The network of capillaries of the glomerular tuft is intimately related to the folding pattern of the glomerular basement membrane (GBM). The peripheral outpocketings of the GBM form a continuous channel system that is open to the mesangium and contains the capillaries. The mechanical stability of this system is largely maintained by the mesangial cells (MCs), which insert alongside the paramesangial aspect of the GBM, most prominently at the turning points of the GBM. Centripetal contraction of MCs generates a constant contractile tone counteracting the centrifugally directed expansile forces resulting from capillary BP.
The structure of the MC-GBM connections was described in 1987,1 but the molecular basis of their elaboration is still insufficiently understood. Kikkawa et al.2 in 2003 described connections of the laminin α5-chain of the GBM to the Lutheran adhesion molecule together with α3β1-integrins in MCs. In this issue of the Journal of the American Society of Nephrology, Zimmerman et al.3 have described a new adhesion complex between MCs and the GBM that is especially prominent at the turning points of the GBM. It consists of nephronectin deposited by podocytes into the GBM and α8β1-integrins enriched in the tips of MC processes inserting to the GBM. These are important findings that will hopefully stimulate research on the relevance of these connections during long-term rearrangements of tuft architecture and in glomerular pathology.
The luminal width of glomerular capillaries is not constant but is subject to long-term changes, likely over weeks4,5; small capillaries increase in width, and large ones decrease. These changes are embedded in rearrangements of tuft architecture, which have so far been poorly studied. Glomerular capillaries consist only of an endothelial tube; there is no complete circumferential basement membrane, and there are no circumferential cells that could, by constriction or relaxation, change the capillary caliper. The width of glomerular capillaries is determined by the shape of the channel-like niches of the GBM. Thus, the only way to vary the width of capillaries consists of changing the shape of these GBM channels.
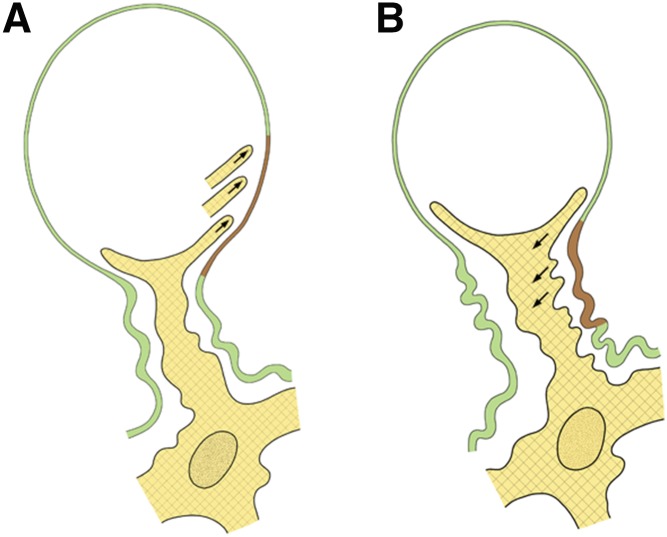
The basic mechanism for changing capillary dimensions seems to consist of changing the extent of the MC-GBM connections, either increasing or decreasing their extent along the inner aspect of the GBM6,7 (Figure 1). Thus, in the case of capillary widening, MC-GBM connections have to be released, and in the case of capillary narrowing, new MC-GBM connections have to be inserted. Thereby, only the width of the GBM channel changes, and the corresponding changes in the capillary lumen will follow and seem to occur by adding or removing endothelial cytoplasmic elements (W. Kriz, unpublished observations).
Figure 1.
Mechanisms of changing the luminal width of glomerular capillaries shown in schematics. (A) Decreasing the capillary lumen (from A to B). Mesangial cell processes extend into the space between the endothelium and the glomerular basement membrane (GBM; arrows) and establish contacts to more peripheral sites of the GBM. By contraction, peripheral parts of the GBM (shown in brown) are pulled centripetally, and as seen in B, they are added to the paramesangial GBM. (B) Increasing the capillary lumen (from B to A). Mesangial cell processes disconnect from the GBM and retract (arrows). Thereby, the most peripheral portions of the paramesangial GBM (shown in brown) are released from the mesangium, and as seen in A, they are added to the peripheral portion leading to capillary expansion driven by the pressure gradient.
It is tempting to suggest that the underlying regulation mirrors changes known from ontogeny.8 Through action of vascular endothelial growth factor and other cytokines, podocytes stimulate the growth of capillary endothelial cells, which in turn, stimulate MCs to establish the folding pattern of the GBM by centripetal contraction of the GBM between capillaries. The deposition of nephronectin by podocytes at an appropriate site within the GBM may critically determine the point for the insertion (or for releasing) of MC processes.
Reducing BP is an essential component of effective treatments that slow the progression of CKD. It has been widely believed that the beneficial effect of reducing BP results from the decrease in the physical stress on podocytes. Podocytes have been considered as a kind of pericyte actively counteracting the pressure-derived expansion of the GBM by the contractile tone of their foot processes.9 This view of the podocyte is no longer viable, because we have learned that the major route of podocyte loss consists of their detachment from the GBM as viable cells. Instead, the complex cytoskeleton of FPs serves as the basis for the attachment of foot processes to the GBM and the maintenance of their interdigitating pattern, including their adaption to changes in the area of the underlying GBM.
The dominant structure generating wall tension to counteract expansion when pressure rises would seem to be the GBM.10,11 The podocytes are situated downstream from the GBM, and thus, they are protected from effects of increases in BP by the GBM, which due to its nonlinear distensibility,12 allows expansions only up to a certain limit. Tensile stress above this limit will not reach podocytes.10,11
This realization draws attention to the fact that tensile stress due to BP will challenge the MC-GBM connections and may locally lead to their breakdown followed by bulging of capillaries and mesangial spaces. This has long been described in many older studies,6,13–16 but its importance has been under-rated in recent decades. The danger specifically to podocytes that result from such local mesangial injuries has not been adequately recognized.
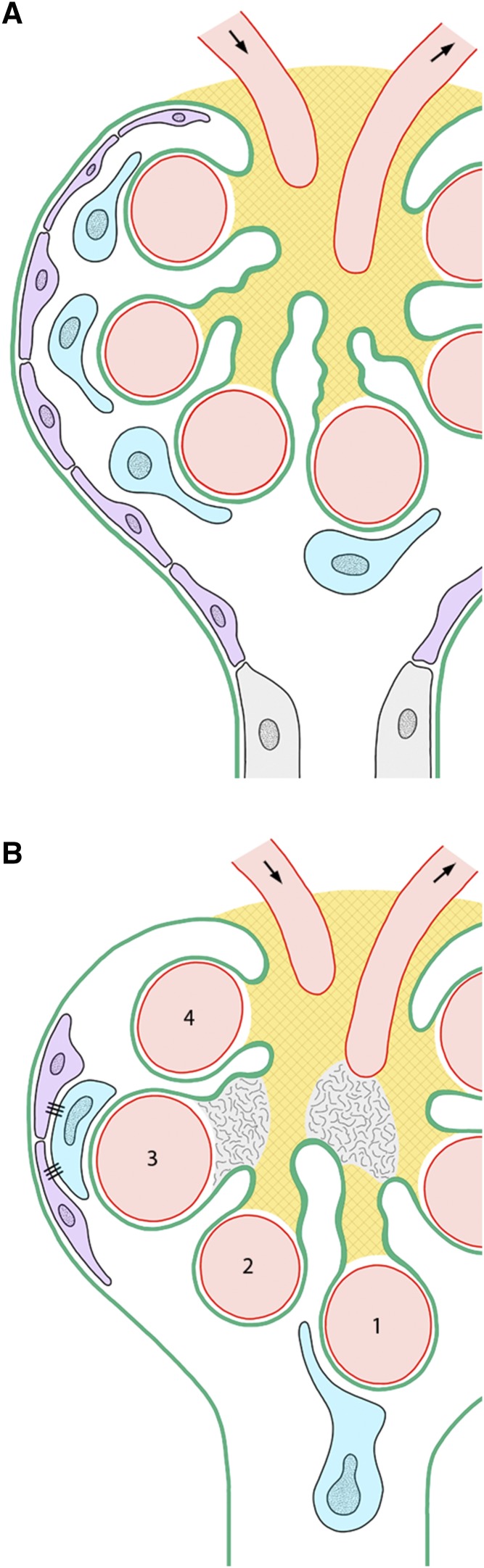
Re-evaluating the problem under this viewpoint, local expansions of the tuft due to local mesangial injury are frequently observed to be topographically associated with podocyte failures. Most prominent are the cases in which a breakdown of MC-GBM connections leads to a displacement of capillaries toward and into the urinary orifice (Figure 2). Here, the corresponding podocytes become exposed to the shear stress of the total filtrate flow draining into the tubule.11 They are subsequently lost by detachment. Examples of this eye-catching situation are generally found in models of secondary FSGS.11,14,15,17,18 Less striking but likely making up the majority of cases are situations in which due to a mesangial failure of its centripetal restraining function, capillaries and corresponding podocytes are shifted radially, coming into contact with the parietal epithelium and forming a kind of tight junction (Figure 2). Contacts of podocytes to parietal cells inevitably start the formation of tuft adhesions19,20 and thus, the first committed lesion for FSGS.
Figure 2.
Consequences of mesangial failures for podocytes shown in schematics. (A) Normal situation. The integrity of the mesangium is indicated by a checker pattern. Podocytes are shown in blue. Parietal epithelial cells are shown in violet. (B) A breakdown of mesangial cell-glomerular basement membrane connections is shown at two sites indicated by the disappearance of the checker pattern. The consequences with respect to capillary 1 consist of a lengthening of the corresponding mesangial axis followed by the prolapse of the capillary and the associated podocyte into the urinary orifice.11,17 With respect to capillary 3, the consequences of a breakdown of mesangial cell-glomerular basement membrane connections consist of a bulging of the mesangium and an expansion of the capillary followed by the peripheral displacement of the associated podocytes, which form contacts to the parietal epithelium. Correspondingly, displacement of capillary 2 would be followed by a tip lesion21 of capillary 4 by a vascular pole–associated tuft adhesion.21
In conclusion, the podocyte lesions that starts the pathway to FSGS seem to be frequently preceded by a failure of the mesangium.
Disclosures
None.
Acknowledgments
The continuous support of my work by the Gotthard Schettler Gesellschaft für Herz und Kreislaufforschung is gratefully acknowledged.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “Nephronectin Regulates Mesangial Cell Adhesion and Behavior in Glomeruli,” on pages 1128–1140.
References
- 1.Sakai T, Kriz W: The structural relationship between mesangial cells and basement membrane of the renal glomerulus. Anat Embryol (Berl) 176: 373–386, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Kikkawa Y, Virtanen I, Miner JH: Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane. J Cell Biol 161: 187–196, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmerman SE, Hiremath C, Tsunezumi J, Yang Z, Finney B, Marciano DK: Nephronectin regulates mesangial cell adhesion and behavior in glomeruli. J Am Soc Nephrol 29: 1128–1140, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elger M, Sakai T, Kriz W: Role of mesangial cell contraction in adaptation of the glomerular tuft to changes in extracellular volume. Pflugers Arch 415: 598–605, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Hakroush S, Moeller MJ, Theilig F, Kaissling B, Sijmonsma TP, Jugold M, et al. : Effects of increased renal tubular vascular endothelial growth factor (VEGF) on fibrosis, cyst formation, and glomerular disease. Am J Pathol 175: 1883–1895, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemley KV, Elger M, Koeppen-Hagemann I, Kretzler M, Nagata M, Sakai T, et al. : The glomerular mesangium: Capillary support function and its failure under experimental conditions. Clin Investig 70: 843–856, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Nagata M, Schärer K, Kriz W: Glomerular damage after uninephrectomy in young rats. I. Hypertrophy and distortion of capillary architecture. Kidney Int 42: 136–147, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Vaughan MR, Quaggin SE: How do mesangial and endothelial cells form the glomerular tuft? J Am Soc Nephrol 19: 24–33, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Shirato I, Sakai T, Kimura K, Tomino Y, Kriz W: Cytoskeletal changes in podocytes associated with foot process effacement in Masugi nephritis. Am J Pathol 148: 1283–1296, 1996 [PMC free article] [PubMed] [Google Scholar]
- 10.Kriz W, Lemley KV: Potential relevance of shear stress for slit diaphragm and podocyte function. Kidney Int 91: 1283–1286, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Kriz W, Lemley KV: Mechanical challenges to the glomerular filtration barrier: Adaptations and pathway to sclerosis. Pediatr Nephrol 32: 405–417, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Janmey PA, Miller RT: Mechanisms of mechanical signaling in development and disease. J Cell Sci 124: 9–18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rennke HG, Anderson S, Brenner BM: Structural and functional correlations in the progression of kidney disease. In: Renal Pathology, edited by Tisher CC, Brenner BM, Philadelphia, Lippincott, 1989, pp 43–66 [Google Scholar]
- 14.Kretzler M, Koeppen-Hagemann I, Kriz W: Podocyte damage is a critical step in the development of glomerulosclerosis in the uninephrectomised-desoxycorticosterone hypertensive rat. Virchows Arch 425: 181–193, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Kriz W, Hosser H, Hähnel B, Simons JL, Provoost AP: Development of vascular pole-associated glomerulosclerosis in the Fawn-hooded rat. J Am Soc Nephrol 9: 381–396, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Dworkin LD, Feiner HD: Glomerular injury in uninephrectomized spontaneously hypertensive rats. A consequence of glomerular capillary hypertension. J Clin Invest 77: 797–809, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedrich C, Endlich N, Kriz W, Endlich K: Podocytes are sensitive to fluid shear stress in vitro. Am J Physiol Renal Physiol 291: F856–F865, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kriz W, Lemley KV: A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol 26: 258–269, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Hir M, Keller C, Eschmann V, Hähnel B, Hosser H, Kriz W: Podocyte bridges between the tuft and Bowman’s capsule: An early event in experimental crescentic glomerulonephritis. J Am Soc Nephrol 12: 2060–2071, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Smeets B, Moeller MJ: Parietal epithelial cells and podocytes in glomerular diseases. Semin Nephrol 32: 357–367, 2012 [DOI] [PubMed] [Google Scholar]
- 21.D’Agati VD: The spectrum of focal segmental glomerulosclerosis: New insights. Curr Opin Nephrol Hypertens 17: 271–281, 2008 [DOI] [PubMed] [Google Scholar]