Abstract
Nonobstructive hydronephrosis, defined as dilatation of the renal pelvis with or without dilatation of the ureter, is the most common antenatal abnormality detected by fetal ultrasound. Yet, the etiology of nonobstructive hydronephrosis is poorly defined. We previously demonstrated that defective development of urinary tract pacemaker cells (utPMCs) expressing hyperpolarization-activated cyclic nucleotide-gated channel 3 (HCN3) and the stem cell marker cKIT causes abnormal ureteric peristalsis and nonobstructive hydronephrosis. However, further investigation of utPMC development and function is limited by lack of knowledge regarding the embryonic derivation, development, and molecular apparatus of these cells. Here, we used lineage tracing in mice to identify cells that give rise to utPMCs. Neural crest cells (NCCs) indelibly labeled with tdTomato expressed HCN3 and cKIT. Furthermore, purified HCN3+ and cKIT+ utPMCs were enriched in Sox10 and Tfap-2α, markers of NCCs. Sequencing of purified RNA from HCN3+ cells revealed enrichment of a small subset of RNAs, including RNA encoding protein kinase 2β (PTK2β), a Ca2+-dependent tyrosine kinase that regulates ion channel activity in neurons. Immunofluorescence analysis in situ revealed PTK2β expression in NCCs as early as embryonic day 12.5 and in HCN3+ and cKIT+ utPMCs as early as embryonic day 15.5, with sustained expression in HCN3+ utPMCs until postnatal week 8. Pharmacologic inhibition of PTK2β in murine pyeloureteral tissue explants inhibited contraction frequency. Together, these results demonstrate that utPMCs are derived from NCCs, identify new markers of utPMCs, and demonstrate a functional contribution of PTK2β to utPMC function.
Keywords: cell biology and structure, genetics and development, hydronephrosis, pacemaker function

Coordinated pyeloureteric peristalsis is critical to the transport of urine from the kidney to the urinary bladder.1,2 Periodic coordinated sequential contractions of the pelvis and ureter are controlled by two distinct populations of urinary tract pacemaker cells (utPMCs).3–5 utPMCs at the pelvic-kidney junction (PKJ) are characterized by expression of hyperpolarization-activated cyclic nucleotide-gated channel 3 (HCN3) and low-voltage–gated T-type calcium channel TTC (also termed CaV 3.1).4,5 utPMCs located adjacent to the smooth muscle layer in the proximal ureter are termed interstitial cells of Cajal–like cells (ICC-LCs) and express cKIT.3,6 ICC-LCs are readily distinguished from cKIT+;CD45+ mast cells also present in the ureter by their spindle-shaped and long cytoplasmic processes as well as the absence of CD45 expression.3,6 The functional contribution of utPMCs to coordinated pyeloureteric contraction was demonstrated by studies in which chemical inhibition or antibody-mediated inhibition of cKIT, HCN3, or TTC in pyeloureteric explant cultures disrupted organized ureteric peristalsis.3–5 Although the physiologic mechanisms by which utPMCs function are poorly studied, Ca2+ signaling and an internal Ca2+ clock are thought to regulate their activity in a manner similar to that of heart and gut PMCs.7–17
Disruption of coordinated ureteric contraction causes nonobstructive hydronephrosis, defined as dilatation of the renal pelvis and ureter without physical obstruction to urinary flow. Previously, we demonstrated that decreased Hedgehog signaling during mouse kidney development results in nonobstructive hydronephrosis.18 Remarkably, mutant mice are also characterized by absent expression of both HCN3 and cKIT.18 These observations suggest that disruption of utPMC development underlies human congenital hydronephrosis.18 However, further analysis of the mechanisms that cause failure of utPMC marker expression in mutant mice with deficient Hedgehog signaling has been limited by lack of knowledge regarding the developmental mechanisms by which utPMCs are generated and the absence of molecular markers that characterize these cells before the midpoint of mouse kidney–urinary tract development (embryonic day [E] E15.5).18
Nonobstructive hydronephrosis constitutes the most common cause of congenital hydronephrosis but has no defined etiology.19–22 Although the molecular and cellular mechanisms that underlie hydronephrosis are largely unknown, perturbations of utPMCs have been associated with human congenital disease. Whereas the expression of HCN3 has not been analyzed, cKIT expression is altered in ureteric tissue isolated from humans and experimental animals with congenital kidney diseases, including vesicoureteral reflux, ureteropelvic junction (UPJ) obstruction, primary obstructive megaureter, and nonobstructive hydronephrosis.23–34 Analysis of proximal ureter tissue sections from children with UPJ obstruction undergoing pyeloplasty demonstrated decreased cKIT expression,24,29,31 although primary obstructive megaureter tissues also exhibit significantly fewer cKIT+ cells compared with control samples.30,33
The critical contribution of HCN3+ and cKIT+ utPMCs to urinary tract health and perturbation of these cells in congenital kidney diseases provides an impetus to elucidate mechanisms that control the specification and differentiation of these cells. Here, we identified the neural crest origin of HCN3+ and cKIT+ utPMCs, isolated single cell type populations from tissue, characterized the molecular signatures of both cell populations, and identified PTK2β as a novel early expressed marker that contributes to pacemaker function.
Results
utPMCs Originate from the Neural Crest Cell Lineage
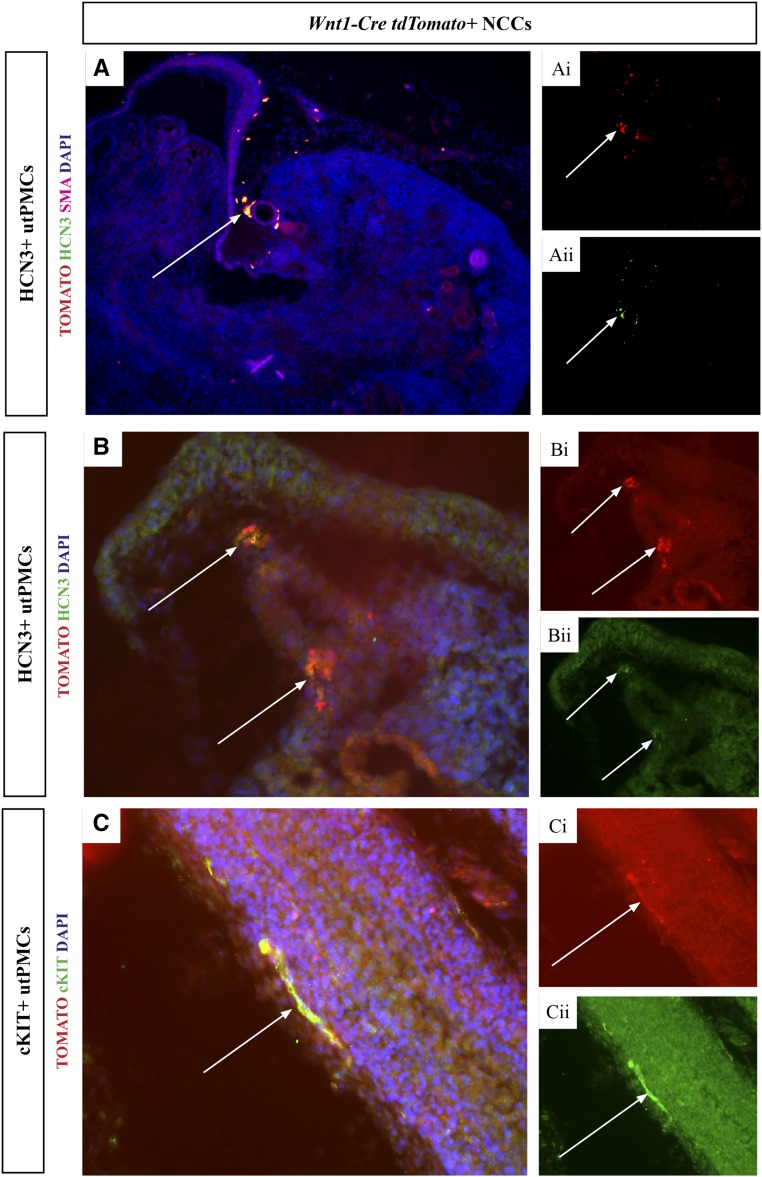
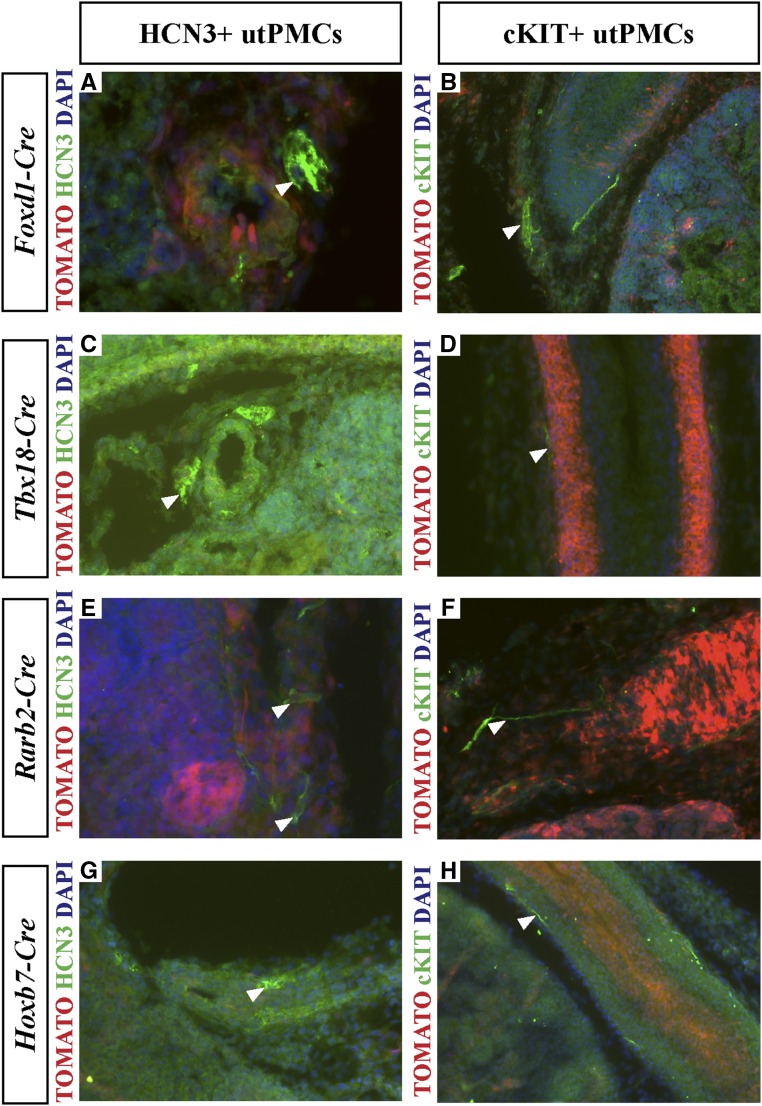
To define the developmental origin of utPMCs, we used lineage tracing in mice. We generated transgenic mice in which the expression of Rosa26tdTomato red fluorescent protein (RFP) (TOMATO) indelibly marked cells in one of five cell lineages that contribute to the kidney and ureter: metanephric mesenchyme, Rarb2-Cre35,36; tailbud mesenchyme, Tbx18-Cre37; neural crest, Wnt1-Cre38,39; renal stroma, Foxd1-Cre40; and ureteric bud, Hoxb7-Cre.41 E18.5 frozen kidney sections, generated from each of the five mouse models, were analyzed for colocalization of TOMATO and cKIT/HCN3 using fluorescence microscopy. First, we imaged tissue sections isolated from mice in which TOMATO is expressed in the neural crest lineage (Wnt1-Cre;RosatdTomato) using fluorescence microscopy. Analysis of lower and higher power images of the area of pacemaker cell localization generated by overlay of TOMATO (red color, Figure 1, Ai and Bi) and HCN3 (green color, Figure 1, Aii and Bii) imaging channels demonstrated colocalization (yellow color) in cells adjacent to the renal artery in the PKJ (Figure 1A [10×], 1B [20×]). Colocalization was also observed between TOMATO and cKIT (Figure 1C). The colocalization was observed in elongated, thin cells adjacent to smooth muscle cells in the ureter, consistent with the spatial pattern of each of TOMATO and cKIT (Figure 1C). In contrast, parallel analyses in mice expressing TOMATO in the Foxd1-derived renal stroma (Figure 2, A and B), tailbud mesenchyme (Figure 2, C and D), metanephric mesenchyme (Figure 2, E and F), and ureteric bud (Figure 2, G and H) failed to reveal any colocalization between TOMATO and cKIT or HCN3 (Figure 2, A–H). Taken together, these data indicate that utPMCs arise from the neural crest but not from metanephric or tailbud mesenchyme, renal stroma, or ureteric bud.
Figure 1.
HCN3+ and cKIT+ utPMCs colocalize with Wnt1-Cre;tdTomato labelled NCCs. (A and B) Lower and higher power images are shown, respectively. (A and B) TOMATO (red color, Ai and Bi) colocalizes with HCN3 (green color, Aii and Bii) generating orange/yellow color in the merged image in PKJ cells adjacent to the renal artery (A and B, arrows). (C) TOMATO (red color, Ci) colocalizes with cKIT (green color, Cii) generating orange/yellow color in the merged image in thin, elongated cells adjacent to the ureteric wall (C, arrows). cKIT, KIT Proto-Oncogene Receptor Tyrosine Kinase; DAPI, 4′,6-Diamidino-2-Phenylindole; HCN3, Hyperpolarization Activated Cyclic Nucleotide Gated Potassium Channel 3; NCC, neural crest cell; SMA, smooth muscle actin; utPMCs, urinary tract pacemaker cells.
Figure 2.
No colocalization is observed between HCN3+ and cKIT+ utPMCs and tdTomato-labeled renal stroma, tailbud mesenchyme, metanephric mesenchyme, or ureteric bud. (A and B) TOMATO-labeled Foxd1-Cre+ renal stromal cells (red color, A and B) do not colocalize with (A) HCN3 or (B) cKIT (green color). (C and D) TOMATO-labeled Tbx18-Cre+ renal tailbud mesenchyme cells (red color, C and D) do not colocalize with (C) HCN3 or (D) cKIT (green color). (E and F) TOMATO-labeled Rarb2-Cre+ metanephric mesenchyme cells (red color, E and F) do not colocalize with (E) HCN3 or (F) cKIT (green color). (G and H) TOMATO-labeled Hoxb7-Cre+ ureteric bud cells (red color, G and H) do not colocalize with (G) HCN3 or (H) cKIT (green color). cKIT, KIT Proto-Oncogene Receptor Tyrosine Kinase; DAPI, 4′,6-Diamidino-2-Phenylindole; HCN3, Hyperpolarization Activated Cyclic Nucleotide Gated Potassium Channel 3; utPMCs, urinary tract pacemaker cells.
FACS-Isolated utPMCs Express Markers Characteristic of Developing Neural Crest Cells
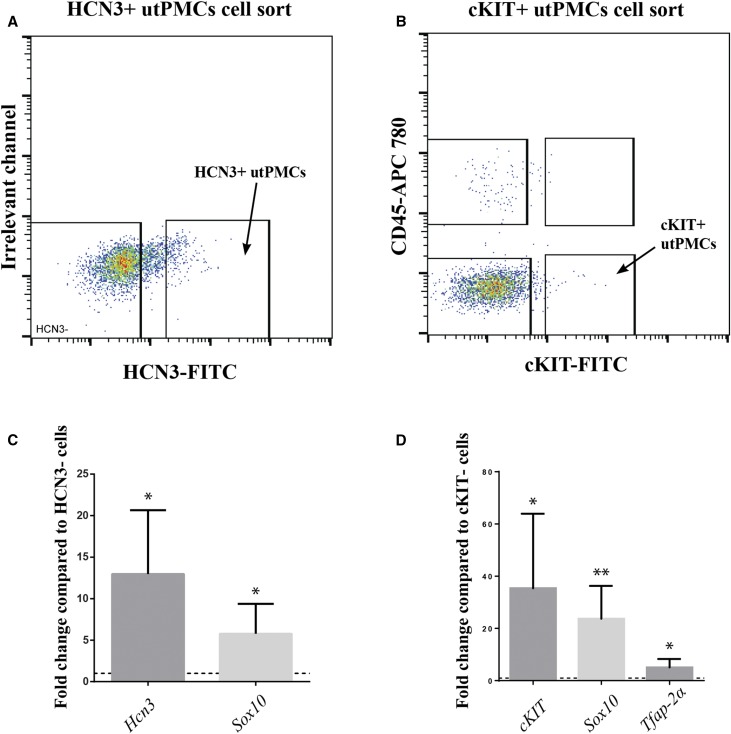
To further study the development and function of utPMCs, we developed a protocol to isolate pure populations of HCN3+ or cKIT+ utPMCs from embryonic tissue. PKJs (for HCN3+ cells) or proximal ureters (for cKIT+ cells) were microdissected from E18.5 wildtype mouse embryos. Tissue, so isolated, was digested and incubated with anti-HCN3 antibodies (to isolate HCN3+ cells), or both anti-cKIT antibodies (to isolate cKIT+ cells) and anti-CD45 antibodies (to select out cKIT+, CD45+ mast cells). Using FACS, HCN3+ cells were isolated with >96% purity, whereas cKIT+ utPMCs were isolated with >94% purity (Figure 3, A and B). FACS indicated that HCN3+ cells constitute approximately 1%, whereas cKIT+ utPMCs constitute approximately 0.25% of the total population of sorted cells (Figure 3, A and B). The identity of isolated cells was confirmed using RT-PCR. Hcn3 was expressed >12-fold higher in FACS-sorted HCN3+ cells compared with HCN3− cells (Figure 3C). cKit expression in cKIT+, CD45− FACS-sorted cells was >35-fold higher compared with that in double negative (cKIT−, CD45−) FACS-sorted cells (Figure 3D). These results provided a basis to investigate the expression of genes characteristic of neural crest in utPMCs compared with sorted HCN3– and cKIT− cells. Sox10 is a transcription factor gene that is expressed in migratory neural crest cells and remains active in neural and melanocyte lineages later in development.42 tfAP-2α is a transcription factor gene required for correct development of multiple neural crest cell derivatives and is also used as a common neural crest marker.43 Our results indicate that Sox10 is highly enriched in HCN3+ utPMCs compared with HCN3− cells (Figure 3C) and that both Sox10 and tfAP-2α are highly enriched in cKIT+ utPMCs compared with double negative cells (Figure 3D).
Figure 3.
Pure populations of utPMCs can be isolated using FACS. (A) HCN3+ utPMCs were isolated from 18.5 embryos using anti-HCN3 antibody. (B) cKIT+ utPMCs were isolated from 18.5 embryos using anti-cKIT antibody and anti-CD45 antibody. (C) FACS-isolated HCN3+ utPMCs are enriched in HCN3 and Sox10 mRNA when compared with HCN3-negative neighboring cells via RT-qPCR. (D) FACS-isolated cKIT+ utPMCs are enriched in cKIT, Sox10, and Tfap-2α mRNA when compared with cKIT-negative neighboring cells via RT-qPCR. *P<0.05; **P<0.01; CD45-APC 780, Allophycocyanin-eFluor 780; cKIT, KIT Proto-Oncogene Receptor Tyrosine Kinase; FITC, Fluorescein Isothiocyanate; HCN3, Hyperpolarization Activated Cyclic Nucleotide Gated Potassium Channel 3; utPMCs, urinary tract pacemaker cells.
RNA Sequencing Identifies Genes Highly Enriched in HCN3+ and cKIT+ utPMCs
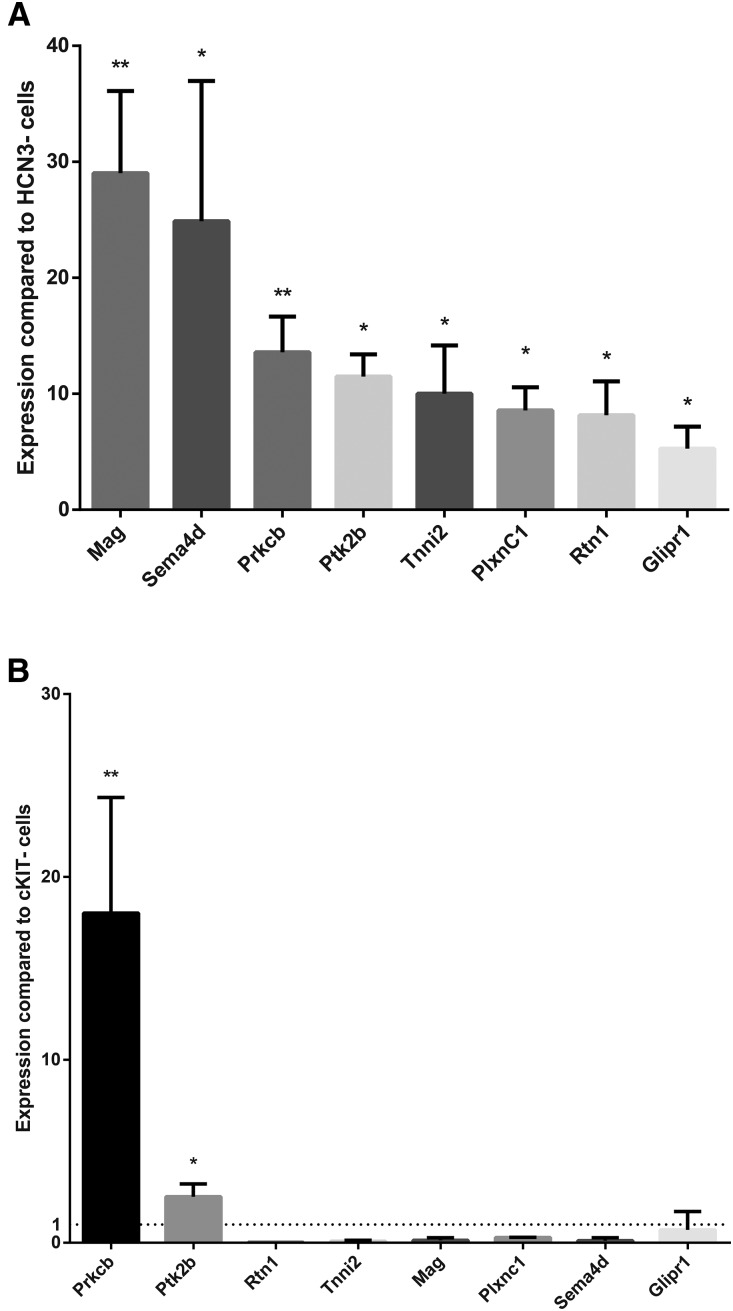
The number of genes that are known to identify utPMCs is very small and includes only HCN3, cKIT, and TTC.3–5 Moreover, the expression of these genes is limited to later stages of embryonic development (E15.5 and later in the mouse). As a step toward identifying novel markers of utPMCs, we sought to perform genome-wide RNA sequencing in HCN3+ and cKIT+ cells. First, RNA was isolated from HCN3+ or cKIT+ cells isolated from E18.5 WT urinary tract tissue. Abundant high-quality RNA could be isolated from HCN3+ cells but not from cKIT+ cells, likely due to the difficulty in isolating large numbers of viable cKIT+ cells. Analysis of HCN3+ cell RNA by RNA sequencing revealed enrichment of a number of novel genes (Gene Ontogeny Consortium [GEO] accession number GSE103400). We selected a subset of these genes for further study on the basis of the following selection criteria: (1) high fold change compared with HCN3− cells (>4-fold), (2) not expressed in other kidney cell types, and (3) published data demonstrating function in non–urinary tract pacemaker cells and/or function in neurons, and/or expression in neural crest–derived cells (Table 1). The expression of these selected genes was validated by RT-qPCR using RNA isolated from FACS-isolated HCN3+ and HCN− cells (Figure 4A). Because HCN3+ and cKIT+ utPMCs both arise from the NCC developmental lineage, we hypothesized that they share the expression of a subset of molecular markers. To test this hypothesis, we utilized RT-qPCR on FACS-sorted cKIT+ cells. Our results reveal that two of the markers for HCN3+ cells (Ptk2β and Prkcβ) are enriched in cKIT+ utPMCs when compared with cKIT− cells (Figure 4B).
Table 1.
Selected genes identified by RNAseq as highly enriched in HCN3+ cells and with a possible role in utPMC function
| Gene | Full Name | Fold Change | Adjusted P Value | RefSeq Gene Description27 |
|---|---|---|---|---|
| Mag | Myelin associated glycoprotein | 26.316 | 1.98E−05 | Member of the Ig-like superfamily expressed in myelinating glial cells. |
| Prkcβ | Protein kinase C β | 25.492 | 5.06E−28 | Member of PKC family that may regulate neuronal functions. |
| Ptk2β | Proline rich tyrosine kinase 2 β | 17.04 | 8.11E−07 | Cytoplasmic protein tyrosine kinase which is involved in calcium-induced regulation of ion channels. |
| Glipr1 | GLI pathogenesis -related 1 | 15.92 | 1.06E−05 | Protein with similarity to both the pathogenesis-related protein (PR) superfamily and the cysteine-rich secretory protein (CRISP) family and expressed in neural crest–derived tissues. |
| Rtn1 | Reticulon 1 | 12.26 | 3.36E−09 | Member of the family of reticulon encoding genes and a marker of neuroendocrine cells and degenerative neuronal tissue. |
| Tnni2 | Troponin I FS | 12.21 | <0.001 | Fast-twitch skeletal muscle protein, a member of the troponin I gene family, a component of the troponin complex which is responsible for the calcium-dependent regulation of striated muscle contraction. |
| Sema4d | Semaphorin 4d | 5.92 | 2.99E−09 | Member of the Semaphorin gene family and a ligand for PlexinB1.28 The Sema4d-PlexinB1 interaction is involved in neural development in the hippocampus.29,30 |
| PlxnC1 | Plexin C1 | 4.128 | <0.001 | A member of the Plexin family, which are transmembrane receptors for Semaphorins, that regulate axon guidance, cell motility, and migration. |
Figure 4.
Eight novel utPMC markers are enriched in HCN3+ utPMCs and two are shared with cKIT+ utPMCs. (A) RT-qPCR on FACS-isolated HCN3+ utPMCs confirms the enrichment of novel marker mRNA when compared with HCN3-negative neighboring cells. (B) RT-qPCR on FACS-isolated cKIT+ utPMCs reveals the enrichment of Prkcb and Ptk2b mRNA when compared with cKIT-negative neighboring cells. *P<0.05; **P<0.01; cKIT, KIT Proto-Oncogene Receptor Tyrosine Kinase; HCN3, Hyperpolarization Activated Cyclic Nucleotide Gated Potassium Channel 3.
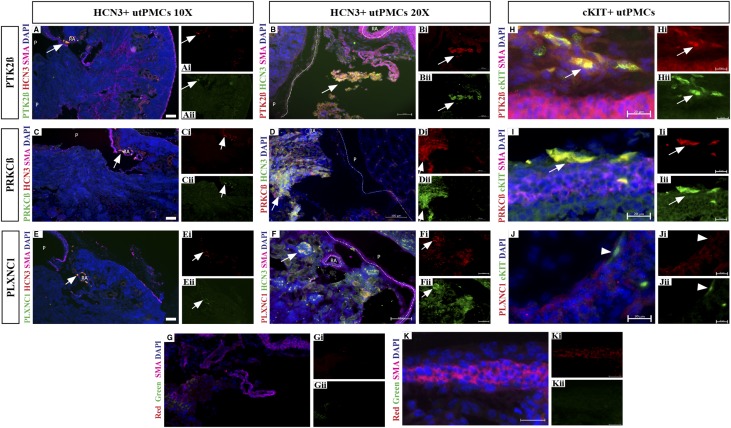
We analyzed the expression of genes highly expressed in HCN3+ and/or cKIT+ cells at the protein level (Figure 5). First, we analyzed the expression of PTK2β and PRKCβ because they are both expressed in HCN3+ and cKIT+ utPMCs (Figure 5, A–D). The PKJ was imaged at lower (Figure 5, A and C) and higher (Figure 5, B and D) power using antibodies specific for HCN3 (Figure 5, Ai, Bi, Cii, and Dii) and either PTK2β (Figure 5, Aii and Bi) or PRKCβ (Figure 5, Cii and Di). Both PTK2β (Figure 5, A and B) and PRKCβ (Figure 5, C and D) colocalized with HCN3. Next, we analyzed the expression of PLEXINC1 (PLXNC1) because it is expressed in neural crest cells early during development and is a cell surface receptor, suggesting a possible functional role in pacemaker cells.44–47 Expression of PLXNC1, imaged at lower and higher power, was observed in the PKJ, albeit with variable intensity (Figure 5, Eii and Fi), and colocalized with HCN3 (Figure 5, Ei and Fii). Colocalization of PLXNC1 and HCN3 was most obvious in areas in which both proteins are highly expressed (Figure 5, E and F). The specificity of antibody-mediated signal was demonstrated using nonspecific IgG instead of antibody specific for HCN3 and specific putative markers (Figure 5G). In these images, SMA is shown via antibody labeled with Alexa Flour 647 (far red) and control antibodies consist of goat or sheep IgG (green color in Figure 5Gii) and rabbit IgG (red color in Figure 5Gi). To confirm that the observed protein colocalization was due to signal from the same cells, confocal microscopy was used to image 0.2-µm optical sections (Supplemental Figure 1). HCN3 and marker (PTK2β, PRKCβ, PLXNC1) signals were observed in the same cells, but PTK2β and PRKCβ were localized to different intracellular compartments than HCN3.
Figure 5.
Novel utPMC markers are enriched in HCN3+ and cKIT+ utPMCs at the protein level in situ. Immunofluorescence analysis revealed colocalization between PTK2β (green, A–Aii; red B–Bii), PRKCβ (green, C–Cii; red, D–Dii), PLXNC1 (green, E–Eii; red, F–Fii), and HCN3 (green, A, C, and E; red, B, D, and F). Colocalization was observed in cells adjacent to the renal artery (RA) outside the pelvis (P) consistent with the location of utPMCs. (G–Gii) Control IgG sections revealed weak/no signal in the red and green channels. Analysis revealed colocalization between cKIT (green, H–Jii), and PTK2β (red, H–Hii) or PRKCβ (red, I–Iii), but not with PLXNC1 (red, J–Jii). cKIT staining was observed in elongated cells in the adventitial layer adjacent to the ureteric muscular layer consistent with the localization of cKIT+ utPMCs. (K–Kii) Control IgG sections revealed weak/no signal in the adventitial layer in red and green channels. Scale bars represent 200 µm (A, C, and E), 100 µm (B, D, F, and G), or 20 µm (H–K). cKIT, KIT Proto-Oncogene Receptor Tyrosine Kinase; DAPI, 4′,6-Diamidino-2-Phenylindole; HCN3, Hyperpolarization Activated Cyclic Nucleotide Gated Potassium Channel 3; PLXNC1, Plexin C1; PRKCβ, Protein Kinase C Beta; PTK2β, Protein Tyrosine Kinase 2 Beta; SMA, smooth muscle actin; utPMCs, urinary tract pacemaker cells.
Next, we analyzed the expression of PTK2β, PRKCβ, and PLXNC1 in cKIT+ utPMCs (Figure 5, H–K). cKIT expression was observed in thin, elongated cells located in the adventitial layer of the ureter (Figure 5, Hii–Jii). Expression of PTK2β (Figure 5Hi) and PRKCβ (Figure 5Ii) was observed in a similar spatial domain. Image overlay revealed colocalization of both PTK2β and PRKCβ with cKIT (Figure 5, H and I). In contrast, PLXNC1 was detected with weak expression in ureteric tissue not including cKIT+ cells (Figure 5Ji). No signal was observed in negative control sections stained with control IgG instead of the primary antibody confirming the specificity of the observed signal (Figure 5K). Taken together, these data demonstrate that PTK2β and PRKCβ are coexpressed with HCN3 and cKIT whereas PLXNC1 is only expressed in HCN+ utPMCs.
PTK2β: A Developmental Marker of utPMCs that Controls Pelvic Contraction
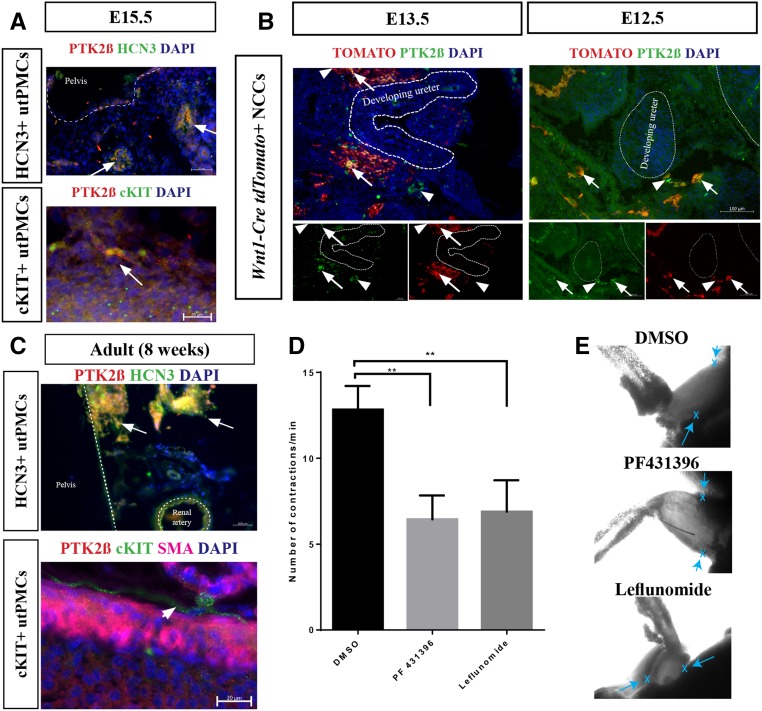
PTK2β, a protein tyrosine kinase, regulates ion channel activity in neurons, senses Ca2+ levels, and interacts with proteins that regulate HCN protein in non–urinary tract tissues.48–52 We investigated the possibility that PTK2β marks utPMCs at stages of development earlier than those during which HCN3 and cKIT are expressed. PTK2β was identified in E15.5 embryonic mouse kidney and ureter by immunofluorescence staining in situ. Results demonstrate that PTK2β is localized in both HCN3+ utPMCs and cKIT+ utPMCs in the PKJ and proximal ureter, respectively (Figure 6A). Next, we studied PTK2β expression at time points earlier than E15.5. Because HCN3 and cKIT are not expressed in utPMCs before E15.5, we colocalized PTK2β with neural crest cells, which give rise to utPMCs, using TOMATO in Wnt1-Cre ROSAtdTomato mice (Figure 6B). Consistent with prior published data, NCCs were identified adjacent to the developing kidney and ureter.39 PTK2β colocalized with a subset of TOMATO-positive cells surrounding the developing ureter and kidney at both E13.5 and E12.5 (Figure 6B, arrows).
Figure 6.
PTK2β is an early expressed utPMC marker that controls ureteric contraction. (A) PTK2β (red color) colocalizes with HCN3 and cKIT (green color) to generate orange/yellow color in the merged images in E15.5 murine renal tissue. (B) PTK2β (green color) colocalizes with a subset of TOMATO-labeled Wnt1-Cre+ NCCs (arrows, red color) surrounding the developing kidney and ureter in E13.5 and E12.5 murine tissue. (C) PTK2β expression (red color) colocalizes with HCN3 but not cKIT (green color) in adult 8-week-old murine renal tissue. (D) PTK2β inhibitors reduce ureteric contraction frequency in an ex vivo assay for pelvis-ureter contractions. (E) Representative still-frames from time-lapse imaging of ex vivo pelvis-ureter explants illustrate the origin (x and arrows) of pyeloureteric contractions at the pelvis in DMSO control as well as in explants treated with PTK2β inhibitors (PF431396 and leflunomide). Representative time-lapse imaging is presented in Supplemental Movies 1–3. **P<0.01; NS indicates P>0.05. cKIT, KIT Proto-Oncogene Receptor Tyrosine Kinase; DAPI, 4′,6-Diamidino-2-Phenylindole; DMSO, Dimethyl sulfoxide; HCN3, Hyperpolarization Activated Cyclic Nucleotide Gated Potassium Channel 3; NCC, neural crest cell; PF431396, PTK2β inhibitor; PTK2β, Protein Tyrosine Kinase 2 Beta; SMA, smooth muscle actin; utPMCs, urinary tract pacemaker cells.
We investigated the functional contribution of PTK2β in urinary tract contraction using an established assay of pelvic-ureteric contraction.4,5 This assay was performed in tissue preparations isolated from 8-week-old mice. At postnatal 8 weeks, PTK2β expression was sustained in HCN3+ utPMCs but lost from cKIT+ utPMCs (Figure 6C). PTK2β function was investigated using two pharmacologic inhibitors, PF (a selective PTK2β inhibitor) and leflunomide (a broader tyrosine kinase inhibitor that is associated with antenatal nonobstructive hydronephrosis).53–57 Pyeloureteric tissue preparations were placed on porous filters, incubated at 37°C for 1 hour, and imaged for contractile activity. Tissues displaying the expected rate of ten contractions/minute were treated with vehicle or PTK2β inhibitor for 1 hour and then imaged again for 5 minutes. Recorded contractions were analyzed for frequency. Results demonstrated a 50% reduction in contraction frequency in explants treated with either PTK2β inhibitor compared with control with no apparent change in the spatial origin of contraction (arrows, Figure 6E, Supplemental Movies 1–3).
Discussion
The developmental and molecular mechanisms that underlie utPMC function in kidney–urinary tract health and disease are poorly understood. HCN3+ and cKIT+ populations of utPMCs function sequentially to initiate and propagate pyeloureteric contractions.2,3,5 Although the presence and spatial organization of utPMCs has been shown to be perturbed in congenital urinary tract malformations and particularly in hydronephrosis,18,23–34 little is known regarding the direct contribution of utPMCs to the pathobiology of these disorders. Lack of knowledge regarding the origin and development of utPMCs limits the ability to investigate these issues. Here, we identify the embryonic origin of utPMCs, showing that HCN3+ and cKIT+ utPMCs arise from Wnt1+ cells. Our results demonstrate that utPMCs arise outside of the nephrogenic field and migrate to take up residence in the PKJ and ureter. Further, using methods we developed to isolated pure populations of HCN3+ and cKIT+ cells, we identified RNAs that are enriched in these cell populations. In so doing, we identified PTK2β as a novel marker of both HCN3+ and cKIT+ cells and show that PTK2β colocalizes with migrating WNT1+ cells as early as E12.5, when these cells are still migrating toward the kidney–urinary tract.39,58 Finally, we demonstrate that PTKb controls pyeloureteric contraction. These findings are consistent with previous observations that WNT1+ neural crest cells give rise to cells that reside in the PKJ and ureteric wall.39 Further, the identification of PTK2β, a Ca+2-dependent tyrosine kinase, is consistent with prior experiments showing that Ca+2 signaling is critical to pyeloureteric contraction.11
A Neural Crest Cell Origin for utPMCs
The neural crest originates at the boundary between the ectoderm and the neural tube in vertebrates.59 Neural crest cells migrate throughout the embryo and contribute to a diverse set of differentiated cell types including craniofacial cartilage and bone, cardiac blood vessels, melanocytes, and peripheral neurons, including the enteric nervous system of the gut.60 Our findings extend our understanding of the neural crest lineage by showing that the neural crest contributes functionally to the kidney–urinary tract. The developmental origin of utPMCs thus differs from ICCs in the gut and HCN4+ pacemakers of the heart, both of which are derived from gut and heart mesenchyme, respectively.61–65 Our findings that HCN3 and cKIT colocalize with Wnt1-driven TOMATO and express Sox10 and Tfap2α, neural crest markers, are consistent with the previous demonstration that cKIT colocalizes with PGP9.5, which is expressed in gut developing neural crest cells.42,43,66–68 Our results are also consistent with previous LacZ lineage-tracing studies, which demonstrated neural crest cells in the metanephros, the proximal ureter, the pelvic-kidney junction, and sparsely in the kidney cortex.39 In these studies, time-lapse analysis of murine neural crest cells marked with Wnt1-dependent LacZ expression demonstrated that neural crest cells surround the metanephric mesenchyme at E11.5.39 By E12.5, fewer neural crest cells border the metanephric mesenchyme and, at E14.5, they are confined to the ureter and renal pelvis.39 Considered in the context of our results, these studies suggest that utPMC precursors migrate into the developing ureter between E11.5 and E14.5 and subsequently undergo their final differentiation. The finding that neural crest cells give rise to utPMCs suggests that they are essential for the functional development of the ureter. Finally, our results provide a basis to re-examine utPMC function in splotch mutants, in which neural crest cells fail to migrate to the developing kidney–urinary tract and in which analysis was limited to embryonic stages before utPMC development.39
Lineage tracing of Wnt1-marked neural crest cells39 in combination with our results shown here also inform our previous published work showing that Hedgehog-GLI signaling in the metanephric mesenchyme of mice controls utPMC development.18 Results now showing that utPMCs are derived from neural crest suggest that HH-GLI signaling in the metanephric mesenchyme acts in a non–cell autonomous fashion to control the specification and differentiation of utPMCs as they migrate into and/or differentiate within the ureter/PKJ. This is consistent with the finding that neural crest cell progenitors that form the cardiac outflow tract are not responsive to Hedgehog directly, but Hedgehog affects their development in a non–cell autonomous manner.69
Molecular Markers of utPMCs
The embryonic development and physiology of utPMCs is largely undefined, in part by a lack of knowledge of the molecular constituents that endow these cells with their particular functions. Here, we used cell purification followed by RNAseq to identify RNAs enriched in HCN3+ cells. Cell isolation and qPCR demonstrated which of these identified RNAs are either shared with cKIT+ cells or are unique to HCN3+ cells. Each of three utPMC markers identified in HCN3+ cells (PLXNC1, SEMA4d, MAG) has been shown to play a role in axonal guidance, neuronal migration, or neural crest cell migration.44,70,71 PLXNC1 and MAG are membrane receptors that engage repulsive or attractive signals (context- and ligand-dependent) to guide cell migration.44,70 SEMA4d is a secreted ligand for Plexin B1 that is released by different tissues to restrict or promote cell migration.71 The demonstrated properties of PLXNC1, SEMA4d, and MAG in nonrenal tissues suggest functional contributions for these proteins in neural crest migration and contribution to the kidney–urinary tract. Three other genes, the expression of which is enriched in HCN3+ cells, display functions distinct from cell migration. RTN1 is a neuroendocrine marker known to regulate protein secretion by neuroendocrine cells.72–74 TNNI2 is involved in muscle cell contractile function.75 Although GLIPR1 function is poorly characterized, it is enriched in some NCC-derived tissues.76 The functions of these proteins in utPMC-mediated functions remain to be defined.
The functions of both PRKCβ and PTK2β in regulating electrical activity in nonrenal tissues suggests that both of these proteins may perform similar functions in both HCN3+ and cKIT+ utPMCs, in which they are enriched. PRKCβ is an isoform of protein kinase C. Protein kinase C, a Ca2+-dependent kinase, regulates heart and gut PMCs, as well as neuron and muscle cell actions.7,77–81 PTK2β is a Ca2+-dependent tyrosine kinase involved in linking Ca2+ signaling to ion channel activity.48 Ca2+ signaling has been described as a major signaling pathway in PMC in the heart, gut, and the urinary tract.8,11,12,14–17 PTK2β has also been shown to interact with SRC kinase, a known regulator of HCN channels.49–51 Our results indicate that pharmacologic inhibition of PTK2β in pyeloureteric explant tissue significantly reduces ureteric contraction frequency, suggesting that PTK2β controls PMC function. Future studies which identify the mechanism by which PTK2β regulates pacemaker activity will shed insight into the physiologic regulation of pacemaker activity.
Clinical Implications
The etiology of human nonobstructive hydronephrosis is poorly defined.82 Prior investigation of genetic defects associated with Congenital Anomalies of the Kidney and Urinary Tract using karyotyping and SNP microarrays identified several microdeletions and duplications associated with hydronephrosis.83,84 However, these studies were limited in that they did not identify individual genes but rather defective chromosomal regions.83,84 The etiology of nonobstructive hydronephrosis is hypothesized to include defects in ureteric peristalsis which could involve defective contractile smooth muscle cell action or defective regulatory utPMC action.18,82,85 Previously, a mouse model with defective smooth muscle cell development linked Tshz3 with defective smooth muscle cell development and nonobstructive hydronephrosis.85 Our published mouse model for nonobstructive hydronephrosis showed that Smo deficiency in the metanephric mesenchyme causes defective utPMC development and nonobstructive hydronephrosis.18 Furthermore, a reduced number of cKIT+ utPMCs has been associated with congenital kidney–urinary tract defects including UPJ obstruction, vesicoureteral reflux, and primary obstructive megaureter.23–30,32–34 Novel utPMC markers described in this study can be utilized in future studies to identify the contribution of utPMC dysfunction to congenital urinary tract disease. Furthermore, because the outcome of nonobstructive hydronephrosis is heterogeneous with the majority of cases stabilizing postnatally and a minority requiring surgery, those markers might serve as prognostic markers to aid in the clinical management of nonobstructive hydronephrosis or therapeutic targets in cases of hydronephrosis.20–22,82
Concise Methods
Mice
Rarb2-Cre mice were mated with Rosa26-tdTomato mice (Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J; The Jackson Laboratory, Bar Harbor, ME) to generate Rarb2-Cre;Rosa26-tdTomato embryos in which the metanephric mesenchyme was indelibly labeled with RFP in Cre-positive mice. In the same way, Tbx18-Cre, Hoxb7-Cre, Wnt1-Cre, and Foxd1-Cre mice were each mated with Rosa26-tdTomato mice to generate embryos in which the tailbud mesenchyme, ureteric bud, neural crest cells, and renal stroma, respectively, were indelibly labeled with RFP in Cre-positive mice. PCR genotyping for Cre recombinase was performed using the following primers. For Rarb2-cre, FoxD1-Cre, HoxB7-Cre, and Wnt1-Cre: CRE Fwd: 5′-AGCGCGATCACATGGTCCTG-3′; CRE Rev: 5′-ACGATCCTGAGACTTCCACACT-3′, which generates a 230-bp product. For Tbx18-cre: TCT Fwd: 5′-CCATCCAACAGCACCTGGGC-3′; TCT Rev: 3′-CCACCATCGGTGCGGGAGATGTCCTTCACT-3′, which generates a 313-bp product. C57BL/6 wildtype mice were mated to generate wildtype embryos for pacemaker cell isolation experiments.
Histologic Analysis
Embryos were collected at E18.5, determined by the number of days after the observation of a copulation plug, with the day of observation corresponding to day 0.5. Experiments using mice were approved by the Animal Ethics Committee at the Hospital for Sick Children and carried out in accordance with the Canadian Council of Animal Care. Immunofluorescence was performed on 4% PFA-fixed, OCT-embedded frozen sections using primary α-CD117 rabbit-monoclonal (1:50 dilution; DAKO), rabbit polyclonal anti-HCN3 (1:200 dilution; Abcam), goat polyclonal anti-PTK2β (1:300 dilution; SCBT), or goat polyclonal anti-TNNI2 (1:150 dilution; SCBT); the appropriate Alexa Flour conjugated secondary antibodies were used at a dilution of 1:1,000 (Thermofisher). Nuclei were visualized using DAPI (1:1,000; Thermo Scientific). Sections were washed and mounted using glass coverslips and hard-set Vectashield (Vector Labs). Images for colocalization experiments were captured with a fluorescence microscope (Zeiss Axio Observer) or an Olympus confocal microscope.
Fluorescence-Activated Cell Sorting
Wildtype C57B6 E18.5 ureters and pelvic-kidney junctions were microdissected using a three-dimensional dissecting microscope and pooled into one sample for cell sorting. Tissue was dissociated using a 1 mg/ml collagenase suspension. Cells were resuspended in sorting buffer with the following antibodies. cKIT: CD117 (cKIT) anti-mouse PE (1:200; eBioscience) to positively select cKIT+ PMC and CD45 anti-mouse APC-eFluor 780 (1:200; eBioscience) to negatively select cKIT+, CD45+ mast cells. Hcn3: α-Hcn3 rabbit-polyclonal (1:400) and secondary Alexa Fluor 488 goat-α-rabbit (1:1,000; Life Technologies). Propidium iodide was used as a dead cell marker (10 mg/ml). Cell sorting was conducted using either MoFlo XDP or MoFlo Astrios Fluorescence Activated Cell Sorting machines. Cells were collected into sorting buffer, centrifuged at 4°C, and resuspended in 150 μl of buffer RLT.
RNA Isolation, Real Time-PCR, and RNAseq
RNA was extracted using an RNEasy Micro Kit (QIAGEN) and cDNA was generated using First Strand cDNA Synthesis (Invitrogen) from total RNA. Real-time PCR was conducted with 2–4 μl of cDNA, SYBR Green PCR Mix (Applied Biosystems), and 5 μM of each primer in a total volume of 20 μl. Primers were designed using NCBI Primer3 software. The sequences of the primers used are attached in Supplemental Table 1. Real-time PCR amplification was conducted using the Applied Biosciences Viia7 Real-Time PCR System. Relative levels of mRNA expression were determined using the ΔΔCT method and normalized by comparison to expression of Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). RNAseq was performed by the Illumina HiSEQ 2500 platform by The Centre for Applied Genomics, The Hospital for Sick Children, Toronto, Canada. RNAseq data has been deposited to GEO (accession number GSE103400).
Ex Vivo Pelvic Ureter Explants
Adult (6–8 weeks) male mice were euthanized using cervical dislocation according to the guidelines of the Canadian Council on Animal Care and immediately put on ice. Urogenital systems were dissected out into ice-cold Tyrode’s solution. The fat tissue surrounding the kidney and ureter was removed using an Olympus stereo microscope, fine tipped tweezers, and needles. The kidney tissue was then cut using a number 10 scalpel so that the papilla, the medulla, and the cortex were cut in half whereas the renal pelvis and ureter remained intact. Pelvis ureter explants were then transferred to a 0.4-µm transwell filter (Falcon) and into a well containing 2 ml of prewarmed (37°C) Tyrode’s solution. Next, 150 µl of Tyrode’s solution was added directly on top of the explant. The plate was then incubated in a humidified 37°C incubator at 5% CO2 on a nutating mixer for 30 minutes. An environmentally controlled Zeiss Axiovert 200m epifluorescence acquisition system fitted with Axiocam HR was used to take bright field time-lapse images of the explants at 15 frames per second to verify that all explants were contracting at comparable frequencies (all outliers were disposed of at this stage). The top media and the media in the well were then replaced with media that contained PTK2β inhibitors (PF431396 or leflunomide,16–18,26 or DMSO as a control). The plate was then returned to the incubator for 60 minutes with upper media changes every 20 minutes before a second set of time-lapse images was captured. The time lapse images were compiled to create videos using Zeiss software (Axiovision or Zen Blue). The number of contractions per minute was quantified manually on the basis of the videos for each group.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism Software (version 6.0f). Data were analyzed using two-tailed paired t test. Statistical significance was indicated by a probability of <0.05. Values are given as mean±SEM.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research and the Kidney Foundation of Canada (to N.D.R.), and a Tier I Canada Research Chair in Developmental Nephrology (to N.D.R.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017090951/-/DCSupplemental.
References
- 1.Lang RJ, Davidson ME, Exintaris B: Pyeloureteral motility and ureteral peristalsis: Essential role of sensory nerves and endogenous prostaglandins. Exp Physiol 87: 129–146, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Feeney MM, Rosenblum ND: Urinary tract pacemaker cells: Current knowledge and insights from nonrenal pacemaker cells provide a basis for future discovery. Pediatr Nephrol 29: 629–635, 2014 [DOI] [PubMed] [Google Scholar]
- 3.David SG, Cebrian C, Vaughan ED Jr, Herzlinger D: c-kit and ureteral peristalsis. J Urol 173: 292–295, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Hurtado R, Bub G, Herzlinger D: The pelvis-kidney junction contains HCN3, a hyperpolarization-activated cation channel that triggers ureter peristalsis. Kidney Int 77: 500–508, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurtado R, Bub G, Herzlinger D: A molecular signature of tissues with pacemaker activity in the heart and upper urinary tract involves coexpressed hyperpolarization-activated cation and T-type Ca2+ channels. FASEB J 28: 730–739, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pezzone MA, Watkins SC, Alber SM, King WE, de Groat WC, Chancellor MB, et al.: Identification of c-kit-positive cells in the mouse ureter: The interstitial cells of Cajal of the urinary tract. Am J Physiol Renal Physiol 284: F925–F929, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Hagiwara N, Irisawa H: Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol 409: 121–141, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torihashi S, Fujimoto T, Trost C, Nakayama S: Calcium oscillation linked to pacemaking of interstitial cells of Cajal: Requirement of calcium influx and localization of TRP4 in caveolae. J Biol Chem 277: 19191–19197, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Takaki M: Gut pacemaker cells: The interstitial cells of Cajal (ICC). J Smooth Muscle Res 39: 137–161, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Niwa N, Yasui K, Opthof T, Takemura H, Shimizu A, Horiba M, et al.: Cav3.2 subunit underlies the functional T-type Ca2+ channel in murine hearts during the embryonic period. Am J Physiol Heart Circ Physiol 286: H2257–H2263, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Lang RJ, Hashitani H, Tonta MA, Parkington HC, Suzuki H: Spontaneous electrical and Ca2+ signals in typical and atypical smooth muscle cells and interstitial cell of Cajal-like cells of mouse renal pelvis. J Physiol 583: 1049–1068, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakatta EG, Maltsev VA, Vinogradova TM: A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ Res 106: 659–673, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh RD, Gibbons SJ, Saravanaperumal SA, Du P, Hennig GW, Eisenman ST, et al.: Ano1, a Ca2+-activated Cl- channel, coordinates contractility in mouse intestine by Ca2+ transient coordination between interstitial cells of Cajal. J Physiol 592: 4051–4068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Sun AY, Kim JJ, Graham V, Finch EA, Nepliouev I, et al.: STIM1-Ca2+ signaling modulates automaticity of the mouse sinoatrial node. Proc Natl Acad Sci U S A 112: E5618–E5627, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nazarov IB, Schofield CJ, Terrar DA: Contributions of cardiac “funny” (f) channels and sarcoplasmic reticulum Ca2+ in regulating beating rate of mouse and guinea pig sinoatrial node. Physiol Rep 3, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi SW, Lee HA, Moon SH, Park SJ, Kim HJ, Kim KS, et al.: Spontaneous inward currents reflecting oscillatory activation of Na+/Ca2+ exchangers in human embryonic stem cell-derived cardiomyocytes. Pflugers Arch 468: 609–622, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Baker SA, Drumm BT, Saur D, Hennig GW, Ward SM, Sanders KM: Spontaneous Ca(2+) transients in interstitial cells of Cajal located within the deep muscular plexus of the murine small intestine. J Physiol 594: 3317–3338, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cain JE, Islam E, Haxho F, Blake J, Rosenblum ND: GLI3 repressor controls functional development of the mouse ureter. J Clin Invest 121: 1199–1206, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudley JA, Haworth JM, McGraw ME, Frank JD, Tizard EJ: Clinical relevance and implications of antenatal hydronephrosis. Arch Dis Child Fetal Neonatal Ed 76: F31–F34, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng AM, Phan V, Geary DF, Rosenblum ND: Outcome of isolated antenatal hydronephrosis. Arch Pediatr Adolesc Med 158: 38–40, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Lee RS, Cendron M, Kinnamon DD, Nguyen HT: Antenatal hydronephrosis as a predictor of postnatal outcome: A meta-analysis. Pediatrics 118: 586–593, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Coelho GM, Bouzada MCF, Pereira AK, Figueiredo BF, Leite MRS, Oliveira DS, et al.: Outcome of isolated antenatal hydronephrosis: A prospective cohort study. Pediatr Nephrol 22: 1727–1734, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Thulesius O, Angelo-Khattar M, Sabha M: The effect of ureteral distension on peristalsis. Studies on human and sheep ureters. Urol Res 17: 385–388, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Solari V, Piotrowska AP, Puri P: Altered expression of interstitial cells of Cajal in congenital ureteropelvic junction obstruction. J Urol 170: 2420–2422, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Ekinci S, Ertunc M, Ciftci AO, Senocak ME, Buyukpamukcu N, Onur R: Evaluation of pelvic contractility in ureteropelvic junction obstruction: An experimental study. Eur J Pediatr Surg 14: 93–99, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Schwentner C, Oswald J, Lunacek A, Fritsch H, Deibl M, Bartsch G, et al.: Loss of interstitial cells of Cajal and gap junction protein connexin 43 at the vesicoureteral junction in children with vesicoureteral reflux. J Urol 174: 1981–1986, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Arena S, Fazzari C, Arena F, Scuderi MG, Romeo C, Nicòtina PA, et al.: Altered ‘active’ antireflux mechanism in primary vesico-ureteric reflux: A morphological and manometric study. BJU Int 100: 407–412, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Kuzgunbay B, Doran F, Bayazit Y, Turunc T, Satar N, Kayis AA: The effects of ureteral obstruction on Cajal-like cells in rats. J Pediatr Urol 5: 269–273, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Zhang Y, Hu J: The expression of Cajal cells at the obstruction site of congenital pelviureteric junction obstruction and quantitative image analysis. J Pediatr Surg 44: 2339–2342, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Kang HJ, Lee HY, Jin MH, Jeong HJ, Han SW: Decreased interstitial cells of Cajal-like cells, possible cause of congenital refluxing megaureters: Histopathologic differences in refluxing and obstructive megaureters. Urology 74: 318–323, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Koleda P, Apoznanski W, Wozniak Z, Rusiecki L, Szydelko T, Pilecki W, et al.: Changes in interstitial cell of Cajal-like cells density in congenital ureteropelvic junction obstruction. Int Urol Nephrol 44: 7–12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yalcin S, Ertunc M, Ardicli B, Kabakus IM, Tas TS, Sara Y, et al.: Ureterovesical junction obstruction causes increment in smooth muscle contractility, and cholinergic and adrenergic activity in distal ureter of rabbits. J Pediatr Surg 48: 1954–1961, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Kart Y, Karakuş OZ, Ateş O, Hakgüder G, Olguner M, Akgür FM: Altered expression of interstitial cells of Cajal in primary obstructive megaureter. J Pediatr Urol 9[6 Pt B]: 1028–1031, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Ji H-Y, Yang Y: The expression of Gli3 and Teashirt3 in the stenotic tissue of congenital pelvi-ureteric junction obstruction in children. Int J Med Sci 13: 412–417, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendelsohn C, Ruberte E, LeMeur M, Morriss-Kay G, Chambon P: Developmental analysis of the retinoic acid-inducible RAR-beta 2 promoter in transgenic animals. Development 113: 723–734, 1991 [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi A, Hirakawa H, Hirata T, Nishino K, Yamaguchi A: Growth phase-dependent expression of drug exporters in Escherichia coli and its contribution to drug tolerance. J Bacteriol 188: 5693–5703, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Tripathi P, Guo Q, Coussens M, Ma L, Chen F: Cre/lox recombination in the lower urinary tract. Genesis 47: 409–413, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Echelard Y, Vassileva G, McMahon AP: Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development 120: 2213–2224, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Itäranta P, Viiri K, Kaartinen V, Vainio S: Lumbo-sacral neural crest derivatives fate mapped with the aid of Wnt-1 promoter integrate but are not essential to kidney development. Differentiation 77: 199–208, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL: Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development 132: 529–539, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Zhao H, Kegg H, Grady S, Truong H-T, Robinson ML, Baum M, et al.: Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol 276: 403–415, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng Y, Cheung M, Abu-Elmagd MM, Orme A, Scotting PJ: Chick sox10, a transcription factor expressed in both early neural crest cells and central nervous system. Brain Res Dev Brain Res 121: 233–241, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Brewer S, Jiang X, Donaldson S, Williams T, Sucov HM: Requirement for AP-2alpha in cardiac outflow tract morphogenesis. Mech Dev 110: 139–149, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Ohta K, Mizutani A, Kawakami A, Murakami Y, Kasuya Y, Takagi S, et al.: Plexin: A novel neuronal cell surface molecule that mediates cell adhesion via a homophilic binding mechanism in the presence of calcium ions. Neuron 14: 1189–1199, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL: Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 424: 398–405, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Xu C, Fan C-M: Allocation of paraventricular and supraoptic neurons requires Sim1 function: A role for a Sim1 downstream gene PlexinC1. Mol Endocrinol 21: 1234–1245, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Pasterkamp RJ, Kolk SM, Hellemons AJCGM, Kolodkin AL: Expression patterns of semaphorin7A and plexinC1 during rat neural development suggest roles in axon guidance and neuronal migration. BMC Dev Biol 7: 98, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, et al.: Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature 376: 737–745, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Zong X, Eckert C, Yuan H, Wahl-Schott C, Abicht H, Fang L, et al.: A novel mechanism of modulation of hyperpolarization-activated cyclic nucleotide-gated channels by Src kinase. J Biol Chem 280: 34224–34232, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Li C-H, Zhang Q, Teng B, Mustafa SJ, Huang J-Y, Yu H-G: Src tyrosine kinase alters gating of hyperpolarization-activated HCN4 pacemaker channel through Tyr531. Am J Physiol Cell Physiol 294: C355–C362, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canobbio I, Cipolla L, Guidetti GF, Manganaro D, Visconte C, Kim S, et al.: The focal adhesion kinase Pyk2 links Ca2+ signalling to Src family kinase activation and protein tyrosine phosphorylation in thrombin-stimulated platelets. Biochem J 469: 199–210, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Zhao M, Finlay D, Zharkikh I, Vuori K: Novel role of Src in priming Pyk2 phosphorylation. PLoS One 11: e0149231, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukushima R, Kanamori S, Hirashiba M, Hishikawa A, Muranaka R-I, Kaneto M, et al.: Teratogenicity study of the dihydroorotate-dehydrogenase inhibitor and protein tyrosine kinase inhibitor Leflunomide in mice. Reprod Toxicol 24: 310–316, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Steeghs N, Nortier JWR, Gelderblom H: Small molecule tyrosine kinase inhibitors in the treatment of solid tumors: An update of recent developments. Ann Surg Oncol 14: 942–953, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Walker DP, Zawistoski MP, McGlynn MA, Li J-C, Kung DW, Bonnette PC, et al.: Sulfoximine-substituted trifluoromethylpyrimidine analogs as inhibitors of proline-rich tyrosine kinase 2 (PYK2) show reduced hERG activity. Bioorg Med Chem Lett 19: 3253–3258, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Pytel D, Sliwinski T, Poplawski T, Ferriola D, Majsterek I: Tyrosine kinase blockers: New hope for successful cancer therapy. Anticancer Agents Med Chem 9: 66–76, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Chambers CD, Johnson DL, Robinson LK, Braddock SR, Xu R, Lopez-Jimenez J, et al.; Organization of Teratology Information Specialists Collaborative Research Group : Birth outcomes in women who have taken leflunomide during pregnancy. Arthritis Rheum 62: 1494–1503, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiese CB, Deal KK, Ireland SJ, Cantrell VA, Southard-Smith EM: Migration pathways of sacral neural crest during development of lower urogenital tract innervation. Dev Biol, 2017. Available at: http://www.sciencedirect.com/science/article/pii/S0012160616308259. Accessed on April 28, 2017 [DOI] [PMC free article] [PubMed]
- 59.Altmann CR, Brivanlou AH: Neural patterning in the vertebrate embryo. Int Rev Cytol 203: 447–482, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Le Douarin NM, Dupin E: Multipotentiality of the neural crest. Curr Opin Genet Dev 13: 529–536, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Lecoin L, Gabella G, Le Douarin N: Origin of the c-kit-positive interstitial cells in the avian bowel. Development 122: 725–733, 1996 [DOI] [PubMed] [Google Scholar]
- 62.Torihashi S, Ward SM, Sanders KM: Development of c-Kit-positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology 112: 144–155, 1997 [DOI] [PubMed] [Google Scholar]
- 63.Young HM: Embryological origin of interstitial cells of Cajal. Microsc Res Tech 47: 303–308, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Stieber J, Herrmann S, Feil S, Löster J, Feil R, Biel M, et al.: The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci U S A 100: 15235–15240, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liang X, Zhang Q, Cattaneo P, Zhuang S, Gong X, Spann NJ, et al.: Transcription factor ISL1 is essential for pacemaker development and function. J Clin Invest 125: 3256–3268, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sidebotham EL, Woodward MN, Kenny SE, Lloyd DA, Vaillant CR, Edgar DH: Assessment of protein gene product 9.5 as a marker of neural crest-derived precursor cells in the developing enteric nervous system. Pediatr Surg Int 17: 304–307, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Iqbal J, Tonta MA, Mitsui R, Li Q, Kett M, Li J, et al.: Potassium and ANO1/ TMEM16A chloride channel profiles distinguish atypical and typical smooth muscle cells from interstitial cells in the mouse renal pelvis. Br J Pharmacol 165: 2389–2408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen MJ, Higashi R, Ohta K, Nakamura K-I, Hashitani H, Lang RJ: Autonomic and sensory nerve modulation of peristalsis in the upper urinary tract. Auton Neurosci 200: 1–10, 2016 [DOI] [PubMed] [Google Scholar]
- 69.Washington Smoak I, Byrd NA, Abu-Issa R, Goddeeris MM, Anderson R, Morris J, et al.: Sonic hedgehog is required for cardiac outflow tract and neural crest cell development. Dev Biol 283: 357–372, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Quarles RH: Myelin-associated glycoprotein (MAG): Past, present and beyond. J Neurochem 100: 1431–1448, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Oinuma I, Ito Y, Katoh H, Negishi M: Semaphorin 4D/Plexin-B1 stimulates PTEN activity through R-Ras GTPase-activating protein activity, inducing growth cone collapse in hippocampal neurons. J Biol Chem 285: 28200–28209, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van de Velde HJ, Roebroek AJ, Senden NH, Ramaekers FC, Van de Ven WJ: NSP-encoded reticulons, neuroendocrine proteins of a novel gene family associated with membranes of the endoplasmic reticulum. J Cell Sci 107: 2403–2416, 1994 [DOI] [PubMed] [Google Scholar]
- 73.Senden NH, Timmer ED, Boers JE, van de Velde HJ, Roebroek AJ, Van de Ven WJ, et al.: Neuroendocrine-specific protein C (NSP-C): Subcellular localization and differential expression in relation to NSP-A. Eur J Cell Biol 69: 197–213, 1996 [PubMed] [Google Scholar]
- 74.Steiner P, Kulangara K, Sarria JCF, Glauser L, Regazzi R, Hirling H: Reticulon 1-C/neuroendocrine-specific protein-C interacts with SNARE proteins. J Neurochem 89: 569–580, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Thijssen VLJL, Ausma J, Gorza L, van der Velden HMW, Allessie MA, Van Gelder IC, et al.: Troponin I isoform expression in human and experimental atrial fibrillation. Circulation 110: 770–775, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Capalbo G, Mueller-Kuller T, Koschmieder S, Klein H-U, Ottmann OG, Hoelzer D, et al.: Endoplasmic reticulum protein GliPR1 regulates G protein signaling and the cell cycle and is overexpressed in AML. Oncol Rep 30: 2254–2262, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Satoh H: Elevation of intracellular Ca2+ concentration by protein kinase C stimulation in isolated single rabbit sino-atrial node cells. Gen Pharmacol 25: 325–332, 1994 [DOI] [PubMed] [Google Scholar]
- 78.Kwan YW, Qi AD: Inhibition by extracellular ATP of L-type calcium channel currents in guinea-pig single sinoatrial nodal cells: Involvement of protein kinase C. Can J Cardiol 13: 1202–1211, 1997 [PubMed] [Google Scholar]
- 79.Southwell BR: Localization of protein kinase C theta immunoreactivity to interstitial cells of Cajal in guinea-pig gastrointestinal tract. Neurogastroenterol Motil 15: 139–147, 2003 [DOI] [PubMed] [Google Scholar]
- 80.Poole DP, Van Nguyen T, Kawai M, Furness JB: Protein kinases expressed by interstitial cells of Cajal. Histochem Cell Biol 121: 21–30, 2004 [DOI] [PubMed] [Google Scholar]
- 81.Furness JB, Hind AJ, Ngui K, Robbins HL, Clerc N, Merrot T, et al.: The distribution of PKC isoforms in enteric neurons, muscle and interstitial cells of the human intestine. Histochem Cell Biol 126: 537–548, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Silay MS, Undre S, Nambiar AK, Dogan HS, Kocvara R, Nijman RJM, et al.: Role of antibiotic prophylaxis in antenatal hydronephrosis: A systematic review from the European Association of Urology/European Society for Paediatric Urology Guidelines Panel. J Pediatr Urol 13: 306–315, 2017 [DOI] [PubMed] [Google Scholar]
- 83.Nicolaides KH, Cheng HH, Abbas A, Snijders RJM, Gosden C: Fetal renal defects: Associated malformations and chromosomal defects. Fetal Diagn Ther 7: 1–11, 1992 [DOI] [PubMed] [Google Scholar]
- 84.Caruana G, Wong MN, Walker A, Heloury Y, Webb N, Johnstone L, et al.: Copy-number variation associated with congenital anomalies of the kidney and urinary tract. Pediatr Nephrol 30: 487–495, 2015 [DOI] [PubMed] [Google Scholar]
- 85.Caubit X, Lye CM, Martin E, Coré N, Long DA, Vola C, et al.: Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development 135: 3301–3310, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.