosis. IgE-mediated food allergies were defined by positive skin test or specific IgE test results with a clinical reaction by history or positive food challenge results to the specific food. Eight control patients without a history of EoE or food allergy were recruited from the allergy and gastroenterology clinics at The Children’s Hospital of Philadelphia.
Nanostring
A cross-sectional validation cohort was used to examine cytokine expression in biopsy tissue. RNA was isolated from esophageal biopsy specimens of patients with EoE-A (diet including cow’s milk), patients with EoE-I (diet excluding cow’s milk), and control patients (mirVana, Ambion, Foster City, California). A custom nCounter Nanostring probe set designed for use with EoE samples, consisting of 283 target genes and 10 housekeeping genes (Nanostring Technologies, Seattle, Washington), was used to quantify RNA transcripts. A total of 100 ng of total RNA was hybridized to the probe sets, and samples were analyzed in the nCounter system according to the manufacturer’s instructions. Analysis was performed in nSolver 3.0 (Nanostring Technologies) Samples with quality control or normalization anomalies were excluded.
Cell Culture and Staining
Peripheral blood mononuclear cells (PMBCs) were collected at the time of endoscopy. PBMCs were isolated by using density gradient separation (Ficoll, GE Healthcare, Little Chalfont, United Kingdom). T-cell activation and intracellular cytokine staining were performed according to previously published methods.24–26 For stimulation assays, PBMCs were resuspended in AIM V medium (Invitrogen–Thermo Fisher Scientific, Philadelphia, Pennsylvania) with 2.5% human serum and 250 µg/mL of bovine milk antigens: α-casein (C6780, 50 µg; Sigma, St Louis, Missouri), β-casein (C6905, 50 µg; Sigma), κ-casein (C0406, 50 µg; Sigma), α-lactalbumin (L5385, 50 µg; Sigma), and β-lactoglobulin (L3908, 50 µg; Sigma) or negative control or α-CD3/CD28 (Dyneabeads–Thermo Fisher) and cultured at 4 × 106 cells in 2 mL in a 5% carbon dioxide incubator. After 2 hours, brefeldin A, 10 µg/mL, was added. After a total of 6 hours, the samples were washed twice in cold phosphate-buffered saline (PBS), labeled with live/dead fixable dead cell stain/Blue Invitrogen (L23105; Invitrogen) according to the manufacturer’s instructions, washed twice in cold PBS, and fixed in 4% paraformaldehyde.
For staining, the following 14-color panel was used: Blue LIVE/DEAD, CD154 phycoerythrin (clone 24–31), IL-4 AF647 (clone MP4-25D2), CD4 BV605 (clone Okt4), CCR5 phycoerythrin–cyanine 7 (clone J418F1), CCR3 peridinin chlorophyll protein complex–cyanine 5.5 (clone 5E8), CD25-BV650 (cone BC96) from Biolegend (San Diego, California) and interferon (IFN) γ Alexa 700 (clone B27), IL-5 APC (clone TRFK-5), CD3-APC-eFluor780 (clone SK7), IL-10 CF594 (clone JES3-19F1), IL-13 V450 (clone JES10-5A2), and CD127 BV785 (clone A019D5) from BD Biosciences (San Jose, California). Cells were stained with monoclonal antibody diluted in PBS, saponin, and nonfat dried milk for 30 minutes, washed twice, and resuspended in PBS for analysis. Parallel samples were stained with isotype-matched controls replacing the anticytokine monoclonal antibodies.
Flow Cytometry
Samples were acquired on an LSR II flow cytometer (BD Biosciences). A total of 3,000,000 to 4,000,000 events were collected to yield 500,000 to 1,000,000 viable CD4 T cells per sample. Analysis was performed by using FlowJo software (TreeStar Inc, Ashton, Oregon). Cell doublets were excluded by using forward scatter area vs height parameters. Viable CD4+ T cells were identified by first gating on typical lymphocyte forward vs side scatter, then Live/Deadneg cells, and finally gating on CD3+CD4+cells (eFig 1). Representative gating for CD154+ ex vivo (eFig 2) after milk and α-CD3CD28 stimulation (eFig 3) and CD154+IL-5+ (eFig 4) is shown.
Statistical Analysis
Statistical significance between groups of data was determined using an unpaired 2-tailWelch t test, Mann-Whitney U test, or analysis of variance and was calculated with a statistical package (GraphPad Prism version 6.00 for Mac OS X, GraphPad Software, San Diego, California). P ≤ .05 was considered statistically significant.
Results
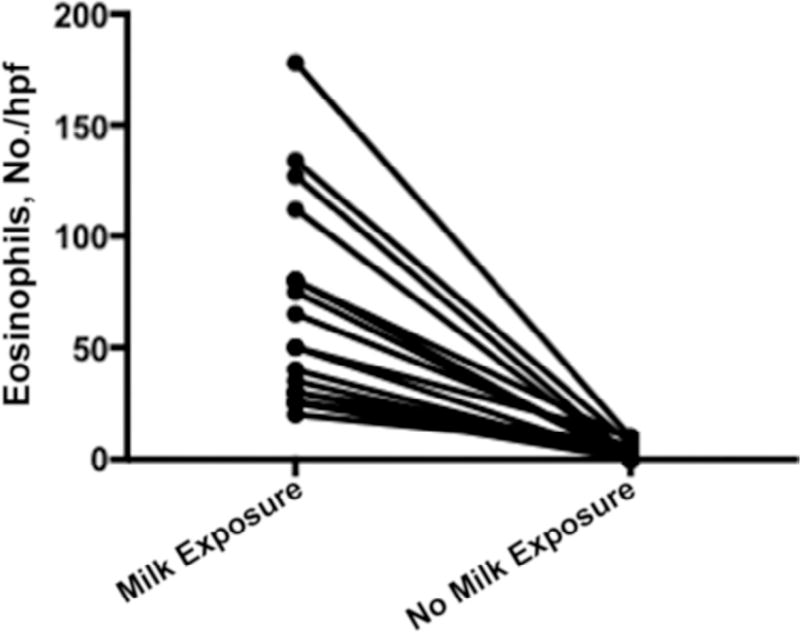
Twenty patients had a mean (SD) age of 11.25 (2.7) years. Similar to the general EoE population,16 they are predominantly males (15 males and 5 females) and all were white (Table 1). They were highly atopic, with 85% having one type of atopic disease, 60% having allergic rhinitis, 55% having asthma, and 25% having atopic dermatitis (Table 1). All patients underwent esophagogastroduodenoscopy with biopsy with and without milk exposure. During milk exposure, the esophageal biopsy specimens had a mean (SD) eosinophil count of 54 (34) eos/hpf, and when milk was removed, the esophageal biopsy specimens had a mean (SD) eosinophil count of 3 (3.04) eos/hpf (P < .001) (Fig 1). In addition, patients with active EoE had a significantly higher mean (SD) peripheral eosinophil count of 430 (190) eosinophil/mm3 (eos/mm3) compared with the same patients not exposed to milk (230 [125] eos/mm3) (P < .02). Most (n = 15) of the group had milk-induced EoE alone. Three patients had milk- and soy-induced EoE, and 2 patients had more than 3 foods inducing their EoE. These 5 patients were strictly avoiding their other food triggers. Five patients (25%) had concomitant IgE-mediated food allergies to mostly peanut and tree nuts, consistent with our previous findings (Table 1).27
Table 1.
Clinical Characteristics of the Study Participants
| Participant No. | Age, y | Sex | Atopy | IgE Food Allergy | EoE Food Trigger(s) |
|---|---|---|---|---|---|
| 1 | 11.12 | Male | AR | None | Milk |
| 2 | 9.55 | Male | As | None | Milk |
| 3 | 13.25 | Male | None | Fish, peanut, TN | Milk, peas, sweet potato |
| 4 | 12.55 | Male | As, AR | None | Milk, soy |
| 5 | 7.82 | Female | As, AR | TN | Milk |
| 6 | 12.54 | Female | None | None | Milk |
| 7 | 5.86 | Male | As, AR | None | Milk, pea, beef, egg |
| 8 | 8.68 | Male | AD, AR, As | None | Milk |
| 9 | 15.25 | Male | AD, AR, As | None | Milk, soy |
| 10 | 5.96 | Male | AR | None | Milk, soy |
| 11 | 12.51 | Male | AS | Egg, fish | Milk |
| 12 | 15.38 | Female | AR | None | Milk |
| 13 | 11.76 | Male | As, AR | NONE | Milk |
| 14 | 10.07 | Male | As | Fish, peanut, TN | Milk |
| 15 | 8.60 | Male | AD | None | Milk |
| 16 | 10.84 | Female | AD, AR, As | Peanut, TN | Milk |
| 17 | 14.56 | Male | AR | None | Milk |
| 18 | 7.98 | Male | As, AD | None | Milk |
| 19 | 14.44 | Male | AR | None | Milk |
| 20 | 10.94 | Female | None | None | Milk |
| Mean (SD) | 11.25 (2.7) | 75% Male | 17/20 Atopic | 5 (25%) IgE-mediated food allergy | NA |
Abbreviations: AD, atopic dermatitis; AR, allergic rhinitis; As, asthma; EoE, eosinophilic esophagitis; NA, not applicable; TN, tree nut.
Figure 1.
Eosinophils per high-power field (hpf). Esophagogastroduodenoscopy with biopsy was performed, and the highest eosinophil counts are shown for patients following a diet that contained milk (milk exposure) and a diet that did not contain milk (no milk exposure).
The 8 control patients were 4 males and 4 females with mean (SD) age of 14.03 (4.04). A total of 75% of the controls were atopic (1 with allergic rhinitis, 1 with atopic dermatitis, 1 with atopic dermatitis and allergic rhinitis, and 3 with asthma and allergic rhinitis) with similar atopic percentages as the patients with EoE.
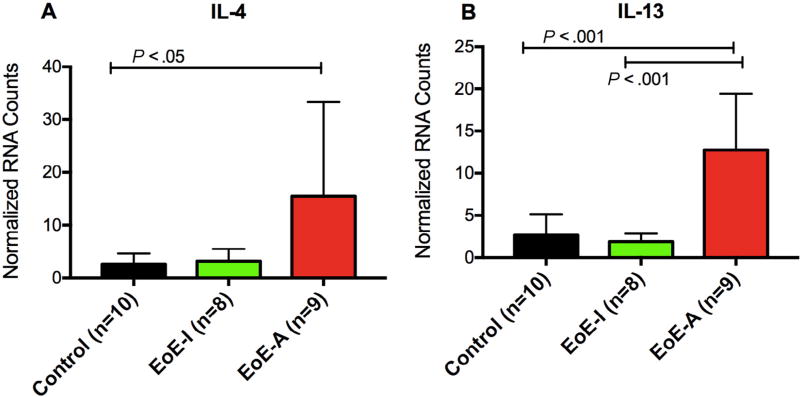
We confirmed the expression of TH2-type cytokines in the esophageal biopsy specimens of patients with active EoE by digital multiplexed gene expression. In a cross-sectional cohort of patients with active EoE while exposed to milk, there is significant upregulation of IL-4 and IL-13 vs controls (P ≤ .05 and .001, respectively) (Fig 2) as well as a trend for increased IL-5 in active EoE biopsy specimens as well (data not shown). In patients with inactive EoE treated with dietary exclusion, including milk, we saw decreased gene expression of IL-13 (P ≤ .001) and a trend for decreased IL-4 expression as well.
Figure 2.
Esophageal expression of TH2 cytokines. TH2 gene expression from esophageal biopsy specimens was assessed by digital multiplexed gene expression assay for patients during active EoE eosinophilic esophagitis (EoE-A) and inactive EOE (EoE-I) as well as controls without EoE. Patients with EoE-A had a higher expression of interleukin (IL) 4 (A) and IL-13 (B). P < .05 was considered to be statistically significant (analysis of variance with Tukey post hoc test).
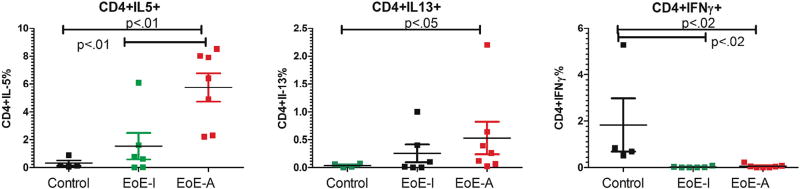
On the basis of the previous finding of TH2 cytokines in the esophageal biopsy specimen, which has been seen by others,28 we explored if a similar phenotype could be identified in PBMCs. We examined for the presence of CD3+CD4+ cells in active, inactive EoE samples and control samples. There was no difference in the number or percentage of T cells (data not shown). Consistent with EoE being a TH2 disease, we found that individuals with active EoE on esophageal biopsy specimens expressed a significant increase in peripheral blood TH2 cells expressing IL-5 for EoE-A vs controls or EoE-I and IL-13 for EoE-A vs controls (Fig 3, A and B). The controls had a significant increase of TH1 cells (IFN-γ) compared with EoE-A or EoE-I (Fig 3C).
Figure 3.
Ex vivo expression of CD4+ cells in patients undergoing esophagogastroduodenoscopy during active EoE eosinophilic esophagitis (EoE-A) and inactive EOE (EoEI). Expression is shown as percentage of positive cells (see eFigures for gating). Patients with milk-induced EoE-A have higher levels of TH2 cytokine (interleukin [IL] 5) (A), IL-13 (B), and decreased TH1 cytokine (interferon [IFN] Ɣ) (C) in CD4+ cells. P < .05 was considered to be statistically significant (Mann-Whitney U test).
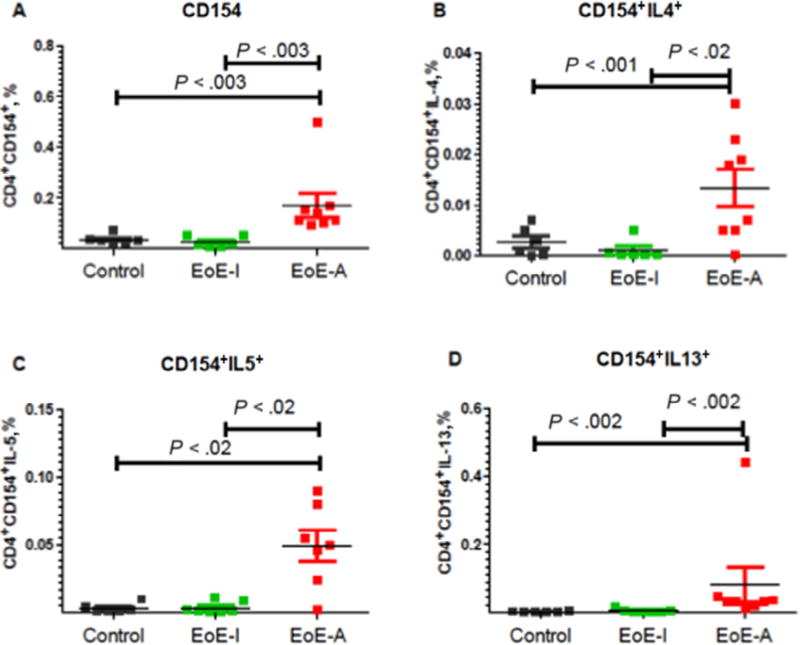
To examine activation of T cells, we use the activation marker CD154+. The participants with active disease on milk had a significantly higher mean (SEM) percentage of CD3+CD4+CD154+ cells (0.17% [0.047%]) compared with healthy controls (0.034% [0.007%]) or patients in the EoE-I group (0.0275% [0.008%]) (P < .002 for EoE-A vs control and for EoE-I vs EoE-A). (Fig 4A and Table 2). Because previous results have indicated that EoE is a TH2-driven disease, we examined whether activated T cells were TH1 or TH2 cytokines defined by the expression of IFN-γ, IL-4, IL-5, and IL-13. We found a significant increase in TH2 (IL-4, IL-5, and IL-13) cytokines (Fig 4, B–D and Table 2) with a decrease in TH1 cytokines (IFN-γ) (Table 2) in the EoE-A compared with EoE-I or control groups.
Figure 4.
Ex vivo expression of CD4+CD154+ T cells in patients undergoing esophagogastroduodenoscopy during active EoE eosinophilic esophagitis (EoE-A) and inactive EOE (EoE-I). Expression is shown as percentage of positive cells (see eFigures for gating). A, Patients with milk-induced EoE-A have increased levels CD154+ cells compared with patients with EoE-I or controls. In addition, patients with EoE-A have an increased TH2 cytokine expression (interleukin [IL] 4) (B), IL-5 (C), and IL-13 (D) in CD4+CD154+ T cells compared with controls or patients with EoE-I. P < .05 is considered to be statistically significant (Mann-Whitney U test).
Table 2.
Expression of CD3+CD4+CD154+ Ex Vivoa
| Cells | Mean (SEM) Positive Cells, % | P Value | |||
|---|---|---|---|---|---|
|
|
|
||||
| Control Patients | EoE-I | EoE-A | Controls vs EoE-A | EoE-I vs EoE-A | |
| CD3+CD4+CD154+ | 0.034 (0.007) | 0.025 (0.008) | 0.17 (0.047) | .002 | .002 |
| CD154+IL-4+ | 0.003 (0.0011) | 0.009 (0.008) | 0.013 (0.008) | .04 | .09 |
| CD154+IL-5+ | 0.0034 (0.001) | 0.0034 (0.001) | 0.049 (0.01) | .01 | .006 |
| CD154+IL-13+ | 0.001 (0.0006) | 0.009 (0.005) | 0.08 (0.05) | .03 | .02 |
| CD154+IFN-γ+ | 0.025 (0.01) | 0.0026 (0.0023) | 0.0009 (0.0004) | .004 | .40 |
Abbreviations: EoE, eosinophilic esophagitis; EoE-A, active EoE; EoE-I, inactive EoE.
Peripheral blood mononuclear cells were collected at the time of endoscopy and analyzed ex vivo.
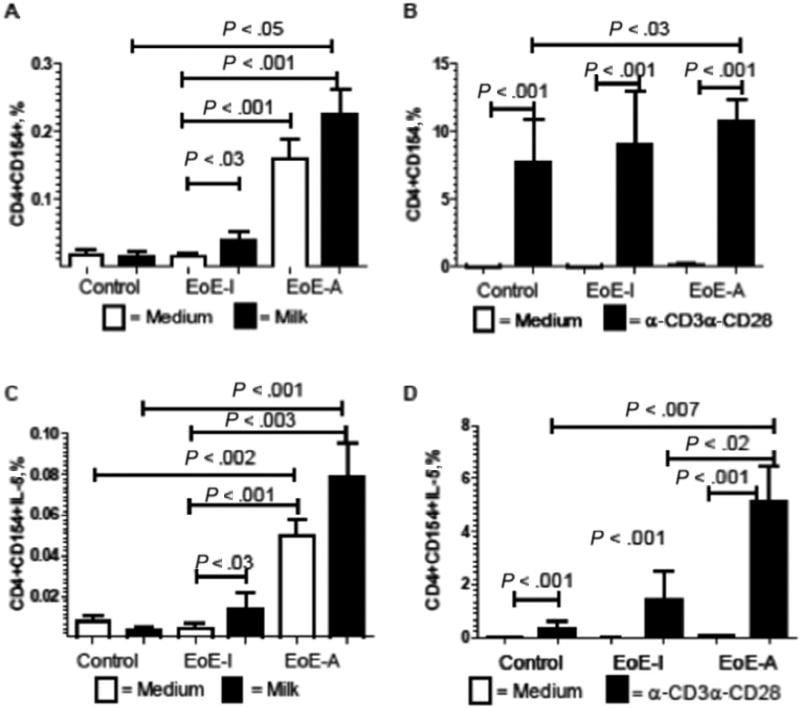
To explore the potential specificity of these T cells, we examined whether the T cells could proliferate to milk antigen. In the control patients, milk antigen did not increase the expression of the CD154+ T cells (Fig 5A). In contrast, the milk sensitive patients with EoE had a statistically significant increase in the percentage of CD3+CD4+CD154+ cells after milk stimulation in the EoE-I group (P < .02) and a nonstatistically significant increase in the EoE-A group (P = .08) (Fig 5A). In fact, the increase in the percentage of T cells was larger in the patients without milk exposure (2.5 times) compared with the patients with milk exposure (1.4 times). These results suggest that the T cells respond to milk and some of the T cells in the active patients with milk exposure might have been already stimulated, leading to the lower-fold increase. Consistent with the TH2 phenotype in EoE, the T-cell expansion was TH2 specific, with a significant increase in CD3+CD4+CD154+IL-5+ cells (Fig 5C) for both populations on a milk diet (baseline: 0.050% [0.008%]; milk stimulation: 0.079% [0.017%]) and without milk exposure (baseline: 0.0045% [0.002%]; milk stimulation: 0.013% [0.008%]). Similarly, the activation was significantly higher in the patients with milk exposure at baseline, indicating activated T cells in the circulation. We also found a similar trend for CD3+CD4+CD154+IL-13+ cells (data not shown). No difference between medium and milk-stimulated cells was observed in IFN-Ɣ levels (data not shown). The overall ability to become activated as measured by CD154+ expression by stimulation of CD3CD28was equivalent in all conditions (Fig 5B). Patients in the EoE-A group expressed significantly more TH2 activated cells measured by IL-5+CD154+ compared with controls (Fig 5D).
Figure 5.
Stimulation of CD3+CD4+CD154+IL-5+ with milk antigens or α-CD3CD28. Peripheral blood mononuclear cells were cultured with milk antigens (250 mg/mL) (A and C) or stimulated with α-CD3CD28 (B and D). Flow cytometry was performed gating on activated T cells measured by CD154+ (A and B) or TH2 activated T cells (CD154+IL-5+) (C and D). Patients with active eosinophilic esophagitis (EoE-A) have higher levels of CD154 activation marker with milk at baseline. Patients with inactive EoE (EoE-I) had increased markers of T-cell activation with milk, whereas T cells were activated in all patients with α-CD3CD28.
Discussion
We examined peripheral blood T cells in milk-induced EoE. These patients have milk-induced disease that meets the Koch postulate with active disease by biopsy and symptoms with milk exposure and normal endoscopy and no symptoms without milk exposure (Fig 1). Consistent with previous results,29 we found TH2 cytokine expression in esophageal biopsy specimens in patients with EoE-A (Fig 2). To determine whether there is systemic involvement in EoE, we examined T cells in PBMCs. We found an increase percentage of TH2 CD4 in EoE-A. This finding is similar to previous results that found increased TH2 cells in EoE and eosinophilic gastrointestinal disorder.24,29 To further characterize these cells, we examined a prototypical marker of T-cell activation, CD154 (CD40L).30 CD154 is a member of tumor necrosis factor α superfamily and plays a key role in costimulation via T-cell priming31 and marker of early T-cell activation and sustained with CD28.32 CD154 activation is seen after activation in birch pollen33 and peanut allergy in a few antigen-specific T cells.34 In our EoE population, we found a significant increase of CD154+ T cells in patients with EoE-A compared with healthy controls or patients with EoE-I (Fig 3 and Table 2) at a level comparable of that observed in IgE-mediated allergy.33,34 We then examined T-cell activation phenotype by examining classic TH2 and TH1 cytokines. The peripheral blood T cells expressed TH2 cytokines, which is consistent with the general thought that EoE is a TH2-driven disease.2 This finding is in agreement with the fact that several molecules have been identified that positively regulate TH2 polarization, such as TSLP, a factor that plays a major role in EoE.35–38 Food allergens, including milk-derived proteins, are weak activators of T cell receptor (TCR) receptor. Feske et al demonstrated that the pattern of cytokine production by T cells was determined by the duration of nuclear residence of NF-AT39 and that sustained NF-AT signaling promoted IFN-γ expression in CD4+ T cells.40 Therefore. weak ligand for TCR tend to produce shorter NF-AT signaling, less IFN-γ production and instead Th2 cytokine release. The Th2 cytokine expression induced in vitro by milk allergens in our experimental system is therefore not surprising in patients who are genetically predisposed to have Th2 reaction being atopic and high producer of TSLP.
The initial data presented here suggest activated T cells maybe a peripheral biomarker for EoE-A. The tremendous number of scopes associated with diet elimination in EoE41,42 and the burden of disease decreases quality of life and increases health care burden. In this study, we use a serum-based test that has the potential to determine disease activity and causative foods. However, additional studies are needed to determine whether this occurs in other food activated EoE and not seen in other active atopic disease.
To further show systemic involvement, we explored specific antigen response. We found a milk-specific antigen response in these milk sensitive patients compared with controls with an increase in CD3+CD4+CD154+ cells after stimulation with milk antigens. We found an increase in CD154 cells after milk stimulation or general stimulation with α-CD3+CD28+. The stimulation was higher in patients with milk exposure compared with those with milk exposure, suggesting that milk sensitive patients with milk exposure already had circulating milk-specific T cells. Not surprising, T cells were a TH2 phenotype with a significant increase of CD154+IL-5+ T cells after milk or α-CD3+CD28+ stimulation. Because standard allergy testing for IgE-mediated allergies has not been able to identify foods in EoE,43 these assays are a potential way to test for foods in EoE. Further studies are necessary to address whether this phenotyping can be used clinically in a larger population, using multiple allergens, such as egg and wheat, to determine the specificity and sensitivity of our testing. Because the IgE test results were negative, the finding of antigen-specific T cells suggests that EoE is a TH2-specific disease. Finally, this novel result raises the possibility that EoE, albeit a local inflammatory process, may be attributable to a more systemic process that implicates a systemic sensitization, resulting in a local manifestation in which a dysfunctional epithelium becomes permissive for disease development. We cannot, however, exclude that EoE is a local disease that leads to peripheral sensitization.
Supplementary Material
Acknowledgments
Funding Sources: The clinical trial is being funded by DBV Technologies and The Children’s Hospital of Philadelphia Allergy Eosinophilic Esophagitis Research Fund. The mechanistic studies are funded by The Children’s Hospital of Philadelphia Allergy Eosinophilic Esophagitis Research Fund. Dr Spergel is funded by the Stuart Starr Endowed Chair in Pediatrics. Dr Cianferoni is funded by 2015 American Academy of Allergy, Asthma & Immunology ART and American Partnership for Eosinophilic Disorders awards. Dr Muir is funded by grant 1K08DK106444-01 from the National Institutes of Health. Dr Ruffner is funded by grant T32-HD043021 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and an American College of Allergy, Asthma, and Immunology Young Faculty Award.
We thank Deirdre Burke and Megan Lewis for recruitment of the study patients and addressing regulatory issues.
Footnotes
Disclosures: Authors have nothing to disclose.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.anai.2017.11.006.
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. e26. doi: 10.1016/j.jaci.2011.02.040. quiz 21–22. [DOI] [PubMed] [Google Scholar]
- 2.Hill DA, Spergel JM. The immunologic mechanisms of eosinophilic esophagitis. Curr Allergy Asthma Rep. 2016;16:9. doi: 10.1007/s11882-015-0592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merves J, Muir A, Modayur Chandramouleeswaran P, Cianferoni A, Wang ML, Spergel JM. Eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2014;112:397–403. doi: 10.1016/j.anai.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherrill JD, Kc K, Wu D, et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol. 2014;7:718–729. doi: 10.1038/mi.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchard C, Stucke EM, Burwinkel K, et al. Coordinate interaction between IL-13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J Immunol. 2010;184:4033–4041. doi: 10.4049/jimmunol.0903069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heimall J, Spergel JM. Filaggrin mutations and atopy: consequences for future therapeutics. Expert Rev Clin Immunol. 2012;8:189–197. doi: 10.1586/eci.11.100. [DOI] [PubMed] [Google Scholar]
- 7.Litosh VA, Rochman M, Rymer JK, Porollo A, Kottyan LC, Rothenberg ME. Calpain-14 and its association with eosinophilic esophagitis. J Allergy Clin Immunol. 2017;139:1762–1771. e7. doi: 10.1016/j.jaci.2016.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112:S118–S127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Spergel JM, Brown-Whitehorn TF, Cianferoni A, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol. 2012;130:461–467. e465. doi: 10.1016/j.jaci.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Arias A, Gonzalez-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology. 2014;146:1639–1648. doi: 10.1053/j.gastro.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Cianferoni A, Spergel J. Eosinophilic esophagitis: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:159–174. doi: 10.1007/s12016-015-8501-z. [DOI] [PubMed] [Google Scholar]
- 12.Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology. 2012;142:1451–1459. e1451. doi: 10.1053/j.gastro.2012.03.001. quiz e1414–1455. [DOI] [PubMed] [Google Scholar]
- 13.Lucendo AJ, Arias A, Gonzalez-Cervera J, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol. 2013;131:797–804. doi: 10.1016/j.jaci.2012.12.664. [DOI] [PubMed] [Google Scholar]
- 14.Molina-Infante J, Arias A, Barrio J, Rodriguez-Sanchez J, Sanchez-Cazalilla M, Lucendo AJ. Four-food group elimination diet for adult eosinophilic esophagitis: a prospective multicenter study. J Allergy Clin Immunol. 2014;134:1093–1099. e1091. doi: 10.1016/j.jaci.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Gonsalves N, Kagalwalla AF. Dietary treatment of eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43:375–383. doi: 10.1016/j.gtc.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48:30–36. doi: 10.1097/MPG.0b013e3181788282. [DOI] [PubMed] [Google Scholar]
- 17.van Rhijn BD, Vlieg-Boerstra BJ, Versteeg SA, et al. Evaluation of allergen-microarray-guided dietary intervention as treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2015;136:1095–1097. e3. doi: 10.1016/j.jaci.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 18.Rocha R, Vitor AB, Trindade E, et al. Omalizumab in the treatment of eosinophilic esophagitis and food allergy. Eur J Pediatr. 2011;170:1471–1474. doi: 10.1007/s00431-011-1540-4. [DOI] [PubMed] [Google Scholar]
- 19.Clayton F, Fang JC, Gleich GJ, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147:602–609. doi: 10.1053/j.gastro.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 20.Maggadottir SM, Hill DA, Ruymann K, et al. Resolution of acute IgE-mediated allergy with development of eosinophilic esophagitis triggered by the same food. J Allergy Clin Immunol. 2014;133:1487–1489. e1481. doi: 10.1016/j.jaci.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Ridolo E, De Angelis GL, Dall’aglio P. Eosinophilic esophagitis after specific oral tolerance induction for egg protein. Ann Allergy Asthma Immunol. 2011;106:73–74. doi: 10.1016/j.anai.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Lucendo AJ, Arias A, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2014;113:624–629. doi: 10.1016/j.anai.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107:83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prussin C, Lee J, Foster B. Eosinophilic gastrointestinal disease and peanut allergy are alternatively associated with IL-5+ and IL-5(−) T(H)2 responses. J Allergy Clin Immunol. 2009;124:1326–1332. e1326. doi: 10.1016/j.jaci.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jyonouchi S, Abraham V, Orange JS, et al. Invariant natural killer T cells from children with versus without food allergy exhibit differential responsiveness to milk-derived sphingomyelin. J Allergy Clin Immunol. 2011;128:102–109. e113. doi: 10.1016/j.jaci.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jyonouchi S, Smith CL, Saretta F, et al. Invariant natural killer T cells in children with eosinophilic esophagitis. Clin Exp Allergy. 2014;44:58–68. doi: 10.1111/cea.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill DA, Dudley JW, Spergel JM. The prevalence of eosinophilic esophagitis in pediatric patients with IgE-mediated food allergy. J Allergy Clin Immunol Pract. 2017;5:369–375. doi: 10.1016/j.jaip.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanchard C, Stucke EM, Rodriguez-Jimenez B, et al. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol. 2011;127:208–217. 217 e201–207. doi: 10.1016/j.jaci.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bullock JZ, Villanueva JM, Blanchard C, et al. Interplay of adaptive th2 immunity with eotaxin-3/c-C chemokine receptor 3 in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:22–31. doi: 10.1097/MPG.0b013e318043c097. [DOI] [PubMed] [Google Scholar]
- 30.Lederman S, Yellin MJ, Krichevsky A, Belko J, Lee JJ, Chess L. Identification of a novel surface protein on activated CD4+ T cells that induces contact-dependent B cell differentiation (help) J Exp Med. 1992;175:1091–1101. doi: 10.1084/jem.175.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 32.Snyder JT, Shen J, Azmi H, Hou J, Fowler DH, Ragheb JA. Direct inhibition of CD40L expression can contribute to the clinical efficacy of daclizumab independently of its effects on cell division and Th1/Th2 cytokine production. Blood. 2007;109:5399–5406. doi: 10.1182/blood-2006-12-062943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith KA, Gray NJ, Cheek E, et al. Characterisation of CD154+ T cells following ex vivo birch allergen stimulation defines a close relationship between T cell subsets in healthy volunteers. BMC Immunol. 2013;14:14. doi: 10.1186/1471-2172-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Pons L, Zhong X, Burks AW. Peanut allergen Ara h 2-specific T cells are activated via Ras-Erk MAP kinase pathway signalling and identified by CD154 expression. Food Agric Immunol. 2011;22 [Google Scholar]
- 35.Cianferoni A, Spergel JM. Immunotherapeutic approaches for the treatment of eosinophilic esophagitis. Immunotherapy. 2014;6:321–331. doi: 10.2217/imt.14.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noti M, Wojno ED, Kim BS, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19:1005–1013. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothenberg ME, Spergel JM, Sherrill JD, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42:289–291. doi: 10.1038/ng.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sleiman PM, Wang ML, Cianferoni A, et al. GWAS identifies four novel eosinophilic esophagitis loci. Nat Commun. 2014;5:5593. doi: 10.1038/ncomms6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feske S, Draeger R, Peter HH, Eichmann K, Rao A. The duration of nuclear residence of NFAT determines the pattern of cytokine expression in human SCID T cells. J Immunol. 2000;165:297–305. doi: 10.4049/jimmunol.165.1.297. [DOI] [PubMed] [Google Scholar]
- 40.Porter CM, Clipstone NA. Sustained NFAT signaling promotes a Th1-like pattern of gene expression in primary murine CD4+ T cells. J Immunol. 2002;168:4936–4945. doi: 10.4049/jimmunol.168.10.4936. [DOI] [PubMed] [Google Scholar]
- 41.AsherWolf W, Huang KZ, Durban R, et al. The six-food elimination diet for eosinophilic esophagitis increases grocery shopping cost and complexity. Dysphagia. 2016;31:765–770. doi: 10.1007/s00455-016-9739-1. [DOI] [PubMed] [Google Scholar]
- 42.Klinnert MD, Silveira L, Harris R, et al. Health-related quality of life over time in children with eosinophilic esophagitis and their families. J Pediatr Gastroenterol Nutr. 2014;59:308–316. doi: 10.1097/MPG.0000000000000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simon D, Cianferoni A, Spergel JM, et al. Eosinophilic esophagitis is characterized by a non-IgE-mediated food hypersensitivity. Allergy. 2016;71:611–620. doi: 10.1111/all.12846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.