Abstract
Dried plum supplementation has been shown to enhance bone formation while suppressing bone resorption. Evidence from previous studies has demonstrated that these responses can be attributed in part to the fruit’s polyphenolic compounds. The purpose of this study was to identify the most bioactive polyphenolic fractions of dried plum with a focus on their osteogenic activity, and to investigate their mechanisms of action under normal and inflammatory conditions. Utilizing chromatographic techniques, six fractions of polyphenolic compounds were prepared from a crude extract of dried plum. Initial screening assays revealed that two fractions (DP-FrA and DP-FrB) had the greatest osteogenic potential. Subsequent experiments using primary bone marrow-derived osteoblast cultures demonstrated these two fractions enhanced extracellular alkaline phosphatase (ALP), an indicator of osteoblast activity, and mineralized nodule formation under normal conditions. Both fractions enhanced bone morphogenetic protein (BMP) signaling, as indicated by increased Bmp2 and Runx2 gene expression and protein levels of phosphorylated Smad1/5. DP-FrB was most effective at upregulating Tak1 and Smad1, as well as protein levels of phospho-p38. Under inflammatory conditions, TNF-α suppressed ALP and tended to decrease nodule formation (p = 0.0674). This response coincided with suppressed gene expression of Bmp2 and the upregulation of Smad6, an inhibitor of BMP signaling. DP-FrA and DP-FrB partially normalized these responses. Our results show that certain fractions of polyphenolic compounds in dried plum upregulate osteoblast activity by enhancing BMP signaling and when this pathway is inhibited by TNF-α, the osteogenic response is attenuated.
Keywords: functional foods, osteoporosis, polyphenol, osteoblast, bone morphogenetic proteins
1. Introduction
Osteoporotic fractures are among the most debilitating health consequences associated with aging [1]. The loss of bone mass and deterioration of bone microstructure that begins in the fifth decade of life leads to a compromise in bone strength and ultimately increases the risk of fracture [2]. Risk factors for osteoporosis tend to be classified into two broad categories—those that interfere with the achievement of a healthy peak bone mass during growth and maturation, and those factors that accelerate loss of bone later in life. Thus, it stands to reason that the most effective osteoporosis prevention and treatment strategies should promote the achievement of a higher peak bone mass as well as reducing the rate at which bone loss occurs.
Approaches involving supplementation of the diet with plant-based foods rich in polyphenolic compounds are considered a promising option. Evidence from pre-clinical and clinical studies has shown that dried plums have potent effects on bone. For example, postmenopausal women consuming dried plum (100 g/day) experienced an increase in spine BMD and a follow-up study with osteopenic women (age 65–79 years) found 50 g/day of dried plum prevented bone loss [3, 4]. These improvements in BMD were attributed to a decrease in bone resorption with the relative rate of bone formation maintained or increased. Findings from animal models of osteoporosis have provided additional insights into the effects of dried plum supplementation on bone metabolism. Dried plum supplementation increased both trabecular and cortical bone in young growing C57BL/6 male mice in a biphasic metabolic manner [5]. Initially, suppression of osteoclast and osteoblast number was observed with a corresponding decrease in bone formation rate, but this was followed by an increase in the bone formation rate by the final study endpoint [5]. Likewise, Shahnazari et al. [6], reported an increase in osteoblast number in young growing mice consuming a dried plum supplemented diet. Further insights into the mechanism through which dried plum affects bone have been provided from the ovarian hormone deficient models. Dried plum supplementation restored BMD and cortical bone in osteopenic, estrogen-deficient rats; a response attributed to an increase in endocortical mineral apposition [7]. Importantly, in both the estrogen deficient and the adult mouse models, the positive effects on bone have been reported in conjunction with systemic anti-inflammatory effects [6, 8]. These clinical and pre-clinical studies provide promising evidence of the osteoprotective effects of dried plum, and yet the bioactive components have remained in question.
Much of the focus on dried plum’s bioactive components has centered on its polyphenolic compounds, especially chlorogenic acid isomers [9]. A recent study published from our laboratory showed that dietary supplementation with a crude ethanol extract of dried plum’s polyphenols accounted for ≥ 90% of the effect of dried plum on bone [10]. In vitro studies utilizing the MC3T3-E1 cell line have demonstrated that this extract induced osteoblast activity and increased mineralization [11]. The results of these in vitro studies combined with in vivo findings suggest that polyphenolic compounds within dried plum may be at least, in part, responsible for dried plum’s effects on bone and that their effects are mediated through their ability to alter the immune response. However, the components of the polyphenolic extract that are responsible for these responses and the mechanisms by which they enhance osteogenesis are not known.
Evidence from one of our previous animal studies indicates that dried plum may enhance osteoblast activity by upregulating BMP signaling [7]. Bmp4 expression was upregulated in osteopenic animals that experienced bone restoration [7]. Increased BMP signaling results in the upregulation of Runx2, the master regulator of osteoblast differentiation, in bone cells [12–14]. Previously, we have shown that an ethanol extract of the total polyphenols from dried plum upregulates Runx2 expression in a pre-osteoblast cell line [11]. Therefore, it is conceivable that polyphenolic compounds in dried plum improve osteoblast activity by stimulating BMP signaling and osteoblast differentiation, as well as enhancing mineralization. Further evidence in support of a potential BMP-mediated response is based on the suppression of osteoblast activity that occurs with increases in TNF-α. Osteoblast activity can be suppressed through the inhibition of BMP signaling when TNF-α is elevated as in the case of early estrogen deficiency or with flares in rheumatoid arthritis [15–20]. Increases in TNF-α reduce the expression and stability of Runx2, which inhibits osteoblast differentiation [15, 16, 18, 20]. Thus it stands to reason that if certain types of polyphenolic compounds found in dried plum have the capacity to alter TNF-α production as we have reported [8, 21], the BMP pathway is a likely target for their actions.
The purpose of this study was two-fold: 1) to begin to examine which components of the crude polyphenolic extract from dried plum are responsible for increasing osteoblast differentiation and function; and 2) to assess whether BMP signaling pathways are altered in response to treatment with these compounds. Due to the role of the inflammatory response in the inhibition of bone formation, a series of studies was designed to investigate the osteoblast response utilizing primary cultures under normal and inflammatory conditions.
2. Materials and Methods
2.1 Isolation of polyphenolic fractions from dried plum
Fractions of polyphenolic compounds were derived from a crude polyphenolic extract of dried plum powder provided by the California Dried Plum Board (Sacramento, CA), using a modification of previously published chromatographic techniques [22]. In short, dried plum powder (500 g) was suspended in 80% methanol and sonicated under nitrogen gas for 20 minutes to derive a total polyphenolic extract. Following filtration, this process was repeated and the extract was subjected to column chromatography using 300 g of HP-20 resin. The HP-20 resin was washed five times with 200 mL of deionized (DI) H20 to remove carbohydrates followed by washing 5× with 200 mL of methanol to elute a polyphenol-rich extract. This extract was again subjected to column chromatography using 200 g of HP-20 resin and fractions eluted with increasing concentrations of methanol (0 – 100%). The elution of DP-FrA was completed with 0% methanol and each subsequent fraction was eluted with increasing increments of 20% methanol (i.e., DP-FrB 20%, DP-FrC 40%, DP-FrD 60%, DP-FrE 80%, and DP-FrF 100%). The weights of the six semi-purified polyphenolic fractions were as follows: DP-FrA, 17.85 g; DP-FrB, 4.42 g; DP-FrC, 3.22 g; DP-FrD, 4.14 g; DP-FrE, 2.16 g; and DP-FrF, 1.39 g.
2.2 Screening the bioactivity of the polyphenolic fractions in enhancing osteoblast activity and function
A commercially available pre-osteoblastic cell line (MC3T3-E1; RIKEN, Japan) was used to screen the effects of the fractions on osteoblast activity and function with the intent to reduce the number of fractions tested in primary bone marrow derived osteoblasts, thereby reducing the number of animals needed for these experiments. MC3T3-E1 cells were seeded at a density of 2.5 × 105 in a 24-well plate in α-modified minimum essential medium (α-MEM; Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY), 2 mM L-glutamine, and 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO). To assess dosage limitations, proliferation assays (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, or MTT) utilizing a dose response of the polyphenolic fractions were completed to determine doses that did not negatively affect cell growth (data not shown).
The ability of the six fractions to enhance osteoblast activity was evaluated by determining the extracellular ALP and mineralization capacity of the MC3T3-E1 cells. Cells were plated at a density of 2.5 × 105 in a 24-well plate in α-MEM supplemented with 10% FBS, 2 mM L-glutamine, and 1% penicillin/streptomycin. When the culture reached ~95% confluence, 25 μg/mL ascorbic acid and 10 mM β-glycerophosphate (Sigma-Aldrich, St. Louis, MO) were added to the media to induce osteogenesis. The media was replaced every 2–3 days until the designated experimental endpoints. Extracellular ALP was analyzed in the media using a fluorometric assay kit (Biovision, Milpitas, CA) following 7 days of MC3T3-E1 treatment with osteogenic media and polyphenolic fractions (5 or 10 μg/mL). The 7-day time point was selected based on ALP production peaking in MC3T3-E1 cells between days 7–14 [23]. Extracellular ALP was expressed relative to the control cells (i.e., 0 μg/mL polyphenolic compounds). Mineralization was assessed using alizarin red S staining, which chelates with calcium cations, following 28 days of treatment with the six fractions (10 μg/mL) in osteogenic media. Staining was accomplished by aspirating the media and fixing the cells with 10% neutral buffered formalin (NBF) for 15 minutes. The cells were then incubated in 40 mM of alizarin red S (pH 4.2) for 20 minutes at room temperature. Excess stain was washed from the wells with DI water and the alizarin red S stain was eluted from the mineralized nodules with 10% acetic acid and heated to 85°C for 10 minutes. Following centrifugation (16,000 × g) to remove cellular debris, the pH of the samples was neutralized with 10% ammonium hydroxide, and alizarin red S was quantified at a wavelength of 405 nm. The fractions and doses that resulted in the greatest increase in ALP activity and nodule mineralization were identified from these screening assays (10 μg/mL of DP-FrA and DP-FrB), which were used in all subsequent experiments with primary osteoblasts, a more physiologically relevant model than the immortalized cell line.
2.3 Activity and mineralization capacity of primary osteoblasts
To prepare primary osteoblast cultures, bone marrow was flushed with sterile phosphate buffered saline (PBS) from the long bones of 4-week-old C57BL/6 female mice (Charles River, Wilmington, MA). For each experiment, cells were pooled from 3–4 mice. All animal procedures were approved by the Oklahoma State University Institutional Animal Care and Use Committee. The bone marrow was cultured in complete α-MEM media which had been supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and 1% penicillin/streptoymycin for two days to allow stromal cell populations to adhere and the non-adherent hematopoietic cell populations to be separated. The media was removed and adherent cells were trypsinized with 0.25% Trypsin-EDTA (Sigma-Aldrich, St. Louis, MO), and collected. Next, the cells were seeded (5 × 105) in a 24-well plate and maintained in complete α-MEM until ~95% confluence before changing to the osteogenic media (i.e., α-MEM complete media supplemented with 50 μg/mL ascorbic acid and 3 mM β-glycerophosphate). Cells were allowed to differentiate in osteogenic media for 7 days to allow for enrichment of an osteogenic cell population prior to treatment with DP-FrA or DP-FrB (10 μg/mL) with or without TNF-α (1 ng/mL) to study the osteoblast response to treatments under normal or inflammatory conditions. The TNF-α dose used was based on previously published data from our lab [11]. Throughout the experiments, the media was replaced every 2–3 days. Each set of experiments were repeated 2–3 times.
To determine the effect of treatment on osteoblast activity and function, extracellular ALP and calcified nodule formation was assessed. Extracellular ALP was analyzed in the media (as described with MC3T3-E1 cells), following 3 and 7 days of treatment with DP-FrA or DP-FrB with 0 or 1 ng/mL TNF-α. Justification for the 3 and 7 day time points was based on our preliminary time course studies that demonstrated ALP peaks in the first 7 days of cultures. To assess nodule formation by primary osteoblasts, von Kossa staining was performed following 14 days of treatment with DP-FrA or DP-FrB (10 μg/mL) with or without TNF-α (1 ng/mL). In contrast to the use of alizarin red S staining in MC3T3-E1 cultures, von Kossa staining is the preferred method for assessing mineralization in primary cells [24]. Briefly, at the end of the study, cells were fixed for 5 mins at room temperature in 10% NBF prior to incubating in 5% silver nitrate for 20 mins under an ultraviolet light. The cells were then washed and incubated in 5% sodium thiosulfate for 3 mins, followed by counterstaining with Nuclear Fast Red solution for 5 mins. The cells were then rinsed and allowed to dry at room temperature prior to imaging using CellSens software (Olympus Life Science, Center Valley, PA). Mineralization area was quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
2.4 Protein analyses
Protein signaling within the BMP pathway was assessed via Western blot. Primary bone marrow-derived osteoblasts were cultured as described previously. To ensure an enriched population of osteoblasts, the bone marrow cells were differentiated in osteogenic media for 7 days prior to treatment with DP-FrA or DP-FrB (10 μg/ml). Total protein was harvested after 15 mins or 1 hour of treatment by washing the cells in sterile PBS and adding radioimmuno-precipitation assay (RIPA) buffer (Life Technologies, Carlsbad, CA) with protease inhibitors (Cell Signaling Technology, Danvers, MA) directly to the wells. These time points were used based on previous reports in the literature [25, 26] evaluating the effects of other phytochemicals on p38 and Erk signaling in primary osteoblast cells. Cells were then dislodged from the well surface with a cell scraper and underwent alternate sonication and vortexing for 60 mins to ensure the liberation of proteins. Following the final vortex, the cells were centrifuged at 16,000 × g for 10 mins to remove cellular debris and the supernatant was collected. Protein concentration was determined using the bicinchoninic acid assay (BCA) assay. Abundance of proteins of interest were analyzed by separating 20 μg of total protein on a denaturing sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and then transferring to a polyvinylidine fluoride (PVDF) membrane. Equal transfer of samples was confirmed with Ponceau staining prior to blocking the membrane with either 5% nonfat milk or 5% bovine serum albumin (BSA) for one hour. The membrane was then incubated at 4°C overnight with antibodies (Cell Signaling Technology, Danvers, MA) to p38, phospho-p38, p44/42 (Erk 1/2), or phospho-p44/42 (phospho-ERK1/2). Actin (Santa Cruz Biotechnology, Dallas, TX) was utilized as a loading control. Following an overnight incubation with primary antibody, the membranes were washed and incubated with secondary antibody for 1 hour prior to signal detection using SuperSignal West (ThermoFisher, Waltham, MA) chemiluminescent substrate. The blots were developed using the ProteinSimple Fluorchem R (San Jose, CA) and the density of the bands was assessed using UN-SCAN-IT gel analysis software (Silk Scientific Inc, Orem, UT). Each experiment was repeated 3–4 times.
2.5 Gene expression analyses
Gene expression analyses were completed to assess alterations in genes in the BMP signaling pathway, as well as genes known to be upregulated with increased bone mineralization. Primary bone marrow derived osteoblasts were cultured for 7 days in osteogenic media and then treated with DP-FrA or DP-FrB (10 μg/ml), with or without TNF-α (1 ng/ml). Total RNA was harvested after 15 mins or 1 hour of treatment to assess early alterations in expression of genes in the BMP pathway, and after 24 hours of treatment to assess alterations in expression of genes known to be upregulated during mineralization activity of osteoblasts based on previous reports [27, 28]. The cells were washed in sterile PBS, and RNA extraction was completed by adding Trizol (Life Technologies, Carlsbad, CA) and following the extraction protocol provided by the manufacturer. To ensure complete collection of cells from the well, a cell scraper was used to dislodge the cells. The concentration of the RNA was determined using spectrophotometry (NanoDrop; Thermo Fisher Scientific, Waltham, MA) and the quality of the 28S and 18S rRNA bands was confirmed via agarose gel electrophoresis. Reverse transcription of cDNA was performed using 2 μg of RNA and the relative abundance of mRNA of interest (Table 1) via real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR; Applied Biosystems, Foster City, CA) using SYBR Green technologies (Life Technologies, Carlsbad, CA). The comparative cycle threshold (CT) method (User Bulletin #2, Applied Biosystems, Foster City, CA) was used to evaluate mRNA expression levels, using glyceraldehyde-3-phosphate dehydrogenase (Gapdh) as a control.
Table 1.
Primer sequences for RT-qPCR.
| Transcript | Sequence (5′-3′) |
|---|---|
| Bmp2 | F: GGA CAT CCG CTC CAC AAA |
| R: GGC GCT TCC GCT GTT T | |
| Runx2 | F: TCT ACA GGC CCT GGT TCT |
| R: ATG TTC CAC TCT CCT CTT CTC TTG | |
| Bsp | F: ACA CCC CAA GCA CAG ACT TTT G |
| R: TCC TCG TCG CTT TCC TTC ACT | |
| Phex | F: GGC ATG ACT GCT GTA AGA TCA GAT |
| R: AGC TCC ATT GAC ATA AGG CAC T | |
| Tak1 | F: CGT CTT CTG CCA GTG AGA TG |
| R: ATC TTT TGC TCT CCA CTT AGC TT | |
| Smad1 | F: GCC CAT GGA CAC GAA CAT G |
| R: TGA ACA TCT CCT CTG CTG ATT TCA | |
| Smad5 | F: AGT GAC AGC AGC ATC TTT GTT CA |
| R: GTG GGA TGG AAG CCA TGG T | |
| Smad6 | F: CTG TCC GAT TCT ACA TTG TCT TAC ACT |
| R: CAT GCT GGC ATC TGA GAA TTC A |
2.6 Characterization of fractions
To begin to determine the composition of the most bioactive fractions, the presence of 14 phytochemicals (12 polyphenolic compounds) known to be present in dried plum (Table 2) was determined in DP-FrA and DP-FrB using liquid chromatography-mass spectrometry (LC/MS) methods previously described [29, 30]. Briefly, compounds detected via MS in the fractions were compared to known standards of the phytochemicals to identify the presence of any of the compounds in the fractions. Quantification of the detected compounds was done using a standard curve derived from the pure standard (Sigma-Aldrich, St. Louis, MO) of the compound.
Table 2.
Assessment of known polyphenolic compounds in DP-FrA and DP-FrB.
| DP-FrA | DP-FrB | |
|---|---|---|
| (mg/100 g total polyphenolic extract) | ||
| Chlorogenic acid | n.d. | n.d. |
| Cryptochlorogenic acid | 16.9 (24.0%) | 86.9 (40.9%) |
| Neochlorogenic acid | 53.6 (76.0%) | 125.7 (59.1%) |
| Caffeic acid | n.d. | n.d. |
| Quinic acid | n.d. | n.d. |
| o-Coumaric acid | n.d. | n.d. |
| m-Coumaric acid | n.d. | n.d. |
| Ferulic acid | n.d. | n.d. |
| Cyanidin 3-rutinoside | n.d. | n.d. |
| Cyanidin 3-glucoside | n.d. | n.d. |
| Quercetin | n.d. | n.d. |
| Rutin | n.d. | n.d. |
| Sorbic acid | n.d. | n.d. |
| 5-Hydroxymethyl-2-furaldehyde | n.d. | n.d. |
DP-FrA, dried plum fraction A; DP-FrB, dried plum fraction B; n.d., not detected. Numbers in parentheses note the percent of total compounds analyzed.
2.7 Statistical analyses
All statistical analyses were performed using SAS Version 9.3 (SAS Institute, Cary, NC). If data were not normally distributed based on univariate analyses, log transformation was completed prior to statistical analysis. To determine the most bioactive fractions in the initial screening experiments with MC3T3-E1 cells, treatment groups were compared using one-way ANOVA. For these experiments, when the overall ANOVA resulted in a significant F test, Bonferroni adjustment was completed due to the large number of comparisons. For all other assays, the effect of polyphenolic fraction treatment was analyzed using one-way ANOVA followed by Fisher’s least significant difference (LSD) post hoc analyses. Each experiment was repeated 2–3 times. All data are reported as means ± standard error (SE).
3. Results
3.1 Identifying the fractions that improve osteoblast activity in MC3T3-E1 cells
To identify the fractions and doses of the dried plum polyphenolic fractions with the greatest effect on osteoblast activity, extracellular ALP activity was assessed in the media of MC3T3-E1 cells. Extracellular ALP activity was upregulated by DP-FrB (p < 0.05) ~2-fold compared to control with treatment at both the 5 μg/mL and 10 μg/mL dose (Figure 1a). Treatment with DP-FrA (10 μg/mL) resulted in a 19.5% increase in ALP activity, but did not differ statistically from control. No other polyphenolic fractions improved extracellular ALP production compared to control and it should be noted that DP-FrE suppressed this response.
Figure 1.
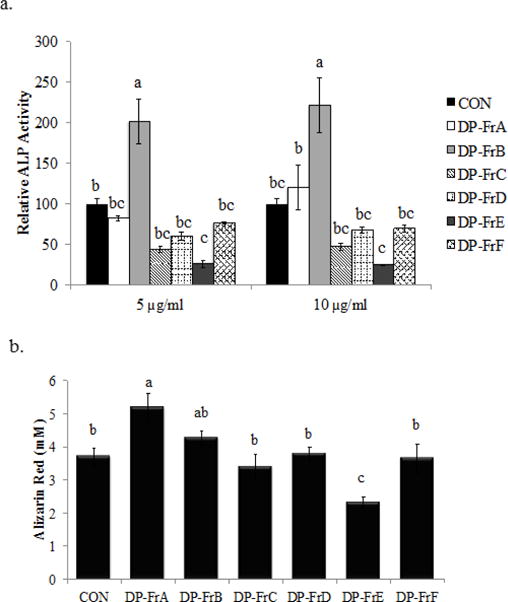
Dried plum polyphenolic fractions DP-FrA and DP-FrB improve osteoblast activity and function in MC3T3-E1 cells. Cells were treated with osteogenic media (alpha-MEM + 10% FBS, 2 mM L-glutamine, 1% penicillin/streptomycin, 25 μg/mL ascorbic acid and 10 mM β-glycerophosphate). a) Extracellular ALP was assessed following 7 days of treatment with DP-FrA and DP-FrB (n=6). b) Calcified nodules were stained with alizarin red following 28 days of treatment with osteogenic media and DP fractions (10 μg/mL) and then the stain was eluted and quantified (n=6). Bars represent the mean ± SE. Bars that do not share the same superscript letter are statistically different from each other, p < 0.05.
Based on the results of the ALP assay in which the most effective dose was 10 μg/mL, next the effects of the polyphenolic fractions on MC3T3-E1 cell function were assessed at that dose. Following 28 days of culture in osteogenic media, treatment with both DP-FrA and DP-FrB increased mineralized nodule formation (p < 0.05) compared to the control. The magnitude of response was greater in DP-FrA than DP-FrB treated cultures (Figure 1b). It is worth noting that DP-FrE treatment decreased (p < 0.05) mineralized nodule formation compared to control and no other treatments altered mineralization.
The results of these screening assays using MC3T3-E1 cells revealed that DP-FrA and DP-FrB at a dose of 10 μg/mL had the greatest potential for increasing osteoblast activity and function. Therefore, the focus of all subsequent experiments was on understanding how DP-FrA and DP-FrB altered primary osteoblasts under normal and inflammatory conditions and the mechanism through which these responses were mediated.
3.2 DP-FrA and DP-FrB enhance ALP activity and mineralization in murine primary bone marrow-derived osteoblasts
The ability of DP-FrA and DP-FrB to enhance osteoblast activity was assessed after 3 and 7 days of treatment. At day 3, ALP activity was significantly increased (i.e., ~2-fold) by both DP-FrA and DP-FrB compared to the control (Figure 2a). ALP continued to be elevated in response to DP-FrB at day 7 compared to the control, but not with DP-FrA (i.e., ~1.5-fold; p = 0.0582). To determine if the increase in ALP translated to improved, mineralized nodule formation, Von Kossa staining was performed following 14 days of treatment. Representative images of von Kossa staining are shown in Figure 2b. Both DP-FrA and DP-FrB significantly increased mineralized nodule formation in bone marrow-derived osteoblasts (Figure 2c).
Figure 2.
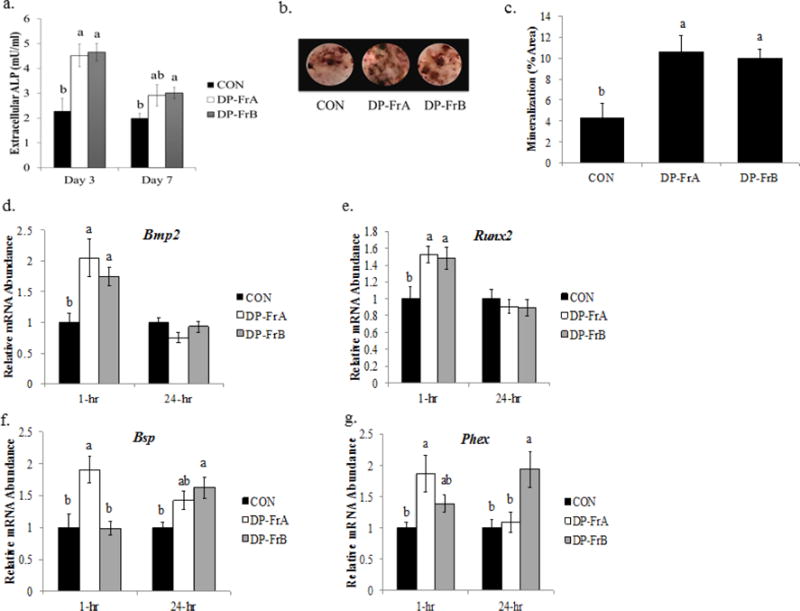
DP-FrA and DP-FrB induce ALP activity and mineralization in primary bone marrow-derived osteoblasts. Primary bone marrow stromal cells were treated with osteogenic media (alpha-MEM + 10% FBS, 2 mM L-glutamine, 1% penicillin/streptomycin, 50 μg/mL ascorbic acid and 3 mM β-glycerophosphate) and allowed to differentiate for 7 days. Cells were then treated with the polyphenolic fractions on day 7 of differentiation. Extracellular ALP a) was assessed in the media following 3 or 7 days of polyphenolic treatment (10 μg/mL). Mineralized nodules were stained using Von Kossa staining following 14 days of treatment with of DP-FrA and DP-FrB (10 μg/mL). b) Representative wells treated with DP-FrA and DP-FrB show increased von Kossa staining and c) increased the percentage of mineralized area (n=6). RNA was extracted following treatment with DP fractions for one hour or 24 hours. Relative mRNA expression of d) Bmp2, e) Runx2, f) Bsp, and g) Phex was assessed using RT-qPCR with Gapdh as a control (n=6). Bars represent the mean ± SE. Bars that do not share the same superscript letter are statistically different from each other, p < 0.05.
Gene expression analyses were performed to assess alterations in the relative abundance of genes that are involved in osteoblast differentiation and mineralization in response to the polyphenolic fractions. Following 1 hour of treatment, both DP-FrA and DP-FrB increased Bmp2 mRNA expression, which stimulates osteoblast differentiation by initiating the BMP/Smad signaling cascade, compared to control (Figure 2d). By 24 hours post-treatment with the polyphenolic fractions, Bmp2 expression normalized to that of the control. Likewise, expression of Runx2, a target of BMP/Smad signaling and an essential transcription factor for activation of genes related to osteoblast differentiation was upregulated by both DP-FrA and DP-FrB compared to control following 1 hour of treatment, and normalized by 24 hours post-polyphenolic fraction treatment (Figure 2e). In addition, Bsp, a phosphoprotein that promotes the mineralization of the extracellular matrix by interacting αvβ3 integrins on osteoblasts, was upregulated by DP-FrA following 1 hour of treatment and by DP-FrB following 24 hours of treatment (Figure 2f). Similarly, Phex, which promotes matrix mineralization by cleaving the inhibitory serine- and arginine-rich peptide of osteopontin that binds tightly to calcium, was also upregulated by DP-FrA after 1 hour of treatment and by DP-FrB after 24 hours of treatment (Figure 2g). These data show that the dried plum polyphenolic fractions can increase bone formation by inducing the gene expression of regulators of differentiation and mineralization activity of osteoblasts.
3.3 BMP and MAPK signaling is enhanced by polyphenolic fractions
Next, gene expression was assessed to examine whether activation of BMP signaling in primary bone marrow-derived osteoblasts could explain the upregulation of Runx2 and osteoblast activity. Following 15 minutes of treatment with the polyphenolic fractions, gene expression of Tak1, a kinase that activates MAPK signaling cascades within the BMP pathway and also phosphorylates Smad1 protein, was upregulated by DP-FrB compared to control (Figure 3a). Expression of Smad1, a transcription factor that induces osteoblast differentiation, was also upregulated by DP-FrB, but not DP-FrA, compared to control (Figure 3b). Smad5 gene expression was not altered by treatment with either dried plum polyphenolic fraction (Figure 3c).
Figure 3.
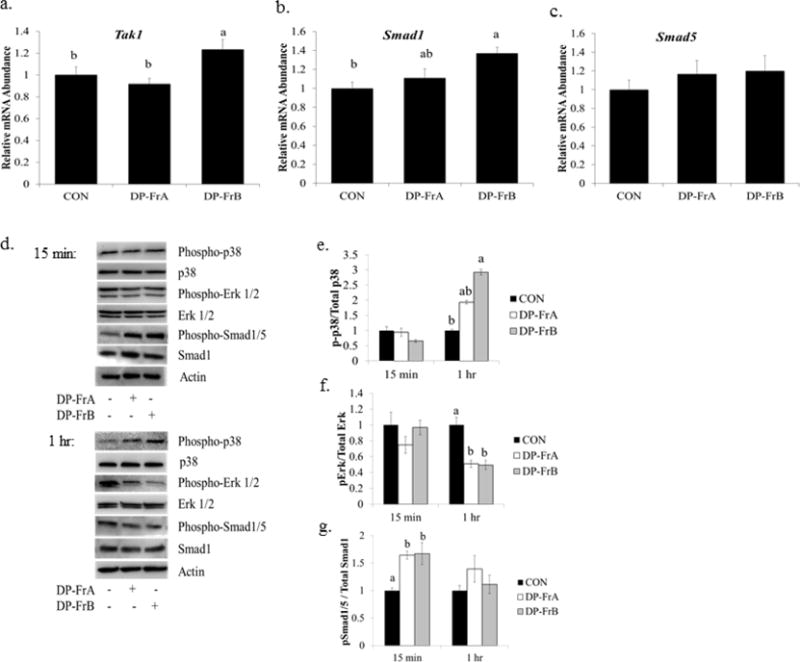
DP-FrA and DP-FrB upregulate BMP signaling in primary bone marrow-derived osteoblasts. Primary bone marrow stromal cells were treated with osteogenic media and allowed to differentiate for 7 days. Cells were then treated with the DP fractions and RNA was extracted after 15 minutes. Relative mRNA expression of a) Tak1, b) Smad1, and c) Smad5 was assessed with qRT-PCR using Gapdh as a control (n=6). Protein was extracted following treatment with the polyphenolic fractions for 15 minutes or 1 hour. d) Representative western blots are shown at each time point. The relative abundance of e) phosphorylated p38, f) ERK, and g) Smad1/5 (n=3) are presented. Bars represent the mean ± SE. Bars that do not share the same superscript letter are statistically different from each other, p < 0.05.
To assess whether proteins within the osteogenic BMP signaling cascade were altered with treatment with DP-FrA and DP-FrB, phosphorylation of p38, Erk, and Smad1/5 were examined over time. Representative blots are shown in Figure 3d. While 15 minutes of treatment with either fraction did not alter activation of p38, which stimulates ALP production and matrix mineralization, 1 hour of treatment with DP-FrB upregulated phosphorylation of p38 (Figure 3e). By 4 hours post-treatment with the fractions, p38 phosphorylation had normalized to the controls (data not shown).
Interestingly, activation of Erk1/2, which is essential for pre-osteoblast proliferation and osteoblast differentiation, but negatively regulates bone mineralization, was decreased after 1 hour of treatment with both DP-FrA and DP-FrB (Figure 3f). Similar to the phospho-p38 response, phosphorylation of Erk1/2 was not altered by DP-FrA or DP-FrB after 15 minutes (Figure 3f) or 4 hours (data not shown) of treatment. Finally, phosphorylation of Smad1/5, which is an important component of BMP signaling, was assessed. Increased phosphorylated Smad 1/5 was observed following 15 minutes of treatment with both DP-FrA (p = 0.0143) and DP-FrB (p = 0.0127) (Figure 3g). However, the abundance of phosphorylated Smad1/5 had normalized by 1 hour. These data indicate that BMP signaling is important early in the osteoblast’s response to the dried plum fractions.
3.4 Effects of polyphenolic fractions on TNF-α-treated primary bone marrow-derived osteoblasts
To examine whether DP-FrA and DP-FrB could induce bone formation in osteoblast under inflammatory conditions, cells were treated with the polyphenolic fractions in the presence of TNF-α. Following 3 and 7 days of treatment, TNF-α suppressed extracellular ALP production compared to an untreated control (Figure 4a). Treatment with dried plum polyphenolic fractions did not improve extracellular ALP compared to TNF-α treated control. Additionally, TNF-α suppressed mineralization activity of osteoblasts by 57%, as indicated by the representative von Kossa staining (Figure 4b); however, this did not reach the level of statistical significance (p=0.0674). Furthermore, treatment with DP-FrA in conjunction with TNF-α resulted in 52% greater mineralized area than that TNF-treated control, while DP-FrB improved mineralized nodule formation by 85% compared to the control treated with TNF-α (Figure 4c). The effects of both fractions resulted in a trend toward improved mineralization with the TNF-treated group and no difference with the negative control. The ALP and von Kossa staining data demonstrate that under inflammatory conditions the polyphenolic fractions were not able to rescue osteoblast ALP activity and while the capacity for DP-FrA and DP-FrB to have anabolic effects on mineralized nodule formation was lost, they were able to maintain normal mineralization.
Figure 4.
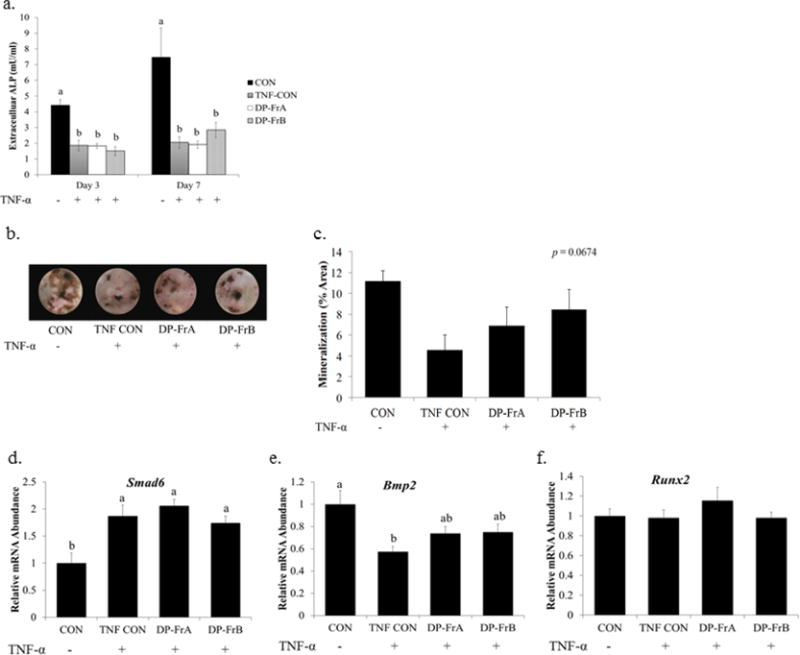
DP fractions failed to rescue primary bone marrow-derived osteoblasts from the detrimental effects of TNF-α. Primary bone marrow stromal cells were treated with osteogenic media and allowed to differentiate for 7 days. Cells were then treated with TNF-α (1 ng/mL) and the DP fractions on day 7 of differentiation. a) Extracellular ALP was measured in the media following 3 or 7 days of treatment. Mineralized nodules were assessed using Von Kossa staining following 14 days of treatment with the DP fractions (10 μg/mL). b) Representative wells treated with DP-FrA and DP-FrB show increased von Kossa staining and c) increased percentage of mineralized area (n=6). RNA was extracted following treatment with DP fractions for 1 hour. Relative mRNA expression of Smad6 (d), Bmp2 (e), and Runx2 (f) was assessed with qRT-PCR using Gapdh as a control. Bars represent the mean ± SE. Bars that do not share the same superscript letter are statistically different from each other, p < 0.05.
To explore why DP-FrA and DP-FrB were limited in their ability to improve osteoblast activity and function under inflammatory conditions, regulators of BMP signaling were examined in primary bone marrow-derived osteoblasts treated with TNF-α and the polyphenolic fractions. Treatment with TNF-α for 1 hour did not significantly affect transcription of Tak1, Smad1, or Smad5 (data not shown). However, mRNA expression of Smad6, an inhibitor of BMP/Smad signaling, was upregulated by TNF-α, and neither DP-FrA or DP-FrB were able to attenuate this response (Figure 4d). Furthermore, Bmp2 expression was suppressed by TNF-α, and DP-FrA and DP-FrB did not fully reverse this suppression (Figure 4e). Runx2 expression was unaltered following 1 hour of treatment with TNF-α and the dried plum fractions (Figure 4f).
3.5 Identification of phenolic acids present in DP-FrA and DP-FrB
Both DP-FrA and DP-FrB were analyzed for the presence of some of the polyphenols previously reported in dried plum extracts. DP-FrA and DP-FrB both contained cryptochlorogenic acid and neochlorogenic acid (Table 2) and both fractions contained a greater percentage of neochlorogenic acid than cryptochlorogenic acid. Chlorogenic acid, caffeic acid, quinic acid, o-coumaric acid, m-coumaric acid, ferulic acid cyanidin 3-rutinoside, cyanidin 3-glucoside, quercetin, rutin, sorbic acid, and 5-hydroxymethyl-2-furaldehyde were not detected in either fraction. This data indicates that neochlorogenic acid and cryptochlorogenic acid may be, at least in part, responsible for the beneficial effects of dried plum on osteoblast activity and function.
4. Discussion
This study identified fractions of a polyphenolic extract from dried plum that enhanced osteoblast activity and function. The two fractions with the greatest solubility in water (Dp-FrA and DP-FrB) were the most bioactive in enhancing ALP activity and mineralized nodule formation in murine primary bone marrow-derived osteoblasts. Both neochlorogenic acid and cryptochlorogenic acid were detected in these fractions, which is interesting in light of Léotoing and colleagues [31] recent report showing that synthetic forms of chlorogenic acid, neochlorogenic acid, and caffeic acid suppressed ALP in primary calvarial pre-osteoblasts. Differences in cell source and stage of maturation, as well as the source of the polyphenols tested (i.e., synthetic polyphenols vs. polyphenol extract) could account for the discrepancies between the two studies. Certainly, it would be premature to attribute the response observed in our studies to these specific compounds, but at the same time they cannot be ruled out as acting alone or in conjunction with other compounds within the two fractions. Isomers of chlorogenic acid are known to have antioxidant and anti-inflammatory properties, as well as the ability to alter cell signaling pathways, including those that involve the kinases JNK, p38 and ERK [9, 32–34]. Less is known about how chlorogenic acid isomers affect other signaling pathways important to osteoblast function, such as BMP signaling.
In the current study, the polyphenolic fractions upregulated the expression of Runx2, the master regulator of osteoblast differentiation, in primary bone marrow-derived pre-osteoblasts under normal conditions. These findings are in agreement with previous in vitro data from our laboratory in which a crude ethanol extract of polyphenols from dried plum enhanced activity of osteoblasts derived from MC3T3-E1 cells by upregulating the expression of Runx2 [11]. It has been demonstrated in vitro that the upregulation of Runx2, and the subsequent increase in osteoblast differentiation and bone formation, occurs as a result of BMP signaling [12–14]. Membrane-bound BMP receptors, activated in pre-osteoblasts by osteogenic BMPs (i.e., BMP2 and BMP4), are serine/threonine kinases that initiate osteoblast differentiation by phosphorylating Smad 1/5/8 and TAK1 [12, 35]. The phosphorylation of Smad1/5/8 induces nuclear translocation of the protein complex, where it acts to induce Runx2 expression [12]. Both of the fractions in the current study significantly upregulated phosphorylation of Smad1/5/8. Furthermore, activation of TAK1 initiates a signaling cascade that results in the phosphorylation and activation of p38 [36]. Phosphorylation of p38 was enhanced by DP-FrB in the current study. Activated p38 enhances osteoblast differentiation by phosphorylating Smad1 at the same site targeted by the BMP receptor, thereby amplifying the BMP signaling cascade. Additionally, p38 phosphorylates and activates Runx2 protein, enhancing the activity of this osteogenic transcription factor [12, 36]. The activation of p38 is essential to inducing osteoblast activity, as treatment with a p38 inhibitor results in downregulation of ALP production and mineralized matrix formation in mouse primary calvarial osteoblasts [37]. Conversely, while the activation of Erk is important in the proliferation of osteoblast precursor cells [38], phosphorylated Erk negatively regulates bone mineralization in vitro [39]. In the current study, treatment with both DP-FrA and DP-FrB resulted in a suppression of Erk phosphorylation, indicating that the polyphenolic fractions increase osteoblast mineralization activity, at least in part, by inhibiting Erk. The increase in Bmp2, Tak1 and Smad1 gene expression, as well as an upregulation in p38 and Smad1/5 phosphorylation, suggests the polyphenolic fractions enhanced BMP signaling in differentiating bone marrow-derived osteoblasts. The upregulation of BMP signaling and the activation of p38 by plant-based polyphenolic compounds have been previously demonstrated in vitro by others as well [40–43].
The increase in osteoblast differentiation due to enhanced BMP signaling resulted in upregulation of mineralized nodule formation in the current study. Bone-forming mature osteoblasts produce collagen, creating an extracellular matrix, as well as non-collagenous proteins, such as bone sialoprotein and osteopontin, in preparation of matrix mineralization [44]. Bone sialoprotein (Bsp), which was upregulated at the mRNA level by both DP-FrA and DP-FrB in the currently study, promotes maturation and mineralization of the extracellular matrix via the interaction of an Arginine-Glycine-Aspartic Acid (RGD) motif with αvβ3 integrins on osteoblasts [45]. Likewise, osteopontin also contains an RGD binding domain, as well as serine- and arginine-rich peptide (ASARM) with a high affinity for calcium, resulting in tight binding of the protein to hydroxyapatite [46]. The binding of the ASARM peptide of osteopontin to hydroxyapatite functions to inhibit crystal growth, indicating the protein’s role in regulating bone mineralization [47, 48]. However, the protease Phex cleaves the ASARM peptide of osteopontin, which eliminates its ability to inhibit mineralization [48]. We have shown here that both DP-FrA and DP-FrB upregulated the expression Phex, which along with the upregulation of Bsp, suggests a mechanism for the observed increased mineralization capacity of osteoblasts treated with these polyphenolic fractions. This increase in mineralized bone formation has also been observed in animal studies and clinical trials following consumption of dried plum [3–5, 7, 49, 50]. This increase in bone formation observed in animal and clinical trials is likely due, at least in part, to the ability of polyphenols in dried plum to upregulate BMP signaling and osteoblast activity.
Further evidence of the role of the fractions in enhancing BMP signaling in bone marrow-derived pre-osteoblasts was observed when the cells were simultaneously treated with TNF-α in the current study. A decline in osteoblast activity, and therefore bone formation, is observed in conditions of inflammation, including that which occurs with estrogen deficiency and rheumatoid arthritis [15, 16, 18]. The inflammatory cytokine, TNF-α, is known to suppress osteoblast differentiation by reducing the expression and stability of Runx2 [15, 16, 18, 20]. TNF-α-upregulates NF-κB signaling, which in turn suppresses BMP signaling by inhibiting the binding of Smad1/5/8 to the Runx2 promoter region [15]. In this study, TNF-α suppressed the expression of Bmp2, as well as ALP activity and mineralized nodule formation, but did not significantly alter Runx2 mRNA expression. Treatment with the fractions resulted in a trend for increased Bmp2 expression and the maintenance of mineralization in the inflammatory environment to the level of the control. BMP signaling was also likely downregulated by the TNF-α-induced upregulation of Smad6 expression, a repressor of BMP-induced Smad signaling [51]. Neither fraction was able to attenuate the increase in Smad6 mRNA levels. Previously, it was demonstrated that a crude ethanol extract of dried plum polyphenols could attenuate the TNF-α-induced decline in osteoblast activity and function using the MC3T3-E1 cell line [11]. Likewise, a crude polyphenolic extract from dried plum reversed bone loss in osteopenic, OVX Sprague Dawley rats [10]. It is possible that a higher dose of the fractions or a combination of the fractions is needed to fully protect the differentiating osteoblasts from the detrimental effects of TNF-α.
One point that merits discussion when studying polyphenolic compounds in vitro is the question of bioavailability of the compounds. While polyphenolic compounds can undergo biotransformation in gastrointestinal tract prior to absorption, the absorption of such compounds has also been reported. For example, Hollman and colleagues [52] showed the absorption of different isoforms of quercetin in subjects with ileostomy (i.e., the absence of colonic bacteria), including quercetin glucosides (~52% of ingested), quercetin rutinosides (~17%), and quercetin aglycones (~24%). While this study does not eliminate the potential for metabolism of the compounds by microbiota residing within the small intestine, these findings do support the notion that the compounds can be absorbed. Moreover, flavonoids have been reported to require conjugation to be transported in the blood, but are then deconjugated prior to tissue storage [53]. These aglycones, which are more likely to be absorbed unaltered, are compounds that are found within tissues in vivo. Thus, it is reasonable to conceive that some of the polyphenolic compounds found in dried plums can be absorbed and reach the tissues to alter osteoblast signaling and function. However, it is important to note that this study should be considered an initial step in an effort to determine if certain type of polyphenolic compounds in dried plums promote greater osteogenic activity and these findings warrant follow-up utilizing animal models.
Based on the results of these studies, it is evident that certain types of compounds in the dried plum extract enhance osteoblast activity and function by enhancing BMP signaling and increase regulators of mineralization. However, these fractions were not able to significantly alter the TNF-α-induced suppression of ALP in differentiation of osteoblasts, which may be due, in part, to a suppression of BMP signaling by Smad6. While the fractions preserved the mineralization capacity of the osteoblast under inflammatory conditions, they were no longer able to induce an anabolic response. Further investigation is needed to determine whether a higher dose of the fractions would be required to induce such a response in the presence of TNF-α. Because the composition of these fractions is likely more than just neochlorogenic acid and cryptochlorogenic acid, it would be premature to infer that the osteogenic activity reported here results from these compounds. Further characterization of the components of DP-FrA and DP-FrB is warranted. Additionally, determining the differences in the polyphenolic profile of DP-FrA and DP-FrB compared to the fractions that had no effect or even suppressed osteoblast activity may provide further insight into the polyphenolic compounds responsible for osteogenesis, but this type of comprehensive assessment of the fractions is beyond the scope of the current project.
Acknowledgments
Funding
This work was supported by the National Institutes of Health 1R21AT006580-01A1 and the Oklahoma Center for Advancement and Technology HR14-126.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Adachi JD, Adami S, Gehlbach S, Anderson FA, Boonen S, Chapurlat RD, et al. Impact of prevalent fractures on quality of life: baseline results from the global longitudinal study of osteoporosis in women; Mayo Clinic Proceedings; Elsevier; 2010. pp. 806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siris E, Brenneman S, Barrett-Connor E, Miller P, Sajjan S, Berger M, et al. The effect of age and bone mineral density on the absolute, excess, and relative risk of fracture in postmenopausal women aged 50–99: results from the National Osteoporosis Risk Assessment (NORA) Osteoporos Int. 2006;17:565–74. doi: 10.1007/s00198-005-0027-4. [DOI] [PubMed] [Google Scholar]
- 3.Hooshmand S, Kern M, Metti D, Shamloufard P, Chai SC, Johnson SA, et al. The effect of two doses of dried plum on bone density and bone biomarkers in osteopenic postmenopausal women: a randomized, controlled trial. Osteoporos Int. 2016 doi: 10.1007/s00198-016-3524-8. [DOI] [PubMed] [Google Scholar]
- 4.Hooshmand S, Chai SC, Saadat RL, Payton ME, Brummel-Smith K, Arjmandi BH. Comparative effects of dried plum and dried apple on bone in postmenopausal women. Br J Nutr. 2011;106:923–30. doi: 10.1017/S000711451100119X. [DOI] [PubMed] [Google Scholar]
- 5.Smith BJ, Graef JL, Wronski TJ, Rendina E, Williams AA, Clark KA, et al. Effects of dried plum supplementation on bone metabolism in adult C57BL/6 male mice. Calcif Tissue Int. 2014;94:442–53. doi: 10.1007/s00223-013-9819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahnazari M, Turner RT, Iwaniec UT, Wronski TJ, Li M, Ferruzzi MG, et al. Dietary dried plum increases bone mass, suppresses proinflammatory cytokines and promotes attainment of peak bone mass in male mice. J Nutr Biochem. 2016;34:73–82. doi: 10.1016/j.jnutbio.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith BJ, Bu SY, Wang Y, Rendina E, Lim YF, Marlow D, et al. A comparative study of the bone metabolic response to dried plum supplementation and PTH treatment in adult, osteopenic ovariectomized rat. Bone. 2014;58:151–9. doi: 10.1016/j.bone.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Rendina E, Lim YF, Marlow D, Wang Y, Clarke SL, Kuvibidila S, et al. Dietary supplementation with dried plum prevents ovariectomy-induced bone loss while modulating the immune response in C57BL/6J mice. J Nutr Biochem. 2012;23:60–8. doi: 10.1016/j.jnutbio.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Nakatani N, Kayano S, Kikuzaki H, Sumino K, Katagiri K, Mitani T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.) J Agric Food Chem. 2000;48:5512–6. doi: 10.1021/jf000422s. [DOI] [PubMed] [Google Scholar]
- 10.Graef JL, Ouyang P, Wang Y, Rendina-Ruedy E, Marlow D, Lucas EA, Smith BJ. Dried Plum Polyphenolic Extract Combined with Vitamin K and Potassium Restores Trabecular and Cortical Bone in Osteopenic Model of Postmenopausal Bone Loss. J Functional Foods. doi: 10.1016/j.jff.2017.12.057. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bu SY, Hunt TS, Smith BJ. Dried plum polyphenols attenuate the detrimental effects of TNF-alpha on osteoblast function coincident with up-regulation of Runx2, Osterix and IGF-I. J Nutr Biochem. 2009;20:35–44. doi: 10.1016/j.jnutbio.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Lee MH, Kim YJ, Kim HJ, Park HD, Kang AR, Kyung HM, et al. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem. 2003;278:34387–94. doi: 10.1074/jbc.M211386200. [DOI] [PubMed] [Google Scholar]
- 13.Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT. BMP signaling is required for RUNX2- dependent induction of the osteoblast phenotype. J Bone Miner Res. 2006;21:637–46. doi: 10.1359/JBMR.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franceschi RT, Xiao G, Jiang D, Gopalakrishnan R, Yang S, Reith E. Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect Tissue Res. 2003;44:109–16. [PMC free article] [PubMed] [Google Scholar]
- 15.Yamazaki M, Fukushima H, Shin M, Katagiri T, Doi T, Takahashi T, et al. Tumor necrosis factor alpha represses bone morphogenetic protein (BMP) signaling by interfering with the DNA binding of Smads through the activation of NF-kappaB. J Biol Chem. 2009;284:35987–95. doi: 10.1074/jbc.M109.070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukai T, Otsuka F, Otani H, Yamashita M, Takasugi K, Inagaki K, et al. TNF-alpha inhibits BMP-induced osteoblast differentiation through activating SAPK/JNK signaling. Biochem Biophys Res Commun. 2007;356:1004–10. doi: 10.1016/j.bbrc.2007.03.099. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Guttridge DC, Tang E, Shi S, Guan K, Wang CY. Suppression of tumor necrosis factor-mediated apoptosis by nuclear factor kappaB-independent bone morphogenetic protein/Smad signaling. J Biol Chem. 2001;276:39259–63. doi: 10.1074/jbc.M105335200. [DOI] [PubMed] [Google Scholar]
- 18.Guo R, Yamashita M, Zhang Q, Zhou Q, Chen D, Reynolds DG, et al. Ubiquitin ligase Smurf1 mediates tumor necrosis factor-induced systemic bone loss by promoting proteasomal degradation of bone morphogenetic signaling proteins. J Biol Chem. 2008;283:23084–92. doi: 10.1074/jbc.M709848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- 20.Kaneki H, Guo R, Chen D, Yao Z, Schwarz EM, Zhang YE, et al. Tumor necrosis factor promotes Runx2 degradation through up-regulation of Smurf1 and Smurf2 in osteoblasts. J Biol Chem. 2006;281:4326–33. doi: 10.1074/jbc.M509430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bu SY, Lerner M, Stoecker BJ, Boldrin E, Brackett DJ, Lucas EA, et al. Dried plum polyphenols inhibit osteoclastogenesis by downregulating NFATc1 and inflammatory mediators. Calcif Tissue Int. 2008;82:475–88. doi: 10.1007/s00223-008-9139-0. [DOI] [PubMed] [Google Scholar]
- 22.Einbond LS, Reynertson KA, Luo X-D, Basile MJ, Kennelly EJ. Anthocyanin antioxidants from edible fruits. Food Chem. 2004;84:23–8. [Google Scholar]
- 23.Beck GR, Jr, Sullivan EC, Moran E, Zerler B. Relationship between alkaline phosphatase levels, osteopontin expression, and mineralization in differentiating MC3T3-E1 osteoblasts. J Cell Biochem. 1998;68:269–80. doi: 10.1002/(sici)1097-4644(19980201)68:2<269::aid-jcb13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Bonewald LF, Harris SE, Rosser J, Dallas MR, Dallas SL, Camacho NP, et al. von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int. 2003;72:537–47. doi: 10.1007/s00223-002-1057-y. [DOI] [PubMed] [Google Scholar]
- 25.Bhargavan B, Gautam AK, Singh D, Kumar A, Chaurasia S, Tyagi AM, et al. Methoxylated isoflavones, cajanin and isoformononetin, have non- estrogenic bone forming effect via differential mitogen activated protein kinase (MAPK) signaling. J Cell Biochem. 2009;108:388–99. doi: 10.1002/jcb.22264. [DOI] [PubMed] [Google Scholar]
- 26.He G, Guo W, Lou Z, Zhang H. Achyranthes bidentata saponins promote osteogenic differentiation of bone marrow stromal cells through the ERK MAPK signaling pathway. Cell Biochem Biophys. 2014;70:467–73. doi: 10.1007/s12013-014-9942-3. [DOI] [PubMed] [Google Scholar]
- 27.Trzeciakiewicz A, Habauzit V, Mercier S, Lebecque P, Davicco M-J, Coxam V, et al. Hesperetin stimulates differentiation of primary rat osteoblasts involving the BMP signalling pathway. J Nutr Biochem. 2010;21:424–31. doi: 10.1016/j.jnutbio.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Tang C-H, Yang R-S, Chien M-Y, Chen C-C, Fu W-M. Enhancement of bone morphogenetic protein-2 expression and bone formation by coumarin derivatives via p38 and ERK-dependent pathway in osteoblasts. Eur J Pharmacol. 2008;579:40–9. doi: 10.1016/j.ejphar.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Prior RL, Wu X, Gu L. Identification and urinary excretion of metabolites of 5-(hydroxymethyl)-2-furfural in human subjects following consumption of dried plums or dried plum juice. J Agric Food Chem. 2006;54:3744–9. doi: 10.1021/jf0601113. [DOI] [PubMed] [Google Scholar]
- 30.Kammalla AK, Ramasamy MK, Inampudi J, Dubey GP, Agrawal A, Kaliappan I. Comparative pharmacokinetic study of mangiferin after oral administration of pure mangiferin and US patented polyherbal formulation to rats. AAPS PharmSciTech. 2015;16:250–8. doi: 10.1208/s12249-014-0206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leotoing L, Wauquier F, Davicco MJ, Lebecque P, Gaudout D, Rey S, et al. The polyphenolic acids of Agen prunes (dried plums) or Agen prune juice concentrates do not account for the protective action on bone in a rat model of postmenopausal osteoporosis. Nutr Res. 2016;36:161–73. doi: 10.1016/j.nutres.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Feng R, Lu Y, Bowman LL, Qian Y, Castranova V, Ding M. Inhibition of activator protein-1, NF-kappaB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J Biol Chem. 2005;280:27888–95. doi: 10.1074/jbc.M503347200. [DOI] [PubMed] [Google Scholar]
- 33.Kim M, Choi SY, Lee P, Hur J. Neochlorogenic Acid Inhibits Lipopolysaccharide-Induced Activation and Pro-inflammatory Responses in BV2 Microglial Cells. Neurochem Res. 2015;40:1792–8. doi: 10.1007/s11064-015-1659-1. [DOI] [PubMed] [Google Scholar]
- 34.Xu JG, Hu QP, Liu Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J Agric Food Chem. 2012;60:11625–30. doi: 10.1021/jf303771s. [DOI] [PubMed] [Google Scholar]
- 35.Ryoo HM, Lee MH, Kim YJ. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene. 2006;366:51–7. doi: 10.1016/j.gene.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Greenblatt MB, Shim JH, Glimcher LH. TAK1 mediates BMP signaling in cartilage. Ann N Y Acad Sci. 2010;1192:385–90. doi: 10.1111/j.1749-6632.2009.05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Y, Chan E, Wang SX, Li B. Activation of p38 mitogen-activated protein kinase is required for osteoblast differentiation. Endocrinology. 2003;144:2068–74. doi: 10.1210/en.2002-220863. [DOI] [PubMed] [Google Scholar]
- 38.Mahalingam CD, Sampathi BR, Sharma S, Datta T, Das V, Abou-Samra AB, et al. MKP1-dependent PTH modulation of bone matrix mineralization in female mice is osteoblast maturation stage specific and involves P-ERK and P-p38 MAPKs. J Endocrinol. 2013;216:315–29. doi: 10.1530/JOE-12-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kono SJ, Oshima Y, Hoshi K, Bonewald LF, Oda H, Nakamura K, et al. Erk pathways negatively regulate matrix mineralization. Bone. 2007;40:68–74. doi: 10.1016/j.bone.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Chen JR, Lazarenko OP, Wu X, Kang J, Blackburn ML, Shankar K, et al. Dietary-induced serum polyphenolic acids promote bone growth via p38 MAPK/beta-catenin canonical Wnt signaling. J Bone Miner Res. 2010;25:2399–411. doi: 10.1002/jbmr.137. [DOI] [PubMed] [Google Scholar]
- 41.Chen JR, Lazarenko OP, Zhang J, Blackburn ML, Ronis MJ, Badger TM. Diet-derived polyphenolic acids regulate osteoblast and adipocyte lineage commitment and differentiation in young mice. J Bone Miner Res. 2014;29:1043–53. doi: 10.1002/jbmr.2034. [DOI] [PubMed] [Google Scholar]
- 42.Trzeciakiewicz A, Habauzit V, Mercier S, Lebecque P, Davicco MJ, Coxam V, et al. Hesperetin stimulates differentiation of primary rat osteoblasts involving the BMP signalling pathway. J Nutr Biochem. 2010;21:424–31. doi: 10.1016/j.jnutbio.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 43.Kim HJ, Kim SH. Tanshinone IIA enhances BMP-2-stimulated commitment of C2C12 cells into osteoblasts via p38 activation. Amino Acids. 2010;39:1217–26. doi: 10.1007/s00726-010-0557-8. [DOI] [PubMed] [Google Scholar]
- 44.Hadjidakis DJ, Androulakis Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–96. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- 45.Gordon JA, Tye CE, Sampaio AV, Underhill TM, Hunter GK, Goldberg HA. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone. 2007;41:462–73. doi: 10.1016/j.bone.2007.04.191. [DOI] [PubMed] [Google Scholar]
- 46.Standal T, Borset M, Sundan A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp Oncol. 2004;26:179–84. [PubMed] [Google Scholar]
- 47.Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, et al. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002;161:2035–46. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Addison WN, Masica DL, Gray JJ, McKee MD. Phosphorylation-dependent inhibition of mineralization by osteopontin ASARM peptides is regulated by PHEX cleavage. J Bone Miner Res. 2010;25:695–705. doi: 10.1359/jbmr.090832. [DOI] [PubMed] [Google Scholar]
- 49.Arjmandi BH, Khalil DA, Lucas EA, Georgis A, Stoecker BJ, Hardin C, et al. Dried plums improve indices of bone formation in postmenopausal women. J Womens Health Gend Based Med. 2002;11:61–8. doi: 10.1089/152460902753473471. [DOI] [PubMed] [Google Scholar]
- 50.Hooshmand S, Brisco JR, Arjmandi BH. The effect of dried plum on serum levels of receptor activator of NF-kappaB ligand, osteoprotegerin and sclerostin in osteopenic postmenopausal women: a randomised controlled trial. Br J Nutr. 2014;112:55–60. doi: 10.1017/S0007114514000671. [DOI] [PubMed] [Google Scholar]
- 51.Shen R, Chen M, Wang YJ, Kaneki H, Xing L, O’Keefe RJ, et al. Smad6 interacts with Runx2 and mediates Smad ubiquitin regulatory factor 1-induced Runx2 degradation. J Biol Chem. 2006;281:3569–76. doi: 10.1074/jbc.M506761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hollman PC, de Vries JH, van Leeuwen SD, Mengelers MJ, Katan MB. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr. 1995;62:1276–82. doi: 10.1093/ajcn/62.6.1276. [DOI] [PubMed] [Google Scholar]
- 53.Perez-Vizcaino F, Duarte J, Santos-Buelga C. The flavonoid paradox: conjugation and deconjugation as key steps for the biological activity of flavonoids. J Sci Food Agric. 2012;92:1822–5. doi: 10.1002/jsfa.5697. [DOI] [PubMed] [Google Scholar]


