Abstract
Renal ammonia metabolism is the primary mechanism through which the kidneys maintain acid-base homeostasis, but the molecular mechanisms regulating renal ammonia generation are unclear. In these studies, we evaluated the role of the proximal tubule basolateral plasma membrane electrogenic sodium bicarbonate cotransporter 1 variant A (NBCe1-A) in this process. Deletion of the NBCe1-A gene caused severe spontaneous metabolic acidosis in mice. Despite this metabolic acidosis, which normally causes a dramatic increase in ammonia excretion, absolute urinary ammonia concentration was unaltered. Additionally, NBCe1-A deletion almost completely blocked the ability to increase ammonia excretion after exogenous acid loading. Under basal conditions and during acid loading, urine pH was more acidic in mice with NBCe1-A deletion than in wild-type controls, indicating that the abnormal ammonia excretion was not caused by a primary failure of urine acidification. Instead, NBCe1-A deletion altered the expression levels of multiple enzymes involved in proximal tubule ammonia generation, including phosphate-dependent glutaminase, phosphoenolpyruvate carboxykinase, and glutamine synthetase, under basal conditions and after exogenous acid loading. Deletion of NBCe1-A did not impair expression of key proteins involved in collecting duct ammonia secretion. These studies demonstrate that the integral membrane protein NBCe1-A has a critical role in basal and acidosis-stimulated ammonia metabolism through the regulation of proximal tubule ammonia-metabolizing enzymes.
Keywords: acidosis, chronic metabolic acidosis, renal tubular acidosis, proximal tubule
Maintenance of acid-base homeostasis is necessary for almost all components of normal health. Chronic metabolic acidosis directly affects almost every organ system, leading to osteopenia and osteoporosis, decreased insulin release and sensitivity, impaired sensitivity to thyroid hormone activity, decreased cardiovascular responsiveness to catecholamines and other vasopressor hormones, increased risk of cardiac arrhythmias, skeletal muscle atrophy and weakness, renal interstitial fibrosis, and increased progression of CKD to ESRD.1–8 Perhaps as a result of these effects, abnormal acid-base homeostasis, whether metabolic acidosis or metabolic alkalosis, is predictive of increased mortality.6,9,10
The kidneys control the metabolic component of acid-base homeostasis through the process of net acid excretion.11–14 Under basal conditions, the major mechanism of net acid excretion involves urinary ammonia excretion,15 and in response to exogenous acid-loading, almost the entire change in net acid excretion occurs as a result of increased ammonia excretion.11,12,15 However, the molecular mechanisms through which the kidneys recognize changes in acid-base status and alter ammonia metabolism are not well understood.
In our study, we examined the role of the proximal tubule basolateral, electrogenic sodium-coupled bicarbonate cotransporter (NBCe1-A), in the regulation of ammonia metabolism under basal conditions and in response to exogenous acid-loading. This protein is the product of the SLC4A4 gene, which encodes NBCe1. There are three major (A, B, and C) and two other (D and E) variants of the SLC4A4 gene. The A variant is expressed in the renal proximal tubule basolateral plasma membrane.16,17 The B variant is expressed primarily in pancreas, and the C variant is expressed primarily in the CNS, but both are also expressed in a number of additional extrarenal sites.18,19 Two minor variants, D and E, have been identified.20 They appear to be expressed at lower levels in most tissues, at least at the gene transcript level, and their physiologic role remains unknown.20
SLC4A4 gene products may have a critical role in renal ammonia metabolism. Deletion of this gene causes severe metabolic acidosis.21,22 Normally, metabolic acidosis increases urinary ammonia excretion and stimulates renal ammonia metabolism. However, deletion of the SLC4A4 gene, despite the accompanying metabolic acidosis, decreases ammonia excretion and causes abnormal expression of multiple enzymes involved in proximal tubule ammonia metabolism.21 In addition to causing severe metabolic acidosis and abnormal ammonia metabolism, SLC4A4 gene deletion also causes 100% mortality before 5 weeks of age.21–23 Consequently, the effect of SLC4A4 gene deletion cannot be studied in adult mice, thereby precluding evaluation of its role in the fully developed kidney as well as its role in response to exogenous acid-base disturbances.
Thus, the purpose of our study was to determine the role of the A variant of NBCe1, NBCe1-A, in renal ammonia metabolism. We chose the A variant because this is the variant shown to be expressed at high levels in the renal proximal tubule.16,24–28 We used mice with specific deletion of the A and D variants of NBCe1 generated using Transcription activator-like effector nuclease (TALEN)-based gene-editing techniques. Because the mouse kidney does not express mRNA for the NBCe1-D variant (L. Fang, I.D. Weiner, unpublished observations), we term these mice NBCe1-A knockout (KO) mice in this report. These mice, despite having early onset severe metabolic acidosis similar to that observed in global NBCe1 deletion mice, do not have early mortality. We also determined the effect of NBCe1-A deletion on basal acid-base homeostasis. Next, we provided an exogenous acid load and determined whether NBCe1-A deletion altered the renal response. Because there was almost complete inhibition of changes in ammonia excretion, we determined the effect of NBCe1-A deletion on the response of renal ammoniagenic enzymes and other key transporters involved in ammonia metabolism.
Results
Verification of NBCe1-A Deletion Mice
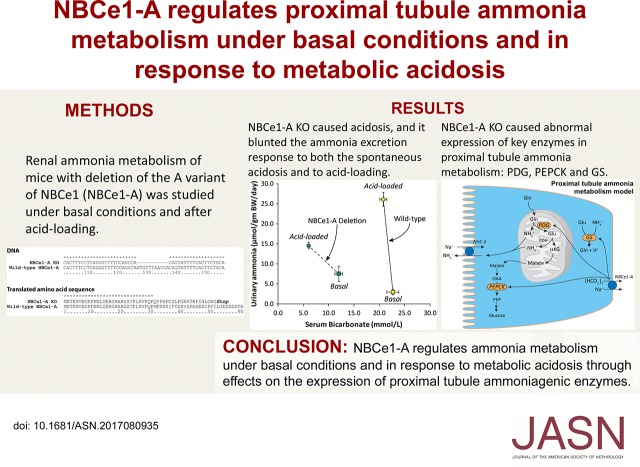
We used mice with specific deletion of the A variant of NBCe1 that were generated using TALEN-based gene-editing techniques. We confirmed NBCe1-A deletion by sequencing the NBCe1 locus at the reported mutation site, which confirmed deletion of nucleotides 128–138 of the coding sequence (Figure 1). Because this is an 11 bp deletion, it causes a frameshift mutation, which in addition to changing downstream amino acid residues, results in a stop codon after residue 52. Thus, this mutation precludes expression of functional NBCe1-A protein. Also, because the NBCe1-A variant has a different 5′ coding sequence in this region than the NBCe1-B and NBCe1-C variants, this genetic change does not alter the coding sequence of the NBCe1-B, NBCe1-C, or NBCe1-E variants of the NBCe1 gene.
Figure 1.
Deletion of 11 bp in the NBCe1-A specific 5` coding sequence causes a downstream frameshift mutation that results in an early stop codon. Top panel shows results of DNA sequencing of NBCe1-A KO mice and WT mice and bottom panel shows the translated amino acid sequence. NBCe1-A KO mice have an 11 bp deletion beginning at nucleotide 128 in the coding sequence for the NBCe1-A gene. This results in a three amino acid deletion in the translated protein followed by a frameshift mutation that results in an early stop codon at amino acid 53. Sequence alignment performed using ClustlX, v 2.1. * indicates an identical nucleotide/amino acid. “Stop” indicates termination of translation because of a stop codon in the mRNA sequence.
We further confirmed NBCe1-A deletion using immunohistochemistry. In wild-type (WT) mice, immunohistochemistry showed basolateral NBCe1-A immunolabel limited to the proximal tubule. Immunolabel intensity was strongest in the proximal convoluted tubule (PCT) in the cortical labyrinth, modest in the cortical proximal straight tubule (PST) in the medullary ray, and not detectable in PST segments in the outer stripe of the outer medulla (OSOM) (Figure 2). No NBCe1-A immunolabel was present in KO mouse kidney. These findings both identify the axial expression of NBCe1-A in the WT mouse kidney for the first time and they validate the generation of mice with NBCe1-A deletion. We also confirm the specificity of the NBCe1-A antibody used in these studies.
Figure 2.
NBCe1-A immunohistochemistry demonstrates normal NBCe1-A expression in WT mouse kidney and the lack of expression in the NBCe1-A KO mouse kidney. Top panels show low-power micrographs of NBCe1-A immunolabel in the WT mouse kidney (left) and NBCe1-A KO kidney (right). Strong NBCe1-A immunoreactivity is present in proximal tubules throughout the cortex in the WT, whereas no detectable immunolabel is present in the KO kidney. Bottom row shows higher-power micrographs of immunolabel in the WT kidney, correlating to the respective rectangles in the upper left panel. In the cortical labyrinth (A), strong basolateral immunolabel limited to the PCT is evident. In the medullary ray in the cortex (B), NBCe1-A immunolabel is limited to the PST, and immunolabel is less intense than in the PCT in the cortical labyrinth. (C) The transition from the corticomedullary junction (top of the panel) to the outer stripe of the outer medulla (OSOM, bottom of the panel). There is a progressive decrease in NBCe1-A immunolabel near the corticomedullary junction such that there is no detectable immunolabel in the PST, or other cells, in the OSOM.
Basal Acid-Base Status in Adult Mice
Because NBCe1-A deletion did not cause early mortality, we were able to determine NBCe1-A’s role in acid-base homeostasis in adult mice. Table 1 shows these results. NBCe1-A deletion caused severe metabolic acidosis. In part, the spontaneous metabolic acidosis may be related to the necessity of NBCe1-A for proximal tubule-mediated, filtered bicarbonate reabsorption. Neither serum Na+ nor K+ concentration was altered significantly by NBCe1-A deletion. Estimates of GFR, BUN, and urea clearance also did not differ significantly. There was no detectable urinary glucose excretion in either WT or NBCe1-A KO mice (n=3 in each genotype). Finally, NBCe1-A deletion mice were smaller in body size than their WT littermates. All analyses included both male and female mice; inclusion of sex in statistical analysis did not change any of these conclusions, either in these analyses or in any of the subsequent electrolyte analyses. Accordingly, data from male and female mice was pooled for data reporting.
Table 1.
Basal physiologic parameters in adult NBCe1-A KO mice
| Parameter | WT | KO | P Value |
|---|---|---|---|
| Weight | 22.5±0.6 (31) | 17.3±0.7 (25) | <0.001 |
| Daily food intake, g | 10.0±0.2 (31) | 9.6±0.3 (25) | NS |
| Urine pH | 6.09±0.06 (30) | 5.15±0.09 (25) | <0.001 |
| Urine ammonia, µmol/d | 85.0±10.7 (29) | 120±18.2 (25) | NS |
| Urine ammonia, µmol/g body wt per day | 3.85±0.50 (29) | 6.61±0.76 (25) | <0.05 |
| Urine titratable acid, µmol/d | 82±13 (8) | 194±32 (8) | <0.01 |
| Urine net acid excretion, µmol/d | 149±25 (8) | 362±75 (8) | <0.05 |
| Serum Na+, mmol/L | 151±1 (8) | 149±1 (8) | NS |
| Serum K+, mmol/L | 3.78±0.08 (8) | 4.03±0.44 (8) | NS |
| Serum HCO3−, mmol/L | 22.7±1.2 (8) | 12.0±0.8 (8) | <0.001 |
| BUN, mg/dl | 27±2 (7) | 27±3 (8) | NS |
| Urea clearance, µl/min | 203±33 (7) | 134±15 (8) | NS |
Values are mean±SEM. Numbers in parentheses are numbers of animals in each group.
Ammonia Excretion under Basal Conditions
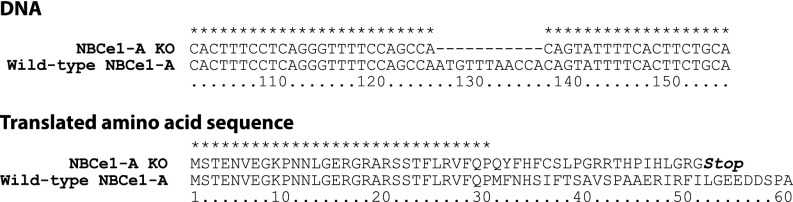
NBCe1-A deletion, despite causing spontaneous and severe metabolic acidosis, did not alter absolute urinary ammonia excretion significantly from that observed in WT littermates (Figure 3). Although the NBCe1-A KO mice were smaller, which resulted in urine ammonia excretion adjusted for body weight to be greater in the KO mice (WT, 3.85±0.50 µmol/g body wt per day; KO, 6.61±0.76 µmol/g body wt per day; P<0.05, n=29 and 25, respectively), absolute food intake, which is the primary determinant of endogenous acid production, was similar. The ability to acidify urine is critical for renal ammonia excretion. Urine pH was significantly lower, i.e., more acidic, in mice with NBCe1-A deletion (Figure 3).
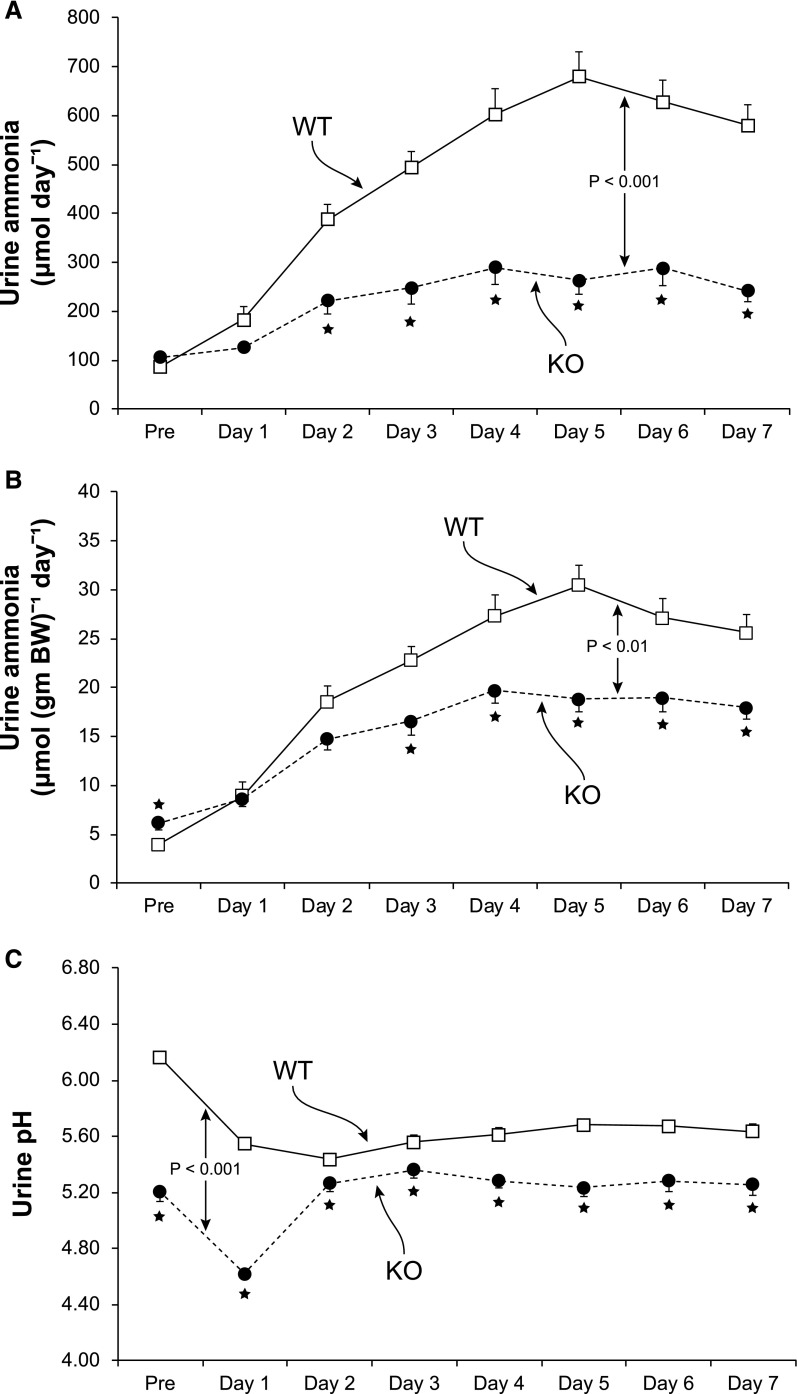
Figure 3.
NBCe1-A KO, which causes spontaneous metabolic acidosis, prevents changes in urinary ammonia excretion despite excretion of urine that is more acidic than in WT mice. (A) Urinary ammonia excretion under basal conditions. There is no significant difference between WT and NBCe1-A KO mice (P=NS; n=29 and 25, respectively). (B) Basal urine pH in NBCe1-A KO mice is significantly more acidic than in WT mice (P<0.001; n=29 and 25, respectively).
Another component of the renal maintenance of acid-base homeostasis involves titratable acid excretion. Under baseline conditions, mice with NBCe1-A deletion excreted more titratable acid than WT mice (KO, 194±32 µmol/d; WT, 82±13 µmol/d; P<0.05, n=8 in each group).
Effect of Metabolic Acidosis on NBCe1-A Expression
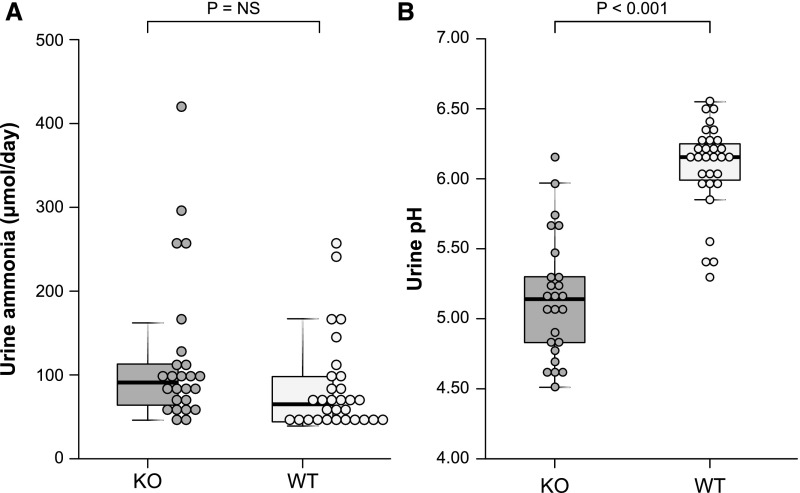
Previous studies have reported that metabolic acidosis increases proximal tubule basolateral Na+-coupled bicarbonate transport, i.e., NBCe1 activity,29 but not NBCe1 protein expression.30,31 Because these studies that showed no change in NBCe1 protein abundance were performed in a different species (rat) than this study (mouse), and because they did not differentiate between NBCe1-A and other NBCe1 variants, we assessed the effect of metabolic acidosis on mouse NBCe1-A protein abundance. Immunoblot analysis showed that metabolic acidosis did not alter mouse NBCe1-A protein expression significantly (Figure 4). Similarly, immunohistochemistry showed no detectable change in the NBCe1-A immunolabel.
Figure 4.
Metabolic acidosis does not alter NBCe1-A protein expression in WT mice. The graph shows immunoblot analysis of renal cortical NBCe1-A protein abundance with quantification. Metabolic acidosis did not alter NBCe1-A protein abundance significantly. The right side of the figure shows NBCe1-A immunolabel in WT mice under basal conditions (A, C, E, and G) and after acid loading (B, D, F, and H). (A and B) Low-power micrographs of NBCe1-A immunolabel under basal conditions and after acid loading (A and B, respectively). Subsequent rows show high-power micrographs in the cortical labyrinth (C and D), cortical medullary ray (E and F), and the OSOM (G and H). Acid loading did not cause a detectable change in NBCe1-A immunolabel in any region. Results are representative of findings in four control and six acid-loaded mice.
Response to an Exogenous Acid Load
A major function of the kidneys is to recognize an exogenous acid load and to respond with increased ammonia excretion. Because NBCe1-A deletion caused an abnormal response to spontaneous metabolic acidosis, we postulated there would also be an abnormal response to an exogenous acid load. To test this, WT and KO mice were provided an exogenous acid load for 7 days. Plasma electrolytes were then obtained (Table 2). Plasma bicarbonate was substantially and significantly lower in KO mice than in WT mice. Moreover, acid loading induced a fall in plasma bicarbonate that was significantly greater in NBCe1-A KO mice (approximately 6 mmol/L) than in WT mice (approximately 2 mmol/L; P<0.03 by ANOVA). Plasma Na+ was unchanged. Plasma K+ was significantly lower in KO mice than in WT mice (P<0.05). Thus, NBCe1-A deletion both causes a spontaneous metabolic acidosis and increases the degree of worsening of metabolic acidosis in response to an exogenous acid load.
Table 2.
Physiologic parameters after acid loading
| Parameter | WT | NBCe1-A KO | P Value |
|---|---|---|---|
| Mean daily body wt, g/d | 21.6±0.5 (23) | 14.3±0.8 (17) | <0.001 |
| Mean daily urine volume, ml/d | 1.87±0.21 (23) | 2.41±0.27 (17) | NS |
| Serum Na+, mmol/L | 150±0 (23) | 149±1 (16) | NS |
| Serum K+, mmol/L | 3.89±0.07 (23) | 3.57±0.13 (16) | <0.05 |
| Serum HCO3−, mmol/L | 20.8±0.7 (23) | 6.1±0.2 (16) | <0.001 |
Numbers in parentheses reflect number of animals studied.
The hallmark of the renal response to an exogenous acid load is increased ammonia excretion. WT mice showed the expected increase in urinary ammonia excretion, with a submaximal increase on day 1, maximal excretion at approximately day 4, and constant and high rates of ammonia during the last 4 days of acid loading (Figure 5). In contrast, the response was significantly different in KO mice (P<0.001 by ANOVA). KO mice had only a minimal change in urinary ammonia excretion on the first day of acid loading, and the absolute increase was substantially less than in WT mice on every day of the acid-loading protocol. Ammonia excretion adjusted for body weight was significantly less in KO mice than in WT mice on days 3–7 of acid loading, and the increase over baseline was significantly less on days 2–7 of acid loading. Over the last 4 days of the acid-loading protocol, when urinary ammonia excretion was stable in both genotypes, the increase over basal ammonia excretion was blunted significantly, averaging only 31%±3% of the absolute increase and 54%±4% of the increase in urinary ammonia adjusted for body weight of that observed in WT mice (P<0.001; n=23 and 17, respectively, for both comparisons).
Figure 5.
NBCe1-A KO significantly impairs the ability to increase urinary ammonia excretion in response to acid-loading despite excretion of urine that is more acidic than in WT mice. (A) Urinary ammonia in response to dietary acid loading. Measurements from the first 2 days in metabolic cages are averaged and shown as “Pre” data. Urinary ammonia excretion in response to the acid loading differed significantly between WT and NBCe1-A KO mice. (B) Urine ammonia adjusted for body weight. On days 3–7 of acid loading, urine ammonia per gram of body weight was significantly less in NBCe1-A KO mice than in WT mice. (C) Urine pH measurements. Urine pH was significantly more acidic in KO mice on each day, both before acid loading and on each day of acid loading. n=23 for WT mice and 17 for KO mice. Overall differences evaluated using general linear modeling with repeated measures analysis. *P<0.05 at each day noted.
Urine pH During Acid Loading
The ability to acidify the urine is critical for ammonia excretion, and the urine pH response differed significantly in the two genotypes (P<0.001 by ANOVA). Urine pH was significantly more acidic in KO mice under baseline conditions and remained so throughout the entire 7 days of acid loading (Figure 5). Over the last 4 days of the acid-loading protocol, when urine pH was stable, urine pH averaged 5.63±0.03 in WT mice and 5.25±0.05 in KO mice (P<0.001; n=23 and 17, respectively). Thus, impaired ammonia excretion in the KO mice cannot be attributed to impaired urine acidification.
Correlation of Serum Bicarbonate and Urinary Ammonia
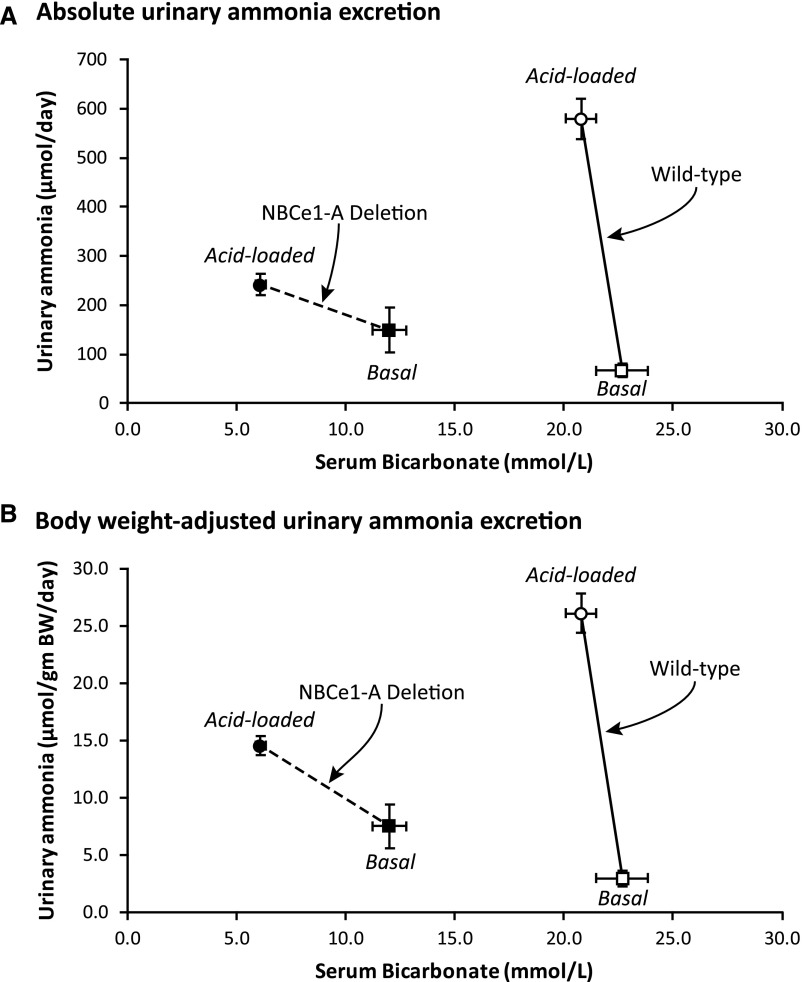
The effects of NBCe1-A deletion on serum bicarbonate and urinary ammonia excretion can be summarized by examining the correlation between the two. Figure 6 shows this correlation. In WT mice, acid loading dramatically increased ammonia excretion with only a minimal change in serum bicarbonate. NBCe1-A KO mice that did not receive an exogenous acid-load had a lower serum bicarbonate than observed in acid-loaded WT mice. Despite this substantial metabolic acidosis, absolute ammonia excretion was not altered significantly and body weight–adjusted ammonia excretion was modestly, but still significantly greater than in WT mice. Acid loading of KO mice caused a further decrease in serum bicarbonate that was of greater magnitude than occurred with acid loading of WT mice. Despite this, the ammonia excretion response was substantially less. Thus, NBCe1-A deletion alters the sensitivity of metabolic acidosis–associated changes in ammonia excretion.
Figure 6.
NBCe1-A deletion changes the correlation between serum bicarbonate and urinary ammonia excretion. This figure shows correlation between serum bicarbonate and urinary ammonia excretion under basal conditions (squares) and after acid loading (circles) in both WT (open symbols) and NBCe1-A KO (filled symbols) mice. (A) shows results using absolute ammonia excretion. NBCe1-A deletion results in a substantial decrease in basal serum bicarbonate without a change in basal ammonia excretion, it causes a greater decrease in plasma bicarbonate after acid loading, and it decreases the change in ammonia excretion in response to acid loading. (B) Results using body weight–adjusted ammonia excretion. Similar results are observed, with the exception that basal ammonia excretion in NBCe1-A KO mice was greater than in WT mice, as described in the text. n=8 for basal measurements in both WT and KO mice, 21 for acid-loaded WT mice and 16 for acid-loaded KO mice.
Ammoniagenic Enzyme Expression
One cause of impaired ammonia excretion despite an intact ability to acidify urine is an abnormality in endogenous ammonia generation. To address this possibility, we determined the effect of NBCe1-A deletion on key proteins involved in mitochondrial (phosphate-dependent glutaminase, PDG) and cytosolic (phosphoenolpyruvate carboxykinase, PEPCK) ammonia generation and cytosolic ammonia recycling (glutamine synthetase, GS).
Effect on PDG
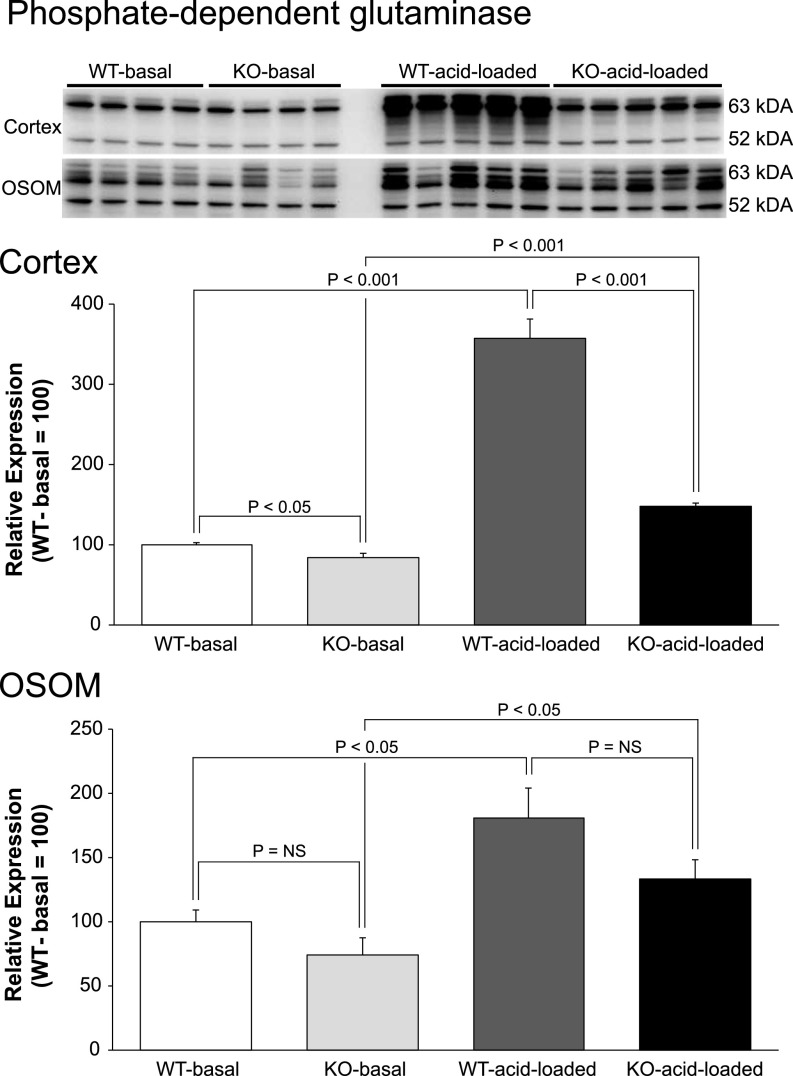
The initial enzyme in renal ammonia generation is the mitochondrial enzyme, PDG, and the normal effect of metabolic acidosis is to increase PDG expression. In contrast, NBCe1-A deletion, as shown in Figure 7, decreased cortical PDG expression significantly both under basal conditions and after exogenous acid-loading, and the magnitude of increase in response to acid loading was significantly blunted compared with the response of WT mice (P<0.001 for each comparison). In the OSOM, despite the spontaneous metabolic acidosis in NBCe1-A KO mice, PDG expression was unchanged compared with WT mice under basal conditions. Because the normal effect of metabolic acidosis is to increase PDG expression, the failure of this increase in NBCe1-A KO with spontaneous metabolic acidosis indicates abnormal expression. Acid loading increased PDG expression in both genotypes. Thus, NBCe1-A deletion caused abnormal PDG expression under basal conditions in both the cortex and OSOM and it blunted the response to exogenous metabolic acidosis in the cortex.
Figure 7.
NBCe1-A deletion alters PDG protein expression under basal conditions and in response to acid-loading. Top panel shows immunoblot analysis of PDG expression in cortex and OSOM of WT and KO mice on normal diet (basal) and after acid loading. Middle panel shows quantification in the cortex, and bottom panel shows quantification in the OSOM. In all panels, quantification is normalized such that mean in WT mice on normal diet is 100. n=4 on normal diet and 5 after acid loading in each group.
Effect on PEPCK
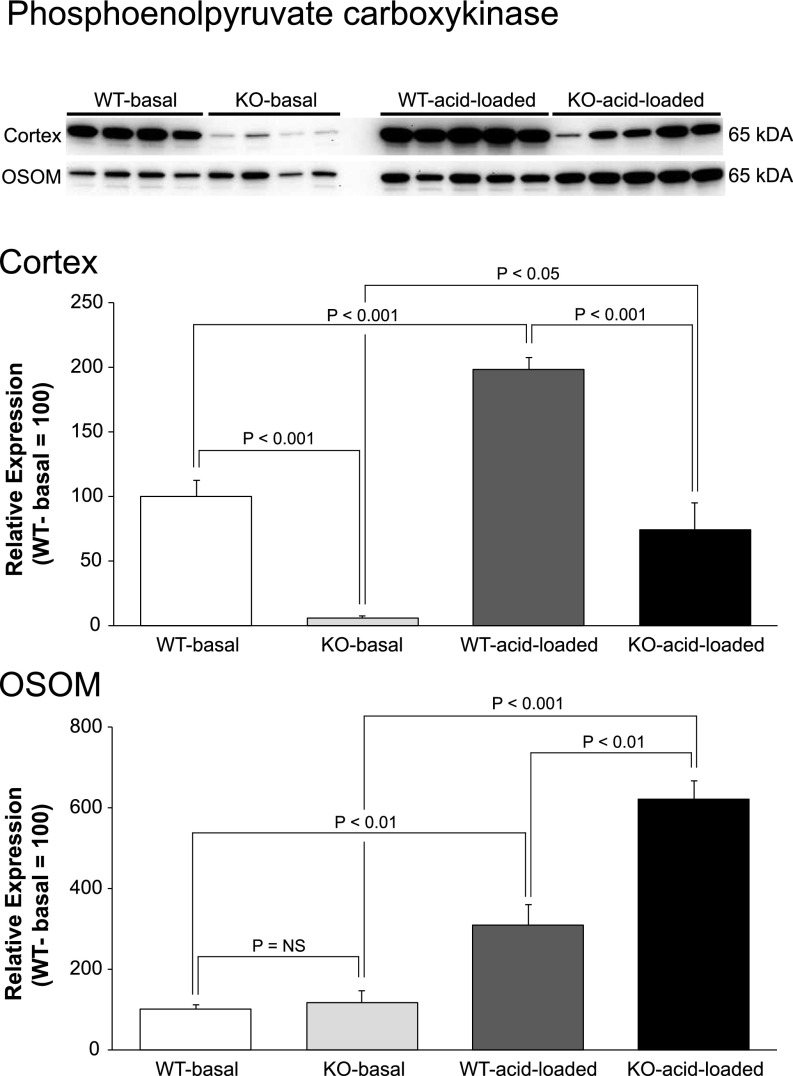
A second critical protein in ammonia metabolism is the cytosolic protein, PEPCK, and metabolic acidosis normally increases PEPCK expression. Figure 8 shows that PEPCK expression was abnormal in NBCe1-A KO mice under both basal and acid-loading conditions. Cortical expression was negligible under basal conditions and remained significantly less after acid loading than in WT mice. In the OSOM under basal conditions, PEPCK expression was not different from WT. Because NBCe1-A KO mice have a spontaneous metabolic acidosis, which normally increases PEPCK expression, the lack of increased PEPCK expression indicates abnormal regulation. Expression after an exogenous acid-load was significantly greater in NBCe1-A KO mice than in WT mice (P<0.001). Thus, NBCe1-A deletion caused abnormal PEPCK expression in both the cortex and OSOM under basal conditions and in the cortex after exogenous acid-loading.
Figure 8.
NBCe1-A deletion alters phoshophoenolpyruvate carboxykinase (PEPCK) protein expression under basal conditions and in response to acid-loading. Top panel shows immunoblot analysis of PEPCK expression in cortex and OSOM of WT and KO mice on normal diet and after acid loading. Middle panel shows quantification in the cortex, and bottom panel shows quantification in the OSOM. In all panels, quantification is normalized such that mean in WT mice on normal diet is 100. n=4 on normal diet and 5 after acid loading in each group.
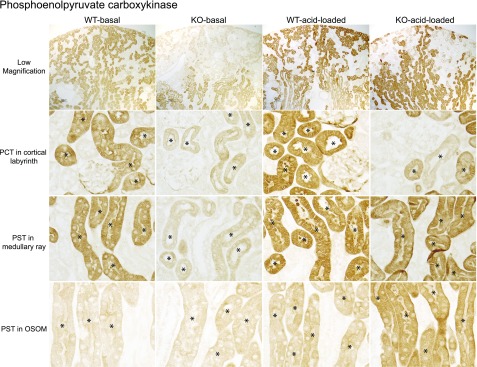
Immunohistochemistry showed that NBCe1-A deletion almost completely inhibited PEPCK expression in PCT and in PST in the medullary ray under basal conditions (Figure 9). In PST in the OSOM, PEPCK expression was similar in WT and KO mice under normal conditions; again, this indicates a failure to respond to the spontaneous metabolic acidosis present in NBCe1-A KO mice. After acid loading, there was continued suppression of the PEPCK immunolabel in PCT. In PST in the medullary ray, expression in response to an exogenous acid load did not differ detectably between NBCe1-A KO and WT. In PST in the OSOM, PEPCK expression increased in both WT and KO mice and immunolabel intensity was greater in KO mice.
Figure 9.
NBCe1-A deletion alters PEPCK immunolabel expression in the proximal tubule under basal conditions and in response to acid-loading. Representative low and high magnification micrographs of PEPCK immunolabel in the kidneys of WT and KO mice on the normal diet (basal) and after acid loading. Top row shows low-power micrographs of kidneys in each of the four experimental group. Second row shows high-power micrographs from the cortical labyrinth showing PCT. Third row shows high-power micrographs from the medullary ray in the cortex, showing cortical PST. Bottom row shows the OSOM with outer medullary PST segments. Findings are representative of results in four mice fed normal diet and six mice after acid loading in each genotype. * indicates proximal tubule.
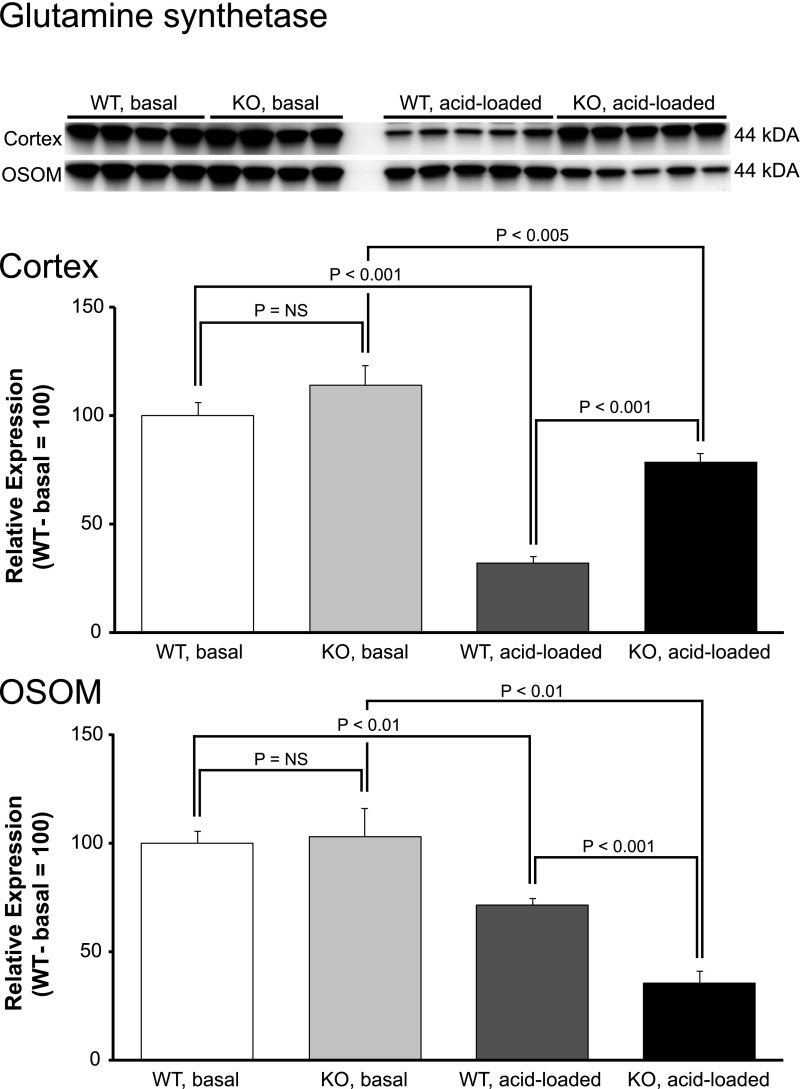
Effect on GS
The proximal tubule can recycle ammonia via the cytosolic enzyme, GS, which decreases net ammonia generation.32–35 Metabolic acidosis normally decreases GS expression.32,34 GS expression in NBCe1-A KO mice was not suppressed despite the basal metabolic acidosis (Figure 10). In the cortex, acid loading decreased GS expression in both WT and KO mice, but expression was significantly greater in KO mice despite more severe acidosis. Thus, cortical GS expression was abnormal both under basal conditions and after acid loading. In the OSOM, NBCe1-A deletion did not alter basal expression, and it accentuated the response to acid loading (P<0.002 by ANOVA). Because metabolic acidosis normally decreases GS expression in this region in WT mice,32,34 this lack of a decrease in the spontaneously acidotic NBCe1-A KO mice indicates abnormal expression under basal conditions.
Figure 10.
NBCe1-A deletion alters glutamine synthetase protein expression under basal conditions and in response to acid-loading. Top panel shows immunoblot analysis of GS expression in cortex and OSOM of WT and KO mice on normal diet (basal) and after acid loading. Middle panel shows quantification in the cortex, and bottom panel shows quantification in the OSOM. In all panels, quantification is normalized such that mean in WT mice on normal diet is 100. n=4 on normal diet and 5 after acid loading in each group.
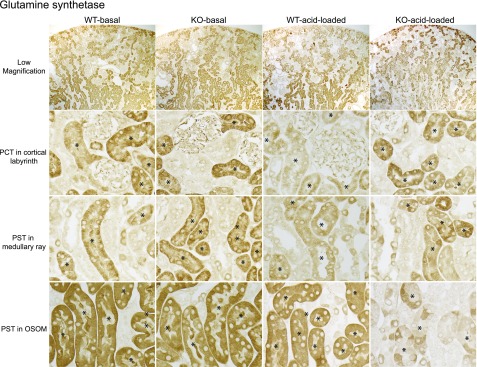
Immunohistochemistry showed findings consistent with the immunoblot analyses (Figure 11). In the PCT, NBCe1-A KO did not alter GS immunolabel detectably under basal conditions, and the decrease caused by acid loading was blunted compared with WT. In PST in the medullary ray basal GS intensity was not detectably altered by NBCe1-A deletion, and the decrease after acid loading was blunted. In PST in the OSOM, the GS immunolabel was not detectably different in KO than in WT mice. After acid loading, the decreased GS expression in response to acid loading was accentuated in NBCe1-A KO mice.
Figure 11.
NBCe1-A deletion alters proximal tubule GS immunolabel expression under basal conditions and in response to acid-loading. Representative low and high magnification micrographs of PEPCK immunolabel in the kidney of WT and KO mice on normal diet and after acid loading. Top row shows low-power micrographs of kidneys in each of the four experimental group. Second row shows high-power micrographs from the cortical labyrinth showing PCT. Third row shows high-power micrographs from the medullary ray in the cortex showing cortical PST. Bottom row shows the OSOM showing outer medullary PST segments. Findings are representative of results in four mice fed normal diet and six mice after acid loading in each genotype.
These findings indicate that NBCe1-A deletion results in dramatically abnormal expression of multiple key proteins involved in renal ammonia generation, during basal conditions, despite spontaneous metabolic acidosis, and after exogenous acid-loading. This effect is most pronounced in the PCTs, which is the site of the greatest expression of basolateral NBCe1-A protein in normal mice.
Rhesus Glycoprotein Expression
The majority of urinary ammonia is secreted by distal epithelial cells through parallel H+ and NH3 secretion.11,36,37 Ammonia secretion involves coordinated transport by the rhesus glycoproteins, Rhesus B Glycoprotein (Rhbg) and Rhesus C Glycoprotein (Rhcg), and genetic deletion of Rhbg and/or Rhcg impairs urinary ammonia excretion in response to an acid load.38–42 The fact that KO mice have a lower urine pH than WT mice, both under basal conditions and after acid loading, excludes a failure of H+ secretion as the primary cause of the decreased urinary ammonia. Furthermore, there was no loss of either Rhbg or Rhcg immunolabel in response to NBCe1-A deletion (Figure 12). Thus, impaired distal ammonia secretion does not appear to be the mechanism through which NBCe1-A deletion alters renal acid-base homeostasis.
Figure 12.
NBCe1-A deletion does not alter Rhbg or Rhcg immunolabel expression either under basal conditions or after acid-loading. Low-power micrographs show no loss of Rhbg or Rhcg immunolabel in NBCe1-A KO mice. Findings are representative of result in four mice fed normal diet and six mice after acid loading in each genotype.
Discussion
This study demonstrates that expression of NBCe1-A is critical for appropriate ammonia metabolism under basal conditions and in response to exogenous acid-loading. Deletion of NBCe1-A caused spontaneous metabolic acidosis. Despite this metabolic acidosis, the expected increase in ammonia excretion was not present. In response to exogenous acid-loading, NBCe1-A deletion almost completely blocked the ability to increase ammonia excretion. This impairment of normal ammonia excretion was associated with, and likely resulted from, abnormal expression of multiple key proteins representative of each of the major components of proximal tubule ammonia generation, mitochondrial ammonia metabolism (PDG), cytosolic enzymatic processes (PEPCK), and cytosolic ammonia recycling (GS). These findings identify NBCe1-A as a key protein necessary for normal proximal tubule ammonia metabolism, both under basal conditions and in response to exogenous acid loads.
The first major finding in this study is that NBCe1-A deletion caused abnormal renal ammonia excretion both under basal conditions and after an exogenous acid load. In the absence of an exogenous acid-load, NBCe1-A deletion caused spontaneous metabolic acidosis that was associated with no significant change in ammonia excretion, indicating a failure of the normal response to metabolic acidosis. Similar findings were observed in mice with deletion of all NBCe1 variants studied 8 days after birth.21 In this previous study, which by necessity examined incompletely developed kidneys, the possibility that NBCe1 deletion delayed renal maturation and that this accounted for the altered ammonia metabolism cannot be excluded. Our study excludes this possibility and, furthermore, extend these previous findings by showing that the A variant, NBCe1-A, is the critical variant involved. Finally, our study shows that a decrease in serum bicarbonate to approximately 12 mmol/L did not alter ammonia excretion in NBCe1-A KO mice, whereas a further decrease did. NBCe1-A deletion also decreased the magnitude of the ammonia response, i.e., the sensitivity, to worsening metabolic acidosis. On each day after initiating an exogenous acid load, the urinary ammonia response was significantly less, averaging only approximately 30% of that observed in WT mice despite the development of significantly worse metabolic acidosis. Thus, NBCe1-A deletion alters the sensitivity of ammonia metabolism to metabolic acidosis.
NBCe1-A deletion alters the ammonia response by impairing the normal regulation of enzymes involved in proximal tubule ammonia metabolism. The expected changes in PDG, PEPCK, and GS, reflecting each of the major enzymatic processes involved in ammonia generation, which should have occurred in response to the spontaneous metabolic acidosis that NBCe1-A deletion causes, were completely blocked throughout the entire proximal tubule. In response to exogenous acid-loading, which normally increases PDG and PEPCK and decreases GS expression, the response of each of these proteins in the PCT was impaired. The PCT is the major site of renal ammonia generation, and it is the major site of increased ammonia generation with an exogenous acid-load.43–45 Thus, NBCe1-A deletion causes abnormal basal and acidosis-stimulated regulation of multiple ammoniagenic enzymes that explains the change in renal ammonia metabolism. In addition, the abnormal regulation of ammonia metabolism likely accentuates the metabolic acidosis that is present. Indeed, the accentuated decrease in serum bicarbonate after acid loading is likely because of the decreased ammonia excretion by NBCe1-A KO mice.
NBCe1-A deletion substantially decreased the ammoniagenic responses to exogenous acid-loading in the PCT, but not in the PST. This axial variation in the role of NBCe1-A in the regulation of ammonia metabolism coincides with axial differences in NBCe1-A expression that occur in rat,16,25–27 rabbit,16,24 human,28 and mouse kidney (this study). Greater NBCe1-A expression in the early portions of the proximal tubule is also consistent with the greater responsiveness of the early proximal tubule ammonia generation and PDG activity to metabolic acidosis than in more distal portions of the proximal tubule.43,45–47 Finally, the absence of NBCe1-A outside of the proximal tubule is consistent with findings that ammonia generation by non-proximal tubule segments are either not regulated at all by metabolic acidosis or that the observed change is much less than occurs in early proximal tubule segments.43,45,46 Thus, the proximal tubule-specific localization and the axial variation in NBCe1-A expression in the proximal tubule correlates with tubule-specific and axial variations in acidosis-stimulated ammonia generation.
There are a number of potential mechanisms through which NBCe1-A might regulate proximal tubule ammonia metabolism. One possibility is through an alteration in intracellular pH, whereby decreased bicarbonate exit in response to NBCe1-A deletion causes intracellular alkalinization. If intracellular pH directly regulates ammoniagenic responses, this mechanism could explain the findings observed. Indeed, peritubular bicarbonate is a major determinant of intracellular pH,48,49 and basolateral sodium-coupled electrogenic bicarbonate transport, i.e., NBCe1-A activity, is the major transporter through which changes in extracellular bicarbonate alter proximal tubule intracellular pH.50,51 Against this possibility is that another key component of the proximal tubule contribution to acid-base homeostasis, filtered bicarbonate reabsorption, is not regulated by intracellular pH.49 These findings relative to bicarbonate reabsorption indicate that intracellular pH is not a “master regulator” of proximal tubule acid-base function. Another possibility is that because NBCe1-A is an electrogenic Na+ transporter, its deletion alters basolateral membrane voltage and/or cytosolic Na+ concentration, either of which could alter intracellular calcium regulation or other membrane voltage-dependent/regulated signaling pathways. Finally, it is possible that NBCe1-A, which has a number of protein–protein interactions with cytoskeletal proteins, directly transduces changes in extracellular bicarbonate, and regulates intracellular signaling pathways. Additional studies will be required to differentiate between these, and possibly other, mechanisms through which NBCe1-A regulates proximal tubule ammonia metabolism.
Three major components of renal acid-base homeostasis occur in the proximal tubule, filtered bicarbonate reabsorption, ammonia generation, and regulation of citrate excretion. Specific integral membrane proteins appear to regulate each of these components. The basolateral integral membrane protein, receptor protein tyrosine phosphatase γ, transduces signals from peritubular bicarbonate and CO2, and regulates proximal tubule bicarbonate reabsorption in response to changes in these acid-base solutes.52 This enables regulation of proximal tubule bicarbonate reabsorption through an intracellular pH-independent mechanism.49 Our study shows that NBCe1-A has a critical role in adult ammonia metabolism, and is consistent with the previous finding that deletion of all NBCe1 variants impairs basal ammonia excretion in 8-day-old pups.21 Regulating citrate excretion is a third critical proximal tubule function in acid-base homeostasis,53,54 and deletion of NBCe1 causes abnormal expression of the major proximal tubule protein regulating urinary citrate excretion, NaDC1.23 Thus, each of the major roles of the proximal tubule in acid-base homeostasis—filtered bicarbonate reabsorption, ammonia metabolism, and NaDC1-mediated citrate reabsorption—are linked to the expression of specific basolateral plasma membrane integral membrane proteins.
Although the collecting duct is the site in which 60%–80% of urinary ammonia is secreted, a primary failure in collecting duct ammonia secretion cannot explain the results observed. Collecting duct ammonia secretion involves parallel H+ and NH3 secretion. Because urine pH was actually more acidic in KO mice under all conditions tested, the inability to generate a luminal acidic pH necessary for ammonia secretion appears highly unlikely. A major component of collecting duct ammonia secretion involve transport by the rhesus glycoproteins, Rhbg and Rhcg, and deletion of either or both of these proteins impairs the ammonia excretion response to metabolic acidosis.38–42,55 However, effects of NBCe1-A deletion on Rhbg and Rhcg do not explain the observed impaired ammonia excretion. First, Rhbg and Rhcg expression was not decreased by NBCe1-A deletion. Second, deletion of Rhbg and Rhcg does not cause spontaneous metabolic acidosis nor does it impair metabolic acidosis-induced ammonia excretion as severely as observed in this study with NBCe1-A deletion, even with combined deletion of both Rhbg and Rhcg.38–42,55 Finally, the genetic deletion in this study involved a protein, NBCe1-A, expressed only in the proximal tubule and not in distal epithelial cells in which Rhbg and Rhcg are expressed; thus, a direct effect of NBCe1-A deletion on Rhbg and Rhcg function is unlikely. Thus, NBCe1-A deletion impairs ammonia excretion in acid-base homeostasis and does so without impairing mechanisms involved in distal ammonia secretion.
When using animal models to study human physiology, considering how accurately these models reflect human physiology is important. Genetic defects in NBCe1 cause the human condition of isolated proximal renal tubular acidosis (RTA); indeed, they are the only known genetic cause.56–61 These individuals develop spontaneous metabolic acidosis, but do not have increased urinary ammonia excretion,62,63 a finding identical to that observed in our study. After an exogenous acid load, their ability to increase urinary ammonia excretion is only approximately 40% of that observed in normal individuals.62 Again, this is almost identical to that observed in our study. In humans, NBCe1 genetic defects are associated with isolated proximal RTA, and not with Fanconi syndrome,59,64 and the mice with NBCe1-A deletion had evidence of isolated proximal RTA and not Fanconi syndrome. Another similarity is that both NBCe1-A KO mice and people with genetic forms of isolated proximal RTA have increased titratable acid excretion under basal conditions.62 However, this difference was modest in people, and net acid excretion, calculated as the sum of urine ammonia and titratable acid excretion, did not differ significantly in people with genetic forms of isolate proximal RTA,62 which differs from the findings in this study. These overall parallel findings regarding ammonia excretion in mice with NBCe1-A deletion and in patients with genetic forms of isolated proximal RTA suggest that our findings are directly relevant to human renal ammonia metabolism.
The spontaneous metabolic acidosis that occurs in response to NBCe1-A deletion likely reflects abnormalities in both proximal tubule filtered bicarbonate reabsorption and ammonia metabolism. NBCe1-A is the primary basolateral bicarbonate exit mechanism present in the PCT and through most of the PST. Its deletion likely impairs filtered bicarbonate reabsorption, leading to increased distal bicarbonate delivery that exceeds distal reabsorptive capacity, resulting in urinary bicarbonate loss. However, this mechanism’s contribution appears to be transient. If it were persistent, then ongoing urinary bicarbonate excretion would necessarily increase urine pH, yet this is not present in either mice with deletion of all NBCe1 variants studied 8 days after birth21 or in adult mice with NBCe1-A deletion (this study). Instead, the change in set-point and sensitivity of renal ammonia metabolism likely results in a change in “steady-state” serum bicarbonate, such that the degree of metabolic acidosis that develops results in sufficient stimulation of ammonia excretion to enable a balance between endogenous acid production and renal net acid excretion.
Additional mechanisms other than NBCe1-A appear to regulate proximal tubule ammonia metabolism. NBCe1-A expression is detectable only in the PCT and PST in the renal cortex in normal mice. Nonetheless, acid loading altered ammoniagenic enzyme expression throughout the entire proximal tubule of NBCe1-A KO. The response to acid loading was suppressed the most in the PCT, where NBCe1-A expression normally is the strongest, consistent with an important role for NBCe1-A in regulation of ammoniagenesis in this segment. However, in the PST in the OSOM, where NBCe1-A is not detectable even in normal mice, acid loading induced changes in ammoniagenic enzyme expression that were either similar to normal or even accentuated in NBCe1-A KO mice compared with WT mice. At present, we do not have sufficient data to identify the mechanisms of NBCe1-A–independent ammonia regulation.
In summary, our study provides substantial new information on, and advances our understanding of renal ammonia metabolism. NBCe1-A expression is necessary for normal ammonia metabolism through which regulation of multiple proximal tubule proteins involved in ammonia generation, including PDG, PEPCK, and GS. Thus, NBCe1-A, an integral membrane protein, is a critical acid-base sensor regulating renal proximal tubule ammonia metabolism under both basal conditions and in response to exogenous metabolic acidosis.
Concise Methods
Animals
We used mice with NBCe1-A–specific deletion generated previously using TALEN-mediated genome editing. We genotyped mice using DNA extracted from tail-clip samples. All breeding involved heterozygous dams with heterozygous sires, and age- and sex-matched WT and KO offspring were used in these studies. The Institutional Animal Care and Use Committees of the University of Florida and the North Florida/South Georgia Veterans Health System approved all animal experiments. All mice were on the C57Bl/6 background strain.
Antibodies
We previously described the PDG and PEPCK antibodies,41 the GS antibodies,35 and the Rhbg and Rhcg antibodies65 used in this study. Antibodies to NBCe1-A were described previously.66
Metabolic Cage Studies
Metabolic cage studies were performed as described previously.32,38–41,55,67,68
Acid Loading
Mice were acid-loaded by addition of 0.4 M HCl to powdered standard rodent chow in a ratio of 1 ml/g chow, as described previously.38–41 The control diet was identical, except we substituted deionized water for HCl.
Electrolyte Measurements
Urine electrolytes, ammonia, titratable acid excretion, and pH and serum Na+, K+, and bicarbonate were measured using standard techniques described previously.41 Urine net acid excretion was calculated as the sum of ammonia and titratable acid excretion. Urine glucose was assessed using Multistix 10 SG Reagent Strips (#2161; Siemens Healthcare GmbH).
Tissue Handling
Mice were anesthetized with inhalant isoflurane anesthesia, and tissues preserved by in vivo cardiac perfusion with PBS (pH 7.4) followed by periodate-lysine 2% paraformaldehyde fixation. Kidney tissue was processed in polyester wax and sectioned as described previously.32,33,38–41,55,67,68 Kidney proteins for immunoblot analysis were obtained using previously described techniques.32,33,38–41,55,67,68
Immunohistochemistry
Immunolocalization was accomplished using standard immunoperoxidase procedures described previously.32,33,38–41,55,67,68
Immunoblot Analysis
Immunoblot analysis was performed using standard techniques.32,33,38–41,55,67,68
Statistical Analyses
Results are presented as mean±SEM. Statistical analysis was performed using ANOVA to determine overall differences, with differences between specified subgroups determined using t test, as detailed previously.33 When repeated measurements over time were obtained, statistical significance for the primary independent variable was determined using general linear model with repeated measures analysis (IBM SPSS Statistics, version 24), followed by analysis at individual time points using t test.33 Sex was included as a dependent variable in all statistical analysis, and when this changed the statistical conclusion this is specifically indicated. P<0.05 was taken as statistically significant. N refers to the number of animals studied.
Disclosures
None.
Supplementary Material
Acknowledgments
These studies were supported by funds from the National Institutes of Health (grants R01-DK045788 and R01-DK107798), the Gatorade Trust Fund, and the Mayo Foundation.
The findings and conclusions in this manuscript do not reflect the official opinions of the Department of Veterans Affairs.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Kraut JA, Madias NE: Metabolic acidosis of CKD: An update. Am J Kidney Dis 67: 307–317, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Wang XH, Mitch WE: Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 10: 504–516, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovesdy CP: Metabolic acidosis and kidney disease: Does bicarbonate therapy slow the progression of CKD? Nephrol Dial Transplant 27: 3056–3062, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Wiederkehr MR, Kalogiros J, Krapf R: Correction of metabolic acidosis improves thyroid and growth hormone axes in haemodialysis patients. Nephrol Dial Transplant 19: 1190–1197, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Weiner ID, Mitch WE, Sands JM: Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin J Am Soc Nephrol 10: 1444–1458, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovesdy CP, Anderson JE, Kalantar-Zadeh K: Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant 24: 1232–1237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W, Abramowitz MK: Metabolic acidosis and the progression of chronic kidney disease. BMC Nephrol 15: 55, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raphael KL, Murphy RA, Shlipak MG, Satterfield S, Huston HK, Sebastian A, et al. ; Health ABC Study : Bicarbonate Concentration, Acid-Base Status, and Mortality in the Health, Aging, and Body Composition Study. Clin J Am Soc Nephrol 11: 308–316, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Wehbe E, Raina R, et al. : Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clin J Am Soc Nephrol 6: 2395–2402, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiner ID, Verlander JW: Ammonia transporters and their role in acid-base balance. Physiol Rev 97: 465–494, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner ID, Verlander JW: Recent advances in understanding renal ammonia metabolism and transport. Curr Opin Nephrol Hypertens 25: 436–443, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamm LL, Nakhoul N, Hering-Smith KS: Acid-base homeostasis. Clin J Am Soc Nephrol 10: 2232–2242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koeppen BM: The kidney and acid-base regulation. Adv Physiol Educ 33: 275–281, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Elkinton JR, Huth EJ, Webster GD Jr, McCANCE RA: The renal excretion of hydrogen ion in renal tubular acidosis. I. quantitative assessment of the response to ammonium chloride as an acid load. Am J Med 29: 554–575, 1960 [DOI] [PubMed] [Google Scholar]
- 16.Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF: Immunolocalization of the electrogenic Na+-HCO-3 cotransporter in mammalian and amphibian kidney. Am J Physiol 276: F27–F38, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Romero MF, Fong P, Berger UV, Hediger MA, Boron WF: Cloning and functional expression of rNBC, an electrogenic Na(+)-HCO3- cotransporter from rat kidney. Am J Physiol 274: F425–F432, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Kurtz I, Zhu Q: Structure, function, and regulation of the SLC4 NBCe1 transporter and its role in causing proximal renal tubular acidosis. Curr Opin Nephrol Hypertens 22: 572–583, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker MD, Boron WF: The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev 93: 803–959, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Xu JY, Wang DK, Wang L, Chen LM: Cloning and identification of two novel NBCe1 splice variants from mouse reproductive tract tissues: A comparative study of NCBT genes. Genomics 98: 112–119, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Handlogten ME, Osis G, Lee HW, Romero MF, Verlander JW, Weiner ID: NBCe1 expression is required for normal renal ammonia metabolism. Am J Physiol Renal Physiol 309: F658–F666, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, et al. : Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3- cotransporter. J Biol Chem 282: 9042–9052, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Osis G, Handlogten ME, Lee H-W, Hering-Smith KS, Huang W, Romero MF, et al. : Effect of NBCe1 deletion on renal citrate and 2-oxoglutarate handling. Physiol Rep 4: e12778, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abuladze N, Lee I, Newman D, Hwang J, Pushkin A, Kurtz I: Axial heterogeneity of sodium-bicarbonate cotransporter expression in the rabbit proximal tubule. Am J Physiol 274: F628–F633, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Endo Y, Yamazaki S, Moriyama N, Li Y, Ariizumi T, Kudo A, et al. : Localization of NBC1 variants in rat kidney. Nephron, Physiol 104: 87–94, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Maunsbach AB, Vorum H, Kwon TH, Nielsen S, Simonsen B, Choi I, et al. : Immunoelectron microscopic localization of the electrogenic Na/HCO(3) cotransporter in rat and ambystoma kidney. J Am Soc Nephrol 11: 2179–2189, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Li C, Kim SW, Ring T, Wen J, Djurhuus JC, et al. : Ureter obstruction alters expression of renal acid-base transport proteins in rat kidney. Am J Physiol Renal Physiol 295: F497–F506, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Yamada H, Yamazaki S, Moriyama N, Hara C, Horita S, Enomoto Y, et al. : Localization of NBC-1 variants in human kidney and renal cell carcinoma. Biochem Biophys Res Commun 310: 1213–1218, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Preisig PA, Alpern RJ: Chronic metabolic acidosis causes an adaptation in the apical membrane Na/H antiporter and basolateral membrane Na(HCO3)3 symporter in the rat proximal convoluted tubule. J Clin Invest 82: 1445–1453, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amlal H, Chen Q, Greeley T, Pavelic L, Soleimani M: Coordinated down-regulation of NBC-1 and NHE-3 in sodium and bicarbonate loading. Kidney Int 60: 1824–1836, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Kwon TH, Fulton C, Wang W, Kurtz I, Frøkiaer J, Aalkjaer C, et al. : Chronic metabolic acidosis upregulates rat kidney Na-HCO cotransporters NBCn1 and NBC3 but not NBC1. Am J Physiol Renal Physiol 282: F341–F351, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Lee HW, Osis G, Handlogten ME, Lamers WH, Chaudhry FA, Verlander JW, et al. : Proximal tubule-specific glutamine synthetase deletion alters basal and acidosis-stimulated ammonia metabolism. Am J Physiol Renal Physiol 310: F1229–F1242, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HW, Osis G, Handlogten ME, Verlander JW, Weiner ID: Proximal tubule glutamine synthetase expression is necessary for the normal response to dietary protein restriction. Am J Physiol Renal Physiol 313: F116–F125, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conjard A, Komaty O, Delage H, Boghossian M, Martin M, Ferrier B, et al. : Inhibition of glutamine synthetase in the mouse kidney: A novel mechanism of adaptation to metabolic acidosis. J Biol Chem 278: 38159–38166, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Verlander JW, Chu D, Lee HW, Handlogten ME, Weiner ID: Expression of glutamine synthetase in the mouse kidney: Localization in multiple epithelial cell types and differential regulation by hypokalemia. Am J Physiol Renal Physiol 305: F701–F713, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiner ID, Verlander JW: Ammonia transport in the kidney by Rhesus glycoproteins. Am J Physiol Renal Physiol 306: F1107–F1120, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiner ID, Hamm LL: Molecular mechanisms of renal ammonia transport. Annu Rev Physiol 69: 317–340, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee HW, Verlander JW, Handlogten ME, Han K-H, Weiner ID: Effect of collecting duct-specific deletion of both Rh B Glycoprotein (Rhbg) and Rh C Glycoprotein (Rhcg) on renal response to metabolic acidosis. Am J Physiol Renal Physiol 306: F389–F400, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bishop JM, Verlander JW, Lee HW, Nelson RD, Weiner AJ, Handlogten ME, et al. : Role of the Rhesus glycoprotein, Rh B glycoprotein, in renal ammonia excretion. Am J Physiol Renal Physiol 299: F1065–F1077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HW, Verlander JW, Bishop JM, Nelson RD, Handlogten ME, Weiner ID: Effect of intercalated cell-specific Rh C glycoprotein deletion on basal and metabolic acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 299: F369–F379, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee HW, Verlander JW, Bishop JM, Igarashi P, Handlogten ME, Weiner ID: Collecting duct-specific Rh C glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 296: F1364–F1375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, et al. : A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Good DW, Burg MB: Ammonia production by individual segments of the rat nephron. J Clin Invest 73: 602–610, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nonoguchi H, Uchida S, Shiigai T, Endou H: Effect of chronic metabolic acidosis on ammonia production from L-glutamine in microdissected rat nephron segments. Pflugers Arch 403: 229–235, 1985 [DOI] [PubMed] [Google Scholar]
- 45.Nonoguchi H, Takehara Y, Endou H: Intra- and inter-nephron heterogeneity of ammoniagenesis in rats: Effects of chronic metabolic acidosis and potassium depletion. Pflugers Arch 407: 245–251, 1986 [DOI] [PubMed] [Google Scholar]
- 46.Curthoys NP, Lowry OH: The distribution of glutaminase isoenzymes in the various structures of the nephron in normal, acidotic, and alkalotic rat kidney. J Biol Chem 248: 162–168, 1973 [PubMed] [Google Scholar]
- 47.Wright PA, Knepper MA: Phosphate-dependent glutaminase activity in rat renal cortical and medullary tubule segments. Am J Physiol 259: F961–F970, 1990 [DOI] [PubMed] [Google Scholar]
- 48.Nakhoul NL, Chen LK, Boron WF: Effect of basolateral CO2/HCO3- on intracellular pH regulation in the rabbit S3 proximal tubule. J Gen Physiol 102: 1171–1205, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Y, Zhao J, Bouyer P, Boron WF: Evidence from renal proximal tubules that HCO3- and solute reabsorption are acutely regulated not by pH but by basolateral HCO3- and CO2. Proc Natl Acad Sci U S A 102: 3875–3880, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alpern RJ: Mechanism of basolateral membrane H+/OH-/HCO-3 transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol 86: 613–636, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alpern RJ, Chambers M: Cell pH in the rat proximal convoluted tubule. Regulation by luminal and peritubular pH and sodium concentration. J Clin Invest 78: 502–510, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Y, Skelton LA, Xu L, Chandler MP, Berthiaume JM, Boron WF: Role of receptor protein tyrosine phosphatase γ in sensing extracellular CO2 and HCO3. J Am Soc Nephrol 27: 2616–2621, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Unwin RJ, Capasso G, Shirley DG: An overview of divalent cation and citrate handling by the kidney. Nephron, Physiol 98: 15–20, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Pajor AM: Citrate transport by the kidney and intestine. Semin Nephrol 19: 195–200, 1999 [PubMed] [Google Scholar]
- 55.Bishop JM, Lee HW, Handlogten ME, Han KH, Verlander JW, Weiner ID: Intercalated cell-specific Rh B glycoprotein deletion diminishes renal ammonia excretion response to hypokalemia. Am J Physiol Renal Physiol 304: F422–F431, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, et al. : The human NBCe1-A mutant R881C, associated with proximal renal tubular acidosis, retains function but is mistargeted in polarized renal epithelia. Am J Physiol Cell Physiol 291: C788–C801, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Dinour D, Chang MH, Satoh J, Smith BL, Angle N, Knecht A, et al. : A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem 279: 52238–52246, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Inatomi J, Horita S, Braverman N, Sekine T, Yamada H, Suzuki Y, et al. : Mutational and functional analysis of SLC4A4 in a patient with proximal renal tubular acidosis. Pflugers Arch 448: 438–444, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Igarashi T, Sekine T, Inatomi J, Seki G: Unraveling the molecular pathogenesis of isolated proximal renal tubular acidosis. J Am Soc Nephrol 13: 2171–2177, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Shiohara M, Igarashi T, Mori T, Komiyama A: Genetic and long-term data on a patient with permanent isolated proximal renal tubular acidosis. Eur J Pediatr 159: 892–894, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, et al. : Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet 23: 264–266, 1999 [DOI] [PubMed] [Google Scholar]
- 62.Brenes LG, Sanchez MI: Impaired urinary ammonium excretion in patients with isolated proximal renal tubular acidosis. J Am Soc Nephrol 4: 1073–1078, 1993 [DOI] [PubMed] [Google Scholar]
- 63.Lemann J Jr, Adams ND, Wilz DR, Brenes LG: Acid and mineral balances and bone in familial proximal renal tubular acidosis. Kidney Int 58: 1267–1277, 2000 [DOI] [PubMed] [Google Scholar]
- 64.Soleimani M, Rastegar A: Pathophysiology of renal tubular acidosis: Core curriculum 2016. Am J Kidney Dis 68: 488–498, 2016 [DOI] [PubMed] [Google Scholar]
- 65.Lee HW, Osis G, Handlogten ME, Guo H, Verlander JW, Weiner ID: Effect of dietary protein restriction on renal ammonia metabolism. Am J Physiol Renal Physiol 308: F1463–F1473, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roussa E, Nastainczyk W, and Thevenod F: Differential expression of electrogenic NBC1 (SLC4A4) variants in rat kidney and pancreas. Biochem Biophys Res Commun 314: 382–389, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Lee HW, Verlander JW, Bishop JM, Handlogten ME, Han KH, Weiner ID: Renal ammonia excretion in response to hypokalemia: Effect of collecting duct-specific Rh C glycoprotein deletion. Am J Physiol Renal Physiol 304: F410–F421, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Han KH, et al. : Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F397–F408, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.