Abstract
Significance
These results demonstrate that accommodation in children is more accurate and less variable when performing a sustained near task with increased cognitive demand. Additionally, children with increased uncorrected hyperopia have less stable accommodative responses, which may have visual implications during sustained near tasks.
Purpose
This study investigated accommodative accuracy (lag) and variability during sustained viewing for passive and active tasks in children and adults with emmetropia and uncorrected hyperopia.
Methods
Lag and variability (root mean square [RMS] and low-frequency component) were measured in 54 children aged 3 to <10 years with mean spherical equivalent (SE) of +1.31±1.05 D (range: −0.37 to +4.58 D) and 8 adults aged 22-32 years with mean SE +0.65±0.62 D (range: −0.13 to +1.15 D). Subjects viewed 20/50 stimuli at 33 cm during both a 10-minute passive and active task. Group 1 (<6 years or non-readers) viewed shapes; Group 2 (≥6 years and reading) and adults read passages.
Results
Groups 1 & 2 had larger lags, RMS, and low-frequency component for passive versus active tasks (P<0.001). Lag and RMS did not differ between tasks in adults (P>0.05), but low-frequency component was larger during passive viewing (P=0.04). Group 1 had significantly higher RMS and low-frequency component than Group 2 and the adults in the passive condition and greater low-frequency component in the active condition. In children, hyperopia was independently associated with RMS and low-frequency component under passive (RMS 95% CI: 0.04–0.15, low-frequency component 95% CI: 0.00011–0.00065) and active (RMS 95% CI: 0.001–0.06, 95% CI: 0.000014–0.00023) viewing.
Conclusions
Accommodation is more accurate and less variable when children are engaged in the task. Children also have more variable accommodation than adults. Additionally, children with greater hyperopia have more variable accommodation during sustained near tasks.
Keywords: accommodation, children, hyperopia, cognition, accommodative variability, accommodative accuracy
The accuracy of the accommodative response is often measured as a single point estimate and referred to as a stable and discrete measure over time. However, the accommodative response is variable and typically fluctuates around the mean response.1 Many factors influence the accuracy and variability of the accommodative response, some of which originate from intrinsic properties of the optical system, such as pupil size,2 and defocus,3,4 while others originate from extrinsic properties of the stimulus and visual environment, such as luminance,5 stimulus size,6–8 and image contrast.1,9
Accommodation is also influenced by neural and cognitive factors such as the sense of nearness10 and an increase in cognitive demand of a task, both of which result in a decrease in accommodative lag.8,11–13 Accommodative variability has been shown to increase with an increase in accommodative demand and subsequent response14–16 and thus if the mean accommodative response increases and is more accurate during a task that requires increased cognitive effort, the accommodative variability would likely increase as well; however, the relationships between cognitive effort and accommodative variability have yet to be studied. Despite potential changes in accommodation secondary to the cognitive demand of the task, most studies of accommodation consist of the subject fixating a detailed picture stimulus,17,18 cartoon movie,14,15 or Maltese cross,19 which all contain a wide spatial frequency distribution but differ in cognitive demands. Hence, it is difficult to compare across studies without accounting for the confounding effect of cognitive demand.
Properties that impact accommodation may be particularly important in the developing visual system, given that young children have, on average, higher levels of hyperopia than older children20, 21 and adults,22 and thus would have larger accommodative demands for any given viewing distance if viewing uncorrected. Thus, understanding the factors that impact the accuracy and stability of the accommodative response is critical to gain perspective into the visual experience of young children with uncorrected hyperopia. Currently the accuracy and variability of the sustained accommodative response in children beyond a 30 second period is unknown as most studies have used time periods of less than 30 seconds.14–16,19,23–25 Sustained accommodation may be of particular concern during the school day while performing near activities and while completing homework.26 Additionally, cognitive demands of near tasks also vary and thus it is important to understand accommodative behavior under different tasks.
The purpose of this study was to investigate accommodative accuracy and accommodative variability for tasks of varying cognitive demand over an extended viewing period in non-myopic children with differing levels of naturally occurring accommodative demands due to refractive error that has been left uncorrected. First, we sought to determine the effect of cognition on accommodative accuracy and accommodative variability during sustained viewing by comparing the accommodative responses during a passive viewing task to an active viewing task of the same duration. These responses were compared to adults with mature visual systems. Secondly, we sought to determine the relationships between uncorrected refractive error and accommodation by evaluating accommodative accuracy and variability over our recruited range of habitually uncorrected refractive error.
METHODS
Study Subjects
Subjects 3 to < 10 years with non-myopic, uncorrected refractive error ranging from emmetropia to moderate hyperopia resulting in a wide range of differing accommodative demands and habitually uncorrected adults < 35 years were recruited from the University of Houston College of Optometry staff, student, and patient populations, and the local community. The study was approved by the University’s institutional review board for the protection of human subjects and followed the tenets of the Declaration of Helsinki. All subjects younger than 18 years provided assent while their parents provided written parental permission to participate in the study and subjects over the age of 18 years provided written informed consent.
Typically developing children, born at ≥ 32 weeks of gestational age with a birth weight of ≥ 2500 grams were invited to participate in the study. Adults <35 years of age who did not wear a refractive correction were also recruited. Subjects were excluded from participation if they had a history of ocular or systemic diagnoses that may impact accommodation, medications known to impact accommodation, or history of developmental delays or behavioral diagnoses, such as attention deficit disorders. Subjects were also ineligible to participate if they had a current or previous refractive correction or had known cycloplegic refractive error ≤−0.50 diopters (D) spherical equivalent, anisometropia >1.00D spherical equivalent, or astigmatism >1.25D cylinder.
All subjects had a complete vision examination. The examination included monocular visual acuity following the electronic visual acuity testing protocol established by the Pediatric Eye Disease Investigator Group,27, 28 and unilateral cover test to identify children with strabismus. Subjects were excluded from data analysis if they did not have typical visual acuity for their age (<20/50 for 3 to < 4 years, <20/40 for 4 to < 5 years, <20/32 for 5 to < 6 years29, 20/25 or worse for subjects ≥ 6 years30), were diagnosed with strabismus or amblyopia (visual acuity >2 lines intraocular difference in the presence of an amblyogenic risk factor),31 or had cycloplegic refractive error ≤−0.50 diopters (D) spherical equivalent, anisometropia >1.00D spherical equivalent, or astigmatism >1.25D cylinder as determined at the study vision examination.
Subjects also had a cycloplegic assessment of refractive error using the Grand Seiko WAM-5500® open-field auto-refractor (RyuSyo Industrial Co., Ltd. Hiroshima, Japan), and a dilated fundus examination to rule out ocular pathology. Three measures of cycloplegic refractive error were obtained for each eye (30 minutes after instillation of 1% cyclopentolate), transformed into power vector notation,32 averaged, then the averaged vectors were back-transformed as the mean sphero-cylinder cycloplegic refractive error. The mean spherical equivalent value was used to classify the refractive error of each eye as the most plus and least plus eye of each subject.
For subjects recruited from the optometry clinic, the cycloplegic assessment of refractive error using the Grand Seiko autorefractor was performed at their routine examination after informed consent was obtained by the investigator (TLR) while all other testing (e.g. visual acuity and cover test) was performed on the day of their laboratory visit. Subjects recruited outside of the optometry clinic completed their vision examination (performed by TLR) on the same day of the laboratory visit with cycloplegia performed after all accommodation measures were completed.
Experimental Set-up
For each experimental task (testing order randomized), all subjects viewed shapes (squares, circles, triangles, stars and arrows), letters, or text that subtended 0.21° (~20/50 sized stimuli at 33 cm) in the vertical dimension (letter size was based on lower case letters such as “a”). The stimuli were displayed on an iPad Air (Apple Inc., Cupertino, CA) (2048×1536 pixels) at 33cm using Keynote (Apple Inc., Cupertino, CA) presentation software and centered in a viewing window of 8.9cm (15.09°) horizontally and 4.6cm (7.94°) vertically for the text and 4.15cm (7.20°) horizontally and 2.2cm (3.8°) vertically for the shape stimuli. The iPad Air was positioned above a beam-splitter (passes infrared light, reflects visible light), which projected the stimulus directly in front of the subject (99.7% Weber contrast through the beam splitter). A photorefractor (PowerRef II, Plusoptix Inc., Atlanta, GA) was located one meter in front of the subject. The stimulus and photorefractor were covered using a curtain throughout the experiment and the room lights were turned out to limit distractions. The subjects were positioned in a headrest to limit head movements and the edge of the curtain was placed to block out any light from the PowerRef II monitor.
Active Viewing Experiment
Subjects performed a cognitively active task (reading story passages or answering questions about displayed shapes) for ten consecutive minutes. Prior to experimental testing, children 6 to <10 years were screened to determine their reading level using the San Diego Quick Assessment test.33 Subjects who were capable of reading the kindergarten level or above read story passages aloud during the active viewing task at each individual’s reading level. Passages from each grade level were obtained from AIMSWEB (Pearson Inc. London, England) and compiled into Keynote presentations and displayed on the iPad Air. At the end of each passage, subjects answered a multiple-choice question to encourage the child to actively attend to the passages throughout the experiment. The adult subjects read from a Master’s Thesis on the topic of economics. To encourage the adults to cognitively attend to the thesis, they were informed prior to the start of the experiment that they would be asked questions regarding the passage at the end of the 10-minute testing period.
All subjects <6 years and subjects ≥6 years unable to read the kindergarten screening words viewed the shape stimuli. The shapes were displayed using a pre-programmed slide presentation made in Keynote. Each slide had 1 to 5 shapes displayed for a total time equal to 2 seconds per shape with the exception that 5 shapes were displayed for 9 seconds. The shapes were evenly spaced with a total stimulus width of 4.4° horizontally and 0.21° vertically. Throughout the trial, the investigator asked the subject questions about each slide ranging in difficulty from naming the shapes, to counting a particular shape, or, for older children in the group, answering questions regarding the spatial distribution of the shapes relative to one another (first, last, before, after). The questions asked varied based upon each subject’s ability to understand and answer the questions. All children who completed the task were able to answer questions regarding the stimulus throughout the duration of the task.
Passive Viewing Experiment
Subjects looked at letters or shapes for ten consecutive minutes. Subjects who viewed shapes during the active viewing task viewed the same shape stimuli in the passive viewing task. Subjects viewed silently and were not asked any questions during the experiment. Subjects ≥ 6 years who read passages and the adults viewed random letters arranged in rows in a separate Keynote® presentation of multiple slides. The subjects were instructed to silently view each letter on each slide beginning at the upper left as though they were reading.
Measures of Accommodation, Eye Position and Pupil Size
Refractive error, eye alignment and pupil size were measured binocularly during the passive and active viewing tasks using the PowerRef II at 25Hz. The method of eccentric photorefraction has been described in detail elsewhere.34, 35 Prior to experimental testing, each individual underwent a trial lens calibration for each eye to obtain more precise measures of refractive error.34
Photorefraction data were filtered offline to eliminate outlying data points that are known to be outside of the working range of the PowerRef II or points that are unlikely to be physiological in nature (i.e.: fluctuations secondary to blinks). Measures of refractive error were removed if the change in focus between two data points were >10D/second,36 refractive error measures were <−6.00D or >+4.00D,35 pupil size was <4mm or >8mm, and gaze position outside of ±10° horizontally or ±5° vertically to eliminate erroneous measures from peripheral refraction37 and as recommended by the manufacturer for the first generation PowerRefractor (Plusoptix Inc., Atlanta, GA).
Raw data traces were examined and regular, periodic changes in the refractive error measures were detected during the reading and passive letter task for horizontal eye movements. The changes in refractive error were consistent with the dynamic changes in the horizontal eye position during the task (Figure 1a). The effect of eye position was not present for the subjects who viewed the shape stimuli as the shapes were placed in the center of the viewing window and had a much smaller total stimulus width, thus requiring small horizontal eye movements. The data with periodic changes in refractive error had a bi-linear distribution with the junction of the two slopes at approximately zero degrees for both the right and left eyes for all subjects (Figure 1b). Piecewise linear regression was performed to determine each slope of the refractive error and eye position functions (slope ≤0° and slope >0°). Each eye position datum point was then multiplied by the slope of the respective regression line (≤0° or >0°) and the product was subtracted from the corresponding refractive error datum point. Prior to any data analysis, the correction factor was applied to each eye of each subject who viewed the reading passages for the active task or the letters during the passive task. The effect of the correction factor for horizontal eye position for one adult subject is shown in Figure 1c and 1d. The data was inspected in a similar fashion for a correlation between pupil size and refractive error measures (data not shown) but no correlation was found.
Figure 1.
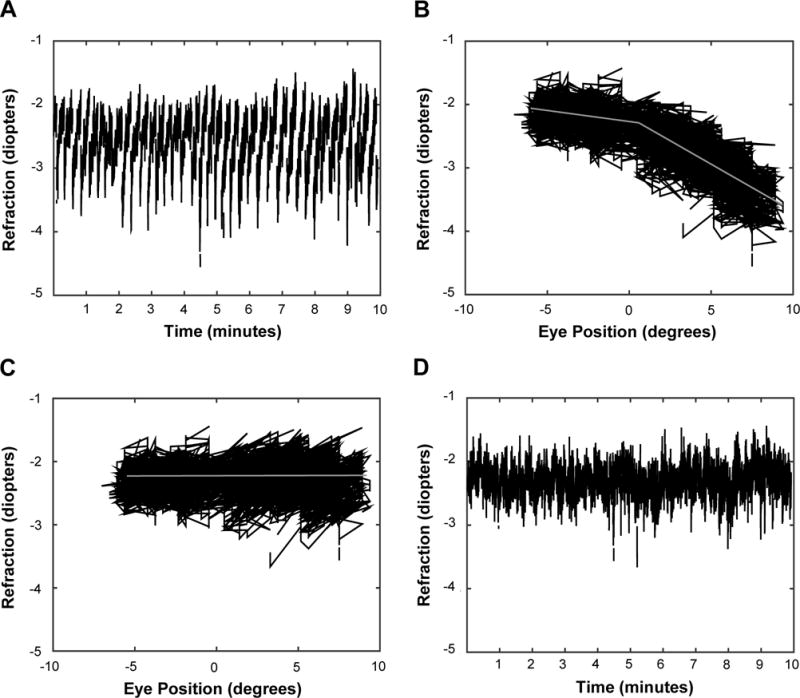
Refractive error and eye position data from the right eye of one adult subject during the cognitive viewing condition. (A) Refractive error of the right eye illustrating the periodic changes in the signal throughout the experiment. (B) Refractive error plotted as a function of horizontal eye position (degrees). The gray lines represent the change in slope of the data when eye position is ≤0° and >0°. Negative degrees represent left gaze position and positive degrees represents right gaze position. (C) Refractive error as a function of eye position after the eye position correction had been applied, illustrating the effectiveness of the correction factor. Negative degrees represent left gaze position and positive degrees represents right gaze position. (D) Refractive error data plotted as a function of time after the eye position correction factor is applied to the data.
Variable Calculations
Accommodative accuracy, total accommodative response, accommodative variability (time and frequency domains), and cycloplegic spherical equivalent refractive error were analyzed for both viewing tasks. The mean measures obtained by the Power Ref II were transposed to obtain a measurement of accommodation (i.e.: −2.50 myopic refraction indicates 2.5 D accommodation).38 Average accommodative accuracy was calculated as the difference between the mean refractive error measures obtained by the PowerRef II and the 3 diopter stimulus demand.36 The average total accommodative response was calculated as the difference between the cycloplegic refractive error in the vertical meridian of the least hyperopic eye obtained from Grand Seiko auto-refraction and the mean refractive error measures obtained by the PowerRef II during the experiment (PowerRef II measures the vertical meridian of the eye in dynamic mode). Thus, the spherical refraction values obtained with the PowerRef II (090-degree meridian of the eye) were combined with the power in the vertical meridian (090) obtained from the average cycloplegic autorefraction (Equation 1)39 to calculate the total accommodative response of the subject during the experimental tasks.
| (1) |
where S is the spherical power, C is the cylindrical power, α is the axis, and 90 represents the desired vertical meridian.
Accommodative variability was calculated in the time and frequency domains from the refractive error data obtained by the PowerRef II during each experiment. Accommodative variability in the time domain was calculated using the root mean square (RMS)24 of the filtered refractive error data. Accommodative variability in the frequency domain was characterized as the area of the curve for the low-frequency component (0-0.6Hz)36 of the Power Spectrum as the low-frequency component represents slow drifts of accommodation that occur over time. The low-frequency component was calculated using the Fast Fourier Transform (FFT) function in Matlab. Prior to running the FFT, missing data points were linearly interpolated and the dc component (i.e. average response) was subtracted from the data. The data were then smoothed using a Gaussian function (sigma=1 standard deviation).
Data Analysis
Subjects were divided into three groups: Group 1-children who viewed the shape stimuli; Group 2-children who read passages; Adults. Refractive error was calculated based upon the spherical equivalent of the least plus eye obtained from cycloplegic auto-refraction and thus the measures of the least plus eye were used for all comparisons. Spherical equivalent refractive error was also compared between the children and adults using two-sample t-test. Analysis was performed using Stata 12.1 and SigmaPlot 13 (Systat Software, San Jose, CA).
A repeated measures ANOVA was used to assess whether each outcome (lag/RMS/low-frequency component), differed by task (passive/active), and whether task effects differed between groups (Group 1/Group 2/Adults). Post-hoc adjustments for multiple comparisons were conducted using the Holm-Sidek method. In the children, multiple linear regression analysis was used to analyze the effect of uncorrected hyperopia on outcomes accommodative lag, RMS, and the low-frequency component for each task (passive and active) with all children grouped together while controlling for stimuli viewed (Group 1 and Group 2) and pupil size.
RESULTS
Seventy-four subjects (66 children and 8 adults) were recruited to participate in the study. Fifty-seven children were cooperative for at least one of the two tasks (3 subjects were uncooperative for the passive task and 4 different subjects were uncooperative for the active task). Group 1 included 31 children (24 children <6 years and 7 children ≥6 years who were non-readers), Group 2 included 23 children, and the adult group included 8 subjects. Descriptive statistics for age, refractive error, accommodative lag, total accommodative response, variability, and pupil size for all groups are found in Table 1. Representative examples of subject data traces and coinciding power spectrum analyses for two children and one adult are shown in Figure 2.
Table 1.
Descriptive statistics measured over the duration of each experimental condition and within each experimental group.
| Group 1 (Children – Symbols) |
Group 2 (Children – Text) |
Adults (Text) |
||||
|---|---|---|---|---|---|---|
| Passive (n = 31) |
Active (n=30) |
Passive (n=23) |
Active (n=23) |
Passive (n=8) |
Active (n=8) |
|
| Age* (years) | 5.0(1.0) | 5.1(1.1) | 7.5(1.2) | 25.3(2.8) | ||
| Spherical Equivalent Refractive Error*‡ (D) | +1.49(0.95) min=−0.37; max=+4.19 |
+1.38(1.00) min=−0.37; max=+4.19 |
+1.15(1.19) min = −0.25; max = +4.58 |
+0.65(0.42) min = −0.13; max = 1.15 |
||
| Accommodative* Lag (D) | 1.54(0.39) | 1.32(0.45) | 1.42(0.53) | 1.23(0.37) | 1.14(0.32) | 1.15(0.36) |
| Total Accommodative Response* (D) | 2.95(1.03) | 3.06(1.21) | 2.73(1.29) | 2.92(1.28) | 2.51(0.39) | 2.50(0.39) |
| RMSˆ (D) | 0.59 (0.39, 0.80) | 0.31 (0.28, 0.43) | 0.33 (0.26, 0.44) | 0.26 (0.22, 0.34) | 0.27 (0.22, 0.41) | 0.22 (0.18, 0.34) |
| LFCˆ (Hz) | 1.28E-03 (5.9E-4, 2.4E-03) |
3.83E-04 (2.2E-04, 5.9E-04) |
3.23E-04 (1.7E-04, 6.2E-04) |
1.93E-04 (1.1E-04, 3.0E-04) |
1.68E-04 (1.1E-04, 4.7E-04) |
1.1E-04 (6.9E-05, 2.6E-04) |
| Pupil Size (mm) | 5.8(0.7) | 5.9(0.7) | 5.9(0.8) | 6.1(0.6) | 5.0(0.6) | 5.6(1.0) |
RMS – Root mean square; LFC – low-frequency component (0-0.6Hz); D – diopters; Hz – hertz; mm – millimeters
Values are reported as mean(standard deviation)
Values are reported as median and interquartile range
Significant difference between mean refractive error of combined Groups 1 & 2 and the adults (two sample t-test, P = 0.0032)
Figure 2.
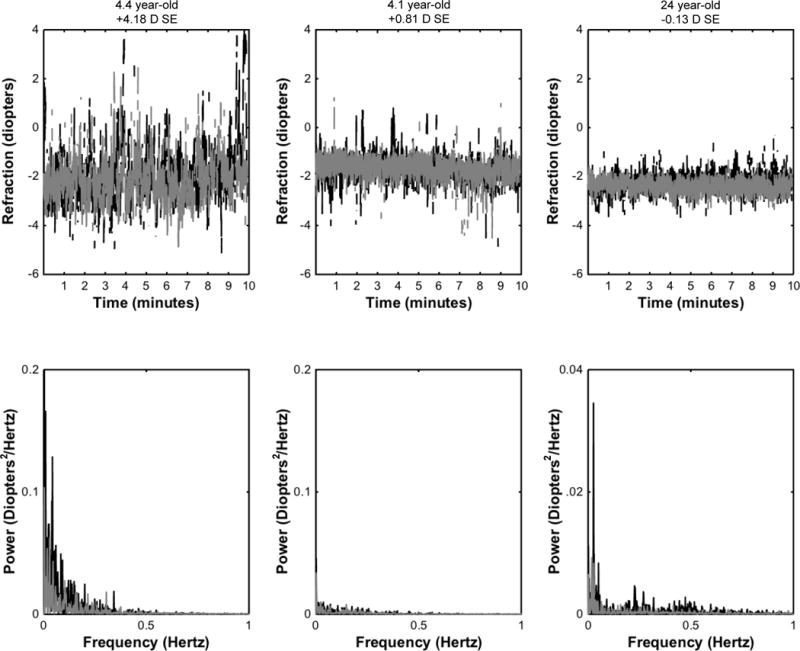
Sample data traces (top row) of the least plus eye’s accommodative response to the stimulus located at 33 cm (3 D demand) from 3 subjects along with coinciding power spectrum (bottom row) obtained by Fourier analysis. The black traces represent the passive condition while the gray traces represent the active conditions. (A) 4.4 year-old with +4.18 D spherical equivalent (SE), (B) 4.1 year-old with +0.81 D SE, and (C) 24 year-old with −0.13 D SE. Note the change in the y-axis scale for the adult subject for the power spectrum analysis.
Summary Characteristics for Cycloplegic Refractive Error and Age
Despite a range of almost +5.00D of uncorrected spherical equivalent hyperopia in the 3 to < 10-year-old children (Table 1), a significant relationship was not detected between refractive error and age (n = 57, r = −0.23, P = 0.089, Pearson’s correlation). However, the children had significantly greater cycloplegic spherical equivalent refractive error (n = 57) than the adults (n = 8) (two sample t-test, P = 0.003).
Accommodative lag, RMS and Low-Frequency Component Comparisons between Groups and Tasks
Results of the repeated-measures ANOVA are found in Table 2 and Fig. 3. Groups 1 and 2 had significantly larger mean accommodative lags (group 1: P < .001, group 2: P = .007), and variability (root mean square and low-frequency component P < .001) during passive viewing compared with active viewing. A significant difference in overall mean accommodative lag and root mean square was not detected in the adults between the passive and active tasks (P < .05). However, the accommodative response was significantly more variable during passive viewing in adults for the low-frequency component (P = .04).
Table 2.
Results from the repeated measures two-factor analysis of variance comparing the mean accommodative lag and mean accommodative variability (time domain [root mean square, RMS] the low-frequency component [LFC, 0-0.6Hz]) between groups and by condition within each group.
| Accommodative Lag | RMS# | LFC# | ||
|---|---|---|---|---|
| Mean Difference (D), p-value | Mean Difference (D), p-value | Mean Difference (D), p-value | ||
| Passive Vs. Active* | Group 1 | 0.26, <0.001 | 0.23, <0.001 | 9.89E-04, <0.001 |
| Group 2 | 0.19, 0.007 | 0.09, <0.001 | 2.71E-04, <0.001 | |
| Adults | <0.01, 0.96 | 0.004, 0.07 | 1.11E-04, 0.04 | |
| Passiveˆ | Group 1 vs. Group 2 | 0.12, 0.336 | 0.23, <0.001 | 1.01-03, <0.001 |
| Group 1 vs. Adults | 0.40, 0.07 | 0.29, <0.001 | 1.21E-03, <0.001 | |
| Group 2 vs. Adults | 0.28, 0.22 | 0.06, 0.24 | 2.0E-04, 0.14 | |
| Activeˆ | Group 1 vs. Group 2 | 0.05, 0.69 | 0.09, 0.05 | 3.0E-04, 0.01 |
| Group 1 vs. Adults | 0.13, 0.83 | 0.11, 0.08 | 3.3E-04, 0.01 | |
| Group 2 vs. Adults | 0.08, 0.87 | 0.02, 0.45 | 3.1E-05, 0.290 | |
RMS – Root mean square, LFC – Low-frequency component (0-0.6 hertz), Hz – hertz, D – diopters
Data log transformed for analysis; reported as arithmetic median differences
positive difference indicates the outcome variable (accommodative lag, RMS or LFC) is largest in the passive condition
positive difference indicates outcome variable is largest in the first group listed
Significant P-values (< 0.05) are in bold
Figure 3.
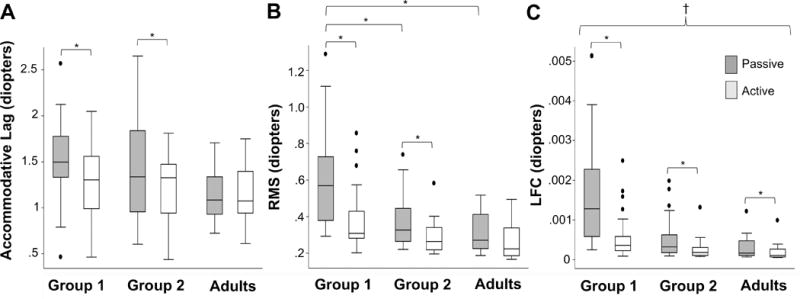
Box-plot diagram showing (A) the average accommodative lag, (B) accommodative variability (time domain, RMS), data is presented in its natural state, and (C) accommodative variability (frequency domain (low frequency component (LFC)), data is presented in its natural state. Group 1 represents children who viewed shape stimuli (n=31), Group 2 represents children who looked at text (n=23). The adults included 8 subjects. Asterisks represent significance of P<0.05. The symbol † is used to indicate significance between Group 1 and both Group 2 and Adults comparisons for both the passive and active viewing tasks.
In comparing across groups, there were no significant differences detected between any group comparisons for accommodative lag in either the passive or active tasks, despite Group 1 and Group 2 having, on average, 0.40 D and 0.28 D larger accommodative lags, respectively, than the adults during the passive task. There were, however, differences detected between groups when evaluating accommodative variability. The children in Group 1 had significantly more variable accommodative responses than both the children in Group 2 and the adults in both the time and frequency domains (RMS: P < 0.001, low-frequency component: P < 0.001) during the passive task and in the frequency domain during the active task (P < 0.001). Significant differences in accommodative variability were not detected between the children in Group 2 and the adults.
Relationships between the Magnitude of Uncorrected Hyperopia and Total Accommodative Response with the Outcomes Average Accommodative Lag, RMS and The Low-frequency Component in Children
Scatter plots of the relationships between the magnitude of uncorrected hyperopia and lag, RMS, and the low-frequency component are shown in Figure 4. The relationship between the subjects’ accommodative behavior (accuracy and variability) and the magnitude of uncorrected hyperopia was evaluated using multivariable regression while adjusting for the stimuli viewed and pupil size. The results are found in Table 3. There was no significant association between increased magnitude of uncorrected hyperopia and accommodative lag in either the passive (95% CI: −0.11 to +0.13) or active (95% CI: −0.19 to +0.02) tasks. There were however, significant relationships between accommodative variability in both the time and frequency domain of both the passive (RMS 95% CI: 0.04–0.15, low-frequency component 95% CI: 1.1E-4–6.5E-4) and active (RMS 95% CI: 0.001–0.06, low-frequency component 95% CI: 1.4E-5–2.3E-4) tasks.
Figure 4.
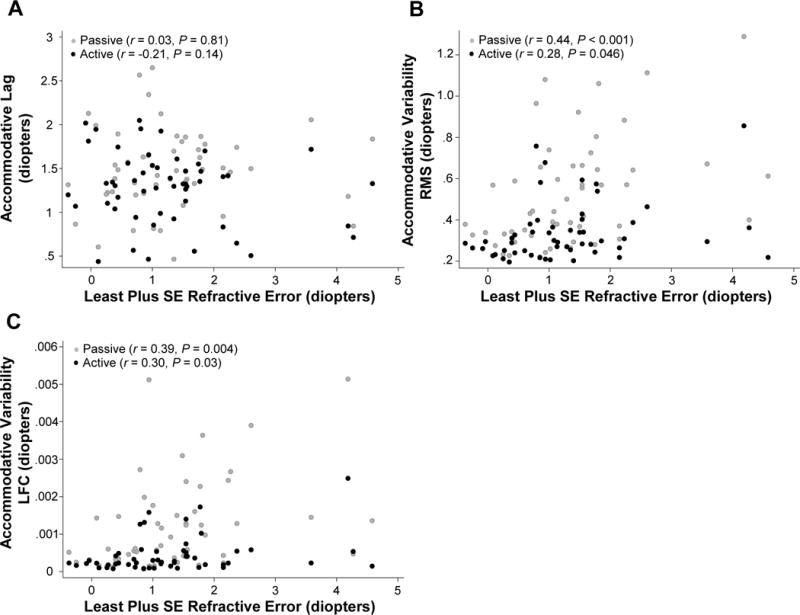
Scatter plots of (A) accommodative lag, (B) accommodative variability (time domain, root mean square, RMS), and (C) accommodative variability (low frequency component, LFC), with least plus spherical equivalent (SE) refractive error in both the passive (gray circles) and active (black circles) viewing tasks for the children.
Table 3.
Multivariable regression models for accommodative lag, accommodative variability (time domain [root mean square, RMS] the low-frequency component [LFC, 0-0.6Hz]) with independent variable uncorrected spherical equivalent hyperopia while adjusting for stimulus type (shapes or letters/text) and pupil size for all children.
| Passive (n = 54) |
Active (n = 53) |
||||||
|---|---|---|---|---|---|---|---|
| Beta-coeffient | 95% CI | p-value | Beta-coeffient | 95% CI | p-value | ||
| Accommodative Lag | Intercept | 1.90 | 0.81–3.00 | 0.001 | 1.53 | 0.46–2.61 | 0.006 |
| Uncorrected Hyperopia | 0.008 | −0.11 to +0.13 | 0.896 | −0.08 | −0.19 to +0.02 | 0.118 | |
| Stimuli | −0.11 | −0.37 to +0.15 | 0.399 | −0.11 | −0.34 to +0.12 | 0.344 | |
| Pupil Size | −0.05 | −0.23 to +0.14 | 0.617 | 0.002 | −0.17 to +0.18 | 0.981 | |
| RMS | Intercept | 1.19 | 0.72–1.66 | <0.001 | 0.93 | 0.62–1.25 | <0.001 |
| Uncorrected Hyperopia | 0.10 | 0.04–0.15 | 0.001 | 0.03 | 0.001–0.06 | 0.042 | |
| Stimuli | −0.21 | −0.32 to −0.10 | <0.001 | −0.07 | −0.14 to −0.002 | 0.043 | |
| Pupil Size | −0.08 | −0.16 to −0.007 | 0.034 | −0.09 | −0.14 to −0.04 | 0.004 | |
| LFC | Intercept | 3.7E-3 | 1.3E-3–6.1E-3 | 0.003 | 2.2E-3 | 1.1E-3–3.3E-3 | <0.001 |
| Uncorrected Hyperopia | 3.8E-4 | 1.1E-4–6.5E-4 | 0.006 | 1.2E-4 | 1.4E-5–2.3E-4 | 0.027 | |
| Stimuli | −9.9E-4 | −1.6E-3 to −4.2E-4 | 0.001 | −2.4E-4 | −4.8E-4 to −4.58E-6 | 0.046 | |
| Pupil Size | −2.7E-4 | −6.7E-4 to +1.3E-4 | 0.179 | −0.26E-4 | −4.4E-4 to −8.7E-5 | 0.004 | |
RMS – Root mean square, LFC – Low-frequency component (0-0.6 hertz)
Significant P-values are reported in bold
It would be expected that uncorrected hyperopia and the accommodative response would be perfectly correlated if all subjects had equivalent accommodative lag, however, as seen in Figure 4, this was not the case. While the magnitude of uncorrected hyperopia was highly correlated with the accommodative response (passive r = 0.92; active r = 0.94, Pearson’s Correlation), because of the range of accommodative lag, the linear regression models regarding stability of the response were repeated to investigate the relationship between the total accommodative response and accommodative variability (Table 4). A significant association was detected between increased total accommodative response and RMS and the low-frequency component in both the passive (RMS 95% CI: 0.01–0.12; low-frequency component 95% CI: 1.1E-5–5.2E-4) and active (RMS 95% CI: 0.01–0.06; low-frequency component 95% CI: 2.7E-5–2.1E-4) tasks.
Table 4.
Multivariable regression models for accommodative variability (time domain [root mean square, RMS] the low-frequency component [LFC, 0-0.6Hz]) with independent variable total accommodative response (calculated in the vertical meridian) while adjusting for stimulus type (shapes or letters/text) and pupil size for all children.
| Passive (n = 54) |
Active (n = 53) |
||||||
|---|---|---|---|---|---|---|---|
| Beta-coefficient | 95% CI | p-value | Beta-coefficient | 95% CI | p-value | ||
| RMS | Intercept | 1.16 | 0.65–1.66 | <0.001 | 0.88 | 0.56–1.20 | <0.001 |
| Total Accommodative Response | 0.06 | 0.01–0.12 | 0.015 | 0.03 | 0.01–0.06 | 0.019 | |
| Stimuli | −0.23 | −0.35 to -0.11 | <0.001 | −0.07 | −0.14 to -0.006 | 0.032 | |
| Pupil Size | −0.84 | −0.17 to −9.5E-4 | 0.048 | −0.09 | −0.14 to −0.39 | 0.001 | |
| LFC | Intercept | 3.5E-3 | 1.0E-3–6.0E-3 | 0.007 | 2.0E-3 | 9.3E-4–3.1E-3 | 0.001 |
| Total Accommodative Response | 2.7E-4 | 1.1E-5–5.2E-4 | 0.041 | 1.2E-4 | 2.7E-5–2.1E-4 | 0.013 | |
| Stimuli | −1.1E-3 | −1.6E-3 to −4.7E-4 | 0.001 | −2.5E-4 | −4.8E-4 to −2.0E-5 | 0.034 | |
| Pupil Size | −2.7E-4 | −6.8E-4 to 1.4E-4 | 0.197 | −2.6E-4 | −4.4E-4 to −8.9E-5 | 0.001 | |
RMS – Root mean square, LFC – Low-frequency component (0-0.6 hertz)
Significant P-values are reported in bold
DISCUSSION
The purpose of this study was to investigate the mean accommodative lag and variability in children between the ages of 3 and < 10 years with a wide-range of habitually uncorrected emmetropic and hyperopic refractive errors, over a sustained, 10-minute viewing period while engaged in tasks of varying cognitive demands. This study demonstrates that an increase in cognition improves both the accuracy and variability of the accommodative response in children (Table 2, Figure 3). This study also demonstrates that under sustained near viewing tasks in children 3 to <10 years, the magnitude of uncorrected hyperopia is associated with increased accommodative variability in both the time and frequency domains during near tasks, independent of the level of cognitive demand of the task or which stimuli the subjects viewed (shapes vs. letters) (Table 3). Our data also suggest that children with greater amounts of uncorrected hyperopia experience more variable accommodation in both the time and frequency domains than children with lesser amounts of hyperopia (Table 3).
Our results are in agreement with other studies that have shown accommodative lag decreases during near viewing tasks that require more cognitive effort.8, 12, 40 However, we found that to be true only for the children, as a significant difference of accommodative lag was not detected between the passive and active tasks in the adults (Table 2, Figure 3). Bullimore & Gillmartin (1988) also did not find a find a difference in accommodative lag between a passive and active task in adults at 33cm.11 It is unclear whether the accommodative system of adults is not as susceptible to cognitive effort as that of children, or if the adults could not help but concentrate during the passive task despite our efforts to use two disparate tasks.
In addition to the effect of cognition on accommodative lag, the results of this study suggest that increased cognitive effort is also associated with a significant decrease in accommodative variability resulting in a more stable accommodative response in both the children (RMS and low-frequency component) and the adults (low-frequency component), despite the overall increase in the mean accommodative response seen in the children (Table 2). Our data also suggest that on average, the stability of the accommodative response improves with age as the youngest children in Group 1 had the most variable responses while the adults had the least variable (Table 1). It is worth noting however that Groups 1 and 2 overlapped at age 6-7 (some 6 year olds and one 7-year-old viewed the shapes while others viewed the letters/text) due to different reading abilities and thus there is ambiguity in the results regarding how much of the differences detected between the groups is due to age versus being due to differences in the stimuli. The effect of cognitive effort on accommodative variability has not been previously investigated, and thus there are no other studies with which to compare our results.
As shown in Figure 3, in children, as the accommodative lag decreased (i.e.: increase in accommodative response) with an increase in cognitive demand during the active task, variability also decreased in both the time (RMS) and frequency domains (low-frequency component). This result is contrary to what one might expect based on previous studies that have found accommodative variability increases with an increase in the accommodative demand and subsequent response when performing within-subject analyses.14–16 In the present study, on average, as the accommodative response increased and became more accurate in the active task, the accommodative response was also more stable. It is feasible, however, that there was not a sufficiently large enough increase in the accommodative response to result in an increase in accommodative variability given that Kotulak and Schor (1986a) found that RMS increased 0.05D/1 D of accommodative response in adults16 and on average the difference in the accommodative responses between the active and passive tasks were 0.26 D for the youngest children and 0.19 D for the older children (Table 2). In addition, when adjusting for stimulus type and considering total accommodative response across children, the multivariable regression shown in Table 4 did demonstrate the expected relationship between increased accommodative response and increased accommodative variability. Therefore, this study also illustrates the importance in differentiating within-subject and between-subject analysis of accommodation, especially when considering associations between the magnitude and variability of the accommodative response. The aforementioned results demonstrate that on an individual level, as the accommodative response increases, resulting in a more accurate accommodative response, the accommodative response also becomes more stable. However, if the magnitude of the response is considered between subjects, children with a greater accommodative response tend to also have more variable responses. Thus, the interpretation of these relationships may differ depending upon whether the comparisons are made within- or between- subjects.
The results of this study differed with other studies that have shown that children with increased levels of uncorrected hyperopia tend to have increased levels of accommodative lag17, 18, 41, 42 as we found that accommodative lag did not increase as a function of the magnitude of uncorrected refractive error. This suggests that children with increased amounts of hyperopia in the absence of amblyopia and strabismus are able to accommodate, on average, a sufficient amount to have a similar average accommodative lag to children with lesser amounts of hyperopia during a 10-minute viewing period. However, our results demonstrated that children with increased magnitudes of uncorrected hyperopia had greater variability of their accommodative response as they have a larger magnitude of hyperopia to overcome (Table 3) than emmetropic and less hyperopic individuals. We confirmed these results by demonstrating that the variability of the accommodative response increased significantly as the magnitude of the total accommodative response increased (Table 4).
While the effect of an increase in the variability in the accommodative response is still unknown, it is reasonable to consider that a less stable accommodative response may impact visual performance, particularly at near where the accommodative demand is greatest. Several studies have described associations between uncorrected hyperopia and poor academic performance,43–46 and deficits in visuocognitive and visuomotor skills.47 Shankar et. al found that children 4 to 7 years of age with uncorrected hyperopia of ≥ +2.00 D in the most hyperopic meridian had worse letter and word recognition, receptive vocabulary, and orthography abilities than children with ≤ +1.50 D.48 The Vision in Preschoolers-Hyperopia in Preschoolers Group reported that uncorrected hyperopia ≥ +4.00 D spherical equivalent or ≥ +3.00D to ≤ +6.00 D spherical equivalent combined with decreased near VA or stereoacuity was significantly associated with poor reading readiness in children 4 and 5 years of age,49 and the Baltimore Reading and Eye Disease Study found that hyperopia ≥ +1.00 D was associated with reduced reading achievement in second and third graders in the Baltimore public schools (Collins M, et al. IOVS 2016, E-Abstract 1536). Although the mechanism by which uncorrected hyperopia negatively impacts visual and cognitive development in children is unknown, it is becoming increasingly clearer that an association exists, at least in some children. Our study has identified a potential contributing factor (e.g.: accommodative variability) as our results show that children with increased magnitudes of uncorrected hyperopia tend to have less stable accommodative response which may impact near visual tasks. More work needs to be done in this area to further understand the many associations identified between accommodation, uncorrected hyperopia and educational outcomes.
Study Limitations
Children were as young as 3 years of age and there may be concern that the youngest subjects did not cooperate throughout the duration of the study given the length of both experimental tasks. However, of the children between the ages 3 to <6 years, only one child recruited was unable to complete either experimental task, and 88% of participants completed the passive viewing task and 84% of participants completed the active viewing task, with 100% of participants ≥ 6 years completing both experiments. Thus, cooperation was not problematic for the vast majority of the young children.
Another limitation of the study concerns the calculation of lag. While relative lens calibrations were performed to account for inter-subject variability in the luminance slope change per diopter, absolute calibrations (absolute off-set) were not performed. Previous authors have analyzed accommodative lag data using photorefraction by calculating a relative change in accommodation from one distance to another. However, this approach relies on the assumption that the subjects fully relax accommodation at the distance stimulus. Given that we were specifically recruiting children with uncorrected hyperopia, it would be incorrect to assume that the children relaxed accommodation for distance, or even had accurate focus to the distance stimulus, making it difficult to perform an absolute calibration. Thus, we accepted the limitation of calculating the accommodative lag from the Power Ref II output for all subjects without an absolute offset calibration. Our decision to do so was also supported by findings that the PowerRefractor (Multichannel Systems version – predecessor to the PowerRef II) compares well to retinoscopy (mean difference = −0.28D (range −0.43 to 0.05 D))50 and the Grand Seiko autorefractor (mean difference at 2.5 D demand = 0.08 ± 0.32 D; mean difference at 5 D demand −0.32 ± 0.48 D),51 and thus we feel that any errors from lack of offset calibration would be small for the 33cm viewing distance.
An additional limitation of the study is that accommodative measures were obtained using the Power Ref II while cycloplegic refraction was measured using the Grand Seiko autorefractor, thus potentially introducing errors into our results when we calculated the total accommodative response if the two instruments were not in agreement. Our decision to accept this limitation was due to the fact that the Power Ref II has a limited working range which is likely not large enough to accurately measure the full magnitude of hyperopia present in some of our subjects. In addition, once the children were under cycloplegia, most of them had pupils that were outside of the operating range of the PowerRef II, making it impossible to obtain a result. Lastly, given that the Power Ref II has been shown to compare well with the Grand Seiko autorefractor for distance and near viewing51 we felt that any errors introduced by combining findings from the two instruments would be minimal.
CONCLUSIONS
Our results suggest that in children, the accommodative response is more variable as the magnitude of uncorrected hyperopia increases. Accommodative variability may affect these children’s ability to perform sustained near tasks.
Acknowledgments
A portion of this work was presented at the Annual American Academy of Optometry Meeting in Anaheim, CA (2016) and at the Annual Association of Research in Vision and Ophthalmology Meeting in Seattle, WA (2016).
Contributor Information
Tawna L. Roberts, Children’s Hospital Vision Center, Akron Children’s Hospital, Akron, OH.
Ruth E. Manny, University of Houston College of Optometry, Houston, Texas.
Julia S. Benoit, University of Houston College of Optometry, Houston, Texas; Texas Institute for Measurement, Evaluation, and Statistics, University of Houston, Houston, Texas.
Heather A. Anderson, University of Houston College of Optometry, Houston, Texas.
References
- 1.Charman WN, Heron G. Fluctuations in Accommodation: A Review. Ophthalmic Physiol Opt. 1988;8:153–64. doi: 10.1111/j.1475-1313.1988.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 2.Hennessy RT, Iida T, Shina K, Leibowitz HW. The Effect of Pupil Size on Accommodation. Vision Res. 1976;16:587–9. doi: 10.1016/0042-6989(76)90004-3. [DOI] [PubMed] [Google Scholar]
- 3.Campbell FW, Westheimer G. Dynamics of Accommodation Responses of the Human Eye. J Physiol. 1960;151:285–95. doi: 10.1113/jphysiol.1960.sp006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tucker J, Charman WN. Reaction and Response Times for Accommodation. Am J Optom Physiol Opt. 1979;56:490–503. doi: 10.1097/00006324-197908000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Tucker J, Charman WN. Depth of Focus and Accommodation for Sinusoidal Gratings as a Function of Luminance. Am J Optom Physiol Opt. 1986;63:58–70. doi: 10.1097/00006324-198601000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Kruger PB, Pola J. Stimuli for Accommodation: Blur, Chromatic Aberration and Size. Vision Res. 1986;26:957–71. doi: 10.1016/0042-6989(86)90153-7. [DOI] [PubMed] [Google Scholar]
- 7.McLin LN, Jr, Schor CM, Kruger PB. Changing Size (Looming) as a Stimulus to Accommodation and Vergence. Vision Res. 1988;28:883–98. doi: 10.1016/0042-6989(88)90098-3. [DOI] [PubMed] [Google Scholar]
- 8.Woodhouse JM, Cregg M, Gunter HL, et al. The Effect of Age, Size of Target, and Cognitive Factors on Accommodative Responses of Children with Down Syndrome. Invest Ophthalmol Vis Sci. 2000;41:2479–85. [PubMed] [Google Scholar]
- 9.Schor CM, Johnson CA, Post RB. Adaptation of Tonic Accommodation. Ophthalmic Physiol Opt. 1984;4:133–7. [PubMed] [Google Scholar]
- 10.Schor CM, Alexander J, Cormack L, Stevenson S. Negative Feedback Control Model of Proximal Convergence and Accommodation. Ophthalmic Physiol Opt. 1992;12:307–18. [PubMed] [Google Scholar]
- 11.Bullimore MA, Gilmartin B. The Accommodative Response, Refractive Error and Mental Effort: 1. The Sympathetic Nervous System. Doc Ophthalmol. 1988;69:385–97. doi: 10.1007/BF00162751. [DOI] [PubMed] [Google Scholar]
- 12.Francis EL, Jiang BC, Owens DA, Tyrrell RA. Accommodation and Vergence Require Effort-to-See. Optom Vis Sci. 2003;80:467–73. doi: 10.1097/00006324-200306000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfield M, Ciuffreda KJ. Proximal and Cognitively-Induced Accommodation. Ophthalmic Physiol Opt. 1990;10:252–6. [PubMed] [Google Scholar]
- 14.Anderson HA, Glasser A, Manny RE, Stuebing KK. Age-Related Changes in Accommodative Dynamics from Preschool to Adulthood. Invest Ophthalmol Vis Sci. 2010;51:614–22. doi: 10.1167/iovs.09-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Candy TR, Bharadwaj SR. The Stability of Steady State Accommodation in Human Infants. J Vis. 2007;7:4.1–16. doi: 10.1167/7.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotulak JC, Schor CM. Temporal Variations in Accommodation during Steady-State Conditions. J Opt Soc Am (A) 1986;3:223–7. doi: 10.1364/josaa.3.000223. [DOI] [PubMed] [Google Scholar]
- 17.Candy TR, Gray KH, Hohenbary CC, Lyon DW. The Accommodative Lag of the Young Hyperopic Patient. Invest Ophthalmol Vis Sci. 2012;53:143–9. doi: 10.1167/iovs.11-8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horwood AM, Riddell PM. Hypo-Accommodation Responses in Hypermetropic Infants and Children. Br J Ophthalmol. 2011;95:231–7. doi: 10.1136/bjo.2009.177378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schultz KE, Sinnott LT, Mutti DO, Bailey MD. Accommodative Fluctuations, Lens Tension, and Ciliary Body Thickness in Children. Optom Vis Sci. 2009;86:677–84. doi: 10.1097/OPX.0b013e3181a7b3ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen G, Tarczy-Hornoch K, McKean-Cowdin R, et al. Prevalence of Myopia, Hyperopia, and Astigmatism in Non-Hispanic White and Asian Children: Multi-Ethnic Pediatric Eye Disease Study. Ophthalmology. 2013;120:2109–16. doi: 10.1016/j.ophtha.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zadnik K, Manny RE, Yu JA, et al. Ocular Component Data in Schoolchildren as a Function of Age and Gender. Optom Vis Sci. 2003;80:226–36. doi: 10.1097/00006324-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Shufelt C, Fraser-Bell S, Ying-Lai M, et al. Refractive Error, Ocular Biometry, and Lens Opalescence in an Adult Population: The Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2005;46:4450–60. doi: 10.1167/iovs.05-0435. [DOI] [PubMed] [Google Scholar]
- 23.Campbell FW, Robson JG, Westheimer G. Fluctuations of Accommodation under Steady Viewing Conditions. J Physiol. 1959;145:579–94. doi: 10.1113/jphysiol.1959.sp006164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray LS, Winn B, Gilmartin B. Accommodative Microfluctuations and Pupil Diameter. Vision Res. 1993;33:2083–90. doi: 10.1016/0042-6989(93)90007-j. [DOI] [PubMed] [Google Scholar]
- 25.Kotulak JC, Schor CM. The Accommodative Response to Subthreshold Blur and to Perceptual Fading During the Troxler Phenomenon. Perception. 1986;15:7–15. doi: 10.1068/p150007. [DOI] [PubMed] [Google Scholar]
- 26.Cotter SA. Management of Childhood Hyperopia: A Pediatric Optometrist’s Perspective. Optom Vis Sci. 2007;84:103–9. doi: 10.1097/OPX.0b013e318031b08a. [DOI] [PubMed] [Google Scholar]
- 27.Moke PS, Turpin AH, Beck RW, et al. Computerized Method of Visual Acuity Testing: Adaptation of the Amblyopia Treatment Study Visual Acuity Testing Protocol. Am J Ophthalmol. 2001;132:903–9. doi: 10.1016/s0002-9394(01)01256-9. [DOI] [PubMed] [Google Scholar]
- 28.Cotter SA, Chu RH, Chandler DL, et al. Reliability of the Electronic Early Treatment Diabetic Retinopathy Study Testing Protocol in Children 7 to <13 Years Old. Am J Ophthalmol. 2003;136:655–61. doi: 10.1016/s0002-9394(03)00388-x. [DOI] [PubMed] [Google Scholar]
- 29.Pan Y, Tarczy-Hornoch K, Cotter SA, et al. Visual Acuity Norms in Pre-School Children: The Multi-Ethnic Pediatric Eye Disease Study. Optom Vis Sci. 2009;86:607–12. doi: 10.1097/OPX.0b013e3181a76e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheiman MM, Hertle RW, Kraker RT, et al. Patching Vs Atropine to Treat Amblyopia in Children Aged 7 to 12 Years: A Randomized Trial. Arch Ophthalmol. 2008;126:1634–42. doi: 10.1001/archophthalmol.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pediatric Eye Disease Investigator Group. A Randomized Trial of Atropine vs. Patching for Treatment of Moderate Amblyopia in Children. Arch Ophthalmol. 2002;120:268–78. doi: 10.1001/archopht.120.3.268. [DOI] [PubMed] [Google Scholar]
- 32.Thibos LN, Wheeler W, Horner D. Power Vectors: An Application of Fourier Analysis to the Description and Statistical Analysis of Refractive Error. Optom Vis Sci. 1997;74:367–75. doi: 10.1097/00006324-199706000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Lapray M, Ross R. Graded Word List – Quick Gauge of Reading Ability. J Reading. 1969;12:305–7. [Google Scholar]
- 34.Schaeffel F, Wilhelm H, Zrenner E. Inter-Individual Variability in the Dynamics of Natural Accommodation in Humans: Relation to Age and Refractive Errors. J Physiol. 1993;461:301–20. doi: 10.1113/jphysiol.1993.sp019515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi M, Weiss S, Schaeffel F, et al. Laboratory, Clinical, and Kindergarten Test of a New Eccentric Infrared Photorefractor (Powerrefractor) Optom Vis Sci. 2000;77:537–48. doi: 10.1097/00006324-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Harb E, Thorn F, Troilo D. Characteristics of Accommodative Behavior During Sustained Reading in Emmetropes and Myopes. Vision Res. 2006;46:2581–92. doi: 10.1016/j.visres.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atchison DA, Pritchard N, Schmid KL. Peripheral Refraction Along the Horizontal and Vertical Visual Fields in Myopia. Vision Res. 2006;46:1450–8. doi: 10.1016/j.visres.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 38.Horwood AM, Riddell PM. The Use of Cues to Convergence and Accommodation in Naive, Uninstructed Participants. Vision Res. 2008;48:1613–24. doi: 10.1016/j.visres.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keating MP. Dioptric Power in an Off-Axis Meridian: The Torsional Component. Am J Optom Physiol Opt. 1986;63:830–8. doi: 10.1097/00006324-198610000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Kruger PB. The Effect of Cognitive Demand on Accommodation. Am J Optom Physiol Opt. 1980;57:440–5. doi: 10.1097/00006324-198007000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Mutti DO. To Emmetropize or Not to Emmetropize? The Question for Hyperopic Development. Optom Vis Sci. 2007;84:97–102. doi: 10.1097/OPX.0b013e318031b079. [DOI] [PubMed] [Google Scholar]
- 42.Tarczy-Hornoch K. Accommodative Lag and Refractive Error in Infants and Toddlers. J AAPOS. 2012;16:112–7. doi: 10.1016/j.jaapos.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grisham JD, Simons HD. Refractive Error and the Reading Process: A Literature Analysis. J Am Optom Assoc. 1986;57:44–55. [PubMed] [Google Scholar]
- 44.Rosner J. Comparison of Visual Characteristics in Children with and without Learning Difficulties. Am J Optom Physiol Opt. 1987;64:531–3. doi: 10.1097/00006324-198707000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Rosner J. The Relationship between Moderate Hyperopia and Academic Achievement: How Much Plus Is Enough? J Am Optom Assoc. 1997;68:648–50. [PubMed] [Google Scholar]
- 46.Williams WR, Latif AH, Hannington L, Watkins DR. Hyperopia and Educational Attainment in a Primary School Cohort. Arch Dis Child. 2005;90:150–3. doi: 10.1136/adc.2003.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atkinson J, Anker S, Nardini M, et al. Infant Vision Screening Predicts Failures on Motor and Cognitive Tests up to School Age. Strabismus. 2002;10:187–98. doi: 10.1076/stra.10.3.187.8125. [DOI] [PubMed] [Google Scholar]
- 48.Shankar S, Evans MA, Bobier WR. Hyperopia and Emergent Literacy of Young Children: Pilot Study. Optom Vis Sci. 2007;84:1031–8. doi: 10.1097/OPX.0b013e318157a67a. [DOI] [PubMed] [Google Scholar]
- 49.Group V-HS. Kulp MT, Ciner E, et al. Uncorrected Hyperopia and Preschool Early Literacy: Results of the Vision in Preschoolers-Hyperopia in Preschoolers (VIP-HIP) Study. Ophthalmology. 2016;123:681–9. doi: 10.1016/j.ophtha.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blade PJ, Candy TR. Validation of the Power Refractor for Measuring Human Infant Refraction. Optom Vis Sci. 2006;83:346–53. doi: 10.1097/01.opx.0000221402.35099.fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aldaba M, Gomez-Lopez S, Vilaseca M, et al. Comparing Autorefractors for Measurement of Accommodation. Optom Vis Sci. 2015;92:1003–11. doi: 10.1097/OPX.0000000000000685. [DOI] [PubMed] [Google Scholar]


