Abstract
AKI is a devastating condition with high morbidity and mortality. The pathologic features of AKI are characterized by tubular injury, inflammation, and vascular impairment. Whether fibroblasts in the renal interstitium have a role in the pathogenesis of AKI is unknown. In this study, we investigated the role of fibroblast-specific β-catenin signaling in dictating the outcome of AKI, using conditional knockout mice in which β-catenin was specifically ablated in fibroblasts (Gli1-β-cat−/−). After ischemia-reperfusion injury (IRI), Gli1-β-cat−/− mice had lower serum creatinine levels and less morphologic injury than Gli1-β-cat+/+ littermate controls. Moreover, we detected fewer apoptotic cells, as well as decreased cytochrome C release; reduced expression of Bax, FasL, and p53; and increased phosphorylation of Akt, in the Gli1-β-cat−/− kidneys. Gli1-β-cat−/− kidneys also exhibited upregulated expression of proliferating cell nuclear antigen and Ki-67, which are markers of cell proliferation. Furthermore, Gli1-β-cat−/− kidneys displayed suppressed NF-κB signaling and cytokine expression and reduced infiltration of inflammatory cells. Notably, loss of β-catenin in fibroblasts induced renal expression of hepatocyte growth factor (HGF) and augmented the tyrosine phosphorylation of c-met receptor after IRI. In vitro, treatment with Wnt ligands or ectopic expression of active β-catenin inhibited HGF mRNA and protein expression and repressed HGF promoter activity. Collectively, these results suggest that fibroblast-specific β-catenin signaling can control tubular injury and repair in AKI by modulating HGF expression. Our studies uncover a previously unrecognized role for interstitial fibroblasts in the pathogenesis of AKI.
Keywords: acute renal failure, apoptosis, fibroblast, signaling, Wnt, HGF
AKI is a devastating clinical condition with high morbidity and mortality, and it is responsible for approximately 2 million deaths each year worldwide.1–3 Over the last two decades, extensive studies have been carried out to elucidate the natural progression, mediators, and patho-mechanisms of AKI, particularly ischemic AKI, in animal models and patients.4–7 Studies show that renal tubular epithelial cells are the primary targets of injury, and depending on the nature and extent of the injurious stimuli, these cells lose functional integrity or die by apoptosis or necrosis. Such tubular injury is accompanied by, and may be initiated or propagated by, renal vascular endothelial dysfunction and the infiltration of proinflammatory cells such as neutrophils, lymphocytes, or macrophages.8–11 If the injury is sublethal, the surviving tubular cells will undergo dedifferentiation, migration, and proliferation, with ultimate restoration of tissue integrity. However, whether fibroblasts in renal interstitium play a role in the pathogenesis of AKI is unknown.
Fibroblasts are situated in the interstitial space between the capillaries and the epithelia throughout the renal parenchyma.12,13 Morphologically, these cells are spindle-shaped and possess multiple cellular processes, which connect them to the tubular and capillary basement membranes.12 Functionally, they produce a proper amount of extracellular matrix that physically bonds different tissue compartments together. Fibroblasts are also major players in regulating tissue hemostasis by secreting a diverse array of cytokines such as hepatocyte growth factor (HGF),14 and produce erythropoietin under hypoxic conditions.12,15,16 In this context, it is conceivable to speculate that dysregulated intrinsic signaling in fibroblasts may have a unique role in regulating tubular injury, repair, and recovery after AKI.
Wnt/β-catenin is an evolutionarily conserved, developmental signal pathway, which is reactivated in adult kidneys after both acute and chronic injury.17–20 Wnt ligands typically transmit their signals through a short range of action.17,21 By acting on nearby neighboring cells, Wnt signal mediates cell-cell communication in local tissue microenvironment. Although sustained activation of this signal cascade is detrimental and promotes kidney fibrosis,22 transient activation is believed to be beneficial and reparative by mitigating initial injury and accelerating subsequent recovery after AKI.23,24 We previously showed that genetic ablation of β-catenin, the principal intracellular mediator of canonic Wnt signaling, in renal tubule aggravates kidney damage after either ischemic or nephrotoxic AKI,23 supporting that tubular activation of Wnt signaling is renoprotective. However, to our surprise, pharmacologic inhibition of β-catenin in vivo by small molecule inhibitor exhibits little or no renal protection against ischemia-reperfusion injury (IRI), implying possibly opposite actions of Wnt signaling in other, nontubular cells, which may negate the beneficial effect of tubular β-catenin in this setting. These speculations prompted us to investigate the role of fibroblast-specific β-catenin signaling in AKI.
In this study, by generating conditional knockout mice in which β-catenin was selectively ablated in interstitial fibroblasts, we demonstrated that fibroblast-specific β-catenin signaling plays a crucial role in controlling tubular injury and repair after AKI. Furthermore, we have uncovered that HGF is a novel downstream target that is negatively regulated by Wnt/β-catenin. Our studies also illustrate an opposite action of β-catenin signaling in different tissue compartments in AKI.
Results
Pharmacologic Inhibition of β-Catenin Does Not Affect the Severity of Kidney Injury after AKI
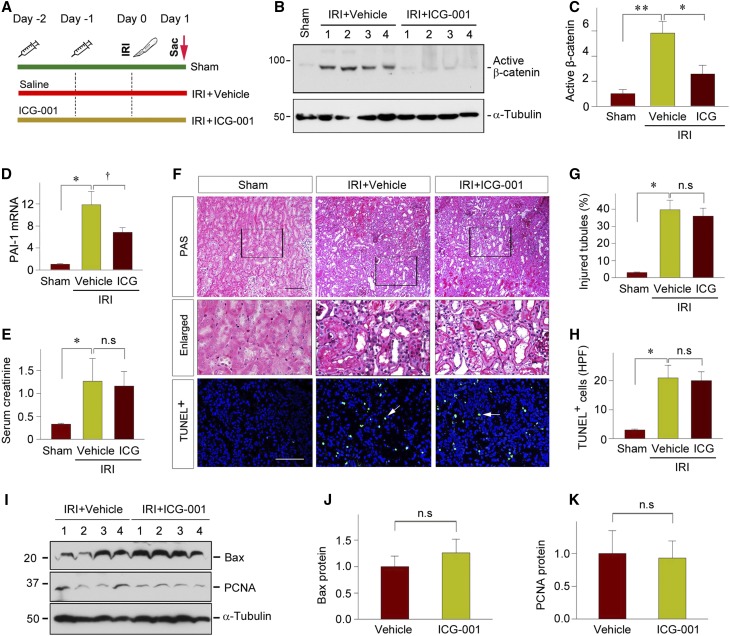
Earlier studies show that tubule-specific deletion of β-catenin aggravates kidney injury after IRI, suggesting a renal protective role of canonic Wnt signaling in the setting of AKI.23,24 To confirm these findings, we used a pharmacologic approach to inhibit β-catenin signaling by injecting a small molecule inhibitor, ICG-001, at 2 days before renal IRI (Figure 1A). As shown in Figure 1, B and C, ICG-001 was able to inhibit β-catenin activation in the kidney after IRI, as demonstrated by western blotting of whole-kidney lysates. Accordingly, ICG-001 repressed renal expression of plasminogen activator inhibitor–1 (PAI-1) (Figure 1D), a downstream target of Wnt/β-catenin signaling.25 To our surprise, inhibition of β-catenin activity in vivo did not affect the severity of kidney damage at 1 day after IRI, as assessed by serum creatinine levels (Figure 1E). Consistently, regardless of the absence or presence of ICG-001, little differences in renal pathology were observed in both groups (Figure 1, F and G). We further examined apoptotic cell death in the kidneys of the mice injected with or without ICG-001. As shown in Figure 1, F and H, terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) staining revealed virtually no differences in cell apoptosis in the mice injected with or without ICG-001. In addition, renal expression of Bax protein, a proapoptotic member of Bcl-2 family,26 was similar in the kidneys receiving vehicle or ICG-001 (Figure 1, G and H). Furthermore, ICG-001 did not affect renal expression of proliferating cell nuclear antigen (PCNA) in the injured kidneys (Figure 1, G and I). Collectively, these data suggest that in sharp contrast to the genetic ablation of tubular β-catenin, pharmacologic inhibition of its activity does not affect the severity of kidney injury after AKI.
Figure 1.
Pharmacologic inhibition of β-catenin signaling has little effect on the severity of AKI. (A) Experimental design. Cartoon syringes indicate the administration of ICG-001. (B and C) ICG-001 blocks β-catenin activation in the kidney at 1 day after IRI. Kidney lysates were immunoblotted with antibodies against active (dephosphorylated) β-catenin and α-tubulin. (B) Representative western blots and (C) quantitative data are presented. Numbers (1–4) indicate each individual animal in a given group. **P<0.01, *P<0.05 (n=6). (D) qRT-PCR analyses reveal that ICG-001 suppressed PAI-1 mRNA expression at 1 day after IRI. *P<0.05 versus shams, †P<0.05 versus vehicles (n=4–6). (E) Serum creatinine level in the mice with or without ICG-001 at 1 day after IRI, compared with shams. *P<0.05 versus shams (n=4–6). (F) Representative micrographs show morphologic injury and apoptosis at 1 day after IRI. Boxed areas are enlarged and presented. Apoptotic cell death was assessed by TUNEL staining. Arrows indicate apoptotic cells. Scale bar, 100 µm. (G and H) Quantitative data on (G) histologic injury and (H) apoptosis are presented. Data are presented as (G) percentage of injured tubules or (H) numbers of apoptotic cells per high power field (HPF), respectively. *P<0.05 versus shams (n=3). (I–K) Western blot analyses of renal expression of Bax and PCNA proteins at 1 day after IRI. Kidney lysates were immunoblotted with specific antibody against Bax, PCNA, and α-tubulin, respectively. (I) Representative western blot and (J and K) quantitative data are presented. Numbers (1–4) indicate each individual animal in a given group (n=6). Sac, sacrifice.
Specific Ablation of β-Catenin in Interstitial Fibroblasts Protects against AKI
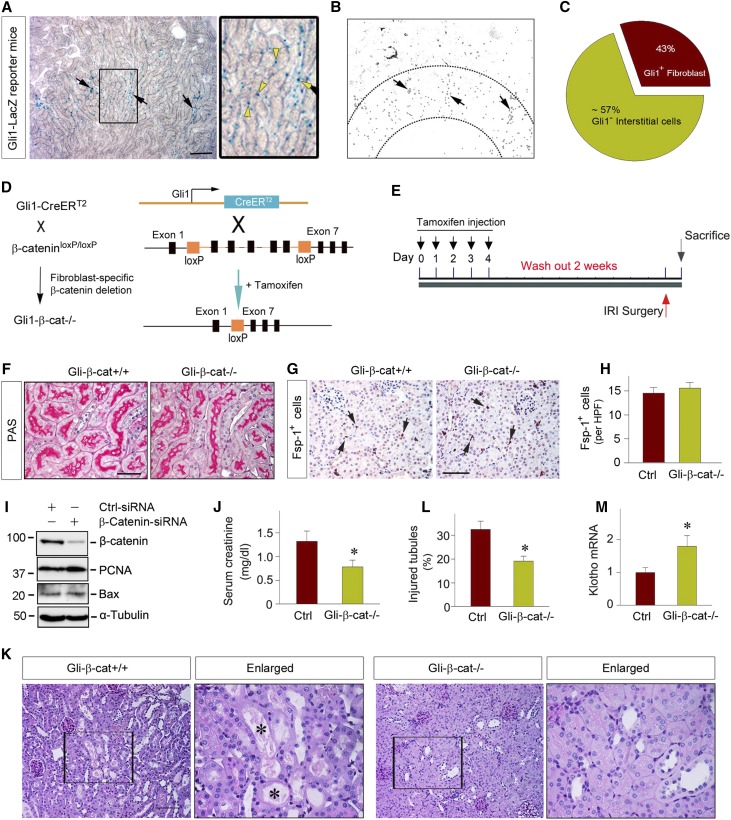
The discrepancy of the AKI outcomes after genetic knockout of tubular β-catenin or its pharmacologic inhibition may suggest an opposite action of β-catenin in other, nontubular cells. To test this hypothesis, we sought to generate conditional knockout mice with fibroblast-specific ablation of β-catenin by mating β-catenin–floxed mice with the Gli1-CreERT2 transgenic mice under the control of endogenous Gli1 promoter, because earlier studies indicate that Gli1 is specifically expressed in activated fibroblasts.27–31 To determine the efficacy and specificity of gene deletion, we examined Gli1 expression by using the Gli1-LacZ knock-in reporter mice, which express a nuclear β-galactosidase under the control of Gli1 promoter. As shown in Figure 2A, 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal) staining revealed significant Gli1+ cells in renal interstitium, particularly in the corticomedullary junction region, at 1 day after IRI. Quantitative determination estimated that about 43% of the total interstitial cell population exhibited a positive nuclear staining for β-galactosidase activity (Figure 2, B and C). As shown previously,29,30 these β-Gal+ cells did not costain with endothelial CD31 and common leukocyte marker CD45. Although most β-Gal+ cells costained with fibroblast marker vimentin, there was a small fraction of vimentin+ cells that did not express β-Gal in renal interstitium, suggesting that not all fibroblasts express Gli1. It was estimated that among vimentin+ fibroblasts, the majority of them (approximately 70%) expressed Gli1-driven β-Gal in the nucleus.
Figure 2.
Specific ablation of β-catenin in fibroblasts reduces kidney injury after AKI. (A–C) Using Gli1-LacZ reporter mice determinates the efficacy and specificity of Gli1-driven gene expression after IRI. X-Gal staining shows the Gli1-LacZ+ cell population and distribution at 1 day after IRI. (A) Boxed area was enlarged. Scale bar, 100 µm. (B) Intensive staining was found in the corticomedullary junction region of the kidney. Arrows indicate positive staining. (C) Pie chart shows the relative distribution of the Gli1-LacZ+ fibroblasts in renal interstitium. (D) Schematic diagram depicts the generation of conditional knockout mice with fibroblast-specific deletion of β-catenin by using Cre-LoxP system. The β-catenin–floxed mice (β-catfl/fl) were crossbred with tamoxifen-inducible Cre transgenic mice under the control of endogenous Gli1 promoter/enhancer elements. Black boxes indicate the exons of the β-catenin gene. Orange boxes denote LoxP site. (E) Experimental design. The mice were injected with tamoxifen at 30 mg/kg body wt for 5 consecutive days to activate Cre recombinase in vivo. After wash out for 2 weeks, the mice were subjected to IRI for 1 day. (F) Representative micrographs show kidney morphology in Gli-β-cat−/− and Gli-β-cat+/+ mice. Sections of PAS staining are shown. Scale bar, 50 µm. (G and H) Ablation of β-catenin does not affect fibroblast survival in the kidney in vivo. Immunostaining for Fsp1 shows similar fibroblast density in Gli-β-cat−/− and Gli-β-cat+/+ kidneys under basal conditions. (G) Representative micrographs and (H) quantitative data are presented. Arrows indicate positive Fsp1 staining. Scale bar, 50 µm. (I) Knockdown of β-catenin in vitro does not affect PCNA and Bax expression in renal fibroblasts. NRK-49F cells were transfected with control or β-catenin–specific siRNA for 3 days. Cell lysates were then immunoblotted with antibodies against β-catenin, PCNA, Bax, and α-tubulin, respectively. (J) Serum creatinine level in the Gli1-β-cat+/+ and Gli1-β-cat−/− mice at 1 day after IRI. *P<0.05 (n=9). (K and L) Representative micrographs show the kidneys at 1 day after IRI in the Gli1-β-cat+/+ and Gli1-β-cat−/− mice. Boxed areas were enlarged and presented. Black asterisks in the enlarged boxed areas indicate the injured tubules. Scale bar, 100 µm. (F) Quantitative assessment of injury is presented. *P<0.05 (n=4). (M) qRT-PCR analyses show an increased Klotho mRNA expression in the Gli1-β-cat−/− mice at 1 day after IRI, compared with the Gli1-β-cat+/+ controls. *P<0.05 (n=9). Ctrl, control; PAS, periodic acid–Schiff.
Figure 2D shows the scheme to generate tamoxifen-inducible, conditional knockout mice with fibroblast-specific deletion of β-catenin (Gli1-β-cat−/−). We used the β-catenin–floxed mice in the same litters as their controls (Gli1-β-cat+/+). After daily injections of tamoxifen for 5 consecutive days (Figure 2E), Cre-mediated recombination was induced in the Gli1+ fibroblasts, as reported previously.30,32,33 We found that Gli1-β-cat−/− mice and their controls were identical in terms of kidney morphology under basal conditions (Figure 2F). Ablation of β-catenin did not affect fibroblast survival and density, as shown by specific staining for fibroblast-specific protein 1 (Fsp1) (Figure 2, G and H). To further confirm this observation, we employed an in vitro approach by using siRNA-mediated knockdown of β-catenin expression in normal rat kidney interstitial fibroblasts (NRK-49F). As shown in Figure 2I, knockdown of β-catenin also did not affect PCNA and Bax expression in NRK-49F cells in vitro.
When subjected to IRI for 1 day, the Gli1-β-cat−/− mice displayed a reduced serum creatinine level, compared with control littermates (Figure 2J). Consistently, Gli1-β-cat−/− kidneys were protected against morphologic injury after AKI, with less cellular debris and proteinous casts in the lumen of individual nephrons (Figure 2K, black asterisks). Quantitative assessment of kidney morphologic injury between control and Gli1-β-cat−/− mice is presented in Figure 2L. In addition, the expression of Klotho, which is rapidly downregulated after AKI,34 was largely preserved in the kidneys of Gli1-β-cat−/− mice, compared with the controls (Figure 2M). Together, it appears clear that fibroblast-specific β-catenin signaling plays a detrimental role in the pathogenesis of AKI after IRI.
Loss of β-Catenin in Fibroblasts Reduces Tubular Cell Apoptosis after AKI
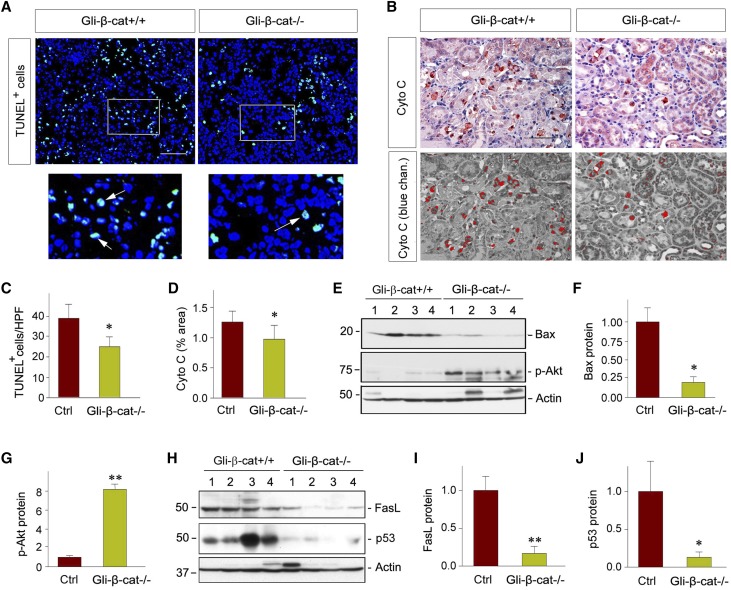
We next examined cell apoptosis in the kidneys of the control and Gli1-β-cat−/− mice after IRI. As shown in Figure 3A, TUNEL staining revealed considerable apoptotic cells in the control kidneys at 1 day after IRI, whereas the frequency of apoptosis in Gli1-β-cat−/− kidneys was significantly reduced under the same conditions (Figure 3A, white arrows in the enlarged boxes). We further studied the cytochrome C release from mitochondria, a key initial step in apoptotic cell death.35,36 As shown in Figure 3B, cytochrome C–positive cells were significantly reduced in the Gli1-β-cat−/− kidneys after IRI, compared with the controls. Immunohistochemical staining images under blue channel clearly showed that the majority of cytochrome C–positive cells were located in the tubular compartment (Figure 3B, bottom panel). Quantitative data on TUNEL+ and cytochrome C+ cells in control and Gli1-β-cat−/− kidneys are presented in Figure 3, C and D, respectively. These results indicate that fibroblast-specific β-catenin signaling is able to control tubular cell apoptosis after AKI.
Figure 3.
Loss of β-catenin in fibroblasts reduces tubular cell apoptosis after AKI. (A) Representative micrographs show apoptotic cells detected by TUNEL staining after IRI at 1 day after IRI. Scale bar, 50 µm. Boxed areas were enlarged. Arrows indicate apoptotic cells. (B) Representative micrographs show renal expression of cytochrome C in the Gli1-β-cat+/+ and Gli1-β-cat−/− mice at 1 day after IRI. Scale bar, 50 µm. The images in the blue channel were shown in the bottom panels. (C and D) Quantitative assessments of apoptotic cells are presented. Data are presented as (C) numbers of apoptotic cells per HPF or (D) area of positive staining of cytochrome C. *P<0.05 (n=4). (E–G) Loss of β-catenin in fibroblasts reduced renal Bax protein expression and activated Akt. (E) Representative western blots and (F and G) quantitative analyses are presented. Numbers (1–4) indicate each individual animal in a given group. **P<0.01, *P<0.05 (n=6). (H–J) Fibroblast-specific ablation of β-catenin suppressed FasL and p53 protein expression. (H) Representative western blots and (I and J) quantitative data are presented. Numbers (1–4) indicate each individual animal in a given group. **P<0.01, *P<0.05 (n=6). Ctrl, control; Cyto C, Cytochrome C
We further investigated the potential mechanisms governing tubular cell death by examining both intrinsic and extrinsic apoptotic pathways.37 As illustrated in Figure 3, E and F, Bax protein was markedly reduced in the Gli1-β-cat−/− kidneys after IRI, when compared with the controls. Because Bax protein is regulated by Akt protein kinase,38 we then examined the phosphorylation status of renal Akt in vivo. As presented in Figure 3, E and G, fibroblast-specific ablation of β-catenin substantially promoted Akt phosphorylation at Serine 473 in Gli1-β-cat−/− mice. In addition, loss of β-catenin in fibroblasts also affected Fas ligand (FasL) and p53 expression in ischemic kidneys, compared with the controls (Figure 3, H–J). Collectively, these results suggest that fibroblast-specific ablation of β-catenin protects kidneys against apoptosis by a multitude of cell survival–regulatory pathways.
Loss of β-Catenin in Fibroblasts Promotes Tubular Regeneration after AKI
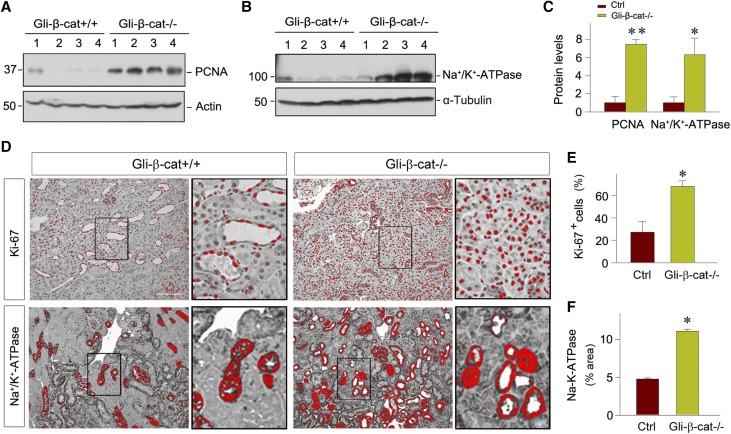
We also examined the effect of fibroblast-specific β-catenin signaling on tubule repair and regeneration, the major cellular events in kidney recovery after AKI.39 As shown in Figure 4A, western blot analyses of whole-kidney lysates revealed significant upregulation of PCNA in Gli1-β-cat−/− kidneys at 1 day after IRI, compared with the controls. These results suggest an increased cell proliferation after AKI in the Gli1-β-cat−/− mice, leading to an enhanced tubule repair and regeneration. Meanwhile, loss of β-catenin in fibroblasts also largely restored renal Na+/K+-ATPase (Figure 4, B–D), a protein that is crucial for the maintenance of cell membrane potential and energy expenditure in renal tubular cells.40 Consistent with these findings, expression of Ki-67, a marker for cell cycle progression, was upregulated in Gli1-β-cat−/− kidneys at 1 day after IRI, compared with the controls (Figure 4, D and E). Notably, the majority of Ki-67+ cells were localized in the renal tubular compartment, rather than renal interstitium. Therefore, fibroblast-specific ablation of β-catenin leads to an enhanced cell proliferation and tubular regeneration after AKI.
Figure 4.
Loss of β-catenin in fibroblasts promotes tubular cell proliferation and tubular regeneration after AKI. (A–C) Western blot analyses show that fibroblast-specific ablation of β-catenin promoted renal PCNA and Na+/K+-ATPase expression at 1 day after IRI. (A and B) Representative western blots and (C) quantitative data are presented. Numbers (1–4) indicate each individual animal in a given group. **P<0.01, *P<0.05 (n=6). (D–F) Representative immunohistochemical micrographs in blue channel show (D) Ki-67 and Na+/K+-ATPase protein expression in Gli1-β-cat+/+ and Gli1-β-cat−/− mice at 1 day after IRI. Kidney sections were immunostained with specific antibodies against Ki-67 and Na+/K+-ATPase. Boxed areas are enlarged. Scale bar, 50 µm. Quantitative determination of (E) positive Ki-67 cells and (F) areas of positive Na+/K+-ATPase are presented. *P<0.05 (n=4). Ctrl, control.
Loss of β-Catenin in Fibroblasts Attenuates Renal Inflammation after AKI
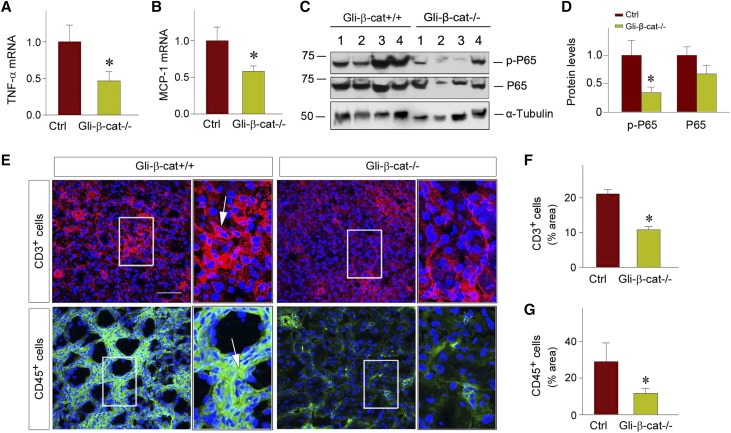
Because inflammation is one major component of AKI, we next assessed the role of fibroblast-specific β-catenin signaling in regulating renal infiltration of inflammatory cells. To this end, we first examined the expression of proinflammatory cytokines in the kidneys of the control and Gli1-β-cat−/− mice at 1 day after IRI. As shown in Figure 5A, quantitative, real-time RT-PCR (qRT-PCR) demonstrated a substantial reduction of renal expression of TNF-α mRNA in Gli1-β-cat−/− kidneys, compared with the controls. In addition, Gli1-β-cat−/− kidneys also exhibited a downregulated mRNA expression of monocyte chemoattractant protein–1 (MCP-1). Consistent with reduced expression of TNF-α and MCP-1, fibroblast-specific ablation of β-catenin also significantly repressed renal expression of the p65 subunit of NF-κB, as shown by western blot analyses. Both total p65 protein and active, phosphorylated p65 at serine 536 were downregulated in Gli1-β-cat−/− kidneys, compared with the controls (Figure 5, C and D).
Figure 5.
Loss of β-catenin in fibroblasts attenuates renal inflammation after AKI. (A and B) qRT-PCR demonstrates a decreased mRNA expression of (A) proinflammatory cytokine TNF-α and (B) MCP-1 in Gli1-β-cat−/− kidneys, compared to Gli1-β-cat+/+ controls. *P<0.05 (n=9). (C and D) Western blot analyses show phosphorylated p65 (p-p65) and total p65 expression in the Gli1-β-cat+/+ and Gli1-β-cat−/− mice at 1 day after IRI. Graphic presentations show the p-p65 and p65 protein abundances in different groups. Relative protein levels over the Gli1-β-cat+/+ controls are reported. *P<0.05 (n=6). (E–G) Immunofluorescence staining revealed a decreased infiltration of CD3+ T cells and CD45+ cells in the kidneys at 1 day after IRI. Boxed areas are enlarged. Arrows indicate positive staining. Scale bar, 50 µm. Quantitative data are presented as the percentage of the areas of (F) CD3+ T cells and (G) CD45+ cells. *P<0.05 (n=4). Ctrl, control.
We further examined renal infiltration of proinflammatory CD3+ T cells and CD45+ leukocytes at 1 day after IRI. As shown in Figure 5, E and F, fibroblast-specific ablation of β-catenin caused fewer infiltrated CD3+ T cells in the kidneys, compared with the controls (Figure 5, E and F). Similarly, fewer CD45+ leukocytes were also observed in the kidneys of Gli1-β-cat−/− mice (Figure 5, E and G). Therefore, it is concluded that fibroblast-specific ablation of β-catenin attenuates renal inflammation after AKI.
Fibroblast-Specific Ablation of β-Catenin Promotes HGF/c-met Signaling In Vivo
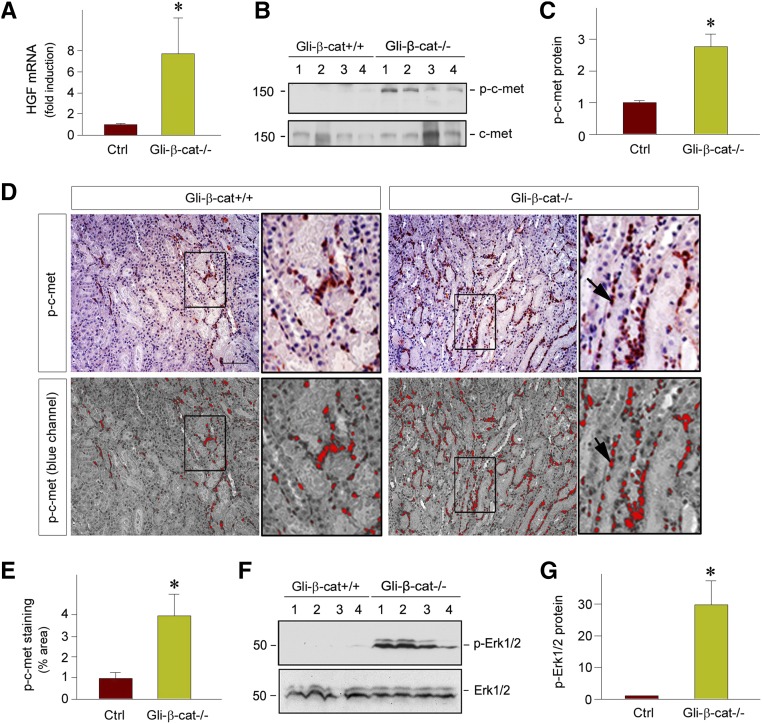
The responses of Gli1-β-cat−/− mice to IRI are largely reminiscent of those in mice with ectopic expression of HGF gene, as previously reported.41 This similarity prompted us to investigate a possible connection between Wnt/β-catenin signaling and HGF expression. To test this hypothesis, we examined renal HGF expression and c-met activation in Gli1-β-cat−/− kidneys. As shown in Figure 6A, qRT-PCR revealed that HGF mRNA expression was dramatically induced in Gli1-β-cat−/− kidneys at 1 day after IRI, compared with the controls. Not surprisingly, this induction of HGF expression led to tyrosine phosphorylation and activation of c-met, the specific transmembrane tyrosine kinase receptor for HGF (Figure 6, B and C). Although total level of c-met protein was unchanged, phosphorylated c-met protein at Tyr1234/Tyr1235 residues was markedly upregulated in Gli1-β-cat−/− kidneys (Figure 6B). Consistently, immunohistochemical staining confirmed more phosphorylated c-met-positive cells in Gli1-β-cat−/− kidneys, compared with the controls (Figure 6, D and E). Notably, phosphorylated c-met–positive cells were found in both tubular and interstitial compartments (Figure 6D).
Figure 6.
Fibroblast-specific ablation of β-catenin promotes HGF/c-met signaling after AKI in vivo. (A) qRT-PCR revealed an increased expression of HGF mRNA in Gli1-β-cat−/− kidneys at 1 day after IRI, compared with the Gli1-β-cat+/+ controls. *P<0.05 (n=6). (B and C) Western blot analyses show an increased HGF receptor, c-met, phosphorylation at Tyr1234/1235 in the Gli1-β-cat−/− kidneys at 1 day after IRI, compared with the Gli1-β-cat+/+ controls. (B) Representative western blot and (C) quantitative data are presented. Numbers (1–4) indicate each individual animal in a given group. *P<0.05 (n=6). (D and E) Representative micrographs show renal localization of phosphorylated c-met in the Gli1-β-cat+/+ and Gli1-β-cat−/− kidneys at 1 day after IRI. The images in the blue channel were shown in the bottom panels. The phosphorylated c-met protein was detected by immunohistochemical staining. Boxed areas are enlarged. Scale bar, 50 µm. (E) Quantitative data are presented as the percentage of the areas of phosphorylated c-met. *P<0.05 (n=4). (F and G) Western blots demonstrate ERK1/2 phosphorylation in the kidneys of the Gli1-β-cat+/+ and Gli1-β-cat−/− mice at 1 day after IRI. (F) Representative western blot and (G) quantitative data are presented. *P<0.05 (n=4). Ctrl, control.
We further examined the activation of extracellular signal–regulated kinase–1 and –2 (ERK1/2), downstream effectors of HGF/c-met signal cascade, in the kidney after AKI. As shown in Figure 6, F and G, active, phosphorylated ERK1/2 was markedly upregulated in Gli1-β-cat−/− kidneys at 1 day after IRI, compared with the controls. Taken together, these results suggest that ablation of β-catenin in fibroblasts is able to induce HGF expression, thereby activating c-met receptor and its downstream signaling.
Pharmacologic Inhibition of β-Catenin in Ksp-β-cat−/− Mice Reduces Kidney Injury after AKI
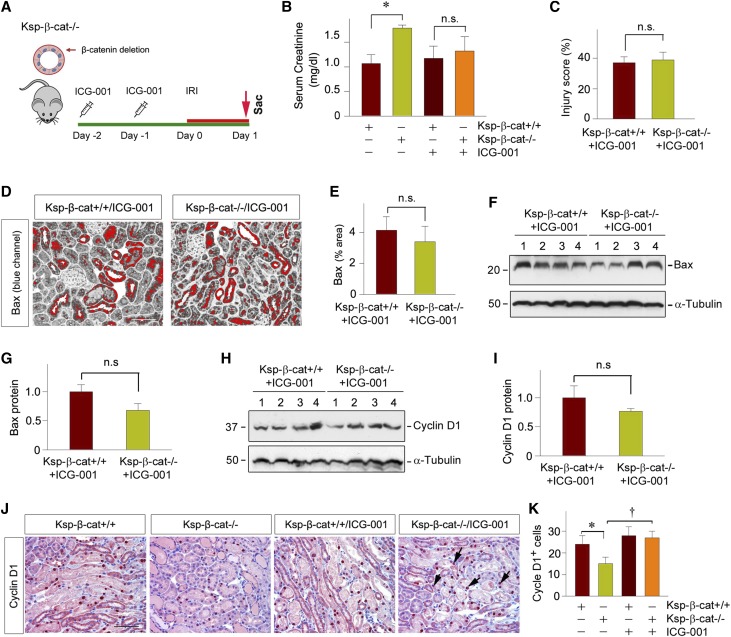
To further confirm the detrimental role of fibroblast-specific β-catenin signaling in the setting of AKI, we studied the effect of pharmacologic inhibition of β-catenin in the nontubular cells by utilizing the Ksp-β-cat−/− conditional knockout mice (Figure 7A), in which β-catenin is specifically deleted in kidney tubular epithelial cells. As shown in Figure 7B, compared with the controls, the Ksp-β-cat−/− mice were more susceptible to IRI and exhibited worsened kidney injury in the absence of ICG-001, consistent with a previous report.23 However, pretreatment with ICG-001 was able to mitigate kidney dysfunction (Figure 7B) and reduce morphologic injury (Figure 7C) in these mice, suggesting that suppression of β-catenin signaling in nontubular cells is able to ameliorate AKI. Consistently, inhibition of nontubular β-catenin by ICG-001 in Ksp-β-cat−/− mice was also able to reduce renal Bax expression, to the level similar to the control mice (Figure 7, D–G). Although ICG-001 did not affect tubular regeneration after IRI in control mice as assessed by cyclin D1 expression, blockade of β-catenin signaling by ICG-001 in nontubular cells in Ksp-β-cat−/− mice was able to promote tubular regeneration (Figure 7, H–K). These results indicate that β-catenin elicits an opposite action in different tissue compartments after AKI, with tubular β-catenin promoting repair, whereas fibroblast β-catenin exacerbates damage.
Figure 7.
Pharmacologic inhibition of β-catenin signaling in Ksp-β-cat mice attenuates kidney injury after AKI. (A) Experimental design. ICG-001 was administrated in conditional knockout mice with tubule-specific ablation of β-catenin 2 days before IRI, and the mice were euthanized at 1 day after IRI. (B) Serum creatinine level in Ksp-β-cat+/+ and Ksp-β-cat−/− mice in the absence or presence of ICG-001 at 1 day after IRI. *P<0.05 (n=8–9). (C) Quantitative assessment of renal injury in Ksp-β-cat+/+ and Ksp-β-cat−/− mice after ICG-001 treatments. Injury score (% of injured tubules) is presented (n=4). (D and E) Representative micrographs showed immunohistochemical staining (blue channel) for Bax in the kidneys at 1 day after IRI. Red color indicates Bax-positive tubules. Scale bar, 50 µm. (E) Quantitative data is presented as the percentage of the areas of Bax staining (n=4). (F and G) Western blot analysis showed little difference in Bax expression between Ksp-β-cat+/+ mice and Ksp-β-cat−/− mice administrated with ICG-001. (F) Representative western blot and (G) quantitative data are presented (n=6). (H and I) Western blot analysis demonstrated Cyclin D1 expression also has no changes between Ksp-β-cat+/+ and Ksp-β-cat−/− mice with ICG-001. (H) Representative western blot and (I) quantitative data are presented (n=6). (J and K) Representative micrographs show renal expression of (J) Cyclin D1 in Ksp-β-cat+/+ and Ksp-β-cat−/− mice in the absence or presence of ICG-001. Scale bar, 50 µm. (K) Quantitative data is presented as positive cell number of Cyclin D1 per HPF. *P<0.05; †P<0.05 (n=4). Sac, Sacrifice.
Wnt/β-Catenin Represses HGF Expression In Vitro
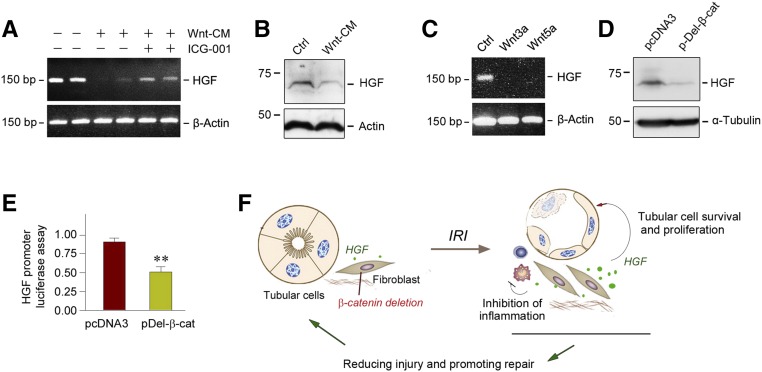
To provide direct evidence for the connection between Wnt/β-catenin and HGF, we studied HGF regulation by Wnts in vitro by using cultured normal rat kidney fibroblast (NRK-49F) cells. As shown in Figure 8, A and B, when NRK-49F cells were incubated with Wnt-enriched conditioned medium (Wnt-CM), both HGF mRNA and protein expression were markedly suppressed. Blockade of β-catenin signaling by ICG-001 restored, at least partially, HGF expression in NRK-49F cells (Figure 8A), suggesting that HGF is a downstream target that is negatively regulated by canonic Wnt signaling. Consistently, HGF gene expression was repressed by purified recombinant Wnt proteins including Wnt3a and Wnt5a in renal interstitial fibroblasts (Figure 8C). To further confirm this finding, we transfected the N-terminally truncated, stabilized β-catenin expression vector (pDel-β-cat) into human cervical cancer cells (C-33A), which have a high level of HGF expression. After 24 hours of incubation, HGF expression was largely abolished in vitro, as presented in Figure 8D.
Figure 8.
Wnt/β-catenin suppresses HGF expression in vitro. (A) RT-PCR analyses show that Wnt-enriched conditioned medium suppressed HGF gene expression in cultured NRK-49F fibroblasts, whereas blockade of Wnt/β-catenin signaling by ICG-001 restored, at least partially, HGF expression in vitro. (B) Western blot analyses reveal that Wnt-enriched conditioned medium inhibited HGF protein expression in NRK-49F cells in vitro. (C) RT-PCR analyses show that recombinant Wnt3a and Wnt5a repressed HGF gene expression in cultured NRK-49F fibroblasts in vitro. (D) Western blot analyses demonstrate that constitutive activation of β-catenin also inhibited HGF expression in human cervical cancer cells (C-33A). C-33A cells were transfected with N-terminally truncated, stabilized β-catenin (pDel-β-cat) or empty vector (pcDNA3), respectively. (E) Expression of stabilized β-catenin suppressed HGF gene transcription in luciferase assay. NRK-49F cells were cotransfected with the pGL3-HGF promoter reporter plasmid (HGF promoter region, −1037 to +56) or pGL3-Basic vector, and N-terminally truncated, stabilized β-catenin expression vector (pDel-β-cat). Luciferase activity was assessed and reported as fold induction over the controls. **P<0.01 versus pcDNA3 control (n=3). (F) Schematic diagram depicts the potential mechanisms accounting for fibroblast-specific β-catenin ablation protecting against tubular injury after AKI by promoting HGF signaling. Ctrl, control.
We finally examined HGF transcriptional regulation by Wnt/β-catenin using a promoter luciferase reporter assay. As shown in Figure 8E, HGF promoter activity was significantly repressed after cotransfection of HGF promoter reporter plasmid (pGL3-HGF) and pDel-β-cat in NRK-49F cells. Taken together, as shown in Figure 8F, these results suggest that fibroblast-specific ablation of β-catenin induces HGF expression, which activates c-met receptor, promotes tubular cell survival and proliferation, and inhibits renal inflammations, thereby leading to renal protection.
Discussion
The pathogenesis of AKI is a complex process, which involves tubular, inflammatory, and vascular components.4,6,42 Whereas the importance of tubular injury, inflammatory infiltration, and vascular impairment in the pathogenesis of AKI is well established, the potential role of resident fibroblasts in renal interstitium in this process has been largely overlooked in the field. In this study, we have demonstrated that fibroblast cells are major players that control tubular injury and repair after ischemic AKI. This conclusion is supported by several lines of evidence, including (1) fibroblast-specific knockout of β-catenin protects kidney tubular cells from injury and apoptosis; (2) loss of β-catenin in fibroblasts induces renal expression of HGF, a cytokine with strong prosurvival and proregenerative potential,14,16 and activates c-met receptor in vivo; and (3) activation of β-catenin in fibroblast cells suppresses HGF expression in vitro. It should be noted that because there is no specific biomarker that can reliably distinguish fibroblasts from pericytes, we used a broad term, “fibroblasts,” which includes both interstitial fibroblasts and capillary pericytes in this study. Our results in this study underscore that fibroblasts are not innocent bystanders in AKI as previously thought. To the best of our knowledge, these studies are the first report that links the severity of AKI to the dysregulation of a fibroblast-originated signaling.
Wnt/β-catenin mediates cellular communication via either paracrine or autocrine mechnaisms.17,21 This signal cascade is activated in the kidneys after both acute and chronic injury. Although copious studies demonstrate that activation of Wnt signaling promotes the onset and progression of CKD,43–45 its role in the setting of AKI is generally thought to be reparative, leading to accelerated recovery after injury.19,24,45 This notion is supported by earlier studies demonstrating that tubule-specific ablation of β-catenin aggravates AKI after IRI or folic acid.23 However, we have shown herein that in sharp contrast to tubular β-catenin signaling, fibroblast-specific β-catenin activation apparently worsens tubular cell injury and kidney dysfunction after IRI. Therefore, even in the same setting of ischemic AKI, β-catenin in different tissue compartments (tubule versus fibroblast) confers opposite actions, leading to disparate outcomes. This viewpoint is further strengthened by pharmacologic inhibition of β-catenin by ICG-001, which displays renal protection after IRI in Ksp-β-cat−/− mice, but not in the control mice (Figures 1 and 7), in which the effects of β-catenin inhibition in tubular and fibroblast cells negated each other. It is worthwhile to point out that because ICG-001 inhibits β-catenin signaling globally, we cannot exclude the possibility that ICG-001 may elicit its beneficial effect in Ksp-β-cat−/− mice by blocking β-catenin in other nontubular cells besides fibroblasts, such as macrophages. However, this is unlikely because previous studies showed that macrophage-derived Wnt7b is renal protective in ischemic AKI.24 Taken together, it is reasonable to conclude that the role of Wnt/β-catenin activation is not only disease stage–dependent (AKI versus CKD) but also cell content–specific (tubule versus fibroblast).
Of many cellular events in AKI, tubular cell apoptosis and inflammatory infiltration are the two most studied pathophysiologic mechanisms.4,6,46 Our data indicate that fibroblast-specific activation of Wnt/β-catenin promotes cytochrome C release from mitochondria and cell apoptosis in tubular epithelial cells, which is mediated by both intrinsic, mitochondria-dependent and extrinsic, death receptor–dependent pathways after IRI. Consistently, loss of β-catenin in fibroblasts is associated with decreased renal expression of Bax, FasL, and p53, as well as increased Akt phosphorylation (Figures 3). Furthermore, loss of β-catenin in fibroblasts also leads to a reduced peritubular infiltration of inflammatory cells, decreased cytokine expression, and inactivated NF-κB signaling (Figure 5), suggesting that fibroblast-specific β-catenin signaling also plays a role in promoting renal inflammation. Of particular interest, loss of β-catenin in fibroblasts leads to an increased expression of PCNA and Ki-67, two markers for cell proliferation, in tubular epithelium after AKI (Figure 4). These data indicate that fibroblast-specific β-catenin signaling hinders kidney repair and regeneration by inhibiting the proliferation of the surviving tubular cells after AKI. Collectively, these data indicate that ablation of β-catenin in a fibroblast-specific fashion renders the kidneys a prosurvival, anti-inflammatory, and proregenerative phenotype, thereby leading to renal protection after AKI.
One of the novel and interesting findings in this study is the identification of HGF as a Wnt/β-catenin target. In vitro, Wnt-CM, individual recombinant Wnt proteins, or transfection with active β-catenin expression vector all repressed HGF mRNA and protein expression in cultured NRK-49F fibroblasts (Figure 8). Contrary to many other Wnt/β-catenin targets, which are positively induced by this signaling,25,47,48 HGF is quite unique in that its expression is suppressed (Figure 8). HGF is a mesenchyme-derived, multifunctional polypeptide originally characterized as a potent mitogen for hepatocytes,49 and it transmits its signal via binding to c-met, a specific transmembrane tyrosine kinase receptor. Among many organs tested in normal adult animals, the kidney is actually the organ with the highest level of HGF expression.14 In the kidneys, tubular cells do not produce HGF but respond to it, leading to an enhanced cell survival, migration, and proliferation. HGF also inhibits inflammatory responses after injury by disrupting NF-κB signaling.50,51 In this regard, it is not surprising that conditional knockout mice with fibroblast-specific ablation of β-catenin phenocopy that with ectopic expression of exogenous HGF gene,41 both of which are manifested by HGF upregulation and autophosphorylation on tyrosine residues of c-met receptor (Figure 6).
Our studies also suggest that a signaling circuit with reciprocal cell-cell communication exists between renal tubule and interstitial fibroblasts in AKI. As recently reported,30,52 injured tubules are the major source of Wnt ligands after kidney injury. Therefore, tubule-derived Wnts after AKI could target fibroblast cells, leading to β-catenin activation. This, in turn, represses HGF expression, and thereby leads to tubular epithelial cell apoptosis and inhibition of proliferation. Consistent with this view, fibroblast-specific loss of β-catenin leads to ERK1/2 activation, tubular cell survival and proliferation, and reduced inflammation. These characteristic features are reminiscent of the prosurvival, anti-inflammatory, and proregenerative properties of HGF. Whether ERK1/2 activation is responsible for the beneficial effect is unknown, because some studies indicate that their activation is actually required for ischemic injury.53,54 Nevertheless, HGF has been shown to promote kidney tubular cell survival and proliferation in vitro and in vivo.14,55 Indeed, mice with conditional knockout c-met receptor in tubular epithelial cells are more susceptible to injury.56 Conversely, expression of exogenous HGF in mice reduces kidney damage and promotes tubular repair and recovery after AKI.42,56 Therefore, the renal protective effects in mice with conditional ablation of β-catenin in fibroblasts are primarily mediated by derepression of HGF/c-met signaling in AKI.
It should be pointed out that the effect of fibroblast β-catenin on tubular injury and repair after AKI, as described in this study, is probably underestimated. This is because not all fibroblasts in the injured kidneys are Gli1-positive.29–31 Using Gli1-LacZ knock-in reporter mice, we show that approximately 43% of the entire interstitial cell population in the kidney at 1 day after IRI was Gli1-positive (Figure 2). There are several major types of cells in renal interstitium after IRI, including CD45+ inflammatory cells, CD31+ endothelial cells, Gli+/vimentin+ fibroblasts, and Gli−/vimentin+ fibroblasts. Because only 70% of the vimentin+ fibroblasts express Gli-driven β-Gal, the role of interstitial fibroblasts in the pathogenesis of AKI perhaps is more likely to be greater than that reported in this study.
In summary, we herein report that fibroblast-specific ablation of β-catenin protects kidneys against AKI by inhibiting apoptosis, attenuating inflammation, and promoting tubular cell proliferation. These reparative effects are likely to be mediated by hyperactive HGF/c-met signaling. These studies suggest that β-catenin signaling in different tissue compartments (tubule versus fibroblast) has opposite function in the same setting of ischemic AKI. Our findings also highlight a critical role for fibroblasts in dictating the outcome of AKI.
Concise Methods
Animal Models
Male C57BL/6J mice weighing about 22–25 g were obtained from the Jackson Laboratories (Bar Harbor, ME). Renal IRI was performed in mice by using an established protocol, as described elsewhere.23 Briefly, bilateral renal pedicles were clamped for 30 minutes using microaneurysm clamps to generate acute injury. During the ischemic period, body temperature was maintained between 36°C and 37.5°C by using a temperature-controlled heating system. For pharmacologic inhibition experiments, mice were given daily intraperitoneal injection of ICG-001-phosphate (kindly provided by Dr. M. Kahn, University of Southern California, Los Angeles, CA) at 5 mg/kg body wt before surgery for 2 days. Mice were euthanized at 1 day after IRI, and serum and kidney tissues were collected for various analyses.
The Gli1-LacZ reporter mice, which harbor a β-galactosidase knock-in mutation that also abolishes endogenous Gli1 gene function, were obtained from the Jackson Laboratories (Stock #008211; Bar Harbor, ME). Homozygous β-catenin–floxed mice (C57BL/6J background) were also purchased from the Jackson Laboratories (Stock #004152), as described previously.23 Transgenic mice (Gli1-CreERT2) that express Cre recombinase fused to a triple mutant form of the human estrogen receptor under the control of endogenous Gli1 promoter/enhancer elements were obtained from Jackson Laboratories (Stock #007913) as well. By mating β-catenin–floxed mice with Gli1-CreERT2 transgenic mice, conditional knockout mice in which β-catenin gene was specifically disrupted in renal Gli1+ fibroblasts/pericytes (genotype β-catfl/fl, Cre±) were created. These mice were crossbred with homozygous β-catenin–floxed mice (genotype β-catfl/fl) to generate offspring with 50% Gli1-β-cat−/− mice and 50% control mice (Gli1-β-cat+/+) within the same litters. A routine PCR protocol was used for genotyping of tail DNA samples with the following primer pairs: Cre transgene, 5′-AGG-TGT-AGA-GAA-GGC-ACT-TAGC-3′ and 5′-CTA-ATC-GCC-ATC-TTC-CAG-CAG-G-3′, which generated a 411-bp fragment; and β-catenin genotyping, 5′-AAGGTA-GAG-TGA-TGA-AAG-TTG-TT-3′ and 5′-CAC-CAT-GTCCTC-TGT-CTA-TTC-3′, which yielded a 324-bp band for the floxed alleles. All animals were born normally at the expected Mendelian frequency, and they were normal in size and did not display any gross physical or behavioral abnormalities. In Gli1-β-cat−/− mice, Cre was exclusively expressed in the Gli1-expressing fibroblasts. Mice were intraperitoneally injected with tamoxifen (T5648; Sigma) at 25 mg/kg body wt for 5 days. After 2 weeks of wash out, these mice then were subjected to IRI for 1 day. Blood and kidneys were collected for various analyses. Animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Cell Culture and Treatment
Normal rat kidney interstitial fibroblast (NRK-49F) cell line was obtained from the American Type Culture Collection (Manassas, VA). Cells were maintained as described previously.57 Human proximal tubular epithelial cell line (HKC-8) was provided by Dr. L. Racusen of the Johns Hopkins University (Baltimore, MD). The Wnt-CM was prepared by transfecting HKC-8 cells with various Wnt expression vectors, as reported previously.22 Serum-starved NRK-49F was treated with Wnt-CM or recombinant human Wnt proteins (StemRD Inc., Burlingame, CA) at different concentrations for various periods of time, as indicated. NRK-49F cells were also transiently transfected with control siRNA or β-catenin–specific siRNA (#6225; Cell Signaling Technology, Danvers, MA) by using Lipofectamine 2000 reagent (Invitrogen, Grand Island, NY). Cells were then collected and subjected to various analyses. Human cervical cancer cell line (C-33A) was kindly provided by Dr. Reza Zarnegar at the University of Pittsburgh.
Determination of Serum Creatinine
Serum was collected from mice at 1 day after IRI. Serum creatinine level was determined by use of a QuantiChrom creatinine assay kit, according to the protocols specified by the manufacturer (BioAssay Systems, Hayward, CA). The level of serum creatinine was expressed as milligrams per 100 ml (dl).
Reverse Transcription and Real-Time PCR
Total RNA isolation and qRT-PCR were carried out by the procedures described previously.29 Briefly, the first-strand cDNA synthesis was carried out by using a Reverse Transcription System kit according to the instructions of the manufacturer (Promega, Madison, WI). qRT-PCR was performed on an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). The PCR reaction mixture in a 25-µl volume contained 12.5 µl 2× SYBR Green PCR Master Mix (Applied Biosystems), 5 µl diluted RT product (1:10), and 0.5 µM sense and antisense primer sets. The PCR reaction was run using standard conditions. After sequential incubations at 50°C for 2 minutes and 95°C for 10 minutes, respectively, the amplification protocol consisted of 40 cycles of denaturing at 95°C for 15 seconds, annealing, and extension at 60oC for 60 seconds. The standard curve was made from series dilutions of template cDNA. The mRNA levels of various genes were calculated after normalizing with β-actin. Primer sequences used for amplifications were presented in Supplemental Table 1.
Western Blot Analysis
Kidney tissues were lysed with radioimmunoprecipitation assay buffer containing 1% NP-40, 0.1% SDS, 100 μg/ml PMSF, 1% protease inhibitor cocktail, and 1% phosphatase I and II inhibitor cocktail (Sigma) in PBS on ice. The supernatants were collected after centrifugation at 13,000 × g at 4°C for 15 minutes. Protein expression was analyzed by western blot analysis as described previously.58 The primary antibodies used were as follows: anti–β-catenin (#610154; BD Transduction Laboratories, San Jose, CA), anti–active β-catenin (#05–665; EMD Millipore, Burlington, MA), anti-Bax (sc-493), anti-PCNA (sc-56), anti-FasL (sc-6237), anti-p53 (sc-126), anti-HGF (sc-7949) (Santa Cruz Biotechnology, Santa Cruz, CA), anti–p-Akt (#4060), anti–p-P65 (#3039), anti-P65 (#8242), anti–p-c-met (#3126), anti–c-met (#3127), anti–p-ERK1/2 (#4370) (Cell Signaling Technology; Danvers, MA), anti–Na+/K+-ATPase (#05–369; Upstate Biotechnology, Lake Placid, NY), anti-Cyclin D1 (RB-9041-P0; Neomarkers, Fremont, CA), anti-ERK1/2 (M5670), anti–α-tubulin (T9026) (Sigma, St. Louis, MO), and anti-actin (MAB1501; EMD Millipore).
Detection of Apoptotic Cells
Apoptotic cell death was determined by using TUNEL staining with DeadEnd Fluorometric Apoptosis Detection System (Millipore, Billerica, MA).
Histology and Immunohistochemical Staining
Paraffin-embedded mouse kidney sections (3-μm thickness) were prepared by a routine procedure. The sections were stained with periodic acid–Schiff staining reagents by standard protocol. Immunohistochemical staining was performed according to the established protocol as described previously.56 The antibodies against cytochrome C (#4280), p-c-met (#3126) (Cell Signaling Technology, Danvers, MA), Ki-67 (ab66155; Abcam, Cambridge, MA), Na+/K+-ATPase (#05–369), Bax (sc-493), and Cyclin D1 (sc-753) (Santa Cruz Biotechnology, Santa Cruz, CA) were used.
Automated Quantitation for Immunostaining
ImageJ Fiji was used for quantifying all immunohistochemistry staining of various proteins,59 including cytochrome C, Ki-67, Na+/K+-ATPase, p-c-met, and Bax. Each image was separated into red, green, and blue channels by using the “RGB Stack” command. To quantify the relative level of each protein marker, the channel with the best separation was selected for the next step. Notably, in this study, the blue channel was chosen because it had the best separation. Then using the “Threshold” tool, the threshold was set for (0, 15) to get the (red) areas where each marker was expressed in high level. The threshold values were chosen manually until each stained marker was highlighted in red but the same thresholds were used for all images for each marker. The percentages of the red areas were calculated with the “Measure” command as the level of each marker. The script in the Supplemental Material was used for automated analysis of all of the immunohistochemistry staining images.
Immunofluorescence Staining and Confocal Microscopy
Kidney cryosections were fixed with 3.7% paraformalin for 15 minutes at room temperature. HKC-8 cells cultured on coverslips were fixed with cold methanol/acetone (1:1) for 10 minutes at −20°C. After blocking with 10% donkey serum for 1 hour, the slides were immunostained with primary antibodies against CD3 (sc-20047) and CD45 (sc-1178) (Santa Cruz Biotechnology, Santa Cruz, CA). These slides were then stained with Cy2- or Cy3-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) and were mounted with Vectashield antifade mounting media using DAPI (4’, 6-diamidino-2-phenylindole, HCl) to visualize the nuclei (Vector Laboratories, Burlingame, CA). The stained slides were viewed under a Leica TCS-SL confocal microscope equipped with a digital camera (Buffalo Grove, IL).
X-Gal Staining
For detecting functional β-galactosidase activity, OCT-embedded kidneys from the Gli1-LacZ knock-in reporter mice were cryosectioned into 7-μm sections and fixed with paraformaldehyde solution and then stained with standard X-Gal for 2 days at 37°C. To quantify LacZ cell number, 100× images were taken of the entire kidney from at least three different mice; the numbers of positive cells were then counted in each image using a manual cell counter from ImageJ (http://rsbweb.nih.gov/ij). The X-Gal staining protocol was generously provided by Dr. Ben Humphreys at the University of Washington in St. Louis, and reported previously.27
Transfection and Luciferase Assay
The effect of Wnt/β-catenin on HGF gene transcription was assessed by cotransfecting with pGL3-HGF promoter reporter plasmid (HGF promoter region, −1037 to +56, kindly provided by Dr. Reza Zarnegar at the University of Pittsburgh) or pGL3-Basic vector,60 and N-terminally truncated, stabilized β-catenin expression vector (pDel-β-cat), by using Lipofectamine 2000 reagent in cultured NRK-49F fibroblasts. An internal control plasmid (0.1 µg) Renilla reniformis luciferase driven under TK promoter (pRL-TK; Promega, Madison, WI) was also cotransfected for normalizing the transfection efficiency. Luciferase assay was performed using a dual luciferase assay system kit according to the manufacturer’s protocols (Promega). Relative luciferase activity (arbitrary units) was reported as fold induction over the controls after normalizing for transfection efficiency.
Statistical Analyses
All data were expressed as mean±SEM. Statistical analyses of the data were performed using SigmaStat software (Jandel Scientific Software, San Rafael, CA). Comparison between groups was made using one-way ANOVA, followed by the Student–Newman–Keuls test. P<0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to the Center for Biologic Imaging at the University of Pittsburgh for the use of their core facilities. We thank Dr. Reza Zarnegar for providing hepatocyte growth factor promoter reporter plasmid and C-33A cell line.
This work was supported by the National Institutes of Health grants DK064005 and DK106049, the National Science Foundation of China grant 81521003, and Guangdong Science Foundation Innovative Group Grant 2014A030312014. H.F. was supported by the National Science Foundation of China grant 31371394. J.X. was supported by the National Science Foundation grant DMS-1462049 and National Institutes of Health grant UL1TR001857.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017080903/-/DCSupplemental.
References
- 1.Vanmassenhove J, Kielstein J, Jörres A, Biesen WV: Management of patients at risk of acute kidney injury. Lancet 389: 2139–2151, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Odutayo A, Wong CX, Farkouh M, Altman DG, Hopewell S, Emdin CA, et al.: AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol 28: 377–387, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu X, Nie S, Liu Z, Chen C, Xu G, Zha Y, et al.: Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol 10: 1510–1518, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Humphreys BD, Bonventre JV: Pathophysiology of acute kidney injury to chronic kidney disease: Maladaptive repair. Contrib Nephrol 174: 149–155, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Sharfuddin AA, Molitoris BA: Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Yang X, Chen C, Teng S, Fu X, Zha Y, Liu H, et al.: Urinary matrix metalloproteinase-7 predicts severe AKI and poor outcomes after cardiac surgery. J Am Soc Nephrol 28: 3373–3382, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinsey GR: Macrophage dynamics in AKI to CKD progression. J Am Soc Nephrol 25: 209–211, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhaun N, Webb DJ: The road from AKI to CKD: The role of endothelin. Kidney Int 84: 637–638, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Ramesh G, Zhang B, Uematsu S, Akira S, Reeves WB: Endotoxin and cisplatin synergistically induce renal dysfunction and cytokine production in mice. Am J Physiol Renal Physiol 293: F325–F332, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB: TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol 19: 923–932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaissling B, Le Hir M: The renal cortical interstitium: Morphological and functional aspects. Histochem Cell Biol 130: 247–262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato Y, Yanagita M: Resident fibroblasts in the kidney: A major driver of fibrosis and inflammation. Inflamm Regen 37: 17, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y: Hepatocyte growth factor and the kidney. Curr Opin Nephrol Hypertens 11: 23–30, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Paliege A, Rosenberger C, Bondke A, Sciesielski L, Shina A, Heyman SN, et al.: Hypoxia-inducible factor-2alpha-expressing interstitial fibroblasts are the only renal cells that express erythropoietin under hypoxia-inducible factor stabilization. Kidney Int 77: 312–318, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Liu Y: Hepatocyte growth factor in kidney fibrosis: Therapeutic potential and mechanisms of action. Am J Physiol Renal Physiol 287: F7–F16, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Nusse R, Clevers H: Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169: 985–999, 2017 [DOI] [PubMed] [Google Scholar]
- 18.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y: Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou D, Tan RJ, Fu H, Liu Y: Wnt/β-catenin signaling in kidney injury and repair: A double-edged sword. Lab Invest 96: 156–167, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD: Wnt4/β-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol 24: 1399–1412, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angers S, Moon RT: Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10: 468–477, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, et al.: Sustained sctivation of Wnt/β-catenin signaling drives AKI to CKD progression. J Am Soc Nephrol 27: 1727–1740, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou D, Li Y, Lin L, Zhou L, Igarashi P, Liu Y: Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury in mice. Kidney Int 82: 537–547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, et al.: Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 107: 4194–4199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He W, Tan R, Dai C, Li Y, Wang D, Hao S, et al.: Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/beta-catenin signaling. J Biol Chem 285: 24665–24675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell A: Apoptosis: Bax to Bak. Nat Rev Mol Cell Biol 2: 6, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Fabian SL, Penchev RR, St-Jacques B, Rao AN, Sipilä P, West KA, et al.: Hedgehog-Gli pathway activation during kidney fibrosis. Am J Pathol 180: 1441–1453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding H, Zhou D, Hao S, Zhou L, He W, Nie J, et al.: Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol 23: 801–813, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou D, Li Y, Zhou L, Tan RJ, Xiao L, Liang M, et al.: Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J Am Soc Nephrol 25: 2187–2200, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou D, Fu H, Zhang L, Zhang K, Min Y, Xiao L, et al.: Tubule-derived wnts are required for fibroblast activation and kidney fibrosis. J Am Soc Nephrol 28: 2322–2336, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramann R, Wongboonsin J, Chang-Panesso M, Machado FG, Humphreys BD: Gli1+ pericyte loss induces capillary rarefaction and proximal tubular injury. J Am Soc Nephrol 28: 776–784, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rauhauser AA, Ren C, Lu D, Li B, Zhu J, McEnery K, et al.: Hedgehog signaling indirectly affects tubular cell survival after obstructive kidney injury. Am J Physiol Renal Physiol 309: F770–F778, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, et al.: Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16: 51–66, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu MC, Shi M, Zhang J, Addo T, Cho HJ, Barker SL, et al.: Renal production, uptake, and handling of circulating αKlotho. J Am Soc Nephrol 27: 79–90, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hüttemann M, Pecina P, Rainbolt M, Sanderson TH, Kagan VE, Samavati L, et al.: The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion 11: 369–381, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahapatra G, Varughese A, Ji Q, Lee I, Liu J, Vaishnav A, et al.: Phosphorylation of cytochrome c threonine 28 regulates electron transport chain activity in kidney: Implications for AMP Kinase. J Biol Chem 292: 64–79, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lockshin RA, Zakeri Z: Programmed cell death and apoptosis: Origins of the theory. Nat Rev Mol Cell Biol 2: 545–550, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Simonyan L, Renault TT, Novais MJ, Sousa MJ, Côrte-Real M, Camougrand N, et al.: Regulation of Bax/mitochondria interaction by AKT. FEBS Lett 590: 13–21, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Bonventre JV: Pathophysiology of AKI: Injury and normal and abnormal repair. Contrib Nephrol 165: 9–17, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Paul SM, Palladino MJ, Beitel GJ: A pump-independent function of the Na,K-ATPase is required for epithelial junction function and tracheal tube-size control. Development 134: 147–155, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai C, Yang J, Liu Y: Single injection of naked plasmid encoding hepatocyte growth factor prevents cell death and ameliorates acute renal failure in mice. J Am Soc Nephrol 13: 411–422, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Chang-Panesso M, Humphreys BD: Cellular plasticity in kidney injury and repair. Nat Rev Nephrol 13: 39–46, 2017 [DOI] [PubMed] [Google Scholar]
- 43.Zhou L, Liu Y: Wnt/β-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat Rev Nephrol 11: 535–545, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan RJ, Zhou D, Zhou L, Liu Y: Wnt/β-catenin signaling and kidney fibrosis. Kidney Int Suppl (2011) 4: 84–90, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawakami T, Ren S, Duffield JS: Wnt signalling in kidney diseases: Dual roles in renal injury and repair. J Pathol 229: 221–231, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Basile DP, Anderson MD, Sutton TA: Pathophysiology of acute kidney injury. Compr Physiol 2: 1303–1353, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, et al.: Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J Am Soc Nephrol 26: 107–120, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y: Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol 20: 1997–2008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michalopoulos GK, Zarnegav R: Hepatocyte growth factor. Hepatology 15: 149–155, 1992 [DOI] [PubMed] [Google Scholar]
- 50.Giannopoulou M, Dai C, Tan X, Wen X, Michalopoulos GK, Liu Y: Hepatocyte growth factor exerts its anti-inflammatory action by disrupting nuclear factor-kappaB signaling. Am J Pathol 173: 30–41, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gong R, Rifai A, Ge Y, Chen S, Dworkin LD: Hepatocyte growth factor suppresses proinflammatory NFkappaB activation through GSK3beta inactivation in renal tubular epithelial cells. J Biol Chem 283: 7401–7410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maarouf OH, Aravamudhan A, Rangarajan D, Kusaba T, Zhang V, Welborn J, et al.: Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J Am Soc Nephrol 27: 781–790, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sáenz-Morales D, Conde E, Escribese MM, García-Martos M, Alegre L, Blanco-Sánchez I, et al.: ERK1/2 mediates cytoskeleton and focal adhesion impairment in proximal epithelial cells after renal ischemia. Cell Physiol Biochem 23: 285–294, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Alderliesten M, de Graauw M, Oldenampsen J, Qin Y, Pont C, van Buren L, et al.: Extracellular signal-regulated kinase activation during renal ischemia/reperfusion mediates focal adhesion dissolution and renal injury. Am J Pathol 171: 452–462, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y: Hepatocyte growth factor promotes renal epithelial cell survival by dual mechanisms. Am J Physiol 277: F624–F633, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Zhou D, Tan RJ, Lin L, Zhou L, Liu Y: Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int 84: 509–520, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu H, Tian Y, Zhou L, Zhou D, Tan RJ, Stolz DB, et al.: Tenascin-C is a major component of the fibrogenic niche in kidney fibrosis. J Am Soc Nephrol 28: 785–801, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y: Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol 24: 771–785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al.: Fiji: An open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seneviratne D, Ma J, Tan X, Kwon YK, Muhammad E, Melhem M, et al. : Genomic instability causes HGF gene activation in colon cancer cells, promoting their resistance to necroptosis. Gastroenterology 148: 181–191.e117, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.