Abstract
Background/Objective
Previous research has focused on associations between dietary fat and body mass index (BMI), but the contributions of different types of fat to BMI remain unclear. The purpose of this study is to estimate whether plasma phospholipid omega-3 (n-3), omega-6 (n-6), or trans fatty acids are associated with BMI at baseline and with subsequent BMI changes over time; and whether total phospholipid n-6 or trans fatty acids modify any association between phospholipid n-3 and BMI.
Methods
Cross-sectional and longitudinal linear mixed models include 6 243 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort. Participants were 45-84 years old, had no history of cardiovascular disease at baseline (2000-2002), and were followed for up to 10 years. Plasma phospholipid fatty acids were measured using fasting plasma samples at baseline. Fully adjusted models include demographics; health behaviors; and other fatty acids (n-3, n-6, trans) as appropriate.
Results
In fully-adjusted models phospholipid n-3 fatty acid levels were inversely associated with baseline BMI (Ptrend <0.001). Baseline BMI was 1.14 (95% CI: 0.71, 1.57) kg/m2 lower among participants with total n-3 values in the highest vs. the lowest quartiles, but was not associated with changes in BMI. Total phospholipid n-6 was positively associated with baseline BMI in partially- but not fully-adjusted models. No overall association was observed between fatty acid levels and changes in BMI. No clear association was observed between trans fatty acids and baseline BMI or BMI change. No effect modification in the association between phospholipid n-3 and baseline BMI or BMI change was observed by either phospholipid n-6 or trans fatty acids.
Conclusion
Phospholipid total and specific n-3 fatty acid levels were inversely associated with BMI at baseline, while associations tended to be positive for total n-6 fatty acids. Significant associations between fatty acid levels and BMI changes were not observed.
Introduction
Previous research has focused on associations among total fat intake, body weight, body mass index (BMI), and weight loss, yet the contributions of different types of fat to BMI remain unclear. Total dietary fat intake does not account for the high levels of obesity found at the population level—as the percentage of energy from dietary fat declined at the end of the 20th century, obesity prevalence rose in the United States;(1) and there is evidence that different types of fat affect body weight differently. While increases in dietary consumption of animal fat, saturated fat, and trans fat have been positively associated with weight gain,(2) there is also evidence that certain fats and fatty acids have beneficial effects on body weight and BMI.(3,4) Of these, the polyunsaturated fatty acids (PUFA) and their two families, omega-3 (n-3) and omega-6 (n-6) fatty acids have garnered particular attention.
Higher intakes of long-chain n-3 PUFAs are well-recognized for their cardiovascular-related benefits.(3) Their potential implications in body weight are less well characterized; however, fish oil, which is high in docosahexaenoic acid (DHA, 22:6 n-3) and eicosapentaenoic acid (EPA, 20:5 n-3), has been associated with reduced body fat mass and increased lipid utilization in healthy adults.(5)
In contrast to the n-3 PUFAs, greater intake of n-6 fatty acids or a high ratio of dietary n-6 to n-3 fatty acids may be responsible for an increase in development of adipose tissue during early stages of life.(6,7) A balance between n-3 and n-6 fatty acids may be essential for weight management, and imbalances may promote weight gain.
Apart from the n-6 and n-3 PUFAs, saturated fat and trans fatty acids may be associated with weight gain and increased abdominal adiposity, as found in animal and human studies.(8,9) Percentage of calories from animal fat, saturated fat, and trans fat has been associated with weight gain over time;(2) and high levels of trans fatty acids in adipose tissue were associated with adult weight gain;(10) however, epidemiologic evidence remains inconsistent.(2)
The purpose of this study is to examine whether selected phospholipid plasma cis and trans unsaturated fatty acids are associated with baseline BMI and with subsequent changes in BMI over time using data from the Multi-Ethnic Study of Atherosclerosis (MESA). We hypothesize that higher baseline phospholipid n-3 PUFA will be associated with lower baseline BMI and with smaller increases in BMI over time, and that the opposite will be true for plasma n-6 and trans fatty acids. We further examine whether phospholipid n-6 or trans fatty acids modify the association between n-3 and BMI and hypothesize that inverse associations between plasma n-3 and BMI at baseline and changes in BMI over time will be stronger among participants with lower levels of phospholipid n-6 and trans fatty acids than among those with higher values.
Methods
Study Population
MESA is a prospective cohort study designed to investigate the prevalence, correlates, and progression of subclinical cardiovascular disease (CVD) in a diverse, population-based sample of 6 814 adults ages 45-84 years at baseline (2000-2002). It has previously been described in detail.(11) Individuals without clinical evidence of CVD were recruited from six communities throughout the United States (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; New York, NY; and St. Paul, MN). All participants self-reported their race/ethnicity as White; Black or African-American; Chinese; or Spanish, Hispanic, or Latino using questions based on the 2000 U.S. Census questionnaire. All participants gave informed consent, and the Institutional Review Boards at all six sites approved this research. Overall, 6 573 participants were included in the baseline analyses after excluding 241 participants missing phospholipid fatty acids measurements and an additional 330 missing diet, activity, or smoking information.
Measurements
Baseline questionnaires included items on age, sex, race/ethnicity, education, and lifestyle factors including diet and physical activity. Dietary intake was measured using a 120-item validated food frequency questionnaire (FFQ) covering usual consumption in the previous year and including foods commonly eaten in Latino and Asian populations.(12–14)
Diet was summarized using the Healthy Eating Index (HEI), a summary dietary quality score developed by the US Department of Agriculture in 2005 that includes 12 diet components.(15) The HEI ranges from 0–100, with higher scores denoting better diet quality. Self-reported physical activity information was collected with a 28-item survey asking participants about the frequency, duration, and intensity of their participation in a variety of activity categories (e.g., work, walking, sports) during a typical week in the past month.(16) Fasting blood was drawn and participant height and weight were measured using standardized procedures during the baseline exam.(11) Serum and EDTA-anticoagulant tubes were collected and processed using a standardized protocol.(11)
Phospholipid Fatty Acid Profile
A venous blood sample was obtained at baseline and stored as EDTA plasma at -70C until chemical analysis. Phospholipid ALA, EPA, DHA, and DPA n-3 fatty acids; linoleic, gamma-linolenic, dihomo gamma-linolenic, and arachidonic n-6 fatty acids; and 16:1, 18:1 and 18:2 trans fatty acids were measured using methodology described by Cao et al.(17) Lipids were extracted from plasma using a mixture of chloroform and methanol. Cholesterol esters, triglycerides, phospholipids, and free fatty acids were separated by thin layer chromatography. Fatty acids from the phospholipid fraction were separated and derivatized to methyl esters and detected by gas chromatography flame ionization. Phospholipid fatty acids were measured in two batches, in February of 2009 and October of 2013, and are presented as a percent of total plasma phospholipid fatty acids. Fatty acids of interest included alpha-linolenic (ALA; 18:3 n-3), eicosapantaenoic (EPA; 20:5 n-3), docosahexaenoic (DHA; 22:6 n-3), and docosapentaenoic (DPA; 22:5 n-3) n-3 fatty acids; linoleic (18:2 n-6), gamma linolenic (18:3 n-6), dihomo gamma-linolenic (20:3 n-6), and arachidonic (20:4 n-6) n-6 fatty acids; and 16:1, 18:1 and 18:2 trans fatty acids. The following representative CVs were obtained (n=20): linoleic acid, 2.6%; ALA, 2.4%; arachidonic acid, 2.4%; EPA, 3.3%; DPA, 2.9%; and DHA, 2.7%.
Statistical Analysis
All analyses were conducted using SAS 9.3 (SAS Institute, Inc., Cary, NC). We estimated associations between plasma phospholipid fatty acids measured at baseline and both baseline BMI and BMI change over time. In addition to total n-3, n-6, and trans fatty acids, we also present results for EPA+DHA and ALA n-3 and linoleic and arachidonic n-6 fatty acids.
When used as the exposure of interest, fatty acid values were divided into quartiles. Fatty acid distributions were checked and all were normal. Many had long, but continuous, tails, and extreme fatty acid values were not found to be influential in sensitivity analyses excluding outliers more than three times the inter-quartile range (IQR) from either end of the IQR. Correlation between individual types of n-3 and n-6 fatty acids and trans fatty acids was modest (all pairwise correlations <|0.40|). BMI was calculated by dividing weight in kilograms by the square of height in meters (kg/m2).
Generalized linear models were fit between fatty acid quartiles and means of baseline BMI. Average annual change in BMI was calculated using longitudinal linear mixed models including random individual intercepts and time slopes and controlling for baseline BMI and years since baseline. Resulting estimates were multiplied by 10 and average estimated 10-year BMI change is presented to approximate the time elapsed between exams 1 and 5. This allowed for the estimation of change in BMI throughout MESA follow-up for all participants with at least two BMI measurements and did not require an Exam 5 visit. These models provide unbiased estimates of linear parameters in the presence of uncorrelated errors between observations with valid estimates of significance levels when errors are normally distributed.(18) Visual inspection of residual plots suggested that residuals for all models were approximately normally distributed.
Adjusted baseline BMI and BMI change models include the following covariates: age; age squared; sex, race/ethnicity; education; MESA study site; phospholipid fatty acid batch; smoking status; METs (metabolic equivalents, or an estimate of the intensity of physical activity relative to sitting quietly(19)) per day of intentional physical activity including walking, conditioning, dance, and individual or team sports; and HEI scores. Covariates were measured at baseline and are included in the models using the categories presented in Table 1, except for age, age squared, and HEI quintiles which were modeled continuously, with age centered on its mean of 62. All covariates in the BMI change models were also interacted with time since baseline.
Table 1. Baseline body mass index (BMI) and change in BMI by baseline characteristics.
| N (%) | Exam 1 BMI N=6 573 Mean (95% CI) |
Change in BMI (Exam 5 – Exam 1) N=4 491 Mean (95% CI) |
|
|---|---|---|---|
| Total | 6 573 (100.0) | 28.3 (28.2, 28.5) | 0.15 (0.08, 0.29) |
| Age | |||
| 44-54 | 1 884 (28.7) | 28.7 (28.5, 29.0) | 0.69 (0.56, 0.81) |
| 55-64 | 1 834 (27.9) | 28.8 (28.5, 29.0) | 0.25 (0.12, 0.38) |
| 65-74 | 1 935 (29.4) | 28.1 (27.9, 28.4) | -0.26 (-0.40, -0.12) |
| 75-84 | 920 (14.0) | 27.1 (26.7, 27.4) | -1.18 (-1.43, -0.92) |
| Sex | |||
| Female | 3 474 (52.9) | 28.7 (28.6, 28.9) | 0.11 (0.00, 0.21) |
| Male | 3 099 (47.2) | 27.9 (27.7, 28.1) | 0.18 (0.07, 0.29) |
| Race | |||
| White | 2 533 (38.5) | 27.7 (27.5, 27.9) | 0.24 (0.12, 0.36) |
| Chinese American | 793 (12.1) | 24.0 (23.6, 24.3) | 0.03 (-0.18, 0.25) |
| Black | 1 802 (27.4) | 30.2 (30.0, 30.5) | -0.07 (-0.22, 0.08) |
| Hispanic | 1 445 (22.0) | 29.5 (29.2, 29.7) | 0.25 (0.09, 0.41) |
| Site | |||
| Forsyth County | 1 036 (15.8) | 28.9 (28.5, 29.2) | 0.13 (-0.06, 0.31) |
| New York | 1 055 (16.1) | 29.0 (28.7, 29.3) | 0.22 (0.04, 0.41) |
| Baltimore | 1 021 (15.5) | 29.4 (29.1, 29.7) | 0.06 (-0.14, 0.27) |
| St. Paul | 1 040 (15.8) | 29.5 (29.1, 29.8) | 0.20 (0.01, 0.38) |
| Chicago | 1 138 (17.3) | 26.7 (26.4, 27.0) | 0.05 (-0.12, 0.22) |
| Los Angeles | 1 283 (19.5) | 27.0 (26.7, 27.3) | 0.17 (-0.02, 0.35) |
| Plasma phospholipid batch | |||
| First | 2 856 (43.5) | 27.9 (27.7, 28.1) | 0.21 (0.09, 0.32) |
| Second | 3 717 (56.6) | 28.7 (28.5, 28.9) | 0.09 (-0.01, 0.19) |
| Education | |||
| Less than high school | 1 186 (18.1) | 28.5 (28.2, 28.8) | 0.02 (-0.18, 0.21) |
| High school/GED | 1 195 (18.2) | 28.9 (28.6, 29.2) | 0.09 (-0.09, 0.27) |
| Some college | 1 861 (28.4) | 28.9 (28.6, 29.1) | 0.18 (0.04, 0.32) |
| College graduate | 2 309 (35.3) | 27.5 (27.3, 27.7) | 0.19 (0.07, 0.31) |
| Smoking status | |||
| Never | 3 296 (50.3) | 28.1 (27.9, 28.3) | 0.12 (0.01, 0.22) |
| Former | 2 398 (36.6) | 28.8 (28.6, 29.0) | 0.00 (-0.12, 0.13) |
| Current | 858 (13.1) | 28.0 (27.7, 28.4) | 0.66 (0.45, 0.87) |
| Physical activity (MET hours of moderate/vigorous activity per week)a | |||
| Tertile 1 (0-6.1) | 2 187 (33.4) | 28.4 (28.1, 28.6) | -0.13 (-0.26, 0.01) |
| Tertile 2 (6.1-14.4) | 2 179 (33.2) | 28.1 (27.8, 28.3) | 0.17 (0.05, 0.30) |
| Tertile 3 (14.5-246.0) | 2 189 (33.4) | 28.5 (28.3, 28.8) | 0.36 (0.23, 0.48) |
| Healthy Eating Index a | |||
| Quintile 1 (23.6-55.3) | 1 252 (20.0) | 29.0 (28.7, 29.3) | 0.20 (0.02, 0.37) |
| Quintile 2 (55.3-61.8) | 1 250 (20.0) | 29.0 (28.7, 29.3) | 0.30 (0.13, 0.48) |
| Quintile 3 (61.8-66.6) | 1 254 (20.0) | 28.4 (28.2, 28.7) | 0.21 (0.04, 0.39) |
| Quintile 4 (66.6-71.7) | 1 252 (20.0) | 27.9 (27.6, 28.2) | 0.15 (-0.02, 0.32) |
| Quintile 5 (71.7-89.2) | 1 251 (20.0) | 27.3 (27.0, 27.6) | -0.09 (-0.25, 0.07) |
Percents may not add up to 100 due to rounding.
Abbreviations: BMI = body mass index, GED = general educational development, MET = metabolic equivalent
Numbers presented in parentheses represent the range for each category.
Estimated reference values are presented for quartile 1 for models of both baseline BMI and estimated 10-year BMI change. For baseline BMI models this estimated value represents the model intercept and for BMI change models this value represents the mean 10-year BMI change among participants with fatty acid values in Q1 for models specified centering all continuous variables at the mean and specifying the first category in Table 1 for all categorical variables. Estimates of baseline BMI and BMI change values for quartiles 2-4 should be added to these reference values to estimate the mean baseline BMI and BMI change values in those quartiles.
Additional models are presented further controlling for total amounts of the fatty acids other than the fatty acid category of interest (total and specific n-3 models control for total n-6 and trans fatty acids; total and specific n-6 models control for total n-3 and trans fatty acids, and trans fatty acids models control for total n-3 and n-6). P-values for trend were estimated using type 3 tests of fixed effects. We examined differences in changes in BMI over time by baseline fatty acid quartile using product terms between the fatty acid quartile indicators and years since baseline. In sensitivity analyses we further included energy in kilocalories/day to the fully-adjusted models. This resulted in the exclusion of an additional 249 participants due to missing energy values and did not importantly change our findings (the largest difference in estimated BMI was 0.12 kg/m/2; however, most estimates were within 0.05 in either direction). Energy intake was not included in our final models; however, results of these sensitivity analyses are presented in Supplementary Table 1.
Interaction between n-3 and n-6 values was assessed by fitting baseline BMI and BMI change models including an interaction term between quartiles of n-3 and n-6 split at the median and reporting the P-value associated with the interaction parameter. The same methodology was used to test for interaction between n-3 trans fatty acids. We chose to split n-6 and trans fatty acids at the median, rather than in quartiles, for ease of interpretation of the interaction results.
Results
Average baseline BMI and change in BMI by demographic and health characteristics are shown in Table 1. Average BMI was 28.3 (95% CI: 28.2, 28.5) kg/m2 at baseline, and BMI increased by an average of 0.15 (95% CI: 0.08, 0.29) kg/m2 through Exam 5. BMIs were highest in participants younger than 65 at baseline, and BMI increased among participants under age 65 at baseline while it decreased among those 65 and older. Baseline BMI was higher in women (28.7, 95% CI: 28.6-28.9) than men 27.9 (95% CI: 27.7-28.1), and both experienced small increases in BMI through Exam 5. Black participants had the highest average BMI at baseline. BMI remained constant for black and Chinese-American participants and increased among white and Hispanic participants. Baseline BMI was lower in Los Angeles (27.1) and Chicago (26.7) than in the other sites (28.8-29.5) but increased only in New York and St. Paul. College graduates had a lower average baseline BMI (27.5) than those with lower levels of education (28.5-28.9), but higher education was associated with BMI increases over time. Never and current smokers had lower baseline BMIs than former smokers but experienced BMI increases over time, particularly current smokers. Baseline BMI was highest among participants reporting the lowest levels of intentional physical activity; however, BMI increased among participants in the second and third tertiles of activity levels. HEI scores were inversely associated with baseline BMI and BMI changes over time.
Table 2 gives baseline BMI and average annual change in BMI by quartile of total and specific forms of n-3 and n-6 fatty acids, and by total trans fatty acids. Phospholipid n-3 fatty acid levels were inversely associated with baseline BMI (Ptrend <0.001) and baseline BMI was 1.07 (95% CI: 0.66, 1.48) kg/m2 lower among participants with total n-3 values in the highest versus the lowest quartiles in partially-adjusted models, and 1.14 kg/m2 (95% CI: 0.71, 1.57) after adjusting for total n-6 and trans fatty acids. This association was particularly strong for alpha linolenic acid, where baseline BMI was 1.47 (95% CI: 1.11, 1.82) kg/m2 lower in the highest versus lowest quartiles, and less strong for phospholipid EPA+DHA, where BMI was 0.90 (95% CI: 0.46, 1.33) kg/m2 lower among participants in the highest compared with the lowest quartile in fully-adjusted models.
Table 2. Baseline body mass index (BMI) and BMI change by quartile of plasma phospholipid total, EPA+DHA, and ALA n-3 fatty acids; total, linoleic, and arachidonic n-6 fatty acids; and total trans-fatty acids.
| Partially-Adjusted Modelsa | Fully-Adjusted Modelsb | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Fatty Acid Quartilesc | Baseline BMI | 95% CI | 10-year BMI Changed | 95% CI | Baseline BMI | 95% CI | 10-year BMI Changed | 95% CI |
| Total n-3e | ||||||||
| Quartile 1 (1.26-4.33)f | 29.30 | 28.51, 30.09 | 0.55 | 0.06, 1.05 | 29.07 | 28.14, 30.01 | 0.22 | -0.36, 0.80 |
|
|
||||||||
| Quartile 2 (4.33-5.34) | -0.12 | -0.47, 0.24 | 0.02 | -0.20, 0.24 | -0.19 | -0.55, 0.17 | 0.01 | -0.21, 0.23 |
| Quartile 3 (5.34-6. 80) | -0.35 | -0.72, 0.03 | 0.13 | -0.10, 036 | -0.44 | -0.83, -0.06 | 0.15 | -0.09, 0.39 |
| Quartile 4 (6.81-23.61) | -1.07 | -1.48, -0.66 | -0.09 | -0.35, 0.16 | -1.14 | -1.57, -0.71 | 0.00 | -0.27, 0.27 |
| Ptrend | <0.001 | 0.24 | <0.001 | 0.46 | ||||
| EPA+DHA | ||||||||
| Quartile 1 (0.80-3.29) f | 29.07 | 28.27, 29.86 | 0.56 | 0.06, 1.08 | 28.84 | 27.90, 29.77 | 0.22 | -0.36, 0.80 |
|
|
||||||||
| Quartile 2 (3.29-4.28) | 0.25 | -0.11, 0.60 | 0.04 | -0.18, 0.26 | 0.18 | -0.19, 0.54 | 0.04 | -0.18, 0.26 |
| Quartile 3 (4.28-5.66) | -0.11 | -0.50, 0.27 | 0.05 | -0.19, 0.28 | -0.21 | -0.60, 0.18 | 0.07 | -0.17, 0.31 |
| Quartile 4 (5.66-22.10) | -0.84 | -1.25, -0.42 | -0.09 | -0.35, 0.16 | -0.90 | -1.33, -0.46 | 0.01 | -0.26, 0.28 |
| Ptrend | <0.001 | 0.60 | <0.001 | 0.92 | ||||
| Alpha linolenic acid (ALA) | ||||||||
| Quartile 1 (0.03-0.12) f | 29.81 | 29.01, 30.60 | 0.42 | -0.08, 0.91 | 29.06 | 28.16, 29.96 | 0.11 | -0.45, 0.67 |
|
|
||||||||
| Quartile 2 (0.12-0.16) | -0.22 | -0.57, 0.13 | 0.05 | -0.17, 0.27 | -0.25 | -0.60, 0.10 | 0.05 | -0.17, 0.27 |
| Quartile 3 (0.16-0.20) | -0.72 | -1.07, -0.38 | 0.24 | 0.02, 0.45 | -0.75 | -1.10, -0.40 | 0.23 | 0.01, 0.44 |
| Quartile 4 (0.20-2.54) | -1.45 | -1.81, -1.10 | 0.14 | -0.09, 0.36 | -1.47 | -1.82, -1.11 | 0.12 | -0.10, 0.34 |
| Ptrend | <0.001 | 0.15 | <0.001 | 0.19 | ||||
| Total n-6g | ||||||||
| Quartile 1 (9.86-33.76) f | 28.44 | 27.59, 29.29 | 0.24 | -0.29, 0.76 | 29.07 | 28.14, 30.01 | 0.22 | -0.36, 0.80 |
|
|
||||||||
| Quartile 2 (33.76-35.81) | 0.50 | 0.15, 0.86 | 0.20 | -0.02, 0.42 | 0.37 | 0.01, 0.72 | 0.18 | -0.04, 0.40 |
| Quartile 3 (35.82-37.70) | 0.58 | 0.22, 0.94 | 0.15 | -0.08, 0.37 | 0.33 | -0.05, 0.70 | 0.13 | -0.11, 0.36 |
| Quartile 4 (37.70-52.76) | 0.42 | 0.03, 0.82 | 0.35 | 0.11, 0.59 | 0.03 | -0.38, 0.45 | 0.32 | 0.07, 0.58 |
| Ptrend | 0.008 | 0.038 | 0.073 | 0.085 | ||||
| Linoleic acid | ||||||||
| Quartile 1 (5.66-17.97) f | 29.48 | 28.66, 30.30 | 0.49 | -0.02, 0.99 | 29.95 | 29.06, 30.84 | 0.47 | -0.08, 1.03 |
|
|
||||||||
| Quartile 2 (17.97-20.05) | -0.05 | -0.40, 0.31 | 0.03 | -0.19, 0.25 | -0.14 | -0.49, 0.21 | 0.02 | -0.20, 0.23 |
| Quartile 3 (20.05-22.27) | -0.29 | -0.65, 0.07 | 0.04 | -0.18, 0.27 | -0.45 | -0.82, -0.09 | 0.02 | -0.20, 0.25 |
| Quartile 4 (22.28-36.13) | -0.83 | -1.21, -0.44 | 0.09 | -0.15, 0.33 | -1.10 | -1.50, -0.71 | 0.07 | -0.18, 0.31 |
| Ptrend | <0.001 | 0.91 | <0.001 | 0.95 | ||||
| Arachidonic acid | ||||||||
| Quartile 1 (1.12-9.87) f | 28.70 | 27.89, 29.52 | 0.48 | -0.02, 0.98 | 28.96 | 28.08, 29.83 | 0.47 | -0.07, 1.02 |
|
|
||||||||
| Quartile 2 (9.87-11.57) | 0.09 | -0.26, 0.44 | 0.06 | -0.16, 0.27 | 0.10 | -0.25, 0.46 | 0.05 | -0.17, 0.27 |
| Quartile 3 (11.57-13.36) | 0.46 | 0.10, 0.82 | 0.02 | -0.20, 0.24 | 0.47 | 0.11, 0.83 | 0.00 | -0.22, 0.22 |
| Quartile 4 (13.36-22.17) | 0.34 | -0.04, 0.72 | 0.11 | -0.12, 0.34 | 0.31 | -0.08, 0.70 | 0.09 | -0.16, 0.33 |
| Ptrend | 0.048 | 0.79 | 0.056 | 0.86 | ||||
| Trans-fatty acids | ||||||||
| Quartile 1 (0.22-1.18) f | 28.74 | 27.95, 29.53 | 0.53 | 0.04, 1.02 | 29.07 | 28.14, 30.01 | 0.22 | -0.36, 0.80 |
|
|
||||||||
| Quartile 2 (1.18-1.63) | 0.46 | 0.08, 0.83 | -0.02 | -0.25, 0.21 | 0.36 | -0.02, 0.74 | -0.03 | -0.26, 0.20 |
| Quartile 3 (1.63-2.15) | 0.56 | 0.17, 0.95 | 0.07 | -0.17, 0.31 | 0.39 | 0.00, 0.78 | 0.05 | -0.19, 0.29 |
| Quartile 4 (2.15-6.50) | 0.01 | -0.39, 0.41 | 0.08 | -0.17, 0.32 | -0.23 | -0.64, 0.18 | 0.07 | -0.18, 0.32 |
| Ptrend | 0.001 | 0.81 | 0.001 | 0.84 | ||||
Abbreviations: ALA = alpha-linolenic acid BMI = body mass index (kg/m2), CI = confidence interval, DHA = docosahexaenoic acid, EPA = eicosapentaenoic acid, HEI = Healthy Eating Index, MESA = Multi-Ethnic Study of Atherosclerosis
Partially-adjusted models include observations from 6 243 participants and control for plasma phospholipid fatty acids batch indicator, baseline age, baseline age squared, sex, race/ethnicity, MESA study site, education, smoking status, HEI, and physical activity.
Fully-adjusted models include observations from 6 243 participants and add adjustment for total n-3 (for total n-6, linoleic, arachidonic and trans fatty acids), total n-6 (for total n-3, EPA+DHA, ALA, and trans fatty acids) and trans fatty acids (for total n-3, EPA+DHA, ALA, total n-6, linoleic, and arachidonic fatty acids) to the partially-adjusted models.
Fatty acid values are expressed as the percent of total plasma phospholipid fatty acids. Numbers presented in parentheses represent the range for each quartile.
BMI change models include interactions between each model covariate and time since baseline exam.
Total n-3 fatty acids include EPA, DHA, docosapentaenoic, and ALA fatty acids.
Values shown in the Quartile 1 rows represent the model intercept for baseline BMI models and the estimated 10-year change in BMI among participants with fatty acid values in lowest quintile for the BMI change models, all evaluated at the mean levels of continuous covariates and the first category in Table 1 for all categorical covariates. Thus the Q1 reference values are the reference against which Q2-Q4 difference or change values are compared. For example, for total n-3 partially adjusted models, the absolute baseline BMI value in Q2 is estimated to be 29.30 -0.12 = 29.18 and the corresponding absolute 10-year BMI change is 0.55 + 0.02 = 0.57.
Total n-6 fatty acids include linoleic, gamma-linolenic, dihomo gamma-linolenic and arachidonic fatty acids.
Baseline levels of phospholipid n-3 fatty acids were not associated with changes in BMI over time.
Baseline BMI was 0.42-0.58 kg/m2 higher among participants with phospholipid n-6 fatty acids in the highest quartiles relative to those with the lowest n-6 values in partially-adjusted models (Ptrend<0.008); however, this association was no longer significant after controlling for total n-3 and trans fatty acids. Arachidonic acid levels were positively associated with baseline BMI (Ptrend =0.048) in partially-adjusted models and marginally associated in fully-adjusted models (P=0.056). Linoleic acid levels were inversely associated with baseline BMI (Ptrend <0.001) in models with and without control for n-3 and trans fatty acids.
Total phospholipid n-6 was positively associated with increased BMI over time (Ptrend =0.038) such that BMI increased by 0.35 kg/m2 more among participants with total n-6 levels in the highest quartile compared with the lowest in partially-adjusted models. This difference attenuated to 0.32 kg/m2 in fully-adjusted models (Ptrend=0.085). Neither linoleic nor arachidonic acid were associated with change in BMI.
Although trans fatty acid levels appeared positively associated with BMI at baseline (Ptrend=0.001 for partially- and fully-adjusted models), results were inconsistent. Baseline BMI was 0.46-0.56 kg/m2 higher among participants whose phospholipid trans fatty acids levels were in the middle quartiles compared with the first in partially-adjusted models, but only the third quartile was different from the first in fully-adjusted models. Phospholipid trans fatty acid values were not associated with changes in BMI over time.
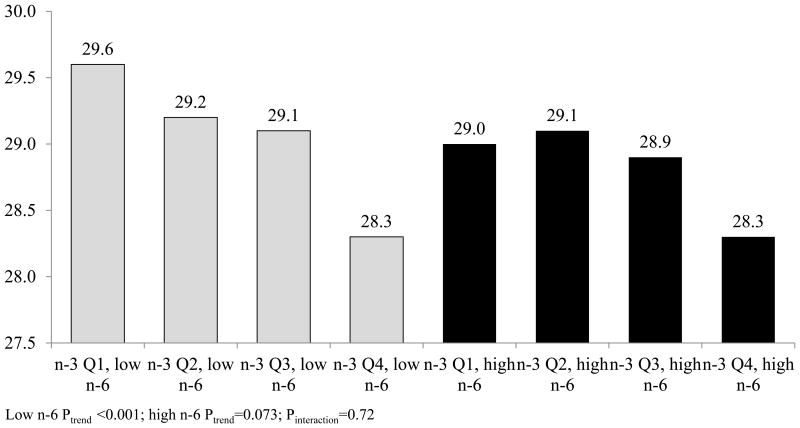
Figure 1 presents results of analyses of quartiles of total phospholipid n-3 and baseline BMI, stratified by phospholipid n-6 (above vs. below the median). No effect modification was observed, and phospholipid n-3 was inversely associated with baseline BMI at phospholipid n-6 values below (Ptrend<0.001) and marginally associated above (Ptrend=0.073) the median (Pinteraction=0.72). Total n-3 levels were not associated with BMI change among participants with total n-6 levels above (Ptrend=0.72) or below (Ptrend=0.13) the median, and no effect modification was observed (Pinteraction=0.34) (data not shown in the figure).
Figure 1. Baseline BMI by quartile of total n-3 phospholipid fatty acids, stratified by total phospholipid n-6 fatty acids.
Abbreviations: ALA = alpha-linolenic acid BMI = body mass index (kg/m2), CI = confidence interval, DHA = docosahexaenoic acid, EPA = eicosapentaenoic acid, MESA = Multi-Ethnic Study of Atherosclerosis.
Figures include observations from 6,243 participants (3,123 below and 3,120 above median n-6). All plasma phospholipid values were collected at baseline and represent percent of total plasma phospholipid fatty acids. Total n-3 plasma phospholipid fatty acids include EPA, DHA, docosapentaenoic, and ALA fatty acids. Total n-6 plasma phospholipid fatty acids include linoleic, gamma-linolenic, dihomo gamma-linolenic and arachidonic fatty acids, split at the median of 35.8%.
Generalized linear models were fit between fatty acid quartiles and means of baseline BMI. Models include the following covariates: age, age squared, sex, race/ethnicity, education, MESA study site, and phospholipid fatty acid batch, smoking status, physical activity, Healthy Eating Index scores, and total plasma phospholipid trans fatty acids.
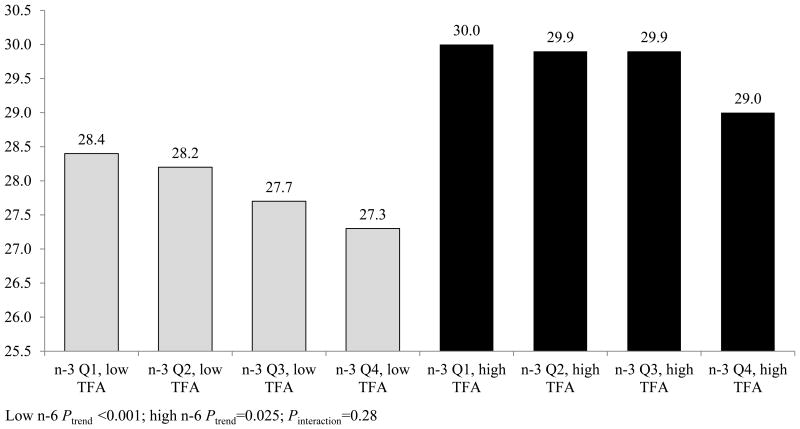
Figure 2 presents the association between quartiles of phospholipid n-3 and baseline BMI, stratified by total trans fatty acids above versus below the median. Total n-3 levels were inversely associated with baseline BMI both above (Ptrend=0.025) and below (Ptrend<0.001) the median; however, no effect modification was observed (Pinteraction=0.28).
Figure 2. Average 10-year change in BMI by quartile of total n-3 phospholipid fatty acids, stratified by total phospholipid n-6 fatty acids.
Abbreviations: ALA = alpha-linolenic acid BMI = body mass index (kg/m2), CI = confidence interval, DHA = docosahexaenoic acid, EPA = eicosapentaenoic acid, MESA = Multi-Ethnic Study of Atherosclerosis.
Figures include observations from 6,243 participants (3,123 below and 3,120 above median n-6). All plasma phospholipid values were collected at baseline and represent percent of total plasma phospholipid fatty acids. Total n-3 plasma phospholipid fatty acids include EPA, DHA, docosapentaenoic, and ALA fatty acids. Total n-6 plasma phospholipid fatty acids include linoleic, gamma-linolenic, dihomo gamma-linolenic and arachidonic fatty acids, split at the median of 35.8%.
Generalized linear models were fit between fatty acid quartiles and means of baseline BMI. Average annual change in BMI was calculated using longitudinal linear mixed models including random individual intercepts and time slopes and controlling for baseline. Resulting estimates were multiplied by 10 to estimate 10-year change in BMI. Models include the following covariates: age, age squared, sex, race/ethnicity, education, MESA study site, and phospholipid fatty acid batch, smoking status, physical activity, Healthy Eating Index scores, and total plasma phospholipid trans fatty acids.
No effect modification was observed in the association between total n-3 fatty acids and change in BMI by total trans fatty acids (Pinteraction=0.35), and n-3 was not associated with change in BMI at trans fatty acid levels above (Ptrend=0.71) or below (Ptrend=0.12) the median (data not shown).
Discussion
Our results suggest that specific phospholipid fatty acid levels are differentially associated with BMI values at baseline, but not with subsequent changes in BMI over time. Higher n-3 concentrations were associated with lower baseline BMI overall, and this association was evident for both EPA+DHA and for ALA; however, in stratified analyses, this association was only marginally significant at n-6 levels above the median. Higher levels of total n-6 fatty acids were associated with higher baseline BMI and positively associated with BMI change over time in partially- but not fully-adjusted models. The inverse association observed between phospholipid n-3 and baseline BMI was evident at n-6 and trans fatty acid values both above and below the median. No clear association was observed between trans-fatty acids and baseline BMI or BMI changes.
Previous work has suggested an inverse association exists between n-3 PUFA and cardiovascular risk,(1,24) including in MESA,(21) but less is known about the association between n-3 PUFA and BMI or obesity. Our findings are consistent with previous animal and limited human studies that have demonstrated inverse associations between n-3 fatty acids and body weight or body fat. In animal studies, higher consumption of long-chain n-3 fatty acids reduced body fat among obese rodents and has been protective against obesity in the presence of an obesogenic diet.(22) Inclusion of ALA (18:3 n-3) in a diet rich in linoleic acid (18:2 n-6) was associated with lower body weight in both mice (23) and normal term human infants, although in this latter study differences in dietary fatty acid composition did not cause significant differences in skinfold thickness.(24)
Our finding of small but statistically significant inverse associations between phospholipid n-3 PUFA and EPA+DHA levels with BMI is consistent with the results of a 2014 systematic review and meta-analysis of randomized controlled trials that reported small reductions in body weight, BMI, and percent body fat in participants taking fish or fish oil supplements compared with controls.(25) In a cross-sectional study of Australian adults, plasma concentrations of n-3 PUFA, particularly EPA and DHA, were inversely associated with BMI and waist and hip circumferences; however, this study included a relatively small sample (N=124), and did not address potential confounding factors including race/ethnicity.(26) By comparison, in our results controlling for several demographic characteristics and health behaviors, the average BMI among participants in the highest quartile of total phospholipid n-3 was 1.14 (95% CI: 0.71, 1.57) kg/m2 lower at baseline compared with participants with phospholipid n-3 values in the lowest quartile.
Several mechanisms have been proposed for the association between n-3 PUFA and favorable associations with body composition. Higher n-3 consumption may be associated with higher overall diet quality and may impact appetite control and satiety. High levels of dietary long-chain n-3 PUFA have been found to modulate satiety in overweight and obese individuals during weight loss.(27) Fatty acids may also interact with neuroendocrine factors such as leptin, ghrelin, and insulin to modulate brain-intestinal loop signals for energy metabolism and appetite control.(26) Higher levels of n-3 PUFA may increase basal fat oxidation, leading to reduced fat mass,(5,28) and have been found to regulate lipid metabolism through inflammation mediators.(29)
We found that higher phospholipid n-6 values were positively associated with baseline BMI and with BMI changes over time in partially-, but not fully-adjusted models. These results are consistent to some extent with previous human and animal studies. In mice, increasing the n-6 PUFA (linoleic acid) proportion in diets from 1% to 8% to reflect changes in diets found in the United States during the 20th century resulted in increased food intake, feed efficiency, and adiposity.(30) Studies in humans suggest that maternal n-6 concentrations, particularly dihomo gamma-linolenic acid, during gestation may be positively associated with children's BMI at age 7,(31) and that higher n-6 fatty acid intake and a higher n-6:n-3 ratio are associated with increases in adipose tissue development during early stages of life.(6,7,32).
Both human and animal studies suggest that long-term intake of trans fatty acids may be associated with weight gain and increased abdominal adiposity.(2,8,9) High levels of trans fatty acids in adipose tissue have been associated with adult weight gain.(10) Epidemiologic evidence remains inconsistent; however, percent of calories from animal, saturated, and trans fats has been associated with weight gain over time.(2) In our results, BMI was 0.39 kg/m2 higher in participants in the third quartile of phospholipid trans fatty acids compared with the lowest; however, there was no significant difference in BMI between the highest and lowest quartiles, and trans fatty acids were not associated with changes in BMI over time.
It should be noted that the follow-up of MESA participants coincided with increasing public awareness of potential negative health consequences of consumption of trans fats, including increased cardiovascular disease risk. This included a 2003 regulation released by the Food and Drug Administration requiring manufacturers to list trans fats on nutrition labels (33) and a 2007 policy statement by the American Public Health Association recommending labeling of trans fats on all commercial food products and a ban on and monitoring of trans fat use in restaurants.(34) Evidence suggests that trans fat intake has declined significantly over the past several years,(35) and baseline levels may not accurately reflect MESA participants' intake throughout follow-up. This would limit our ability to detect an association between baseline phospholipid trans fatty acids and changes in BMI over time.
This study features several important strengths, including the use of a large, multi-ethnic cohort with longitudinal follow-up of several thousand participants over several years, which allowed for the examination of the association between several specific phospholipid fatty acids and BMI at baseline and BMI changes over time. Using plasma phospholipid values avoids the limitations of estimating dietary fatty acid intake from self-reported measures such as food frequency questionnaires, although fatty acid metabolism likely differs between people and plasma levels are not an exact substitute for dietary intake. Plasma levels also do not differentiate dietary from supplemental intake of fatty acids; however, plasma phospholipid levels of the major n-6, n-3 and trans fatty acids are useful biomarkers of their dietary intakes.(36,37) Using BMI values measured by study staff at repeated clinic visits avoids potential bias associated with the use of self-reported height and weight measures. Detailed baseline questionnaires allowed for the control of several demographic characteristics and health behaviors.
Our results should be considered in the context of potential limitations. Although BMI was calculated based on repeated measures of height and weight, phospholipid data were only available at baseline and our results do not reflect any changes in phospholipid fatty acid distribution throughout follow-up. Further, because individual fatty acid values are measured as a percent of total fatty acids, higher values for one fatty acid implies lower values of others and we are not able to estimate associations between absolute fatty acid values and BMI. Although BMI is a reasonable measure of body fatness at a population level, it does not accurately capture individual body composition, such as lean mass, visceral fat, or excess body fatness. The large sample size allowed for adjustment for several potential confounding factors, including diet quality and physical activity; however, the possibility of residual confounding cannot be ruled out.
Although our results require further confirmation in other longitudinal studies and do not distinguish body metabolism from food and supplemental intake, they suggest that total and specific phospholipid n-3 PUFA are inversely associated with BMI in cross-sectional analyses, as is linoleic acid. In contrast, associations tended to be positive for total n-6 fatty acids, arachidonic acid, and trans fatty acids. Total n-6 fatty acids were marginally positively associated with increases in BMI over time; however, no significant associations between fatty acid levels and BMI changes over time were observed.
Supplementary Material
Acknowledgments
This research was supported by P60MD002249 from the National Institute on Minority Health and Health Disparities (to TAH); by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest.
Supplementary Information: Supplementary information is available at International Journal of Obesity's website.
References
- 1.Willett WC. Dietary fat plays a major role in obesity: no. Obes Rev. 2002 May 1;3(2):59–68. doi: 10.1046/j.1467-789x.2002.00060.x. [DOI] [PubMed] [Google Scholar]
- 2.Field AE, Willett WC, Lissner L, Colditz GA. Dietary Fat and Weight Gain Among Women in the Nurses' Health Study. Obesity. 2007 Apr 1;15(4):967–76. doi: 10.1038/oby.2007.616. [DOI] [PubMed] [Google Scholar]
- 3.Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis. 2006 Nov;189(1):19–30. doi: 10.1016/j.atherosclerosis.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, et al. Omega-6 Fatty Acids and Risk for Cardiovascular Disease A Science Advisory From the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009 Feb 17;119(6):902–7. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 5.Couet C, Delarue J, Ritz P, Antoine JM, Lamisse F. Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int J Obes. 1997 Dec 18;21(8):637–43. doi: 10.1038/sj.ijo.0800451. [DOI] [PubMed] [Google Scholar]
- 6.Ailhaud G, Guesnet P. Fatty acid composition of fats is an early determinant of childhood obesity: a short review and an opinion. Obes Rev. 2004 Feb 1;5(1):21–6. doi: 10.1111/j.1467-789x.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- 7.Innis SM. Metabolic programming of long-term outcomes due to fatty acid nutrition in early life. Matern Child Nutr. 2011 Apr 1;7:112–23. doi: 10.1111/j.1740-8709.2011.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kavanagh K, Jones KL, Sawyer J, Kelley K, Carr JJ, Wagner JD, et al. Trans Fat Diet Induces Abdominal Obesity and Changes in Insulin Sensitivity in Monkeys. Obesity. 2007 Jul 1;15(7):1675–84. doi: 10.1038/oby.2007.200. [DOI] [PubMed] [Google Scholar]
- 9.Koh-Banerjee P, Chu NF, Spiegelman D, Rosner B, Colditz G, Willett W, et al. Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9-y gain in waist circumference among 16 587 US men. Am J Clin Nutr. 2003 Oct 1;78(4):719–27. doi: 10.1093/ajcn/78.4.719. [DOI] [PubMed] [Google Scholar]
- 10.Dahm CC, Gorst-Rasmussen A, Jakobsen MU, Schmidt EB, Tjønneland A, Sørensen TIA, et al. Adipose Tissue Fatty Acid Patterns and Changes in Anthropometry: A Cohort Study. PLoS ONE. 2011 Jul 21;6(7):e22587. doi: 10.1371/journal.pone.0022587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AVD, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am J Epidemiol. 2002 Nov 1;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 12.Nettleton JA, Rock CL, Wang Y, Jenny NS, Jacobs DR. Associations between dietary macronutrient intake and plasma lipids demonstrate criterion performance of the Multi-Ethnic Study of Atherosclerosis (MESA) food-frequency questionnaire. Br J Nutr. 2009 Oct;102(08):1220–1227. doi: 10.1017/S0007114509382161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, et al. Validity and Reproducibility of a Food Frequency Interview in a Multi-Cultural Epidemiologic Study. Ann Epidemiol. 1999 Jul;9(5):314–24. doi: 10.1016/s1047-2797(98)00070-2. [DOI] [PubMed] [Google Scholar]
- 14.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990 Jan 1;43(12):1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 15.Guenther PM, Reedy J, Krebs-Smith SM, Reeve BB. Evaluation of the Healthy Eating Index-2005. J Am Diet Assoc. 2008 Nov;108(11):1854–64. doi: 10.1016/j.jada.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, et al. The Association Between Physical Activity and Subclinical Atherosclerosis The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009 Feb 15;169(4):444–54. doi: 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and Clearance of Omega-3 Fatty Acids in Erythrocyte Membranes and Plasma Phospholipids. Clin Chem. 2006 Dec 1;52(12):2265–72. doi: 10.1373/clinchem.2006.072322. [DOI] [PubMed] [Google Scholar]
- 18. [cited 2017 Jan 5];PROC GLM: Statistical Assumptions for Using PROC GLM :: SAS/STAT(R) 9.2 User’s Guide. (Second). [Internet] Available from: http://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm#statug_glm_sect026.htm.
- 19.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000 Sep;32(9):S498–516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 20.Kris-Etherton PM, Harris WS, Appel LJ, Committee for the N Fish Consumption, Fish Oil, Omega-3 Fatty Acids, and Cardiovascular Disease. Circulation. 2002 Nov 19;106(21):2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 21.de O Otto MC, JHY Wu, Baylin A, Vaidya D, Rich SS, Tsai MY, et al. Circulating and Dietary Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2013 Dec 19;2(6):e000506. doi: 10.1161/JAHA.113.000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckley JD, Howe PRC. Anti-obesity effects of long-chain omega-3 polyunsaturated fatty acids. Obes Rev. 2009 Nov 1;10(6):648–59. doi: 10.1111/j.1467-789X.2009.00584.x. [DOI] [PubMed] [Google Scholar]
- 23.Massiera F, Saint-Marc P, Seydoux J, Murata T, Kobayashi T, Narumiya S, et al. Arachidonic acid and prostacyclin signaling promote adipose tissue development a human health concern? J Lipid Res. 2003 Feb 1;44(2):271–9. doi: 10.1194/jlr.M200346-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Jensen CL, Prager TC, Fraley JK, Chen H, Anderson RE, Heird WC. Effect of dietary linoleic/alpha-linolenic acid ratio on growth and visual function of term infants. J Pediatr. 1997 Aug;131(2):200–9. doi: 10.1016/s0022-3476(97)70154-9. [DOI] [PubMed] [Google Scholar]
- 25.Bender N, Portmann M, Heg Z, Hofmann K, Zwahlen M, Egger M. Fish or n3-PUFA intake and body composition: a systematic review and meta-analysis. Obes Rev. 2014 Aug 1;15(8):657–65. doi: 10.1111/obr.12189. [DOI] [PubMed] [Google Scholar]
- 26.Micallef M, Munro I, Phang M, Garg M. Plasma n-3 polyunsaturated fatty acids are negatively associated with obesity. Br J Nutr. 2009 Nov;102(09):1370–1374. doi: 10.1017/S0007114509382173. [DOI] [PubMed] [Google Scholar]
- 27.Parra D, Ramel A, Bandarra N, Kiely M, Martínez JA, Thorsdottir I. A diet rich in long chain omega-3 fatty acids modulates satiety in overweight and obese volunteers during weight loss. Appetite. 2008;51(3):676–680. doi: 10.1016/j.appet.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Kunesová M, Braunerová R, Hlavatý P, Tvrzická E, et al. The Influence of n-3 Polyunsaturated Fatty Acids and Very Low Calorie Diet during a Short-term Weight Reducing Regimen on Weight Loss and Serum Fatty Acid Composition in Severely Obese Women. Physiol Res. 2006;55(1):63–72. doi: 10.33549/physiolres.930770. [DOI] [PubMed] [Google Scholar]
- 29.Tai CC, Ding ST. N-3 polyunsaturated fatty acids regulate lipid metabolism through several inflammation mediators: mechanisms and implications for obesity prevention. J Nutr Biochem. 2010 May;21(5):357–63. doi: 10.1016/j.jnutbio.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Alvheim AR, Malde MK, Osei-Hyiaman D, Hong YH, Pawlosky RJ, Madsen L, et al. Dietary Linoleic Acid Elevates Endogenous 2-AG and Anandamide and Induces Obesity. Obesity. 2012 Oct 1;20(10):1984–94. doi: 10.1038/oby.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Vries PS, Gielen M, Rizopoulos D, Rump P, Godschalk R, Hornstra G, et al. Association between polyunsaturated fatty acid concentrations in maternal plasma phospholipids during pregnancy and offspring adiposity at age 7: The MEFAB cohort. Prostaglandins Leukot Essent Fat Acids PLEFA. 2014 Sep 1;91(3):81–5. doi: 10.1016/j.plefa.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Muhlhausler BS, Ailhaud GP. Omega-6 polyunsaturated fatty acids and the early origins of obesity. Curr Opin Endocrinol Diabetes Obes. 2013 Feb;20(1):56–61. doi: 10.1097/MED.0b013e32835c1ba7. [DOI] [PubMed] [Google Scholar]
- 33.Food and Drug Administration, HHS. Food labeling: trans fatty acids in nutrition labeling, nutrient content claims, and health claims. Final rule Fed Regist. 2003 Jul 11;68(133):41433–506. [PubMed] [Google Scholar]
- 34. [cited 2016 Jun 30];Restricting trans Fatty Acids in the Food Supply. [Internet] Available from: http://www.apha.org/policies-and-advocacy/public-health-policy-statements/policy-database/2014/07/23/09/40/restricting-trans-fatty-acids-in-the-food-supply.
- 35.Honors MA, Harnack LJ, Zhou X, Steffen LM. Trends in Fatty Acid Intake of Adults in the Minneapolis-St Paul, MN Metropolitan Area, 1980–1982 Through 2007–2009. J Am Heart Assoc. 2014 Oct 24;3(5):e001023. doi: 10.1161/JAHA.114.001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baylin A, Campos H. The use of fatty acid biomarkers to reflect dietary intake. Curr Opin Lipidol. 2006 Feb;17(1):22–7. doi: 10.1097/01.mol.0000199814.46720.83. [DOI] [PubMed] [Google Scholar]
- 37.Hodge AM, Simpson JA, Gibson RA, Sinclair AJ, Makrides M, O'Dea K, et al. Plasma phospholipid fatty acid composition as a biomarker of habitual dietary fat intake in an ethnically diverse cohort. Nutr Metab Cardiovasc Dis. 2007 Jul 1;17(6):415–26. doi: 10.1016/j.numecd.2006.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.