Inheriting two apolipoprotein L1 gene (APOL1) renal risk variants accounts for the majority of the excess risk for nondiabetic ESRD in individuals with recent African ancestry.1 APOL1 renal risk variants are common in blacks in the United States, with about 13% carrying two variants (defining the high-risk genotype), whereas 39% have one variant and 48% have no variant. In contrast, APOL1 renal risk variants are virtually absent in nonadmixed European, Asian, and Hispanic populations. Approximately 20% of those with the APOL1 high-risk genotype ultimately develop CKD, supporting the postulate that modifying factors are necessary to trigger development of nephropathy.2
The effect of having two APOL1 renal risk variants extends beyond native kidney disease. Kidney transplants from deceased black donors with the APOL1 high-risk genotype fail more quickly than allografts from donors with zero or one APOL1 renal risk variant.3 The outcomes of kidneys from deceased black donors with zero or one APOL1 renal risk variant approximate those of kidneys from white donors.3,4 Furthermore, serum creatinine concentrations are higher in recipients with functioning kidneys from donors with the APOL1 high-risk genotype, raising concerns that additional allografts will also fail over longer intervals.3,5 The poorer outcome of these allografts is independent of the ethnicity of the recipient, indicating that the effect of the genotype travels with the kidney.6 Nonetheless, such as is the case for native kidney disease, the association of the APOL1 high-risk genotype with a poor outcome is far from universal. Indeed, most allografts from deceased black donors with the APOL1 high-risk genotype function well for prolonged intervals, again suggesting that modifying factors initiate or accentuate renal damage.
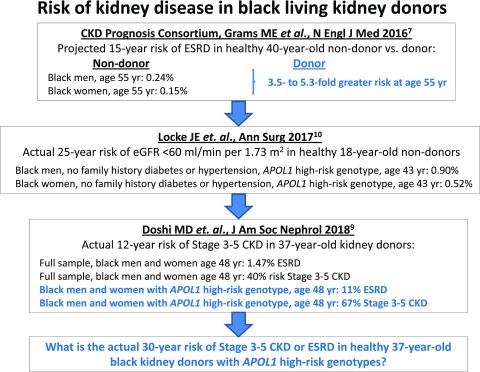
More recently, the kidney transplant community has begun to examine a possible effect of APOL1 renal risk variants on outcomes in living donor transplantation. Living black kidney donors more often develop ESRD than donors from other racial groups, with frequencies about 3.5- to 5.3-fold higher than in age- and sex-matched whites7 (Figure 1). Patient reports have described the loss of kidneys from living black donors with the APOL1 high-risk genotype several years after transplantation due to proteinuric disease, with subsequent development of ESRD in the donor.8 These reports raise the question: does the presence of the APOL1 high-risk genotype adversely affect postdonation renal function in black living kidney donors?
Figure 1.
Higher risk of advanced CKD or ESRD in living kidney donors with the high-risk APOL1 genotype, compared to low risk genotypes.
In this issue of the Journal of the American Society of Nephrology, Doshi et al.9 addressed that question by studying a cohort of 136 black living kidney donors with mean age of 37 years and mean follow-up of 12 years. Nineteen (14%) patients had the APOL1 high-risk genotype, a frequency similar to that in the general black population. They found that the mean eGFR before nephrectomy was significantly lower in APOL1 high-risk donors than in donors with zero or one APOL1 renal risk variant. This difference suggests that deterioration of renal clearance function started before donation, although without clinical manifestations that would preclude acceptance as a kidney donor. Nephrectomy decreased eGFR by 25–30 ml/min per 1.73 m2 in both donor subgroups, but the decline in eGFR after recovery from nephrectomy was more rapid in APOL1 high-risk donors. Of concern, both donors who developed ESRD due to proteinuric kidney disease had an APOL1 high-risk genotype, comprising 11% of this donor subgroup. Furthermore, stage 3 or worse CKD developed in 67% of APOL1 high-risk donors compared with 36% of donors with zero or one APOL1 renal risk variant (Figure 1). A subset of 115 donors was matched with nondonor controls from the Coronary Artery Risk Development in Young Adults (CARDIA) Study for baseline age, sex, systolic BP, family history of ESRD in first degree relatives, APOL1 genotype, and duration of follow-up. The annual decrements in eGFR in nondonors and donors after nephrectomy, grouped by APOL1 genotype, were similar. At the end of the study, severity of microalbuminuria was similar in donors and nondonors but worse in APOL1 high-risk donors versus APOL1 low-risk donors. Prevalence of hypertension at the end of the study did not associate with the APOL1 high-risk genotype. Hypertension developed in nearly one half of donors in both subgroups, was often untreated, and was more common than in nondonors.
These data, although preliminary and from a small sample, raise concerns for the long-term kidney health of black living kidney donors. Although APOL1 high-risk living donors had a follow-up eGFR of 57 ml/min per 1.73 m2, their mean age after 12 years of follow-up was only 48 years. A continued 1.1 ml/min per 1.73 m2 per year (95% confidence interval, 0 to 2.3 ml/min per 1.73 m2 per year) postnephrectomy decline in eGFR will likely culminate in stage 4 or 5 CKD for a sizable number of donors. ESRD is probably more likely in younger donors with their extra postnephrectomy years and greater potential exposure to additional kidney disease risk factors, such as diabetes, obesity, and nephrotoxic agents. An analysis in a larger sample of the CARDIA Study participants examined the risk for CKD in young adults10 (Figure 1). A cohort of 3438 participants (48% black; mean age of 24.8 years) who satisfied criteria for potential kidney donation was used to assess the effect of the APOL1 genotype on kidney function over a 25-year span. The presence of two APOL1 renal risk variants increased the risk of the eGFR decreasing below 60 ml/min per 1.73 m2 by fivefold in blacks compared with their white counterparts and by 2.5- to threefold compared with blacks with no APOL1 renal risk variants. The APOL1 two-renal-risk-variant genotype was the strongest of all 11 risk factors for development of CKD stage 3 or higher. Furthermore, presence of one APOL1 renal risk variant also increased risk of CKD, although to a lesser degree.
Considering APOL1 genotypes may be less critical in older candidates for living donor nephrectomy. Some older individuals with two APOL1 renal risk variants will already manifest kidney disease and will not satisfy screening criteria. Moreover, they will presumably encounter fewer opportunities to develop renal injury or initiate APOL1-associated kidney damage given their shorter life expectancies.
The findings of Doshi et al.9 should heighten our concern about an adverse effect of the APOL1 two-renal-risk-variant genotype on the long-term kidney health of black living kidney donors. Without a uniformly consistent poor outcome, the effect of APOL1 and the biochemical implications remain unclear. Additional studies are needed to clarify this apparent effect. Ideally, these studies will also uncover other factors that modify the risk for CKD/ESRD in some individuals with two APOL1 renal risk variants. The newly launched National Institutes of Health prospective APOL1 Long-Term Kidney Transplantation Outcomes Network (APOLLO) Study is addressing these issues. The APOLLO Study is evaluating allograft survival of kidneys from deceased and living black donors and the long-term renal outcomes of black living donors. In the meantime, black donor candidates should be informed of the association of APOL1 renal risk variants with CKD/ESRD. Although it is not yet possible to quantify the risk in an individual, young two APOL1 renal risk variant donors with a family history of ESRD, hypertension, or other risk factors likely face the highest risk. All donors should be counseled to adopt a healthy lifestyle after nephrectomy, with avoidance of tobacco, control of weight, regular assessments of kidney health, and periodic monitoring of blood glucose and BP (with treatment of hypertension). We must learn from the candidates their perception of benefits and risks of donation for themselves and the recipients. The amount of risk that is acceptable to a donor will vary between individuals and by relationship of the donor to the recipient. We anticipate that future investigations will clarify how an APOL1-associated process that can culminate in a proteinuric kidney disease reduces eGFR in its early stages, often with minimal albuminuria. This new knowledge will hopefully lead to novel approaches to preserve kidney function.
Disclosures
Wake Forest University Health Sciences and B.I.F. have rights to an issued United States patent related to APOL1 genetic testing. B.I.F. is a consultant for Ionis Pharmaceuticals. B.A.J. reports no disclosure relevant to this work.
Acknowledgments
The authors thank Dr. Roslyn Mannon, Dr. Jayme Locke, Dr. Amber Reeves-Daniel, and Dr. Robert Stratta for their critical review of this manuscript.
Grant support was received from National Institutes of Health grants R01 DK084149 (to B.I.F.), R01 DK070941 (to B.I.F.), R01009055 (to B.I.F. and B.A.J.), U01 DK116041 (to B.I.F.), and U01 DK115997 (to B.A.J.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “APOL1 Genotype and Renal Function of Black Living Donors,” on pages 1309–1316.
References
- 1.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. : Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman BI, Skorecki K: Gene-gene and gene-environment interactions in apolipoprotein L1 gene-associated nephropathy. Clin J Am Soc Nephrol 9: 2006–2013, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman BI, Pastan SO, Israni AK, Schladt D, Julian BA, Gautreaux MD, et al. : APOL1 genotype and kidney transplantation outcomes From deceased African American donors. Transplantation 100: 194–202, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, et al. : The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant 11: 1025–1030, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman BI, Locke JE, Reeves-Daniel AM, Julian BA: Apolipoprotein L1 gene effects on kidney transplantation. Semin Nephrol 37: 530–537, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee BT, Kumar V, Williams TA, Abdi R, Bernhardy A, Dyer C, et al. : The APOL1 genotype of African American kidney transplant recipients does not impact 5-year allograft survival. Am J Transplant 12: 1924–1928, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grams ME, Sang Y, Levey AS, Matsushita K, Ballew S, Chang AR, et al. ; Chronic Kidney Disease Prognosis Consortium: Kidney-failure risk projection for the living kidney-donor candidate. N Engl J Med 374: 411–421, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kofman T, Audard V, Narjoz C, Gribouval O, Matignon M, Leibler C, et al. : APOL1 polymorphisms and development of CKD in an identical twin donor and recipient pair. Am J Kidney Dis 63: 816–819, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Doshi MD, Ortigosa-Goggins M, Garg AX, Li L, Poggio ED, Winkler CA, et al. : APOL1 genotype and renal function of black living donors. J Am Soc Nephrol 29: 1309–1316, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locke JE, Sawinski D, Reed RD, Shelton B, MacLennan PA, Kumar V, et al. : Apolipoprotein L1 and chronic kidney disease risk in young potential living kidney donors [published online ahead of print February 9, 2017]. Ann Surg doi:10.1097/SLA.0000000000002174 [DOI] [PMC free article] [PubMed] [Google Scholar]