Abstract
Purpose
To explore the use of polyvinylpyrrolidone (PVP) for simulated materials with tissue-equivalent dielectric properties.
Methods
PVP and salt were used to control, respectively, relative permittivity and electrical conductivity in a collection of 63 samples with a range of solute concentrations. Their dielectric properties were measured with a commercial probe and fitted to a 3D polynomial in order to establish an empirical recipe. The material’s thermal properties and MR spectra were measured.
Results
The empirical polynomial recipe (available at http://www.amri.ninds.nih.gov/phantomrecipe.html) provides the PVP and salt concentrations required for dielectric materials with permittivity and electrical conductivity values between ~ 45 and 78, and 0.1 to 2 S/m, respectively, from 50 MHz to 4.5 GHz. The second (solute concentrations) and seventh (frequency) order polynomial recipe provided less than 2.5% relative error between the measured and target properties. PVP’s side peaks in the spectra were negligible and unaffected by temperature changes.
Conclusion
PVP-based phantoms are easy to prepare, non-toxic, and their semi-transparency makes air bubbles easy to identify. The polymer can be used to create simulated material with a range of dielectric properties, negligible spectral side peaks and long T2 relaxation time, which are favorable in many MR applications.
Keywords: High-field MRI, MR phantoms, tissue equivalent materials, electrical conductivity, relative permittivity
Introduction
Materials used as a substitute for biological tissues are ubiquitous fixtures in various electromagnetic applications. Wireless technologies utilize a standardized Specific Anthropomorphic Mannequin (SAM) head phantom with realistic anatomical and dielectric properties as a test bed to measure radiofrequency dosimetry in specific absorption rate (SAR) compliance testing (1). Similarly, materials with dielectric properties that are matched to those of biological tissues are essential reference standards in Magnetic Resonance Imaging (MRI) for quantitative techniques, protocol and radiofrequency coil development, and thermometry. A characteristic that is shared among electromagnetic applications is that the fields interact and are indeed shaped by the object itself. This interaction underscores the importance of tissue-mimicking objects, or phantoms, in system calibrations and other measurements that precede in vivo application (2).
In MRI, dielectric phantoms have received greater interest as the static main field in whole body devices has progressed from 1.5 Tesla in the 1980’s to 10.5 Tesla today (3,4). Stronger magnets operate at higher frequencies, which are associated with shorter wavelengths that result in more pronounced interactions between the object and electromagnetic fields (5–7). These interactions cause constructive and destructive interference patterns that depend on the shape and dielectric properties of the object. Traditional MRI phantoms that replicate tissue relaxation properties (T1 and T2) (8–14) may not be appropriate for certain image protocol development at high field strengths if they yield an electromagnetic field distribution that is not representative of in vivo situations. The purpose of this work is to prepare and characterize phantoms with tissue-equivalent dielectric properties that are suitable for MRI applications.
Given that MRI routinely probes the proton nucleus, a water-based phantom is preferred. While human tissues have dielectric properties that cover a wide range, most soft tissues and liquids (i.e., excluding adipose tissue and cortical bone) have an electrical conductivity greater than water and a relative permittivity less than water at frequencies above 100 MHz (15). Ionic compounds, such as salt, are commonly used to increase electrical conductivity in tissue-mimicking materials, while various substances, such as alcohol, sucrose, or polymers, have been used to reduce permittivity to the desired value (16–29). Alcohol and sucrose can be unfavorable because they introduce extraneous spectral components that complicate the MR signal evolution and can reduce the relaxation times. These are particularly undesirable characteristics in MR thermometry techniques based on proton resonance frequency shift (PRF), because the measurement precision is directly proportional to T2* (30) and nonlinearity between temperature and signal phase adds significant complexity to the model (31,32).
As an alternative, we explored polyvinylpyrrolidone (PVP) to tune the permittivity in dielectric phantoms (33–37). PVP is a non-toxic (38), readily available, and water-soluble polymer that, unlike alcohol and sucrose, generates negligible extraneous spectral components. The main aim of this work was to introduce a parametric recipe for PVP-based tissue-equivalent phantoms with a range of dielectric properties. We additionally reported thermal properties, which are expected to be relevant in thermometry or dosimetry experiments.
Methods
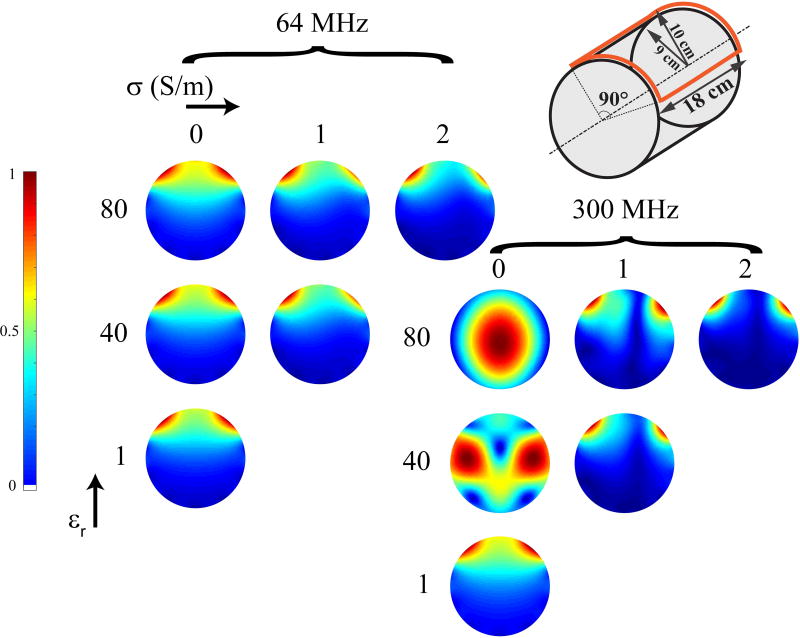
To motivate the use of tissue-equivalent phantoms in MRI, we performed full-wave simulations using an analytic framework based on dyadic Green’s functions (5,39) with objects of various dielectric properties. In particular, we simulated the |B1−| field of a surface loop coil in the central transverse plane of a head-sized cylindrical phantom. Simulations were performed at 64 and 300, with the object’s electrical conductivity set to 0, 1, and 2 S/m and relative permittivity set to 1, 40, and 80. For brevity, the term “conductivity” will refer to electrical conductivity in the remainder of the manuscript. Figure 1 illustrates how the object’s dielectric properties influence the |B1−| field pattern at 64 MHz. This effect is even more pronounced as the operating frequency increases, where features, such as the so-called dielectric brightening and B1 twisting, are clearly visible indicating the presence of interference patterns and conduction currents in the sample (5,21,40–42).
Fig. 1.
Simulated |B1−| maps in central transverse plane of a head-sized cylindrical object (18 cm diameter and 18 cm length) generated with a surface loop coil (18 cm length and 90° aperture) at 64 and 300 MHz for different dielectric properties of the phantom. Already at 64 MHz, |B1−| distortions due to the phantom’s properties are visible and are enhanced at 300 MHz. The color scale in each map was set independently to highlight distinctive field patterns. Maps for objects with low permittivity and high conductivity were omitted because these properties are generally not encountered in vivo.
In light of these observations, the use of phantoms that mimic the dielectric properties of tissues is indispensable at high field. In this work PVP is used to control permittivity in water-based phantoms. We acquired proton NMR spectra in two samples doped with 34% PVP and 49% sucrose respectively on a 500 MHz spectrometer (AV-500, Bruker, Billerica, MA). Spectra were measured over a temperature range 25–35°C with a step of 1°C to determine the temperature coefficient α, which expresses the sensitivity of the material’s resonance frequency to its temperature.
We proceeded to prepare candidate phantoms with a range of solute concentrations to investigate their relation to electrical properties over a range of frequencies. Specifically, we used three ingredients:
-
-
Distilled water as solvent
-
-
NaCl (Sodium Chloride S9625 – anhydrous, free-flowing, Redi-DriTM, ACS reagent, ≥ 99%, Sigma-Aldrich®, St. Louis, MO) to control conductivity.
-
-
Polyvinylpyrrolidone (PVP40 - average mol wt 40.000 g mol−1, Sigma-Aldrich®, St. Louis, MO) to control permittivity.
The ingredients were carefully weighed and mixed together. No heat was required to dissolve the solutes. The solutions were allowed to homogenize for ~ 48 hours at room temperature.
A collection of sixty three 125 ml samples were prepared with PVP concentration that ranged from 0% to 50% at intervals of 6.25% and NaCl concentration between 0% to 1.5% at intervals of 0.25%. All concentrations are reported as w/w, relative to the mass of the solution. Nine extra samples were prepared with PVP concentration 37.5 % to 50% and NaCl concentration 2% to 7% to expand coverage to lower permittivity and higher conductivity values.
The dielectric properties of each sample were measured with a probe and electronic calibration module that was recommended by the manufacturer to improve measurement stability (models 85070E and 85093C, Agilent, Santa Clara, CA) from 50 MHz–4.5 GHz with 5 MHz step at 21°C. Since the manufacturer does not recommend the use of the probe at frequencies below 200 MHz, we performed measurements on materials with known dielectric properties (saline solutions with various concentrations, methanol and 2-propanol) (43–46) to estimate probe accuracy. The data were fitted to a 3D polynomial using customized Matlab software (version R2013b, with supporting parallel computing toolbox; Mathworks, Natick, MA), where the dimensions represent NaCl and PVP concentrations and frequency. In our experience, polynomial fitting was appropriate because of its robustness to measurement noise, feasibility to incorporate supplemental data points, and a parameterized output recipe. In addition, to avoid a result that was numerically dominated by one particular input variable while disregarding the others, all inputs (PVP concentration, NaCl concentration, and frequency) were normalized to approximately unity before polynomial fitting was performed.
As a step toward recipe validation, we compared the predicted and measured dielectric properties of two additional phantoms with properties that targeted the human heart and skeletal-muscle and at 300 MHz and 500 MHz, respectively (15). To investigate the stability of the solutions, the dielectric properties of a 25% PVP / 1% NaCl and a 50% PVP / 1% NaCl phantom were measured over the course of one year. During this time, they were stored in sealed plastic containers at room temperature. No preservatives were added.
The dielectric properties of three samples were measured over the range of temperatures 25 – 45 °C to determine their dependency on temperature. The samples contained the same amount of PVP (25%) and increasing amount of NaCl (0%, 0.75% and 1.5%).
We developed a gelling procedure in order to make the phantoms suitable for MR thermometry measurements. Gel phantoms perform better in MR thermometry, local SAR calculation or dosimetry because they mitigate heat convection, which can lead to underestimation of heating due to RF power deposition. As gelling agent we used Select Agar (A5054, Sigma-Aldrich®, St. Louis, MO) with a concentration of 2% of total volume. Agar was added to the PVP and salt solution and the mixture was heated to 85°C while stirring until complete dissolution of the agar. Finally, the material was allowed to cool to room temperature. For phantoms with high PVP concentrations (40% or higher), a hot water bath is recommended to slow down the cooling process and avoid air bubbles. Volumetric heat capacity and heat diffusivity were measured on PVP gel phantoms using a thermal properties analyzer (KD2 probe, Decagon Devices Inc., Pullman, WA).
To assess T2* in phantoms with different permittivity-controlling solutes, multi-echo gradient echo (GRE) images were acquired in three PVP-based and three sucrose-based gel phantoms with dielectric properties of heart, muscle and white matter. The images were acquired on a 7 Tesla whole body MR system (MAGNETOM, Siemens Healthineers, Erlangen, Germany) with twelve linearly spaced echo times ranging from 6.3 to 60.6 ms.
Results
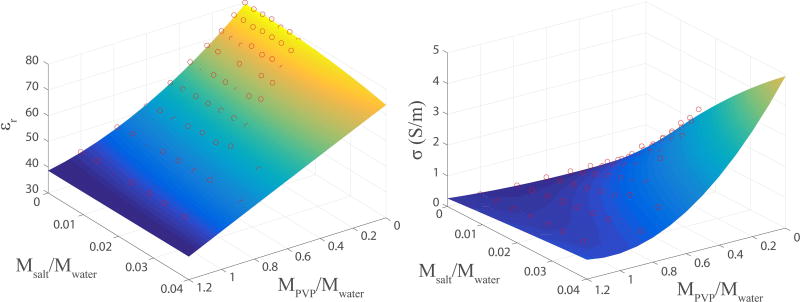
The dielectric measurements in reference materials were in good agreement with predicted values in the frequency range tested here (see Supporting Table S1 and Figure S1), indicating that the PVP dielectric recipe is valid over the same range (50 MHz to 4.5 GHz). The master dielectric recipe was empirically developed using the following 3D polynomial orders to capture data dynamics while reducing sensitivity to measurement noise: 2th order on PVP concentration, 2th order on NaCl concentration, and 7th order on frequency. The polynomial function shows good agreement with the measured data (Figure 2). This function is reimplemented in GNU Octave (http://www.gnu.org/software/octave/) and is available as a web application at http://www.amri.ninds.nih.gov/phantomrecipe.html.
Fig. 2.
The 3D polynomial fitted surfaces for relative permittivity (left) and conductivity (right) at 300 MHz are well-matched to the experimental measurements (red circles) with relative RMSE of 0.011 for permittivity and absolute RMSE of 0.061 S/m for conductivity. The absolute error is reported for conductivity since values close to zero dominated the relative error.
The fitted second order polynomial surfaces are shown in Figure 2 at 300 MHz. The polynomial functions are well-fitted to the data points with relative error 1.1% for relative permittivity and absolute error 0.06 S/m for conductivity. The absolute error is reported for conductivity since values close to zero dominated the relative error.
Interdependence between the solute concentration and dielectric properties was observed. In particular, as the concentration of PVP increases the amount of NaCl needed to achieve a given conductivity value increases as well. The relative errors in the predicted dielectric properties for the 7 T (300 MHz) muscle phantom and the 11.7 T (500 MHz) heart phantom used to validate the recipe were below 2.3%.
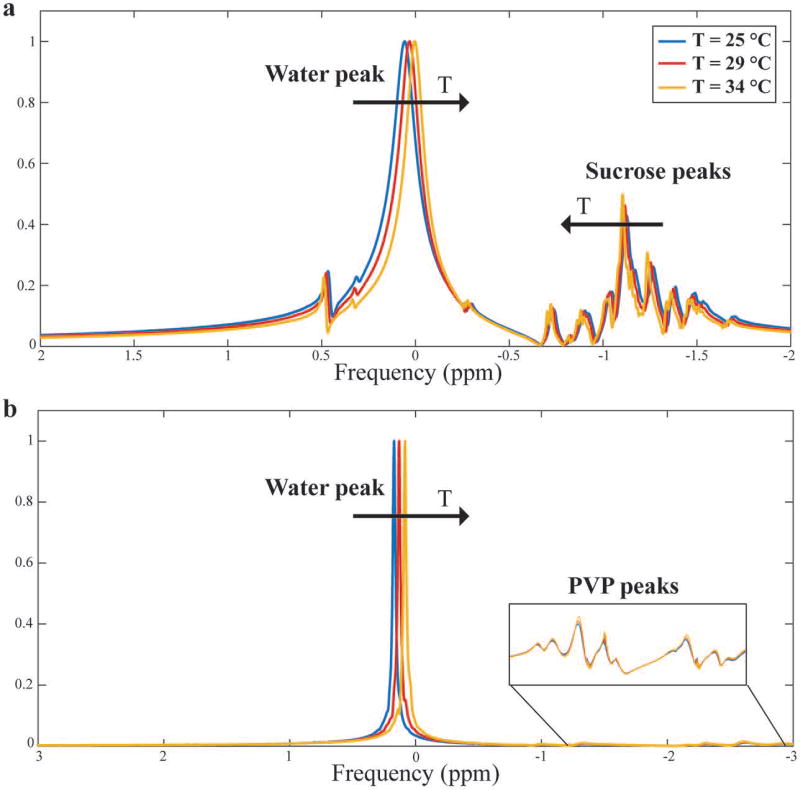
The presence of multiple side peaks in the NMR spectrum, which is typical of sucrose-based phantoms, is minimal in PVP phantoms, whose spectra are similar to that of pure water (Figure 3). This represents an important advantage of PVP over sucrose-based phantoms in thermometry experiments. The dependence between temperature and resonance frequency is also shown in Figure 3. As the temperature increases, the water peak shifts, as expected, in both phantoms. The difference between the two phantoms is in the side peaks shift; sucrose’s side peaks move towards water’s, while PVP’s side peaks do not shift.
Fig. 3.
Sucrose-based (a) and PVP-based (b) phantom NMR spectra at different temperatures. The amplitudes were normalized to the water peak. Sucrose is characterized by multiple side peaks that shift towards the water’s peak as the temperature increases leading to a complicated phase evolution. On the other hand, PVP’s side peaks are considerably smaller and unaffected by temperature changes, making the solute more suitable for MRI applications.
The temperature coefficient of the PVP phantom was – 0.0106 ppm/°C, which is comparable to that of pure water (47). The value for the sucrose phantom was – 0.0085 ppm/°C, suggesting interchange between the two species. To account for system-related global frequency shift during the experiment, the temperature coefficients were calculated using the frequency difference between the water peak and largest solute side peak in consecutive acquisitions.
The relative permittivity of the 25% PVP / 1% NaCl sample was initially 64.0 and 61.4 after 6 months where it remained stable 12 months after preparation. The 50% PVP / 1% NaCl sample showed a more variability; its permittivity went from 43.4, to 40.82 after 6 months and 35.0 after 12 months. Its conductivity was decreased from 0.36 S/m, to 0.33 S/m after 6 months and 0.26 S/m after 12 months. No degradation in purity or homogeneity was visually observed.
The 25% PVP phantom permittivity varied approximately linearly with temperature in the range 25–40°C with slope ~ −0.1°C−1 at 300 MHz. The sensitivity of conductivity to temperature increased with NaCl concentration; the linear dependence was 0.001, 0.010 and 0.023 S/m°C for 0%, 0.75% and 1.5% NaCl, respectively. Similar volumetric heat capacity and heat diffusivity values were measured in the heart, muscle and white matter PVP gel phantoms (3.48 ± 0.21 MJ/m3K and 0.13 ± 0.01 mm2/s, respectively).
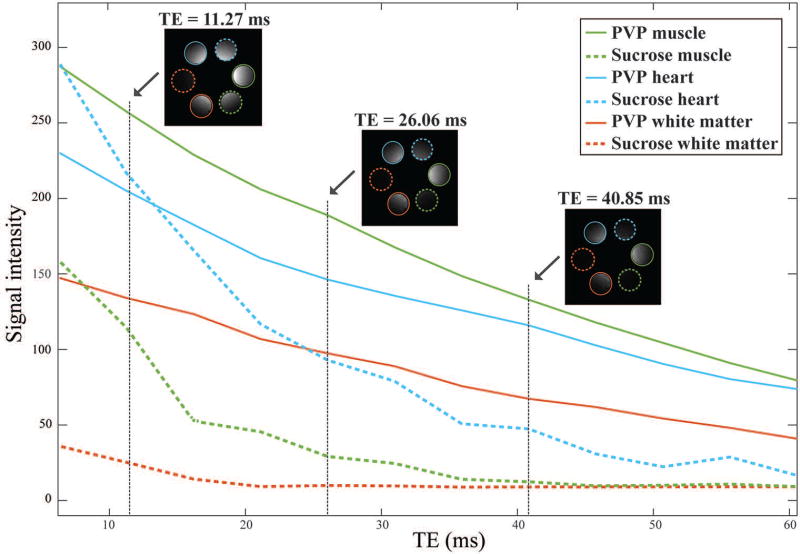
Recipes to simulate heart, muscle, and white matter tissues at 300 MHz are listed in Table 1. Figure 4 shows that the effective T2* is much longer for PVP-based phantoms compared to sucrose-based phantoms with the same dielectric properties, which translates in higher SNR and makes PVP phantoms advantageous for many MR applications.
Table 1.
Recipe for PVP-based and sucrose-based phantoms with dielectric properties of heart, muscle and white matter at 300 MHz.
| Heart | Muscle | White Matter | ||||
|---|---|---|---|---|---|---|
| PVP-based | Sucrose-based | PVP-based | Sucrose-based | PVP-based | Sucrose-based | |
| Solute (% w/w) | 17.1 | 29.4 | 33.9 | 48.9 | 44.3 | 63.7 |
| NaCl (% w/w) | 0.7 | 0.9 | 1.1 | 1.9 | 1.7 | 1.9 |
| Target εr | 69.3 | 58.2 | 43.8 | |||
| Target σ (S/m) | 0.9 | 0.77 | 0.41 | |||
Fig. 4.
Signal intensity as a function of TE for three PVP-based (solid lines) and sucrose-based (dotted lines) gel phantoms mimicking muscle (green), heart (blue) and white matter (red). The MR signal decays more slowly in the PVP phantoms compared to sucrose phantoms with same dielectric properties. The water bath in which the phantoms were surrounded has been masked out for clarity. The non-uniform signal intensity within each phantom is due primarily to the characteristic B1+ profile at 300 MHz.
Discussion and Conclusions
This work explored PVP as a means to create materials with a range of dielectric properties that represent various human tissues: from 40 to 78 for relative permittivity and from 0.2 to 2 S/m for conductivity. Our measurements were packaged into an empirical recipe that provides the required solute concentration for a desired set of dielectric properties and operating frequency. We focused on materials with relative permittivity less than that of water, which are relevant for most soft tissues at frequencies greater than 200 MHz. PVP proved to be a suitable solute to reduce the permittivity of water from its baseline to values as low as approximately 40. Although values of relative permittivity below 40 may be relevant to replicate certain tissues or combinations thereof, we found ~50% w/w PVP concentration approached the solubility limit of the polymer in water by manual mixing or standard magnetic stirring devices. Higher concentrations of PVP in water could be possibly prepared with more sophisticated mixing tools. While this work focuses on MRI applications, the parametric recipe covers a wide frequency band (50 MHz to 4.5 GHz) that can serve other technologies.
The recipe and dielectric values reported in this work are based on PVP with a molecular weight of 40.000 g mol−1. PVP phantom properties are expected to be influenced by molecular weight, for which a wide range is readily available. This extra degree of freedom could offer the opportunity to tune dielectric parameters in addition to others properties such as viscosity (48,49).
The liquid PVP phantoms are expected to be useful for MRI or electric field measurements that require electromagnetic patterns representative of those in vivo. The phantoms can be gelled using agar for example, which has a negligible effect on dielectric properties. A gelled phantom provides a static medium that prevents heat convection, which is useful for local specific absorption rate, dosimetry, and MR thermometry measurements.
In PRF-based MR thermometry, the difference in the signal phase from two gradient-echo acquisitions is related to local heating by the material’s temperature sensitivity, which is well-known for water (α ≈ − 0.01 ppm/°C) (47). The signal in a dual-species material such as water doped with PVP or sucrose is:
where Aw and As are the proportions of the water and secondary components, t the echo time, the inverse of T2*, and ψ the global offset frequency (31,32). Signals from the nth spectral peak are represented by normalized amplitude ρn and frequency ωn, where the amplitudes sum to unity. For the PVP phantom whose spectra is illustrated in Figure 3b, the minor secondary peaks (Aw/As = 7.6) result in a roughly linear relationship between ωw and the phase of S, or likewise between ωw and the temperature change through α. In contrast, Aw/As = 2.3 for the sucrose phantom in Figure 3a, which gives added weight to the second term of the above signal equation and makes it more difficult to determine ωw. Another important advantage of PVP phantoms is that PRF-based MR thermometry can be performed with improved precision (30) because they have longer T2* values than sucrose phantoms with equivalent dielectric properties (see Figure 4). Longer T2* also implicates in higher SNR that can be beneficial for EP mapping techniques (50–54).
While the goal of this study was to develop phantoms with specific dielectric properties, a phantom that additionally replicates in vivo relaxation properties may be valuable for comprehensive MRI protocol development. Ingredients such as agarose can be used to control T2, while gadolinium (11), nickel or copper (9,55,56) or manganese chloride (57,58) have been utilized to modify T1. However, these ingredients additionally influence conductivity, which necessitates a more complex recipe than that proposed here to simultaneously achieve the desired conductivity, permittivity, and T1 and T2.
The reader should be aware that PVP, as is the case with many polymers, is prone to degradation over long periods of time. We observed a drop in the electrical properties in samples with high PVP concentration (>30% w/w) after one year, while the low PVP concentration samples were more stable. One hypothesis is that dielectric instability may be due to the formation of crosslinks along the polymer chain, which reduces mobility.
In conclusion, we developed an empirical recipe for PVP–NaCl-based phantoms that mimic tissue dielectric properties by fitting direct measurements to a polynomial equation. The recipe has been made publically available to facilitate phantom preparation by other investigators. PVP phantoms have several advantageous properties including straightforward fabrication, its non-toxic character and semi-transparency that makes air bubbles easy to identify. Their application in MR thermometry is valuable because of their long T2* values and single spectral peak.
Supplementary Material
Supporting Table S1. Comparison between measured and theoretical dielectric properties of four known materials.
Supporting Fig. S1. Measured and theoretical real and imaginary part of methanol complex permittivity over the frequency range 50 MHz – 4.5 GHz
Acknowledgments
The authors thank Christopher Collins for insightful reference materials. The Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net) at New York University School of Medicine is supported by NIH/NIBIB grant number P41 EB017183.
Footnotes
This work was presented in part in “Sugar free tissue-mimicking MRI phantoms for improved signal-to-noise ratio” 24th Scientific Meeting of the International Society for Magnetic Resonance in Medicine (ISMRM), Singapore, 7–13 May 2016, p. 2239 and in “Design and construction of a tissue-mimicking phantom to validate electrical properties mapping techniques based on magnetic resonance” URSI Atlantic Radio Science Conference (AT-RASC), Gran Canaria (Canary Islands), 18–22 May 2015, p. K03.1.
References
- 1.Beard BB, Kainz W. Review and standardization of cell phone exposure calculations using the SAM phantom and anatomically correct head models. Biomed Eng Online. 2004;3(1):34. doi: 10.1186/1475-925X-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang QX, Wang J, Collins CM, Smith MB, Zhang X, Ugurbil K, Chen W. Phantom design method for high-field MRI human systems. Magnetic resonance in medicine. 2004;52(5):1016–1020. doi: 10.1002/mrm.20245. [DOI] [PubMed] [Google Scholar]
- 3.Moser E. Ultra-high-field magnetic resonance: Why and when? World journal of radiology. 2010;2(1):37–40. doi: 10.4329/wjr.v2.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ugurbil K. Magnetic Resonance Imaging at Ultrahigh Fields. Ieee T Bio-Med Eng. 2014;61(5):1364–1379. doi: 10.1109/TBME.2014.2313619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaidya MV, Collins CM, Sodickson DK, Brown R, Wiggins GC, Lattanzi R. Dependence of B1+ and B1− Field Patterns of Surface Coils on the Electrical Properties of the Sample and the MR Operating Frequency. Concepts in magnetic resonance Part B. Magnetic resonance engineering. 2016;46(1):25–40. doi: 10.1002/cmr.b.21319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins CM, Wang Z. Calculation of radiofrequency electromagnetic fields and their effects in MRI of human subjects. Magnetic resonance in medicine. 2011;65(5):1470–1482. doi: 10.1002/mrm.22845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoult DI. The principle of reciprocity in signal strength calculations - a mathematical guide. Concepts in Magnetic Resonance. 2000;12(4):173–187. [Google Scholar]
- 8.Blechinger JC, Madsen EL, Frank GR. Tissue-mimicking gelatin-agar gels for use in magnetic resonance imaging phantoms. Medical physics. 1988;15(4):629–636. doi: 10.1118/1.596219. [DOI] [PubMed] [Google Scholar]
- 9.Christoffersson JO, Olsson LE, Sjoberg S. Nickel-Doped Agarose-Gel Phantoms in Mr Imaging. Acta Radiol. 1991;32(5):426–431. [PubMed] [Google Scholar]
- 10.Freed M, de Zwart JA, Loud JT, El Khouli RH, Myers KJ, Greene MH, Duyn JH, Badano A. An anthropomorphic phantom for quantitative evaluation of breast MRI. Medical physics. 2011;38(2):743–753. doi: 10.1118/1.3533899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikemoto Y, Takao W, Yoshitomi K, Ohno S, Harimoto T, Kanazawa S, Shibuya K, Kuroda M, Kato H. Development of a human-tissue-like phantom for 3.0-T MRI. Medical physics. 2011;38(11):6336–6342. doi: 10.1118/1.3656077. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura K, Kato H, Kuroda M, Yoshida A, Hanamoto K, Tanaka A, Tsunoda M, Kanazawa S, Shibuya K, Kawasaki S, Hiraki Y. Development of a tissue-equivalent MRI phantom using carrageenan gel. Magnetic resonance in medicine. 2003;50(5):1011–1017. doi: 10.1002/mrm.10619. [DOI] [PubMed] [Google Scholar]
- 13.Mustafi D, Peng B, Heisen M, Wood AM, Buurman J, czmar GSK. World of phantoms: Reference standards for bench to breast MRI; 17th Annual Meeting & Exhibition of ISMRM (International Society for Magnetic Resonance in Medicine); Honolulu, Hawaii. 2009; p. 2104. [Google Scholar]
- 14.Mitchell MD, Kundel HL, Axel L, Joseph PM. Agarose as a tissue equivalent phantom material for NMR imaging. Magnetic resonance imaging. 1986;4(3):263–266. doi: 10.1016/0730-725x(86)91068-4. [DOI] [PubMed] [Google Scholar]
- 15.Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Physics in medicine and biology. 1996;41(11):2251–2269. doi: 10.1088/0031-9155/41/11/002. [DOI] [PubMed] [Google Scholar]
- 16.Park SM, Nyenhuis JA, Smith CD, Lim EJ, Foster KS, Baker KB, Hrdlicka G, Rezai AR, Ruggieri P, Sharan A, Shellock FG, Stypulkowski PH, Tkach J. Gelled versus nongelled phantom material for measurement of MRI-induced temperature increases with bioimplants. Ieee T Magn. 2003;39(5):3367–3371. [Google Scholar]
- 17.Ianniello C, Brown R, Angellotti V, Grivo B, Sodickson DK, Massa R, Lattanzi R. Design and construction of a tissue-mimicking phantom to validate electrical properties mapping techniques based on magnetic resonance. 1st URSI Atlantic Radio Science Conference (URSI AT-RASC); 18–22 May 2015; ExpoMeloneras Convention Centre; Gran Canaria. 2015. [Google Scholar]
- 18.Chou CK, Chen GW, Guy AW, Luk KH. Formulas for preparing phantom muscle tissue at various radiofrequencies. Bioelectromagnetics. 1984;5(4):435–441. doi: 10.1002/bem.2250050408. [DOI] [PubMed] [Google Scholar]
- 19.Kato H, Ishida T. Development of an agar phantom adaptable for simulation of various tissues in the range 5–40 MHz. Physics in medicine and biology. 1987;32(2):221–226. doi: 10.1088/0031-9155/32/2/006. [DOI] [PubMed] [Google Scholar]
- 20.Guy AW. Analyses of Electromagnetic Fields Induced in Biological Tissues by Thermographic Studies on Equivalent Phantom Models. Ieee T Microw Theory. 1971;Mt19(2):205-&. [Google Scholar]
- 21.Neves AL, Leroi L, Cochinaire N, Abdeddaim R, Sabouroux P, Vignaud A. Mimicking the Electromagnetic Distribution in the Human Brain: A Multi-frequency MRI Head Phantom. Applied Magnetic Resonance. 2017;48(3):213–226. [Google Scholar]
- 22.Graedel NN, Polimeni JR, Guerin B, Gagoski B, Wald LL. An anatomically realistic temperature phantom for radiofrequency heating measurements. Magnetic resonance in medicine. 2015;73(1):442–450. doi: 10.1002/mrm.25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanda MY, Ballen M, Salins S, Chou CK, Balzano Q. Formulation and characterization of tissue equivalent liquids used for RF densitometry and dosimetry measurements. Ieee T Microw Theory. 2004;52(8):2046–2056. [Google Scholar]
- 24.Stauffer PR, Rossetto F, Prakash M, Neuman DG, Lee T. Phantom and animal tissues for modelling the electrical properties of human liver. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2003;19(1):89–101. doi: 10.1080/0265673021000017064. [DOI] [PubMed] [Google Scholar]
- 25.Hartsgrove G, Kraszewski A, Surowiec A. Simulated biological materials for electromagnetic radiation absorption studies. Bioelectromagnetics. 1987;8(1):29–36. doi: 10.1002/bem.2250080105. [DOI] [PubMed] [Google Scholar]
- 26.Chew KM, Sudirman R, Seman N, Yong CY. Human brain phantom modeling based on relative permittivity dielectric properties. International Conference on Biomedical Engineering and Biotechnology, iCBEB 2012; Macau, China; 2012; pp. 817–820. [Google Scholar]
- 27.Takahiro Sunaga, Hiroo Ikehira, Shigeo Furukawa, Mitsuru Tamura, Eiji Yoshitome, Takayuki Obata, Hiroshi Shinkai, Shuji Tanada, Hajime Murata, Sasaki Y. Development of a dielectric equivalent gel for better impedance matching for human skin. Bioelectromagnetics. 2003;24:214–217. doi: 10.1002/bem.10080. [DOI] [PubMed] [Google Scholar]
- 28.Duan Q, Duyn JH, Gudino N, de Zwart JA, van Gelderen P, Sodickson DK, Brown R. Characterization of a dielectric phantom for high-field magnetic resonance imaging applications. Medical physics. 2014;41(10):102303. doi: 10.1118/1.4895823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito K, Furuya K, Okano Y, Hamada L. Development and characteristics of a biological tissue-equivalent phantom for microwaves. Electronics and Communications in Japan, Part 1. 2001;84(4):2000. [Google Scholar]
- 30.Rieke V, Butts Pauly K. MR thermometry. Journal of magnetic resonance imaging : JMRI. 2008;27(2):376–390. doi: 10.1002/jmri.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deniz CM, Brown R, de Zwart JA, Collins CM, Sodickson DK. Multi-Channel Array Safety Using Least Squares Fitting based MR Thermometry. ISMRM Safety in MRI Workshop; Washington, DC. 2014. [Google Scholar]
- 32.de Zwart JA, van Gelderen P, Duan Q, Gudino N, Deniz CM, Alon L, H DJ. Improved MR thermometry in the presence of non-water proton signals. 23rd Scientific Meeting, ISMRM, At Toronto. 2015;1868 [Google Scholar]
- 33.Sarode AV, Kumbharkhane AC. Dielectric relaxation and thermodynamic properties of polyvinylpyrrolidone using time domain reflectometry. Polym Int. 2012;61(4):609–615. [Google Scholar]
- 34.Shinyashiki N, Matsumura Y, Miura N, Yagihara S, Mashimo S. Dielectric Study of Water-Structure in Polymer-Solution. J Phys Chem-Us. 1994;98(51):13612–13615. [Google Scholar]
- 35.Shinyashiki N, Asaka N, Mashimo S, Yagihara S. Dielectric Study on Dynamics of Water in Polymer Matrix Using a Frequency-Range 10(6)–10(10) Hz. J Chem Phys. 1990;93(1):760–764. [Google Scholar]
- 36.Busselez R, Arbe A, Cerveny S, Capponi S, Colmenero J, Frick B. Component dynamics in polyvinylpyrrolidone concentrated aqueous solutions. J Chem Phys. 2012;137(8):084902. doi: 10.1063/1.4746020. [DOI] [PubMed] [Google Scholar]
- 37.Choudhary S, Sengwa RJ. Dielectric spectroscopy and viscosity studies of aqueous poly(ethylene oxide) and poly(vinyl pyrrolidone) blend. Indian J Phys. 2011;85(11):1591–1602. [Google Scholar]
- 38.Nair B. Final Report On the Safety Assessment of Polyvinylpyrrolidone (PVP) Int J Toxicol. 1998;17(4_suppl):95–130. [Google Scholar]
- 39.Lattanzi R, Sodickson DK. Ideal current patterns yielding optimal signal-to-noise ratio and specific absorption rate in magnetic resonance imaging: computational methods and physical insights. Magnetic resonance in medicine. 2012;68(1):286–304. doi: 10.1002/mrm.23198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tropp J. Image brightening in samples of high dielectric constant. Journal of magnetic resonance. 2004;167(1):12–24. doi: 10.1016/j.jmr.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Collins CM, Liu W, Schreiber W, Yang QX, Smith MB. Central brightening due to constructive interference with, without, and despite dielectric resonance. Journal of magnetic resonance imaging : JMRI. 2005;21(2):192–196. doi: 10.1002/jmri.20245. [DOI] [PubMed] [Google Scholar]
- 42.Bomsdorf H, Helzel T, Kunz D, Roschmann P, Tschendel O, Wieland J. Spectroscopy and imaging with a 4 tesla whole-body MR system. NMR in biomedicine. 1988;1(3):151–158. doi: 10.1002/nbm.1940010308. [DOI] [PubMed] [Google Scholar]
- 43.Schwan HP. Electrical properties of tissue and cell suspensions. Adv Biol Med Phys. 1957;5:147–209. doi: 10.1016/b978-1-4832-3111-2.50008-0. [DOI] [PubMed] [Google Scholar]
- 44.Gregory AP, Clarke RN. Tables of the complex permittivity of dielectric reference liquids at frequencies up to 5 GHz NPL Report MAT 23 2009 [Google Scholar]
- 45.Jorsdan BP, Sheppard RJ, Szwarnowski S. The dielectric properties of formamide, ethanediol and methanol. J Phys D Appl Phys. 1978;11:695–701. [Google Scholar]
- 46.Anderson C. Baylor University; 2006. Determining the complex permittivity of materials with the waveguide-cutoff method [phD dissertation] [Google Scholar]
- 47.Hindman JC. Proton Resonance Shift of Water in Gas and Liquid States. J Chem Phys. 1966;44(12):4582-&. [Google Scholar]
- 48.Shinyashiki N, Imoto D, Yagihara S. Broadband dielectric study of dynamics of polymer and solvent in poly(vinyl pyrrolidone)/normal alcohol mixtures. The journal of physical chemistry B. 2007;111(9):2181–2187. doi: 10.1021/jp065414e. [DOI] [PubMed] [Google Scholar]
- 49.Sengwa RJ, Sankhla S. Chain length effect on dynamical structure of poly(vinyl pyrrolidone)-polar solvent mixtures in dilute solution of dioxane studied by microwave dielectric relaxation measurement. Pramana-J Phys. 2006;67(2):375–381. [Google Scholar]
- 50.Katscher U, Voigt T, Findeklee C. Electrical conductivity imaging using magnetic resonance tomography. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3162–3164. doi: 10.1109/IEMBS.2009.5334031. [DOI] [PubMed] [Google Scholar]
- 51.Voigt T, Katscher U, Doessel O. Quantitative conductivity and permittivity imaging of the human brain using electric properties tomography. Magnetic resonance in medicine. 2011;66(2):456–466. doi: 10.1002/mrm.22832. [DOI] [PubMed] [Google Scholar]
- 52.Sodickson DK, Alon L, Deniz CM, Brown R, Zhang B, Wiggins GC, Cho GY, Ben Eliezer N, Novikov DS, Lattanzi R, Duan Q, Sodickson L, Zhu Y. Local Maxwell Tomography Using Transmit-Receive Coil Arrays for Contact-Free Mapping of Tissue Electrical Properties and Determination of Absolute RF Phase. ISMRM; Melbourne, Australia. 2012; p. 387. [Google Scholar]
- 53.Sodickson DK, Alon L, Deniz CM, Ben-Eliezer N, Cloos M, Sodickson LA, Collins CM, Wiggins GC, Novikov DS. Generalized Local Maxwell Tomography for Mapping of Electrical Property Gradients and Tensors. ISMRM 21st Annual Meeting & Exhibition; Salt Lake City, Utah, USA. 2013; p. 4175. [Google Scholar]
- 54.Ianniello C, Brown R, Cloos M, Duan Q, Walczyk J, Wiggins G, Sodickson D, Lattanzi R. Sugar free tissue-mimicking MRI phantoms for improved signal-to-noise ratio. ISMRM 24th annual meeting & exhibition; Singapore. May 7–13, 2016; 2016. p. 2239. [Google Scholar]
- 55.Kraft KA, Fatouros PP, Clarke GD, Kishore PR. An MRI phantom material for quantitative relaxometry. Magnetic resonance in medicine. 1987;5(6):555–562. doi: 10.1002/mrm.1910050606. [DOI] [PubMed] [Google Scholar]
- 56.Howe FA. Relaxation times in paramagnetically doped agarose gels as a function of temperature and ion concentration. Magnetic resonance imaging. 1988;6(3):263–270. doi: 10.1016/0730-725x(88)90400-6. [DOI] [PubMed] [Google Scholar]
- 57.Kurmis AP, Slavotinek JP, Barber C, Fazzalari NL. Determining disk hydration status with a MnCl2-based MR model. Radiologic technology. 2008;79(6):507–513. [PubMed] [Google Scholar]
- 58.Kurmis AP, Barber C, Slavotinek JP, Fazzalari NL. A MnCl2-based MR signal intensity linear response phantom. Radiologic technology. 2007;79(2):119–125. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table S1. Comparison between measured and theoretical dielectric properties of four known materials.
Supporting Fig. S1. Measured and theoretical real and imaginary part of methanol complex permittivity over the frequency range 50 MHz – 4.5 GHz