Summary
Background
Tuberculosis is a leading cause of global childhood mortality; however, interventions to detect undiagnosed tuberculosis in children are underused. Child contact tracing has been widely recommended but poorly implemented in resource-constrained settings. WHO has proposed a pragmatic screening approach for managing child contacts. We assessed the effectiveness of this screening approach and alternative symptom-based algorithms in identifying secondary tuberculosis in a prospectively followed cohort of Ugandan child contacts.
Methods
We identified index patients aged at least 18 years with microbiologically confirmed pulmonary tuberculosis at Old Mulago Hospital (Kampala, Uganda) between Oct 1, 1995, and Dec 31, 2008. Households of index patients were visited by fieldworkers within 2 weeks of diagnosis. Coprevalent and incident tuberculosis were assessed in household contacts through clinical, radiographical, and microbiological examinations for 2 years. Disease rates were compared among children younger than 16 years with and without symptoms included in the WHO pragmatic guideline (presence of haemoptysis, fever, chronic cough, weight loss, night sweats, and poor appetite). Symptoms could be of any duration, except cough (>21 days) and fever (>14 days). A modified WHO decision-tree designed to detect high-risk asymptomatic child contacts was also assessed, in which all asymptomatic contacts were classified as high risk (children younger than 3 years or immunocompromised [HIV-infected]) or low risk (aged 3 years or older and immunocompetent [HIV-negative]). We also assessed a more restrictive algorithm (ie, assessing only children with presence of chronic cough and one other tuberculosis-related symptom).
Findings
Of 1718 household child contacts, 126 (7%) had coprevalent tuberculosis and 24 (1%) developed incident tuberculosis, diagnosed over the 2-year study period. Of these 150 cases of tuberculosis, 95 (63%) were microbiologically confirmed with a positive sputum culture. Using the WHO approach, 364 (21%) of 1718 child contacts had at least one tuberculosis-related symptom and 85 (23%) were identified as having coprevalent tuberculosis, 67% of all coprevalent cases detected (diagnostic odds ratio 9.8, 95% CI 6.8–14.5; p<0.0001). 1354 (79%) of 1718 child contacts had no symptoms, of whom 41 (3%) had coprevalent tuberculosis. The WHO approach was effective in contacts younger than 5 years: 70 (33%) of 211 symptomatic contacts had coprevalent disease compared with 23 (6%) of 367 asymptomatic contacts (p<0.0001). This approach was also effective in contacts aged 5 years and older: 15 (10%) of 153 symptomatic contacts had coprevalent disease compared with 18 (2%) of 987 asymptomatic contacts (p<0.0001). More coprevalent disease was detected in child contacts recommended for screening when the study population was restricted by HIV-serostatus (11 [48%] of 23 symptomatic HIV-seropositive child contacts vs two [7%] of 31 asymptomatic HIV-seropositive child contacts) or to only culture-confirmed cases (47 [13%] culture confirmed cases of 364 symptomatic child contacts vs 29 [2%] culture confirmed cases of 1354 asymptomatic child contacts). In the modified algorithm, high-risk asymptomatic child contacts were at increased risk for coprevalent disease versus low-risk asymptomatic contacts (14 [6%] of 224 vs 27 [2%] of 1130; p=0.0021). The presence of tuberculosis infection did not predict incident disease in either symptomatic or asymptomatic child contacts: in symptomatic contacts, eight (5%) of 169 infected contacts and six (5%) of 111 uninfected contacts developed incident tuberculosis (p=0.80). Among asymptomatic contacts, incident tuberculosis occurred in six (<1%) of 795 contacts infected at baseline versus four (<1%) of 518 contacts uninfected at baseline, respectively (p=1.00).
Interpretation
WHO’s pragmatic, symptom-based algorithm was an effective case-finding tool, especially in children younger than 5 years. A modified decision-tree identified 6% of asymptomatic child contacts at high risk for subclinical disease. Increasing the feasibility of child-contact tracing using these approaches should be encouraged to decrease tuberculosis-related paediatric mortality in high-burden settings, but this should be partnered with increasing access to microbiological point-of-care testing.
Funding
National Institutes of Health, Tuberculosis Research Unit, AIDS International Training and Research Program of the Fogarty International Center, and the Center for AIDS Research.
Introduction
Tuberculosis is a leading cause of global childhood mortality;1 however, screening interventions to detect undiagnosed tuberculosis in children are underused.2–4 This is especially clear in high-burden settings where scarce health resources have largely concentrated on diagnosing adults with smear-positive tuberculosis.5,6 As a consequence, case detection of tuberculosis in children is poor in these settings, with some reports estimating that two-thirds of paediatric cases are unreported and undiagnosed globally.7 These detection rates are especially concerning because more than 20% of untreated children with tuberculosis die.8 If treated, however, these children are very likely to survive,8,9 suggesting that improving case detection and treatment might decrease childhood mortality.
Household tracing of child contacts has been widely recommended by global health organisations and disease experts for more than a decade10–12 because child contacts have a high yield of both Mycobacterium tuberculosis infection and disease.13–16 Despite this, contact tracing has been implemented poorly or not at all in high-burden, low-income settings.11,17 For example, fewer than 20% of contacts of tuberculosis patients diagnosed in Ethiopia in 2013 were programmatically screened for disease.18 As a consequence, a large proportion (often more than 90%19) of child contacts eligible for preventive therapy remain unidentified by tuberculosis-control services.20 In many low-incidence settings, provision of preventive therapy is considered standard of care and is broadly implemented. In high-incidence settings, programmatic implementation of tracing household-exposed children has been difficult because of resource intensiveness and poor access to diagnostic tests, including radiographical imaging, microbiological examinations, and tuberculin skin testing.3,17,21,22 Alternative screening approaches to detect childhood tuberculosis that can be implemented in high-burden settings are needed to increase case detection and prevent progression to disease.
In an attempt to make implementation of child contact tracing more feasible, WHO published pragmatic symptom-based guidelines for the management of child contacts of individuals with tuberculosis in high-burden settings in 200610 and updated these in 2014.23,24 These guidelines state that child contacts of individuals with tuberculosis should be screened for tuberculosis using a set of tuberculosis-specific symptoms chosen to discriminate disease from infection. On the basis of the presence of symptoms, children could be treated for disease if diagnosed or offered preventive therapy for presumed tuberculosis infection, especially in very young or immunocompromised children. This symptom-based approach might be a feasible and efficient programmatic avenue for National Tuberculosis Programs with scarce resources or without access to diagnostic testing to identify high-risk child contacts. Other research groups have suggested modifications to the WHO guidelines4 or have developed and assessed alternative symptom-based algorithms25,26 in an attempt to improve the diagnostic performance of contact screening at the programmatic level. There is a shortage of large, prospective validations of these recommendations,24 and the role of HIV infection has not been investigated. Whether these algorithms are useful in sub-Saharan Africa, where HIV is common and paediatric tuberculosis represents a large proportion of all cases,7,27,28 remains uncertain.
To fill this knowledge gap, we estimated the concordance with the WHO guidelines for secondary tuberculosis in a large, prospectively followed Ugandan cohort of child household contacts exposed to tuberculosis. Uganda is one of 22 high-tuberculosis-burden countries globally, and the community prevalence of tuberculosis around Kampala has been reported to be as high as 1%.29 We also assessed two proposed alternative screening recommendations, including a more expansive, modified WHO decision-tree analysis4 and a more restrictive algorithm assessing only children with chronic cough and one other tuberculosis-related symptom.
Methods
Study design and participants
The study design has been described previously.15,30,31 Briefly, we identified patients with newly diagnosed pulmonary tuberculosis aged at least 18 years at Old Mulago Hospital (Kampala, Uganda) from Oct 1, 1995, to Dec 31, 2008. Index cases were microbiologically confirmed based on growth of M tuberculosis in culture, with at least one household contact and an assessment through a physical examination and medical history.
Households of index tuberculosis cases were visited by field workers within 2 weeks of diagnosis of the index case. A household was defined as a group of people living within one residence who shared meals together and identified a head of family who made decisions for the household. Contacts were defined as any individual who had spent at least 7 consecutive days in the same household as the index case in the 3 months preceding diagnosis. House hold members completed a baseline sociodemographic questionnaire and a physical examination to collect data on age, sex, relationship to the index case, education level, past tuberculosis disease, and household characteristics. Previous BCG vaccination was assessed through inspecting deltoid scars and then confirmed with medical records when available.
Index cases and contacts were offered HIV testing with an ELISA (Cambridge BiosScience, Worcester, MA, USA). This assay was done after informed consent from parents or guardians of the index cases and contacts and pre-test and post-test counselling. Tuberculosis infection in contacts was identified with tuberculin skin testing, done by placing 0.1 mL of 5 tuberculin units of purified protein derivative (Tubersol, Connaught Laboratories, ON, Canada) on the volar surface of the left forearm using the Mantoux method. Two field workers independently read the transverse diameter of palpable induration within 48–72 h using digital callipers to reduce digit preference. Children with a negative tuberculin skin test were retested within 3 months; those testing positive on the second test were considered to be infected at baseline.
The study protocol was reviewed and approved by the National HIV/AIDS Research Committee of Makerere University (Kampala, Uganda) and the institutional review board at University Hospitals Cleveland Medical Center (Cleveland, OH, USA). Informed consent was obtained for all index cases and household contacts. Parents or guardians of child contacts provided written consent in addition to verbal assent from the children.
Outcomes
The two main outcomes were coprevalent and incident tuberculosis, assessed in household contacts through clinical, radiographical, and microbiological examinations over a period of 2 years. Disease rates were compared among children younger than 16 years.
The diagnosis of childhood tuberculosis in this study has been described elsewhere15,31 and further details are in the appendix. Briefly, all household contacts were assessed for tuberculosis through a medical examination and anteroposterior chest radiographs were examined independently by two experienced pulmonary physicians. Specimen microscopy and mycobacterial culture were obtained if the child was younger than 6 years, symptomatic, had suggestive chest radiograph findings, clinical suspicion of tuberculosis, or was HIV-seropositive. If a sputum sample could not be collected, alternative sites (including gastric aspiration, nasopharyngeal aspiration, pleural fluid, cerebrospinal fluid, or lymph node aspiration, as clinically indicated) were tested. Culture was done (Lowenstein Jensen slants, BACTEC) in triplicate at the baseline assessment, at sick visits (ie, if the participant felt ill or had a health issue such as a rash and arrived at the clinic requesting an examination), and regular follow-up visits.
Classification of pulmonary tuberculosis was done using international consensus guidelines.32 Confirmed tuberculosis was defined as bacteriological confirmation from at least one specimen at respiratory and non-respiratory sites. Unconfirmed tuberculosis was defined as a clinical illness consistent with tuberculosis based on at least two of the following: results of chest radiography consistent with pulmonary tuberculosis, chest radiograph consistent with tuberculosis, or a response to anti-tuberculosis therapy. Unlikely tuberculosis was defined as children in whom bacteriological confirmation was not obtained and criteria for unconfirmed tuberculosis were not met. Coprevalent disease was defined as confirmed or unconfirmed tuberculosis identified at baseline or within 3 months; incident tuberculosis was defined as confirmed or unconfirmed tuberculosis that occurred among contacts classified as without coprevalent disease.33 9-month isoniazid prophylaxis was offered to disease-free child contacts if they were younger than 5 years, HIV-infected, or had a positive tuberculin skin test at enrolment. Adherence and completion of preventive therapy was monitored for clinical purposes only but not for the study. The cohort was followed at 6-month intervals for 2 years.
WHO guidelines23 state that child contacts with tuberculosis-related symptoms, including chronic cough, fever, night sweats, haemoptysis, weight loss, and loss of appetite (appendix), should be screened for tuberculosis and, if diagnosed, treated. The guidelines also recommend that children without disease but younger than 5 years or who are immunocompromised should be offered isoniazid preventive therapy.23 Chronic cough was defined as a continuous, non-remitting cough present for more than 3 weeks. Fever was defined as a body temperature of more than 38°C for 14 days, after exclusion of common causes such as malaria or pneumonia. Weight loss was defined as reporting of weight loss or failure to thrive with confirmatory evidence from the child’s growth chart. Haemoptysis was defined as the expectoration of blood. Night sweats and loss of appetite were self-reported from children and parents. Asymptomatic contacts included children with cough duration of less than 3 weeks or fever duration of less than 2 weeks.
Statistical analysis
Contacts were included in the analysis if they were younger than 16 years. Coprevalent and incident tuberculosis was estimated among all child contacts and then separately for each included characteristic in the WHO algorithm using standard 2 × 2 contingency tables. The number of characteristics present in each child was calculated and disease outcomes were computed for contacts with each number of symptoms. We assessed the number needed to screen (NNS) to detect one coprevalent case and the proportion of the total number of diseased cases detected by screening.
To assess the effectiveness of the algorithms in different subgroups, we stratified our cohort in several ways. First, child contacts were grouped by age (0–4 and 5–15 years) and algorithm effectiveness was assessed in each age group separately. Second, to assess whether the symptom-based algorithm would effectively detect secondary disease in HIV-endemic and non-endemic settings, we assessed coprevalent and incident tuberculosis in HIV-seropositive and HIV-seronegative child contacts separately. We stratified the results by HIV-serostatus and compared the yield of coprevalent and incident disease. Last, we did a secondary analysis using only culture-confirmed child cases instead of the more comprehensive diagnostic definition used in the primary analysis.
We used two-sided p values and 95% CIs to assess statistical significance in all analyses. When comparing the different approaches, we used either Pearson χ2 or McNemar tests for the difference between two proportions. Cochran-Armitage tests were used to test for trends between disease outcomes and the presence of multiple symptoms. Diagnostic odds ratios (ORs) for coprevalent tuberculosis were calculated for each symptom individually, the presence of any symptom, and other relevant characteristics. Because ruling out active disease, thus avoiding inappropriately administering preventive therapy, is an important screening criterion, we also reported the negative predictive value for each outcome using the algorithm. As a secondary analysis, we assessed other symptom-based screening algorithms. A modified WHO decision-tree4 suggests screening all symptomatic contacts regardless of age or immuno competency; asymptomatic contacts should be grouped as either high risk (age <3 years or immunocompromised) or low risk (aged ≥3 years and immunocompetent) children. We also assessed the effectiveness of a more restrictive, symptom-based approach (chronic cough with another tuberculosis-related symptom). For each approach, we calculated rates of coprevalent and incident tuberculosis in child contacts and the proportion of the total number of disease cases detected.
In the WHO guidelines, HIV testing and counselling is recommended among contacts with an HIV source case (recommendation 24).23 Because this recommendation is based on “very low-quality evidence”, we compared the yield of HIV-testing in child contacts stratified by the HIV-serostatus of the index case and the age of the contact.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
3048 household contacts were enrolled in the study (figure 1), of whom 1718 were aged less than 16 years and included in this analysis (further reference to contacts in the manuscript refers to these child contacts). Most contacts had a positive tuberculin skin test using cutoffs of 10 mm or more (1056 [62%]) or 5 mm or more (1216 [71%]) and were BCG vaccinated (1325 [77%] of 1718, table 1, table 2).
Figure 1. Study profile of symptom-based approaches assessed for child contact investigation, Kampala, Uganda*.
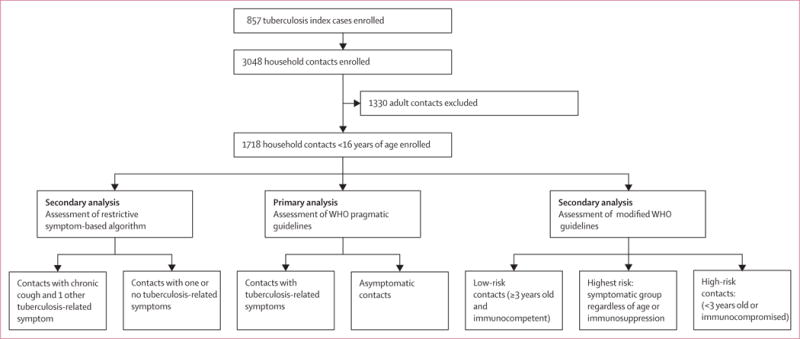
*Symptomatic contacts included contacts with any tuberculosis-related symptoms, including chronic cough, fever, night sweats, haemoptysis, weight loss, and loss of appetite. Chronic cough was defined as a continuous, non-remitting cough present for >3 weeks. Fever was defined as body temperature of >38°C for 14 days, after exclusion of common causes such as malaria or pneumonia. Weight loss was defined as reporting of weight loss or failure to thrive with confirmatory evidence from the child’s growth chart. Haemoptysis was defined as the expectoration of blood from the lung airways or parenchyma. Night sweats and loss of appetite were self-reported by children and parents. Asymptomatic contacts included children with cough of <3 weeks’ duration or fever of <2 weeks’ duration, or both.
Table 1.
Demographic characteristics of household child contacts of individuals with tuberculosis
| Household child contacts of individuals with tuberculosis (n=1718) | |
|---|---|
| Household contact characteristics | |
|
| |
| Age (years) | 7 (3–11) |
| Age group (years) | |
| 0–4 | 578 (34%) |
| 5–15 | 1140 (66%) |
| Sex | |
| Male | 582 (34%) |
| Female | 1136 (66%) |
| BCG vaccinated* | 1325 (77%) |
| Exposure to another individual with tuberculosis† | 161 (9%) |
| Relation to index case | |
| Child | 1028 (60%) |
| Sibling | 104 (6%) |
| Other‡ | 571 (33%) |
| Missing information | 15 (<1%) |
| Past tuberculosis | 11 (<1%) |
| Closeness to index case | |
| Share bed | 192 (11%) |
| Share room, not bed | 896 (52%) |
| Different room | 610 (36%) |
| Missing information | 20 (1%) |
|
| |
| Index case characteristics§ | |
|
| |
| Age (years) | 30 (25–37) |
| Age group (years) | |
| 18–29 | 789 (46%) |
| 30–39 | 589 (34%) |
| 40–49 | 238 (14%) |
| ≥50 | 75 (4%) |
| Missing information | 27 (2%) |
| Sex | |
| Male | 795 (47%) |
| Female | 923 (53%) |
| Cigarette smoker | 302 (18%) |
| Cavitary disease¶ | 992 (58%) |
| Cough duration (days) | 90 (45–120) |
| HIV-seropositive | 754 (44%) |
|
| |
| Household characteristics | |
|
| |
| Housing type | |
| Multifamily household | 834 (49%) |
| Single family household | 877 (51%) |
| Missing data | 7 (<1%) |
| Charcoal or fire smoke exposure | |
| Inside household | 372 (22%) |
| Outside household | 1262 (74%) |
| None | 57 (3%) |
| Missing data | 27 (2%) |
| Ventilation (≤1 window per room)‖ | 1532 (89%) |
| Density, individuals per home (IQR) | 6 (4–8) |
| Household size (individuals per home) | |
| 1–5 | 717 (42%) |
| 6–10 | 818 (48%) |
| >10 | 183 (11%) |
Data are median (IQR) or n (%). Percentages refer to within-characteristic column totals among household contacts of index patients with tuberculosis. Percentages might not total 100% because within-column percentages were rounded to the nearest integer.
Assessed through BCG scar and verified by medical records when available.
Refers to exposure to another individual with tuberculosis other than the index case in the previous 12 months before the baseline visit.
Includes other relatives, such as grandparents, grandchildren, aunts, uncles, and cousins. Also includes non-relatives living in the household.
Number and percentage of household contacts exposed to the index case characteristic in the left-hand column.
Radiographic imaging results were graded by an experienced clinician using the 1961 National Tuberculosis Association classification system.34
Windows must be to the outside.
Table 2.
Yield of coprevalent tuberculosis in 1718 household child contacts of individuals with tuberculosis, stratified by WHO’s health screening guideline for the management of child contacts
| Total* | Coprevalent tuberculosis†
|
||||
|---|---|---|---|---|---|
| Diseased contacts (%) | NNS‡ | Percentage of all paediatric tuberculosis cases in child contacts | Diagnostic odds ratio (95% CI) | ||
| All child contacts | 1718 | 126 (7%) | 14 | 100% | NA |
|
| |||||
| Tuberculosis-related symptoms§ | |||||
| Poor appetite | 52 | 13 (25%) | 4 | 10% | 4.7 (2.4–9.0) |
| Chronic cough | 254 | 60 (24%) | 4 | 48% | 6.6 (4.5–9.7) |
| Weight loss | 105 | 35 (33%) | 3 | 28% | 8.5 (5.3–13.4) |
| Fever | 114 | 35 (31%) | 3 | 28% | 7.4 (4.7–11.6) |
| Night sweats | 52 | 12 (23%) | 4 | 10% | 4.1 (2.1–8.0) |
| Haemoptysis | 7 | 1 (17%) | 6 | <1% | 2.9 (0.3–24.7) |
|
| |||||
| Children with ≥1 tuberculosis-related symptom§ | 364 | 85 (23%) | 4 | 67% | 9.8 (6.8–14.5) |
|
| |||||
| Number of tuberculosis-related symptoms§ | |||||
| 0 | 1354 | 41 (3%) | 34 | 33% | 1 (reference) |
| 1 | 225 | 43 (19%) | 5 | 34% | 7.6 (4.8–11.9) |
| 2 | 80 | 22 (28%) | 4 | 18% | 12.1 (6.8–21.7) |
| 3 | 40 | 13 (33%) | 3 | 10% | 15.4 (7.4–32.0) |
| 4 | 17 | 5 (29%) | 3 | 4% | 13.3 (7.4–32.0) |
| 5 | 2 | 2 (100%) | 1 | 4% | NA |
|
| |||||
| Children with any symptom other than chronic cough or fever for >2 weeks | 161 | 42 (26%) | 4 | 33% | 6.2 (4.1–9.4) |
|
| |||||
| Tuberculin skin test ≥5 mm | 1216 | 105 (9%) | 12 | 83% | 2.1 (1.3–3.4) |
|
| |||||
| Tuberculin skin test ≥10 mm | 1056 | 92 (9%) | 12 | 73% | 1.7 (1.2–2.6) |
|
| |||||
| HIV-seropositive | 54 | 13 (24%) | 4 | 10% | 3.7 (1.9–7.1) |
Data are n (%) or n (95% CI). NNS=number needed to screen. NA=not applicable.
Participants with missing values for characteristics included in the WHO guideline were assumed to not have the characteristic in our main analysis.
Coprevalent tuberculosis was defined as the identification of tuberculosis at or within 3 months of the baseline household visit.
Number of child contacts in the specified row that was needed to screen to detect one coprevalent tuberculosis case. This was calculated by dividing 100 by the row-specific disease prevalence–eg, 7% of all child contacts had coprevalent tuberculosis. Therefore, 14 contacts needed to be screened to detect one coprevalent tuberculosis case in the total child contact cohort (n=1718). Numbers have been rounded up.
Participants were included in the pragmatic guideline if they had one tuberculosis-related symptom (chronic cough, fever, night sweats, haemoptysis, weight loss, or loss of appetite).
After baseline clinical examinations and microbiological testing, 126 contacts (7%) were diagnosed with coprevalent tuberculosis (table 2, figure 2), and after a 2-year follow-up, 24 children (1%) were diagnosed with incident tuberculosis (figure 2). One child was diagnosed with extrapulmonary tuberculosis. Among incident cases, the date of diagnosis was available for 17 incident cases; ten (59%) were diagnosed in the first year of follow-up and seven (41%) after 1 year. Of these 150 cases of coprevalent and incident tuberculosis, 95 (63%) were microbiologically confirmed with a positive sputum culture. Among 1592 child contacts without baseline tuberculosis, information about preventive therapy was known in 1060 (67%), of whom 563 were given preventive therapy with isoniazid. Of those given therapy, 554 (98%) remained free from tuberculosis over follow-up. Preventive therapy was given to around 60% of contacts younger than 5 years and around 52% of HIV-seropositive children (there are substantial missing values for these numbers).
Figure 2. Tuberculosis-related outcomes* stratified by WHO’s symptom-based approach for the management of child contacts†.
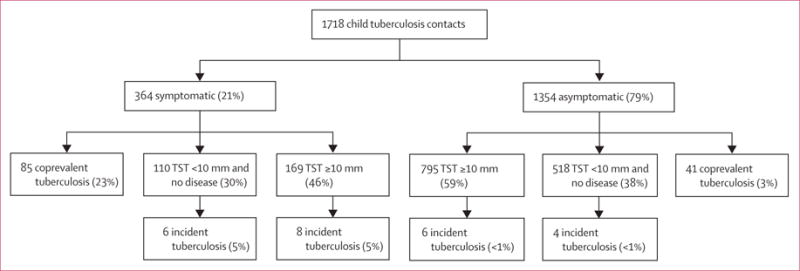
TST=tuberculin skin test. *Individuals with coprevalent disease were excluded from analyses of incident disease. In this analysis, tuberculosis infection was defined as a tuberculin skin-test induration response of ≥10 mm. Percentages might not total 100% because within-characteristic percentages were rounded to the nearest integer. †Participants were included in the pragmatic guideline if they had one tuberculosis-related symptom including chronic cough, fever, night sweats, haemoptysis, weight loss, and loss of appetite. Chronic cough was defined as a continuous, non-remitting cough present for >3 weeks. Fever was defined as body temperature of >38°C for 14 days, after exclusion of common causes such as malaria or pneumonia. Weight loss was defined as reporting of weight loss or failure to thrive with confirmatory evidence from the child’s growth chart. Haemoptysis was defined as the expectoration of blood from the lung airways or parenchyma. Night sweats and loss of appetite were self-reported by children and parents. Asymptomatic contacts included children with cough of <3 weeks’ duration or fever of <2 weeks’ duration, or both. The difference between coprevalent tuberculosis among symptomatic and asymptomatic contacts was statistically different (23% vs 3%, p<0.0001). Contacts with tuberculosis infection (tuberculin skin-test induration ≥10 mm) did not result in increased risk for incident tuberculosis (among symptomatic contacts, 5% vs 5% incident tuberculosis rates among contacts infected and uninfected at baseline, p=0.80; among asymptomatic contacts, <1% vs <1% incident tuberculosis rates among contacts infected and uninfected at baseline, p=1.0)
364 children (21% of 1718 child contacts) had at least one tuberculosis-related symptom (table 2, appendix). The most common symptom was chronic cough. Of 1414 child contacts (82% of the cohort) tested for HIV, 54 (4%) tested positive. The clinical characteristics of children with tuberculosis were similar regardless of whether they were identified by the WHO guideline or not, except that children recommended for screening were younger (p=0.005) and more likely to have an HIV-seropositive mother (p=0.015; appendix).
The proportion of child contacts with coprevalent tuberculosis ranged from 17% (one of seven) with haemoptysis to 33% (35 of 105) with weight loss (table 2). Of 364 contacts with at least one tuberculosis-related symptom, 85 (23%) had coprevalent pulmonary tuberculosis (OR 9.8, 95% CI 6.8–14.5; table 2, figure 2). In the overall sample, the NNS to detect one coprevalent case was 14 children. Among child contacts recommended for screening using the WHO algorithm, the NNS to detect one case was four children, whereas among contacts not recommended for screening (ie, those who did not have any tuberculosis-related symptoms), the NNS was 34 (p<0.0001).
Using the WHO approach, 21% of child contacts would be screened and 67% of coprevalent cases would be detected. Among 1354 children without symptoms, coprevalent tuberculosis was detected in 41 (3%; p<0.0001). The negative predictive value to detect coprevalent tuberculosis was 97% (95% CI 96.2–97.7; appendix). The WHO approach was effective in identifying coprevalent tuberculosis in contacts younger than 5 years old (33% vs 6%, p<0.0001 for symptomatic vs asymptomatic contacts; figure 3). However, the differences in coprevalent disease were also present among symptomatic versus asymptomatic children aged 5 years and older (10% vs 2%, p<0.0001), suggesting that the WHO approach was also effective in this age group.
Figure 3. Tuberculosis-related outcomes* in children screened following the WHO symptom-based approach for the management of child contacts, stratified by the age of the child†.
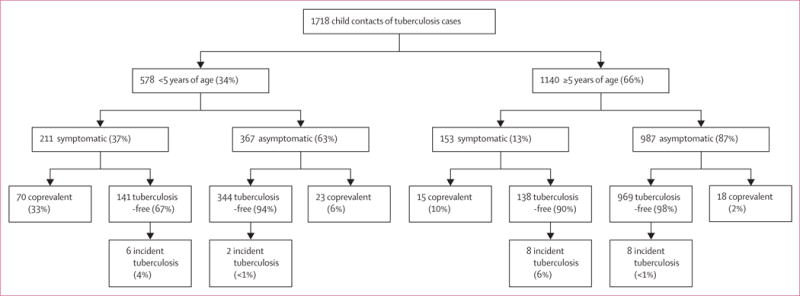
*Individuals with coprevalent disease were excluded from analyses of incident disease. In this figure, tuberculosis-free indicates contacts free from tuberculosis, but these contacts might have had tuberculosis infection. Percentages might not total 100% because within-characteristic percentages were rounded to the nearest integer. †Participants were included in the pragmatic guideline if they had one tuberculosis-related symptom including chronic cough, fever, night sweats, haemoptysis, weight loss, and loss of appetite. Chronic cough was defined as a continuous, non-remitting cough present for >3 weeks. Fever was defined as body temperature of >38°C for 14 days, after exclusion of common causes such as malaria or pneumonia. Weight loss was defined as reporting of weight loss or failure to thrive with confirmatory evidence from the child’s growth chart. Haemoptysis was defined as the expectoration of blood from the lung airways or parenchyma. Night sweats and loss of appetite were self-reported by children and parents. Asymptomatic contacts included children with cough of <3 weeks’ duration or fever of <2 weeks’ duration, or both. The difference between coprevalent tuberculosis among symptomatic and asymptomatic contacts was statistically different for children younger than 5 years (33% vs 6%, p<0.0001) and aged 5 years or older (10% vs 2%, p<0.0001).
The WHO approach successfully identified disease outcomes in both HIV-seropositive and HIV-seronegative child contacts (figure 4; symptomatic vs asymptomatic p<0.0001). When we restricted our diagnostic definition of tuberculosis to only culture-confirmed disease (appendix), 95 children were diagnosed with both coprevalent and incident tuberculosis. For coprevalent disease, 47 (13%) with tuberculosis-related symptoms had confirmed disease compared with 29 (2%) without symptoms (p<0.0001).
Figure 4. Tuberculosis-related outcomes* in children screened following the WHO symptom-based approach for the management of child contacts, stratified by the HIV-serostatus of the child†.
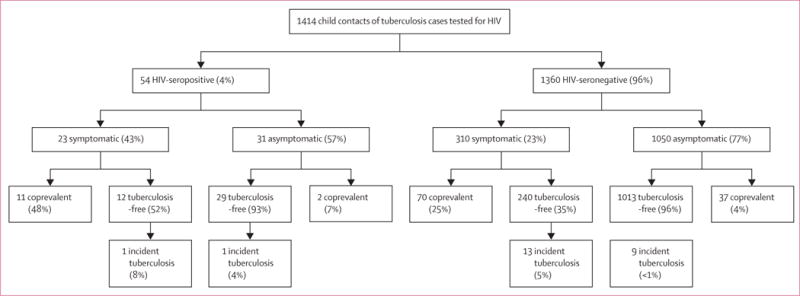
*Individuals with coprevalent disease were excluded from analyses of incident disease. In this figure, tuberculosis-free indicates contacts free of tuberculosis, but these contacts might have had tuberculosis infection. Percentages might not total 100% because within-characteristic percentages were rounded to the nearest integer. †In this flowchart, infants with tuberculosis-related symptoms (including chronic cough, fever, night sweats, haemoptysis, weight loss, and loss of appetite,) were included to be screened in the algorithm. Chronic cough was defined as a continuous, non-remitting cough present for >3 weeks. Fever was defined as body temperature of >38°C for 14 days, after exclusion of common causes such as malaria or pneumonia. Weight loss was defined as reporting of weight loss or failure to thrive with confirmatory evidence from the child’s growth chart. Haemoptysis was defined as the expectoration of blood from the lung airways or parenchyma. Night sweats and loss of appetite were self-reported by children and parents. Asymptomatic contacts included children with cough of <3 weeks’ duration and/or fever of <2 weeks’ duration. This analysis included only 1414 child contacts who took an HIV test. The difference between coprevalent tuberculosis among symptomatic and asymptomatic contacts was statistically different for HIV-seropositive children (48% vs 7%, p<0.0001) and HIV-seronegative children (23% vs 4%, p<0.0001).
In 511 children younger than 5 years or HIV-seropositive without baseline tuberculosis, nine (2%) developed tuberculosis over follow-up (figure 5). Incident tuberculosis occurred in two (5%) of 41 HIV-seropositive contacts (one of 12 symptomatic contacts and one of 29 asymptomatic contacts, figure 4). Among disease-free child contacts with tuberculosis-related symptoms, 14 (5%) of 279 contacts developed incident tuberculosis over follow-up. Among children without symptoms, ten (<1%) of 1324 developed incident tuberculosis. Tuberculosis infection slightly increased the risk of development of incident disease in our study population (figure 2). In symptomatic contacts, eight (5%) of 169 infected contacts developed incident tuberculosis and six (5%) of 111 uninfected contacts developed disease (p=0.80). Among asymptomatic contacts, incident tuberculosis occurred in six (<1%) of 795 contacts infected at baseline versus four (<1%) of 518 contacts uninfected at baseline, respectively (p=1.00). When using only microbiological confirmation for paediatric diagnosis, 12 children would be classified as developing disease during follow-up. Seven of these children were symptomatic while five were asymptomatic (appendix).
Figure 5. Tuberculosis-related outcomes* in children screened following a modified WHO decision-tree† classifying asymptomatic child contacts into high-risk and low-risk groups‡.
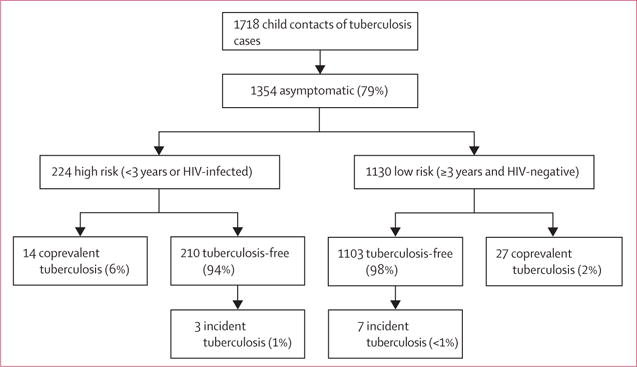
*Individuals with coprevalent disease were excluded from analyses of incident disease. In this figure, tuberculosis-free indicates contacts free of tuberculosis, but these contacts might have had tuberculosis infection. Percentages might not total 100% because within-characteristic percentages were rounded to the nearest integer. †This algorithm was first proposed by Marais and colleagues.4 ‡Children with tuberculosis-related symptoms are shown in figure 2 and included children with either chronic cough, fever, night sweats, haemoptysis, weight loss, or loss of appetite. Chronic cough was defined as a continuous, non-remitting cough present for >3 weeks. Fever was defined as body temperature of >38°C for 14 days, after exclusion of common causes such as malaria or pneumonia. Weight loss was defined as reporting of weight loss or failure to thrive with confirmatory evidence from the child’s growth chart. Haemoptysis was defined as the expectoration of blood from the lung airways or parenchyma. Night sweats and loss of appetite were self-reported by children and parents. Asymptomatic contacts included children with cough of <3 weeks’ duration or fever of <2 weeks’ duration, or both. The modified decision-tree, which proposes to group asymptomatic contacts into high-risk and low-risk groups, symptomatic contacts were at highest-risk for disease (23% and 5% coprevalent and incident disease; p<0.05 vs both other groupings). High-risk asymptomatic contacts had higher rates of coprevalent disease compared with low-risk asymptomatic contacts (6% versus 2%, p<0.01) and similar rates of incident disease (1% vs <1%, p=0.23). If both symptomatic and high-risk asymptomatic contacts were screened and followed up, 79% and 71% of all coprevalent and incident cases, respectively, would be assessed.
Using a modified WHO algorithm that classified asymptomatic contacts into high-risk and low-risk groups (figure 5), symptomatic contacts were deemed at highest-risk for disease (23% [NNS four] and 5% [NNS 20] for coprevalent and incident disease; p<0.0001 for coprevalent comparisons and 0.045 for incident comparisons). High-risk asymptomatic contacts had higher rates of coprevalent disease compared with low-risk asymptomatic contacts (6% vs 2%, p=0.0021) but similar rates of incident disease (pexact=0.23). If both symptomatic and high-risk asymptomatic contacts were screened and followed up, 79% and 71% of all coprevalent and incident cases, respectively, would be assessed (appendix).
When using a more restrictive symptom algorithm, only 108 child contacts would have been screened compared with the 394 from the main analysis or 588 for the modified WHO algorithm that includes both symptomatic and high-risk asymptomatic children. Compared with the WHO approach, the restrictive algorithm would detect a higher disease yield (32% [NNS three] vs 23% [NNS four], p<0.01) but substantially fewer numbers of the total coprevalent disease burden (27% versus 68%, p<0.0001). Similar results were seen for incident disease (10% vs 5% disease yield, p=0.13; and 29% vs 63% of the total number of cases, p<0.0001).
HIV infection was noted almost exclusively in child contacts of HIV-seropositive tuberculosis cases (47 [89%] of all 53 HIV-seropositive contacts who were tested for HIV; 47 [8%] of 624 child contacts of HIV-seropositive cases vs six [<1%] of 756 child contacts of HIV-seronegative cases; appendix). This relationship was magnified in contacts younger than 10 years (22 [10%] of 211 vs one [<1%] of 315 for age <5 years, p<0.0001; 19 [10%] of 198 vs three [1%] of 230 for age 5–9 years, p<0.0001). Contacts aged 10 years or older with an HIV-seropositive index case were not at a statistically increased risk of HIV-infection (six [3%] of 215 vs two [1%] of 211, p=0.29).
Discussion
Paediatric deaths from tuberculosis occur largely because of an absence of validated and implementable health interventions to identify undiagnosed children in high-burden settings.1 In this large prospective cohort study including 1718 Ugandan child contacts of tuberculosis cases, we found that screening child contacts using a symptom-based approach recommended by WHO detected around 70% of cases of coprevalent tuberculosis while screening 22% of child contacts. The yield of coprevalent tuberculosis in children identified by the WHO approach was higher in all subgroups, including HIV-seropositive and HIV-seronegative children as well as very young children and adolescents. Incident tuberculosis was higher in child contacts identified by the symptom-based approach and when stratified by several important subgroups. Last, we assessed a modified algorithm that attempted to identify high-risk asymptomatic contacts and showed that more than 6% of this group presented with coprevalent disease in our study population. If symptomatic and high-risk symptomatic child contacts were screened in our study, approximately 80% of coprevalent tuberculosis would be assessed.
Few previous studies have assessed WHO guidelines for household contact tracing of children.24,35 Our study extended findings from earlier studies in several ways. First, symptomatic contacts had higher rates of coprevalent tuberculosis in both HIV-seropositive and HIV-seronegative contacts than asymptomatic contacts with and without HIV, suggesting the usefulness of the WHO symptom-based approach in settings with varying burdens of HIV. Second, in our study the WHO algorithm was effective among children with microbiologically confirmed disease. A larger proportion (>60%) of child tuberculosis cases in our study were confirmed microbiologically through sputum culture than other paediatric tuberculosis studies.36 This was the result of a systematic, protocol-driven approach that collected culture samples at multiple sites and timepoints, collected culture in triplicate, and used inclusive criteria for microbiological testing. In previous validation studies, and in most studies investigating childhood tuberculosis, tuberculosis was exclusively diagnosed either clinically or radiographically, both of which have limited specificity. Other than young age, there were no significant differences between diagnosed paediatric tuberculosis cases with and without tuberculosis-related symptoms in our study, suggesting that this algorithm might detect tuberculosis in very young children who are notoriously difficult to diagnose microbiologically.
Previous studies assessing the WHO guidelines diagnosed only a few incident cases24 or had a cross-sectional study design.35 We diagnosed a total of 24 incident cases after 2 years of follow-up. Our study was not designed to estimate the efficacy of isoniazid preventive therapy so there might have been confounding by indication, where contacts who were offered and accepted preventive therapy were at highest risk for primary progressive disease. This confounding might have underestimated incident tuberculosis among symptomatic children in our main analysis and among high-risk asymptomatic child contacts in the modified WHO decision-tree analysis. Because of ethical reasons, offering and providing preventive therapy in this hyperendemic setting was necessary.
The WHO guidelines are pragmatic in nature and therefore imperfect. Other symptom-based diagnostic algorithms have also been proposed for childhood tuberculosis to optimise contact tracing. To prioritise the identification of asymptomatic child contacts at high risk for secondary tuberculosis disease, Marais and colleagues4 proposed a modification to the WHO guidelines. With this modified decision-tree, we showed that 6% of high-risk asymptomatic children had coprevalent disease and that if both symptomatic and high-risk asymptomatic children were screened, almost eight of ten secondary cases would be assessed. For national and subnational tuberculosis programmes with sufficient resources, this approach might be beneficial and detect high-risk children with low chances of diagnosis through current screening measures. A more restrictive symptom-based algorithm was also assessed. Unsurprisingly, this algorithm was more effective in detecting coprevalent and incident disease as it restricts screening to only the highest-risk contacts. However, when using this approach, our results suggested a sizeable proportion of child contacts would be left undiagnosed. There is no one-size-fits-all approach for childhood case detection because national tuberculosis programmes have distinct health resources and childhood tuberculosis epidemics. We present results from all proposed algorithms of which we were aware and suggest that local disease dynamics need to be assessed alongside these results.37
Alarmingly, in contacts younger than 10 years of HIV-seropositive index cases, more than 10% were themselves HIV-seropositive. This association with young age is likely due to mother-to-child HIV transmission. This result highlights the need for integrated tuberculosis and HIV management, HIV testing among high-risk children and pregnant women, and antiretroviral therapy in pregnant women.
In 2014, there were an estimated 7.5 million household child contacts of diagnosed tuberculosis cases globally38 representing a large pool of children at high risk for progressive disease (incident tuberculosis) and death.13,39 These results suggest that the WHO’s symptom-based approach,10,23 in combination with a modified decision-tree,4 might be useful in resource-constrained settings to improve implementation of contact tracing. Symptoms can be assessed without specialised clinicians, little information is needed to inform if further assessment or treatment are needed, and most information can be validly self-reported by parents or guardians. Cost-effectiveness analyses could also be useful to further inform policy;40 however, in our study population, very few contacts needed to be screened to detect a single case under the proposed approach, which suggests high-resource efficiency.
Although these algorithms might be useful now in addressing the problem of paediatric tuberculosis in resource-constrained settings, their performance characteristics can and should be improved. Symptomatology algorithms often have to trade off between sensitivity and specificity. As symptoms become less inclusive, sensitivity worsens but specificity improves; and conversely, as symptoms become more inclusive, sensitivity improves but specificity worsens. The current repertoire of diagnostic testing (eg, GeneXpert, chest radiography, tuberculin skin tests, or interferon-gamma assays) is unlikely to improve household contact investigations because they are expensive or unavailable in the many clinics that have to handle contact asssessments.22,23 Accurate and reliable point-of-care diagnostic tests that can be readily used and implemented in resource-constrained settings are urgently needed to improve household case detection of children.41 If access to high-quality diagnostic tools improves, the proposed algorithms might be more effective.
Our analysis had several limitations. For example, although most symptoms were validated, others such as night sweats and poor appetite were self-reported and could have been subject to social desirability and recall biases. This bias would probably bring the association between our analyses and tuberculosis outcomes towards the null. Tuberculosis diagnosis in an asymptomatic exposed child with a culture-confirmed test is an area of open debate in the scientific literature (especially if chest radiographical examinations are clear). Theoretically, if these children do not actually have tuberculosis and are in some other stage of the disease spectrum (ie, tuberculosis infection), as suggested by some investigators,42 this would bias our assessments of these algorithms towards the null. In the analysis of incident disease, it is possible that differential loss to follow-up might introduce a selection bias in the analysis.
Last, our findings might be distinct from those in communities with low tuberculosis or HIV burden. However, tuberculosis and HIV prevalence is high in many African and low-income countries; poor nutrition and socioeconomic status were common in our study population and are ubiquitous across sub-Saharan African communities, suggesting that these results might be applicable to other areas in Africa with high tuberculosis prevalence.
In conclusion, a symptom-based algorithm approach23 and a modified decision-tree4 detected approximately 70% and 80% of all secondary tuberculosis disease in Ugandan child contacts despite screening a small proportion of these contacts. This approach might be useful in resource-constrained settings to improve implementation of child contact tracing but should be partnered with increasing access to microbiological point-of-care testing.
Supplementary Material
Research in context.
Evidence before this study
We did a systematic search of PubMed for articles published in English and Spanish up to August, 2017. The search included the terms “(child* OR pediatric OR infant) AND (tuberculosis OR TB) AND (symptom*)”. We found two important studies, one in Indonesia and one in South Africa, which assessed WHO’s symptom-based pragmatic guideline. The first used a cross-sectional design and diagnosed paediatric cases through chest radiographical examinations. The second was a prospective cohort study and diagnosed paediatric tuberculosis using clinical symptoms and radiographical examinations (all microbiological tests were negative). Both studies reported a large difference in coprevalent disease rates between children included and not included in the guideline. After 1 year of follow-up, the second study identified four incident tuberculosis cases; two (3%) in contacts recommended for screening and two (1%) in contacts not recommended for screening (p=0.59). Neither study measured HIV-infection and therefore were unable to assess its influence on the diagnostic value of the guideline. A review proposed a modification to the WHO pragmatic guideline, including separating out child contacts into three groups: symptomatic contacts, high-risk asymptomatic contacts, and low-risk asymptomatic contacts. We were unable to find any studies that assessed this modified WHO decision-tree. The additional yield given by tuberculin skin testing child contacts has been debated. An important large contact study from The Gambia showed that tuberculin skin testing was useful for detecting secondary tuberculosis in asymptomatic contacts.
Added value of this study
The present study is the second prospective cohort to assess WHO’s pragmatic guideline for detecting secondary tuberculosis disease in child contacts. Our study extends findings from earlier studies by showing statistically higher rates of secondary disease in symptomatic contacts regardless of the HIV-serostatus of child contacts, suggesting that the algorithm might be useful in areas with high and low burdens of HIV. We were also able to show that the algorithm worked when applied only to culture-confirmed paediatric cases, giving increased validity to our findings. Last, we showed in our Ugandan child-contact cohort that tuberculin skin testing was not complementary to detect additional coprevalent or incident tuberculosis cases in these algorithms. This has important practical implications because tuberculin is often unavailable in sub-Saharan Africa because of national and global shortages. The value of tuberculin skin testing in other settings where HIV and tuberculin infection rates are lower than what we have shown requires further elucidation.
Implications of all the available evidence
Although the guidelines are pragmatic and therefore imperfect in nature, these results support scale-up of WHO’s symptom-based guideline, in combination with a modified version designed to detect high-risk asymptomatic contacts, for the management of child contacts in resource-constrained settings such as sub-Saharan Africa. Cost-effectiveness analyses might be useful to further inform policy; however, in our study population, very few contacts needed to be screened to detect a single case (4.5 needed to screen) under the proposed approach, suggesting high resource efficiency. The current repertoire of diagnostic testing (ie, GeneXpert, chest radiography, tuberculin skin tests, and interferon-gamma assays) is unlikely to improve household contact investigations because they are expensive or unavailable in the many clinics that must handle contact evaluations. Accurate and reliable point-of-care diagnostic tests for paediatric tuberculosis infection and disease that can be readily used and implemented in resource-constrained settings are urgently needed to improve household case detection of children. If available, the proposed algorithms might be able to achieve greater effectiveness.
Acknowledgments
This study was partly supported by the Tuberculosis Research Unit, established with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NO1-AI-95383 and HHSN266200700022C/NO1-AI-70022); the AIDS International Training and Research Program of the Fogarty International Center (TW-00011); and the Center for AIDS Research (AI 36219). LM and CCW were partly supported by an investigator-initiated grant (AI 093856) and a diversity supplement grant (3R01AI093856-05W1) from the National Institute of Allergy and Infectious Diseases. We thank the many participants and families of participants who gave time and dedication to health research, and acknowledge the invaluable contributions made by Albert Maganda, Hussein Kisingo, Yusuf Mulumba, Deborah Nsamba, Janet Mukose, Barbara Kyeyune, Gladys Mpalanyi, Brenda Okware, Augustine Banyanga, Mary Nsereko, Mary Rutaro, Harriet Mayanja-Kizza, Bonnie Thiel, Mark Breda, and Dennis D Dobbs. We also acknowledge contributions made by study medical officers, health visitors, and data clerks and thank the staff at the National Tuberculosis Treatment Centre, Mulago Hospital, the Ugandan National Tuberculosis and Leprosy Program, and the Uganda Tuberculosis Investigation Bacteriological Unit, Wandegeya, for their contributions to this study.
Footnotes
See Online for appendix
Contributors
LM conceived the study, analysed the data, and wrote the first draft of the manuscript, and was the main investigator responsible for interpretation of the results. LM, CMS, LLM, SC, WHB, MLJ, CCW, and SZ contributed to study and data management. YS, AH, CMS, LLM, SC, and CCW assisted LM with the analytical plan and data analysis. All authors were involved in interpretation of the study results, subsequent manuscript drafting for important intellectual content, reviewing and editing of the manuscript, and approval of the final version of the manuscript.
Declaration of interests
CMS and WHB report grants from the National Institutes of Health. All other authors declare no competing interests.
References
- 1.Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Glob Health. 2017;5:e898–906. doi: 10.1016/S2214-109X(17)30289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.du Cros P, Nyang’wa BT, Gale M, Venis S, Ford N. Counting children: comparing reporting for paediatric HIV and tuberculosis. Bull World Health Organ. 2011;89:855. doi: 10.2471/BLT.11.098582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill PC, Rutherford ME, Audas R, van Crevel R, Graham SM. Closing the policy-practice gap in the management of child contacts of tuberculosis cases in developing countries. PLoS Med. 2011;8:e1001105. doi: 10.1371/journal.pmed.1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marais BJ, Pai M. New approaches and emerging technologies in the diagnosis of childhood tuberculosis. Paediatr Respir Rev. 2007;8:124–33. doi: 10.1016/j.prrv.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Maher D, Chaulet P, Spinaci S, Harries A. Treatment of tuberculosis: guidelines for national programmes. 2nd. Geneva: 1997. For World Health Organization Global tuberculosis programme. WHO/TB/97.220. [Google Scholar]
- 6.Borgdorff MW, Floyd K, Broekmans JF. Interventions to reduce tuberculosis mortality and transmission in low-and middle-income countries. Bull World Health Organ. 2002;80:217–27. [PMC free article] [PubMed] [Google Scholar]
- 7.Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health. 2014;2:e453–59. doi: 10.1016/S2214-109X(14)70245-1. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins HE, Yuen CM, Rodriguez CA, et al. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:285–95. doi: 10.1016/S1473-3099(16)30474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osman M, Lee K, Du Preez K, Dunbar R, Hesseling AC, Seddon JA. Excellent treatment outcomes in children treated for tuberculosis under routine operational conditions in Cape Town. Clin Infect Dis. 2017;65:1444–52. doi: 10.1093/cid/cix602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Geneva: World Health Organization; 2006. [PubMed] [Google Scholar]
- 11.Graham SM, Triasih R. More evidence to support screening of child contacts of tuberculosis cases: if not now, then when? Clin Infect Dis. 2013;57:1693–94. doi: 10.1093/cid/cit647. [DOI] [PubMed] [Google Scholar]
- 12.Hsu KH. Contact investigation: a practical approach to tuberculosis eradication. Am J Public Health Nations Health. 1963;53:1761–69. doi: 10.2105/ajph.53.11.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:359–68. doi: 10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]
- 14.Martinez L, Shen Y, Mupere E, Kizza A, Hill PC, Whalen CC. Transmission of Mycobacterium tuberculosis in households and the community: a systematic review and meta-analysis. Am J Epidemiol. 2017;185:1327–39. doi: 10.1093/aje/kwx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaganath D, Zalwango S, Okware B, et al. Contact investigation for active tuberculosis among child contacts in Uganda. Clin Infect Dis. 2013;57:1685–92. doi: 10.1093/cid/cit645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandalakas AM, Ngo K, Ustero PA, et al. BUTIMBA: intensifying the hunt for child TB in Swaziland through household contact tracing. PLoS One. 2017;12:e0169769. doi: 10.1371/journal.pone.0169769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutherford ME, Ruslami R, Anselmo M, et al. Management of children exposed to Mycobacterium tuberculosis: a public health evaluation in West Java, Indonesia. Bull World Health Organ. 2013;91:932–41a. doi: 10.2471/BLT.13.118414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gebregergs GB, Alemu WG. Household contact screening adherence among tuberculosis patients in Northern Ethiopia. PLoS One. 2015;10:e0125767. doi: 10.1371/journal.pone.0125767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Wyk SS, Reid AJ, Mandalakas AM, et al. Operational challenges in managing isoniazid preventive therapy in child contacts: a high-burden setting perspective. BMC Public Health. 2011;11:544. doi: 10.1186/1471-2458-11-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. Global tuberculosis report 2016. Geneva: World Health Organization; 2016. [Google Scholar]
- 21.Rutherford ME, Hill PC, Triasih R, Sinfield R, van Crevel R, Graham SM. Preventive therapy in children exposed to Mycobacterium tuberculosis: problems and solutions. Trop Med Int Health. 2012;17:1264–73. doi: 10.1111/j.1365-3156.2012.03053.x. [DOI] [PubMed] [Google Scholar]
- 22.Pande T, Pai M, Khan FA, Denkinger CM. Use of chest radiography in the 22 highest tuberculosis burden countries. Eur Respir J. 2015;46:1816–19. doi: 10.1183/13993003.01064-2015. [DOI] [PubMed] [Google Scholar]
- 23.WHO. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- 24.Triasih R, Robertson CF, Duke T, Graham SM. A prospective evaluation of the symptom-based screening approach to the management of children who are contacts of tuberculosis cases. Clin Infect Dis. 2015;60:12–18. doi: 10.1093/cid/ciu748. [DOI] [PubMed] [Google Scholar]
- 25.Bonnet M, Kyakwera C, Kyomugasho N, et al. Prospective cohort study of the feasibility and yield of household child tuberculosis contact screening in Uganda. Int J Tuberc Lung Dis. 2017;21:862–68. doi: 10.5588/ijtld.16.0889. [DOI] [PubMed] [Google Scholar]
- 26.Egere U, Togun T, Sillah A, et al. Identifying children with tuberculosis among household contacts in The Gambia. Int J Tuberc Lung Dis. 2017;21:46–52. doi: 10.5588/ijtld.16.0289. [DOI] [PubMed] [Google Scholar]
- 27.Donald PR. Childhood tuberculosis: out of control? Curr Opin Pulm Med. 2002;8:178–82. doi: 10.1097/00063198-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Martinez L, le Roux DM, Barnett W, Stadler A, Nicol MP, Zar HJ. Tuberculin skin test conversion and primary progressive tuberculosis disease in the first 5 years of life: a birth cohort study from Cape Town, South Africa. Lancet Child Adolesc Health. 2017 doi: 10.1016/S2352-4642(17)30149-9. published online Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez L, Sekandi JN, Castellanos ME, Zalwango S, Whalen CC. Infectiousness of HIV-seropositive patients with tuberculosis in a high-burden African setting. Am J Respir Crit Care Med. 2016;194:1152–63. doi: 10.1164/rccm.201511-2146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez L, Handel A, Shen Y, et al. A prospective validation of a clinical algorithm to detect tuberculosis in child contacts. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201706-1210LE. published Oct 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guwatudde D, Zalwango S, Kamya MR, et al. Burden of tuberculosis in Kampala, Uganda. Bull World Health Organ. 2003;81:799–805. [PMC free article] [PubMed] [Google Scholar]
- 32.Graham SM, Cuevas LE, Jean-Philippe P, et al. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis. 2015;61(suppl 3):S179–87. doi: 10.1093/cid/civ581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41:140–156. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Thoracic Society. National Tuberculosis Association of the USA diagnostic standards and classification of tuberculosis. New York: National Tuberculosis Association; 1961. [Google Scholar]
- 35.Kruk A, Gie RP, Schaaf HS, Marais BJ. Symptom-based screening of child tuberculosis contacts: improved feasibility in resource-limited settings. Pediatrics. 2008;121:e1646–52. doi: 10.1542/peds.2007-3138. [DOI] [PubMed] [Google Scholar]
- 36.Kunkel A, zur Wiesch PA, Nathavitharana RR, Marx FM, Jenkins HE, Cohen T. Smear positivity in paediatric and adult tuberculosis: systematic review and meta-analysis. BMC Infect Dis. 2016;16:282. doi: 10.1186/s12879-016-1617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theron G, Jenkins HE, Cobelens F, et al. Data for action: collection and use of local data to end tuberculosis. Lancet. 2015;386:2324–33. doi: 10.1016/S0140-6736(15)00321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuen C, Jenkins H, Chang R, Mpunga J, Becerra M. Two methods for setting child-focused tuberculosis care targets. Public Health Action. 2016;6:83–96. doi: 10.5588/pha.16.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomes VF, Andersen A, Wejse C, et al. Impact of tuberculosis exposure at home on mortality in children under 5 years of age in Guinea-Bissau. Thorax. 2011;66:163–67. doi: 10.1136/thx.2010.141309. [DOI] [PubMed] [Google Scholar]
- 40.Mandalakas AM, Hesseling AC, Gie RP, Schaaf H, Marais BJ, Sinanovic E. Modelling the cost-effectiveness of strategies to prevent tuberculosis in child contacts in a high-burden setting. Thorax. 2013;68:247–55. doi: 10.1136/thoraxjnl-2011-200933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zar HJ, Connell TG, Nicol M. Diagnosis of pulmonary tuberculosis in children: new advances. Expert Rev Anti Infect Ther. 2010;8:277–88. doi: 10.1586/eri.10.9. [DOI] [PubMed] [Google Scholar]
- 42.Loveday M, Sunkari B, Marais BJ, Master I, Brust JC. Dilemma of managing asymptomatic children referred with ‘culture-confirmed’ drug-resistant tuberculosis. Arch Dis Child. 2016;101:608–13. doi: 10.1136/archdischild-2015-310186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


