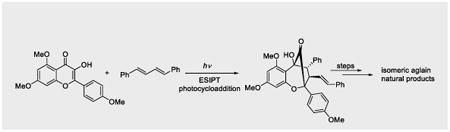
Abstract
Selective ESIPT photocycloaddition of 3-hydroxyflavones with trans, trans-1,4-diphenyl-1,3-butadiene is described. Using this methodology, total syntheses of the natural products (±)-foveoglin A and (±)-perviridisin B have been accomplished. Enantioselective ESIPT photocycloaddition using TADDOLs as chiral hydrogen-bonding additives provided access to (+)-foveoglin A. Mechanistic studies have revealed the possibility for a photoinduced electron transfer (PET) pathway.
Keywords: ESIPT photocycloaddition, Total synthesis, Photoinduced electron transfer, Asymmetric photoreaction
Graphical abstract
Selective ESIPT photocycloaddition of 3-hydroxyflavones with trans,trans-1,4-diphenyl-1,3-butadiene has been achieved which enabled the total syntheses of isomeric aglain natural products foveoglin A and perviridisin B. Mechanistic studies have revealed the possibility of a photoinduced electron transfer (PET) pathway.
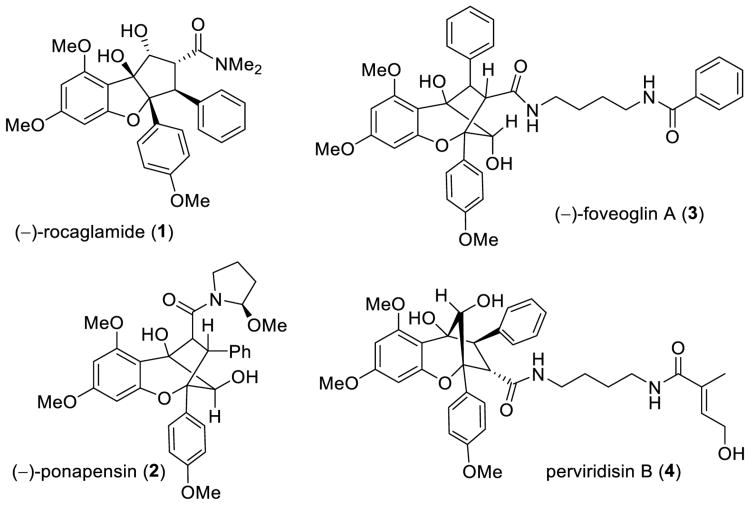
Flavagline (rocaglate) natural products are compounds[1] which are biosynthetically derived from a flavonoid and a cinnamic amide derivative and include the cyclopenta[b]benzofuran rocaglamide (1)[2] and the cyclopenta[b,c]benzopyran (aglain) ponapensin (2)[3] (Figure 1). Both natural and synthetic rocaglates have been shown to exhibit anticancer and antiviral activities.[4] Due to their structural complexity and intriguing biological activities, a number of groups have reported chemical syntheses of rocaglates and analogues.[5] Previously, we reported a biomimetic approach towards these compounds in which 3-hydroxyflavone (3-HF) 5 undergoes ESIPT (excited state intramolecular proton transfer)-mediated (3+2) photocycloaddition[6] with dipolarophiles including methyl cinnamate 6a.[7] Although this approach rapidly provides access to rocaglates, recent isolation reports of the aglain derivatives foveoglin A (3)[8] and perviridisin B (4),[9] as well as the related aglapervirisins,[10] suggested new challenges to employ ESIPT photocycloaddition to access their isomeric core structures in comparison to ponapensin (2).
Figure 1.
Rocaglamide (1), ponapensin (2), and the isomeric aglain natural products (3) and (4).
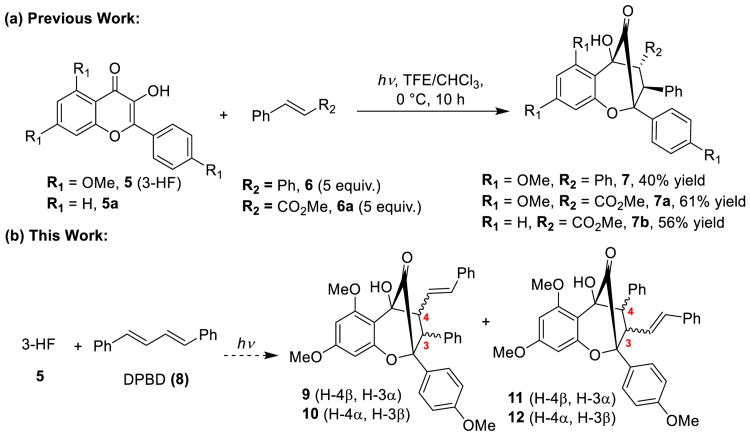
We have previously reported that trans-stilbene 6may be used as a dipolarophile in ESIPT photocycloadditions of 3-hydroxyflavones 5 (Scheme 1).[11] In the current study, we have evaluated trans,trans-1,4-diphenyl-1,3-butadiene (DPBD, 8) as dipolarophile. By extending the conjugation of the reaction partner, we expected that four possible isomers could be formed (Scheme 1b). In particular, preferential formation of 11/12 vs. 9/10 would provide access to aglain scaffolds required for syntheses of 3 and 4 wherein the styrene moiety serves as an amide precursor.
Scheme 1.
(3+2)-Photocycloaddition of 3-hydroxyflavones with dipolarophiles 6, 6a, and 8.
We first examined photocycloadditions of 3-hydroxyflavone 5 and DPBD 8 (Scheme 2). Upon photoirradiation of 5 and 8 using TFE/CHCl3 (3:7) in a continuous photoflow reactor,[5c] only diastereomers 11 and 12 were produced (d.r. = 5:1). The inseparable mixture was treated with NaBH4 to afford the corresponding secondary alcohols (d.r.= 5:1) and isolation of the major product 13 in 44% yield (2 steps). Compound 13 was subsequently acylated to provide the (±)-p-bromobenzoate (±)-14 whose structure was established by single crystal X-ray diffraction.[12]
Scheme 2.
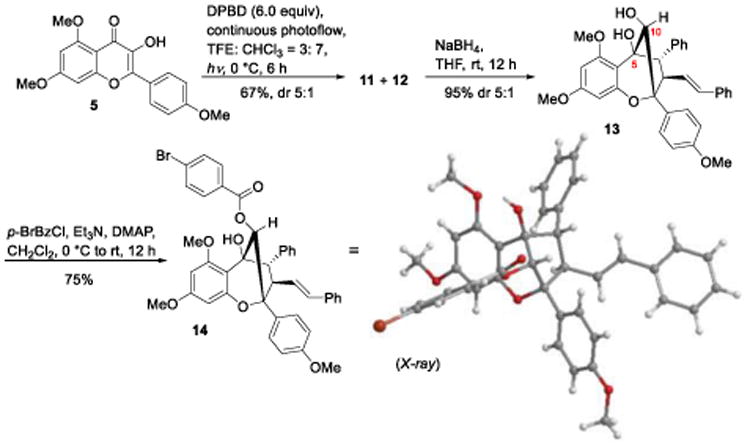
ESIPT photocycloaddition of 3-HF 5 with DPBD 8.
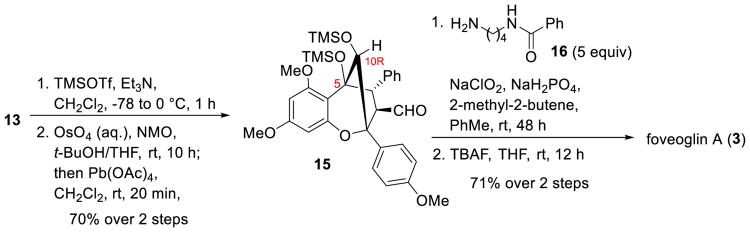
The total synthesis of (±)-foveoglin A (3) was next accomplished as illustrated in Scheme 3. Bis-trimethylsilyl protection of 13 to block cleavage of the C5-C10 bond followed by OsO4-catalyzed dihydroxylation and subsequent oxidative cleavage of the derived diol using Pb(OAc)4 provided aldehyde 15 (70% yield, 2 steps). Compound 15 was further treated with amine 16 to generate the corresponding imine which was transformed to an amide using oxidative amidation conditions.[13] Subsequent silyl deprotection using tetrabutylammonium fluoride furnished (±)-foveoglin A (3).
Scheme 3.
Total synthesis of (±)-foveoglin A.
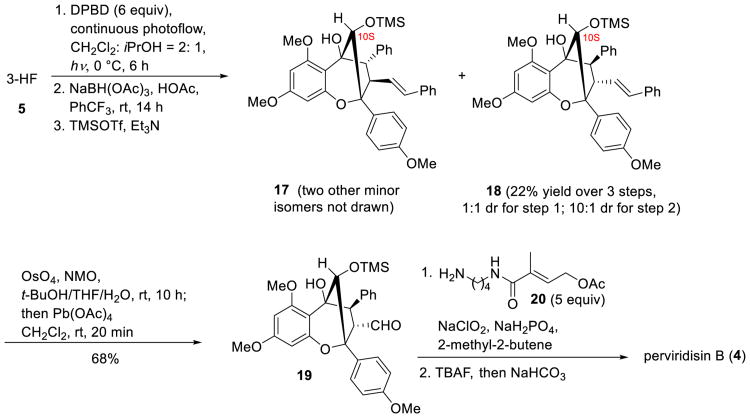
In order to achieve the synthesis of the congener perviridisin B (4), practical access to the exo-diastereomer 12 was required. After thorough evaluation of conditions, flow photocycloaddition of 5 and 8 using 2:1 CH2Cl2:isopropanol as solvent afforded a 52% yield of photocycloadducts 11 and 12 (d.r. = 1:1). The corresponding 10S-alcohol epimers were produced in 96% yield and in 10:1 diastereoselectivity by reduction with NaBH(OAc)3 in trifluorotoluene. Subsequent trimethylsilyl protection furnished the mono-silylated product 18 which was subjected to oxidative double bond cleavage to afford aldehyde 19 in 68% yield. Oxidative amidation[13] of 19 with amine 20, followed by TMS deprotection and in situ acetate hydrolysis, afforded (±)-perviridisin B (4) (Scheme 4).
Scheme 4.
Total synthesis of (±)-perviridisin B.
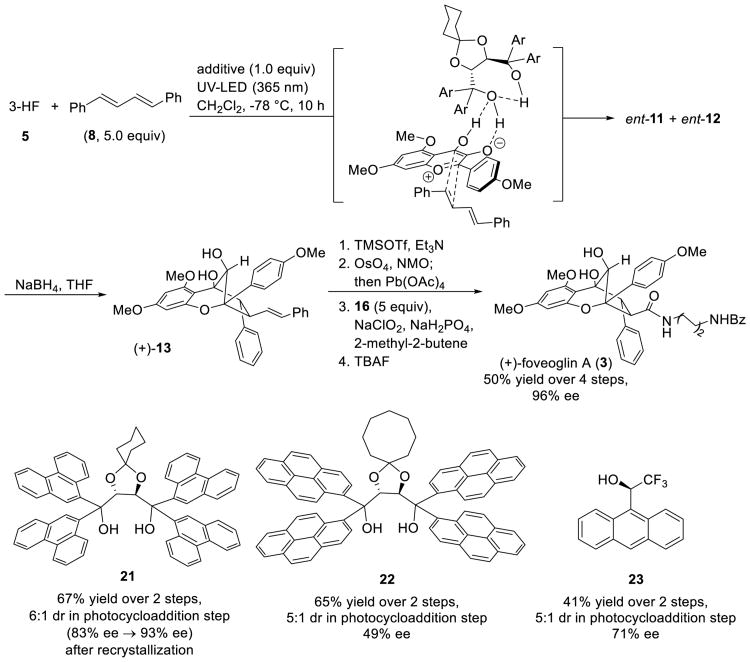
We next studied the corresponding enantioselective ESIPT photocycloaddition using chiral hydrogen-bonding additives.[14] Although we have reported use of TADDOL derivatives to mediate enantioselective ESIPT photocycloaddition of 3-hydroxyflavones, thus far dipolarophiles have been limited to cinnamates. Use of DPBD 8 which lacks the ester moiety as a potential hydrogen-bonding acceptor in comparison to dipolarophile 6a provides further support for our proposal that host-guest complexation of 3-HF 5 by hydrogen-bonding additives controls enantioselectivity.[14a] In the presence of a stoichiometric amount of a hydrogen-bonding additive, 3-HF 5 and DPBD 8 were irradiated for 10 h at -78 °C. Limited solubility of DPBD 8 at low temperatures was observed which prompted us to dilute the reaction (0.005 M) to achieve homogeneity. Using TADDOL 21 as a stoichiometric additive, we obtained two inseparable diastereomers which were reduced with NaBH4 to obtain major diastereomer 13 for enantiomeric excess (e.e.) determination. As maintaining low temperature is helpful for enantioselectivity,[14] and to overcome significant heat release from use of traditional UV lamp, a UV-LED (λ = 365 nm) was chosen as a light source[15] After condition optimization,[16] TADDOL 21 was found to be an optimal additive to provide compound (+)-13 in 67% yield and 83% e.e., whereas TADDOL 22 was able to facilitate the photocycloaddition in a similar yield and in 49% e.e. (Scheme 5). Interestingly, use of the chiral trifluoroethanol, Pirkle's alcohol 23, led to 13 in 71% e.e. Although Pirkle's alcohol 23 was previously reported as an NMR chiral shift reagent,[17] this study represents the first application of Pirkle's alcohol as a chiral hydrogen bonding additive.
Scheme 5.
Enantioselective ESIPT photocycloaddition and asymmetric synthesis of (+)-foveoglin A.
Due to difficulties in achieving absolute stereochemistry assignment of (+)-13 or (+)-14 by X-ray crystal structure analysis, the absolute configuration of (+)-13 was determined by VCD analysis[18] of p-bromobenzoate (+)-14.[16] Further conversion of (+)-13 (Scheme 5) afforded (+)-foveoglin A in 96% ee ([α]D[20] -30.0 natural (c 0.16, CHCl3), [α]D[23] +25.3 synthetic (c 0.16, CHCl3)). The absolute configuration of natural (-)-foveoglin A (3) is in agreement with the aglain natural product, (-)-ponapensin (2).[3]
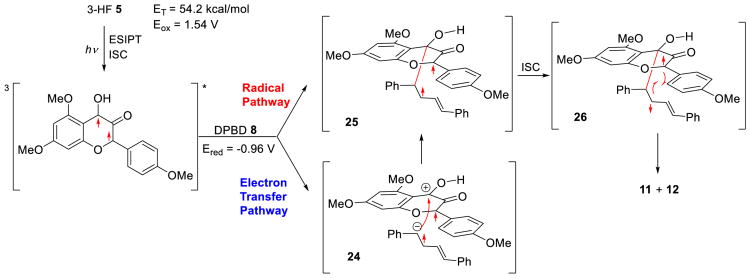
Mechanistic studies were next conducted to provide a rationale for the selectivity of the photocycloaddition. In polar protic solvents (e.g. trifluoroethanol), dual fluorescence emission (fluorescence of the normal state N and tautomer state T) was observed both at room temperature and 77 K[16] Based on the fast ESIPT process and the singlet state energy for 5, singlet energy transfer from excited 5 to 8 was ruled out.[20] As determined by the phosphorescence spectrum, the lowest triplet excited state of 3-HF 5 was found to be ∼54 kcal/mol[16] In addition, using the Rehm-Weller equation,[21] the feasibility for photoinduced electron transfer (PET)[22] initiating the photocycloaddition from the triplet excited state was ascertained. The free energy for electron transfer from the triplet excited state was exergonic (ΔGET = -5.3 kcal/mol) suggesting a facile PET pathway initiating the photocycloaddition. To ascertain that photoexcitation of 3-HF 5 rather than DPBD 8 is crucial for photoreactivity, a control experiment using a purple LED (λmax = 395 nm) as light source for selective excitation of 5 was found to cleanly produce the photocycloadduct.[16] As shown in Figure 2, the triplet excited state of 5 may initiate the photocycloaddition via radical and/or electron transfer pathways. Formation of the triplet diradical 25 either by direct addition of 8 to 35* or through radical ion pair 24 followed by subsequent intersystem crossing (ISC) leads to singlet diradical 26. Ring closure of 26 affords 11 and 12. The selectivity in the system is dictated by the electronics (due to charge transfer interactions) as well as the approach of 8 towards [3]5T* Based on our photophysical measurements,[16] we did not observe an electron donor-acceptor (EDA) complex[23] between 5 and 8 in the ground state. The lack of a ground state EDA complex is expected as triplet state 5 initiates the electron transfer pathway.
Figure 2.
Proposed mechanism for ESIPT photocycloaddition of 3-HF 5 and DPBD 8.
To further evaluate dipolarophile scope, we synthesized a number of unsymmetrical DPBDs and tested their feasibility in ESIPT photocycloaddition.[16] Use of the electron deficient pentafluorophenyl DPBD 27[24] cleanly afforded photocycloadducts which were subjected to the previously described reduction, silylation, and alkene cleavage sequence to afford two aldehyde products 30 and 15 in a 4:1 ratio (Scheme 6). This outcome indicates that the pentafluorophenyl moiety of 27 may stabilize negative charge after formation of a radical ion pair (cf. 24, Figure 2) which appears to dominate the selectivity for the photocycloaddition. Additionally, a π-π stacking (donor-acceptor) interaction (cf. 28, Scheme 6) between 5 and 27 also appears to orient photocycloaddition site-selectivity. This selectivity was also observed with other fluorinated dipolarophiles including bis-trifluoromethylphenyl DPBD and pentafluorostilbene.[16]
Scheme 6.
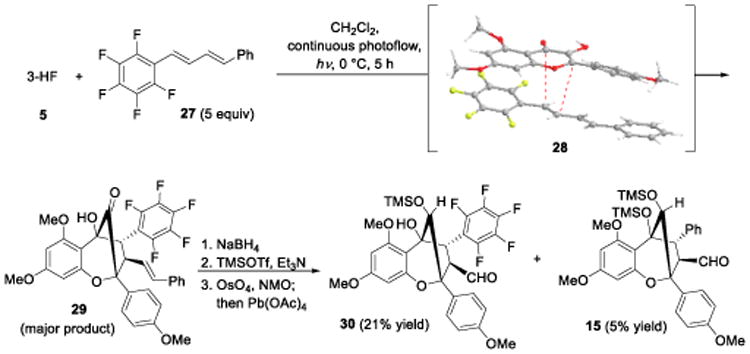
Use of pentafluorophenyl dipolarophile 27 in ESIPT photocycloaddition.
In summary, total syntheses of the aglain natural products foveoglin A (3) and perviridisin B (4) have been accomplished employing selective ESIPT photocycloaddition of 3-HF 5 with DPBD 8. Enantioselective ESIPT photocycloaddition was also demonstrated using chiral hydrogen-bonding additives to access (+)-foveoglin A. Mechanistic studies were conducted leading to the identification of a photoinduced electron transfer (PET) pathway. ESIPT photocycloadditions of 3-HF 5 with substituted, unsymmetrical DPBDs have also been conducted leading to site-selective photocycloadditions. Further applications of ESIPT photocycloaddition towards the syntheses of complex natural products and derivatives are currently in progress and will be reported in due course.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (R35 GM118173) for research support. J.S. and A.C. thank NDSU for support. J.S. and A.C. thank the National Science Foundation (CHE-1465075) for the purchase of Combiflash and solvent purification systems. We thank Dr. Jeffrey Bacon (Boston University) for X-ray crystal structure analysis and Dr. Sergey Shilov (Bruker Optics, Billerica, MA) for VCD data acquisition. NMR (CHE-0619339) and MS (CHE-0443618) facilities at Boston University are supported by the NSF and Work at the BU-CMD is supported by R24 GM111625.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.a) Ebada SS, Lajkiewicz NJ, Porco JA, Jr, Li-Weber M, Proksch P. Prog Chem Org Nat Prod. 2011;94:1–58. doi: 10.1007/978-3-7091-0748-5_1. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Basmadjian C, Thuaud F, Ribeiro R, Désaubry L. Future Med Chem. 2013;5:2185–2197. doi: 10.4155/fmc.13.177. [DOI] [PubMed] [Google Scholar]; c) Pan L, Woodward JL, Lucas DM, Fuchs JR, Kinghorn AD. Nat Prod Rep. 2014;31:924–939. doi: 10.1039/c4np00006d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King ML, Chiang CC, Ling HC, Fugita E, Ochiai M, McPhail AT. J Chem Soc, Chem Commun. 1982:1150–1151. [Google Scholar]
- 3.Lajkiewicz NJ, Roche SP, Gerard B, Porco JA., Jr J Am Chem Soc. 2012;134:13108–13113. doi: 10.1021/ja305342f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Chu J, Cencic R, Wang W, Porco JA, Jr, Pelletier J. Mol Cancer Ther. 2015;15:136–141. doi: 10.1158/1535-7163.MCT-15-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu S, Wang W, Brown LE, Qiu C, Lajkiewicz N, Zhao T, Zhou J, Porco JA, Jr, Wang TT. EBioMedicine. 2015;2:1600–1606. doi: 10.1016/j.ebiom.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Thuaud F, Bernard Y, Turkeri G, Dirr R, Aubert G, Cresteil T, Baguet A, Tomasetto C, Svitkin Y, Sonenberg N, Nebigil CG, Désaubry L. J Med Chem. 2009;52:5176–5187. doi: 10.1021/jm900365v. [DOI] [PubMed] [Google Scholar]; b) Stone SD, Lajkiewicz NJ, Whitesell L, Hilmy A, Porco JA., Jr J Am Chem Soc. 2015;137:525–530. doi: 10.1021/ja511728b. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wang W, Cencic R, Whitesell L, Pelletier J, Porco JA., Jr Chem Eur J. 2016;22:12006–12010. doi: 10.1002/chem.201602953. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) El Sous M, Khoo ML, Holloway G, Owen D, Scammells PJ, Rizzacasa MA. Angew Chem. 2007;119:7981–7984. doi: 10.1002/anie.200702700. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;46:7835–7838. [Google Scholar]; d) Yueh H, Gao Q, Porco JA, Jr, Beeler AB. Bioorg Med Chem. 2017 doi: 10.1016/j.bmc.2017.06.010. http://dx.doi.org/10.1016/j.bmc.2017.06.010. [DOI] [PMC free article] [PubMed]
- 6.Reviews on complex molecule synthesis using photochemical reactions: Bach T, Hehn JP. Angew Chem. 2011;123:1032–1077. doi: 10.1002/anie.201002845.Angew Chem Int Ed. 2011;50:1000–1045. doi: 10.1002/anie.201002845.Kärkäs MD, Porco JA, Jr, Stephenson CRJ. Chem Rev. 2016;116:9683–9747. doi: 10.1021/acs.chemrev.5b00760..
- 7.Gerard B, Jones G, II, Porco JA., Jr J Am Chem Soc. 2004;126:13620–13621. doi: 10.1021/ja044798o. [DOI] [PubMed] [Google Scholar]
- 8.Salim AA, Chai H, Rachman I, Riswan S, Kardono LBS, Farnsworth NR, Carcache-Blanco EJ, Kinghorn AD. Tetrahedron. 2007;63:7926–7934. doi: 10.1016/j.tet.2007.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan L, Acuña UM, Li J, Jena N, Ninh TN, Pannell CM, Chai H, Fuchs JR, Carcache de Blanco EJ, Soejarto DD, Kinghorn AD. J Nat Prod. 2013;76:394–404. doi: 10.1021/np3007588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An F, Wang X, Wang H, Li Z, Yang M, Luo J, Kong L. Sci Rep. 2016;6 doi: 10.1038/srep20045. 2016. [DOI] [Google Scholar]
- 11.Roche SP, Cencic R, Pelletier J, Porco JA., Jr Angew Chem. 2010;122:6683–6688. doi: 10.1002/anie.201003212. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:6533–6538. doi: 10.1002/anie.201003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CCDC 1523489 (14) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif. See the Supporting Information for complete experimental details.
- 13.Goh KS, Tan C. RSC Adv. 2012;2:5536–5538. [Google Scholar]
- 14.For asymmetric photoreactions utilizing hydrogen-bonding templates, see: Gerard B, Sangji S, O'Leary DJ, Porco JA., Jr J Am Chem Soc. 2006;128 doi: 10.1021/ja062621j.Maturi MM, Bach T. Angew Chem. 2014;126:7793–7796.Angew Chem Int Ed. 2014;53:7661–7664. doi: 10.1002/anie.201403885.Vallavoju N, Selvakumar S, Jockusch S, Sibi MP, Sivaguru J. Angew Chem. 2014;126:5710–5714. doi: 10.1002/anie.201310940.Angew Chem Int Ed. 2014;53:5604–5608. doi: 10.1002/anie.201310940..
- 15.Kuznetsov DM, Mukhina OA, Kutateladze AG. Angew Chem. 2016;128:7102–7105. doi: 10.1002/anie.201602288. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2016;55:6988–6991. doi: 10.1002/anie.201602288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Please see the Supporting Information for complete experimental details.
- 17.Pirkle WH, Sikkenga DL, Pavlin MS. J Org Chem. 1977;42:384–387. [Google Scholar]
- 18.For a review on determination of absolute configuration of chiral molecules using VCD, see: Stephens PJ, Devlin FJ, Pan JJ. Chirality. 2008;20:643–663. doi: 10.1002/chir.20477..
- 19.a) Itoh M, Tanimoto Y, Tokumura K. J Am Chem Soc. 1983;105:3339–3340. [Google Scholar]; b) Brewer WE, Studer SL, Standiford M, Chou PT. J Phys Chem. 1989;93:6088–6094. [Google Scholar]
- 20.Bennett JA, Birge RR. J Chem Phys. 1980;73:4234–4246. [Google Scholar]
- 21.Rehm D, Weller A. Isr J Chem. 1970;8:259–271. [Google Scholar]
- 22.For recently reported photoinduced electron transfer reactions, see: Kranz DP, Griesbeck AG, Alle R, Perez-Ruiz R, Neudörfl JM, Meerholz K, Schmalz HG. Angew Chem. 2012;124:6102–6106. doi: 10.1002/anie.201201222.Angew Chem Int Ed. 2012;51:6000–6004. doi: 10.1002/anie.201201222.Guo W, Lu LQ, Wang Y, Wang YN, Chen JR, Xiao WJ. Angew Chem. 2015;127:2293–2297. doi: 10.1002/anie.201408837.Angew Chem Int Ed. 2015;54:2265–2269. doi: 10.1002/anie.201408837.Deng Y, Wei XJ, Wang H, Sun Y, Noël T, Wang X. Angew Chem. 2017;129:850–854.Angew Chem Int Ed. 2017;56:832–836.Silvi M, Verrier C, Rey YP, Buzzetti L, Melchiorre P. Nat Chem. 2017 doi: 10.1038/nchem.2748..
- 23.a) Foster R. J Phys Chem. 1980;84:2135–2141. [Google Scholar]; b) Arceo E, Jerberg ID, Álvarez-Fernández A, Melchiorre P. Nat Chem. 2013;5:750–756. doi: 10.1038/nchem.1727. [DOI] [PubMed] [Google Scholar]
- 24.a) Vishnumurthy K, Row TNG, Venkatesan K. Photochem Photobiol Sci. 2002;1:427–430. doi: 10.1039/b202557d. [DOI] [PubMed] [Google Scholar]; b) Gdaniec M, Jankowski W, Milewska MJ, Poloñski T. Angew Chem. 2003;115:4033–4036. doi: 10.1002/anie.200351432. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:3903–3906. doi: 10.1002/anie.200351432. [DOI] [PubMed] [Google Scholar]; c) Liu J, Boarman KJ, Wendt NL, Cardenas LM. Tetrahedron Lett. 2005;46:4953–4956. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.