Abstract
Purpose
To develop and evaluate a novel dynamic contrast-enhanced imaging technique called RACER-GRASP (Respiratory-weighted, Aortic Contrast Enhancement-guided and coil-unstReaking Golden-angle RAdial Sparse Parallel) MRI that extends GRASP to include automatic contrast bolus timing, respiratory motion compensation and coil-weighted unstreaking for improved imaging performance in liver MRI.
Methods
In RACER-GRASP, aortic contrast enhancement (ACE)-guided k-space sorting and respiratory-weighted sparse reconstruction are performed using ACE and respiratory motion signals extracted directly from the acquired data. Coil-unstreaking aims to weight multicoil k-space according to streaking artifact level calculated for each individual coil during image reconstruction, so that coil elements containing a high level of streaking artifacts contribute less to the final results. Self-calibrating GRAPPA operator gridding (GROG) was applied as a pre-reconstruction step to reduce computational burden in the subsequent iterative reconstruction. RACER-GRASP was compared with standard GRASP reconstruction in a group of volunteers and patients referred for clinical liver MR examination.
Results
Compared to standard GRASP, RACER-GRASP significantly improved overall image quality (average score: 3.25 v.s. 3.85) and hepatic vessel sharpness/clarity (average score: 3.58 v.s. 4.0), and reduced residual streaking artifact level (average score: 3.23 v.s. 3.94) in different contrast phases. RACER-GRASP also enabled automatic timing of the arterial phases.
Conclusion
The ACE-guided sorting, respiratory motion compensation and coil-unstreaking introduced by RACER-GRASP improve upon the imaging performance of standard GRASP for free-breathing dynamic contrast-enhanced MRI of the liver.
Keywords: RACER-GRASP, coil-unstreaking, radial imaging, streaking artifact, free breathing, compressed sensing, DCE-MRI, liver
Introduction
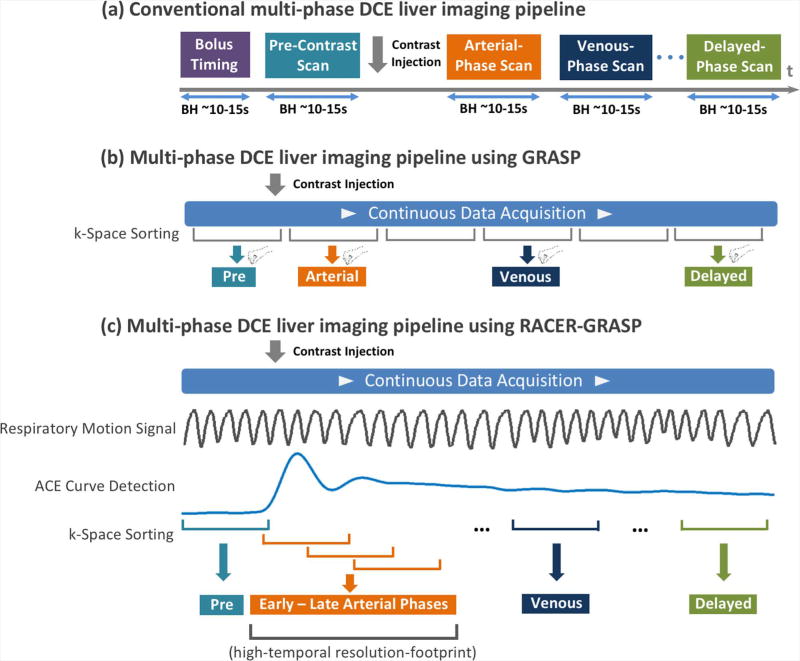
Dynamic contrast-enhanced MRI (DCE-MRI) is a valuable imaging technique for identifying and characterizing tumors and other lesions (1). It is widely used in oncology studies, and is an integral part of routine clinical imaging protocols (2). In a typical clinical DCE-MRI scan, multiple 3D image sets must be acquired in different contrast-enhancement phases, and rapid imaging is required to capture the passage of contrast media in the vascular system and through the organs such as the liver. To ensure accurate detection of contrast-enhancement phases, a separate bolus tracking step is generally performed in the beginning of each scan, from which an aortic contrast enhancement (ACE) curve is obtained from a manually placed region or interest (ROI) in the aorta. The curve is then used to guide the subsequent DCE acquisitions. For example, in a clinical liver DCE-MRI exam, an arterial phase, a venous phase and an equilibrium phase are usually acquired 5–10 seconds, ~60 seconds and ~180 seconds after the peak aortic enhancement, respectively. Moreover, to avoid respiratory-motion-induced artifacts, the multi-phase 3D images are normally acquired during multiple breath-holds in abdominopelvic exams. This punctuated imaging pipeline, as shown in Figure 1a, is cumbersome, and often fails in patients with impaired breath-hold capabilities.
Figure 1.
(a) Conventional multi-phase DCE liver imaging workflow. Separate 3D image volumes are acquired in desired contrast-enhancement phases during multiple breath-holds. A bolus timing step is performed before the DCE scan to ensure optimal detection of desired contrast phases. (b) Multi-phase DCE liver imaging using GRASP. Data are continuously acquired under free breathing, and images can be reconstructed with flexible temporal resolutions by grouping a specific number of consecutive spokes as each contrast phase. However, the optimal detection of desired contrast-enhanced phases is not guaranteed, and radiologists must manually select their desired contrast phases. (c) Multi-phase DCE liver imaging using RACER-GRASP. A respiratory motion signal and a ACE (Aortic Contrast Enhancement) signal are extracted from the acquired k-space to guide k-space sorting and image reconstruction. The ACE information can ensure optimal detection of desired contrast phases.
In order to address abovementioned limitations, several methods have recently been proposed to improve DCE-MRI acquisitions from conventional breath-hold snapshots to a continuous free-breathing streaming process, combining innovative sampling schemes with advanced reconstruction and motion compensation strategies. These methods can be roughly classified into two categories based on their sampling strategies. For the first category, data are acquired using a golden-angle interleaved 3D Cartesian sampling scheme. For example, Zhang et al have developed a DCE-MRI framework combining golden-angle Cartesian sampling (3) with respiratory motion-weighted (a.k.a soft-gating) compressed sensing reconstruction for abdominal imaging in pediatric patients (4). Liu et al and Zhu et al have also developed a similar imaging framework for DCE-MRI of the wrist (5) and the brain (6), respectively. For the second category, data are acquired using golden-angle non-Cartesian (often radial) trajectories. For example, Golden-angle RAdial Sparse Parallel (GRASP) MRI (7) is one example combining stack-of-stars sampling with multicoil compressed sensing reconstruction for free-breathing DCE-MRI. In all these methods, DCE-MR data are acquired continuously using a golden-angle reordering scheme (8), and a contrast-enhanced image series can be reconstructed with flexible temporal resolutions to meet different clinical needs.
Although these novel methods have all demonstrated promising imaging performance, they all suffer from various challenges. On one hand, Cartesian imaging is more robust to system imperfections (e.g., off-resonance and/or gradient delay), and allows easier and faster image reconstruction compared to radial imaging. However, a major limitation of Cartesian sampling is the sensitivity to motion (9), even with a golden-angle rotation scheme that repeatedly samples the center of k-space (10). On the other hand, radial sampling is more sensitive to system imperfections, and images are often contaminated by strong streaking artifacts in case of undersampling. Although these streaks normally arise from outer portions of the field of view (FOV), due to gradient nonlinearity effects and/or insufficient fat suppression resulting from magnetic field inhomogeneity (11), they often spread throughout the entire image with strong signal intensity, and are difficult to remove completely with conventional reconstruction algorithms. In addition, despite improved motion-robustness, radial sampling is not free of motion artifacts, and residual respiratory blurring is often observed in abdominal exams, particularly in patients with irregular and/or deep breathing patterns. Moreover, radial imaging suffers from increased computation burden, particularly in iterative reconstruction where the gridding and re-gridding operations are often incorporated in the iterative process (7,12). Last but not least, since conventional bolus tracking is not used in above mentioned continuous DCE-MRI methods for simplicity purpose, reconstructed images may suffer from suboptimal capture of desired phases, particularly the arterial phase representing the fast contrast wash-in period. Without bolus information, radiologists also have to manually select their desired image phases from a series of contrast-enhanced image volumes, which is time-consuming and cumbersome.
Several attempts have been made during the past few years to address some of these challenges. These approaches include: a) contrast bolus tracking using the center of k-space (13); b) extension of GRASP to XD-GRASP (eXtra-Dimensional GRASP) to reduce residual motion effects by reconstructing a multidimensional respiratory motion-resolved image-set (14); and c) exclusion of “poor” coil elements containing a high level of streaking artifacts before image reconstruction (11,15,16). Although these methods can partially solve some challenges, they all have various limitations. For example, k-space center represents the average signal throughout the entire FOV, and they do not reflect the true contrast bolus in an artery. The XD-GRASP technique, despite its improved image quality (17), requires prolonged reconstruction time and a sufficient amount of k-space measurements in each contrast phase to reconstruct an extra respiratory dimension. Approaches that aim to exclude “poor” coil elements require a reliable clustering algorithm to identify coil elements contaminated by strong streaking artifacts. Incorrect clustering may lead to loss of image information, or insufficient streak removal. Moreover, when the number of coils is small, some coil elements may contain both streaks and useful image content, which imposes an additional challenge in selection of unwanted coils.
In this study, we developed a new DCE-MRI framework called Respiratory-weighted, Aortic Contrast Enhancement-guided and coil-unstReaking GRASP (RACER-GRASP) MRI, which represents a unified solution to efficiently address all the limitations described above, with the aim of improving the robustness of rapid and continuous free-breathing DCE-MRI for routine clinical use. As part of RACER-GRASP, we also proposed new algorithms for automatic extraction of contrast bolus and respiratory motion signals from the acquired radial k-space data, and an approach for automated removal of strong streaking artifacts in radial imaging in general. The performance of the proposed framework was demonstrated for DCE-MRI of the liver in a cohort of healthy volunteers and patients.
Methods
RACER-GRASP Framework
RACER-GRASP is an extension of GRASP to include ACE-guided k-space sorting (with a role similar to conventional bolus timing), respiratory-weighted image reconstruction (for reduction of motion-induced blurring), and coil-unstreaking (for removal of coil-related streaking artifacts). RACER-GRASP aims to deliver a new free-breathing DCE-MRI pipeline as shown in Figure 1c, which enables motion-weighted and streaking-artifact-reduced reconstruction to capture clinically relevant contrast phases and information. In the following subsections, we describe the algorithms for automatic extraction of ACE and respiratory motion signals, then the coil-unstreaking process, and, finally, detailed steps for implementation of RACER-GRASP.
Automatic ACE Signal detection
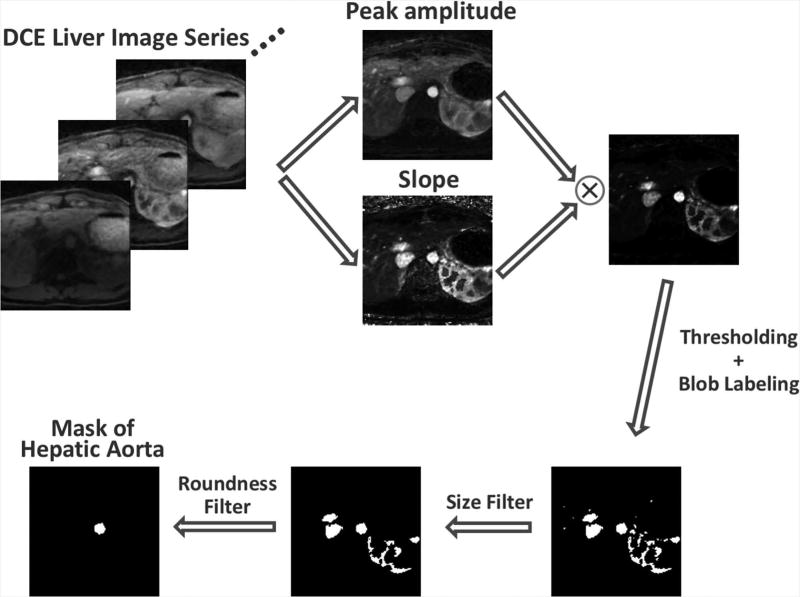
The ACE signal can be extracted from an axial DCE liver image series based on the facts that i) contrast agent flows through the aorta at the level of the origin of the celiac artery (and hepatic artery), causing its signal to brighten both more fully and more quickly than other regions/organs at the same level; and ii) the aorta has a more nearly circular cross-section than other types of tissues or organs. Figure 2 outlines the algorithm for automatic ACE signal detection. For each slice, a low spatial resolution but high temporal resolution image series is generated first with standard gridding reconstruction (see Figure S1 in the supplementary materials), from which a peak amplitude image and a slope image are generated using the following equations to represent the contrast enhancement peak and slope in each voxel:
| [1] |
| [2] |
For each voxel, S(t) represents the signal intensity at a time point t, S0 is the baseline signal intensity averaged from the first five pre-contrast phases (~15 seconds), S(t)/S0 is the signal enhancement ratio, and tp represents the time to reach peak enhancement. After multiplication of the peak amplitude and slope images, a thresholding process is performed, followed by a blob labeling process to generate distinguishable 2D-connected components (18). A size filter is then applied to remove the blobs that are too big (e.g., >300 pixels) or too small (e.g., <20 pixels) to be the aorta. The blob with the highest circularity, calculated using the following equation, is selected as the final mask for the aorta in the current slice:
| [3] |
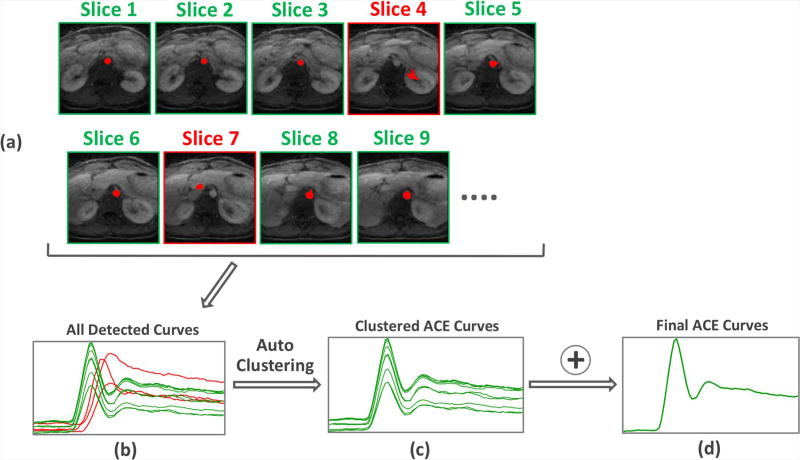
To improve robustness and reliability of the algorithm, this detection process is repeated for the central 16 slices, as shown in Figure 3. A clustering algorithm (19) is then applied to identify the dominant curve pattern, which will be the ACE signal given that the aorta is present in most slices. For more details about the clustering process, please see Figure S2 in the supplementary materials.
Figure 2.
The pipeline for ACE (Aortic Contrast Enhancement) signal detection. For each slice, a low spatial resolution but high temporal resolution image series is generated with view sharing and standard gridding reconstruction, from which a height image and a slope image are generated to represent the contrast enhancement height and slope of each voxel. After multiplication of the height and slope images, a thresholding process is performed, followed by a blob labeling process to generate distinguishable 2D-connected components. A size filter is applied to keep only the blobs that are big enough to be the aorta. The blob with the highest circularity is selected as the final mask of hepatic aorta for this slice.
Figure 3.
Signal clustering to find the averaged ACE signal and to improve the robustness and reliability of the algorithm. The detection process is repeated for the central 16 slices. The dominant curve pattern generated among all 16 slices is identified, which is the ACE signal given that the hepatic aorta is presented in most slices.
Automatic Respiratory Motion Detection
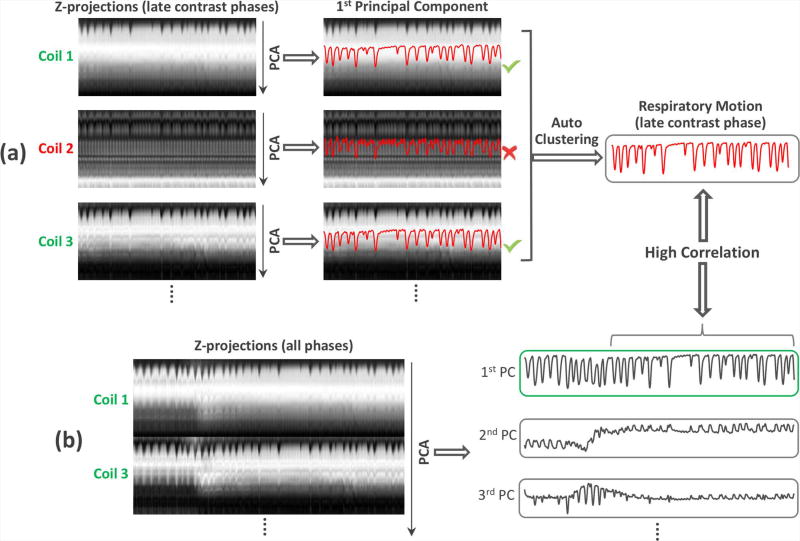
Figure 4 shows the pipeline for automatic respiratory motion detection from an axial DCE liver dataset. In the first step, z-projection profiles of the entire imaging volume are computed by performing a 1D partition-direction fast Fourier transform (FFT) on the series of central k-space points (kx=ky=0). The signal intensity of each projection is normalized between 0 and 1 so that the effect of contrast enhancement can be partially compensated. In the second step, we aim to identify the appropriate coils with a clear respiratory motion pattern. Specifically, for each coil element, principal component analysis (PCA) is performed to determine the most common signal variation mode in the late contrast phase (in order to avoid the influence of contrast-enhancement), as shown in Figure 4a. For coil elements that have an appropriate respiratory pattern, the first principal component (PC) is expected to represent respiratory motion. Given that respiratory motion is the dominant pattern among all coils, the clustering algorithm already used for the ACE curve detection can be reused to identify desired coil elements, and to extract a respiratory motion signal for the late contrast-enhancement phase automatically. In the third step, the selected coils are concatenated together and PCA is performed again to determine the most common signal variation mode for all contrast phases, as shown in Figure 4b. The PC that has the highest correlation with the late contrast phase respiratory motion signal extracted in the previous step is then selected as the final respiratory motion signal (Figure 4b), followed by demodulation of the contrast enhancement envelope as described in (14).
Figure 4.
The pipeline for automatic respiratory motion detection. (a) Z-projection profiles of the entire imaging volume are computed by performing a 1D partition-direction FFT on the series of central k-space points (kx=ky=0). For each coil-element, principal component analysis (PCA) is performed to determine the most common signal variation mode in the late contrast phase. For coil elements that have a good representation of respiratory pattern, their first principal components (PC) are expected to represent respiratory motion. Thus, the clustering algorithm shown in Figure 3 can be used to identify those “good” coil elements and to extract a respiratory motion signal for the late contrast-enhancement phase. (b) The selected “good” coils are concatenated together and PCA is performed to determine the most common signal variation mode for all contrast phases. The PC that has the highest correlation with the late contrast phase respiratory motion signal extracted in the previous step is selected as the final respiratory motion signal.
Coil Unstreaking
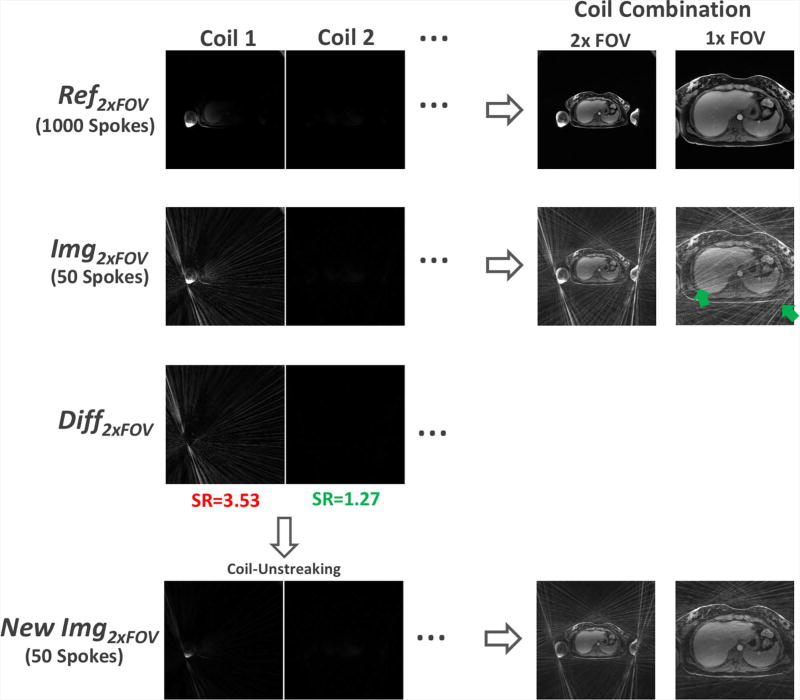
The concept of streak ratio was previously introduced (11) for evaluating the degree of streaking artifacts in radial images, so that coils with a high streak ratio can be excluded for image reconstruction. In this work, we use a variation of the streak ratio to weight the contribution of each coil, instead of using a simple binary decision for inclusion or exclusion. Our streak ratio is defined in the following way. As shown in Figure 5, Ref2×FOV refers to multicoil magnitude images reconstructed using a number of spokes (e.g., 800–1000) that are sufficient to reconstruct artifact-free images with two-fold (2×) oversampled FOV; Img2×FOV refers to multicoil magnitude images reconstructed using a small number of spokes (e.g., 50) with 2× oversampled FOV, in which a high level of artifact is presented due to heavy undersampling; The difference of Ref2×FOV and Img2×FOV (i.e., Diff2×FOV) can then be used to quantify the artifact level in each coil. Here, Ref2×FOV and Img2×FOV are generated using spokes from the late contrast phase to avoid the influence of contrast enhancement. Then, the streak ratio of the kth coil (rk) can be calculated as:
| [4] |
where Ref1×FOV represents the cropped central half of Ref2×FOV excluding the oversampled region, and N indicates the total number of coil elements. The streak ratio vector [r1, r2, …, rN] is normalized such that its minimum element is one. It should be noted that the denominator in Equation 4 is calculated using multicoil images with cropped FOV so that the energy of streaking artifacts generated in the peripheral region of the FOV (e.g., the arms) can be properly preserved.
Figure 5.
The steps for streak ratio calculation and coil-unstreaking. Ref2×FOV refers to multicoil images reconstructed using a number of spokes (e.g., 1000) that are sufficient to reconstruct artifact-free images with 2× oversampled FOV; Img2×FOV refers to multicoil images reconstructed using a small number of spokes (e.g., 40) with 2× oversampled FOV, in which a high level of artifact is presented due to heavy undersampling (green arrows); Diff2×FOV is the difference of Ref2×FOV and Diff2×FOV for quantifying the artifact level created in each coil and a streak ratio index can be calculated for each coil element. The streak ratio is then incorporate into iterative reconstruction, so that the contribution of each coil to the final image is weighted based on its streaking artifacts level (i.e., coil elements with a high streak ratio contribute less to the final result, and vice versa). In order to avoid generation of excessive inhomogeneity in the reconstructed images, the coil-unstreaking process is only performed in the coils whose streak ratio is greater than a threshold (labelled with red color).
In the next step, instead of excluding those coils contaminated by strong streaking artifacts, we propose a so-called “coil-unstreaking” process which incorporates the streak ratio information into iterative reconstruction. For example, in the context of iterative reconstruction, coil-unstreaking can be formulated as follows, so that the contribution of each coil to the final image is weighted by its streak ratio (i.e., coil elements with a high streak ratio contribute less to the final result, and vice versa).
| [5] |
where R represents the streak-ratio weights to adjust the contribution of each coil in the data consistency term. The kth element of R (i.e., the weight for the kth coil) is obtained as
| [6] |
Here, R is adjusted such that the coil-unstreaking process is only performed in the coils whose streak ratio is greater than a threshold (empirically set to 1.3 in this work) to avoid excessive inhomogeneity in the reconstructed images.
RACER-GRASP Reconstruction
Given the detected ACE signal, respiratory motion signal, and streak ratio, RACER-GRASP reconstruction is performed for multi-phase liver MRI with the following steps:
K-space data are automatically sorted at the temporal positions of [−30, −15, 0, +5, +10, +15, +22, +30, +45, +60, +90, +120, +150, +180] seconds with a temporal footprint of ~15 seconds, which is a temporal resolution normally used in routine clinical studies. Here, 0s indicates the peak aortic enhancement position and −/+ indicates whether the current phase is before or after the peak aortic enhancement. The phase at −30s is selected as the pre-contrast phase, the phases at +5s, +10s and +15s are selected as the early to late arterial phases, the phase at +60s is selected as the portal venous phase, and the phase at +180s is selected as the equilibrium phase. Note that in clinical workflow, all phases can be automatically exported for a radiologist based on their time stamps without user interaction.
Each contrast phase is binned into multiple motion phases spanning from end-expiration to end-inspiration using the extracted respiratory motion signal, as shown in Figure S3 (supplementary materials) for an example of four respiratory phases.
- Multicoil radial k-space data in each sorted dynamic phase are gridded onto a Cartesian grid using self-calibrating GRAPPA operator gridding (GROG) (20–22) (Equation 7), such that the subsequent iterative reconstruction can be performed directly on the Cartesian space.
Here, yr is the acquired radial k-space, G is the self-calibrated GROG operator (21), and yc is the gridded Cartesian k-space.[7] - Image reconstruction is performed by solving the following equation, with each motion phase and each coil weighed differently (23) in order to reduce the contribution of “motion-corrupted” k-space measurements (e.g., inspiratory phases) and streaking-artifact-contaminated coil elements, respectively, to the final images.
Here, F is the FFT operator, C represents the coil sensitivities, × is the image-series to be reconstructed, and T is a temporal sparsifying transform. W represents motion weights for a total of n respiratory phases, and the weight in the tth motion phase is calculated as follows, with an exponential decay from expiration (the 1st phase) to inspiration (the nth phase) as suggested in (3,4).[8]
When coil compression is used, multicoil images are mixed together, Thus, implementation of Equation 8 is accomplished as[9]
where a pre-processing step is applied as follows for coil-unstreaking and coil-compression.[10]
Here, ỹc is coil-weighted, compressed Cartesian k-space after GROG, C̃ represents compressed coil sensitivities after coil-unstreaking, and A is an operator performing coil compression.[11]
Volunteers and Patients
In this IRB-approved and HIPAA-compliant study, five healthy volunteers (age=34.8±12.9 years, 4 females) were recruited to participate. Written informed consent was obtained from all volunteers before MR scans. In addition, MR raw data from twenty-two patients (age=77.5±15.2 years, 14 females) who were referred for clinical DCE liver MRI examination at our institute from 02/2016 to 01/2017, and were imaged using a radial T1-weighted sequence, were retrospectively collected for the study. The written informed consent was waived and this was approved by the IRB. The patient demographics are shown in Table S1 in the supplementary materials.
Data Acquisition
All volunteer imaging was performed on a clinical 3.0T MRI scanner (MAGNETOM Prisma, Siemens Healthineers, Germany) in a transverse orientation using a fat-saturated stack-of-stars golden-angle radial imaging sequence as previously described in (24). Relevant imaging parameters were: TR/TE = 3.6/1.6 ms, matrix size = 256×256×48, FOV = 350×350×240 mm3, acquired voxel size = 1.37×1.37×5.0 mm3, flip angle = 12°. 80% partial Fourier was applied along the slice dimension and a total of 1222 spokes were acquired for each partition, resulting in a total scan time of 190 seconds. For each scan, a weight-based half dose injection (0.1 mmol per kg of body weight) of Magnevist (Bayer Healthcare) was performed 20 seconds after the start of data acquisition, at a rate of 2 mL/sec.
All patient datasets were acquired on a clinical 3.0T MRI scanner (MAGNETOM Skyra, Siemens Healthineers, Germany) in a transverse orientation using the same sequence. Relevant imaging parameters were: TR/TE = 4.12/1.81 ms, matrix size = 256×256×(46–56), FOV = 385×385×(230–280) mm2, acquired voxel size = 1.5×1.5×5.0 mm3, flip angle = 12°. 80% partial Fourier was applied along the slice dimension and a total of 1904 spokes were acquired for each partition, resulting in an averaged total scan time of 345.2±29 seconds. For each scan, a weight-based half dose injection (0.1 mmol per kg of body weight) of Gadavist (Bayer Healthcare) was performed ~15–20 seconds after the start of data acquisition, at a rate of 2 mL/sec.
One volunteer (female, 28 years) was also scanned using a clinical breath-held Cartesian DCE-liver imaging protocol in a second visit after ~5 months with similar imaging parameters: FOV = 350×350×216 mm3, acquired voxel size = 1.37×1.37×5.0 mm3, flip angle = 12° and 80% partial Fourier was applied along the slice dimension. The images were acquired during four breath-holds to acquire four clinical-relevant contrast phases, and the total scan time was ~5–6 minutes including breath-hold instructions, the bolus timing run and the waiting time between scans.
Image Reconstruction
RACER-GRASP sorted every contrast phase into four respiratory phases, and with the parameter b in Equation 9 empirically selected as 4. For comparison, standard GRASP reconstruction with consecutive k-space sorting was also performed with the same temporal resolution as in RACER-GRASP. GROG was employed in both GRASP and RACER-GRASP to reduce computation burden. All reconstructions were performed using a non-linear conjugate gradient algorithm in a server equipped with 256 GB RAM, and two 6-Gb NVIDIA GPU cards. Coil sensitivity maps were estimated from a static 3D image volume reconstructed using all the acquired data using the adaptive combination approach (25). Regularization parameters were empirically selected for each reconstruction type, and were fixed for all datasets. A first-order finite difference operation along the temporal dimension was chosen as the sparsifying transform. Following FFT along the slice dimension, the reconstruction of each 4D DCE dataset (x-y-z-contrast) was implemented slice-by-slice in Matlab (Mathworks, MA) and was accelerated using GPUs. The source code for RACER-GRASP, including the algorithms for extracting ACE and respiratory motion signals, are available for free download from http://cai2r.net/resources/software.
Image Quality Assessment
For both standard GRASP and RACER-GRASP results, the first phase was selected as the pre-contrast phase. For each GRASP dataset, an abdominal radiologist with 10 years of post-fellowship experience (H.C.) manually selected the best early-arterial, late-arterial and portal venous phases. For RACER-GRASP, the phases reconstructed at +5s, +10s and +15s were automatically selected as the early to late arterial phases, and, for purposes of comparison, the radiologist assigned one of them as the best early-arterial phase and assigned another one of them as the best late-arterial phase. The phase reconstructed at +60s was automatically selected as the venous phase.
All selected contrast phases from both GRASP and RACER-GRASP datasets were randomized for a paired visual evaluation. Each pair consisted of GRASP and RACER-GRASP images from the same subject, with matching contrast phase, presented in a randomized order. Each pair was independently evaluated by two board-certified abdominal radiologists (K.S. and C.H., with 9 and 2 years of post-fellow experience, respectively) blinded to the reconstruction schemes. For pre-contrast and venous phases, the readers scored the overall image quality, hepatic vessel sharpness/clarity and streaking artifact level of each image-set based on a 5-point scale (5=excellent/no streak; 4=good/mild streak; 3=adequate/moderate streak; 2=poor/extreme streak; 1=non-diagnostic/unreadable). For the early arterial phase, the readers scored the overall image quality, streaking artifact level, and capture/timing of arterial phases on the same 5-point-scale. The vessel sharpness was not assessed for this phase because of reduced vessel-tissue contrast in this phase. For the late arterial phase, it was noticed that timing differed between GRASP and RACER-GRASP (e.g., the late arterial phase selected in GRASP could be too late, with liver and portal vein already enhanced), resulting in inappropriate comparison of image quality. Thus, the readers only scored the capture/timing for the late arterial phase.
Statistical Analysis
For each image quality assessment category in each contrast phase, the reported scores were averaged from two readers to yield a mean ± standard deviation. A nonparametric paired two-tailed Wilcoxon signed-rank test was used to compare the scores, with a P value less than 0.05 indicating statistical significance. The inter-reader variability was assessed using the Bland-Altman analysis.
Results
The average time for reconstructing the whole 4D DCE-MR datasets was 12.59±1.98 and 12.33±1.19 minutes (calculated for the iterative reconstruction process) for GRASP and RACER-GRASP, respectively, both with GROG and GPU acceleration. This reconstruction speed represents a substantial improvement compared to previously reported results in (7). Table 1 summarizes the readers’ scores for different assessment categories. RACER-GRASP achieved systematic improvement over GRASP, and statistical significance was reached in all categories except for the hepatic vessel sharpness/clarity in portal venous phase. RACER-GRASP also enabled significantly improved capture of both early and late arterial phases. The inter-reader variability is shown in Figure S4 (supplementary materials).
Table 1.
Qualitative comparison between standard GRASP and RACER-GRASP for different contrast phases. RACER-GRASP achieved systematic improvement in all assessment categories. Statistical significance was reached in all categories except for the hepatic vessel sharpness/clarity in the portal venous phase.
| Overall Image Quality |
Hepatic Vessel Sharpness/Clarity |
Streaking Artifact Level |
Capture of the Arterial Phase |
||
|---|---|---|---|---|---|
| Pre-Contrast Phase | GRASP | 3.31±0.90 | 3.26±0.78 | 3.33±0.82 | N/S |
| RACER-GRASP | 3.80±0.76‡ | 3.80±0.79‡ | 3.93±0.66‡ | N/S | |
| Portal Venous Phase | GRASP | 3.57±0.70 | 3.89±0.71 | 3.43±0.70 | N/S |
| RACER-GRASP | 4.15±0.52‡ | 4.20±0.50 | 4.19±0.54‡ | N/S | |
| Early Arterial Phase | GRASP | 2.87±0.78 | N/S | 2.93±0.74 | 3.72±0.86 |
| RACER-GRASP | 3.60±0.62‡ | N/S | 3.69±0.68‡ | 4.11±0.51‡ | |
| Late Arterial Phase | GRASP | N/S | N/S | N/S | 2.83±0.67 |
| RACER-GRASP | N/S | N/S | N/S | 3.78±0.79‡ |
Scoring criterial:
For overall image quality, hepatic vessel sharpness and capture of arterial phases:
5=excellent, 4=good, 3=adequate, 2=poor, 1=non-diagnostic.
For streaking artifacts level:
5=no streaks, 4=mild streaks, 3=moderate streaks, 2=extreme streaks, 1=unreadable.
The difference between RACER-GRASP and GRASP was statistical significance N/S: Not Scored
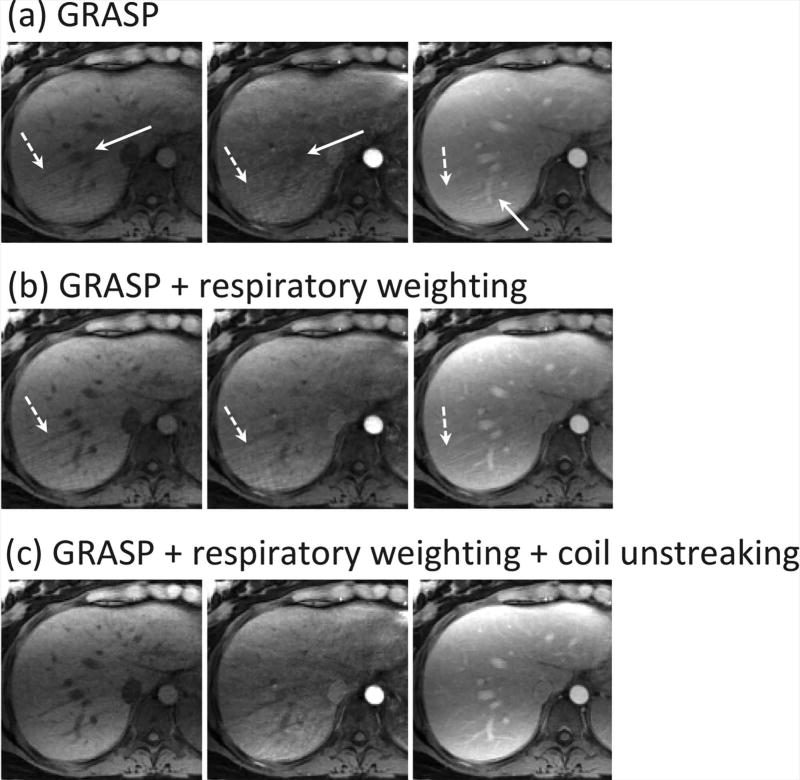
Figure 6 shows a comparison of different reconstruction schemes in three representative contrast phases in a volunteer dataset. GRASP (a) suffered from both residual motion burring (solid white arrows) and residual streaking artifacts (dashed white arrows). Although respiratory-weighted reconstruction (b) improved image quality and resolved fine details in the liver, the residual streaking artifacts were still observed. With additional coil-unstreaking (c), residual streaking artifacts can be removed and image quality can be further improved in all phases.
Figure 6.
A comparison of different reconstruction schemes in three representative contrast phases. (a) GRASP suffered from both residual motion burring (while straight-line arrows) and residual streaking artifacts (while dashed-line arrows) that were generated from certain peripheral regions (e.g., the arms) in the FOV. (b) Respiratory-weighted reconstruction improved image quality and resolved fine details in the liver. However, residual streaking artifacts were still observed. (c) Respiratory-weighted and coil-unstreaking reconstruction removed residual streaking artifacts and achieved further improved image quaity in all phases.
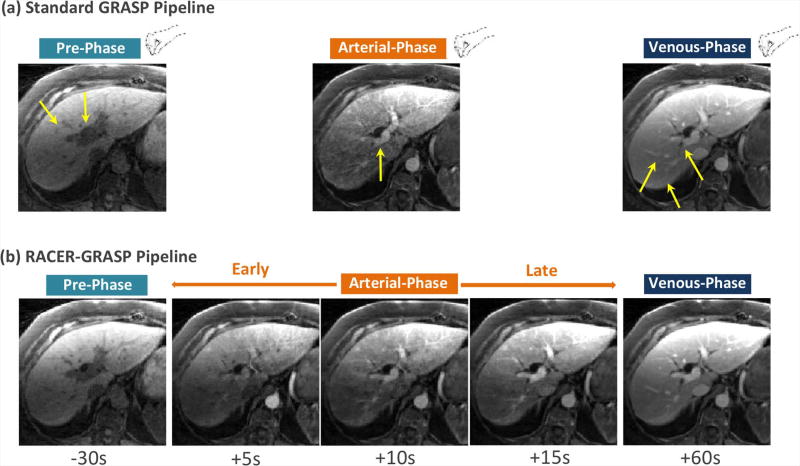
Figure 7 shows a comparison of the standard GRASP pipeline with the proposed RACER-GRASP pipeline in a patient dataset. Standard GRASP required manual selection of desired contrast phases by a radiologist, and images suffered from residual motion blurring (solid yellow arrows). RACER-GRASP, on the other hand, improved reconstruction of all contrast phases, capture of the arterial phases, and the vessel sharpness/clarity, and enabled automatic selection of desired contrast phases.
Figure 7.
A comparison of standard GRASP pipeline (a) with the proposed RACER-GRASP pipeline (b). Standard GRASP requires manual selection of desired contrast phases that suffered from residual motion blurring effects (yellow straight-line arrows) and residual streaking artifacts (yellow dashed-line arrows). On the other hand, RACER-GRASP enabled improved reconstruction of all phases, better capture of the arterial phases, higher vessel sharpness/clarity, and could automatically export desired contrast phases for a radiologist.
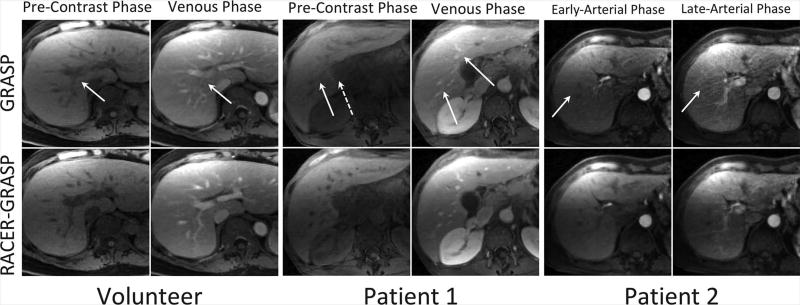
Figure 8 shows a side-by-side comparison of the pre-contrast, venous and arterial phases between GRASP and RACER-GRASP in both volunteer and patients. RACER-GRASP achieved clearly improved overall image quality, better vessel sharpness and reduced streaking artifacts level, while residual motion blurring (solid white arrows) and streaking artifacts (dashed white arrows) were observed in the GRASP images. Meanwhile, RACER-GRASP enabled better capture of the arterial phases, while the late arterial phase in GRASP was not captured accurately (i.e., the liver parenchyma and the portal vein had already started enhancing). Comparisons of arterial phases in two additional patients are shown in Figure S5 (supplementary materials), with similar improvements achieved by RACER-GRASP in all assessment categories.
Figure 8.
A side-by-side comparison of the pre-contrast phase, venous phase and arterial phases between GRASP (top row) and RACER-GRASP (bottom row) in both volunteer and patients. RACER-GRASP achieved clearly improved overall image quality, better vessel sharpness and reduced streaking artifacts level, while residual motion blurring (while straight-line arrows) and streaking artifacts (while dashed-line arrows) were observed in the GRASP images. Particularly, RACER-GRASP enabled better capture of the arterial phases in the patient images while the late arterial phase in GRASP was not captured accurately (the liver already started enhancing).
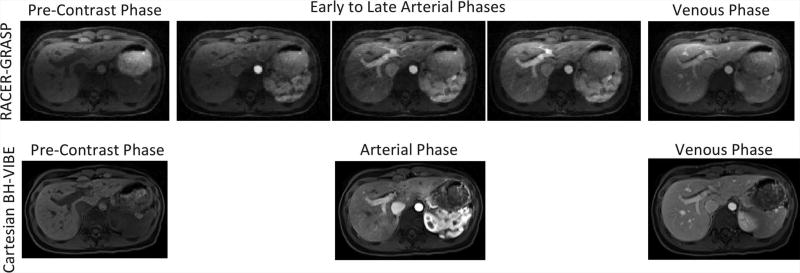
Figure 9 shows a comparison of RACER-GRASP with conventional breath-held liver imaging (clinical reference). Both techniques yielded multiphase liver images that are adequate for clinical use (assessed by an expert body radiologist (H.C.)), although the breath-held results show superior visual image quality in this volunteer. However, RACER-GRASP was able to provide more clinical relevant information by reconstructing multiple arterial phases from early to late enhancement.
Figure 9.
A comparison of RACER-GRASP with conventional breath-held VIBE liver imaging, which serves as the clinical reference. While the breath-held images show superior visual image quality, RACER-GRASP was able to provide more clinical relevant information by reconstructing multiple arterial phases from early to late enhancement.
Discussion
RACER-GRASP offers a unified solution to efficiently address many of the limitations in existing imaging approaches and represents a robust imaging framework for rapid and continuous free-breathing DCE-MRI of the liver. RACER-GRASP represents the latest step of the evolution of GRASP-based techniques, where the initial idea of rapid continuous imaging during free-breathing was improved by several new components to make it more robust for clinical practice. ACE-guided k-space sorting ensures capture of desired contrast phases without the need for a separate bolus timing step; respiratory motion-weighted reconstruction achieves improved vessel sharpness/clarity and reduced motion blurring effects without increasing reconstruction time; coil-unstreaking enables automatic removal of residual streaking artifacts; and GROG avoids gridding and re-gridding during iterative reconstruction, which has been shown to substantially reduce computational burden (22). Preliminary implementations of the RACER-GRASP reconstruction algorithm can achieve processing times of ~10–15 minutes with superior imaging performance to standard GRASP; and it is expected that the reconstruction times can be further reduced with sufficient hardware support and further optimized implementation.
With automatic bolus timing from the acquired data, RACER-GRASP reconstructs three arterial phases centered at 5, 10 and 15 seconds after peak aortic enhancement. Therefore, there is little chance for missing an arterial phase, which is the most important contrast-enhancement phase in DCE liver imaging. The contrast dynamics from early to late arterial phases also provides radiologists with more relevant information. Meanwhile, the ACE information enables automatic selection of desired contrast phases for radiologists, and allows reconstruction of a smaller number of contrast phases in the slow contrast wash-out period, reducing reconstruction burden.
Residual streaking artifact remains one of the major challenges in radial imaging. These artifacts usually originate from edge and outside regions of the FOV in case of undersampling. They often carry strong signal intensity and can cause severe degradation of image quality. Multiple receiver coils with different spatial sensitivities can be used to localize the sources of these artifacts, and thus one can identify “poor” coil elements that are contaminated by streaking artifacts. Coil-unstreaking represents an easy and efficient way for automatic streaking artifact reduction in radial imaging. It aims to weight multicoil k-space according to the streaking artifacts level calculated for each individual coil during image reconstruction, so that coil elements with a high level of streaking artifacts contribute less to the final results. Thus, coil-unstreaking does not need to cluster coil elements and can also potentially avoid insufficient or excessive removal of coil elements.
Robust implementation of RACER-GRASP requires an ACE signal and a respiratory motion signal to guide k-space sorting and image reconstruction, respectively. Two algorithms were proposed in this work for automatic detection of the required signals, and they were successfully demonstrated in all volunteers and patients. The algorithm for ACE signal detection can also be applied to other applications, such as DCE-MRI of the prostate (see Figure S6 in the supplementary materials). The algorithm for respiratory motion detection is an extension of that proposed in (14). Unlike many methods that select respiratory motion signals based on their frequency spectrum in a pre-defined frequency range (e.g., 0.1–0.5 Hz), our new algorithm does not rely upon this assumption. Therefore, it could be more robust for imaging of patients with different respiratory patterns.
The respiratory-weighted (or soft-gated) reconstruction represents a simple but effective approach to handle respiratory motion. Despite certain SNR loss due to the weighting process, it is more flexible, more efficient and can be more robust towards various respiratory patterns compared to motion correction approaches that based on a registration algorithm (26). The respiratory-weighted reconstruction implemented in this study shares some similarities with the previously proposed XD-GRASP approach (14). They both sort acquired k-space data into multiple respiratory phases. However, since XD-GRASP explicitly reconstructs an extra motion dimension, it leads to higher computational burden and requires sufficient k-space measurements in each motion phase to ensure good reconstruction performance, which limits achievable temporal resolution. An example comparing XD-GRASP with RACER-GRASP can be seen in the supplementary materials (Figure S7), and a more comprehensive comparison was recently reported in (27), which suggested that respiratory-weighted reconstruction achieved comparable image quality to XD-GRASP in multiphase liver imaging without increasing computation burden.
This first feasibility study was carried out in a small number of volunteers and a group of retrospectively collected patient datasets. It is a limitation that a comparison of RACER-GRASP with conventional breath-held Cartesian liver imaging was only performed in one subject. Although the breath-held results showed superior image quality in this healthy young volunteer, RACER-GRASP is expected to be more useful for patients who have impaired capabilities to hold their breath. Meanwhile, RACER-GRASP provides an easier acquisition pipeline comparing to breath-hold acquisitions, and this could greatly improve the entire experience of DCE-MRI both for physicians and for patients. Although RACER-GRASP showed adequate performance in this study, evaluation of its applicability in a larger cohort of patients in a comparison with conventional breath-held imaging would be necessary to further validate the robustness of this technique. This is currently underway and we are in the process of translating this new framework into clinical practice (28). In additional to multiphase liver imaging, it would also be interesting to evaluate the performance of this framework for free-breathing perfusion imaging with high-temporal-resolution. The arterial input function (AIF), which is normally required for pharmacokinetic modeling and is usually obtained from a manually selected ROI, can be automatically extracted using our proposed framework.
Conclusion
The combination of novel physiological (contrast bolus, respiratory motion) and physical (coil streaks) information in RACER-GRASP provides a unified solution for rapid, motion-robust, information-driven and computationally-efficient DCE-MRI of the liver. The possibility of capturing desired contrast-enhanced phases without a separate bolus timing run and the automatic reduction of streaking and motion artifacts are major steps towards simplified DCE-MRI examinations that can be applied in a wide range of routine clinical applications.
Supplementary Material
Acknowledgments
Grant support: National Institutes of Health: P41 EB017183; R01 EB018308
This work was supported in part by the NIH (P41 EB017183 and R01 EB018308) and was performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R), a NIBIB Biomedical Technology Resource Center. Li Feng would like to thank Ye Tian and Prof. Edward DiBella from the University of Utah for sharing the GROG source code and support in implementing the self-calibrated GRAPPA operator gridding algorithm.
References
- 1.Knopp MV, Giesel FL, Marcos H, von Tengg-Kobligk H, Choyke P. Dynamic contrast-enhanced magnetic resonance imaging in oncology. Top Magn Reson Imaging. 2001;12(4):301–308. doi: 10.1097/00002142-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 2. https://radiopaedia.org/articles/mri-protocols.
- 3.Cheng JY, Zhang T, Ruangwattanapaisarn N, Alley MT, Uecker M, Pauly JM, Lustig M, Vasanawala SS. Free-breathing pediatric MRI with nonrigid motion correction and acceleration. Journal of magnetic resonance imaging : JMRI. 2015;42(2):407–420. doi: 10.1002/jmri.24785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang T, Cheng JY, Potnick AG, Barth RA, Alley MT, Uecker M, Lustig M, Pauly JM, Vasanawala SS. Fast pediatric 3D free-breathing abdominal dynamic contrast enhanced MRI with high spatiotemporal resolution. Journal of magnetic resonance imaging : JMRI. 2015;41(2):460–473. doi: 10.1002/jmri.24551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Pedoia V, Heilmeier U, Ku E, Su F, Khanna S, Imboden J, Graf J, Link T, Li X. High-temporospatial-resolution dynamic contrast-enhanced (DCE) wrist MRI with variable-density pseudo-random circular Cartesian undersampling (CIRCUS) acquisition: evaluation of perfusion in rheumatoid arthritis patients. NMR Biomed. 2016;29(1):15–23. doi: 10.1002/nbm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, Guo Y, Lingala SG, Lebel RM, Law M, Nayak KS. GOCART: GOlden-angle CArtesian randomized time-resolved 3D MRI. Magnetic resonance imaging. 2016;34(7):940–950. doi: 10.1016/j.mri.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magnetic resonance in medicine. 2014;72(3):707–717. doi: 10.1002/mrm.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE transactions on medical imaging. 2007;26(1):68–76. doi: 10.1109/TMI.2006.885337. [DOI] [PubMed] [Google Scholar]
- 9.Axel L, Summers RM, Kressel HY, Charles C. Respiratory effects in two-dimensional Fourier transform MR imaging. Radiology. 1986;160(3):795–801. doi: 10.1148/radiology.160.3.3737920. [DOI] [PubMed] [Google Scholar]
- 10.Feng L, Chandarana H, Zhao T, Bruno M, Sodickson DK, Otazo R. Golden-Angle Sparse Liver Imaging: Radial or Cartesian Sampling?. Proceedings of the 21st Annual Meeting of ISMRM; Honolulu, HI. 2017; p. 1285. [Google Scholar]
- 11.Xue Y, Yu J, Kang HS, Englander S, Rosen MA, Song HK. Automatic coil selection for streak artifact reduction in radial MRI. Magnetic resonance in medicine. 2012;67(2):470–476. doi: 10.1002/mrm.23023. [DOI] [PubMed] [Google Scholar]
- 12.Block KT, Uecker M, Frahm J. Undersampled radial MRI with multiple coils. Iterative image reconstruction using a total variation constraint. Magnetic resonance in medicine. 2007;57(6):1086–1098. doi: 10.1002/mrm.21236. [DOI] [PubMed] [Google Scholar]
- 13.Grimm G, Feng L, Forman C, Hutter J, Kiefer B, Hornegger J, Block K. Automatic Bolus Analysis for DCE-MRI Using Radial Golden-Angle Stack-of-stars GRE Imaging. Proceedings of the 21st Annual Meeting of ISMRM; Salt Lake City, Utah. 2013; p. 696. [Google Scholar]
- 14.Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magnetic resonance in medicine. 2016;75(2):775–788. doi: 10.1002/mrm.25665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimm R, Forman C, Hutter J, Kiefer B, Hornegger J, Block K. Fast Automatic Coil Selection for Radial Stack-Of-Stars GRE Imaging. Proceedings of the 21st Annual Meeting of ISMRM; Salt Lake City, Utah. 2013; p. 3786. [Google Scholar]
- 16.Holme HC, Frahm J. Sinogram-based coil selection for streak artifact reduction in undersampled radial real-time magnetic resonance imaging. Quantitative imaging in medicine and surgery. 2016;6(5):552–556. doi: 10.21037/qims.2016.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandarana H, Feng L, Ream J, Wang A, Babb JS, Block KT, Sodickson DK, Otazo R. Respiratory Motion-Resolved Compressed Sensing Reconstruction of Free-Breathing Radial Acquisition for Dynamic Liver Magnetic Resonance Imaging. Investigative radiology. 2015;50(11):749–756. doi: 10.1097/RLI.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Yao J, Thomasson D. Automatic determination of arterial input function for dynamic contrast enhanced MRI in tumor assessment. Med Image Comput Comput Assist Interv. 2008;11(Pt 1):594–601. doi: 10.1007/978-3-540-85988-8_71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang T, Cheng JY, Chen Y, Nishimura DG, Pauly JM, Vasanawala SS. Robust self-navigated body MRI using dense coil arrays. Magnetic resonance in medicine. 2016;76(1):197–205. doi: 10.1002/mrm.25858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seiberlich N, Breuer FA, Blaimer M, Barkauskas K, Jakob PM, Griswold MA. Non-Cartesian data reconstruction using GRAPPA operator gridding (GROG) Magnetic resonance in medicine. 2007;58(6):1257–1265. doi: 10.1002/mrm.21435. [DOI] [PubMed] [Google Scholar]
- 21.Seiberlich N, Breuer F, Blaimer M, Jakob P, Griswold M. Self-calibrating GRAPPA operator gridding for radial and spiral trajectories. Magnetic resonance in medicine. 2008;59(4):930–935. doi: 10.1002/mrm.21565. [DOI] [PubMed] [Google Scholar]
- 22.Tian Y, Erb KC, Adluru G, Likhite D, Pedgaonkar A, Blatt M, Kamesh Iyer S, Roberts J, DiBella E. Evaluation of Pre-reconstruction Interpolation Methods for Iterative Reconstruction of Radial k-Space Data. Med Phys. 2017 Aug;44(8):4025–4034. doi: 10.1002/mp.12357. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson KM, Block WF, Reeder SB, Samsonov A. Improved least squares MR image reconstruction using estimates of k-space data consistency. Magnetic resonance in medicine. 2012;67(6):1600–1608. doi: 10.1002/mrm.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Block KT, Chandarana H, Milla S, Bruno M, Mulholland T, Fatterpekar G, Hagiwara M, Grimm G, Geppert C, Kiefer B, Sodickson DK. Towards routine clinical use of radial stack-of-stars 3d gradient-echo sequences for reducing motion sensitivity. Journal of the Korean Society of Magnetic Resonance in Medicine. 18(2):87–106. [Google Scholar]
- 25.Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magnetic resonance in medicine. 2000;43(5):682–690. doi: 10.1002/(sici)1522-2594(200005)43:5<682::aid-mrm10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Feng L, Benkert T, Block KT, Sodickson DK, Otazo R, Chandarana H. Compressed sensing for body MRI. Journal of magnetic resonance imaging : JMRI. 2017;45(4):966–987. doi: 10.1002/jmri.25547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benkert T, Feng L, Bittman M, Ream J, Sodickson DK, Otazo R, Block KT, Chandarana H. Method of Choice to Increase the Motion-Robustness for Free-Breathing Applications: Self-Gating, Motion-Weighting, or Extra-Dimensional Reconstruction. Proceedings of the 21st Annual Meeting of ISMRM; Honolulu, HI. 2017; p. 3927. [Google Scholar]
- 28.Block KT, Grimm G, Feng L, Otazo R, Chandarana H, Bruno M, Geppert C, Sodickson DK. Bringing Compressed Sensing to Clinical Reality: Prototypic Setup for Evaluation in Routine Applications. Proceedings of the 21st Annual Meeting of ISMRM; Salt Lake City. 2013; p. 3809. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.