Abstract
Central venous catheters (CVCs) contribute disproportionately to bloodstream infection (BSI) and, by extension, to infection-related hospitalization, mortality, and health care costs in patients undergoing dialysis. Recent product advancements may reduce BSIs, but a sufficiently powered comparative-effectiveness study is needed to facilitate evidence-based patient care decisions. In a 13-month, prospective, cluster-randomized, open-label trial, we compared BSI rates in facilities using ClearGuard HD antimicrobial barrier caps (ClearGuard group) with those in facilities using Tego hemodialysis connectors plus Curos disinfecting caps (Tego+Curos group). Forty DaVita dialysis facilities in the United States were pair-matched by BSI rate, number of patients using CVCs, and geographic location, and then cluster randomized 1:1. We enrolled all adult patients undergoing dialysis with CVCs at these facilities, except those allergic to heparin or chlorhexidine. Overall, 1671 patients participated in the study, accruing >183,000 CVC-days. The study outcome was positive blood culture (PBC) rate as an indicator of BSI rate. We calculated results at the cluster level and adjusted for the facility cluster effect. During a 3-month run-in period immediately before study interventions, the groups had similar BSI rates (P=0.8). During the 13-month intervention period that immediately followed, the ClearGuard group had a BSI rate significantly lower than that of the Tego+Curos group (0.28 versus 0.75 PBCs per 1000 CVC-days, respectively; P=0.001). No device-related adverse events were reported. In conclusion, compared with Tego connectors plus Curos caps, ClearGuard HD antimicrobial barrier caps significantly lowered the rate of catheter-related BSIs in patients undergoing hemodialysis using CVCs, representing an important advancement in hemodialysis patient care.
Keywords: hemodialysis access, vascular access, dialysis access, hemodialysis
Central venous catheters (CVCs) are used in only 19% of dialysis procedures in the United States, but are responsible for 70% of vascular access–related bloodstream infections (BSIs).1 In addition, BSI is the second leading cause of death among patients undergoing hemodialysis who use CVCs for their vascular access. Devices are now available that may significantly reduce BSI rates. However, comparative effectiveness data for these devices have not been available to allow evidence-based patient care decisions.
This randomized comparative-effectiveness study evaluated two interventions designed to mitigate the risk of catheter-related BSI: ClearGuard HD Antimicrobial Barrier Cap (Pursuit Vascular, Inc.), versus the combination of Tego Needlefree Hemodialysis Connector (ICU Medical, Inc.) used in combination with Curos Disinfecting Cap for Tego (3M Healthcare). These devices are hereinafter referred to as the ClearGuard, Tego, and Curos, respectively.
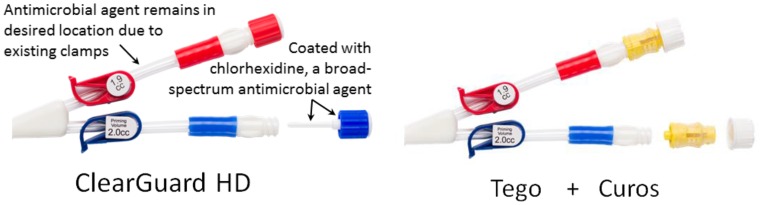
The devices studied are shown in Figure 1 and modes of action are summarized in Table 1. ClearGuard is used in the same manner as a standard CVC cap. However, ClearGuard has a rod and threads containing a dry chlorhexidine coating. Once the cap is attached to the catheter hub, the dry chlorhexidine coating dissolves into the lock solution proximal to the clamp to kill >99.99% of common pathogenic organisms.2 Because chlorhexidine is an antibiotic-free antimicrobial, the risk for developing resistant organisms is low compared with antibiotics.3,4 ClearGuard was previously shown to significantly lower catheter-related bloodstream infection (CRBSI) rates when compared with standard CVC caps in a prospective randomized study of 2470 patients receiving hemodialysis.5
Figure 1.
ClearGuard is a single-piece design that applies antimicrobial inside and outside the CVC hub versus Tego+Curos, which is a two-piece design that applies antimicrobial only to the outside of the Tego.
Table 1.
Modes of action for the ClearGuard HD cap, Tego connector, and Curos cap
| Attribute | ClearGuard | Tego | Curos |
|---|---|---|---|
| Kills bacteria inside of hub | ✓ | ||
| Kills bacteria on outside of hub | ✓ | ✓ | |
| “Closed” system (opened once per week) | ✓ |
✓, indicates attribute is present.
Tego is designed to prevent bacteria from getting inside the CVC using a mostly “closed” system.6 Tego was previously evaluated in a retrospective study, which concluded that Tego may reduce the risk of CRBSI.7 For additional protection, Curos is used with Tego. Curos kills organisms on the outside surface of Tego using 70% isopropanol alcohol. Curos was previously shown in vitro to kill infection-causing organisms.8,9 The combination of Tego+Curos was used in this study because it is expected to perform better than Tego alone. ClearGuard is designed to kill bacteria inside and outside the catheter hub, whereas Tego+Curos kills bacteria on the outside only.
Results
Patient Flow and Demographics
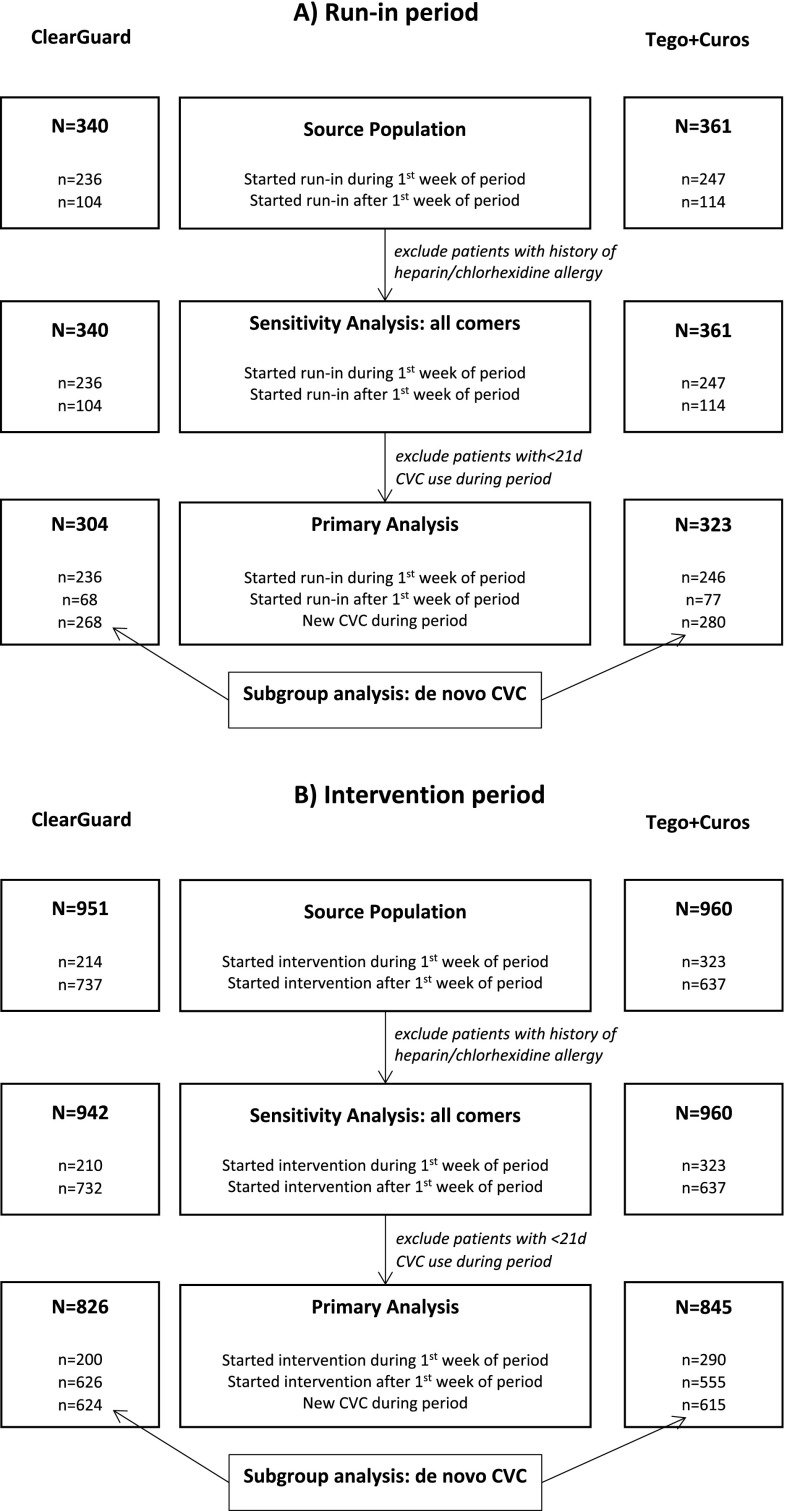
Forty dialysis facilities were enrolled in the study, with 20 in the ClearGuard group and 20 in the Tego+Curos group. All facilities remained in the study throughout its duration. All patients with CVCs dialyzing in participating facilities were enrolled in the study with the exception of nine who were excluded due to history of heparin allergy (none were excluded for chlorhexidine allergy). A flow diagram for enrollment is shown in Figure 2. There were no protocol deviations or device-related adverse events reported during the study. There was one protocol change to increase the intervention period from 12 to 13 months.
Figure 2.
Flow of subjects through the study. (A) Run-in and (B) intervention periods.
A total of 627 patients (304 ClearGuard, 323 Tego+Curos) qualified for primary analysis of the run-in phase of the study. Characteristics of participants in the two study groups (Table 2) were reasonably balanced, with the exception of race (35% versus 46% black, respectively; omnibus P=0.02) and diabetes (55% versus 64%, respectively; P=0.02).
Table 2.
Patient demographics during the run-in and intervention periods
| Characteristic | All | ClearGuard Group | Tego+Curos Group | P Value |
|---|---|---|---|---|
| Run-in period (Aug 2015–Oct 2015) | ||||
| No. of facilities | 40 | 20 | 20 | |
| No. of CVC patients | 627 | 304 | 323 | |
| Age, yr | 62.9±15.6 | 63.7±15.6 | 62.2±15.5 | 0.2 |
| Sex (% men) | 308 (49) | 145 (48) | 163 (50) | 0.5 |
| Race | 0.02 | |||
| White | 255 (41) | 133 (44) | 122 (38) | |
| Black | 256 (41) | 107 (35) | 149 (46) | |
| Hispanic | 73 (12) | 37 (12) | 36 (11) | |
| Other | 43 (7) | 27 (9) | 16 (5) | |
| Missing | 0 (0) | 0 (0) | 0 (0) | |
| Diabetes | 376 (60) | 168 (55) | 208 (64) | 0.02 |
| Dialysis vintage, yr | 2.2±3.9 | 2.2±4.2 | 2.2±3.6 | 0.9 |
| Intervention period (Nov 2015–Nov 2016) | ||||
| No. of facilities | 40 | 20 | 20 | |
| No. of CVC patients | 1671 | 826 | 845 | |
| Age, yr | 62.8±14.9 | 63.7±14.4 | 62.0±15.3 | 0.02 |
| Sex (% men) | 856 (51) | 421 (51) | 435 (51) | 0.8 |
| Race | <0.001 | |||
| White | 778 (47) | 414 (50) | 364 (43) | |
| Black | 621 (37) | 267 (32) | 354 (42) | |
| Hispanic | 171 (10) | 83 (10) | 88 (10) | |
| Other | 98 (6) | 60 (7) | 38 (5) | |
| Missing | 3 (0) | 2 (0) | 1 (0) | |
| Diabetes | 998 (60) | 477 (58) | 521 (62) | 0.1 |
| Dialysis vintage, yr | 1.7±3.2 | 1.6±3.3 | 1.8±3.2 | 0.2 |
Values for categoric variables are given as number (percentage); values for continuous variables, as mean±SD.
A total of 1671 patients (826 ClearGuard, 845 Tego+Curos) qualified for primary analysis of the intervention phase of the study. Again, characteristics of participants were reasonably balanced (Table 2) with the exception of age (63.7 versus 62.0 years, respectively; P=0.02) and race (32% versus 42% black, respectively; omnibus P<0.001).
Primary Analysis
Run-In Period
During the 3-month run-in period, 18 positive blood cultures (PBCs) occurred during 18,739 CVC-days in the ClearGuard group, and 22 PBCs occurred during 20,454 CVC-days in the Tego+Curos group, corresponding to rates of 1.02 and 1.08 per 1000 CVC-days, respectively. Between-group differences in rates were nonsignificant (P=0.8).
Intervention Period
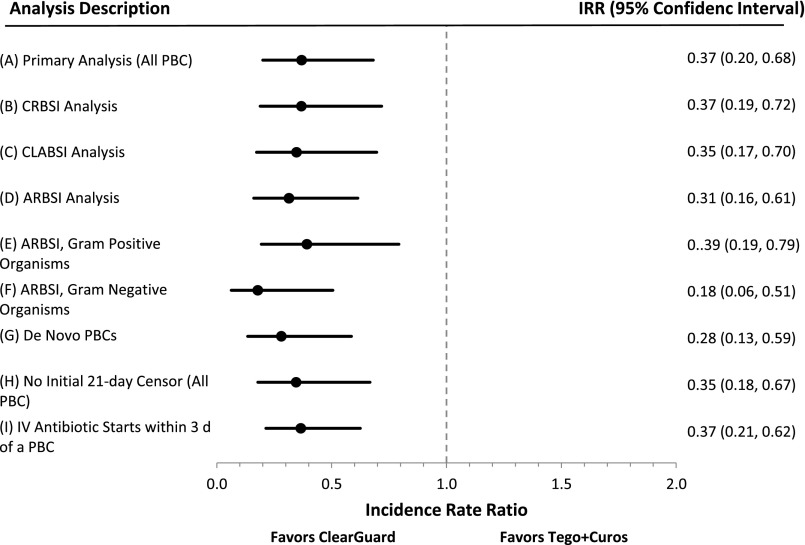
During the 13-month intervention period, 23 PBCs occurred during 83,064 CVC-days in the ClearGuard group, and 75 PBCs occurred during 100,042 CVC-days in the Tego+Curos group, corresponding to rates of 0.28 and 0.75 PBCs per 1000 CVC-days, respectively. Twenty-one and 63 unique patients experienced PBCs, respectively. The incidence rate ratio (IRR) was 0.37 (P=0.001) favoring ClearGuard (Figure 3A, Table 3).
Figure 3.
Study results demonstrate that ClearGuard caps are superior to Tego+Curos for reducing bloodstream infection across all nine analyses. Summary of IRRs (dots) and 95% confidence intervals (whiskers), ClearGuard facilities versus Tego+Curos facilities, for (A) primary analysis and (B–I) exploratory sensitivity analyses. Estimates<1 favor ClearGuard.
Table 3.
Summary of analyses results: ClearGuard facilities versus Tego+Curos facilities, for primary analysis and exploratory sensitivity analyses
| Variable | Clinical Study Results and Exploratory Analyses | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Primary Analysis (All PBC) | CRBSI Analysis | CLABSI Analysis | ARBSI Analysis | ARBSI Analysis, Gram-Positive Organisms | ARBSI Analysis, Gram-Negative Organisms | De Novo CVC Analysis | No Initial 21-d Censoring Analysis (All PBC) | IV Antibiotic Starts within 3 d of a PBC Analysis | |
| Number of patients in analysis | |||||||||
| Combined | 1671 | 1671 | 1671 | 1671 | 1671 | 1671 | 1239 | 1902 | 1671 |
| ClearGuard | 826 | 826 | 826 | 826 | 826 | 826 | 624 | 942 | 826 |
| Tego+Curos | 845 | 845 | 845 | 845 | 845 | 845 | 615 | 960 | 845 |
| Cumulative duration (CVC-days) | |||||||||
| Combined | 183,106 | 178,296 | 183,106 | 183,106 | 183,106 | 183,106 | 115,321 | 216,379 | 183,106 |
| ClearGuard | 83,064 | 81,834 | 83,064 | 83,064 | 83,064 | 83,064 | 55,504 | 99,433 | 83,064 |
| Tego+Curos | 100,042 | 96,462 | 100,042 | 100,042 | 100,042 | 100,042 | 59,817 | 116,946 | 100,042 |
| Number of events | |||||||||
| Combined | 98 | 42 | 76 | 87 | 57 | 31 | 58 | 119 | 73 |
| ClearGuard | 23 | 10 | 17 | 18 | 14 | 4 | 12 | 27 | 17 |
| Tego+Curos | 75 | 32 | 59 | 69 | 43 | 27 | 46 | 92 | 56 |
| Event rate (events per 1000 CVC-days) | |||||||||
| Combined | 0.54 | 0.24 | 0.42 | 0.48 | 0.31 | 0.17 | 0.50 | 0.55 | 0.40 |
| ClearGuard | 0.28 | 0.12 | 0.20 | 0.22 | 0.17 | 0.05 | 0.22 | 0.27 | 0.20 |
| Tego+Curos | 0.75 | 0.33 | 0.59 | 0.69 | 0.43 | 0.27 | 0.77 | 0.79 | 0.56 |
| IRR | 0.37 | 0.37 | 0.35 | 0.31 | 0.39 | 0.18 | 0.28 | 0.35 | 0.37 |
| 95% LCI | 0.20 | 0.19 | 0.17 | 0.16 | 0.19 | 0.06 | 0.13 | 0.18 | 0.21 |
| 95% UCI | 0.68 | 0.72 | 0.70 | 0.61 | 0.79 | 0.51 | 0.59 | 0.67 | 0.62 |
| P value | 0.001 | 0.003 | 0.003 | <0.001 | <0.01 | 0.001 | <0.001 | 0.002 | <0.001 |
IRRs<1 favor ClearGuard. 95% LCI, lower 95% confidence interval; 95% UCI, upper 95% confidence interval.
Exploratory Analyses
CRBSI Analysis
The term CRBSI, as used in the literature, ranges from surveillance definitions (all PBCs occurring in patients with CVCs) to rigorous clinical definitions (defined by precise laboratory findings identifying the CVC as the source of the infection).10,11 In this study, the link between the PBC and a true CVC-related BSI is as follows. The analysis, CRBSI is defined as: (1) analysis is performed on a per-patient basis (maximum of one PBC per patient) to ensure no duplicate counting of the same infection; (2) to better ensure that PBCs were related to the CVC, only PBCs designated as access-related on the National Healthcare Safety Network (NHSN) forms of patients with CVCs were included; (3) to help rule out skin contamination, only PBCs with recognized pathogens (no common commensals) were included; and (4) PBCs with polymicrobial growth were excluded because this may be an indication of contamination. The denominator is the number of days at-risk per study protocol. The resulting IRR was 0.37 (P=0.003) favoring ClearGuard (Figure 3B, Table 3).
Central Line–Associated Bloodstream Infections Analysis
The central line–associated bloodstream infections (CLABSI) analysis was on the basis of the NHSN CLABSI definition.12 In this analysis, the PBC (numerator) must either be (1) a recognized pathogen and not related to an infection at another site, or (2) a common commensal from two blood draws, not related to an infection at another site, and patient has at least one of: fever, chills, or hypotension. The denominator is the number of days at-risk per study protocol. The resulting IRR was 0.35 (P=0.003) favoring ClearGuard (Figure 3C, Table 3).
Access-Related Bloodstream Infections Analysis
The access-related bloodstream infections (ARBSI) analysis was on the basis of the Center for Disease Control (CDC) NHSN definition for ARBSI.13 The numerator is PBCs with the suspected source reported as the vascular access or uncertain. The denominator is the number of days at-risk per study protocol. The resulting IRR was 0.32 (P<0.001) favoring ClearGuard (Figure 3D, Table 3).
Organism Analysis
ARBSI events were analyzed according to organism type. When considering only ARBSIs comprising Gram-positive organisms, the IRR was 0.40 (P=0.01) favoring ClearGuard (Figure 3E, Table 3). For ARBSIs comprising only Gram-negative organisms, the IRR was 0.19 (P=0.001) favoring ClearGuard (Figure 3F, Table 3). There were too few fungi PBCs to perform an analysis (no events in the ClearGuard group). The IRR for multidrug resistant organisms was 0.60 (P=0.5); there were 4 PBCs in the ClearGuard group and 8 PBCs in the Tego+Curos group.
De Novo CVCs Analysis
To account for potential latent effects due to colonization of catheters before entering the study, a subgroup analysis was performed among patients entering the study with a new CVC (called de novo CVC); thus, all patients in this subgroup start with a CVC vintage of zero. The resulting IRR was 0.28 (P<0.001) favoring ClearGuard (Figure 3G, Table 3).
Sensitivity Analysis—No Initial 21-Day Censor
Because of the biologic latency between CVC inoculation and eventual BSI, the primary analysis did not consider infections that occurred within 21 days of patient start (Figure 4), and thereby also excluded patients who had <21 CVC-days. To understand potential implications of this a priori analytic choice a sensitivity analysis was conducted in which all patients with CVCs were considered from their study start. The resulting IRR was 0.35 (P=0.002) favoring ClearGuard (Figure 3H, Table 3).
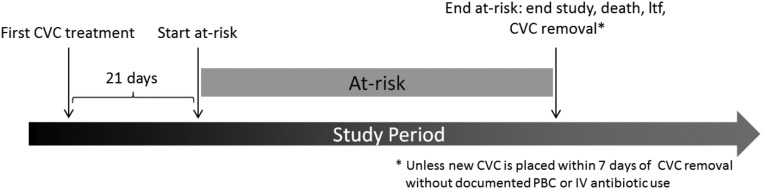
Figure 4.
Each patient's at-risk time is dependent on when the patient began and ended CVC use during the study period.
Intravenous Antibiotic Analysis
An analysis was performed to investigate whether an increase in antibiotic use could be responsible for the decreased infection rates. The rate of intravenous (IV) antibiotic starts decreased by 0.6 per 1000 CVC-days from run-in period to intervention period, with the greatest decrease in the ClearGuard group. In addition, an analysis was performed to investigate whether there was a corresponding decrease in the rate of IV antibiotics associated with PBCs. The resulting IRR was 0.37 (P<0.001) favoring ClearGuard (Figure 3I, Table 3).
Other Analyses
Additional exploratory analyses were performed to observe trends in areas where the study was not designed to have sufficient power. CVC exchange rate was not statistically different in the ClearGuard group versus Tego+Curos (0.94 versus 1.03 per 1000 CVC-days, respectively; P=0.8). CVC removal rate was similar between the two groups (7.57 versus 7.56 events per 1000 CVC-days, respectively; P=0.9). Thrombolytic use rate was not significantly different between the two groups (1.84 versus 1.89 per 1000 CVC-days, respectively; P=0.9). Hospital admissions for BSI were analyzed using the dialysis facilities’ records of admission (no other hospital records were available); the rate of hospitalizations for BSI was lower in the ClearGuard group versus Tego+Curos (0.06 versus 0.11 per 1000 CVC-days, respectively), but the difference was not statistically significant (IRR=0.55; P=0.5). There were no deaths within 30 days of a PBC in the ClearGuard group, and three deaths in the Tego+Curos group; however, these results were statistically insignificant.
Lock solutions were not required to be reported. However, they were recorded in 33% of all procedures. Within both groups, the vast majority (>95%) of procedures used saline as the lock solution.
Discussion
This study demonstrated that use of ClearGuard resulted in a significantly lower BSI rate versus use of Tego+Curos.
During the 3-month run-in period, there was no statistical difference in PBC rates between the two groups. This suggests that groups were well balanced in terms of BSI risk and that differences in BSI rates seen during the intervention phase of the study can be more confidently ascribed to study interventions. The BSI rates were lower during intervention period versus run-in period in both groups, which is expected because ClearGuard and Curos are both designed to reduce BSI rates.
This study’s primary analysis, along with the exploratory analyses, demonstrated that in a real-world hemodialysis setting use of ClearGuard was superior to use of Tego+Curos at reducing PBCs, CRBSI, CLABSI, Gram-positive infections, Gram-negative infections, and IV antibiotic starts related to PBCs in patients undergoing hemodialysis using CVCs.
A de novo subgroup analysis considered comparative effects of the study interventions in the context of newly placed CVCs. Such a scenario may be a better indicator of the effects of interventions if each were adopted as standard of care (i.e., whereby over time all CVCs would be treated with the ascribed intervention from the outset). Although the effects in this subgroup cannot be formally compared with the effects of the primary analysis, it is reassuring that effects were at least as great and, in fact, numerically more potent (IRR=0.28; P<0.001).
In the primary analysis, at-risk time began 21-days after first receipt of study intervention. This choice was prespecified and was adopted to account for biologic latency of infections particularly in the setting of preexisting CVCs. Nonetheless, it is reassuring that the comparative benefit of ClearGuard was at least as great (IRR=0.35; P=0.002) in a sensitivity analysis in which the 21-day lagged start of at-risk time was not used and all patients were considered from the first date of study intervention.
Randomized clinical trials have been conducted using antimicrobial and antibiotic lock solutions.14–17 This study is substantially larger than the referenced analyses (>3.5× larger than the largest individual referenced study and >2× larger than either meta-analysis). Although observational comparisons to external populations are not ideal, it is reaffirming to note that ClearGuard results are comparable to the best lock solutions (rate=0.28 versus 0.34 per 1000 CVC-days in ClearGuard versus Liu meta-analysis, respectively), but with several advantages: (1) ClearGuard antimicrobial is confined proximally to the CVC clamp, thus being prevented from entering the bloodstream as occurs with antimicrobial lock solutions18,19; (2) ClearGuard dose is fixed, thus eliminating risk of lock solution misadministration and the associated complications of dysgeusia and even death20–25; (3) ClearGuard antimicrobial is antibiotic-free so the risk of developing drug-resistant organisms is presumably lower than with antibiotic locks; and (4) ClearGuard is FDA cleared for use in patients receiving hemodialysis and may be used with heparin, saline, or citrate lock solution.2
The study results are likely broadly generalizable to United States dialysis facilities because (1) this study was conducted in a real-world manner with broad inclusionary requirements, (2) the control group was formidable (Tego+Curos using best-practice infection prevention techniques), (3) the ClearGuard performance is consistent with an independent study conducted at another large dialysis provider using a different control group,5 and (4) the primary results are consistent with the exploratory analyses which scrutinized potential contamination, sources of infection, and censoring effects.
Our findings demonstrate that ClearGuard caps are statistically superior to Tego+Curos for reducing CRBSI, representing an important advancement in improved patient care.
Concise Methods
Design
This was a prospective, cluster-randomized comparative effectiveness trial of ClearGuard versus Tego+Curos among patients dialyzing with CVCs.
The study was conducted at 40 dialysis facilities. All facilities had previously used Tego before inclusion in the study. Also, all facilities had indicated willingness to adhere to treatment allocation upon eventual randomization and all underwent a 30-minute training session describing procedures necessary to both study arms.
These 40 facilities were pair matched on the basis of: (1) prestudy BSI rate, as reported to the CDC NHSN from February to July of 2015; (2) the number of patients with a CVC; and (3) geographic location. Within each matched pair, one facility was randomly allocated to ClearGuard and the other to Tego+Curos using a computer-generated algorithm.
The unit of randomization was the facility and all eligible patients within the study were treated according to the corresponding intervention. Eligible patients were those who dialyzed using a CVC. Beyond this, exclusions were made only for known hypersensitivity to heparin (nine patients) or to chlorhexidine (none).
The study was approved by New England Independent Review Board (IRB# 15–281), which also granted a patient informed consent waiver. The informed consent waiver was important for conducting the study in a pragmatic manner, adherence to the prescribed intervention, and broad inclusion.
Run-In Phase
Upon randomization, facilities entered into a 3-month run-in phase. During this phase both study arms were treated according to facility standard policy (including use of Tego). The purpose of this period was to assess whether BSI rates were equivalent between study arms before institution of study interventions.
Intervention Phase
After the run-in period, facilities entered a 13-month intervention phase. During this phase, patients in the Tego+Curos group began including Curos (and continued using Tego). Patients in the ClearGuard group converted from Tego to ClearGuard. Patients with a CVC received the facility’s assigned intervention at their first dialysis session after the study start date, and new patients with CVCs coming into the facility were added as appropriate throughout the study.
In the Tego+Curos group, Curos was replaced each session and Tego was replaced once per week according to existing facility protocols. In the ClearGuard group, ClearGuard caps were replaced each session.
Aside from CVC capping, all patients were treated according to local standard of care and existing physician orders. This included standard surveillance for potential BSI, which adhered to the NHSN guidelines. All blood cultures were sent to and processed by a single clinical lab (DaVita Labs, Deland, FL). As is standard policy, blood culture results were reported into the electronic health record in automated fashion, from which they were abstracted for analysis.
Outcomes and Analysis
The primary study outcome was blood culture positivity rate. This was calculated by dividing the cumulative number of PBCs (as defined according to NHSN guidelines) by CVC time at-risk (Figure 4). To avoid double-counting the same BSI, patients were censored using the CDC’s NHSN-recommended 21-day rule: a PBC is counted only if it occurred 21 days or more after a previously reported PBC in the same patient.26 To account for biologic latency between catheter seeding and clinical manifestation of BSI, at-risk time began on day 21 after first receipt of study intervention and continued until end of study, death, CVC removal, or loss to follow up; by extension, patients treated with a CVC for <21 days were excluded from analysis. In addition, multiple exploratory analyses were conducted using DaVita electronic records, which include microbiology, NHSN surveillance, and other records.
The IRRs and the corresponding 95% confidence intervals for the final analysis were calculated using a Poisson regression model with a log link function and the natural logarithm of patient-days at risk as an offset and adjusted for the facility cluster effect, where each matched facility pair was considered a cluster.
Disclosures
This study was supported by Pursuit Vascular Inc. S.M.B. is an employee of DaVita Clinical Research; D.B.V.W. and L.N. are employees of DaVita Inc.; L.E.L., D.P.K., and R.J.Z. are employees of Pursuit Vascular Inc. and may have patents, board membership, and/or stock ownership. Pursuit Vascular sponsored the study and thus paid DaVita Clinical Research, the clinical research organization of DaVita, for conducting the study. No additional financial relationships exist between DaVita and Pursuit Vascular.
Supplementary Material
Acknowledgments
We extend our sincere appreciation to the teammates in >2000 DaVita clinics who work every day to take care of patients and also to ensure the extensive data collection on which our work is based. We thank all of the DaVita Clinical Research teammates who contributed to this work. We thank NAMSA (North American Science Associates Inc.) for performing the statistical analysis.
An abstract of this study was published as a late-breaking clinical trial in the National Kidney Foundation Spring Clinical Meetings, Orlando, Florida, April 18–22, 2017.
Trial registration (www.clinicaltrials.gov): NCT02593149.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Targeting Zero Infections in Dialysis: New Devices, Yes, but also Guidelines, Checklists, and a Culture of Safety,” on pages 1083–1084.
References
- 1.Nguyen DB, Shugart A, Lines C, Shah AB, Edwards J, Pollock D, et al.: National healthcare safety network (NHSN) dialysis event surveillance report for 2014. Clin J Am Soc Nephrol 12: 1139–1146, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Food and Drug Administration: K131060 ClearGuard HD hemodialysis catheter luer end cap, 2013. Available at: www.accessdata.fda.gov/cdrh_docs/pdf13/k131060.pdf. Accessed December 12, 2017
- 3.Wand ME, Bock LJ, Bonney LC, Sutton JM: Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob Agents Chemother 61: e01162–16, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlett CD, Millar EV, Crawford KB, Cui T, Lanier JB, Tribble DR, et al.: Prevalence of chlorhexidine-resistant methicillin-resistant Staphylococcus aureus following prolonged exposure. Antimicrob Agents Chemother 58: 4404–4410, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hymes JL, Mooney A, Van Zandt C, Lynch L, Ziebol R, Killion D: Dialysis catheter-related bloodstream infections: A cluster-randomized trial of the ClearGuard HD antimicrobial barrier cap. Am J Kidney Dis 69: 220–227, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Medical ICU: Inc.: Tego needlefree connector, 2017. Available at: http://www.icumed.com/products/specialty/renal-systems/tego-connector.aspx. Accessed December 12, 2017
- 7.Brunelli SM, Njord L, Hunt AE, Sibbel SP: Use of the Tego needlefree connector is associated with reduced incidence of catheter-related bloodstream infections in hemodialysis patients. Int J Nephrol Renovasc Dis 7: 131–139, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.3M: Protect ports and ensure peace of mind, 2017. Available at: http://www.3m.com/3M/en_US/company-us/all-3m-products/~/All-3M-Products/Health-Care/Medical/Curos/?N=5002385+8707795+8707798+8711017+8717585+3294857497&rt=r3. Accessed December 12, 2017
- 9.Sweet MA, Cumpston A, Briggs F, Craig M, Hamadani M: Impact of alcohol-impregnated port protectors and needleless neutral pressure connectors on central line-associated bloodstream infections and contamination of blood cultures in an inpatient oncology unit. Am J Infect Control 40: 931–934, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Miller LM, Clark E, Dipchand C, Hiremath S, Kappel J, Kiaii M, et al. : Hemodialysis tunneled catheter-related infections. Can J Kidney Health Dis 3: 2054358116669129, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, et al.: Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious diseases society of America. Clin Infect Dis 49: 1–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC): National Healthcare Safety Network (NHSN) patient safety component manual, 2017. Cpt 4 device-associated module BSI. Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf. Accessed December 12, 2017
- 13.Centers for Disease Control and Prevention: Dialysis event surveillance manual. Available at: https://www.cdc.gov/nhsn/PDFs/pscManual/Dialysis-Manual.pdf. Accessed December 20, 2017
- 14.Jaffer Y, Selby NM, Taal MW, Fluck RJ, McIntyre CW: A meta-analysis of hemodialysis catheter locking solutions in the prevention of catheter-related infection. Am J Kidney Dis 51: 233–241, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Zhang AQ, Cao L, Xia HT, Ma JJ: Taurolidine lock solutions for the prevention of catheter-related bloodstream infections: A systematic review and meta-analysis of randomized controlled trials. PLoS One 8: e79417, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maki DG, Ash SR, Winger RK, Lavin P; AZEPTIC Trial Investigators : A novel antimicrobial and antithrombotic lock solution for hemodialysis catheters: A multi-center, controlled, randomized trial. Crit Care Med 39: 613–620, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Grudzinski A, Agarwal A, Bhatnagar N, Nesrallah G: Benefits and harms of citrate locking solutions for hemodialysis catheters: A systematic review and meta-analysis. Can J Kidney Health Dis 2: 13, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polaschegg HD: A novel method for measuring catheter lock spillage: An in vitro study. Artif Organs 36: 824–829, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Schilcher G, Schneditz D, Ribitsch W, Horina JH, Hoenigl M, Valentin T, et al.: Loss of antimicrobial effect of trisodium citrate due to ‘lock’ spillage from haemodialysis catheters. Nephrol Dial Transplant 29: 914–919, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Reidenberg BE, Wanner C, Polsky B, Castanheira M, Shelip A, Stalleicken D, et al. : Postmarketing experience with Neutrolin® (taurolidine, heparin, calcium citrate) catheter lock solution in hemodialysis patients [published online ahead of print December 6, 2017]. Eur J Clin Microbiol doi: 10.1007/s10096-017-3157-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.FDA: FDA issues warning on tricitrasol dialysis catheter anticoagulant. In: Food and Drug Administration. FDA Talk Paper, 14. T00–T16, Rockville, MD, Food and Drug Administration, 2000 [Google Scholar]
- 22.Punt CD, Boer WE: Cardiac arrest following injection of concentrated trisodium citrate. Clin Nephrol 69: 317–318, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Willicombe MK, Vernon K, Davenport A: Embolic complications from central venous hemodialysis catheters used with hypertonic citrate locking solution. Am J Kidney Dis 55: 348–351, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Polaschegg HD, Sodemann K: Risks related to catheter locking solutions containing concentrated citrate. Nephrol Dial Transplant 18: 2688–2690, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Schilcher G, Scharnagl H, Horina JH, Ribitsch W, Rosenkranz AR, Stojakovic T, et al.: Trisodium citrate induced protein precipitation in haemodialysis catheters might cause pulmonary embolism. Nephrol Dial Transplant 27: 2953–2957, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention: Dialysis event protocol, 2015. Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual/8pscdialysiseventcurrent.pdf. Accessed December 12, 2017
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.