Abstract
Background
Some obese adults are not afflicted by the metabolic abnormalities often associated with obesity [the “metabolically healthy obese” (MHO)], however, they may be at increased risk of developing cardiometabolic abnormalities in the future. Little is known about the relative incidence of individual components of metabolic syndrome (MetSyn).
Methods
We used data from a multi-center, community-based cohort aged 45–64 years at recruitment [the Atherosclerosis Risk in Communities (ARIC) study] to examine the first appearance of any MetSyn component, excluding waist circumference. Body mass index (BMI, kg/m2) and cardiometabolic data were collected at four triennial visits. Our analysis included 3,969 adults who were not underweight and free of the components of MetSyn at the initial visit. Participants were classified as metabolically healthy normal weight (MHNW), over weight (MHOW) and MHO at each visit. Adjusted hazard ratios (HR) and 95% confidence intervals were estimated with proportional hazards regression models.
Results
The relative rate of developing each risk factor was higher among MHO than MHNW with the strongest association noted for elevated fasting glucose [MHO vs. MHNW, HR: 2.33 (1.77, 3.06)]. MHO was also positively associated with elevated triglycerides [HR: 1.63 (1.27, 2.09)], low HDL-C [HR: 1.68 (1.32, 2.13)] and elevated blood pressure [HR: 1.54 (1.26, 1.88)]. A similar, but less pronounced pattern was noted among the MHOW vs. MHNW.
Conclusions
We conclude that even among apparently healthy individuals, obesity and overweight are related to more rapid development of at least 1 cardiometabolic risk factor, and that elevations in blood glucose develop most rapidly.
Keywords: metabolically healthy obese, cardiometabolic risk factors, metabolic syndrome
Introduction
Although the association between obesity and markers of chronic disease risk is well documented,1 a subgroup of individuals with high BMI have metabolic markers that fall within the normal range.2 These so-called metabolically healthy obese (MHO) and metabolically healthy overweight (MHOW) exhibit normal blood pressure and lipids, and possess a higher degree of insulin sensitivity and glucose control than their unhealthy counterparts.3 According to one study2 the metabolically healthy represent over 30% of obese [body mass index (BMI) >=30 kg/m2] and over 50% of overweight (BMI >=25 kg/m2 and <30 kg/m2) U.S. adults. Some researchers have suggested that this subset of obese individuals with a favorable metabolic profile exhibits a risk of chronic disease similar to those of normal-weight (BMI < 25.0 kg/m2),4, 5 although this observation remains controversial.6
While previous work, including one study from our group,7 has shown an increased incidence of overt metabolic syndrome among the MHO,7–9 there has been little work considering the incidence of individual components of MetSyn among the MHO. Most of the previous studies on individual components have been conducted in populations living in Asia and the results from these studies may therefore not be broadly generalizable as the relationships between body size, body composition, and health outcomes may be different in this group compared to other racial/ethnic groups.10 In one cohort of adult Korean men free from all metabolic abnormalities, obesity was associated with increased incidence of dyslipidemia, pre-hypertension, fatty liver and elevated HOMA-IR.11 Other studies in metabolically healthy Asian populations that considered more limited sets of cardiometabolic outcomes have reported positive associations of obesity with incident hypertension8, 12, 13 and type 2 diabetes.8, 14, 15 Studies in Spain,16, 17 the United States,4, 18 United Kingdom,19, 20 Australia,21 and Israel,22 have also described an increased risk of diabetes among the MHO. The relationship between MHO and dyslipidemia (low high density lipoprotein (HDL) and high triglycerides) has been largely unexplored.
To date, we know of no study that has compared the incidence of multiple individual cardiometabolic abnormalities between obese and normal weight males and females in U.S. populations who were initially free of any component of MetSyn. A comprehensive understanding of the evolution of cardiometabolic risk factors, in particular which ones develop most rapidly, would provide valuable insight into the course of the metabolically healthy obese population as well as suggest which conditions may be sentinel events for development of the clustering of these metabolic abnormalities. This would be of interest to clinicians and public health professionals when designing and implementing primary prevention efforts in this population.
The primary objective of our current study is to compare the incidence of cardiometabolic abnormalities between the metabolically healthy normal weight (MHNW), MHOW, and MHO using data from a community-based cohort of white and African-American adults. We examined the incidence of individual cardiometabolic abnormalities (dyslipidemia, elevated blood pressure, glucose dysregulation) as well as the overall incidence of any component. We also examined effect modification of these associations by race, age, sex and physical activity.
Methods
Study Population
Our analysis used data from the Atherosclerosis Risk in Communities (ARIC) Study, a prospective cohort study of the etiology of atherosclerosis in adult white and African-American men and women. The ARIC study was conducted in four U.S. communities (Forsyth County, NC; Jackson, MS; suburban Minneapolis, MN; Washington County, MD)23 and was approved by the Institutional Review Boards at each site.
Data Collection
The total ARIC study sample included 15,792 men and women aged 45 to 64 years. The first visit occurred between 1987 and 1989 with follow-up visits approximately every 3 years (1990–1992, 1993–1995 and 1996–1998). Participation rates at the 2nd–4th visits were 93%, 86% and 81%, respectively. At each visit participants answered interviews administered by trained study personnel using standardized questionnaires that assessed sociodemographic and lifestyle factors relevant to the etiology of cardiovascular disease. At the first and third visits usual diet was assessed using a modification of the 66-item food frequency questionnaire developed by Willett,24 while physical activity was assessed using a modified version of the Baecke questionnaire.25
Physiological and anthropometric measures were collected in clinic visits. Blood was collected from an antecubital vein into a vacuum tube with ethylenediamine tetraacetic acid (for lipids) or a serum separator gel (for glucose). Triglycerides, high density lipoprotein (HDL) and serum glucose were assayed using enzymatic methods, dextran-magnesium precipitation and hexokinase/glucose-6-phosphate dehydrogenase, respectively.23 Three blood pressure measurements were obtained using a random-zero sphygmomanometer and the last two measurements were averaged. Body weight was measured using a calibrated scale with subjects in scrub suits without shoes and height was measured using a ruler.
Outcomes
Outcomes were the incidence of individual components of MetSyn as defined by the National Cholesterol Program’s Adult Treatment Panel III (ATP III) guidelines:26 1) elevated triglycerides: >=150 mg/dL; 2) low HDL cholesterol, men: <40 mg/dL, women: <50 mg/dL; 3) elevated blood pressure: >=130 or >=85 mm Hg; 4) elevated fasting glucose: >=110 mg/dL. Additionally, subjects were considered to meet the criteria for each of the above listed components if they were taking medications to manage the corresponding condition. Elevated waist circumference (>40 inches for men, >35 inches for women according to the ATP III guidelines26) was not considered because most obese individuals, and few normal weight individuals, would satisfy this criterion by virtue of body size and so it may not be a direct marker of metabolic dysfunction.
Exposure
The exposure for this analysis was body mass index (BMI), defined as the ratio of weight in kilograms to squared height in meters using the height at visit 1 and the measured weight collected at the four visits. We categorized BMI according to standard cutpoints of 18.5–<25.0 (normal weight), 25.0–<30.0 (overweight) and >=30.0 (obese).
Participants were sequentially excluded from the analysis if they had any component of MetSyn at visit 1, excluding elevated waist circumference (n=11,046 total; which included 4,365 with elevated triglycerides, 5,824 with low HDL-C, 7,220 with elevated blood pressure, and 3,287 with elevated fasting glucose), were underweight or missing BMI at baseline (n=265), missing covariate data (n=51) or had non-fasting blood glucose data at visit 1 (n=554). Our final sample included 3,969 subjects who were free from any of the previously described cardiometabolic abnormalities, and not taking medications for them, at the initial ARIC visit.
Statistical Analysis
Hazard ratios (HR) and 95% confidence intervals (CI) for the association of BMI category with development of at least one risk factor, and each individual cardiometabolic risk factor, were estimated using a Weibull model for interval-censored time-to-event data.27 Interval censored methods were used because the outcomes for this analysis were assessed according to the ARIC visit schedule and thus the specific dates of onset were unknown. For each of the four individual outcomes (elevated triglycerides, low HDL cholesterol, high blood pressure, elevated glucose) and for the outcome of any one or more of the above, a dataset that included multiple records per subject was created (one observation per subject per interval, with a minimum of 1 and a maximum of 3 observations per subject) with a binary variable indicating if the outcome had occurred within the corresponding interval or not. Subjects were right censored if they did not achieve the outcome by their last observed follow-up. For analyses of the individual cardiometabolic risk factors, subjects were also censored if they developed any of the other risk factors before the one under consideration as this would violate our definition of metabolically healthy for subsequent outcomes. We also considered analyses that allowed for recurrent events28 (e.g. individuals who were metabolically unhealthy in visit 2, then were healthy again for visit 3 and thus at risk of another event by visit 4), but sparsity of the data prevented these models from converging. Since the Weibull model was estimated in the accelerated failure time metric we converted the parameters to hazard ratios by multiplying the negative of each coefficient by the shape parameter and calculated standard errors by the delta method. The primary exposure, BMI category at the beginning of each interval, was treated as a time-varying covariate as its value was allowed to change at each follow-up visit. All models were adjusted for age (continuous), sex (male, female), race (white, African-American), education level (less than high school, high school graduate or vocational school, attended college), smoking status (never, former, current), alcohol use (never/rare, former, light, medium, heavy) and leisure time physical activity [tertiles of Baecke index25]. Additional adjustment by total caloric intake did not materially change the effect estimates and so it was excluded from the final models. To assess the trend with respect to continuous BMI and incidence of 1 or more MetSyn component we used restricted quadratic splines, linear splines and simple linear coding of BMI with the spline knots at the mid- and end-points of the BMI categories (22.75, 25.0, 27.5, 30.0 kg/m2); nested models were compared using the likelihood ratio test.29 We also plotted the unadjusted cumulative probability of developing at least one MetSyn component, which is analogous to an adjusted Kaplan-Meier failure curve for continuous time-to-event data. To assess multiplicative interaction by race, age, sex, physical activity and BMI at age 25 years we included product terms for BMI category and race group (black, white), age group (45–54 years, 55–64 years), sex (male, female), physical activity (tertiles), and BMI at age 25 years (≥25 vs. <25; dichotomized to avoid small strata). BMI at 25 years was calculated from self-reported recalled weight at age 25 and measured height at visit 1.30 These were compared to models without the interaction terms using the likelihood ratio test with a significance level of 5%. To assess the joint associations of overall body size and central adiposity over the follow-up we also report associations of combinations of BMI and elevated waist circumference (WC; categorized as ≥102 cm for males, ≥88 cm for females) relative to the single reference group with normal weight and normal waist circumference. The statistical analysis was performed with the “survival” package31, 32 in R 3.3.1.33
Results
Characteristics of the study sample at visit 1 are shown in Table 1. The number of individuals initially considered metabolically healthy was lower in the obese group than the normal weight group, with 2,062 individuals in the MHN group at visit 1 and 458 individuals in the MHO group. The mean age at visit 1 was similar across BMI categories, and African-American race was more common among MHOW and MHO individuals. Greater education, and greater prevalence of current smoking was somewhat more common among MHN individuals. Physical activity levels tended to be higher among the MHN and MHOW individuals compared to the MHO. The number of individuals who developed a metabolic abnormality by visit 2 but became free from all conditions by visit 3 were: 89 (of 413) for elevated triglycerides, 180 (of 580) for low HDL-C, 86 (of 557) for elevated BP, and 113 (of 432) for elevated fasting glucose (data not in tables). Of these, there were 34 second episodes (by visit 4) of elevated triglycerides, 59 of low HDL-C, 45 for elevated BP and 29 elevated fasting glucose.
Table 1.
Characteristics [n (%)] of 3,969 subjects included in the analysis, according to visit 1 body mass index (BMI).The Atherosclerosis Risk InCommunities (ARIC) Study, 1987–1998.
| Body Mass Index (kg/m2) at visit 1 | ||||
|---|---|---|---|---|
| 18.5–<25.0 | 25.0–<30.0 | ≥30.0 | ||
| N | 2,062 | 1,449 | 458 | |
| Triglycerides (mg/dL)* | 80.9 (26.5) | 86.8 (27.1) | 91.1 (26.4) | |
| HDL-C (mg/dL)* | 65.8 (16.0) | 58.8 (14.0) | 58.5 (11.9) | |
| Systolic blood pressure (mmHg)* | 108.2 (10.7) | 111.5 (9.8) | 114.2 (9.2) | |
| Diastolic blood pressure (mmHg)* | 67.1 (8.1) | 69.7 (7.5) | 71.2 (6.9) | |
| Fasting Glucose (mg/dL)* | 93.8 (7.1) | 96.0 (6.7) | 96.3 (6.9) | |
| Waist circumference (cm)* | 82.8 (7.6) | 94.9 (7.1) | 108.3 (11.1) | |
| Age* | 52.9 (5.6) | 53.0 (5.6) | 52.9 (5.6) | |
| Female | 1,417 (68.7) | 736 (50.8) | 298 (65.1) | |
| African-American | 271 (13.1) | 307 (21.2) | 148 (32.3) | |
| Center | ||||
| Forsyth County, NC | 665 (32.3) | 352 (24.3) | 84 (18.3) | |
| Jackson, MS | 228 (11.1) | 270 (18.6) | 136 (30.0) | |
| Suburban Minneapolis, MN | 680 (32.9) | 473 (32.6) | 121 (26.4) | |
| Washington County, MD | 489 (23.7) | 354 (24.4) | 117 (25.6) | |
| Education | ||||
| Less than high school | 263 (12.8) | 256 (17.7) | 99 (21.6) | |
| High school | 851 (41.3) | 586 (40.4) | 186 (40.6) | |
| College | 948 (46.0) | 607 (41.9) | 173 (37.8) | |
| Smoking | ||||
| Never | 935 (45.3) | 647 (44.7) | 255 (55.7) | |
| Former | 600 (29.1) | 525 (36.2) | 138 (30.1) | |
| Current | 527 (25.6) | 277 (19.1) | 65 (14.2) | |
| Alcohol use | ||||
| Never/rare | 843 (40.9) | 561 (38.7) | 236 (51.5) | |
| Former | 266 (12.9) | 210 (14.5) | 76 (16.6) | |
| Light | 231 (11.2) | 173 (11.9) | 44 (9.6) | |
| Medium | 361(17.5) | 313(21.6) | 57(12.5) | |
| Heavy | 359 (17.4) | 192 (13.3) | 45 (9.8) | |
| Physical Activity(Baecke Index) | ||||
| 1st Tertile (0–2) | 661 (32.1) | 497 (34.4) | 207 (45.4) | |
| 2nd Tertile (2–2.75) | 642 (31.2) | 439 (30.4) | 128 (28.1) | |
| 3rd Tertile (2.75-) | 758 (36.8) | 510 (35.3) | 121 (26.5) | |
| Overweight at age 25 years (%) | 97 (4.7) | 325 (22.5) | 212 (46.7) | |
Mean (standard deviation)
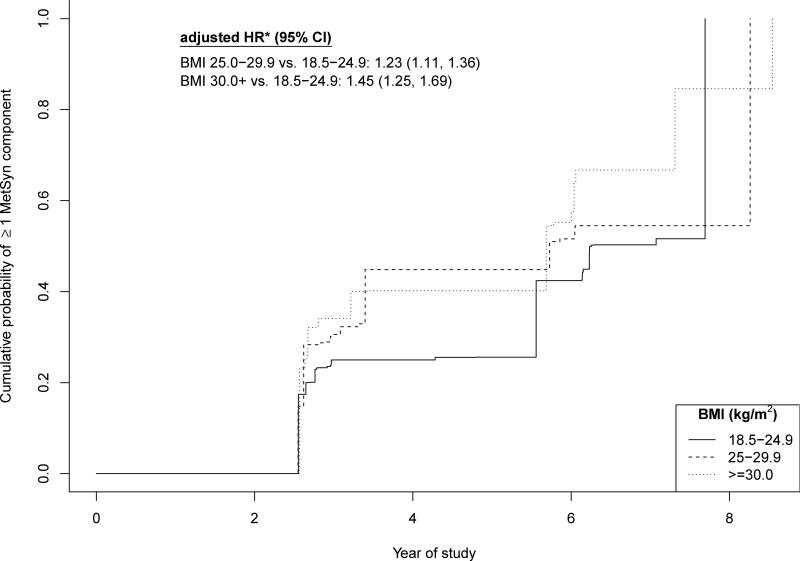
As shown in Figure 1, the risk of developing one or more cardiometabolic abnormalities over 9 years of follow-up among the MHOW was 23% higher compared to the MHN [HR: 1.23 (1.11, 1.36)] and 45% higher for the MHO compared to the MHN [HR: 1.45 (1.25, 1.69)]. In analyses that considered the separate components of MetSyn individually, the MHO developed each of the components faster than the MHN individuals. The MHO developed elevated triglycerides [HR: 1.63 (1.27, 2.09)], low HDL-C [HR: 1.68 (1.32, 2.13)] and high blood pressure [HR: 1.54 (1.26, 1.88)] more than 50% faster than the MHN (Table 2). The most pronounced effect was observed for elevated glucose, with the rate of developing this component in the MHO more than twice that of the MHN [HR: 2.33 (1.77, 3.06)]. A less pronounced, although consistent increase in rate of developing these components was also observed among the MHOW.
Figure 1.
Unadjusted estimated cumulative probability and adjusted hazard ratios for incidence of 1 or more MetSyn component over 4 ARIC visits among metabolically healthy individuals, by BMI level. The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1998.
* Hazard ratios adjusted for age, sex, field center, race, alcohol use, smoking, education, physical activity.
Table 2.
Hazards ratios (95% confidence intervals) for incidence of each component of MetSyn (excluding elevated waist circumference). The Atherosclerosis Risk In Communities (ARIC) Study, 1987–1998.
| Elevated Triglycerides | Low HDL-C | High Blood Pressure | Elevated Glucose | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| BMI (kg/m2) | Events/ Person-Years† |
Hazard Ratio (95% CI) |
Events/ Person-Years† |
Hazard Ratio (95% CI) |
Events/ Person-Years† |
Hazard Ratio (95% CI) |
Events/ Person-Years† |
Hazard Ratio (95% CI) |
| 18.5–<25.0 | 300/10,122 | 1. | 313/10,128 | 1. | 425/10,116 | 1. | 153/10,116 | 1. |
| 25.0–<30.0 | 264/6,907 | 1.37 (1.16, 1.61) | 314/6,907 | 1.37 (1.17, 1.61) | 357/6,895 | 1.20 (1.04, 1.38) | 214/6,907 | 1.69 (1.38, 2.07) |
| ≥30.0 | 90/2,082 | 1.63 (1.27, 2.09) | 107/2,082 | 1.68 (1.32, 2.13) | 150/2,051 | 1.54 (1.26, 1.88) | 89/2,067 | 2.33 (1.77, 3.06) |
Adjusted for age, sex, field center, race, alcohol use, smoking, education, physical activity.
Total amount of time ascribed to subjects in all 3-year intervals for the corresponding BMI value.
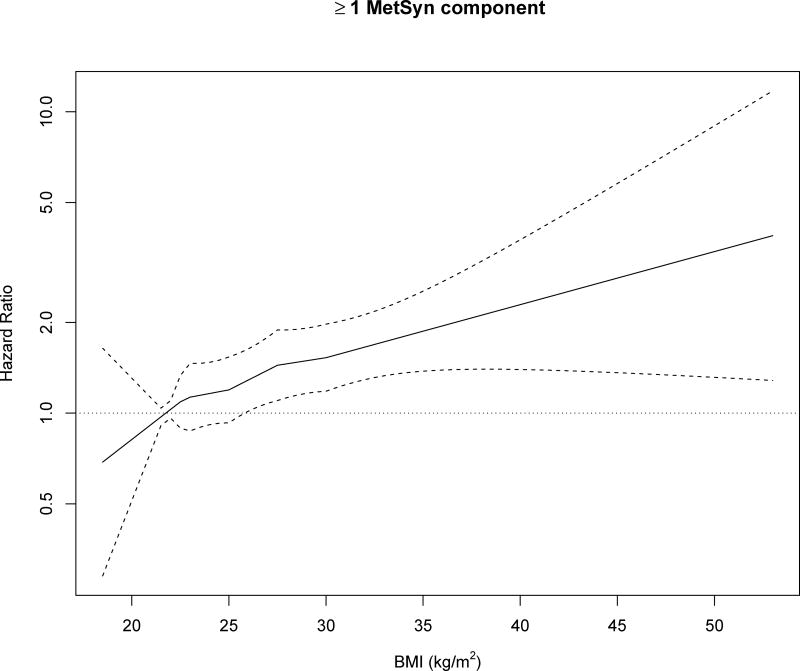
In the trend analysis the linear spline coding was adequate to describe the relationship between BMI and any MetSyn component when compared to the restricted quadratic spline and simple linear coding. Figure 2 shows the estimated hazard ratios and 95% confidence intervals from the linear spline model for an increase in BMI from a reference level of 21.75 kg/m2 (the midpoint of the normal weight range). Overall the incidence of at least 1 MetSyn component increased with increasing BMI across the entire range.
Figure 2.
Adjusted hazard ratios for incidence of 1 or more MetSyn component over 4 ARIC visits among metabolically healthy individuals, according to BMI using linear spline coding with knots at 22.75, 25.0, 27.5, 30 kg/m2. The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1998.
* Adjusted for age, sex, field center, race, alcohol use, smoking, education, physical activity.
There was no significant effect modification of the association between body size and incidence of MetSyn components in the metabolically healthy by age group, sex or BMI at age 25 years (all p-values for test of interaction > 0.05; Table 3). We observed significant interaction between BMI and race for the rate of developing low HDL-C and elevated fasting glucose, with the associations more pronounced among whites than blacks. This was also reflected by a significant interaction between BMI and race when considering the incidence of at least one cardiometabolic abnormality [black MHOW HR: 1.03 (0.78, 1.35), MHO HR: 1.08 (0.80, 1.47); white MHOW HR: 1.32 (1.18, 1.47), MHO HR: 1.57 (1.32, 1.87); p-interaction<0.001 (data not presented in table)]. We also did not observe heterogeneity of the relationship of BMI and any incidence of any of the MetSyn components by physical activity (all p-values for test of interaction > 0.05; data not shown).
Table 3.
Effect modification of association of BMI and MetSyn components (excluding waist circumference) by race, age, sex and BMI at 25 years. The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1998.
| Hazard Ratio* (95% Confidence Interval) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Elevated Triglycerides | Low HDL-C | High Blood Pressure | Elevated Glucose | |||||
| Race | ||||||||
|
|
||||||||
| BMI (kg/m2) | Black | White | Black | White | Black | White | Black | White |
|
| ||||||||
| 18.5–<25.0 | 1. | 1. | 1. | 1. | 1. | 1. | 1. | 1. |
| 25.0–<30.0 | 1.52 (0.86, 2.69) | 1.37 (1.15, 1.62) | 1.06 (0.68, 1.66) | 1.51 (1.28, 1.78) | 0.87 (0.61, 1.24) | 1.30 (1.12, 1.52) | 1.62 (1.02, 2.56) | 1.96 (1.57, 2.45) |
| ≥30.0 | 1.20 (0.61, 2.35) | 1.73 (1.33, 2.24) | 0.84 (0.48, 1.47) | 1.95 (1.52, 2.51) | 1.14 (0.78, 1.67) | 1.67 (1.32, 2.11) | 1.63 (0.96, 2.78) | 2.72 (2.00, 3.70) |
| p-interaction† | 0.23 | <0.001 | 0.09 | <0.001 | ||||
|
| ||||||||
| Age | ||||||||
|
|
||||||||
| BMI (kg/m2) | 45–54 years | 55–64 years | 45–54 years | 55–64 years | 45–54 years | 55–64 years | 45–54 years | 55–64 years |
|
| ||||||||
| 18.5–<25.0 | 1. | 1. | 1. | 1. | 1. | 1. | 1. | 1. |
| 25.0–<30.0 | 1.31 (1.10, 1.55) | 1.31 (1.05, 1.64) | 1.39 (1.18, 1.63) | 1.31 (1.06, 1.61) | 1.16 (0.99, 1.36) | 1.29 (1.06, 1.56) | 1.57 (1.30, 1.90) | 1.83 (1.41, 2.38) |
| ≥30.0 | 1.50 (1.18, 1.91) | 1.06 (0.72, 1.57) | 1.40 (1.11, 1.78) | 1.51 (1.11, 2.06) | 1.39 (1.12, 1.73) | 1.30 (0.96, 1.77) | 1.70 (1.31, 2.21) | 1.85 (1.30, 2.63) |
| p-interaction† | 0.20 | 0.71 | 0.52 | 0.61 | ||||
|
| ||||||||
| Sex | ||||||||
| BMI (kg/m2) | Male | Female | Male | Female | Male | Female | Male | Female |
|
| ||||||||
| 18.5–<25.0 | 1. | 1. | 1. | 1. | 1. | 1. | 1. | 1. |
| 25.0–<30.0 | 1.32 (1.00, 1.75) | 1.40 (1.14, 1.72) | 1.24 (0.97, 1.58) | 1.49 (1.22, 1.83) | 1.33 (1.06, 1.68) | 1.11 (0.92, 1.34) | 1.44 (1.10, 1.90) | 2.02 (1.51, 2.71) |
| ≥30.0 | 1.86 (1.18, 2.93) | 1.52 (1.14, 2.03) | 1.75 (1.14, 2.67) | 1.63 (1.24, 2.14) | 1.49 (0.99, 2.25) | 1.55 (1.23, 1.95) | 2.16 (1.42, 3.27) | 2.53 (1.77, 3.61) |
| p-interaction† | 0.77 | 0.53 | 0.58 | 0.38 | ||||
|
| ||||||||
| BMI at age 25 years | ||||||||
|
|
||||||||
| BMI (kg/m2) | 18.5–<25.0 | ≥25.0 | 18.5–<25.0 | ≥25.0 | 18.5–<25.0 | ≥25.0 | 18.5–<25.0 | ≥25.0 |
|
| ||||||||
| 18.5–<25.0 | 1. | 1. | 1. | 1. | 1. | 1. | 1. | 1. |
| 25.0–<30.0 | 1.31 (1.10, 1.57) | 2.30 (1.21, 4.38) | 1.36 (1.15, 1.61) | 1.62 (0.90, 2.93) | 1.10 (0.95, 1.29) | 1.63 (0.99, 2.70) | 1.65 (1.33, 2.06) | 2.26 (1.07, 4.76) |
| ≥30.0 | 1.78 (1.34, 2.36) | 2.10 (1.05, 4.20) | 1.71 (1.28, 2.28) | 1.82 (0.96, 3.43) | 1.52 (1.19, 1.93) | 1.56 (0.91, 2.69) | 2.39 (1.71, 3.34) | 2.98 (1.38, 6.43) |
| p-interaction† | 0.14 | 0.78 | 0.12 | 0.71 | ||||
Adjusted for age, sex, field center, race, alcohol use, smoking, education, physical activity.
P-value from likelihood ratio test for interaction between BMI and corresponding variable.
As shown in Table 4, the combination of obesity and elevated waist circumference, compared to normal weight and normal waist circumference was most strongly associated with increased risk of low HDL-C [HR: 1.77 (1.39, 2.24); p-interaction: 0.03] and high blood pressure [HR: 1.57 (1.28, 1.93); p-interaction: 0.02]. Qualitatively, a similar pattern was observed for elevated triglycerides [obese/elevated WC vs. normal weight/normal WC, HR: 1.68 (1.30, 2.16)] and elevated glucose [obese/elevated WC vs. normal weight/normal WC, HR: 2.45 (1.86, 3.23)].
Table 4.
Associations of cross-classified BMI/waist circumference categorieson risk of developing MetSyn components. The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1998.
| Hazard Ratio* (95% Confidence Interval) | ||
|---|---|---|
| Elevated Triglycerides | ||
|
| ||
| BMI (kg/m2) | Normal WC† | Elevated WC† |
|
| ||
| 18.5–<25.0 | 1. | 1.13 (0.84, 1.54) |
| 25.0–<30.0 | 1.45 (1.16, 1.80) | 1.35 (1.09, 1.65) |
| ≥30.0 | 1.18 (0.41, 3.38) | 1.68 (1.30, 2.16) |
| p-interaction§ | 0.48 | |
|
| ||
| Low HDL-C | ||
|
| ||
| Normal WC† | Elevated WC† | |
|
| ||
| 18.5–<25.0 | 1. | 1.10 (0.79, 1.51) |
| 25.0–<30.0 | 1.49 (0.84, 1.65) | 1.29 (1.06, 1.57) |
| ≥30.0 | 0.57 (0.11, 2.97) | 1.77 (1.39, 2.24) |
| p-interaction§ | 0.03 | |
|
| ||
| High Blood Pressure | ||
|
| ||
| Normal WC† | Elevated WC† | |
|
| ||
| 18.5–<25.0 | 1. | 0.97 (0.74, 1.26) |
| 25.0–<30.0 | 1.41 (1.18, 1.68) | 1.02 (0.84, 1.24) |
| ≥30.0 | 0.58 (0.15, 2.31) | 1.57 (1.28, 1.93) |
| p-interaction§ | 0.02 | |
|
| ||
| Elevated Glucose | ||
|
| ||
| Normal WC† | Elevated WC† | |
|
| ||
| 18.5–<25.0 | 1. | 1.01 (0.61, 1.67) |
| 25.0–<30.0 | 1.71 (1.34, 2.19) | 1.65 (1.27, 2.14) |
| ≥30.0 | 0.76 (0.19, 2.99) | 2.45 (1.86, 3.23) |
| p-interaction§ | 0.08 | |
Adjusted for age, sex, field center, race, alcohol use, smoking, education, physical activity.
Elevated waist circumference (updated at each follow-up) defined as ≥102 cm among men or ≥88 cm among women.
P-value from likelihood ratio test for interaction between BMI and indicator of elevated waist circumference.
Discussion
In our sample of white and African-American adults free from any component of MetSyn, we observed an increase in risk of developing at least one cardiometabolic abnormality over 9 years of follow-up. There was a consistent increase in the rate of development of MetSyn components among the MHO, relative to MHNW, with glucose dysregulation developing the most rapidly. The results were largely consistent across age groups, although MHO and MHOW females tended to develop these MetSyn components more rapidly than their male counterparts.
In a previous analysis in the ARIC cohort we reported that obesity was associated with 4.5 times the risk of developing incident MetSyn over the 3 follow-up visits.7 However, that study focused on MetSyn as a single outcome (attainment of 3 or more of the MetSyn characteristics) and so did not consider the effect of body size on individual cardiometabolic factors. To our knowledge, this current report is the only study of multiple components of metabolic health among the MHO in a United States cohort. The report by Chang et al. is the only other study to comprehensively consider individual components of MetSyn in a manner comparable to our analysis, but their study was in a Korean sample and limited to males.11 The associations that they reported for elevated triglycerides and low HDL-C were similar in magnitude to those that we observed among men when we stratified our analysis by sex. However, in contrast to our findings, they reported increases in risk of 13% for elevated glucose and 85% for elevated blood pressure among the MHO compared to the MHNW11 which are notably different than the 104% and 29% increases, respectively, that we found among males. One factor that may be related to these discrepancies is that the lower end of the age range in the Chang study was 15 years less than in the ARIC cohort, and so their analysis included more younger men. Additionally, they excluded individuals with evidence of fatty liver on ultrasound or elevated homeostasis model assessment-insulin resistance (HOMA-IR) and thus may have removed individuals who were more likely to present with glucose dysregulation in the short follow-up period. In a recent letter, Bell and colleagues describe incidence of individual MetSyn components in the Whitehall II study of British adults.20 Although they reported that the MHO were significantly more likely to develop insulin resistance, high blood glucose and hypertension over increasingly longer periods of follow-up, their classification of MHO status was defined at the baseline visit and thus their analysis did not account for individuals changing MHO classification over their follow-up as in our present analysis and in the study by Chang et al.11
Most of the other studies of incident metabolic abnormalities focused exclusively on incident diabetes, with associations more pronounced than what we report here for elevated glucose, with many reporting relative risks between 2 and 4.4, 14–16, 18, 19, 21, 22 The strongest association was noted in the study by Hwang et al. in which the MHO had more than 11 times the risk of diabetes than MHN subjects.8 All studies that examined elevated blood pressure or hypertension among the MHO reported significant increases compared to MHN.8, 11–13
A potential difference between our analysis and previous ones, especially those that did not examine multiple abnormalities, is that for each outcome we administratively censored those who developed any of the other MetSyn components at the event time, as they no longer met the criteria for metabolic health in the subsequent follow-up periods. For example, when considering hypertension as an outcome, if a subject had developed elevated triglycerides at visit 2 while remaining free of hypertension they would have been censored for blood pressure at visit 2 (but counted as an event in the triglyceride analysis). These individuals may have gone on to subsequently develop additional components (e.g. low HDL-C), but we would not have counted the later events. This approach was chosen to maintain a consistent definition of metabolic health across all of the follow-up periods. Retaining individuals who develop some components of MetSyn would create heterogeneity in the exposed group (MHO) across the follow-up. Because MHO are at greater risk of developing each of the components of MetSyn, that group would grow less healthy over time, making the interpretation of the effect of MHO unclear due to its changing definition.
We did not observe statistically significant effect modification by age, sex, physical activity, or BMI at age 25, although there was a more pronounced association between obesity and rate of incident low HDL-C and elevated glucose among whites compared to blacks. These differences are consistent with previous observations that at a given BMI white individuals carry more visceral adipose tissue than blacks34, 35 and that visceral adiposity may be more relevant for identifying individuals in these race groups who are at high risk for developing metabolic abnormalities.36 Our results suggest that the etiology of the transition of the MHO state to a metabolically unhealthy state may differ by race. Because these different cardiometabolic abnormalities may influence specific outcomes differently (e.g. myocardial infarction and diabetes), these findings may have implication for identification of subgroups of the MHO who are higher risk for particular diseases.
Our findings regarding the relationship between the joint association of BMI and waist circumference suggest that body composition may play an important role in the transition between healthy to unhealthy status.
Although the clinical and public health utility of understanding obesity phenotypes has been discussed previously,3, 37 an unresolved issue is the lack of consensus regarding the definition of metabolic health.38, 39 This inconsistency both hinders the clinical application of this concept and makes results difficult to interpret across studies.40 The clustering of cardiometabolic risk factors that defines MetSyn is believed to be driven by insulin resistance41 and so attempts to refine the definition have included direct markers of insulin resistance4, 12 and inflammation2, 42 to more precisely characterize a high-risk obese phenotype. However, in studies that considered multiple definitions of metabolic health, authors have noted that associations are qualitatively similar regardless of the definition. Using data from the Framingham Offspring Study, Meigs et al. reported comparable associations between obesity phenotypes and the outcomes of type 2 diabetes and cardiovascular disease using two different definitions of metabolic health: 1) absence of MetSyn (2 or less out of the 5 components) or 2) HOMA-IR below the 75th percentile.4 In a Korean population, odds ratios for development of hypertension comparing MHO to MHN ranged from 1.46–1.5812 when metabolic health was defined according to the ATP-III definition (omitting waist circumference, as we did), the criteria of Wildman et al.2 and Karelis et al.42 (both including C-reactive protein), and the HOMA-IR definition used by Meigs et al.4 Given the consistency of these associations across these different definitions we expect our results to be robust to the classification of metabolic health, however refinement of the definition of metabolic health should remain a priority for future research.
Strengths of our study include its relatively large sample size and standardized assessments of measured anthropometric characteristics and cardiometabolic outcomes. While these strengths are important, our results should be interpreted in light of a few limitations. As mentioned previously, research into the MHO phenotype is currently limited by lack of a consistent definition of metabolic health.39, 40, 43 This implies some inconsistency in defining the target population, and could muddle the interpretation of these findings since some marginally unhealthy individuals could be classified as healthy. While the age range of the ARIC study corresponds to that for which cardiovascular disease is most relevant, we were limited in our ability to investigate these associations among older, or younger individuals. Additionally, although 9 years of follow-up is longer than most of the previous studies, additional follow-up would allow for more detailed of how these associations may change over time. Finally, individuals may transition in and out of unhealthy status becoming repeatedly at risk of metabolic abnormalities, but there were too few second events among such individuals in these data to allow us to investigate this, which is a common issue in recurrent event analysis.28 Studies of transitory states of metabolic health would require longer follow-up than we had available.
In conclusion, among a cohort of adults who were free of the components of MetSyn (except waist circumference), we observed that excess weight was associated with more rapid development of a cardiometabolic abnormality compared to normal weight individuals. Our results add to the evidence that these factors are strongly associated with excess adiposity among U.S. adults, even among those who may appear initially free from these conditions. Future research should seek to understand the increase in chronic disease risk in this subpopulation of obese subjects as well as potential behavioral and lifestyle interventions aimed to achieve stable cardiometabolic risk factors.
Acknowledgments
none
Financial support: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 2.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168(15):1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 3.Phillips CM. Metabolically healthy obesity: definitions, determinants and clinical implications. Reviews in endocrine & metabolic disorders. 2013;14(3):219–27. doi: 10.1007/s11154-013-9252-x. [DOI] [PubMed] [Google Scholar]
- 4.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91(8):2906–12. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 5.Rhee EJ, Lee MK, Kim JD, Jeon WS, Bae JC, Park SE, et al. Metabolic health is a more important determinant for diabetes development than simple obesity: a 4-year retrospective longitudinal study. PloS one. 2014;9(5):e98369. doi: 10.1371/journal.pone.0098369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Annals of internal medicine. 2013;159(11):758–69. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw PT, Monda KL, Stevens J. Metabolic syndrome in healthy obese, overweight, and normal weight individuals: the Atherosclerosis Risk in Communities Study. Obesity (Silver Spring) 2013;21(1):203–9. doi: 10.1002/oby.20248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang LC, Bai CH, Sun CA, Chen CJ. Prevalence of metabolically healthy obesity and its impacts on incidences of hypertension, diabetes and the metabolic syndrome in Taiwan. Asia Pac J Clin Nutr. 2012;21(2):227–33. [PubMed] [Google Scholar]
- 9.Achilike I, Hazuda HP, Fowler SP, Aung K, Lorenzo C. Predicting the development of the metabolically healthy obese phenotype. Int J Obes (Lond) 2015;39(2):228–34. doi: 10.1038/ijo.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.W. H. O. Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 11.Chang Y, Ryu S, Suh BS, Yun KE, Kim CW, Cho SI. Impact of BMI on the incidence of metabolic abnormalities in metabolically healthy men. Int J Obes (Lond) 2011 doi: 10.1038/ijo.2011.247. [DOI] [PubMed] [Google Scholar]
- 12.Kang YM, Jung CH, Jang JE, Hwang JY, Kim EH, Park JY, et al. The Association of Incident Hypertension with Metabolic Health and Obesity Status: Definition of Metabolic Health Does Not Matter. Clin Endocrinol (Oxf) 2016 doi: 10.1111/cen.13074. [DOI] [PubMed] [Google Scholar]
- 13.Lee SK, Kim SH, Cho GY, Baik I, Lim HE, Park CG, et al. Obesity phenotype and incident hypertension: a prospective community-based cohort study. J Hypertens. 2013;31(1):145–51. doi: 10.1097/HJH.0b013e32835a3637. [DOI] [PubMed] [Google Scholar]
- 14.Jung CH, Lee MJ, Kang YM, Jang JE, Leem J, Hwang JY, et al. The risk of incident type 2 diabetes in a Korean metabolically healthy obese population: the role of systemic inflammation. J Clin Endocrinol Metab. 2015;100(3):934–41. doi: 10.1210/jc.2014-3885. [DOI] [PubMed] [Google Scholar]
- 15.Heianza Y, Kato K, Kodama S, Suzuki A, Tanaka S, Hanyu O, et al. Stability and changes in metabolically healthy overweight or obesity and risk of future diabetes: Niigata wellness study. Obesity (Silver Spring) 2014;22(11):2420–5. doi: 10.1002/oby.20855. [DOI] [PubMed] [Google Scholar]
- 16.Soriguer F, Gutierrez-Repiso C, Rubio-Martin E, Garcia-Fuentes E, Almaraz MC, Colomo N, et al. Metabolically healthy but obese, a matter of time? Findings from the prospective Pizarra study. J Clin Endocrinol Metab. 2013;98(6):2318–25. doi: 10.1210/jc.2012-4253. [DOI] [PubMed] [Google Scholar]
- 17.Navarro-Gonzalez D, Sanchez-Inigo L, Fernandez-Montero A, Pastrana-Delgado J, Alfredo Martinez J. Are all metabolically healthy individuals with obesity at the same risk of diabetes onset? Obesity (Silver Spring) 2016;24(12):2615–2623. doi: 10.1002/oby.21667. [DOI] [PubMed] [Google Scholar]
- 18.Aung K, Lorenzo C, Hinojosa MA, Haffner SM. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J Clin Endocrinol Metab. 2014;99(2):462–8. doi: 10.1210/jc.2013-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinnouho GM, Czernichow S, Dugravot A, Nabi H, Brunner EJ, Kivimaki M, et al. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J. 2015;36(9):551–9. doi: 10.1093/eurheartj/ehu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell JA, Hamer M, Batty GD, Singh-Manoux A, Sabia S, Kivimaki M. Incidence of Metabolic Risk Factors Among Healthy Obese Adults: 20-Year Follow-Up. Journal of the American College of Cardiology. 2015;66(7):871–3. doi: 10.1016/j.jacc.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appleton SL, Seaborn CJ, Visvanathan R, Hill CL, Gill TK, Taylor AW, et al. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: a cohort study. Diabetes Care. 2013;36(8):2388–94. doi: 10.2337/dc12-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Twig G, Afek A, Derazne E, Tzur D, Cukierman-Yaffe T, Gerstein HC, et al. Diabetes risk among overweight and obese metabolically healthy young adults. Diabetes Care. 2014;37(11):2989–95. doi: 10.2337/dc14-0869. [DOI] [PubMed] [Google Scholar]
- 23.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 24.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 25.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 26.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 27.Lindsey JC, Ryan LM. Tutorial in biostatistics methods for interval-censored data. Stat Med. 1998;17(2):219–38. doi: 10.1002/(sici)1097-0258(19980130)17:2<219::aid-sim735>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 28.Amorim LD, Cai J. Modelling recurrent events: a tutorial for analysis in epidemiology. Int J Epidemiol. 2015;44(1):324–33. doi: 10.1093/ije/dyu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witte JS, Greenland S. A nested approach to evaluating dose-response and trend. Ann Epidemiol. 1997;7(3):188–93. doi: 10.1016/s1047-2797(96)00159-7. [DOI] [PubMed] [Google Scholar]
- 30.Stevens J, Truesdale KP, Wang CH, Cai J, Erber E. Body mass index at age 25 and all-cause mortality in whites and African Americans: the Atherosclerosis Risk in Communities study. J Adolesc Health. 2012;50(3):221–7. doi: 10.1016/j.jadohealth.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Therneau TM, Grambsh PM. Modeling survival data: extending the Cox model. Springer; New York: 2000. [Google Scholar]
- 32.Therneau TM. A package for survival analysis in S. (2.38) 2015 https://cran.r-project.org/package=survival.
- 33.R Core Team. R: A Language and Environment for Statistical Computing. 3.3.1. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 34.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, Ravussin E, et al. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91(1):7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring) 2011;19(2):402–8. doi: 10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katzmarzyk PT, Heymsfield SB, Bouchard C. Clinical utility of visceral adipose tissue for the identification of cardiometabolic risk in white and African American adults. Am J Clin Nutr. 2013;97(3):480–6. doi: 10.3945/ajcn.112.047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradshaw PT, Stevens J. Invited commentary: limitations and usefulness of the metabolically healthy obesity phenotype. Am J Epidemiol. 2015;182(9):742–4. doi: 10.1093/aje/kwv178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karelis AD, Brochu M, Rabasa-Lhoret R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab. 2004;30(6):569–72. doi: 10.1016/s1262-3636(07)70156-8. [DOI] [PubMed] [Google Scholar]
- 39.Rey-Lopez JP, de Rezende LF, Pastor-Valero M, Tess BH. The prevalence of metabolically healthy obesity: a systematic review and critical evaluation of the definitions used. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2014;15(10):781–90. doi: 10.1111/obr.12198. [DOI] [PubMed] [Google Scholar]
- 40.Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35(7):971–81. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- 41.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 42.Karelis AD, Rabasa-Lhoret R. Inclusion of C-reactive protein in the identification of metabolically healthy but obese (MHO) individuals. Diabetes Metab. 2008;34(2):183–4. doi: 10.1016/j.diabet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud'homme D, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90(7):4145–50. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]