Abstract
Purpose of review
Aggressive behavior has adaptive value in many natural environments; however, it places substantial burden and costs on human society. For this reason, there has long been interest in understanding the neurobiological basis of aggression. This interest, and the flourishing of neuroimaging research in general, has spurred the development of a large and growing scientific literature on the topic. As a result, a neural circuit model of aggressive behavior has emerged that implicates interconnected brain regions that are involved in emotional reactivity, emotion regulation, and cognitive control.
Recent findings
Recently, behavioral paradigms that simulate provocative interactions have been adapted to neuroimaging protocols, providing an opportunity to directly probe the involvement of neural circuits in an aggressive interaction. Here we review neuroimaging studies of simulated aggressive interactions in research volunteers. We focus on studies that use a well-validated laboratory paradigm for reactive physical aggression and examine the neural correlates of provocation, retaliation, and evaluating punishment of an opponent.
Summary
Overall, the studies reviewed support the involvement of neural circuits that support emotional reactivity, emotion regulation, and cognitive control in aggressive behavior. Based on a synthesis of this literature, future research directions are discussed.
Keywords: Aggression, Neurobiology, Laboratory paradigms, Violence, Functional magnetic resonance imaging, fMRI, Neural circuits
Introduction
A rich literature supports the role of biological and neurobiological factors in aggressive behavior. Genetic factors account for variation in aggressive behavior [1–3], and neurotransmitters acting centrally in the brain are thought to facilitate or constrain aggression. Lesion and neuroimaging studies point to the role of abnormal brain structure and function in aggressive and antisocial behavior [4]. This literature has begun to converge on a neural circuit model of aggression in humans [5, 6••, 7, 8••] that includes diverse circuits (i.e., brain regions that interact to comprise a network). The circuits hypothesized to play a role in aggression overlap with those that support emotional response and arousal, emotional regulation, and cognitive control.
Studies of the neural circuitry of aggression have focused on structural brain differences between healthy and aggressive participants (e.g., [4, 9]) or on functional differences in brain activity observed while subjects completed standard tasks assessing emotional or cognitive processes (such as viewing emotional faces) [4, 10••, 11, 12]. Within the past few years, laboratory paradigms that simulate aggressive interactions behaviorally (paradigms which have been used in aggression research for decades) have been adapted to functional magnetic resonance imaging (fMRI), allowing researchers to study the neural basis of aggressive behavior in vivo in real time. This research provides an opportunity to evaluate hypotheses about the neural underpinnings of aggression; to more fully characterize the “normal” neural circuitry of reactive aggression in healthy human subjects; and to identify patterns of neural activity that may be abnormal in individuals with pathological (i.e., severe and chronic) aggression. This review will (1) review the literature on neural circuits implicated in aggression and (2) compare these with findings from fMRI adaptations of aggression-simulating paradigms. The review will focus specifically on reactive aggression paradigms that simulate physically provocative aggressive social interactions.
Current neural circuit models of emotion highlight the involvement of and interconnections between cortical structures of the prefrontal and medioprefrontal cortices, subcortical regions, and striatal brain regions [13, 14]. Brain regions within these neural circuits are involved in generating emotions (i.e., emotional responses) and regulating emotions. Key structures involved in generating emotions include the ventromedial prefrontal cortex (i.e., orbitofrontal cortex; OFC), amygdala, ventral striatum, and insula. The amygdala plays as a key role in processing emotions, detecting environmental threats, arousal, and facilitating the stress response [15]. The amygdala is also believed to facilitate behavioral and autonomic responses to threat [16–20]. Rich structural and functional interconnections facilitate the amygdala’s involvement in a variety of social and emotional processes [15, 19]. The insula, also implicated in emotional responding, appears to encode viscerosensory information from the body along a posterior-anterior gradient [21, 22]. The insula is also involved in cognitive functions such as detecting and redirecting cognitive resources toward responding to salient events [23]. The ventral striatum (VS) facilitates learning about how cues predict rewards and reinforcement [13]. The VS responds to both primary and secondary reinforcers (e.g., food and money; [24, 25]) and even to abstract social rewards [26]. The OFC’s role in emotional experience includes integrating affective information from the amygdala, ventral striatum, and other regions to track the affective value of specific stimuli within the current context [27]. This valuation function of the OFC comes into play in processes that are relevant to aggression including emotional valuation and decision-making.
Emotion regulation (ER) involves using regulation strategies to modify ongoing emotional experience. Broadly, emotion regulation processes are supported by functionally distinct but interconnected regions of the prefrontal cortex (PFC) and subcortical brain regions that are involved in emotional processes. Important regions for emotion regulation include the OFC, dorsomedial PFC (DMPFC), dorsolateral PFC (DLPFC), ventral lateral PFC (VLPFC), rostral anterior cingulate cortex (rACC), and dorsal ACC (dACC). Based in part on basic research findings, a model has been proposed in which anatomical connections between cortical and subcortical regions facilitate both bottom-up (“feedforward”) and top-down (“feedback”) emotional processes [28], with feedback mechanisms supporting emotion regulation. Finally, connections between cortical regions (i.e., cortico-cortical connections) are also implicated in emotion regulation. Anatomical and functional connections between the OFC and other PFC, limbic, sensory, and striatal regions support the OFC’s hypothesized roles in downregulating negative affect [29], integrating sensory input [30], and representing the value of action outcomes [31].
Cognitive control (CC) describes a group of cognitive processes that support the flexible pursuit of goals through mechanisms such as performance monitoring and behavioral adjustment [32]. CC includes distinct facets such as attentional vigilance, initiation of behavior, inhibition, flexibility, planning, and working memory. Aspects of cognitive control are tapped by standardized behavioral paradigms including: Stroop and Flanker tasks (conflict monitoring); Stop and Go/No-Go tasks (inhibition), and others. A recent meta-analysis of cognitive control neuroimaging studies found support for a hierarchical network comprising both an overarching component and specific subcomponents [33]. Niendam and colleagues found that several brain regions are activated across facets of executive cognitive function: lateral and medial PFC; superior, middle, and inferior frontal gyri; OFC and DLPFC; medial ACC; superior and inferior parietal lobes; temporal regions; subcortical regions (thalamus, caudate, putamen); and cerebellum. The most robust activations were in the DLPFC, ACC, parietal lobe, and precuneus. Classically, emotional functions (e.g., assessing emotional information and emotional responding) of the ACC have been considered to be localized more in the rostral subdivision (rACC), while cognitive functions (motor control, error detection, conflict monitoring) are associated with the dorsal subdivision (dACC; [34]); however, their roles are probably not mutually exclusive [35].
What is the evidence that the neural circuits that support emotional arousal, emotion regulation, and cognitive control also mediate aggressive behavior? Aggressive behavior is often observed under conditions of threat or aversive stimulation [36, 37]. Viewing angry faces has been shown to activate the OFC, ACC, DMPFC, and insula [38, 39]. The OFC is also engaged when subjects participate in anger-inducing script driven imagery (regional cerebral blood flow; rCBF; [40]) or imagine responding aggressively to provocation [41]. Damage to frontal lobe regions is associated with behavioral changes including impulsiveness and aggressiveness [42, 43], and acquired injury to medial and orbitofrontal brain areas, in particular, is associated with aggressive behavior [44]. Patients with lesions to the ventral frontal lobes experience increased anger and impairments in recognizing emotional facial expressions and emotional vocal expressions [45]. These patients also show deficits in adaptive decision-making and deficient physiological responses during decision-making [46]. Evidence also implicates the amygdala in aggressive behavior. Early studies of amygdala lesions show effects on aggressive behavior [47–49]. Some fMRI studies have found angry faces, specifically, to activate the amygdala [50, 51], while others have not (see [38]). Nomura et al. (2004) found that fMRI blood oxygen level dependent (BOLD) signal change in the amygdala correlated positively with subjects’ perceptions of anger intensity. These authors also observed that activity in the right amygdala was functionally inversely correlated with activity in the right inferior frontal gyrus, supporting the notion of cortico-limbic downregulation of emotion.
In humans, chronic, severe aggression is associated with emotional dysregulation. Pathological aggression, as evidenced by meeting criteria for intermittent explosive disorder (IED), is highly comorbid with psychiatric disorders characterized by emotional disturbance (depression), hyper-arousal (posttraumatic stress disorder), and emotional lability (borderline personality disorder; [52, 53]). Individuals with IED also report more negative emotionality, greater affective lability, and impulsive decision-making than non-aggressive psychiatrically healthy individuals [54, 55]. Functional neuroimaging studies point to differences between healthy and aggressive individuals in emotional and cognitive control neural networks. Coccaro et al. (2007) found that aggressive individuals with IED showed decreased OFC and increased amygdala activity when viewing angry faces compared to healthy subjects. Healthy subjects also showed greater BOLD response to emotional faces generally in regions of prefrontal cortex including rostral and dorsal medial prefrontal cortex, middle frontal gyrus, and superior frontal gyrus. Similar to the earlier study by Nomura et al. (2004), healthy subjects showed inverse functional connectivity between the amygdala and the OFC while viewing emotional faces; however, IED subjects showed no significant functional connectivity between these regions, suggesting that aggressive subjects may have impaired connectivity in the cortico-limbic pathway thought to support emotion regulation. The amygdala and connectivity (but not OFC) findings have since been replicated [11]. With respect to cognitive control neural circuitry, Raine and colleagues found that, while both affective and predatory murderers showed higher glucose metabolism during a cognitive control task (continuous performance task; CPT) in right subcortical regions (including the amygdala) than healthy control subjects, only affective murderers showed decrease prefrontal brain functioning (medial and lateral; [56]). The affective murderers also showed lower ratios of prefrontal to subcortical functioning. In another study, Raine and colleagues (1997) observed lower glucose metabolism among violent offenders compared to control subjects during the CPT, despite the absence of any performance differences (response errors) on the task. In another study, Meyer-Lindenberg and colleagues (2006) found that male carriers of a low transcribing monoamine oxidase (MAO-L) gene, who are at greater risk for aggressive and violent behavior, showed lower dACC activity during a flanker task compared to MAO-H carriers [57]. In sum, there is evidence from standard neuroscience tasks that aggressive individuals show hyper-reactivity in limbic brain regions that mediate emotional arousal and hypo-reactivity in brain regions that mediate emotion regulation and cognitive control [6••, 8••, 10••, 58].
Laboratory Aggression Paradigms
Laboratory-based paradigms for aggression were developed to address the need to study aggressive behavior systematically, efficiently, and under highly controlled and safe conditions. These paradigms solve many of the challenges to studying aggression, including the low-base rate of the behavior, stigma, and heterogeneity in its expression. A classic paradigm that has served as the basis for several neuroimaging studies is the Taylor Reaction-Time Task [59]. In this paradigm, the research subject interacts via computer with an “opponent,” who is actually fictitious. The participant and opponent engage in an interactive cover task (a reaction-time competition) that draws attention away from the true purpose of the study—to observe aggressive behavior—thus minimizing the potential influence of social desirability motives. The subject and ostensible opponent compete in a series of RT trials, and the loser of each trial receives an aversive stimulus (e.g., fingertip shock) the intensity of which is set by the other person. Adaptations to the task have used other aversive stimuli such as loud noise blasts or monetary deductions. Meta-analyses support the validity of the approach and the sensitivity to individual differences and experimental manipulations [37, 60].
Several studies have translated the Taylor Reaction-Time (TRT) into event-related fMRI designs that retain key aspects of the original task. Task implementation varies across studies, but all adaptations include a provocation by the opponent, retaliation by the subject, and an outcome phase in which punishment is administered to the loser of the competitive task. Based on the existing literature, we expect that, across studies, provocation will invoke neural circuitry related to experiencing emotions (emotional processing, autonomic arousal) and emotion regulation; retaliation is expected to involve brain regions implicated in cognitive control (including rostral and dorsal regions of ACC) and motor movements. Finally, we expected that trait aggression will be associated with increased reactivity to provocation in amygdala and insula and decreased activity in prefrontal regions that support emotion regulation.
Functional Neuroimaging of Aggression Paradigms
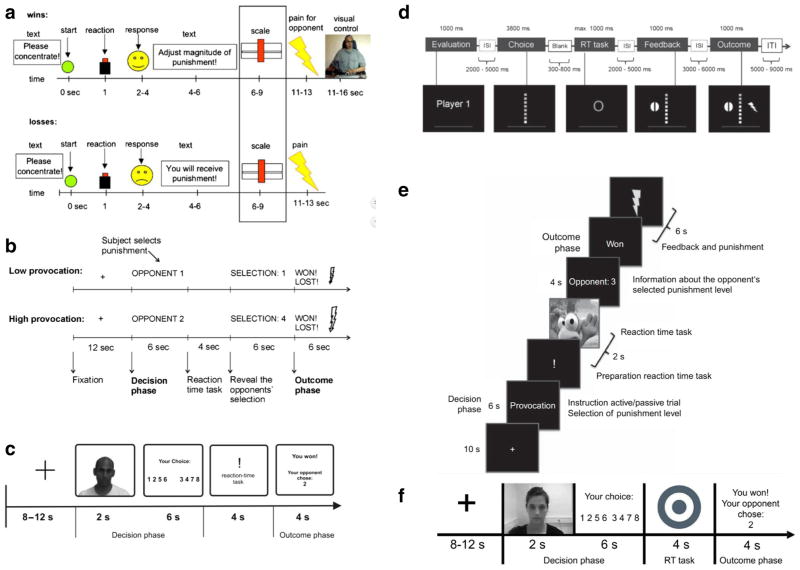
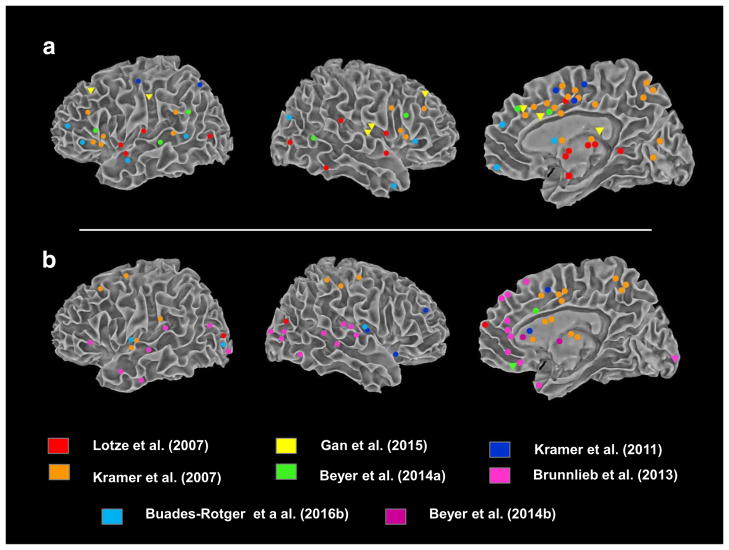
We found nine studies that adapted the TRT to the fMRI environment. All of the studies were conducted in healthy or non-selected individuals. Studies have included male or female participants or both, with age ranges in the 20s to 30s. Four studies examined individual differences relevant to aggression: psychopathy [61•]; self-reported trait aggression [62•]; empathy and trait anger [63•]; and emotional reactivity (fear potentiated startle; [64•]). Although all studies employed a provocation, retaliation, and an outcome phase, seven studies specifically examined provocation effects on neural activity. Five of these examined the effect of provocation on the decision (retaliation) phase [62•, 64•, 65•, 66•, 67•]; two on the outcome phase [65•, 66•]; and two during the provoking event [61•, 68•]. Seven studies assessed neural correlates of retaliation, although approaches varied (see Table 1). Three studies included pharmacological manipulations (vasopressin [63•]; tryptophan depletion [62•]; alcohol [68•]) and one examined endogenous hormones [66•]. Studies employed whole-brain analyses, ROI analyses, or both. Figure 1a–f shows the trial schemas for the studies. Provocation- and retaliation-related activations reported in the studies are listed in Table 1. Peak activations are also plotted in Fig. 2 for provocation and retaliation. Below we review the results of the studies, focusing on (1) brain activations and functional connectivity related to provocation, aggressive retaliation, and outcome evaluation; and (2) the relationship between individual differences and task-related patterns of neural activity. Drug and hormone effects will not be discussed.
Table 1.
Peak activations from reported in eight fMRI studies of simulated aggressive interactions
| Provocation | Retaliation | |
|---|---|---|
| n = 7 | n = 7 | |
| (nos. 1, 2, 3, 5, 6, 7, 8) | (nos. 1, 2, 3, 4, 5, 7, 8) | |
| Prefrontal regions/insula | ||
| Orbitofrontal cortex (OFC) | 7 | 4,5– |
| Inferior frontal gyrus (IFG) | 1257,7a | 34 |
| Middle frontal gyrus | 2 | 35 |
| Superior frontal gyrus | 5 | |
| Lateral/dorsolateral PFC (DLPFC) | 1 | |
| Ventrolateral PFC (VLPFC) | 1 R, 5 B | 1 R |
| Insula | 12,6– | 13 |
| Limbic/subcortical regions | ||
| Amygdala | 1,7a | 17 |
| Thalamus | 1b25,6– | 2 |
| Globus pallidus | 8 | |
| Caudate | 78 | 138 |
| Putamen | 12 | 2 |
| Medial prefrontal regions | ||
| Mediofrontal gyrus | 2357,7a | 34 |
| Medial cingulate | 13 | 15 |
| Dorsomedial PFC (DMPFC) | 6– | 1 |
| Rostral anterior cingulate (rACC) | 2 | 4 |
| Dorsal ACC (dACC) | 235,6– | 2345 |
| Posterior cingulate gyrus | 7a | |
| Motor cortex | 235 | 12 |
| Premotor/supplementary motor cortex (SMA) | 37 | 1 |
| Secondary somatosensory cortex | 1 | 1 |
| Temporal regions | ||
| Inferior temporal gyrus | 7 | 4 |
| Middle temporal gyrus | 57,7a | 4 |
| Superior temporal gyrus/sulcus | 1257,7a | 247 |
| Temporal pole | 5 | 14 |
| Fusiform gyrus | 5 | 14 |
| Temporo-parietal junction (TPJ) | 1,6– | |
| Hippocampus | 8 | 4 |
| Parietal regions | ||
| Inferior parietal lobe | 2 | 12 |
| Superior parietal lobe | 5 | |
| Supramarginal gyrus | 2 | |
| Precuneus | 23 | 24 |
| Occipital regions | ||
| Occipital lobe | 1 | 147 |
| Cuneus | 2 | 2 |
| Lingual gyrus | 125 | 4–P |
| Cerebellum | 127 | 24 |
For studies with drug administration, activations are reported for the placebo group or for main effects across groups. Unless specified by (–), brain region activations are positive. Peak activations were from contrasts and parametric analyses and whole-brain and region-of-interest (ROI) analyses, using labels reported by the original authors,
1 Lotze et al. (2007), 2 Kramer et al. (2007), 3 Kramer et al. (2011), 4 Brunnleib et al. (2013), 5 Beyer et al. (2014a), 6 Gan et al. (2015), 7 Buades-Rotger et al. (2016b), 8 Beyer et al. (2014b), R right, B bilateral, P passive > active contrast
Analyses were conducted on the outcome phase
Thalamus-hypothalamus
Fig. 1.
Trial schemas for fMRI adaptations of the Taylor Reaction-Time Task. a Reprinted from Lotze et al. [61•], with permission from Elsevier. b Kramer et al. [62•, 67•]. c Reprinted from Beyer et al. [65•], with permission from Oxford University Press. d Gan et al. [68•]. e Brunnleib et al. [63•] and Buades-Rotger et al. [85•]. f Buades-Rotger et al. [66•]. Beyer et al. [64•] used a paradigm similar to that in c but without faces
Fig. 2.
Summary of peak activations reported in fMRI studies of the TRT. Activations are plotted in MNI space using authors’ reported coordinates or figures. Activations reported without coordinates (e.g., Lotze (2007)) are presented in Table 1. Cortical activations were projected using SUMA. Results include whole-brain and ROI findings. a Provocation-related activations. Circles = high > low provocation by the opponent. Triangles represent activations to low>high provocation. For Lotze et al., only parametric modulations are presented (see Results and Table 1). In all studies except Lotze (2007) and Gan (2015), provocation effect was analyzed during decision-making. Provocation effects of Buades-Rotges on outcome phase are not displayed (see Table 1 for list). b Retaliation-related activations. Circles = high > low retaliation selections by the participant. In Brunnlieb (2013), results reflect “active” trials (in which a selected punishment would be administered) versus “passive” trials (in which the selected punishment would not be administered). In Kramer (2011), coordinates represent areas whose activity in the provocation phase correlated with behavioral aggression on the task
Provocation
Provocation engaged diverse brain regions including limbic regions (amygdala, insula); subcortical regions that mediate arousal (thalamus, hypothalamus); regions involved in emotion regulation and cognitive control (DLPFC, VLPFC, medial PFC, and ACC); regions that support social cognition (temporo-parietal junction (TPJ), precuneus), movement preparation, and movement (pre-supplementary motor cortex [pre-SMA], SMA, and motor cortex); and occipital regions that support visual processing and attention (cuneus, lingual gyrus, fusiform gyrus). While these regions were affected by provocation, only a few encode the threat value of provocation per se. It is worth noting that in most of the studies, the effect of provocation on neural activity was observed during decision-making when subjects were deciding the level of punishment to set for the opponent. Only two of five studies examined provocation independent of decision-making [61•, 68•].
Models of emotional behavior place the amygdala at the center as a key region involved in detecting threats and facilitating responses. It is notable that only two studies observed an effect of provocation on amygdala activity [61•, 66•]. Lotze and colleagues employed a unique task design that separated provocation from decision-making while Buades-Rotger et al.’s (2016b) result was found using a task that showed angry face videos of the opponent selecting the punishment level. In that study, the neural effect was evaluated during the decision-making phase, which included and immediately followed the angry face presentation. Their sample was all female. With respect to the lack of amygdala findings, it is possible that, in general, the cognitive demands associated with decision-making about retaliation diminished or suppressed activity in the amygdala, perhaps by diverting attention or by downregulating the amygdala [69]. Furthermore, studies may have “missed” amygdala activity by focusing on a time frame of the task other than the actual provoking event when the amygdala response was less robust. Lotze’s finding is particularly compelling in that it shows parametric modulation of the amygdala by the intensity of provocation. In three studies, ROI analyses were used to detect provocation effects in the amygdala, but these yielded null results [63•, 65•, 66•]. Using angry faces and videos to enhance the ecological validity of the task in most cases did not result in amygdala findings [64•, 65•] nor did accounting for individual differences in emotional reactivity (fear-potentiated startle; [64•]). It is possible that amygdala response habituated rapidly during the task [70]. Another possibility is that the amygdala may encode provocation but that this activity does not influence decision-making about retaliation, at least within the time frames examined in these studies.
The insula was more frequently engaged by provocation than the amygdala. Two studies found positive modulation of the insula by provocation [61•, 67•], and one found negative modulation [68•]. Two of these studies observed the activation during stand-alone provocation, while one [67•] observed activation during decision-making. This insula is known to be engaged by experimental tasks that induce emotional responses, including anger [17]. However, the insula also appears to be preferentially active during cognitively demanding emotional tasks compared to passive emotional viewing tasks [17]. The insula has been linked to diverse emotional, cognitive, and regulatory functions including bodily and emotional interoception and monitoring conflict and awareness of errors [21]. Given its role in awareness and its sensitivity to emotional events and cognitive demands, it has been proposed that the insula serves as a saliency detector that interacts with other brain regions to direct attention and working memory resources toward relevant targets and to modulate autonomic and behavioral responding to events [23]. With respect to aggression, the findings here suggest that insula is sensitive to provocation and may influence decision-making under conditions of provocation.
The most robust provocation-related activity was observed in the medial prefrontal cortex and anterior cingulate cortex (mPFC/ACC), where effects were concentrated in anterior-dorsal ACC (adACC) and posterior-dorsal ACC regions (pdACC; [71]). In the majority of the studies, activations were positively related to provocation intensity. In Kramer et al. [67•], activity in medial frontal gyrus was related to provocation even while controlling for retaliation intensity, a potential confound. In contrast to other studies, Gan et al. (2015) observed less activation in the mPFC/ACC for high compared to low provocation. The finding did not seem to be attributable to alcohol effects. Two features are prominent in the pattern of activation in the mPFC/ACC. First, Lotze and colleagues (2007) found only a single activation in this region (in the medial cingulate). Rather, their provocation-related activations were localized to subcortical structures including caudate, putamen, and thalamus. Given that their analyses focused on actual provocation, the authors may have observed neural activity at an earlier stage of processing. As a result, their task appears to better capture “bottom-up” provocation effects that are more closely related to emotion generation and the initial threat response [13, 72]. Second, activations found in the medial wall of the frontal cortex are located more ventrally and posteriorly around the dACC. While some evidence has implicated this region in more cognitively oriented processes—and the rACC in emotional processes—another model posits that dorsal regions of mPFC/ACC is involved in detecting emotional conflicts, emotion appraisal, and emotion expression, while the rACC is more closely linked to emotion regulation [71]. According to this model, the observed pattern of activations observed would suggest that provocation elicits stronger emotional responses, generates greater emotional conflict, and/or instigates more intense appraisal processes. Kramer et al. (2011) also found that more intense provocation was associated with activity in motor cortex during retaliation.
In three studies, provocation was found to modulate activity in the thalamus, two positively [61•, 67•] and one negatively [68•]. Located in the diencephalon between cerebral cortex and midbrain, the thalamus acts to relay sensory and motor information between sensory, subcortical (e.g., amygdala) and cortical brain structures. The thalamus is proposed to relay sensory information to the amygdala and may also mediate top-down regulation of emotion by prefrontal cortex [14, 73]. Thalamus is regularly engaged in studies that elicit emotion [14] and is known to be involved in arousal and regulatory processes. In previous studies, the thalamus was engaged during anger induction [74]; furthermore, affective murderers have been found to have greater right thalamic activity during a CPT task compared to healthy subjects [75].
Other brain areas that were modulated by provocation include temporoparietal regions that support social cognitive processes such as theory of mind (TPJ; [76]) and occipital regions that support visual processing and are sensitive to emotional information that captures attention [77]. Although these regions do not represent core emotional neural circuitry, their functions support adaptive responding to threats and emotionally salient events. Accordingly, modulation of these regions by provocation is overall consistent with the notion that provocation engages emotional neural circuitry, which in turn interacts with and recruits engagement of other neural circuits.
Only one study showed an effect of provocation on the OFC. Buades-Rotger et al. (2016b) found using whole-brain analyses that videos of angry faces during provocation and decision-making recruited the OFC. Furthermore, connectivity (which was positive) between amygdala and OFC ROIs was reduced in the angry condition relative to neutral. Given the hypothesized role of the OFC in cortico-limbic models of emotion regulation, this finding may reflect reduced top-down control when evaluating provocative stimuli. Other studies did not observe OFC modulation by provocation even using ROI analyses and ecologically valid stimuli [65•, 68•]. One difficulty in imaging the OFC region is the loss of signal due to the region’s proximity to the air/tissue interfaces of the sinuses. The present findings may therefore fail to capture the extent of OFC involvement in provocation and retaliation. More extensive activation was observed in the nearby ventral lateral PFC (inferior frontal gyrus). Four studies reported modulation of this region by provocation both alone and during decision-making (see Table 1). The VLPFC has been associated with emotion regulation (reducing negative emotions), behavioral inhibition, and avoidance conditioning [13, 78–80].
Retaliation
Retaliatory behavior engaged many brain regions that subserve diverse functional domains (Table 1; Fig. 2b). The authors used varying approaches to evaluate the neural correlates of retaliation, including examining simple contrasts of punishment selection (high versus low); using contrasts that control for the level of provocation; contrasts between administered and symbolic punishments; parametric neural correlates of retaliation intensity; and regression of average behavioral aggression on neural contrasts. This variability across studies may contribute to the heterogeneous activations observed for retaliation across studies. As with provocation, the region most consistently implicated in retaliation behavior was the mPFC/ACC, where activations were distributed across both rACC (including subgenual and pregenual cortex) and dACC, and along dorsal and rostral medial PFC (dmPFC and rmPFC). In the study by Lotze et al. (2007), the DMPFC was parametrically modulated by the intensity of retaliation. Kramer et al. (2007) found that retaliation against both opponents and the computer activated left IFC, rACC, and dACC, as well as bilateral anterior insula. Retaliation also activated the dACC even when controlling for provocation intensity. Overall, the findings across studies fit with a model of emotion regulation/cognitive control that implicates the ACC [71]. The direction of activations (positive in all cases except OFC and lingual gyrus) indicates that more intense retaliation was associated with larger BOLD response in medial prefrontal and other regions. It is worth noting that most of the activations in rostral portions of ACC were reported by one study [63•], in which the contrast was between active and passive trials. On active trials the selected punishment would be administered to the opponent. On passive trials, the selected punishment would be displayed but not administered. Retaliation-related activations reported in three other studies were located more posteriorly in the dACC.
Two studies found that activity in the OFC was modulated during retaliation. Brunnlieb (2013) found that OFC activity was greater on active versus passive trials. The meaning of this contrast in the OFC is somewhat ambiguous but may reflect the greater decision-making (i.e., valuation) demands on trials in which retaliation selection was meaningful versus merely symbolic or communicative. Using ROI analysis, Beyer et al. (2014a) found that average retaliation selection on the task was negatively correlated with OFC reactivity to provocation in the left medial OFC (and marginally in the right medial OFC). The decision-making stage included both the provocation and the retaliation decision. In other words, subjects who were more aggressive toward the opponent engaged less OFC in response to angry versus neutral faces when deciding on a retaliation response. This result recalls the finding by Coccaro et al. (2007) that aggressive subjects showed less OFC response to angry faces than did non-aggressive subjects. Although overall few of the studies reviewed found activation in OFC, other studies using social exchange paradigms, which are sometimes employed as models of reactive aggression, have observed modulation of OFC activity such that increasing economic punishment of an opponent is associated with decreased activity in OFC (e.g., [81, 82]). When Lotze et al. (2007) and Beyer et al. (2014a) examined the neural correlates of retaliation level parametrically within-subjects, they found effects in the ACC/mPFC rather than OFC (Fig. 2b). This suggests that ACC/mPFC may have a more significant role in the decision to retaliate during a provoked aggressive encounter. Buadas-Rotger (2016b) found, in whole brain analyses, that angry-versus-neutral face reactivity in the superior temporal gyrus (STG) correlated with task-related aggressive behavior. Amygdala reactivity extracted from ROIs also correlated with aggression and the BOLD response peaked earlier compared to STG. The authors applied a test of mediation to the extracted cluster values and found that STG reactivity mediated the relationship between amygdala reactivity and task-related aggression. Previous studies associated STG activity with appraising unfair offers, threat detection, and mentalizing [66•]. Other regions of activation (lateral PFC, temporal lobe, parietal cortex) were activated heterogeneously across studies (see Fig. 2b).
Reward Value of Aggression
Researchers have been interested in whether aggression invokes activity in reward-related brain areas, which might suggest that aggression is reinforcing. Such effects could explain why many individuals behave aggressively in spite of negative consequences and social prohibitions against aggression. Research on economic exchanges has found that punishing unfair behavior of a confederate is associated with neural responses in the striatum, including ventral striatum and dorsal striatum (caudate and putamen) [82–84]. Several of the studies reviewed here examined whether punishing a provocative opponent revealed striatal involvement. Not surprisingly, winning versus losing RT trials was associated with ventral striatum activity in some studies (e.g., [63•, 67•]). Several studies also looked for reward-related neural responses in the retaliation and outcome phases. Lotze and colleagues (2007) observed dorsal striatal (caudate) activity when subjects set the punishment level for the opponent (which always occurred on winning trials). Kramer et al. (2007) found that caudate and putamen were associated with selecting the punishment for the opponent during the retaliation phase, controlling for provocation intensity. They also found that winning the RT trial evoked VS activity (outcome phase) but saw no difference in VS response between wins against a human confederate compared to the computer, suggesting that VS activity was more closely tied to avoiding punishment rather than punishing an opponent. Kramer et al. (2011) found that provocation-related caudate activity during the outcome phase early in the task correlated with later provoked aggression.
Brunnlieb et al. (2013) sought to disentangle reward-related effects of punishing the opponent and avoiding punishment. They compared “active trials” in which the subject could administer a punishment to their opponent (and avoid punishment) by winning and “passive” trials in which their selection would not be administered (only revealed) and they could avoid punishment by winning. The authors observed winning-related VS activity during the outcome phase but found no difference between active and passive trials, reflecting a lack of reward-related activity specifically related to the opponent receiving punishment. In a reanalysis of the same data using VS ROIs, Buades-Rotger, Brunnlieb, and colleagues (2016a) found that punishing the opponent activated VS more so than avoiding punishment alone, providing the first evidence of ventral striatal involvement in punishing the opponent [66•]. This activity occurred during the outcome phase when the previously selected punishment was delivered. Beyer et al. (2014b) found that provocation-modulated caudate activity during the decision phase correlated with behavioral aggression on the task. Using ROI analyses, Gan et al. (2015) found that modulation of the VS during provocation correlated with overall provoked aggressive behavior (i.e., aggression toward the provocative versus mild opponent) in both their placebo and alcohol groups [68•]. Here it should be noted that alcohol is known to affect VS. Buades-Rotger (2016a) conducted connectivity analyses of the VS during the outcome phase (during which the trial winner was revealed and the previously-selected punishment was administered). Using seed regions in VS, the authors found that winning and the opponent being punished (versus winning while avoiding punishment) was linked to stronger connectivity between VS and OFC, a region known to play a role in tracking subjective rewards. Punishment was also associated with enhanced VS connectivity with motor regions, although no motor response was required at this stage of the trial. Finally, punishing was associated with enhanced VS-insula/thalamus connectivity and the strength of this relationship was related to provoked aggressive behavior on the task (i.e., retaliation selections on active minus passive trials). The meaning of this latter contrast is somewhat ambiguous; however, it may indicate the role of the VS and thalamus in facilitating motivated aggressive behavior. Trend-level negative correlations between VS-SMA connectivity and RT times raise the possibility that VS-SMA connectivity might facilitate faster responding on active (punishment) trials; although again, the reward activity and RTs occurred at different points in the trial.
In reviewing the literature on striatal activity in economic games, White et al. (2014) suggested that distinct roles of the ventral and dorsal striatum within the larger cortical-basal ganglia circuit may be important in interpreting the findings from such tasks. Specifically, these authors suggested that activity in dorsal striatum may indicate preparation for motor behavior in the form of retaliatory punishment, possibly reflecting action selection or preparation to start or stop movement. In their study, as did others, White and colleagues (2014) observed increased activity in caudate (but not VS) associated with punishment selection [82, 83]. The current review also found evidence from multiple studies that dorsal striatum is engaged during retaliation, in contrast to ventral striatum. Although we cannot rule out the possibility that the striatum is involved in signaling reward during aggression, particularly given that the dorsal striatum may also participate in reward-related functions, overall clear evidence that aggression is rewarding at the neural level is lacking. Future studies may help to clarify this relationship. It is also worth noting that subjective feelings of reward have not been assessed during these tasks. Not surprisingly, winning, losing, punishing the opponent, avoiding punishment, watching as punishment is administered, and receiving punishment were associated with activity in other brain regions that are involved in social cognition (e.g., TPJ), emotion and emotion regulation, and cognitive functions (e.g., middle frontal gyrus, ACC).
Pathological Aggression
As reviewed, a robust literature points to abnormal brain structure and function in individuals who engage in recurrent destructive aggression. These differences are hypothesized to center around hyper-responsivity in emotional circuitry (e.g., amygdala) and hypo-responsivity in emotion regulation (ER)/ cognitive control (CC) circuitry (e.g., PFC; [6••, 8••, 10••, 58]). Several of the studies reviewed assessed the relationship between trait aggression—or related constructs—and task-related behavioral aggression and neural activity. Kramer et al. (2011) found that individuals who displayed more provoked aggression on the task showed greater provocation-related activity during decision-making in brain regions that not only support emotional reactivity (insula) but also increased activity in ER and CC regions (inferior frontal gyrus (IFG), ACC). The authors also examined whether self-reported trait aggression was associated with activity in these clusters but found no evidence this was the case [62•]. This finding partially supports the cortico-limbic model of pathological aggression with respect to hyper-responsivity of subcortical regions. However, because task-related behavior and trait aggression may be confounded, we cannot be sure whether the retaliation results reflect only the neural correlates of retaliation or trait differences in pathological aggression. Beyer et al. (2014a) found that subjects who were more aggressive on the task showed less OFC reactivity to angry faces, using an ROI analysis. By contrast, Buades-Rotger et al. (2016b) found greater amygdala reactivity to angry faces in subjects who were aggressive on the task, using ROI analyses. Both of these findings are consistent with the cortico-limbic model of aggression. However, task-related aggressive behavior may not reflect differences in trait aggression, particularly in healthy, unselected samples like those employed in the studies we reviewed. Accordingly, these results may point to neural correlates of retaliation behavior but not necessarily pathologically aggressive behavior. Based on significant first-level MRI findings, Kramer et al. (2011) examined whether provocation-related reactivity in clusters located in caudate, IFG, and ACC during retaliation was related to trait aggression; however, no significant relationships emerged.
In two studies, Coccaro et al. have found that aggressive individuals show decrease or inverse (i.e., positive) frontal-limbic connectivity patterns compared to healthy individuals [10•, 11]. Only two studies explored connectivity during the TRT task, focusing on seed regions (ROIs) in the ventral striatum, OFC, and amygdala. Buadas-Rotger et al. (2016b) observed task-related OFC-amygdala connectivity; however, individual differences in aggression on the task did not modulate the strength of the connectivity. Buades-Rotger et al. (2016a) found that trait anger was associated with positive connectivity between VS and IFG/INS when applying punishment versus avoiding punishment [85•]. This appears to be a novel finding regarding connectivity in angry individuals, but one that is overall consistent with the notion of diminished top-down (i.e., inverse) control of limbic regions in aggressive individuals.
Discussion
Studies using functional magnetic resonance imaging of aggressive interactions (using the Taylor Reaction-Time Task) together support a neural circuit model of aggressive behavior that includes activity within and connectivity between brain regions that support emotional processes including emotion regulation and cognitive control. Real-world aggression is a complex multi-determined behavior, so too are the aggression paradigms described, which, like actual aggression, tap multiple psychological processes.
The nine studies reviewed overall provide evidence consistent with a neurobiological model of normal reactive aggression that implicates neural circuitry that mediates emotional reactivity, emotion regulation, and cognitive control. The studies in general also support the cortico-limbic model of pathological aggression that implicates hyper-reactivity in emotional brain areas and deficient activity and regulation by regions involved in regulating emotions and behavior. A few surprising findings emerged from the review of these studies. First, the studies revealed fewer than expected findings with respect to the amygdala. This may be attributable to aspects of the task designs and analytic approaches. However, activations in other regions implicated in emotional arousal, responding, and regulation (e.g., insula, thalamus) were replicated across studies and did, in some instances, correlate with trait aggression. Second, some support, though also less than expected, was found for the role of the OFC in aggressive behavior. Overall, there is greater evidence for the involvement of dorsal medial regions of prefrontal cortex in aggressive interactions. In particular, there was considerable evidence that mPFC/ACC activity modulates aggressive retaliation; however, there was very little evidence that differences in trait aggression are related to functioning of this region during an aggressive interaction. This is in part because analyses related to pathological aggression have focused on ROIs in the OFC and amygdala and not whole brain data or mPFC ROIs. Finally, the studies pointed toward the role of the dorsal striatum in aggression. Although the reported findings are heterogeneous in nature, there is enough evidence of striatal involvement in aggression to warrant further study.
Limitations and Future Directions
Overall, there are some limitations in the conclusions we can draw from these studies. First, in striving for greater ecological validity, studies using fMRI TRT adaptations may sacrifice some internal validity with respect to testing very specific hypotheses about neural mechanisms. Now that there is a literature on real-time neural correlates of reactive aggression, studies may begin to focus on more specific neural mechanisms that influence aggression, as can be seen in the approach taken by Buades-Rotger et al. (2016a). Relatedly, none of the studies we reviewed included specific instructions directing subjects to regulate their emotional responses. Therefore, we are limited in the conclusions we can draw about emotion regulation that may have occurred during the tasks. Activations across prefrontal and medial prefrontal regions, as well as connectivity findings, suggest that these tasks may be sensitive to directed emotion regulation effects. Future studies are needed further examine the role of specific emotion regulation processes during reactive aggression. Relatedly, in their current form, tasks may not be optimized to isolate specific cognitive control mechanisms, a limitation that may be addressed in future studies.
Future neurobiological research on aggression would benefit from an expanded focus beyond the role of the OFC and amygdala. There is sufficient evidence from the studies reviewed and others that more diverse brain regions play an important role in aggression. Further connectivity analyses are warranted. In addition, approaches that model causality such as Granger causality and dynamic causal modeling would provide novel information on the timing of neural events in aggressive behavior and pathological aggression. Continuing to optimize tasks designs may be productive and address unexpected (lack of) findings, for example, in the amygdala and OFC. fMRI scanning parameters that address signal dropout around the OFC may be important for this area of research. Similarly, now that there is a body of evidence to suggest emotional and cognitive neural circuit involvement in aggression, there is a need to pose more specific questions about the neural mechanisms underlying reactive aggressive. This could be addressed through new or altered task designs and the application of novel statistical contrasts. To better understand how altered neural circuit functioning relates to pathological aggressive behavior, there is a need to conduct studies in samples selected for high levels of aggression. Analyses of trait aggression in unselected or healthy populations may be underpowered and results in type 2 statistical errors (false negative findings). A related concern involves disentangling neural activity related to retaliation and activity that is altered in subjects with high trait aggression, as these two constructs are confounded in most studies. Finally, larger sample sizes may be needed to resolve heterogeneity across studies.
Conclusions
Neuroimaging studies of realistic aggressive interactions provide an opportunity to evaluate hypotheses about the role of various neural circuits in aggressive behavior. We found nine studies to date that probe the neural circuitry of aggression in real time using behavioral paradigms that simulate physically provocative aggressive interactions. These studies support models of aggressive behavior that implicate emotional and cognitive control neural circuitry. Our review of this literature synthesizes empirical findings and suggests future directions for research into the neural mechanisms of provoked aggressive behavior.
Acknowledgments
Royce Lee reports receiving grant support from the National Institute of Health (1R21MH083309). Jennifer R. Fanning reports receiving grant support from the National Institute of Health (1K23MH109824-01A1). Emil F. Coccaro received a grant from the Pritzker-Pucker Family Foundation.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Dr. Jennifer R. Fanning, Dr. Sarah Keedy, Dr. Mitchell E. Berman, Dr. Royce Lee, and Dr. Emil F. Coccaro declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
This article is part of the Topical Collection on Personality and Impulse Control Disorders
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Coccaro EF, et al. Heritability of aggression and irritability: a twin study of the Buss—Durkee Aggression Scales in adult male subjects. Biol Psychiatry. 1997;41(3):273–84. doi: 10.1016/s0006-3223(96)00257-0. [DOI] [PubMed] [Google Scholar]
- 2.Rhee SH, Waldman ID. Genetic and environmental influences on aggression. In: Shaver PR, Mikulincer M, editors. Human aggression and violence: Causes, manifestations, and consequences. American Psychological Association; Washington DC: 2011. pp. 143–63. [Google Scholar]
- 3.Yeh MT, Coccaro EF, Jacobson KC. Multivariate behavior genetic analyses of aggressive behavior subtypes. Behav Genet. 2010;40(5):603–17. doi: 10.1007/s10519-010-9363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a me-ta-analysis. Psychiatry Res Neuroimaging. 2009;174(2):81–8. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee R, Fanning JR, Coccaro EF. Clinical neuroscience of impulsive aggression. In: Schmahl C, Friedel R, Phan KL, editors. Neurobiology of personality disorders. New York: Oxford University Press; 2017. [Google Scholar]
- 6••.Coccaro EF, et al. Corticolimbic function in impulsive aggressive behavior. Biol Psychiatry. 2011;69(12):1153–9. doi: 10.1016/j.biopsych.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Fanning JR, Coccaro EF. Neurobiology of impulsive aggression. In: Kleespies PM, editor. The Oxford handbook of behavioral emergencies and crises. New York: Oxford University Press; 2016. [Google Scholar]
- 8••.Siever LJ. Neurobiology of aggression and violence. Am J Psychiatr. 2008;165(4):429–42. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coccaro EF, et al. Frontolimbic morphometric abnormalities in intermittent explosive disorder and aggression. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2016;1(1):32–8. doi: 10.1016/j.bpsc.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 10••.Coccaro EF, et al. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry. 2007;62(2):168–78. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 11.McCloskey MS, et al. Amygdala hyperactivation to angry faces in intermittent explosive disorder. J Psychiatr Res. 2016;79:34–41. doi: 10.1016/j.jpsychires.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Marsh AA, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatr. 2008;165(6):712–20. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- 13.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N YAcad Sci. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kober H, et al. Functional grouping and cortical–subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 16.LeDoux JE, et al. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 1988;8(7):2517–29. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phan KL, et al. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 18.Phan KL, et al. Functional neuroimaging studies of human emotions. CNS spectrums. 2004;9(4):258–66. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- 19.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. (Miller 2003) [DOI] [PubMed] [Google Scholar]
- 20.Whalen PJ, et al. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 1998;18(1):411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1) doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 22.Kurth F, et al. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214(5–6):519–34. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist. 2004;10(3):260–8. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- 25.Sescousse G, et al. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuro-imaging studies. Neurosci Biobehav Rev. 2013;37(4):681–96. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58(2):284–94. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, et al. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuro-imaging studies. Neurosci Biobehav Rev. 2011;35(5):1219–36. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghashghaei H, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–79. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 29.Banks SJ, et al. Amygdala–frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2(4):303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Öngür D, Price J. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 31.Hare TA, et al. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28(22):5623–30. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ridderinkhof KR, et al. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 33.Niendam TA, et al. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, & Behavioral Neuroscience. 2012;12(2):241–68. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 35.Mohanty A, et al. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44(3):343–51. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- 36.Berkowitz L. On the formation and regulation of anger and aggression: a cognitive-neoassociationistic analysis. Am Psychol. 1990;45(4):494. doi: 10.1037//0003-066x.45.4.494. [DOI] [PubMed] [Google Scholar]
- 37.Bettencourt B, et al. Personality and aggressive behavior under provoking and neutral conditions: a meta-analytic review. Psychol Bull. 2006;132(5):751. doi: 10.1037/0033-2909.132.5.751. [DOI] [PubMed] [Google Scholar]
- 38.Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanat-omy of emotions: a meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3(3):207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- 39.Blair RJR, et al. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122(5):883–93. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- 40.Dougherty DD, et al. Anger in healthy men: a PET study using script-driven imagery. Biol Psychiatry. 1999;46(4):466–72. doi: 10.1016/s0006-3223(99)00063-3. [DOI] [PubMed] [Google Scholar]
- 41.Pietrini P, et al. Neural correlates of imaginal aggressive behavior assessed by positron emission tomography in healthy subjects. Am J Psychiatry. 2000;157(11):1772–81. doi: 10.1176/appi.ajp.157.11.1772. [DOI] [PubMed] [Google Scholar]
- 42.Brower MC, Price B. Neuropsychiatry of frontal lobe dysfunction in violent and criminal behaviour: a critical review. J Neurol Neurosurg Psychiatry. 2001;71(6):720–6. doi: 10.1136/jnnp.71.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hesdorffer DC, Rauch SL, Tamminga CA. Long-term psychiatric outcomes following traumatic brain injury: a review of the literature. J Head Trauma Rehabil. 2009;24(6):452–9. doi: 10.1097/HTR.0b013e3181c133fd. [DOI] [PubMed] [Google Scholar]
- 44.Grafman J, et al. Frontal lobe injuries, violence, and aggression a report of the vietnam head injury study. Neurology. 1996;46(5):1231. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- 45.Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia. 1996;34(4):247–61. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 46.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 47.Klüver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Arch Neurol Psychiatr. 1939;42(6):979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- 48.Eichelman B. The limbic system and aggression in humans. Neurosci Biobehav Rev. 1983;7(3):391–4. doi: 10.1016/0149-7634(83)90044-1. [DOI] [PubMed] [Google Scholar]
- 49.Siegel A, Victoroff J. Understanding human aggression: new insights from neuroscience. Int J Law Psychiatry. 2009;32(4):209–15. doi: 10.1016/j.ijlp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Morris JS, Öhman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393(6684):467–70. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- 51.Nomura M, et al. Functional association of the amygdala and ventral prefrontal cortex during cognitive evaluation of facial expressions primed by masked angry faces: an event-related fMRI study. NeuroImage. 2004;21(1):352–63. doi: 10.1016/j.neuroimage.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 52.Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psychiatr. 2012;169(6):577–88. doi: 10.1176/appi.ajp.2012.11081259. [DOI] [PubMed] [Google Scholar]
- 53.Kessler RC, et al. The prevalence and correlates of DSM-IV intermittent explosive disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2006;63(6):669–78. doi: 10.1001/archpsyc.63.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fetick KC, McCLoskey MS, Look AE, Cocaro EF. Emotion regulation deficits in intermittent explosive disorder. Aggress Behav. 2014 doi: 10.1002/AB.21566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puhalla AA, et al. Negative urgency and reward/punishment sensitivity in intermittent explosive disorder. J Affect Disord. 2016;201:8–14. doi: 10.1016/j.jad.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 56.Raine A, et al. Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behavioral Sciences & the Law. 1998;16(3):319–32. doi: 10.1002/(sici)1099-0798(199822)16:3<319::aid-bsl311>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 57.Meyer-Lindenberg A, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci. 2006;103(16):6269–74. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science. 2000;289(5479):591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 59.Taylor SP. Aggressive behavior and physiological arousal as a function of provocation and the tendency to inhibit aggression. J Pers. 1967;35(2):297–310. doi: 10.1111/j.1467-6494.1967.tb01430.x. [DOI] [PubMed] [Google Scholar]
- 60.Anderson CA, Bushman BJ. External validity of “trivial” experiments: the case of laboratory aggression. Rev Gen Psychol. 1997;1(1):19. [Google Scholar]
- 61•.Lotze M, et al. Evidence for a different role of the ventral and dorsal medial prefrontal cortex for social reactive aggression: an interactive fMRI study. NeuroImage. 2007;34(1):470–8. doi: 10.1016/j.neuroimage.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 62•.Kramer UM, et al. An fMRI study on the role of serotonin in reactive aggression. PLoS One. 2011;6(11):1–8. doi: 10.1371/journal.pone.0027668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63•.Brunnlieb C, et al. Vasopressin modulates neural responses during human reactive aggression. Soc Neurosci. 2013;8(2):148–64. doi: 10.1080/17470919.2013.763654. [DOI] [PubMed] [Google Scholar]
- 64•.Beyer F, et al. Emotional reactivity to threat modulates activity in mentalizing network during aggression. Soc Cogn Affect Neurosci. 2014;9(10):1552–60. doi: 10.1093/scan/nst146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Beyer F, et al. Orbitofrontal cortex reactivity to angry facial expression in a social interaction correlates with aggressive behavior. Cerebral cortex. 2014 Sep;:3057–3063. doi: 10.1093/cercor/bhu101. [DOI] [PubMed] [Google Scholar]
- 66•.Buades-Rotger M, et al. Endogenous testosterone is associated with lower amygdala reactivity to angry faces and reduced aggressive behavior in healthy young women. Scientific Reports. 2016:6. doi: 10.1038/srep38538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Krämer UM, et al. Tit-for-tat: the neural basis of reactive aggression. NeuroImage. 2007;38(1):203–11. doi: 10.1016/j.neuroimage.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 68•.Gan G, et al. Neural and behavioral correlates of alcohol-induced aggression under provocation. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2015;40(13):2886–96. doi: 10.1038/npp.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Dillen LF, Heslenfeld DJ, Koole SL. Tuning down the emotional brain: an fMRI study of the effects of cognitive load on the processing of affective images. NeuroImage. 2009;45(4):1212–9. doi: 10.1016/j.neuroimage.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 70.Breiter HC, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–87. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 71.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hesse E, et al. Early detection of intentional harm in the human amygdala. Brain. 2016;139(1):54–61. doi: 10.1093/brain/awv336. [DOI] [PubMed] [Google Scholar]
- 73.Das P, et al. Pathways for fear perception: modulation of amygdala activity by thalamo-cortical systems. NeuroImage. 2005;26(1):141–8. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 74.Kimbrell TA, et al. Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biol Psychiatry. 1999;46(4):454–65. doi: 10.1016/s0006-3223(99)00103-1. [DOI] [PubMed] [Google Scholar]
- 75.Raine A, Buchsbaum M, LaCasse L. Brain abnormalities in murderers indicated by positron emission tomography. Biol Psychiatry. 1997;42(6):495–508. doi: 10.1016/S0006-3223(96)00362-9. [DOI] [PubMed] [Google Scholar]
- 76.Saxe R, Kanwisher N. People thinking about thinking people: the role of the temporo-parietal junction in “theory of mind”. NeuroImage. 2003;19(4):1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- 77.Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9(12):585–94. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 78.Aron AR, et al. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27(44):11860–4. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delgado MR, et al. Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Neuroeconomics. 2009:72. doi: 10.3389/neuro.08.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci. 2010;14(6):268–76. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.White SF, et al. Punishing unfairness: rewarding or the organization of a reactively aggressive response? Hum Brain Mapp. 2014;35(5):2137–47. doi: 10.1002/hbm.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.White SF, et al. Callous-unemotional traits modulate the neural response associated with punishing another individual during social exchange: a preliminary investigation. J Personal Disord. 2013;27(1):99–112. doi: 10.1521/pedi.2013.27.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Quervain DJ, et al. The neural basis of altruistic punishment. Science. 2004;305(5688):1254. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- 84.Strobel A, et al. Beyond revenge: neural and genetic bases of altruistic punishment. NeuroImage. 2011;54(1):671–80. doi: 10.1016/j.neuroimage.2010.07.051. [DOI] [PubMed] [Google Scholar]
- 85•.Buades-Rotger M, et al. Winning is not enough: ventral striatum connectivity during physical aggression. Brain Imaging and Behavior. 2016;10(1):105–14. doi: 10.1007/s11682-015-9370-z. [DOI] [PubMed] [Google Scholar]