Abstract
Urinary concentrating ability is central to mammalian water balance and depends on a medullary osmotic gradient generated by a countercurrent multiplication mechanism. Medullary hyperosmolarity is protected from washout by countercurrent exchange and efficient removal of interstitial fluid resorbed from the loop of Henle and collecting ducts. In most tissues, lymphatic vessels drain excess interstitial fluid back to the venous circulation. However, the renal medulla is devoid of classic lymphatics. Studies have suggested that the fenestrated ascending vasa recta (AVRs) drain the interstitial fluid in this location, but this function has not been conclusively shown. We report that late gestational deletion of the angiopoietin receptor endothelial tyrosine kinase 2 (Tie2) or both angiopoietin-1 and angiopoietin-2 prevents AVR formation in mice. The absence of AVR associated with rapid accumulation of fluid and cysts in the medullary interstitium, loss of medullary vascular bundles, and decreased urine concentrating ability. In transgenic reporter mice with normal angiopoietin-Tie2 signaling, medullary AVR exhibited an unusual hybrid endothelial phenotype, expressing lymphatic markers (prospero homeobox protein 1 and vascular endothelial growth factor receptor 3) as well as blood endothelial markers (CD34, endomucin, platelet endothelial cell adhesion molecule 1, and plasmalemmal vesicle–associated protein). Taken together, our data redefine the AVRs as Tie2 signaling–dependent specialized hybrid vessels and provide genetic evidence of the critical role of AVR in the countercurrent exchange mechanism and the structural integrity of the renal medulla.
Keywords: angiopoietin, Tie2, ascending vasa recta, lymphatic, countercurrent exchange, fluid homeostasis
Renal blood flow accounts for approximately 20% of total cardiac output in humans, but the kidneys constitute <1% of body mass. Not surprisingly, the renal vasculature is one of the most complex among highly vascularized organs, and it supports the many activities of the kidneys that include plasma filtration, maintenance of fluid, electrolyte and acid-base balance, and endocrine functions.1,2 The various vascular beds of the kidney differ in expression of endothelial markers, presence or absence of fenestrations, variable mural cell coverage, and architectural organization.3 In particular, the renal medullary vasculature consists of fenestrated ascending vasa recta (AVR) and capillary plexus along with the nonfenestrated descending vasa recta (DVR).4 This vasculature plays a major role in maintaining hyperosmolarity of the medullary interstitium to facilitate fluid resorption and promote fluid recycling and homeostasis. The proximity of the AVR and DVR is believed to be central to the countercurrent exchange mechanism, because it enables transport of solute and water while preventing washout of the osmotic gradient in the inner medullary interstitium, which is needed for maximal urine concentration ability.2,5,6 To date, studies with different transgenic mouse lines with knockout of various transporters and channels have confirmed the importance of the nephrons and DVR for urinary concentrating capacity.7 However, no such results have been reported for the AVR.
Despite the critical roles of renal vasculature, very little is known about how most renal vascular beds develop and differentiate. This major knowledge gap must be filled to permit development of targeted vascular therapies and accelerate development of vascularized organoids and bioengineered kidneys. To date, genetic knockout studies in mice have led to the identification of cytokines and growth factors, which are relevant to renal vascular development, including the angiopoietins.8 The angiopoietin ligands Angpt1 and Angpt2 and their cognate receptor tyrosine kinase endothelial tyrosine kinase 2 (Tie2)/Tek are essential for the development and homeostasis of blood and lymphatic vasculature.9,10 Angpt1-mediated activation of Tie2 supports endothelial cell proliferation, survival, cell-cell junction formation, and regulation of mural cell recruitment, thereby promoting blood vessel quiescence.11–13 The specific role of Angpt2 is cell context dependent. In the blood vasculature, Angpt2 can antagonize Tie2 activation, promoting permeability, sprouting, destabilization, and regression of blood vessels.14–19 In contrast, during lymphatic development, Angpt2 serves as the major endogenous Tie2 agonist.20,21 In the renal vasculature, deletion of Angpt1 at embryonic day E10.5 leads to partial defects in the specialized capillary bed of the glomerulus, with dilated capillary loops and disorganization of the glomerular basement membrane observed at E17.5.22 Angpt2 deletion leads to disorganization of the cortical peritubular capillaries.23 In the anterior chamber of the eye, both angiopoietin ligands have been shown to cooperate in activation of Tie2 signaling, which is required for formation of Schlemm canal (SC), an unusual specialized hybrid vessel with both blood vessel and lymphatic-like characteristics.24
Using a variety of newly generated transgenic mouse lines, we show that loss of both Angpt1 and Angpt2 or the receptor Tie2 at embryonic day E16.5 leads to a cystic renal phenotype and urinary concentrating defect, the result of a dramatic loss of AVR. Furthermore, we show that most, if not all, AVRs have a unique molecular phenotype characterized by lymphatic and blood venous molecular markers. These findings show that the AVR represent hybrid vessels with lymphatic-like features that are needed to perform the crucial role of interstitial fluid drainage in the medulla, a region devoid of classic lymphatic vasculature.
Results
Lineage Tracing of Tie2-Expressing Cells
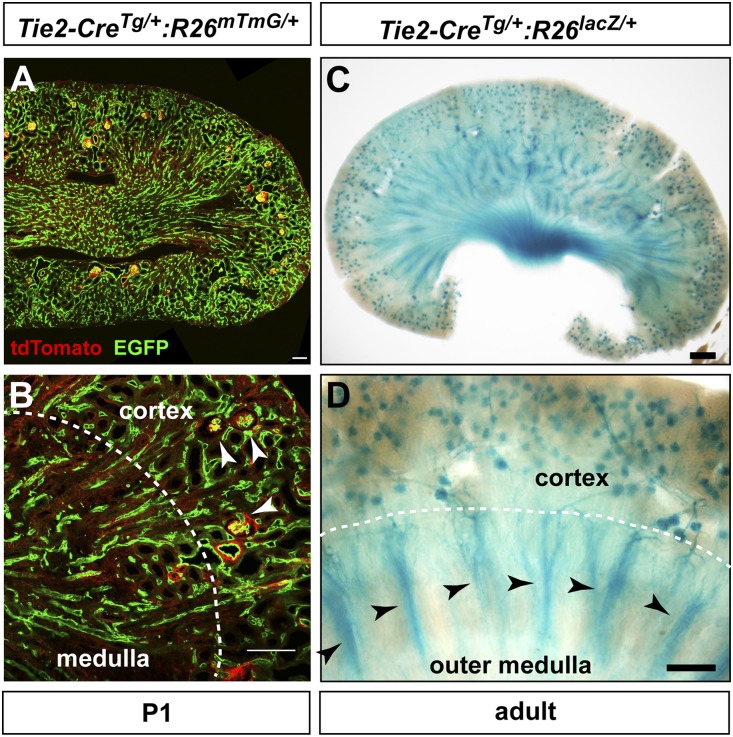
To better understand the importance of angiopoietin-Tie2 signaling in the kidney, we first assessed the renal pattern of Tie2 promoter activity. Without a suitable Tie2 antibody for tissue immunolabeling, we genetically labeled cells derived from the Tie2-expressing lineage by intercrossing Tie2-Cre and reporter transgenic mice (Rosa26mTmG and Rosa26lacZ, which results in enhanced green fluorescent protein (EGFP) and β-galactosidase expression in the presence of Cre activity). At P1, Tie2-CreTg/+:R26mTmG/+ hemizygotes show EGFP expression pattern consistent with the distribution of glomerular and peritubular capillaries (Figure 1, A–C). EGFP colocalizes with most, if not all, Emcn-expressing vessels in both the cortex and the medulla (Supplemental Figure 1). In adult Tie2-CreTg/+:R26lacZ/+ mice, β-galactosidase enzymatic activity reveals a pattern of Tie2-expressing cell derivatives that closely recapitulates the microvascular organization of the kidney, including prominent staining in glomeruli and medullary vascular bundles (Figure 1D). These findings confirm that Tie2-expressing cells give rise to these specialized renal vascular beds.
Figure 1.
Lineage-tracing analysis reveals Tie2 expression pattern in the renal microvasculature. (A) Cells with prior or current Tie2 promoter activity observed as EGFP fluorescence in a P1 animal doubly hemizygous for the transgenes Tie2-Cre and Rosa26mTmG (R26mTmG). (B) Higher magnification pattern of Tie2 promoter activity in a P1 kidney showing EGFP expression in glomeruli (white arrowheads) and peritubular capillaries within the cortex and medulla. (C) LacZ histochemical staining of a vibratome-sectioned adult kidney from a mouse hemizygous for both Tie2-Cre and Rosa26lacZ (R26lacZ) transgenes showing Tie2 promoter activity consistent with the renal vascular network. (D) Cortical and outer medullary lacZ staining pattern in an adult kidney showing prior or current Tie2-Cre activity in glomeruli, cortical peritubular capillaries, medullary capillary plexus, and medullary vascular bundles (black arrowheads). Scale bars, 100 μm.
Late Gestational Deletion of ANGPT-TIE2 Signaling Leads to Renal Cysts
Using a Tet-inducible Cre recombination strategy, we inactivated Angpt1, Angpt2, and Tie2 at late gestation (E16.5) in mice. This overcomes the early lethality of constitutive loss of these genes and allows us to study their relevance in kidney development and function. Homozygous conditional knockout mutants are born within expected Mendelian ratios and live up until adulthood. They are referred to from hereon as follows: Angpt1 knockout (A1ΔE16.5), Angpt2 knockout (A2ΔE16.5), Angpt1/Angpt2 double knockout (A1/A2ΔE16.5), and Tek/Tie2 knockout (Tie2ΔE16.5). Other genotypes were classified as controls and did not manifest overt renal phenotypes. A1/A2ΔE16.5 mutant mice have 92% reduction in Angpt1 and 84.5% reduction in Angpt2 mRNA transcript levels in the kidneys compared with control littermates as determined by quantitative RT-PCR analysis (Supplemental Figure 2, A and B). Tie2 protein levels in the kidneys were diminished 66.8% in Tie2ΔE16.5 mutant mice compared with kidneys from their control cohorts (Supplemental Figure 2, C and D). Adult (8–10 weeks old) A1/A2ΔE16.5 and Tie2ΔE16.5 mutant mice compared with control littermates have 49.5% and 26.9% reduction, respectively, in GFR, an index of diminished renal function (Figure 2, A and B). However, neither A1/A2ΔE16.5 nor Tie2ΔE16.5 mutants show overt proteinuria and have insignificant urinary albumin-to-creatinine ratios relative to controls, despite having markedly reduced GFR (Supplemental Figure 3, A and B). Consistently, A1/A2ΔE16.5 and Tie2ΔE16.5 mutants have normal glomerular histology and ultrastructure (Supplemental Figure 3, C and D).
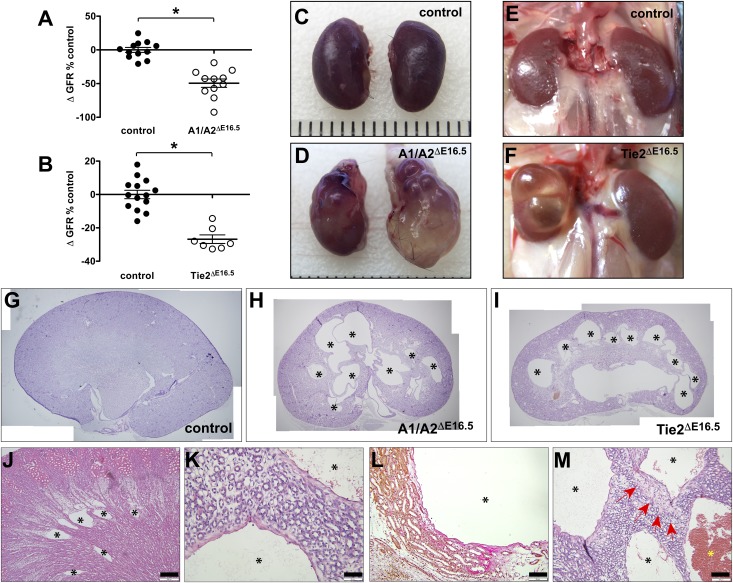
Figure 2.
Renal cysts develop in the absence of Angpt1/2-Tie2 signaling. (A) Reduced GFR in A1/A2ΔE16.5 mutants. (B) Reduced GFR in Tie2ΔE16.5 mutants. Asterisks in A and B denote statistical significance at P<0.05. Gross kidney appearances in adult mice showing renal cysts in (D and F) compound Angpt1/2 (A1/A2ΔE16.5) and Tie2 (Tie2ΔE16.5) knockout mutants in comparison with (C and E) their control littermates, respectively. (G–I) Stitched images of hematoxylin and eosin histology of adult kidneys showing multiple cysts (asterisks) in A1/A2ΔE16.5 and Tie2ΔE16.5 mutants. (J) Medullary microcyst (asterisks) development in a P10 Tie2ΔE16.5 kidney. (K) Higher magnification image showing squamous cells lining renal cysts. (L) Van Gieson staining reveals collagen matrix accumulation around cysts (shown from a representative A1/A2ΔE16.5 kidney). (M) Occasional hemorrhage in renal cysts (yellow asterisk) shown from a representative of hematoxylin and eosin–stained A1/A2ΔE16.5 kidney. Specimen ages: 10 weeks in A, B, and E–I; 20 weeks in C, D, and K–M; and P10 in J. Scale bars, 100 μm in J–M.
Unexpectedly, kidneys dissected from adult A1/A2ΔE16.5 and Tie2ΔE16.5 mutant animals were grossly cystic (Figure 2, C–F). Histologic analysis reveals the presence of multiple macrocysts in 8- to 10-week-old A1/A2ΔE16.5 and Tie2ΔE16.5 mutants (Figure 2, G–I). As early as postnatal age P2, microcysts start to emerge with variable penetrance within the renal outer medulla, although these medullary microcysts become clearly prominent at age P10 in kidneys from A1/A2ΔE16.5 and Tie2ΔE16.5 mutants (Figure 2J). However, in adult mutant mice, huge cysts have distorted the renal parenchyma, making the exact location of the cystic lesions obscure. The cysts are typically lined with flattened cells, which in adult mutants, show increased matrix deposition (Figure 2, K and L). Cellular infiltration, likely from inflammatory macrophages, and erythrocyte-filled cysts are occasionally seen in severely cystic mutant kidneys (Figure 2M). In contrast, singular A1ΔE16.5 or A2ΔE16.5 mutants do not develop renal cysts, indicating that a compound loss of Angpt1 and Angpt2 is necessary to mitigate cyst formation.
Renal Cysts Express Myofibroblast but Not Epithelial or Endothelial Markers
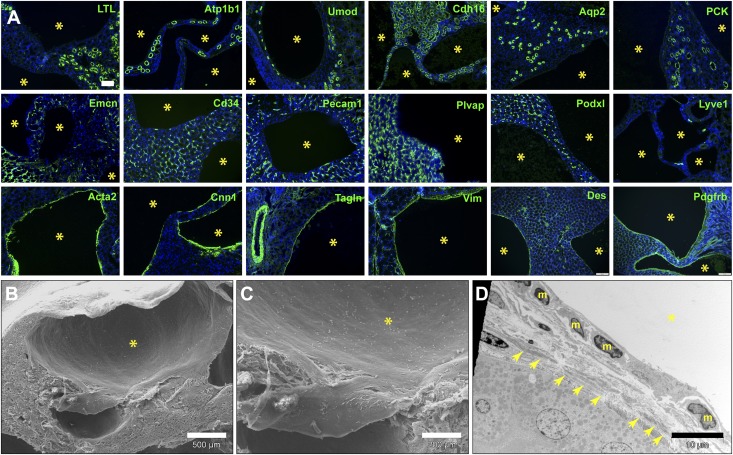
We performed multiple marker analyses through antibody and lectin staining of kidney sections to characterize the renal cysts found in A1/A2ΔE16.5 and Tie2ΔE16.5 mutants (Figure 3A). The cysts lack expression of multiple epithelial tubule markers: Na+/K+-ATPase, Lotus tetraglobulus lectin for proximal tubules, uromodulin for the thick ascending limb of the loop of Henle, Ksp-cadherin for distal tubules, and aquaporin-2 and pancytokeratin for collecting ducts. The cysts also do not express the common blood endothelial cell markers Emcn, Cd34, and Pecam1. Podxl, normally expressed by peritubular capillaries and large caliber renal blood vessels, and Plvap/PV1, which is expressed in diaphragm-containing capillaries, were also negative in the renal cysts. The lymphatic endothelial marker Lyve1 is also absent in the cysts. In contrast, several myofibroblast markers strongly and uniformly stain the cysts, including α-smooth muscle actin (Acta2), calponin-1 (Cnn1), SM22α/transgelin (Tagln), vimentin (Vim), desmin, and PDGFRβ receptor. Microcysts in younger (P2–P5) A1/A2ΔE16.5 and Tie2ΔE16.5 mutant animals also show prominent expression of the same myofibroblast markers but do not show prominent expression of epithelial or endothelial markers.
Figure 3.
Renal cysts arising from loss of Angpt1/2-Tie signaling uniformly express myofibroblast markers. (A) Representative immunofluorescence analysis of renal cysts (yellow asterisks) found adult A1/A2ΔE16.5mutants showing lack of expression of epithelial tubule–specific (L. tetraglobulus lectin [LTL], Na+/K+-ATPase [Atp1b1], uromodulin [Umod], Ksp-cadherin [Cdh16], aquaporin-2 [Aqp2], and pancytokeratin [PCK]) and endothelial (Emcn, Cd34, Pecam1, Plvap, Podxl, and Lyve1) markers but strong expression of myofibroblast markers (Acta2, Cnn1, Tagln, Vim, desmin [Des], and PDGFRβ receptor [Pdgfrb]). (B and C) Scanning electron and (D) transmission electron micrographs of renal cysts (yellow asterisks) in an adult Tie2ΔE16.5 kidney showing the squamous mesenchymal morphology of cells lining cysts (nuclei labeled m). Fibrillar deposits are also visible underneath the cyst linings (arrows). Scale bars, 100 μm in A; 500μm in B; 200 μm in C; 10 μm in D.
Ultrastructural imaging of the renal cysts in Tie2ΔE16.5 mutant animals reveals that the cysts are lined by nonciliated squamous cells (Figure 3, B–D). These flattened cells are relatively smooth and lack luminal villous brush border–like projections. The presence of fibrillar matrix directly underneath these spindle-shaped cyst wall cells is evident in transmission electron micrographs of Tie2ΔE16.5 mutant kidneys (Figure 3D).
Angpt1/2-Tie2 Signaling Affects Patterning of Renal Vasculature
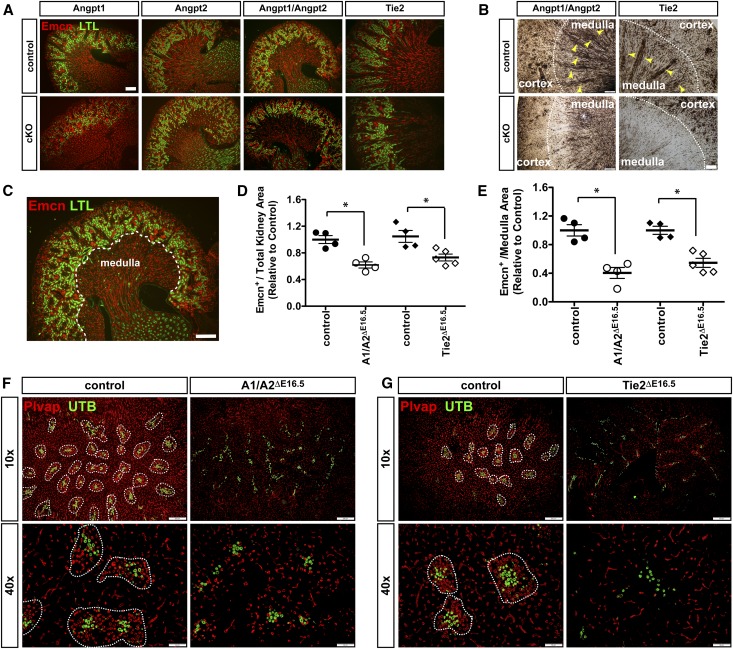
Serial sectioning of postnatal P5 kidneys followed by staining for Emcn and L. tetraglobulus lectin (to demarcate the cortex from the medulla) reveals that Emcn+ve vessels are notably sparse in A1/A2ΔE16.5 and Tie2ΔE16.5 but are not notably sparse in singular mutants A1ΔE16.5 or A2ΔE16.5, particularly within the outer medulla (Figure 4A). A striking paucity of Emcn+ve outer medullary vascular bundles is observed in P14 kidneys from both A1/A2ΔE16.5 and Tie2ΔE16.5 kidneys (Figure 4B). Quantification of Emcn-stained areas relative to total tissue area indicates that overall vascular density is reduced by 38.3% and 26.9% in A1/A2ΔE16.5 and Tie2ΔE16.5 relative to control littermates, respectively (Figure 4, C and D). More pronounced microvascular rarefaction is observed within the medulla, where Emcn+ve-stained areas are reduced by 59.6% and 45.4% in A1/A2ΔE16.5 and Tie2ΔE16.5 mutants, respectively, compared with controls (Figure 4, C and E).
Figure 4.
AVRs are lost and renal vascular density is reduced upon attenuation of Angpt1/2-Tie2 signaling. (A) Sagittal sections of P5 kidneys doubly stained for Emcn and L. tetraglobulus lectin (LTL) showing attenuated vascular density in the absence of both Angpt1 and Angpt2 together and Tie2 but not in singular loss of Angpt1 or Angpt2. (B) Immunohistochemical staining for Emcn in adult kidneys showing persistence of renal vascular density reduction in A1/A2ΔE16.5 and Tie2ΔE16.5 mutants and notable loss of medullary vascular bundles (yellow arrowheads). cKO, homozygous conditional knockout mutant. (C) Representative sagittal section of a P5 kidney indicating the demarcation (white dotted line) of cortical and medullary regions on the basis of LTL-stained proximal tubules for quantification of Emcn staining density. (D) Emcn staining area normalized to total tissue area in fully sectioned kidneys showing reduced total renal vascular densities in A1/A2ΔE16.5 and TieΔE16.5 mutant compared with control littermates. (E) Emcn staining density indicating significant medullary vascular reduction in A1/A2ΔE16.5 and TieΔE16.5 mutants relative to controls. (F) Plvap and UTB coimmunostaining of transverse kidney sections at the level of the outer medulla showing diminished Plvap-stained microvasculature in a representative kidney from an A1/A2ΔE16.5 mutant. AVR clusters but not the DVRs are lost in A1/A2ΔE16.5 mutants. (G) Diminished Plvap-stained microvasculature in the outer medulla in representative kidney from a TieΔE16.5 mutant. Similar to A1/A2ΔE16.5 mutants, TieΔE16.5 mutants have distinctive loss of AVRs but not the DVRs. (F and G) Vascular bundles formed by Plvap+ve AVR and UTB+ve DVR are encircled. Scale bars: 200 μm in A–C, F, upper panel, and G, upper panel; 50 μm in F, lower panel, and G, lower panel. Statistically significant means (P<0.05) are denoted by asterisks in D and E.
We then stained kidney sections with Plvap, an integral protein of fenestral diaphragms found in AVR and interbundle capillary plexus.25 Consistently, as seen after Emcn staining, Plvap+ve vessels are scant in the outer medulla of kidneys from both A1/A2ΔE16.5 and Tie2ΔE16.5 in P14 pups (Figure 4, F and G). Plvap staining reveals that vessel rarefaction occurs within the medullary vascular bundles and interbundle capillary plexus of mutant kidneys. In contrast, the abundance of UTB+ve DVR clusters is comparable between mutants and control kidneys (Figure 4, F and G). Thus, the disappearance of vascular bundles is due to specific loss of the AVR. Angpt1/2-Tie2 signaling is, therefore, essential for the establishment of the AVR and the interbundle capillary plexus.
Urine Concentrating Ability Is Reduced in the Absence of Angpt1/2-Tie2 Signaling
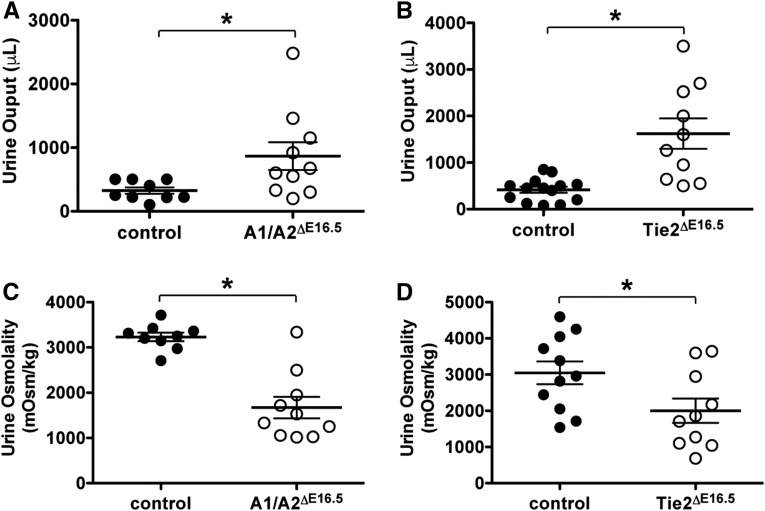
The AVR, DVR, and medullary capillary plexus play important roles in fluid recycling and concentration of urine. The pronounced simplification of the medullary capillary bed due to inactivation of Angpt1/2-Tie2 signaling, therefore, led us to suspect that fluid resorption through medullary vessels could be compromised. Indeed, A1/A2ΔE16.5 and Tie2ΔE16.5 mutant mice kept in metabolic cages have increased urinary outputs (2.7- and 3.9-fold higher compared with controls, respectively) within a 24-hour period (Figure 5, A and B). Additionally, urine obtained from A1/A2ΔE16.5 and Tie2ΔE16.5 mutant mice is significantly more dilute relative to that of controls on the basis of osmolality measurements (approximately 52% and 66% of controls, respectively) (Figure 5, C and D). These findings, therefore, strongly implicate Angpt1/2-Tie2 signaling in indirect regulation of urine concentration and fluid homeostasis.
Figure 5.
A1/A2ΔE16.5 and Tie2ΔE16.5 mutants have urine concentration defects. Total urine collected within a 24-hour period showing increased urine output from (A) A1/A2ΔE16.5 and (B) Tie2ΔE16.5 mutants. Osmolality measurements on urine samples showing dilute urine production from (C) A1/A2ΔE16.5 and (D) Tie2ΔE16.5 mutants. Statistically significant means (P<0.05) are denoted by asterisks.
AVRs Atypically Express Lymphatic Markers Prox1 and VEGFR3 and Depend on Angpt1/2-Tie Signaling for Development
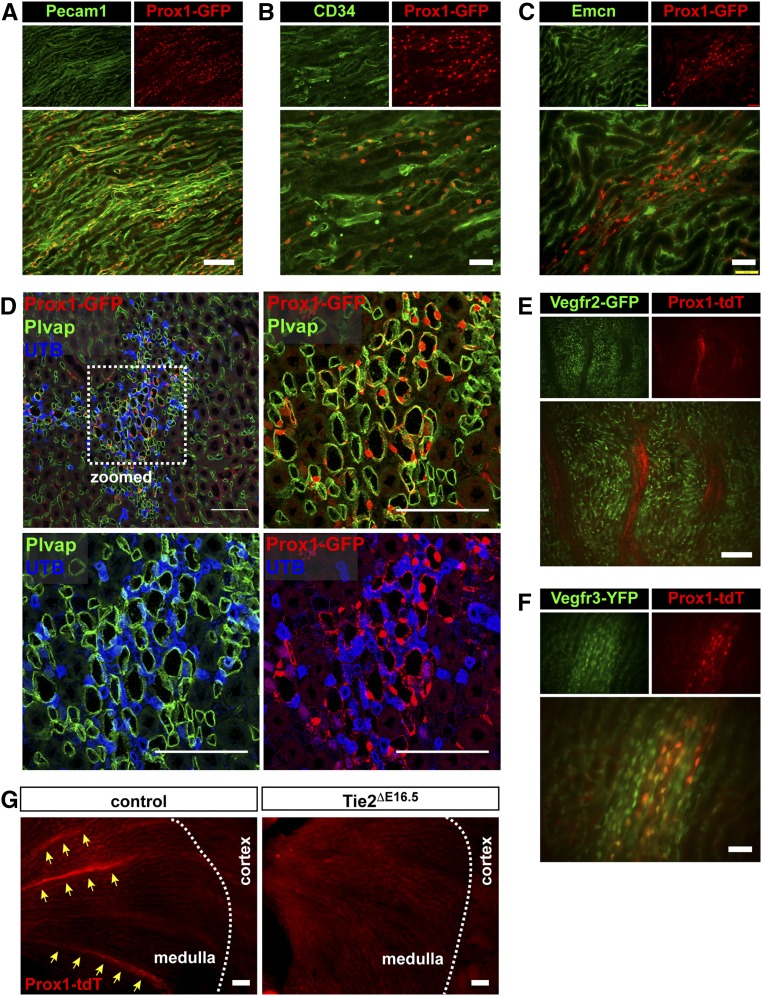
On the basis of previous studies from our laboratory, where we identified a key role of angiopoietin-Tie2 signaling in the formation of lymphatic-like SC in the eye,24 we wondered if any of the vessels lost in the kidney also exhibited lymphatic or hybrid endothelial characteristics. We incorporated Prox1 fluorescent (EGFP or tdTomato) transgenes into our Tie2 conditional genetic crosses to assess potential restructuring of Prox1+ve lymphatic or hybrid vessels. Similar to lymphatic vessels and the SC,26–29 we found that the AVRs were genetically labeled with Prox1. Prox1 reporter activity was found coexpressed with Pecam1, CD34, and Emcn in vascular bundles in the outer medulla of control animals (Figure 6, A–C). More importantly, these bundled vessels expressing both PV1 and Prox1 promoter activity are distinct from nearby Prox−ve/PV1−ve/UTB+ve DVR (Figure 6D). These Prox+ve vessels, therefore, are identical to the AVRs. Unlike the capillary plexus, VEGFR2 expression is absent in these Prox1+ve AVR vessels (Figure 6E). Instead, expression of promoter activities for Prox1 and VEGFR3 (markers strongly expressed in lymphatic vessels28) colocalizes in the AVR (Figure 6F). However, the AVRs lack expression of other canonical lymphatic markers, such Lyve1 and podoplanin (Pdpn)30,31 (Supplemental Figure 4). Interestingly, these Prox+ve medullary vascular bundles are dramatically lost in kidneys from Tie2ΔE16.5 mutants (Figure 6G). In contrast, Lyve1+ve/Pdpn+ve arcuate lymphatic vessels remain present in kidneys from A1/A2ΔE16.5 and Tie2ΔE16.5 mutants. Altogether, these findings indicate that the AVRs are Tie2-dependent hybrid vessels with intermediate properties of blood and lymphatic endothelial cells.
Figure 6.
The AVR bundles lost on inactivation of Angpt1/2 signaling express Prox1 and Vegfr3. In Prox1-GFP reporter mice, vessels within medullary vascular bundles coexpress GFP with (A) Pecam1, (B) CD34, (C) Emcn, and (D) Plvap but not UTB. (E) TdTomato and GFP expressions in Prox1-tdTomato (Prox1-tdT)/Vegfr2-GFP double-reporter mice do not overlap. Prox1-tdT+ve medullary vessels do not express GFP. (F) In Prox1-tdT/Vegfr3-YFP double-reporter mice, YFP expression overlaps with tdTomato in medullary vascular bundles. (G) Prox1+ve AVR vessels are lost in kidneys from Tie2ΔE16.5 mutants. Scale bars: 100μm in A, D, F, and G; 50 μm in B, C, and E.
Angpt1/2-Tie Signaling Regulates the Patterning of the Renal Interstitium
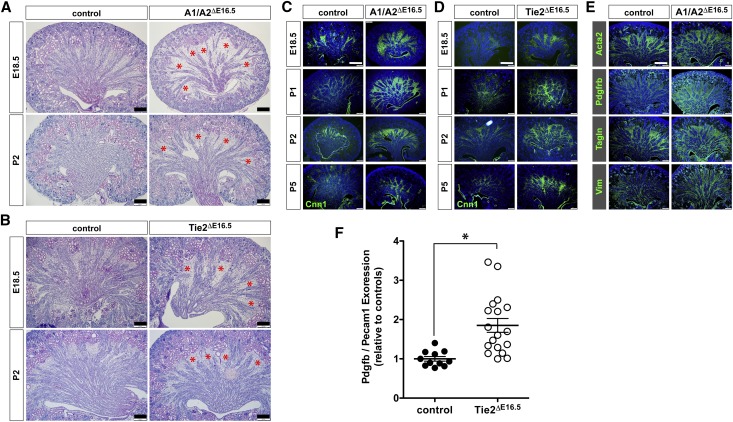
Histologic examination of late embryonic kidneys (E18.5) and postnatal P2 kidneys reveals a notable expansion of the medullary interstitium concomitant with the thinning of vascular arrays in A1/A2ΔE16.5 and Tie2ΔE16.5 mutants (Figure 7, A and B). We, therefore, inferred that loss of vascular bundles could have resulted in altered patterning of perivascular support cells and the surrounding interstitium. We first analyzed the renal expression of the mesenchymal marker Cnn1 during late gestation (E18.5) and perinatally (P1, P2, and P5). We found that Cnn1 has markedly broader expression in all four time points examined in both A1/A2ΔE16.5 and Tie2ΔE16.5 mutants consistent with the histologic findings (Figure 7, C and D). Similar elevated and widened expression patterns are seen with other myofibroblast markers that we found expressed in renal cysts, such as Acta2, Pdfgfrb, Tagln, and Vim (Figure 7E). In kidneys of P1 pups, transcripts for Pdgfb were elevated 85% relative to controls (Figure 7F). Thus, loss of Angpt1/2-Tie2 signaling upregulates the expression of PDGFB, a growth factor that likely promotes the hyperproliferation of PDGFRβ-expressing medullary interstitial or perivascular cells. Intriguingly, the same myofibroblast markers are also the ones that we found expressed by the squamous cells lining the cysts of kidneys from A1/A2ΔE16.5 and Tie2ΔE16.5 mutants. In adult control animals, the expressions of the myofibroblast markers Acta2, Cnn1, and Tagln are restricted to smooth muscles covering large caliber blood vessels, whereas Vim expression in addition is also found strongly expressed in podocytes (Supplemental Figure 5).
Figure 7.
A1/A2ΔE16.5 and Tie2ΔE16.5 mutants have abnormal expansion of the renal interstitium. (A and B) Periodic acid–Schiff histology of E18.5 and P2 kidney sections showing similar expansion of medullary interstitium (red asterisks) in A1/A2ΔE16.5 and Tie2ΔE16.5 mutants. (C and D) Immunofluorescence staining for the interstitial marker Cnn1 showing expansion of the interstitium at E18.5, P1, P2, and P5 in A1/A2ΔE16.5 and Tie2ΔE16.5 mutants. (E) Immunofluorescence staining for other myofibroblast markers (Acta2, PDGFRβ receptor [Pdgfrb], Tagln, and Vim) notably expressed in the medullary interstitium as seen in P1 kidneys showing increased expression of these markers in the kidneys of A1/A2ΔE16.5 mutants. (F) Renal expression of Pdgfb is increased in the absence of Tie2 signaling on the basis of quantitative RT-PCR analysis. Pdgfb relative to Pecam1 gene expression as normalized to controls from P1 kidneys. Statistically significant means (P<0.05) are denoted by an asterisk. Scale bars: 200 μm in A and B; 400 μm in C–E.
Discussion
Excess interstitial fluid is generally removed by the lymphatic system and recirculated back into the systemic blood vasculature. In the kidney, definitive lymphatic vessels surround hilar renal arteries and cortical interlobular and arcuate arteries, and they are found within the renal capsule.32,33 Paradoxically, conventional lymphatic vessels are absent in the renal medulla,32–34 although it has a great need for clearance of interstitial fluid. Instead of these vessels, the renal medulla is thought to rely on fenestrated AVRs and their connection to the arcuate veins for efficient removal of medullary interstitial fluid and preservation of the crucial medullary osmotic gradient.3,6,35–37 The specialized features of the AVR, such as abundant fenestrations, lack of mural cell coverage, and strategic organization into vascular bundles in the outer medulla, are believed to contribute to their high hydraulic conductance.4,38–41 However, the molecular basis of development and differentiation of the AVR have not been reported previously. Furthermore, formal genetic proof has not been provided to show their role in renal medullary fluid removal.
This study shows that angiopoietin-Tie2 signaling is vital for the normal development of medullary microcirculation and in particular, the AVR. When genetically attenuated, angiopoietin-Tie2 signaling results in the absence of vascular bundles of the outer medulla and the rarefaction of surrounding capillary plexus. Although lineage tracing studies revealed that Tie2-expressing cells give rise to the majority, if not all, of the vessels within the medullary vascular bundles, the AVRs but not the DVRs seem to be absent when Tie2 signaling is lost at E16.5. This finding suggests a time-sensitive requirement for Angpt-Tie2 signaling in AVR formation.
Using different transgenic fluorescent reporter mice, we identified expression in the AVR of the transcription factor Prox1, a master regulator of lymphatic cell fate.26,27 Similarly, characterization of reporter mice indicated that the AVRs also coexpress VEGFR3, a tyrosine kinase receptor required for lymphangiogenesis.26,42,43 In addition to the expression of both Prox1 and VEGFR3, markers normally ascribed to lymphatic vessels,28 the AVRs also express blood endothelial–specific markers (Emcn, CD34, Pecam1, and Plvap). Conversely, the AVRs do not express other canonical lymphatic markers, such as Lyve1 and Pdpn.30,31 Notably, VEGFR2, the expression of which is typical of many blood vessels, including peritubular renal capillaries,44 is undetectable in the AVR. Thus, the AVRs seem to represent unusual hybrid vessels that carry molecular features of both lymphatic and blood vascular endothelial cells. The expression of Prox1 and VEGFR3 in the AVR together with their abundant fenestration help explain the relatively high hydraulic conductivity of the AVR and their role in the reuptake of large volumes of interstitial fluid4,38,39,41—features typically found in lymphatics. Prox1 is known to regulate Vegfr3 expression positively,45 whereas VEGFR3 receptor activity has been implicated in vascular remodeling and widening of vessel diameters to accommodate increased fluid flow.46,47 The activation of VEGFR3 by fluid shear stress has been postulated to contribute to the regulation of vessel caliber.48 Indeed, the AVRs are approximately 60% wider in diameter than the DVRs.36,40,41
The hybrid phenotypic and molecular characteristics of the AVR closely resemble those found in SC, a vessel that drains back into the venous system the aqueous humor produced in the anterior chamber of the eye. Recently, the SC has been described as a hybrid vessel expressing Prox1, VEGFR3, CD34, Emcn, and Plvap but not Lyve1 or Pdpn.29,49 Intriguingly, the development of the AVR and the SC depends on cooperative agonist functions of Angpt1 and Angpt2 on Tie2 activation.11,14,21,24 In contrast, with blood vascular development, Angpt1-dependent Tie2 activation is antagonized by Angpt2.11,14,21,24 The context-dependent requirement for Angpt1 and Angpt2 in AVR development, therefore, is consistent with its hybrid phenotype.
As predicted, mice that lack AVRs exhibited reduced urinary concentrating capacity. Unexpectedly, however, the mice also developed a dramatic cystic phenotype. Analysis of multiple markers indicated that the cysts are not derived from vascular or epithelial cells. Instead, the cysts are lined by squamous mesenchymal PDGFRβ+ve cells that uniformly express multiple myofibroblast markers (Cnn1, Tagln, Acta2, and Vim). Interestingly, inactivation of angiopoietin-Tie2 signaling results in expansion of PDGFRβ+ve and myofibroblast-like mesenchyme in the renal medulla. One plausible explanation for the increase in PDGFRβ+ve medullary mesenchyme is the upregulation of the Pdgfb gene encoding the PDGFRβ ligand PDGFB, which we observed in Tie2ΔE16.5 mutant animals. Excessive PDGFB levels in conjunction with increased interstitial fluid flow could also facilitate the differentiation of the interstitial mesenchyme into myofibroblasts.50,51 In venous malformations linked to hyperactivation of Tie2, perivascular support is diminished, whereas endothelial expression of Pdgfb is suppressed, a finding that indicates that Tie2 negatively regulates the expression of Pdgfb.52 The cysts progressively enlarge with age, suggesting fluid accumulation. We posit that these cysts represent collections of interstitial fluid entrapped by myofibroblast-like cell derivatives of the medullary interstitium or perivascular cells. The broadening of the medullary interstitium with concomitant impaired circulatory drainage could contribute to altered fluid dynamics in the medulla and subsequent fluid retention. The renal cysts are also reminiscent of the dramatic buphthalmos (bulging of the eyes) that results from development failure of the SC and impaired aqueous humor drainage due to loss-of-function mutations in angiopoietin-Tie2 signaling,24,53 providing additional parallels between function of AVR and SC.
Overall, our data redefine the AVRs as hybrid vessels highly analogous to SC in the eye with molecular features of both lymphatic and blood endothelia. The angiopoietin-Tie2 signaling pathway is essential to the development of these hybrid vessels. Given the susceptibility of the medullary microcirculation to injury in diseases, such as ischemia-reperfusion AKI,54 and the fact that Tie2 signaling can be targeted clinically (e.g., with recombinant angiopoietins or mimetics),55,56 our findings pave the way for the development of novel therapeutic renoprotective strategies and suggest that kidney structure and function should be investigated in patients with glaucoma caused by mutations in the TEK/TIE2 gene.53
Concise Methods
Conditional floxed alleles of murine genes for Angpt1, Angpt2, and Tek/Tie2 were described previously.24 These genes were bred accordingly with the transgenes R26-rtTAand tetO-Cre, allowing for doxycycline-induced whole-body inactivation of the floxed alleles. Doxycyline (0.5% [wt/vol]) was administered in drinking water of time-mated dams from the 16th day of gestation (E16.5) until pups were weaned (P21). Renal Tie2 expression was mapped by breeding Tie2-Cre with R26-LacZ or R26-mTmG reporter mice. Transgenic Prox1-EGFP and Prox1-tdTomato, Vegfr3-YFP, and Vegfr2-GFP reporter mice were used to identify Prox1+ve, Vegfr3+ve, and Vegfr2+ve vessels. Mouse genotyping was done by PCR. Mouse rearing, husbandry, and phenotyping were carried out following ethical procedures approved by the Institutional Animal Care and Use Committee of Northwestern University. Additional methods are included in Supplemental Material.
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to the staff of the Center for Comparative Medicine of Northwestern University for assistance in animal care. We are indebted to Douglas Holmyard (Mount Sinai Hospital, Toronto, Canada) for help with electron microscopy. We are thankful to Dr. Peter S. Aronson (Yale University) for his gift of Ksp-cadherin antibody. We would like to acknowledge Douglas Fambrough (Johns Hopkins University) and Eugene Butcher (Stanford University) for antisera against Na+/K+-ATPase and Plvap, respectively, which were obtained through the Developmental Studies Hybridoma Bank (University of Iowa). We express our gratitude to Thomas Pannebecker (University of Arizona) for helpful suggestions on immunostainings of the vasa recta.
Imaging work was performed at the Northwestern University Center for Advanced Microscopy, and it was generously supported by National Cancer Institute Cancer Center support grant P30 CA060553 (to the Robert H. Lurie Comprehensive Cancer Center). This work was funded with research grants from the National Institutes of Health: grants 1R01GM120592-01 (R.V.S.), R01-DK41707 (to J.D.K. and J.M.S.), 1R01HL124120-01 (to S.E.Q.), R01EY025799 (to S.E.Q.), and 5T32DK108738-02 (to S.E.Q.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Unique Gene Expression in Developing Ascending Vasa Recta: A Tale of Tie,” on pages 1073–1074.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017090962/-/DCSupplemental.
References
- 1.Munger KA, Maddox DA, Brenner BM, Kost CK: The renal circulations and glomerular ultrafiltration. In: Brenner & Rector’s the Kidney, 10th Ed., edited by Skorecki K, Chertow GM, Marsden PA, Taal MW, Yu ASL, Philadelphia, Elsevier, 2016, pp 83–111 [Google Scholar]
- 2.Pallone TL, Chunhua C: Renal cortical and medullary microcirculations: Structure and function. In: Seldin and Giebisch’s the Kidney, 5th Ed., edited by Alpern RJ, Moe OW, Caplan MJ, London, Academic Press, 2013, pp 803–857 [Google Scholar]
- 3.Kriz W, Kaissling B: Structural organization of the mammalian kidney. In: Seldin and Giebisch’s the Kidney, 5th Ed., edited by Alpern RJ, Moe OW, Caplan MJ, Amsterdam, Elsevier, 2013, pp 595–691 [Google Scholar]
- 4.Schwartz MM, Karnovsky MJ, Vehkatachalam MA: Ultrastructural differences between rat inner medullary descending and ascending vasa recta. Lab Invest 35: 161–170, 1976 [PubMed] [Google Scholar]
- 5.Pallone TL, Turner MR, Edwards A, Jamison RL: Countercurrent exchange in the renal medulla. Am J Physiol Regul Integr Comp Physiol 284: R1153–R1175, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Sands JM, Layton HE: Advances in understanding the urine-concentrating mechanism. Annu Rev Physiol 76: 387–409, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Fenton RA, Knepper MA: Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 87: 1083–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Sequeira Lopez ML: The origin and regulation of the renal vasculature. In: Kidney Development, Disease, Repair and Regeneration, edited by Little MH, London, Elsevier, 2016, pp 146–162 [Google Scholar]
- 9.Eklund L, Kangas J, Saharinen P: Angiopoietin-Tie signalling in the cardiovascular and lymphatic systems. Clin Sci (Lond) 131: 87–103, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augustin HG, Koh GY, Thurston G, Alitalo K: Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10: 165–177, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD: Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87: 1171–1180, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N: Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol 10: 513–526, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, Olsen BR, Alitalo K: Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol 10: 527–537, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD: Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277: 55–60, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Ziegler T, Horstkotte J, Schwab C, Pfetsch V, Weinmann K, Dietzel S, Rohwedder I, Hinkel R, Gross L, Lee S, Hu J, Soehnlein O, Franz WM, Sperandio M, Pohl U, Thomas M, Weber C, Augustin HG, Fässler R, Deutsch U, Kupatt C: Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis [published online ahead of print July 1, 2013]. J Clin Invest doi:10.1172/JCI66549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roviezzo F, Tsigkos S, Kotanidou A, Bucci M, Brancaleone V, Cirino G, Papapetropoulos A: Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther 314: 738–744, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, Kutschera S, Bartels S, Appak S, Besemfelder E, Terhardt D, Chavakis E, Wieland T, Klein C, Thomas M, Uemura A, Goerdt S, Augustin HG: Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest 122: 1991–2005, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hakanpaa L, Sipila T, Leppanen VM, Gautam P, Nurmi H, Jacquemet G, Eklund L, Ivaska J, Alitalo K, Saharinen P: Endothelial destabilization by angiopoietin-2 via integrin β1 activation. Nat Commun 6: 5962, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG: The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci 118: 771–780, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD: Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell 3: 411–423, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Dellinger M, Hunter R, Bernas M, Gale N, Yancopoulos G, Erickson R, Witte M: Defective remodeling and maturation of the lymphatic vasculature in angiopoietin-2 deficient mice. Dev Biol 319: 309–320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, Quaggin SE: Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest 121: 2278–2289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitera JE, Woolf AS, Gale NW, Yancopoulos GD, Yuan HT: Dysmorphogenesis of kidney cortical peritubular capillaries in angiopoietin-2-deficient mice. Am J Pathol 165: 1895–1906, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomson BR, Heinen S, Jeansson M, Ghosh AK, Fatima A, Sung HK, Onay T, Chen H, Yamaguchi S, Economides AN, Flenniken A, Gale NW, Hong YK, Fawzi A, Liu X, Kume T, Quaggin SE: A lymphatic defect causes ocular hypertension and glaucoma in mice. J Clin Invest 124: 4320–4324, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan J, Pannabecker TL: Architecture of inner medullary descending and ascending vasa recta: Pathways for countercurrent exchange. Am J Physiol Renal Physiol 299: F265–F272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G: An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 21: 1505–1513, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wigle JT, Oliver G: Prox1 function is required for the development of the murine lymphatic system. Cell 98: 769–778, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Castro EC, Galambos C: Prox-1 and VEGFR3 antibodies are superior to D2-40 in identifying endothelial cells of lymphatic malformations–A proposal of a new immunohistochemical panel to differentiate lymphatic from other vascular malformations. Pediatr Dev Pathol 12: 187–194, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Kizhatil K, Ryan M, Marchant JK, Henrich S, John SW: Schlemm’s canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol 12: e1001912, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG: LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 144: 789–801, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D: Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: Podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 154: 385–394, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell RD, Keyl MJ, Shrader FR, Jones EW, Henry LP: Renal lymphatics: The internal distribution. Nephron 5: 454–463, 1968 [DOI] [PubMed] [Google Scholar]
- 33.Holmes MJ, O’Morchoe PJ, O’Morchoe CC: Morphology of the intrarenal lymphatic system. Capsular and hilar communications. Am J Anat 149: 333–351, 1977 [DOI] [PubMed] [Google Scholar]
- 34.Kriz W, Dieterich HJ: The lymphatic system of the kidney in some mammals. Light and electron microscopic investigations. Z Anat Entwicklungsgesch 131: 111–147, 1970 [PubMed] [Google Scholar]
- 35.Gottschalk CW, Mylle M: Micropuncture study of the mammalian urinary concentrating mechanism: Evidence for the countercurrent hypothesis. 1959. J Am Soc Nephrol 8: 153–164, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Pallone TL, Zhang Z, Rhinehart K: Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol 284: F253–F266, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Sands JM, Layton HE, Fenton RA: Urine concentration and dilution. In: Brenner & Rector’s the Kidney, 10th Ed., edited by Skorecki K, Chertow GM, Marsden PA, Taal MW, Yu ASL, Philadelphia, Elsevier, 2016, pp 258–280 [Google Scholar]
- 38.Holliger C, Lemley KV, Schmitt SL, Thomas FC, Robertson CR, Jamison RL: Direct determination of vasa recta blood flow in the rat renal papilla. Circ Res 53: 401–413, 1983 [DOI] [PubMed] [Google Scholar]
- 39.Zimmerhackl B, Robertson CR, Jamison RL: Fluid uptake in the renal papilla by vasa recta estimated by two methods simultaneously. Am J Physiol 248: F347–F353, 1985 [DOI] [PubMed] [Google Scholar]
- 40.Pallone TL: Resistance of ascending vasa recta to transport of water. Am J Physiol 260: F303–F310, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Pallone TL, Work J, Jamison RL: Resistance of descending vasa recta to the transport of water. Am J Physiol 259: F688–F697, 1990 [DOI] [PubMed] [Google Scholar]
- 42.Iljin K, Karkkainen MJ, Lawrence EC, Kimak MA, Uutela M, Taipale J, Pajusola K, Alhonen L, Halmekytö M, Finegold DN, Ferrell RE, Alitalo K: VEGFR3 gene structure, regulatory region, and sequence polymorphisms. FASEB J 15: 1028–1036, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K: Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA 92: 3566–3570, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kappel A, Rönicke V, Damert A, Flamme I, Risau W, Breier G: Identification of vascular endothelial growth factor (VEGF) receptor-2 (Flk-1) promoter/enhancer sequences sufficient for angioblast and endothelial cell-specific transcription in transgenic mice. Blood 93: 4284–4292, 1999 [PubMed] [Google Scholar]
- 45.Srinivasan RS, Escobedo N, Yang Y, Interiano A, Dillard ME, Finkelstein D, Mukatira S, Gil HJ, Nurmi H, Alitalo K, Oliver G: The Prox1-Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev 28: 2175–2187, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baeyens N, Nicoli S, Coon BG, Ross TD, Van den Dries K, Han J, Lauridsen HM, Mejean CO, Eichmann A, Thomas JL, Humphrey JD, Schwartz MA: Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point [published online ahead of print February 2, 2015]. Elife doi:10.7554/eLife.04645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baeyens N, Schwartz MA: Biomechanics of vascular mechanosensation and remodeling. Mol Biol Cell 27: 7–11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coon BG, Baeyens N, Han J, Budatha M, Ross TD, Fang JS, Yun S, Thomas JL, Schwartz MA: Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J Cell Biol 208: 975–986, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herrnberger L, Ebner K, Junglas B, Tamm ER: The role of plasmalemma vesicle-associated protein (PLVAP) in endothelial cells of Schlemm’s canal and ocular capillaries. Exp Eye Res 105: 27–33, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Boor P, Ostendorf T, Floege J: PDGF and the progression of renal disease. Nephrol Dial Transplant 29[Suppl 1]: i45–i54, 2014 [DOI] [PubMed] [Google Scholar]
- 51.Ng CP, Hinz B, Swartz MA: Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci 118: 4731–4739, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Uebelhoer M, Nätynki M, Kangas J, Mendola A, Nguyen HL, Soblet J, Godfraind C, Boon LM, Eklund L, Limaye N, Vikkula M: Venous malformation-causative TIE2 mutations mediate an AKT-dependent decrease in PDGFB. Hum Mol Genet 22: 3438–3448, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Souma T, Tompson SW, Thomson BR, Siggs OM, Kizhatil K, Yamaguchi S, Feng L, Limviphuvadh V, Whisenhunt KN, Maurer-Stroh S, Yanovitch TL, Kalaydjieva L, Azmanov DN, Finzi S, Mauri L, Javadiyan S, Souzeau E, Zhou T, Hewitt AW, Kloss B, Burdon KP, Mackey DA, Allen KF, Ruddle JB, Lim SH, Rozen S, Tran-Viet KN, Liu X, John S, Wiggs JL, Pasutto F, Craig JE, Jin J, Quaggin SE, Young TL: Angiopoietin receptor TEK mutations underlie primary congenital glaucoma with variable expressivity. J Clin Invest 126: 2575–2587, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, Yasunaga K, Kim KT, Kim I, Choi HH, Kim W, Kim SH, Park SK, Lee GM, Koh GY: COMP-Ang1: A designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci USA 101: 5547–5552, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han S, Lee SJ, Kim KE, Lee HS, Oh N, Park I, Ko E, Oh SJ, Lee YS, Kim D, Lee S, Lee DH, Lee KH, Chae SY, Lee JH, Kim SJ, Kim HC, Kim S, Kim SH, Kim C, Nakaoka Y, He Y, Augustin HG, Hu J, Song PH, Kim YI, Kim P, Kim I, Koh GY: Amelioration of sepsis by TIE2 activation-induced vascular protection. Sci Transl Med 8: 335ra55, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.