Abstract
Infants born at very low gestational age contribute disproportionately to neonatal morbidity and mortality. Advancements in antenatal steroid therapies and surfactant replacement have favored the survival of infants with ever-more immature lungs. Despite such advances in medical care, cardiopulmonary and neurological impairment prevail in constituting the major adverse outcomes for neonatal intensive care unit (NICU) survivors. With no single effective therapy for either the prevention or treatment of such neonatal disorders, the need for new tools to treat and reduce risk of further complications associated with extreme preterm birth is urgent. Mesenchymal stem/stromal cell (MSC)-based approaches have shown promise in numerous experimental models of lung injury relevant to neonatology. Recent studies have highlighted that the therapeutic potential of MSCs is harnessed in their secretome, and that the therapeutic vector therein is represented by the exosomes released by MSCs. In this review, we summarize the development and significance of stem cell-based therapies for neonatal diseases, focusing on preclinical models of neonatal lung injury. We emphasize the development of MSC exosome-based therapeutics and comment on the challenges in bringing these promising interventions to clinic.
Introduction
Infants born at very low gestational age contribute disproportionately to neonatal morbidity and mortality, and are at increased risk of developing several diseases including, periventricular leukomalacia (PVL), retinopathy of prematurity (ROP), hypoxic-ischemic encephalopathy (HIE) and bronchopulmonary dysplasia (BPD) (1–3). With no single effective therapy for either the prevention or treatment of such neonatal disorders, the need for new tools to treat and reduce risk of further complication associated with extreme preterm birth is urgent.
Recently, Stoll and coworkers investigated the 20-year survival and outcome trend of >34,000 preterm infants, and found that the frequency of several morbidities associated with prematurity has decreased, yet between 2009 and 2011 the prevalence of BPD had increased across infants of 22–27 weeks of gestational age (1). Consequently, BPD is the most common complication of pre-term birth, affecting ~35% of infants born at ≤28 week’s gestation. This trend is likely attributed to advancements in medical care that favor the survival of extremely preterm infants. As a result, the modern day neonatal intensive care unit (NICU) witnesses the presentation of ever-more immature lungs, complicating the challenge of preventing and treating neonatal lung injury (4).
BPD is a multifactorial chronic lung disorder that occurs almost exclusively in preterm infants receiving supplemental oxygen and mechanical ventilation. It is characterized by lung growth arrest, alveolar simplification, impaired blood vessel development and abnormal pulmonary function (5). Infants with BPD exhibit abnormalities in lung function that often begin at birth and persist through infancy, childhood and into adolescence. In turn, young adults who were former BPD infants may present with increased incidence of reactive airway disease and, premature lung dysfunction with ‘emphysema-like’ features of lung disease (6, 7). Moreover, the development of pulmonary hypertension in moderate to severe cases of BPD is linked to increased mortality (8, 9). Collectively, BPD is no longer considered a disease specific for the neonatal period, but a multifactorial condition with lifelong consequences.
Lack of therapies for bronchopulmonary dysplasia
Current NICU treatment strategies aim to not only support the survival of the preterm infant, but minimize further lung injury and facilitate recovery (for recent review, see (10)). Aside from the notable improvements in ventilation strategies, surfactant supplementation and the use of corticosteroids, there has been a lack of new therapies that have had a dramatic effect in preventing/treating BPD. Currently, active research avenues are exploring the most efficient method of surfactant administration; synthetic versus animal-derived surfactant; time and dose optimization for corticosteroid treatment and further advancements in ventilation techniques (11). Despite the necessity for continuing optimization, it is fair to speculate that classical pharmacological therapies and ‘subtle’ technological improvements may have only a modest impact on BPD outcomes. Moreover, in multi-factorial disorders like BPD, it is unlikely that therapies targeting a single pathway will achieve a significant clinical impact. Indeed, inhaled nitric oxide (12) and vitamin A supplementation (13) are just some of the alternative drug approaches that have failed to consistently produce effective clinical outcomes. Thus, there is a burgeoning need for novel therapeutic approaches to restore homeostasis and allow regular developmental growth. Stem cell based-therapies represent one such approach which holds great promise. Herein, we will comment on the development of mesenchymal stem/stromal cell (MSC) -based therapies in experimental models of neonatal lung injury, focusing on MSC-exosome therapeutics.
Early evidence of depletion and/or dysregulation of endogenous progenitor/stem cell populations in neonatal lung injury
It is widely acknowledged that the preterm infant is exposed to several risk factors that result in tissue injury across multiple organs, but an understanding of the mechanisms underlying tissue simplification is incomplete. Common mechanisms of pathogenesis include inflammation, infection, ischemia/reperfusion and nitro-oxidative stress. In addition, genetic susceptibility and nutritional deprivation play a crucial role in exacerbating injury (14, 15). The combination of such risk factors may lead to arrest of normal organ growth. Emerging evidence suggests that loss of endogenous stem/progenitor cells required for normal cell differentiation and tissue repair may also underlie the pathobiology of such injury (14, 16, 17).
The lungs are equipped with an arsenal of endogenous stem/progenitor cell populations that are readily mobilized upon cellular injury, proliferating and differentiating to actively repair and regenerate injured tissue (recently reviewed, (15, 18, 19)). Previous studies have found that exposure to hyperoxia (80% O2) reduces endothelial progenitor cell (EPC, defined as CD45−/Sca–1+/CD133+/VEGFR–2+) levels in the circulation, bone marrow, and lungs of neonatal mice (20).
In the clinical setting, Bozyk et al, showed that lung resident MSCs isolated from the tracheal aspirates of preterm neonates with respiratory distress, demonstrated increased expression of proinflammatory cytokines (19). In accordance, Popova et al found the presence of MSCs in the tracheal aspirates of preterm infants (<33 weeks gestational age), to be significantly associated with subsequent development of BPD (20). Although the biological role of tracheal aspirate MSCs in lung homeostasis and repair remains unclear, these studies imply that both depletion and/or dysfunction of endogenous progenitor/stem cells can increase the risk of BPD. Lung-resident MSCs are a multipotent stem cell population. It is believed that such tissue resident stem cells are key regulators of pulmonary homeostasis and play an important role in regulating the lung immune and repair responses in processes such as inflammation, angiogenesis, and fibrosis (15, 18, 19). Previous studies have shown that treatment of adult mice with bleomycin was associated with a significant loss of endogenous lung MSCs, fibrosis, inflammation and pulmonary arterial hypertension. Interestingly, transplantation of isolated ‘control’ lung-MSCs attenuated the bleomycin-associated pathology and mitigated the development of pulmonary arterial hypertension via restoration of the effector T-cell response (21).
However, there are ‘two sides to every coin’. In addition to their reparative properties, studies have shown that lung MSCs, under specific conditions can mediate pathogenic changes in the lung, a process governed by their immediate microenvironment. For example, Chow and coworkers reported that increased oxidative stress, induced via a targeted depletion of extracellular superoxide dismutase, modulated the programming of resident lung MSCs, causing them to contribute to pulmonary microvascular remodeling (22). Furthermore, it was recently shown that transforming growth factor (TGF)-β expression within the lungs of premature infants stimulates MSCs to differentiate into myofibroblasts. Such reports highlight the importance of the microenvironment in regulating MSC function (23).
The bulk of the literature surrounding pulmonary progenitor/stem cells is derived from preclinical research that often involves mice. Compared to the mouse, less information is known about endogenous progenitor/stem cell populations in human lung. However, findings from preclinical studies and small clinical cohorts to date provide early evidence that loss and/or dysregulation of endogenous progenitor/stem cell populations that facilitate growth, development and repair of developing organs, could underpin the pathophysiology of neonatal disorders such as BPD. Collectively, such findings provided a foundation to explore stem cell-based therapies to treat and/or prevent neonatal lung injury. Indeed, further studies and continuing progress in this field is essential for our understanding of the molecular mechanisms susceptible to pulmonary perinatal/neonatal stress.
Mesenchymal stem (stromal) cell-therapy in bronchopulmonary dysplasia
Transplantation of different stem cell types, including endothelial colony forming cells (ECFCs) and human amnion epithelial cells (hAECs) have shown promise in preclinical models for the prevention and/or treatment of BPD and other major sequelae of prematurity. Such studies have also demonstrated profound anti-inflammatory, neuroprotective and functional benefits in preclinical models of neonatal hypoxia-ischemia, cerebral palsy and stroke (for recent reviews see (15, 24–30)). Herein, we will focus on therapeutic applications using MSCs and their secreted products.
MSCs are non-hematopoietic adult stem cells that can be successfully propagated in vitro. Methods for isolating MSCs from several human tissues, including bone marrow (BMSCs), Wharton’s jelly (WJMSCs), umbilical cord blood and adipose tissue are well documented (31, 32). With no known specific MSC marker, MSCs are defined by their adherence to plastic, the demonstration of in vitro differentiation potential to mesodermal lineage, and conformity to a well characterized surface marker panel (33).
MSC treatment was shown to be efficacious in a number of animal models of lung disease, (reviewed in (28)). Using experimental models of BPD, we (34) and others (35) have shown that MSC treatment blunts hyperoxia-induced lung inflammation, improves lung architecture, ameliorates vascular remodeling, improves exercise capacity and increases survival rates. Promising preclinical data (reviewed in(29)) and the demonstration of safety in several clinical trials on MSC treatment in adult lung diseases (36, 37) have recently brought MSC therapy into clinical development for newborn lung disease. Specifically, in a single-center, phase I dose-escalation feasibility trial, nine preterm infants (25.3 ± 0.9 weeks of gestational age) who were predicted to be at highest risk of BPD were enrolled and treated with a bolus endotracheal dose of allogeneic human umbilical cord blood MSCs (10 – 20 million cells/kg) at 10.4 ± 2.6 postnatal days. MSC-therapy was well-tolerated, the trial demonstrated feasibility and short-term safety in preterm neonates and phase II trials are now in progress (https://clinicaltrials.gov, NCT01897987). Although functional endpoints are beyond the scope of phase I clinical trials, it was noticed that MSC administration was associated with a reduction in inflammatory markers from baseline in the tracheal aspirates of treated infants, and lower BPD severity from the historical case-matched comparison group (38).
It is important to note that, although clinical trials are progressing, we lack complete understanding of the mechanism(s) underlying the therapeutic properties of MSCs. Earlier hope that MSCs could repair lung tissue by engrafting with high efficiency in the injured lung and transdifferentiating to pneumocytes was subsequently proven to be rather premature and claims supporting it were based on equivocal evidence.
The substantial physiologic improvements in the recipient lung following MSC transplantation, in the absence of significant engraftment of donor cells has led to the generally accepted notion that the MSC therapeutic action is mainly through paracrine mechanisms (34, 39, 40). In an experimental model of ‘severe’ hyperoxia-induced BPD, we previously assessed the therapeutic capacity of BMSCs and their respective secretome in parallel. Interestingly, we found that treatment with the BMSC secretome (specifically BMSC-conditioned media), provided a superior therapeutic capacity over MSCs themselves, providing greater protection in preventing alveolar simplification and preserving the lung architecture (34).
In a different study using a similar ‘severe’ model of hyperoxia induced-BPD, our group demonstrated that a bolus dose of MSC-conditioned media, delivered intravenously after 14 days of hyperoxia exposure (75% O2), blunted lung inflammation, reversed pulmonary hypertension and vascular pruning, ameliorated lung fibrosis, and improved lung architecture (41). In accordance, the Thébaud laboratory also demonstrated that the therapeutic capacity of MSCs was harnessed in their secretome (42). They noted that the BMSC-conditioned media accelerated wound healing and enhanced endothelial cord formation in vitro and that human umbilical cord MSC-conditioned media afforded short- and long-term therapeutic benefits without adverse lung effects in a rat model of hyperoxia-induced BPD (35, 43, 44).
Although the paracrine mode of MSC action is a generally accepted notion, we have not yet clearly defined the precise bioactive moiety responsible for their therapeutic efficacy. The complex MSC secretome contains proteins of diverse functions, nucleic acids, morphogens, free fatty acids, genetic information (including small non-coding RNA) and membrane components (45). Over the last several years a number of these bioactive molecules have been reported to represent the active component(s) of the MSC secretome, but, in general, studies attempting to recapitulate the effects of MSC-conditioned media in vivo through the administration of the isolated candidate molecule did not yield consistent and reproducible results. This led to the hypothesis that the therapeutic vector of the MSC secretome was represented by moieties of higher molecular complexity, and in particular, the subclass of extracellular vesicles (EVs) termed exosomes. Recent validation of this hypothesis in several preclinical studies creates the promise that exosomes may represent a novel and exciting approach to treating neonatal lung diseases.
Extracellular vesicles: nomenclature and classification
Exosomes are submicron (~30–150 nm), plasma membrane enclosed EVs. Originally assumed to represent a mechanism for the cell to jettison unwanted moieties, the secretion of EVs, and particularly the subclass generated via the endosomal sorting machinery, is now considered to represent an effective method for cell-to-cell communication via the transfer of cellular components (15, 46–48). As our understanding of exosome biogenesis has improved, it has become apparent that during their formation, exosomes can associate with an assortment of ‘bioactive’ cargo from their parental cell. In turn, exosomes are at the forefront of active research themes for diagnostic, prognostic and therapeutic applications across multiple disciplines (49).
As the consequence of a relatively new multi-disciplinary research field, the nomenclature and classification of these vesicles remains abstract. Typically, EVs are categorized into three subclasses: exosomes (~30 – 150 nm in diameter), microvesicles (~100 nm – 1 μm in diameter) and apoptotic bodies (> 1 μm). These sub-classes of vesicles were grouped based on their biogenesis and biophysical properties (such as size, density and predominant protein markers). Exosome biogenesis and nomenclature has been extensively reviewed (45, 46, 50–53).
With limitations in current technology, the field currently lacks the ability to analyze exosomes at a single vesicle level. Thus, it is becoming increasingly difficult to uphold one terminology over another. Arguably, as a consequence of no ‘gold-standard’ methods for EV isolation, characterization and quantification, and a concomitant concern within the field about the inappropriate application and interpretation of studies, the International Society for Extracellular Vesicles (ISEV) previously urged the widespread adoption of the generic term EV when referring to secreted vesicles (50). Utilizing the ‘generic’ EV terminology demands a complementary EV biophysical characterization (for example, assessment of predominant protein markers, density, vesicle quantification, morphology, particle:protein ratio) to help specify the EV population in question. However, we argue that a comprehensive characterization is a quest that is seldom executed in previous/concurrent literature. Moreover, lack of EV characterization coupled with ‘poor’ isolation methods that are prone to co-isolate non-EV material often obfuscates the true potential and biological activity of EVs.
For the purposes of this review we will just note that it can be particularly misleading to attempt to classify EV subpopulations by size alone, since isolation manipulations often break up larger EVs, generating an artificial heterogeneity in the smaller size subclasses. Herein, we will adopt the nomenclature chosen by the cited articles, and support nomenclature with their respective isolation methods where appropriate.
Mesenchymal stem/stromal cell-exosome therapy in experimental bronchopulmonary dysplasia
The therapeutic capacity of MSC-‘exosomes’ derived from different organs, have been tested in various disease models, demonstrating a similar or even superior functional capacity to MSCs themselves, reducing myocardial infarction size (54, 55), aiding repair of kidney injury (56), and orchestrating neurological protection by the transfer of mircroRNA (57). A summary of MSC-exosome based approaches for the treatment of different disease models is provided in Table 1. Previously, our group was the first to demonstrate that exosomes mediate the cytoprotective effect of MSCs in the lung, using a mouse model of hypoxia-induced pulmonary hypertension (58). Here, administration of MSC-exosomes isolated by size exclusion chromatography and characterized through the presence of established exosome markers (including Alix and TSG101, Flotilin-1 and tetraspanins (CD81, CD9)) and transmission electron microscopy, protected against the elevation of right ventricular systolic pressure and the development of right ventricular hypertrophy after 3 weeks of hypoxic exposure (8% O2). Importantly, exosome-depleted CM had no effect. BMSC-exosome treatment was also able to abrogate early hypoxic macrophage influx and down-regulate hypoxia-activated inflammatory pathways, thus recapitulating the anti-inflammatory actions of MSCs (58, 59).
Table 1. Summary of MSC-‘exosome’ based therapeutics.
Ultracentrifugation (UC). 100,000 – 120,000 × g (100K – 120K × g). SEC (size exclusion chromatography). ExoQuick and ExoELISA refer to a commercially available exosome isolation kit and CD63 capture (exosome) ELISA, respectively (Systems Biosciences,CA). Table adapted from (72).
| Disease | MSC-product ‘nomenclature’ | Final isolation step | Dose assessment | Dose | Ref |
|---|---|---|---|---|---|
| Bronchopulmonary dysplasia | Exosomes | Density Cushion | Cell equivalent | 0.5 × 106 | (60) |
| Pulmonary hypertension | Exosomes | SEC | Protein | 0.1 – 10 μg | (58) |
| Pulmonary hypertension | Exosomes | UC (100K × g) | Protein | 25 μg | (81) |
| Acute lung injury | Microvesicles | UC (100K × g) | Cell equivalent | 1.5 × 10 | (82) |
| Silicosis | Exosomes | Sucrose gradient | Protein | 40 μg | (83) |
| Pneumonia | Microvesicles | UC (100K × g) | Cell equivalent | 9 × 10 | (84) |
| Myocardial infarction | Exosomes | ExoQuick | Cell equivalent | 4 × 10 | (85) |
| Myocardial infarction | EVs | UC (100K × g) | Protein | 80 μg | (86) |
| Myocardial infarction | Exosomes | ExoQuick | Protein | 80 μg | (87) |
| Ischemia/reperfusion | Exosomes | HPLC | Protein | 0.4 μg | (54) |
| Ischemia/reperfusion | Exosomes | HPLC | Protein | 0.4 – 0.8 μg | (55) |
| Traumatic brain injury | EVs | Anion exchange chromatograph | Protein | 30 μg | (88) |
| Laser-induced retinal injury | Exosomes | UC (110K × g) | Protein | 10 μg | (89) |
| Optical nerve crush | Exosomes | UC (100K × g) | ExoELISA | 3 × 10 | (90) |
| Stroke | EVs | UC (110K × g) | Cell equivalent | 2 × 10 | (91) |
| Stroke | Exosomes | UC (100K × g) | Protein | 100 μg | (92) |
| Cardiotoxin injury | EVs | UC (100K × g) | Protein | 5 μg | (61) |
| Drug-induced liver injury | Exosomes | UC (100K × g) | Protein | 0.4 μg | (93) |
| Liver fibrosis | Exosomes | UC (100K × g) | Protein | 250 μg | (94) |
| Colitis | EVs | UC (100K × g) | Protein | 50 – 200 μg | (95, 96) |
| Wound healing | Exosomes | UC (100K × g) | Protein | 160 μg | (97) |
| Wound healing | Exosomes | UC (120K × g) | Protein | 100 μg | (96) |
| Ischemia/reperfusion | Microvesicles | UC (100K × g) | Protein | 100 μg | (98) |
| Acute kidney injury | Microvesicles | UC (100K × g) | Protein | 100 μg | (99) |
More recently, our group assessed the effect of highly purified human MSC-exosomes, isolated by density on iodixanol (OptiPrep®) gradients, in an established neonatal mouse model of hyperoxia-induced BPD. Here, we subjected newborn FVB mice to hyperoxia (75% O2) for 7 days (postnatal day 1–7) and administered a bolus intravenous dose of MSC-exosomes at postnatal day 4. We found that a single dose of purified human MSC-exosomes (the amount produced by 0.5 × 106 MSCs over 36 hours under standard tissue culture conditions) significantly improved lung morphology and pulmonary development, decreased lung fibrosis, ameliorated pulmonary vascular remodeling and restored the hyperoxia-induced loss of pulmonary blood vessels. Importantly, we found that MSC-exosome treatment improved lung function and alleviated associated pulmonary hypertension as assessed at postnatal day 42, long after the hyperoxic lung injury. The therapeutic efficacy of exosomes was comparable for preparations obtained from MSC cultures derived from either human bone marrow or human umbilical cord Wharton’s Jelly. This observation opens the possibility that WJMSCs, a resource far more abundant and easier obtainable than BMSCs may expedite large scale, good manufacturing practice (GMP) production of MSC exosomes for upcoming clinical trials (60).
Mechanisms by which EVs and their respective vesicle subtypes induce their therapeutic effects remain incompletely understood. With beneficial effects in diverse disease models it is reasonable to suggest that a single ‘bioactive’ molecule/pathway cannot account for their entire therapeutic capacity. It is well reported that MSC-EVs harbor a wealth of bioactive cargo. We hypothesis that such ‘bioactive’ cargo may synergistically play a role, however, with such heterogeneity in EV secretion, the precise bioactive ingredient maybe disease/model specific.
Considering this, recent focus has been on the immunomodulatory capacity of MSC-exosomes as an attempt to decipher the cellular mechanisms responsible for their therapeutic action. We recently showed that treatment with MSC-exosomes (isolated by density gradient), blunts hyperoxia-induced inflammation, an effect that, at least in part is mediated via modulation of lung macrophage phenotype. Specifically, WJMSC-exosomes suppressed the levels of proinflammatory (M1) associated markers such as Il-6 and Tnfα and altered the expression proremodeling (M2) markers (such as Cd206 and Arginase-1) both in vitro and in vivo (60). In accordance, recent studies have shown that BMSC-EVs (isolated by differential ultracentrifugation, last step; 100,000 × g for 90 minutes) modulates the macrophage transcriptome to favor an anti-inflammatory, M2-like phenotype, both in vitro and in vivo in a murine model of cardiotoxin (CTX)-induced skeletal muscle injury (61). Although the above studies highlight a potent immunomodulatory effect following MSC-exosome treatment, one can only conclude an association. The precise ‘bioactive’ modalities within the exosomes/EVs that are responsible for such effects remain unclear. A schematic representation of the immunomodulatory capacity of MSC-exosomes is highlighted in Figure 1.
Figure 1. Schematic of postulated therapeutic action of MSC exosomes in hyperoxia-induced BPD.
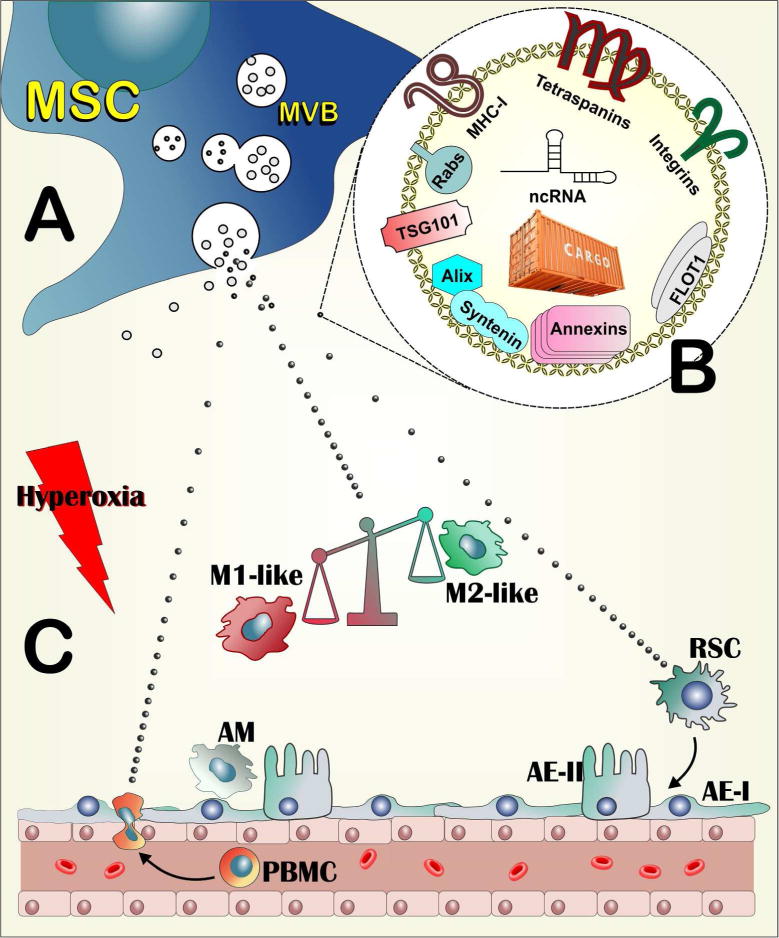
(A). MSCs routinely generate exosomes in multivesicular bodies (MVB) through the endocytic pathway. The majority of produced exosomes represent jettisoning of unwanted moieties by the cell and probably have no discernable function (larger grey symbols). Upon specific organismal or environmental cues, MSCs also produce a subpopulation of exosomes that harbor the therapeutic activity (small black symbols). (B). The therapeutic exosomes harbor cell surface components arguably involved in targeting recipient cells (Tetraspanins, Integrins) or immunomodulation, such as MHC-I. They also contain molecules associated with the pathways of their biogenesis, such as Rabs, TGS101, Alix Syntenin, Annexins and FLOT1. Their cargo includes small non-coding RNAs, but also macromolecular modules yet to be characterized. (C). The hyperoxic insult creates an inflammatory environment in the lung, which activates the alveolar macrophages (AM) and recruits circulating monocytes (PBMC) to the alveolar space. The main function of MSC exosomes is to induce a shift in macrophage polarization, tilting the balance from a destructive, M1-like inflammatory state to an anti-inflammatory, M2-like state. Additional actions of exogenously administered MSC exosomes could be the direct or indirect inhibition of PBMC recruitment to the injured lung and the direct or indirect enhancement of the activity of lung resident stem cells (RSC), leading to faster healing of injured tissue. AE-I and AE-II : Alveolar epithelial cells type I and type II respectively.
The concept that the therapeutic vector of MSCs is represented by exosomes is well supported by evidence, but we should be very cautious to assume that one specific mechanism could be operative in all disease models. With this in mind, human BMSC-EVs, isolated from conditioned media by sequential differential ultracentrifugation (final spin; 100,000 × g for 2 hours), have also been shown to exert anti-inflammatory and immunomodulatory effects on injured lung tissue, enhancing the resolution of pulmonary edema by upregulating sodium-dependent alveolar fluid clearance in experimental models of acute respiratory distress syndrome (ARDS) (50–52). Such reports suggest that the mechanism of action of MSCs may require cell-to-cell contact and mitochondrial transfer through tunneling nanotubes (34), or mitochondrial transfer through larger EV subclasses (greater than 500 nm in diameter), such as microvesicles (56). It is fair to assume that, depending on the particular injury and the specific microenvironment, MSCs may use a variety of mechanisms to repair injured tissue and restore pulmonary homeostasis, and that the exosome merely represents one weapon in the arsenal of the MSCs artillery.
Challenges in bringing of exosome based-therapeutics
With such promising preclinical findings in numerous disease models, investigators are now tasked with developing a safe, feasible and reproducible MSC-exosome therapy. However, the goal of bringing exosome based-therapies to clinical trials and assessing the actual potential of exosome based-therapeutics is met with significant challenges. Arguably, the major hurdles are the ‘poor’ methodologies for exosome isolation and characterization and the absence of standardization in exosome purification. Akin to MSC-therapy, difficulties in designing robust measures of exosome potency, and the challenges of industrial scale-up will complicate smooth transition to clinical development and must be resolved at an early phase. Herein we comment on the potential for therapeutic application of MSC-exosomes, outlining relevant issues to facilitate their development and provide guidance on the current challenges in bringing exosome based-therapies to clinic.
Establishing a preclinical model
The choice of animal model is critical in the development of exosome based-therapeutics. Demonstrating efficacy at the preclinical stage is pivotal in determining the transition into clinical development. For neonatal disorders such as BPD, several animal models have been developed and continue to be refined with the aim of mimicking the pathological pulmonary characteristics noted in lungs of neonates with BPD (62). Arguably BPD is most commonly modeled in mice. Mice have relatively short gestation times (~20 days) allowing studies that monitor saccular and alveolar lung development to be conducted relatively quickly. Importantly, mice delivered at term are in the saccular stage of lung development. Thus, the developmental stage of the mouse lung at birth resembles that in the human preterm neonate (born at 24 to 28 weeks gestation) (63), in turn making the newborn mouse an excellent model to study human developmental lung injury. However, it is important to note that, when term mice are born (in the saccular stage), these newborn rodent pups are competent for proper gas exchange and do not require oxygen supplementation as a life-saving intervention; this is in marked contrast to preterm human neonates (62, 63).
The economic sustainability of the model is also important. In comparison to large animal models, small animals (for example rodents) require less exosomes per body weight. Thus, in addition to rodent models, future studies should consider larger animal models, such as the fetal lamb BPD model (64). Although the premature lamb will be larger than the gestational aged-matched preterm infant counterpart, alternative routes of administration (i.e. endotracheal) are possible, as is the ability to ventilate and investigate combination therapies, for example, MSC-exosomes combined with surfactant supplementation, thus mimicking more closely the current standard operating procedures in the NICU.
Characterization of tissue/cell origin
Several open questions remain unanswered. Is the production of exosomes the same for all MSCs, independent of their tissue of origin? How do donor demographics and different cell culture conditions effect resultant MSC exosome? Such questions need to be addressed to facilitate the development of MSC-exosome therapeutics and standardize product formulations.
Donor-to-donor variability remains a prominent challenge. Indeed, BMSCs obtained from older donors have slower proliferation and reduced differentiation potential in vitro (31, 65). Borghesi et al, screened different umbilical cord-MSC clones for acquired genomic imbalances during in vitro expansion. Using array-comparative genomic hybridization they found two out of eleven umbilical cord-MSC cultures carried genomic imbalances, generating genetic mosaicism at intermediate passages (66). Collectively, such uncertainties will likely affect the therapeutic capacity of MSCs, impacting on the characteristics and thus the potency of their resultant exosomes. It is therefore necessary to address these variables and exercise caution when designing MSC-based protocols.
Exosome heterogeneity
Although, the field currently lacks exact tools to distinguish vesicles from different routes of biogenesis, recent studies have shown that MSCs release different exosome subpopulations that differ in biophysical, proteomic and RNA repertoires. Specifically, Kowal and coworkers found that large-, medium- and small-sized EVs can be isolated by sequential centrifugation steps. Among the small-EVs (where exosomes should fractionate), four subcategories were defined by their degree of CD63, CD9, and CD81 tetraspanin enrichment (67). It is fair to speculate that such exosome subtypes will mediate different effects on targets cells. Thus, separation techniques that can distinguish between different exosome and/or small EV subtypes, may help identify the functional subpopulation and enrich for the active species. This will not only result in more potent preparations, but the resultant homogenous population will facilitate the deciphering of the molecular mechanisms underlying exosome function on their targets.
Assessing exosome potency assay/dose evaluation
Determining the optimal tool to reliably assess exosome dose; the appropriate time window for exosome administration; dosing frequency and the most effective route of administration to achieve maximal therapeutic benefits without adverse effects, are significant issues to resolve. Such issues will be disease/model specific. However, with no standardized method to quantify exosomes, investigators currently rely on several different methods to assess exosome dosage. Typical quantitative methods include reporting cell equivalents, protein concentration and/or specialized quantitative analytical measurements by instruments such as dynamic light scattering (DLS), tunable resistive pulse sensing (TRPS) and nanoparticle tracking analysis (NTA), with each technique subject to their own advantages and limitations. Beyond the scope of this review, exosome quantification methods are discussed in the following reviews (68–71)).
An exosome potency assay would be a valuable tool in overcoming the inconsistencies in batch-to-batch variation and could also bypass the limitations surrounding current exosome quantification methods. Investigators could consider using an exosome potency assay to standardize practices and minimize biological variation between MSC-exosome preparations. To date, candidate MSC-exosome potency assays are arguably centered around T cell proliferation and/or macrophage polarization assays (72). Importantly, the chosen exosome potency assay needs to be fit-for-purpose, employ relevant functional end-points, and demonstrate a clear correspondence between in vitro potency (as measured by the chosen assay) and in vivo efficacy. The examples of assays above are based on the immunomodulatory properties of MSC exosomes and they can return a metric of this property. This metric could be proportional to in vivo efficacy, since it appears that one of the major effects of exosome treatment in the lung models of disease is indeed immunomodulation. Nevertheless, this represents an extreme oversimplification of the issue, and the establishment of a proper exosome potency assay based on function will require careful design and even more careful validation. In the interim, in-depth exosome characterization may establish correlations between efficacy and specific exosome markers (for example, expression of exosomal protein marker TSG101 or CD63). In turn, subsequent surrogate markers of exosome potency/dose maybe initially considered. Such ‘fingerprinting assays’ may provide quality control and batch-to-batch consistency (73).
Lung-on-a-chip and organoid platforms
Improving preclinical predictions of experimental drug responses is critical to minimize failures and optimize success in clinical trials. Recent advancements in cell biology, bioengineering and microfluidics have enabled the development of functional models of human organs, coined ‘organs-on-chips’ (74, 75). In addition to gaining further insight into MSC-exosome mechanism-of-action, the application of technologies such as lung-on-a-chip could provide a powerful tool for assessing MSC-exosome potency and to support preclinical assays with greater predictive power. The lung-on-a-chip microdevice could incorporate pulmonary epithelium, endothelium and immune cells in a microenvironment consisting of a culture medium or blood/air interface and mechanical stretching mimicking conditions prevalent at the early stages of disease.
Under certain culture conditions, macrophages, basal, stromal, secretory and type II cells can give rise to three-dimensional, self-organizing structures known as organoids. Although preclinical data shows that MSC-exosome therapy results in robust physiological benefits, the biodistribution and in vivo metabolic fate of MSC-exosomes remains difficult to assess. In turn, just as target cell interactions remains unclear, the effect of experimental drugs, such as MSC-exosome therapy in ‘whole lung’ systems would allow insight to ‘off-target’ cellular effects (76). Although such technologies are aimed to closely mimic in vivo conditions, when using such platforms to assess the potency of experimental drugs, fit-for-purpose functional endpoints need to be established.
In vivo metabolic fate and biodistribution
For future exosome therapeutic applications technological advances are needed to help improve our understanding of the in vivo fate of exosomes. Numerous studies have assessed the biodistribution of exosomes in mice. Recently, Wiklander et al, (77) and others (78, 79) labeled exosomes with near-infrared lipophilic dyes and tracked the exosomes in vivo fate following intravenous, intraperitoneal or subcutaneous injection. They found that the route of administration and the dose dictated the in vivo biodistribution pattern. However, in addition to dye dilution via target cell fusion, a potential limitation of using lipophilic dyes to track exosomes is the disparity in half-life between exosomes and the dye. At present, with each labeling method harboring its advantages and limitations, a multimodal approach is encouraged.
Manufacturing, Toxicology and safety
In comparison to cell-based therapies in regenerative medicine, MSC-exosome treatment is more attractive for clinical development. Reasons being are manifold. MSC-exosomes are ‘perceived’ to be less immunogenic than their parental cells, as assessed by lower amounts of MHC-II (57). This may make MSC-exosomes less likely to invoke an immunological response in a foreign host. In addition, by replacing the administration of live cells with their secreted exosomes, many of the safety concerns and limitations associated with adoptive-transplantation of viable replicating cells are also mitigated. Furthermore, emerging evidence suggests that exosomes are nebulizable and can be cryopreserved at −20°C for six months with no loss to their biochemical activity.
The challenge of manufacturing a safe and reproducible medicinal product is complex. The regulatory landscape for exosome-based therapeutics is still evolving and typical toxicology testing approaches may not be considered appropriate for such biologic medicines due to complex bioactive properties that may include tumorigenicity and unknown off-target activities. With this in mind, we suggest that patients who receive MSC-exosome therapy are closely monitored for several years for the theoretical risk of tumor formation. Notwithstanding the theoretical potential of tumor formation, a recent meta-analysis looking at >1000 adult subjects has shown that there is no incidence of increased tumor risk up to 60 months post MSC (cell-therapy) treatment (80). To avoid unwanted immunological events and monitor off-target effect, upon advancing to clinical studies, investigators could monitor the immunogenicity of exosome-based therapies. Open questions include: do MSC-exosomes from different cell sources and donors possess the same immunomodulatory capacity? Are MSC-exosomes susceptible to cytotoxicity by the endogenous T-cells or natural killer cells of the host?
Summary
Stem cell approaches such as MSC-therapy have shown promise in numerous preclinical models relevant to neonatology. Such studies provided the platform for early phase clinical trials to study the feasibility and safety of MSCs in preterm infants at risk of developing BPD. Recent data have demonstrated that the therapeutic capacity of MSCs is comprised in their secretome, and that the major therapeutic vector therein is represented by the exosomes the MSCs release. Indeed, exosome-based therapeutics may represent the next generation drug delivery system, providing an unparalleled efficacy for the treatment of numerous diseases lacking efficient pharmacotherapy. However, their clinical application and development remains hampered by technological, mechanistic and safety issues. Careful consideration of the key issues raised in this review, may help in bringing exosome-based therapeutics a step closer to the clinic.
Acknowledgments
Financial Support: This work was supported in part by NIH grants RO1 HL085446 & RO1 HL055454 (SK) and a United Therapeutics Corp. Sponsored Research Grant (SK & SAM).
Footnotes
Author’s Contributions: GRW and SAM participated in manuscript writing. SK and SAM contributed to final article editing & approval.
Disclosure statement: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Reference list
- 1.Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manuck TA, Rice MM, Bailit JL, et al. Preterm neonatal morbidity and mortality by gestational age: A contemporary cohort. Am J Obstet Gynecol. 2016;215:103.e1–103.e14. doi: 10.1016/j.ajog.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fanaroff AA, Stoll BJ, Wright LL, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147.e141–147.e148. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Zysman-Colman Z, Tremblay GM, Bandeali S, et al. Bronchopulmonary dysplasia – trends over three decades. Paediatr Child Health. 2013;18:86–90. doi: 10.1093/pch/18.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 6.Schmalisch G, Wilitzki S, Roehr CC, et al. Development of lung function in very low birth weight infants with or without bronchopulmonary dysplasia: Longitudinal assessment during the first 15 months of corrected age. BMC Pediatr. 2012;12:37. doi: 10.1186/1471-2431-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1:728–742. doi: 10.1016/S2213-2600(13)70118-8. [DOI] [PubMed] [Google Scholar]
- 8.Khemani E, McElhinney DB, Rhein L, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: Clinical features and outcomes in the surfactant era. Pediatr. 2007;120:1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 9.del Cerro MJ, Sabaté Rotés A, Cartón A, et al. Pulmonary hypertension in bronchopulmonary dysplasia: Clinical findings, cardiovascular anomalies and outcomes. Pediatr Pulmonol. 2014;49:49–59. doi: 10.1002/ppul.22797. [DOI] [PubMed] [Google Scholar]
- 10.Jain D, Bancalari E. Bronchopulmonary dysplasia: Clinical perspective. Birth Defects Res A Clin Mol Terato. 2014;100:134–144. doi: 10.1002/bdra.23229. [DOI] [PubMed] [Google Scholar]
- 11.Collins JJP, Tibboel D, de Kleer IM, et al. The future of bronchopulmonary dysplasia: emerging pathophysiological concepts and potential new avenues of treatment. Front Med. 2017;4:61. doi: 10.3389/fmed.2017.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mercier J-C, Hummler H, Durrmeyer X, et al. Inhaled nitric oxide for prevention of bronchopulmonary dysplasia in premature babies (EUNO): a randomised controlled trial. Lancet. 2010;376:346–354. doi: 10.1016/S0140-6736(10)60664-2. [DOI] [PubMed] [Google Scholar]
- 13.Couroucli XI, Placencia JL, Cates LA, et al. Should we still use vitamin A to prevent bronchopulmonary dysplasia. J Perinatol. 2016;36:581–585. doi: 10.1038/jp.2016.76. [DOI] [PubMed] [Google Scholar]
- 14.Borghesi A, Cova C, Gazzolo D, Stronati M. Stem cell therapy for neonatal diseases associated with preterm birth. J Clin Neonatol. 2013;2:1–7. doi: 10.4103/2249-4847.109230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitsialis SA, Kourembanas S. Stem cell-based therapies for the newborn lung and brain: Possibilities and challenges. Semin Perinatol. 2016;40:138–151. doi: 10.1053/j.semperi.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borghesi A, Massa M, Campanelli R, et al. Circulating Endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2009;180:540–546. doi: 10.1164/rccm.200812-1949OC. [DOI] [PubMed] [Google Scholar]
- 17.Baker CD, Balasubramaniam V, Mourani PM, et al. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Eur Respir J. 2012;40:1516–1522. doi: 10.1183/09031936.00017312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foronjy RF, Majka SM. The potential for resident lung mesenchymal stem cells to promote functional tissue regeneration: understanding microenvironmental cues. Cells. 2012;1:874. doi: 10.3390/cells1040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wansleeben C, Barkauskas CE, Rock JR, et al. Stem cells of the adult lung: their development and role in homeostasis, regeneration, and disease. Wiley Interdiscip Rev Dev Biol. 2013;2:131–148. doi: 10.1002/wdev.58. [DOI] [PubMed] [Google Scholar]
- 20.Balasubramaniam V, Mervis CF, Maxey AM, et al. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1073–L1084. doi: 10.1152/ajplung.00347.2006. [DOI] [PubMed] [Google Scholar]
- 21.Jun D, Garat C, West J, et al. The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector T-cell proliferation. Stem Cells. 2011;29:725–735. doi: 10.1002/stem.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow K, Fessel JP, Kaoriihida S, et al. Dysfunctional resident lung mesenchymal stem cells contribute to pulmonary microvascular remodeling. Pulm Circ. 2013;3:31–49. doi: 10.4103/2045-8932.109912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popova AP, Bozyk PD, Goldsmith AM, et al. Autocrine production of TGF-β1 promotes myofibroblastic differentiation of neonatal lung mesenchymal stem cells. A Am J Physiol Lung Cell Mol Physiol. 2010;298:L735–L743. doi: 10.1152/ajplung.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagaishi K, Mizue Y, Chikenji T, Otani M, Nakano M, Konari N, Fujimiya M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. 2016;6:34842. doi: 10.1038/srep34842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lunn JS, Sakowski SA, Hur J, Feldman EL. Stem Cell Technology for Neurodegenerative Diseases. Annals of neurology. 2011;70:353–361. doi: 10.1002/ana.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Einstein O, Ben-Hur T. The changing face of neural stem cell therapy in neurologic diseases. Archives of Neurology. 2008;65:452–456. doi: 10.1001/archneur.65.4.452. [DOI] [PubMed] [Google Scholar]
- 27.Duelen R, Sampaolesi M. Stem Cell Technology in Cardiac Regeneration: A Pluripotent Stem Cell Promise. EBioMedicine. 2017;16:30–40. doi: 10.1016/j.ebiom.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kourembanas S. Expanding the pool of stem cell therapy for lung growth and repair. Circulation. 2014;129:2091–2093. doi: 10.1161/CIRCULATIONAHA.114.009727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kourembanas S. Stem cell-based therapy for newborn lung and brain injury: feasible, safe, and the next therapeutic breakthrough? J Pediatr. 2014;164:954–956. doi: 10.1016/j.jpeds.2014.01.064. [DOI] [PubMed] [Google Scholar]
- 30.Weiss DJ. Concise Review: Current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells. 2014;32:16–25. doi: 10.1002/stem.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romanov YA, Darevskaya AN, Merzlikina NV, et al. Mesenchymal stem cells from human bone marrow and adipose tissue: isolation, characterization, and differentiation potentialities. Bull Exp Biol Med. 2005;140:138–143. doi: 10.1007/s10517-005-0430-z. [DOI] [PubMed] [Google Scholar]
- 32.Pirjali T, Azarpira N, Ayatollahi M, et al. Isolation and characterization of human mesenchymal stem cells derived from human umbilical cord wharton’s jelly and amniotic membrane. Int J Organ Transplant Med. 2013;4:111–116. [PMC free article] [PubMed] [Google Scholar]
- 33.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 34.Aslam M, Baveja R, Liang OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Haaften T, Byrne R, Bonnet S, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180:1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss DJ, Casaburi R, Flannery R, et al. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143:1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L-T, Ting C-H, Yen M-L, et al. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci. 2016;23:76. doi: 10.1186/s12929-016-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang YS, Ahn SY, Yoo HS, et al. Mesenchymal Stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr. 2014;164:966–972.e966. doi: 10.1016/j.jpeds.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Jackson MV, Morrison TJ, Doherty DF, et al. Mitochondrial Transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34:2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang X, Ding Y, Zhang Y, et al. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23:1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 41.Hansmann G, Fernandez-Gonzalez A, Aslam M, et al. Mesenchymal stem cellmediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. 2012;2:170–181. doi: 10.4103/2045-8932.97603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fung ME, Thébaud B. Stem cell-based therapy for neonatal lung disease—it’s in the juice. Pediatr Res. 2014;75:2–7. doi: 10.1038/pr.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waszak P, Alphonse R, Vadivel A, et al. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells Dev. 2012;21:2789–2797. doi: 10.1089/scd.2010.0566. [DOI] [PubMed] [Google Scholar]
- 44.Pierro M, Ionescu L, Montemurro T, et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 2013;68:475–484. doi: 10.1136/thoraxjnl-2012-202323. [DOI] [PubMed] [Google Scholar]
- 45.Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 46.van der Pol E, Böing AN, Harrison P, et al. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 47.Rashed M, Bayraktar EK, Helal G, et al. Exosomes: From garbage bins to promising therapeutic targets. Int J Mol Sci. 2017;18:538. doi: 10.3390/ijms18030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willis GR, Kourembanas S, Mitsialis SA. Therapeutic applications of extracellular vesicles: perspectives from newborn medicine. Methods Mol Biol. 2017;1660:409–432. doi: 10.1007/978-1-4939-7253-1_34. [DOI] [PubMed] [Google Scholar]
- 49.Lässer C. Exosomes in diagnostic and therapeutic applications: biomarker, vaccine and RNA interference delivery vehicle. Expert Opin Biol Ther. 2015;15:103–117. doi: 10.1517/14712598.2015.977250. [DOI] [PubMed] [Google Scholar]
- 50.Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013;2:10.3402. doi: 10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Pol E, Böing AN, Gool EL, et al. Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J Thromb Haemost. 2016;14:48–56. doi: 10.1111/jth.13190. [DOI] [PubMed] [Google Scholar]
- 52.Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 53.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 54.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Reis LA, Borges FT, Simões MJ, et al. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS ONE. 2012;7:e44092. doi: 10.1371/journal.pone.0044092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xin H, Katakowski M, Wang F, et al. MicroRNA-17–92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. 2017;48:747–753. doi: 10.1161/STROKEAHA.116.015204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang OD, Mitsialis SA, Chang MS, et al. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells. 2011;29:99–107. doi: 10.1002/stem.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willis GR, Fernandez-Gonzalez A, Anastas J, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lo Sicco C, Reverberi D, Balbi C, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: Endorsement of macrophage polarization. Stem Cells Transl Med. 2017;6:1018–1028. doi: 10.1002/sctm.16-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nardiello C, Mižíková I, Morty RE. Looking ahead: where to next for animal models of bronchopulmonary dysplasia? Cell and Tissue Res. 2017;367:457–468. doi: 10.1007/s00441-016-2534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berger J, Bhandari V. Animal models of bronchopulmonary dysplasia. The term mouse models. Am J Physiol Lung Cell Mol Physiol. 2014;307:L936–L947. doi: 10.1152/ajplung.00159.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albertine KH. Utility of large-animal models of BPD: chronically ventilated preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2015;308:L983–L1001. doi: 10.1152/ajplung.00178.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choudhery MS, Badowski M, Muise A, et al. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12:8. doi: 10.1186/1479-5876-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borghesi A, Avanzini MA, Novara F, et al. Genomic alterations in human umbilical cord–derived mesenchymal stromal cells call for stringent quality control before any possible therapeutic approach. Cytotherapy. 2013;15:1362–1373. doi: 10.1016/j.jcyt.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Kowal J, Arras G, Colombo MJ et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koritzinsky EH, Street JM, Star RA, et al. Quantification of Exosomes. J Cell Physiol. 2017;232:1587–1590. doi: 10.1002/jcp.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rupert DLM, Claudio V, Lässer C, et al. Methods for the physical characterization and quantification of extracellular vesicles in biological samples. Biochim Biophys Acta. 2017;1861:3164–3179. doi: 10.1016/j.bbagen.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 70.Chia BS, Low YP, Wang Q, et al. Advances in exosome quantification techniques. TrAC Trends Anal Chem. 2017;86:93–106. [Google Scholar]
- 71.Akers JC, Ramakrishnan V, Nolan JP, et al. Comparative analysis of technologies for quantifying extracellular vesicles (EVs) in clinical cerebrospinal fluids (CSF) PLoS One. 2016;11:e0149866. doi: 10.1371/journal.pone.0149866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Willis GR, Kourembanas S, Mistalis SA. Towards exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front Cardiovasc Med. 2017 doi: 10.3389/fcvm.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reiner AT, Witwer KW, van Balkom BWM, et al. Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles. Stem Cells Transl Med. 2017;6:1730–1739. doi: 10.1002/sctm.17-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Konar D, Devarasetty M, Yildiz DV, et al. Lung-on-a-chip technologies for disease modeling and drug development. Biomed Eng Comput Biol. 2016;7:17–27. doi: 10.4137/BECB.S34252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 2015;14:248–260. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barkauskas CE, Chung M-I, Fioret B, et al. Lung organoids: current uses and future promise. Development. 2017;144:986–997. doi: 10.1242/dev.140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiklander OPB, Nordin JZ, Loughlin A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takahashi Y, Nishikawa M, Shinotsuka H, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165:77–84. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 79.Ohno S-i, Takanashi M, Sudo K, et al. Systemically injected exosomes targeted to egfr deliver antitumor microrna to breast cancer cells. Mol Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harron DWG. The textbook of pharmaceutical medicine. Blackwell Publishing Ltd; Oxford: 2007. Technical requirements for registration of pharmaceuticals for human use: the ICH process; pp. 552–564. [Google Scholar]
- 81.Aliotta JM, Pereira M, Wen S, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res. 2016;110:319–330. doi: 10.1093/cvr/cvw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu Y-g, Feng X-m, Abbott J, et al. Human mesenchymal stem cell microvesicles for treatment of e.coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Phinney DG, Di Giuseppe M, Njah J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Monsel A, Zhu Y-g, Gennai S, et al. Therapeutic effects of human mesenchymal stem cell–derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192:324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu B, Kim HW, Gong M, et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Crdio. 2015;182:349–360. doi: 10.1016/j.ijcard.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bian S, Zhang L, Duan L, et al. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med. 2014;92:387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 87.Teng X, Chen L, Chen W, et al. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and antiinflammation. Cell Physiol Biochem. 2015;37:2415–2424. doi: 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- 88.Kim D-k, Nishida H, An SY, et al. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A. 2016;113:170–175. doi: 10.1073/pnas.1522297113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu B, Shao H, Su C, Jiang Y, et al. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci Rep. 2016;6:34562. doi: 10.1038/srep34562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mead B, Tomarev S. BMSC-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl Med. 2017;6:1273–1285. doi: 10.1002/sctm.16-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Doeppner TR, Herz J, Görgens A, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4:1131–1143. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xin H, Li Y, Cui Y, et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tan CY, Lai RC, Wong W, et al. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5:76. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li T, Yan Y, Wang B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–54. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang J, Liu X-X, Fan H, et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One. 2015;10:e0140551. doi: 10.1371/journal.pone.0140551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fang S, Xu C, Zhang Y, Xue C, et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal micrornas suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem Cells Transl Med. 2016;5:1425–1439. doi: 10.5966/sctm.2015-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. doi: 10.1186/s12967-015-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zou X, Zhang G, Cheng Z, et al. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther. 2014;5:40. doi: 10.1186/scrt428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bruno S, Grange C, Collino F, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]


