Abstract
Objectives
The location and mechanisms of the initial generation of autoantibodies to citrullinated (cit) and non-cit proteins/peptides during the natural history of rheumatoid arthritis (RA) development is incompletely understood. Herein, we sought to explore individual antibody responses to cit and non-cit proteins/peptides in the sputum and associations with neutrophil extracellular traps (NETs) in subjects At-Risk for the future development of RA.
Methods
We studied 41 RA-free subjects At-Risk for RA based on familial or serologic risk factors, 20 classified RA subjects and 22 healthy controls. Serum and sputum were evaluated using a bead-based array for IgG reactivity to 29 cit proteins/peptides and 21 non-cit proteins/peptides. Cut-off levels for antibody positivity were established in a separate control group. Sputum NET levels were measured using sandwich ELISAs that quantitate DNA-myeloperoxidase and DNA-neutrophil elastase complexes.
Results
In At-Risk subjects, antibody responses to cit-fibrinogen, cit-apolipoprotein-E and cit-fibronectin were highly prevalent. The most cit specific sputum antibodies in At-Risk subjects were to fibrinogen, vimentin and peptides of fibrinogen A and apolipoprotein A1. Patterns of sputum autoantibody positivity differed between At-Risk and RA subjects. In At-Risk subjects, increasing sputum NET levels significantly correlated with several cit and some non-cit antibody reactivities.
Conclusions
These findings suggest that sputum antibody reactivity to particular cit and non-cit proteins/peptides is specific for At-Risk and RA subjects, and their association with NETs may be a key feature of early RA-related autoimmunity in the lung. These findings further support the hypothesis that the lung plays a role in early RA-related autoimmunity.
Keywords: Rheumatoid arthritis, ACPA, anti-CCP, lung, NETs
INTRODUCTION
Citrullination is the post-translational modification of peptidyl-arginine to peptidyl-citrulline that is catalyzed through peptidylarginine deiminase (PAD) enzymes(1). Citrullination is a normal physiologic process that can be upregulated during inflammation(2). In addition, citrullination of certain self-antigens has been associated with autoimmune responses characterized by antibodies to citrullinated (cit) protein/peptide antigens (ACPAs)(3).
Serum ACPAs are strongly associated with rheumatoid arthritis (RA). In RA, ACPA reactivity has been identified to a number of different cit-protein/peptide antigens. Antibody reactivity to the native non-cit counterparts of these antigens has also been identified in many RA patients(4–9). Furthermore, serum antibody reactivity to cit and non-cit antigens has been identified prior to the onset of clinically-apparent synovitis during a ‘preclinical’ phase of RA development(10–12), and several studies demonstrate that an increasing number of cit-antigens are targeted as individuals transition from preclinical to classified RA(13, 14). These findings suggest that a key part of RA development is early reactivity to a limited number of cit as well as non-cit antigens with expansion/epitope spreading over time as inflammatory arthritis and classified RA develops.
As the goals in RA management shift to include the prevention of arthritis(15), it is necessary to further understand the mechanisms underlying the initial development of antibody responses in RA and identify relevant immune targets for prevention. Several lines of evidence suggest that one major site where RA-related antibodies may be initially generated is the lung mucosa(16–19). Relevant to this study, our group has demonstrated through induced sputum testing that ACPA, as characterized by anti-cyclic cit peptide (CCP) antibodies, are generated in the lung in a portion of subjects with classified RA and individuals with an increased risk of developing RA in the future(17, 19). However, individual sputum antibody reactivities to cit and non-cit peptides/proteins have not been characterized in these subjects.
In addition to understanding early autoantibody targets in sputum, it is also important to understand potential mechanisms that could generate these autoantibody responses. One mechanism of particular interest is the formation of neutrophil extracellular traps (NETs). NET formation (termed NETosis) can externalize cit and non-cit proteins that can be taken up by antigen presenting cells and could lead to antibody generation(8). NETs can also serve as antigenic targets for RA-related autoantibodies (20, 21). Furthermore, our group has identified a strong positive correlation between sputum levels of anti-CCP and NETs in subjects At-Risk for RA(19), but the individual sputum autoantibodies associated with NETosis have not been previously identified. Therefore, in this study, we sought to explore antibody responses to individual cit and non-cit proteins/peptides as well as associations with NETosis in the sputum of subjects At-Risk for RA.
METHODS
Study Subjects
Subjects were recruited from the Studies of the Etiology of RA (SERA) Lung Study that is described in detail elsewhere(17, 19). Briefly, SERA is a cohort designed to study the natural history of RA through the prospective study of individuals who have an increased risk of developing RA in the future(22). The SERA Lung Study was designed to study biomarkers of autoimmunity in the lung during different phases of RA development by collecting blood and sputum samples from subjects At-Risk for RA from the SERA cohort, as well as subjects with classified RA and healthy control subjects. For the current study, randomly selected stored samples were included from SERA Lung Study subjects who participated between January 2011 and December 2014.
At-Risk subjects
We included 41 subjects without current or prior inflammatory arthritis who were At-Risk for developing RA based on a familial or serologic risk. Specifically, we included 30 subjects with a ‘Familial’ risk of RA that was defined as having a first-degree relative (FDR) with RA. Four of 30 (13%) FDRs were serum anti-CCP positive (CCP2 and/or CCP3.1). We also included 11 subjects with a ‘Serologic’ risk of RA that was defined as having serum anti-CCP positivity (CCP2 and/or CCP3.1) in the absence of inflammatory arthritis identified through community health fair, clinic or research-based blood test screening.
RA subjects
We included 20 subjects with established RA who met RA classification criteria based on medical chart review(23, 24). All were serum anti-CCP positive (CCP2 and/or CCP3.1), and 16/20 (80%) were currently or previously taking disease modifying anti-rheumatic drugs or biologics.
Healthy Controls
We included 44 healthy controls who were recruited through local advertisement and were without RA or inflammatory arthritis, did not have an FDR with RA and were serum anti-CCP negative (CCP2 and CCP3.1). These 44 subjects were randomly split into two groups of 22. One group was used to establish cut-off levels for antibody positivity (Cut-off Controls) and the other was used for comparative analyses (Comparator Controls).
Study visit
All subjects had a paired collection of blood and sputum at their study visit. Subjects without RA underwent a joint-focused interview and 66-count joint examination to assure the lack of clinically-evident inflammatory arthritis. Standardized questionnaires were used to obtain demographic information and self-reported histories of smoking and chronic lung disease.
Genetic testing
Blood was tested for the presence of alleles containing the shared epitope (SE) using previously described methodologies(22). The alleles considered to contain the SE were as follows: DRB1*04:01, 04:04, 04:05, 04:08, 04:09, 04:10, and DR1 01:01 and 01:02.
Sputum collection and processing
Induced sputum was collected using established protocols(17, 19). Briefly, subjects underwent a 15-minute inhalation of nebulized hypertonic saline. To minimize salivary contamination, subjects underwent an oral wash prior to inhalation of saline, and during the sputum collection, subjects were instructed to spit any saliva into a separate container and only use the sputum collection cup when producing a sample from coughing. In addition, sputum samples used in this study demonstrated <10 squamous epithelial cells/high power field on light microscopy or <80% squamous epithelial cells on cell differential, consistent with lower airway origin of the sample(25, 26).
After collection, the sputum sample underwent weight-based dilution with phosphate buffered saline (PBS) followed by mechanical syringe-based homogenization. Samples then underwent centrifugation, and the sputum supernatant was used for all biomarker testing.
Anti-cit and non-cit antibody testing
Serum and sputum were tested for IgG reactivity to 29 individual cit-proteins/peptides using a bead-based array developed in the laboratory of W.H.R. and described in detail elsewhere(3, 9, 27). These cit-proteins/peptides were selected based on antigens reported in the literature as well as identified in immune complexes isolated from synovial tissue from RA patients(28) and are listed in Supplemental Table 1. For 21 of the 29 cit-proteins/peptides, a non-cit/native equivalent was also tested. Antigens were conjugated to spectrally distinct beads (Bio-Plex) and incubated with the subject’s sample for 30 minutes at room temperature. After washing, beads were incubated with PhycoErythrin (PE)-conjugated anti-human-IgG antibody and incubated at room temperature. Beads were then passed through a detector (Luminex200, Bio-Plex-Software V.5.0, Bio-Rad) that quantified the amount of antibody bound to each bead in fluorescence intensity (FI). All antibody testing was performed in a blinded fashion.
We established a positive cut-off level in sputum and serum for each anti-cit and non-cit protein/peptide antibody that was above the 95th percentile in the 22 Cut-off Controls (i.e. present in <5%). This definition for a positive autoantibody has been previously recommended in the American College of Rheumatology RA classification criteria(24).
To test reproducibility of the antigen array for sputum, 10 sputum samples were randomly selected to undergo repeated testing for antibody levels to cit-antigens in a blinded fashion and included samples from RA, At-Risk and Control subjects. The mean coefficient of variation was calculated for each of the 29 antibodies to cit antigens, and the mean (SD) for the mean coefficient of variation was 7.2% (1.7).
Anti-CCP testing
Clinical ACPA testing commonly uses commercially-available anti-CCP assays, although these assays cannot distinguish individual ACPAs. In this study, serum was tested for anti-CCP using commercial ELISA assays: CCP3.1 (IgG/IgA, Inova Diagnostics, San Diego, CA, USA) and CCP2 (IgG, Axis-Shield, Dundee, United Kingdom). Serum positivity was determined based on the manufacturer’s recommended cut-off level (≥20 units for CCP3.1 and >5 units for CCP2).
Sputum was also tested for anti-CCP, but limited to the CCP2 assay (IgG). Because CCP2 is an IgG-only assay, it was comparable with the isotype reactivity detected by our antigen array testing. CCP2 results in ELISA units were calculated using the standard curve provided by the manufacturer followed by multiplication of the sputum dilution factor. We established a cut-off level for sputum anti-CCP2 positivity using the same approach used for determining positive/negative levels of serum and sputum anti-cit and non-cit antibodies (i.e. above the 95th percentile in Cut-off Controls).
Rheumatoid factor (RF) testing
Sputum was tested for RF isotypes (IgA, IgG and IgM) using commercial ELISA assays (Inova Diagnostics, San Diego, CA, USA). Using the same approach applied to other sputum antibody levels, we established a cut-off level for sputum RF positivity that was above the 95th percentile in the Cut-off Control group.
NET complexes
Twenty-four At-Risk subjects and 33 healthy Controls who had an adequate volume of sputum available underwent NET testing. NETs were quantified in the sputum cell-free supernatant by measuring NET-specific protein/nucleic acid complexes using a sandwich ELISA that detects DNA-myeloperoxidase (MPO) and DNA-neutrophil elastase (NE) complexes as previously described(29). Briefly, for DNA-MPO complexes, high-binding 96-well ELISA microplates were incubated overnight at 4°C with mouse anti-human MPO (clone 4A4; AbD, Serotec) in coating buffer from the Cell Death Detection ELISA kit (Roche). After blocking with 1% BSA, plates were incubated overnight at 4°C with 10% sputum in blocking buffer, washed and anti-DNA-POD (monoclonal anti-DNA antibody from mouse clone MCA-33 conjugated with peroxidase, Roche) was added for 1.5 hours at room temperature. TMB substrate (Sigma) was then added and absorbance measured at 450nm after addition of stop reagent (Sigma). Because this assay detects binding of protein complexes and not antibodies, amplification of RF is unlikely. Similar methods were used for DNA-NE complexes with the antibody used to coat plates being rabbit anti-NE (Calbiochem). In addition, after overnight incubation with sputum, plates were incubated for 1 hour at room temperature with mouse anti-dsDNA monoclonal antibody (Millipore), followed by anti-mouse IgG-HRP conjugate (Biorad). To control for plate-to-plate variation, the same healthy control samples were included on every plate and an OD index was calculated based on the average of the healthy control samples’ absorbance at 450nm. All samples were run in duplicate.
Statistical Analysis
Subject characteristics were compared between groups using Kruskal-Wallis testing for age and Chi-square/Fishers Exact test for dichotomous variables. Regression models were used to compare associations between sputum antibody positivity (logistic) and levels (linear) while accounting for covariates. The median number of positive antibodies in serum and sputum were compared using nonparametric matched pair analyses. Cohen’s kappa coefficient (κ) was used to compare agreement between sputum and serum antibody positivity. Because antibody levels followed a non-normal distribution, Kruskal-Wallis (3 groups) and Mann-Whitney testing (2 groups) were used to compare antibody levels between groups and Spearman’s correlation coefficient was used to determine correlation between antibody and NET levels. Because each antibody had a unique range of reactivity, when comparing levels between different antibodies, we normalized each antibody by dividing the antibody level by the median level in the Cut-Off Control group for that particular antibody. In addition, when comparing ratios of cit- to non-cit antibody levels, we used these normalized values.
A p-value of <0.05 was considered significant except for analyses that compared each of the 29 individual antibodies. For these analyses, we used Bonferroni correction to account for multiple comparisons and a p-value of ≤0.002 (0.05 divided by 29 comparisons) was considered significant. All analyses were performed using SPSS software, version 23 and figures were generated using GraphPad Prism version 7.
RESULTS
Demographics
At-Risk and RA subjects were older and marginally more often male than Controls, and RA subjects were more likely to be current smokers (Table 1). Other demographic characteristics were not significantly different between groups.
Table 1.
Subject characteristics
| Cut-Off Controls (N=22) | Comparator Controls (N=22) | At-Risk1 (N=41) | RA (N=20) | p-value2 | |
|---|---|---|---|---|---|
| Age, median (range) | 37 (23–65) | 31 (22–65) | 54 (29–79) | 57 (36–75) | <0.01 |
| Female, N (%) | 20 (91) | 18 (82) | 27 (66) | 13 (65) | 0.10 |
| Non-Hispanic white, N (%) | 15 (71) | 16 (73) | 30 (73) | 11 (58) | 0.66 |
| Ever-smoker, N (%) | 5 (23) | 6 (27) | 14 (34) | 10 (50) | 0.26 |
| Current smoker, N (%) | 0 (0) | 1 (5) | 1 (2) | 4 (20) | 0.02 |
| ≥1 Shared Epitope allele, N (%) | 8 (36) | 10 (46) | 20 (49) | 12 (67)3 | 0.29 |
| Chronic lung disease, N (%) | 3 (14) | 1 (5) | 10 (24) | 4 (20) | 0.23 |
| Serum anti-CCP (+), N (%) | 0 (0) | 0 (0) | 15 (37) | 20 (100) | <0.01 |
| ≥1 Sputum ACPA4 (+), N (%) | 4 (18) | 1 (5) | 18 (44) | 15 (75) | <0.01 |
| ≥1 Serum ACPA4 (+), N (%) | 8 (36) | 6 (27) | 23 (56) | 19 (95) | <0.01 |
Comparing At-Risk subjects who were included based on Familial vs. Serologic risk, there was no significant difference in the characteristics listed in the table except that serum anti-CCP positivity was higher in Serologic At-Risk subjects (100% vs. 13%, p<0.01). Notably, there was no difference in the prevalence of ≥1 sputum ACPA positive (Familial 47% vs. Serologic 36%, p=0.73)
P-value compares the median age (Kruskal-Wallis) or prevalence of positivity for other characteristics (Chi-square/Fishers) between all groups.
Only 18 of 20 had DNA available for SE testing.
ACPA defined as a positive antibody response to 1 of the 29 cit proteins/peptides tested in this study.
Sputum antibody responses to cit proteins/peptides
Overall, 18/41 (43.9%) At-Risk and 15/20 (75.0%) RA subjects demonstrated ≥1 positive sputum antibody to 1 of the 29 cit proteins/peptides tested (Table 1). This prevalence was significantly higher in At-Risk and RA subjects compared to Controls (p<0.01 for both). Using logistic regression and adjusting for age, At-Risk and RA subjects were more likely to have ≥1 positive sputum antibody to a cit protein/peptide compared to Controls (At-Risk: OR=21.3, 95% CI 1.8–253.1; RA: OR=79.1, 95% CI 5.5–1143.3). In addition, the total number of sputum antibodies to cit proteins/peptides was significantly higher in At-Risk and RA subjects compared to Controls (Supplemental Figure 1).
Within the At-Risk subjects, the most prevalent sputum antibody responses to cit proteins/peptides were directed to cit-fibrinogen (34.1%), cit-apolipoprotein E (31.7%), cit-fibronectin (31.7%), cit-histone 2B (29.3%) and cit-histone 2A (26.8%) (Table 2 and Supplemental Table 2). When accounting for multiple comparisons, anti-cit-fibrinogen, anti-cit-apolipoprotein E and anti-cit-fibronectin were significantly more prevalent in At-Risk subjects compared to Controls (p≤0.002).
Table 2.
Most prevalent sputum antibody responses to cit proteins/peptides in At-Risk and RA subjects
| N, (%) | HC (N=22) | AR (N=41) | RA (N=20) | AR v. HC p-value1 | RA v. HC p-value1 | AR v. RA p-value1 |
|---|---|---|---|---|---|---|
| Cit-fibrinogen | 0 (0) | 14 (34) | 5 (25) | 0.001 | 0.018 | 0.564 |
| Cit-apolipoprotein E | 0 (0) | 13 (32) | 3 (15) | 0.002 | 0.099 | 0.222 |
| Cit-fibronectin | 0 (0) | 13 (32) | 3 (15) | 0.002 | 0.099 | 0.222 |
| Cit-histone 2B | 0 (0) | 12 (29) | 8 (40) | 0.005 | 0.001 | 0.402 |
| Cit-histone 2A | 1 (5) | 11 (27) | 7 (35) | 0.043 | 0.018 | 0.511 |
| Cit-filaggrin (48–65) cyclic | 0 (0) | 4 (10) | 8 (40) | 0.288 | 0.001 | 0.013 |
| Cit-fibrinogen A (616–635) cyclic | 0 (0) | 5 (12) | 6 (30) | 0.153 | 0.007 | 0.153 |
| Cit-fibronectin (1029–1042) | 0 (0) | 7 (17) | 6 (30) | 0.086 | 0.007 | 0.247 |
| Cit-clusterin (231–250)cyclic | 0 (0) | 6 (15) | 6 (30) | 0.083 | 0.007 | 0.156 |
p-value compares the prevalence of positivity between two groups using chi-square or Fishers Exact testing as appropriate. p≤0.002 (bolded values) is considered significant based on Bonferroni correction.
Abbreviations: HC=Healthy Controls, AR=At-Risk, RA=Rheumatoid Arthritis, Cit=citrullinated
Within RA subjects, the most prevalent sputum antibodies to cit proteins/peptides were directed to cit-histone 2B (40.0%), cit-filaggrin 48–65 cyclic (40.0%), cit-histone 2A (35.0%), cit-fibrinogen A 616–635 cyclic (30.0%), cit-fibronectin 1029–1042 (30.0%) and cit-clusterin 231–250 cyclic (30.0%) (Table 2 and Supplemental Table 2). Sputum antibodies to cit-histone 2B and anti-cit-filaggrin 48–65 cyclic were significantly more prevalent in RA compared to Controls (p≤0.002). Notably, antibodies to cit-histone 2B and cit-histone 2A were among the most prevalent sputum antibody responses in both At-Risk and RA subjects.
Sputum antibody responses to non-cit proteins/peptides
In addition to anti-cit antibodies, antibodies to non-cit/native proteins have been identified in the serum of preclinical and early classified RA subjects(4–9). These data suggest that early autoimmunity may be to native proteins initially, with evolution to cit epitopes over time through epitope spreading. Therefore, it is important to understand antibody reactivity to both cit and non-cit antigens during different phases of RA development.
Overall, we identified ≥1 positive sputum antibody to a non-cit protein/peptide in 23/41 (56.1%) At-Risk and 13/20 (65.0%) RA subjects. When compared to Controls, sputum positivity for ≥1 antibody to a non-cit antigen was significantly higher in At-Risk (56% vs. 14%, p<0.01) and RA subjects (65% vs. 14%, p<0.01).
Comparison of sputum antibody responses to cit and non-cit proteins/peptides
For the 21 antibodies that had a cit and non-cit antigen counterpart, in subjects with sputum antibody positivity to cit proteins/peptides, we considered a cit/non-cit ratio >1 to be consistent with cit specificity. In At-Risk subjects with a positive sputum antibody to any cit protein/peptide, a high level of cit-specificity (≥75% of subjects) was demonstrated for the proteins fibrinogen and vimentin and the peptides apolipoprotein A1 231–248, fibrinogen A 556–575 cyclic and fibrinogen A 582–599 (Table 3). In RA subjects with a positive sputum antibody to any cit protein/peptide, a high level of cit-specificity (≥75% of subjects) was demonstrated for the proteins histone 2A, histone 2B and vimentin and the peptides histone 2A/a-2 1–20, fibrinogen A 41–60 cyclic, fibrinogen A 556–575 cyclic and fibrinogen A 616–635 cyclic. In RA compared to At-Risk subjects, there was a non-significant trend toward a higher prevalence of cit-specificity in RA subjects for fibrinogen A 616–635 cyclic and filaggrin 48–65 cyclic. In addition, in the one Comparator Control who had two positive sputum antibodies to cit proteins/peptides, both antibody responses demonstrated cit-specificity with a cit/non-cit ratio >1.
Table 3.
Prevalence of sputum anti-cit antibody level being higher than sputum anti-non-cit antibody level in subjects with sputum antibody positivity for each cit protein/peptide1
| Cit Proteins, N2 (%) | At-Risk (N=41) | RA (N=20) | p-value3 |
|---|---|---|---|
| Apolipoprotein A1 | 1/7 (14) | 1/3 (33) | 1.0 |
| Histone 2A | 5/11 (45) | 6/7 (86) | 0.15 |
| Histone 2B | 5/12 (42) | 6/8 (75) | 0.20 |
| Fibrinogen | 11/14 (79) | 3/5 (60) | 0.57 |
| Vimentin | 6/8 (75) | 4/4 (100) | 0.52 |
| Cit Peptides, N (%) | |||
| Apolipoprotein A1 231–248 | 3/4 (75) | 1/3 (33) | 0.49 |
| Biglycan 247–266 cyclic | 2/4 (50) | 2/4 (50) | 1.0 |
| Clusterin 231–250 cyclic | 2/6 (33) | 3/6 (50) | 1.0 |
| Histone 2A/a-2 1–20 | 1/2 (50) | 3/3 (100) | 0.40 |
| Histone 2B/a 62–81 cyclic | 2/3 (67) | 0/2 (0) | 0.40 |
| Enolase 1A 5–21 | 2/8 (25) | 2/5 (40) | 1.0 |
| Fibrinogen A 41–60 cyclic | 2/3 (67) | 3/4 (75) | 1.0 |
| Fibrinogen A 211–230 cyclic | 2/4 (50) | 1/3 (33) | 1.0 |
| Fibrinogen A 556–575 cyclic | 4/5 (80) | 3/3 (100) | 1.0 |
| Fibrinogen A 582–599 | 3/4 (75) | 0/1 (0) | 0.40 |
| Fibrinogen A 616–635 cyclic | 2/5 (40) | 6/6 (100) | 0.06 |
| Fibrinogen B 54–72 | 2/5 (40) | 1/4 (25) | 1.0 |
| Fibrinogen B 246–267 | 1/5 (20) | 1/5 (20) | 1.0 |
| Filaggrin 48–65 cyclic | 0/4 (0) | 5/8 (63) | 0.08 |
| Vimentin 1–16 | 2/5 (40) | 1/4 (25) | 1.0 |
| Vimentin 58–77 cyclic | 1/3 (33) | 3/5 (60) | 1.0 |
All cit/non-cit ratios were calculated using the normalized antibody level that was divided by the median level for that antibody in the Cut-Off Control group.
N/N results display the number with a cit/non-cit ratio >1 divided by the total number of subjects with a positive sputum antibody response to that particular protein or peptide.
P-value calculated using Fisher’s Exact test comparing At-Risk and RA subjects.
Comparison of sputum ACPA positivity by array and anti-CCP2 positivity
We found that 6/6 (100%) sputum anti-CCP2 positive At-Risk and 11/12 (92%) sputum anti-CCP2 positive RA subjects demonstrated ≥1 positive sputum antibody to a cit protein/peptide on the antigen array tested. All of these sputum anti-CCP2 positive At-Risk and RA subjects with a positive antibody response to a cit protein/peptide demonstrated cit-specificity to at least one of those antigens based on a cit/non-cit ratio >1. Of the 35 At-Risk and 4 RA subjects who were sputum anti-CCP2 negative, 12/35 (34%) and 4/8 (50%) demonstrated ≥1 positive sputum antibody to a cit protein/peptide. In these 12 anti-CCP2 negative At-Risk and 4 anti-CCP2 negative RA subjects who had a positive antibody response to a cit protein/peptide, 11/12 At-Risk and 4/4 RA demonstrated cit-specificity to at least one of those antigens.
Sputum antibody positivity in subjects stratified by serum antibody status
Using the 29 cit proteins/peptides tested, we identified 18/44 (44%) At-Risk and 19/20 (95%) RA subjects who had ≥1 positive antibody to a cit antigen in serum. The majority of these serum antibody responses demonstrated cit-specificity (Supplemental Table 3). In addition, in At-Risk subjects, antibodies to the proteins fibrinogen and vimentin as well as peptides fibrinogen A 582–599 and fibrinogen A 556–575 cyclic demonstrated high cit specificity in sputum as well as serum.
Following these experiments, we stratified At-Risk subjects based on serum antibody positivity to cit antigens. The rationale for this stratification is that more of the At-Risk subjects who have already developed a systemic RA-related autoimmune response can be assumed to be further along the pathway to developing classifiable RA compared to serum antibody negative individuals, although even amongst the serum ACPA positive subjects, not all will develop classifiable RA. In these analyses and using the 29 cit protein/peptide antigens, we found ≥1 positive sputum antibody in 6/18 (33.3%) serum antibody negative and 12/23 (52.2%) serum antibody positive At-Risk subjects. The prevalence of each sputum anti-cit antibody is listed in Supplemental Table 4. Within At-Risk subjects, there was overall poor agreement between sputum and serum antibody positivity to cit antigens (κ≤0.25). However, there was higher agreement for cit-enolase-1A 5–21 (κ=0.55), where 4/8 (50%) sputum anti-cit-enolase-1A 5–21 positive At-Risk subjects were also serum positive.
Within the At-Risk subjects who were negative for serum antibodies to cit antigens, the most prevalent sputum antibodies were directed to cit-fibrinogen (27.8%), cit-apolipoprotein E (22.2%), and cit-fibronectin (22.2%). Similarly, within At-Risk subjects who were positive for any serum antibody response to a cit protein/peptide, the most prevalent sputum antibodies were to cit-fibrinogen (39.1%), cit-apolipoprotein E (39.1%) and cit-fibronectin (39.1%), but also included cit-histone 2B (39.1%), cit-histone 2A (34.8%), cit-enolase-1A 5–21 (30.4%), cit-apolipoprotein E 277–296 cyclic (30.4%) and cit-vimentin (30.4%). These rates were all higher compared to Controls (all p≤0.05), with significantly higher prevalence (p≤ 0.002) for cit-fibrinogen, cit-apolipoprotein E, cit-fibronectin and cit-histone 2B.
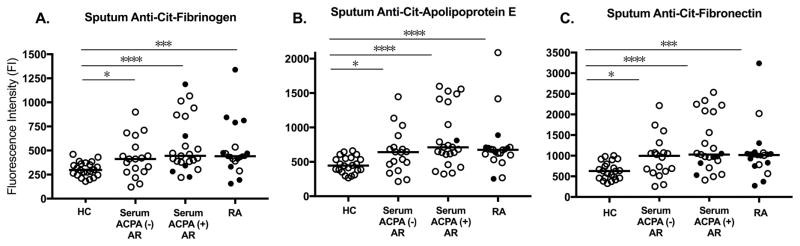
In addition to prevalence of positivity, the median sputum level of anti-cit-fibrinogen, anti-cit-apolipoprotein E and anti-cit-fibronectin was significantly higher in serum antibody positive At-Risk subjects and RA subjects compared to Controls (Figure 1).
Figure 1. Sputum antibody levels to cit proteins by subject group.
The figure depicts the levels of sputum anti-citrullinated fibrinogen (Panel A), anti-citrullinated apolipoprotein E (Panel B), and anti-citrullinated fibronectin (Panel C) in Comparator Healthy Controls (HC, N=22), Serum ACPA(−) At-Risk subjects (N=18), Serum ACPA(+) At-Risk subjects (N=23) and RA subjects (N=20). P-values compare median sputum antibody levels between groups (Mann-Whitney U). * p≤0.01, *** p≤0.001, **** p≤0.0001. ● = serum positive for the depicted ACPA,
 = serum negative for the depicted antibody, Median antibody level. Abbreviations: HC=Comparator Healthy Control group, ACPA=Antibody response to citrullinated protein/peptide antigen based on antibody positivity to any of the 29 cit antigens tested in the study, AR=At-Risk, Cit=citrullinate
= serum negative for the depicted antibody, Median antibody level. Abbreviations: HC=Comparator Healthy Control group, ACPA=Antibody response to citrullinated protein/peptide antigen based on antibody positivity to any of the 29 cit antigens tested in the study, AR=At-Risk, Cit=citrullinate
Sputum NET levels and ACPA positivity in At-Risk subjects
Overall, sputum NET levels were higher in At-Risk subjects with ≥1 positive antibody response to a cit or non-cit antigen in sputum (N=15) compared to sputum antibody negative Controls (N=27). Specifically, the median (IQR) in ODindex for sputum DNA-MPO was 6.3 (3.7–8.1) vs. 3.7 (3.1–4.4) (p<0.01) and for DNA-NE was 4.9 (2.6–12.2) vs. 3.5 (2.6–5.0) (p=0.06). These differences are in line with our prior published data comparing NET levels in sputum anti-CCP positive At-Risk and Control subjects(19).
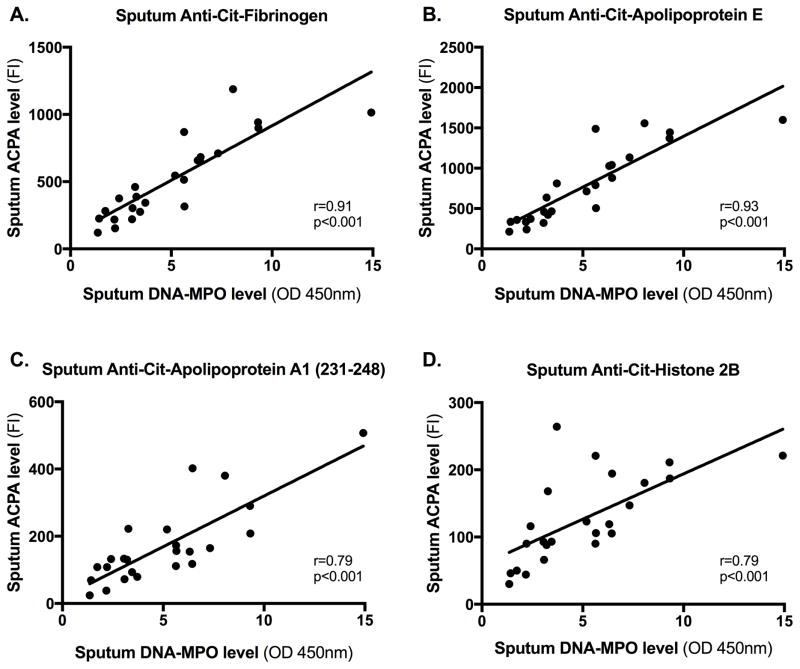
We also determined correlations between sputum levels of NET complexes and antibodies to cit and non-cit proteins/peptides using a conservative approach that identified significant associations only if two separate NET assays (DNA-MPO and DNA-NE) demonstrated a significant correlation after accounting for multiple comparisons (p≤0.002). With this approach, we found a significant positive correlation between sputum NET levels and the majority of antibody responses to cit proteins/peptides [27/29 (93%), Table 4 and Figure 2]. Furthermore, after adjusting for smoking that has been associated with NETosis(30, 31), sputum NET levels remained significantly associated with 15/29 antibodies including antibodies to the cit-fibrinogen, cit-apolipoprotein A1 231–248 and fibrinogen A 582–599 that also had demonstrated high sputum cit-specificity in At-Risk subjects (Table 4). The following antigens also demonstrated significant associations between sputum NET levels and the antibody responses to the cit antigen, but not the non-cit antigen, after adjusting for smoking: histone 2A/a-2 1–20, fibrinogen A 211–230 cyclic, fibrinogen B 36–52 and vimentin 58–77 cyclic.
Table 4.
Correlations between sputum levels of antibodies to cit proteins/peptides and sputum NET levels in At-Risk subjects
| DNA-MPO | DNA-NE | |||
|---|---|---|---|---|
| Cit-Proteins** | r-value | p-value* | r-value | p-value* |
| Apolipoprotein A1 | 0.86 | <0.001 | 0.66 | <0.001 |
| Apolipoprotein E§ | 0.93 | <0.001 | 0.73 | <0.001 |
| Histone 2A | 0.80 | <0.001 | 0.69 | <0.001 |
| Histone 2B | 0.79 | <0.001 | 0.66 | <0.001 |
| Fibrinogen§ | 0.91 | <0.001 | 0.74 | <0.001 |
| Fibronectin | 0.90 | <0.001 | 0.72 | <0.001 |
| Vimentin | 0.79 | <0.001 | 0.67 | <0.001 |
| Cit-Peptides** | ||||
| Apolipoprotein A1 231–248§ | 0.79 | <0.001 | 0.72 | <0.001 |
| Apolipoprotein E 277–296 cyclic§ | 0.89 | <0.001 | 0.80 | <0.001 |
| Biglycan 247–266 cyclic | 0.76 | <0.001 | 0.66 | <0.001 |
| Clusterin 221–240 cyclic | 0.81 | <0.001 | 0.65 | <0.001 |
| Clusterin 231–250 cyclic | 0.74 | <0.001 | 0.67 | <0.001 |
| Histone 2A/a-2 1–20§ | 0.78 | <0.001 | 0.70 | <0.001 |
| Histone 2A/a-2 1–20 cyclic§ | 0.75 | <0.001 | 0.70 | <0.001 |
| Histone 2B/a 62–81 cyclic | 0.80 | <0.001 | 0.68 | <0.001 |
| Enolase 1A 5–21§ | 0.91 | <0.001 | 0.77 | <0.001 |
| Fibrinogen A 27–43§ | 0.83 | <0.001 | 0.74 | <0.001 |
| Fibrinogen A 41–60 cyclic | 0.72 | <0.001 | 0.62 | 0.001 |
| Fibrinogen A 211–230 cyclic§ | 0.80 | <0.001 | 0.69 | <0.001 |
| Fibrinogen A 556–575 cyclic | 0.65 | <0.001 | 0.56 | 0.004 |
| Fibrinogen A 582–599§ | 0.81 | <0.001 | 0.71 | <0.001 |
| Fibrinogen A 616–635 cyclic§ | 0.86 | <0.001 | 0.77 | <0.001 |
| Fibrinogen B 36–52§ | 0.84 | <0.001 | 0.73 | <0.001 |
| Fibrinogen B 54–72§ | 0.83 | <0.001 | 0.74 | <0.001 |
| Fibrinogen B 246–267 | 0.78 | <0.001 | 0.72 | <0.001 |
| Fibronectin 1029–1042 | 0.64 | <0.001 | 0.58 | 0.003 |
| Filaggrin 48–65 cyclic§ | 0.81 | <0.001 | 0.71 | <0.001 |
| Vimentin 1–16 | 0.81 | <0.001 | 0.70 | <0.001 |
| Vimentin 58–77 cyclic§ | 0.84 | <0.001 | 0.76 | <0.001 |
P-value calculated using Spearman’s correlation coefficient.
The following non-citrullinated proteins/peptides were significantly correlated with DNA-MPO and DNA-NE after adjusting for ever-smoking: Apolipoprotein A1, Fibrinogen, Histone 2A, Apolipoprotein A1 231–248, Enolase 1A 5–21, Fibrinogen A 582–599, Fibrinogen A 616–635 cyclic, Fibrinogen B 54–72 and Filaggrin 48–65 cyclic.
Indicates the citrullinated proteins/peptides that remained significantly correlated with DNA-MPO and DNA-NE (p≤0.002) after adjusting for ever-smoking.
Figure 2. Correlations between sputum antibody levels and NET levels in At-Risk subjects.
The figure depicts on the y-axis levels of sputum ACPA [anti-citrullinated fibrinogen (Panel A), anti-citrullinated apolipoprotein E (Panel B), anti-citrullinated apolipoprotein A1 (231–248) (Panel C) and anti-citrullinated fibronectin (Panel D)] and on the x-axis levels of sputum NETs as measured by DNA-MPO complexes in At-Risk subjects. Spearman’s correlation coefficient was used to calculate the R and P-values in each Panel. Abbreviations: ACPA=Antibody to citrullinated protein/peptide antigen, Cit=citrullinated, FI= fluorescence intensity, DNA-MPO=deoxyribonucleic acid-myeloperoxidase, OD 450nm= optical density at 450 nanometers
Of note, a non-cit antigen was not available for testing the following antigens that had significant associations between sputum antibodies to cit proteins/peptides and NET levels: apolipoprotein E, apolipoprotein E 277–296 cyclic, histone 2A/a-2 1–20 cyclic and fibrinogen A 27–43. Also, there was no significant difference in age, sex, race, SE positivity or smoking history between the 24 At-Risk subjects who underwent NET testing and the 17 who did not (data not shown).
We also examined the 33 healthy Controls who had sputum NET testing. There was a significant correlation (p≤0.002) between sputum NET levels and levels of antibodies to the following peptides: cit-biglycan 247–266 cyclic, cit-clusterin 221–240 cyclic, cit-clusterin 231–250 cyclic, cit-fibrinogen A 556–575 cyclic, cit-fibrinogen B 54–72, cit-fibrinogen B 246–267, cit-filaggrin 48–65 cyclic, cit-histone 2A/a-2 1–20 and cit-histone 2B/a 62–81 cyclic.
Correlations of sputum NET levels and RF
To determine if the relationships seen between NET levels and anti-cit/non-cit antibodies were limited to these antibodies or involved other autoantibody systems, we evaluated the correlation of sputum NET levels and sputum RF isotypes in the 9 At-Risk subjects who were negative for sputum anti-cit and non-cit antibodies. We found a significant correlation between RF-IgA and NET levels using both NET assays in sputum (DNA-MPO p<0.01; DNA-NE p=0.04). However, there was no significant association between NET levels and RF-IgM or RF-IgG (For RF-IgM, DNA-MPO p=0.24, DNA-NE p=0.61; For RF-IgG, DNA-MPO p=0.10, DNA-NE p=0.27). These results suggest that there is not a general association between autoantibodies and NETs in sputum. Of interest, these findings are in line with prior studies that report RF-IgA is strongly associated with NET formation, whereas RF-IgG is weakly and RF-IgM is not associated with NET formation(32).
DISCUSSION
Understanding the development of ACPA is a critical step in understanding the overall etiology and pathogenesis of RA. Our prior work has demonstrated the presence of anti-CCP antibodies in the sputum of subjects At-Risk for RA(17, 19). In this study, we demonstrate for the first time that a subset of individual antibody responses to cit and non-cit antigens are detectable in the sputum of subjects At-Risk for RA, including At-Risk subjects who are serum antibody negative and thereby informative of very early RA-related autoantibody development. We also demonstrate that sputum antibodies to both cit and non-cit antigens are significantly more prevalent in At-Risk and RA subjects compared to Controls. Furthermore, several sputum antibodies strongly correlate with increasing NET levels in subjects At-Risk for RA. Altogether, these data suggest that sputum antibody responses to particular cit and non-cit antigens, in association with higher levels of local NETosis in the lung, may be a key feature of early RA-related autoimmunity.
In this study, we found several sputum antibodies of particular interest in subjects At-Risk for RA. Sputum anti-cit-fibrinogen, anti-cit-apolipoprotein E and anti-cit-fibronectin antibodies were more prevalent in At-Risk subjects, including serum ACPA negative At-Risk subjects, suggesting that these proteins may represent the earliest antigen targets of antibodies generated in the lung in subjects At-Risk of RA. In particular, anti-cit-fibrinogen demonstrated high cit-specificity in sputum and serum of At-Risk subjects. In the lung, protein levels of fibrinogen, apolipoprotein E and fibronectin are known to be increased in the setting of inflammation and injury(33–35), and lung tissue plasma cells in RA patients have demonstrated production of anti-cit-fibrinogen antibodies(36). Linking these concepts as well as based on our prior work demonstrating increased airways inflammation in At-Risk subjects(16, 19), it may be that increased levels of these proteins promote the generation of an autoimmune response in a portion of subjects At-Risk of RA. In addition to fibrinogen, antibodies directed to vimentin, fibrinogen A 582–599 and fibrinogen A 556–575 cyclic also demonstrated cit specificity in the sputum and serum of At-Risk subjects, and antibodies to cit-enolase 1A 5–21 uniquely demonstrated agreement between the sputum and serum in At-Risk subjects. While these findings are cross-sectional, they suggest that these antibody responses may play a role in transitions from localized mucosal to systemic autoimmunity.
An additional sputum antibody of interest is anti-cit-histone 2B that was the only sputum antibody to a cit antigen that was significantly more prevalent in both serum antibody positive At-Risk and RA subjects compared to Controls. Antibodies directed to cit-histone 2B also demonstrated higher cit-specificity in the sputum of RA subjects. Together with data in murine models demonstrating that NET-associated anti-cit-histone 2B antibodies were associated with development of arthritis(37), these data suggest that anti-cit-histone 2B in the sputum may be relevant in transitions from At-Risk to classified RA.
We also found that the individual sputum antibodies most prevalent in At-Risk subjects differed from those most prevalent in classified RA. While this finding could be related to RA treatment effects, it may also reflect epitope spreading or changes in the lung during the evolution of RA-related autoimmunity. Moreover, we found that the number of autoantigens demonstrating high cit specificity increased in the sputum from At-Risk to RA subjects and to a larger degree in the serum. While epitope spreading is well described in the serum during the preclinical period of RA(13, 38), our findings suggest that epitope spreading may also occur in the lung, although longitudinal studies are needed to test this hypothesis.
Recently, our group described a significant correlation between sputum levels of anti-CCP and NET remnants(19). In this study, we expand on that finding to demonstrate that several sputum antibodies to cit as well as non-cit antigens significantly correlate with sputum NET levels in At-Risk subjects. Sputum NETs correlated with antibodies to cit-histone and cit-vimentin peptides but not their non-cit counterpart, and it is of interest that these are two cit-proteins that have been identified in the protein cargo of NETs induced in RA patients’ neutrophils(20). In this study, we cannot determine whether immunogenic proteins on NETs triggered local autoantibody generation or if local autoantibodies triggered increased NETs. However, findings from several recent studies support the hypothesis that NETs could trigger ACPA, including that NETs can themselves trigger immune responses associated with autoimmunity(39), that NETs in lupus patients can alter the structure and function of proteins including apolipoproteins(40), and that the uptake of NET proteins by antigen presenting cells is associated with systemic ACPA(8).
We also identified correlations between sputum NETs and antibodies to cit-antigen targets that have not been previously described in NETs. Additional studies are needed, but this finding could be due to unique protein cargo in the NETs generated by At-Risk subjects, the release of PAD enzymes from NETs which can result in citrullination of nearby proteins that could trigger ACPA(41) or cross-reactivity of ACPAs to several cit-antigen targets as has been demonstrated in RA patients(42–44). Although, it is of note, that while some ACPAs in RA demonstrate cross reactivity to multiple cit-antigens, others demonstrate non-overlapping cit antigen binding(43). We also found that multiple anti-cit antigen antibodies and RF-IgA but not RF-IgM and RF-IgG were associated with markers of NETosis in sputum suggesting that NETs are preferentially associated with certain antibodies. Overall, these data support the importance of additional studies that can specifically address the possibility of antibody cross-reactivity in the sputum as well as the role of NETs in specific antibody responses.
Furthermore, we identified correlations between sputum NETs and several sputum antibodies to cit and non-cit antigens in healthy Controls. While the sputum antibody levels were significantly lower in Controls compared to At-Risk or RA subjects, these findings suggest the possibility that even in individuals without a known risk for RA, low levels of antibody responses to cit or non-cit antigens may be associated with mucosal NETosis, perhaps reflecting a role for antibodies to cit or non-cit antigens in normal regulation of mucosal processes, that in some cases may become dysregulated leading to greater mucosal immunity and ultimately systemic inflammation and classified RA. Each of these potential mechanisms and hypotheses could improve our understanding of the early phases of ACPA development and should be explored in future studies.
In this study, we also found that At-Risk and RA subjects had higher sputum reactivity to non-cit proteins/peptides compared to Controls. This finding suggests that broad antibody reactivity to both non-cit and cit antigens occurs in the lung of these subjects, but that more cit specific reactivity is generated toward certain antigens and is more prevalent in subjects with RA. These findings support the hypothesis that early localized autoimmunity may be directed to native proteins, with evolution to cit epitopes occurring through epitope spreading(4–6, 45). However, it is also possible that tolerance is initially broken to cit proteins, and that the autoimmune responses to epitopes intramolecularly spreads to native epitopes on the same proteins. Longitudinal studies are needed to understand the temporal relationship between antibodies to cit and non-cit proteins/peptides.
There are several caveats to our study, including the cross-sectional design that limits inferences regarding the evolution of autoantibody responses over time. In addition, there is the potential for oral-pharyngeal contamination of sputum, and antibody translocation from the circulation. However, we believe the novel cross-sectional findings in this study are important to support longitudinal studies. In addition, as described in the Methods section, due to our careful collection of sputum that minimizes salivary contamination, and our previous demonstration of minimal translocation of antibodies from serum to sputum(17, 19), we believe that the findings herein reflect lung and airway biology. Furthermore, the antigen array we used was based on proteins known to be relevant in the RA joint, and while there are shared RA-related antigens between the lung and joints(46), it may be that to more deeply understand initial breaks in tolerance related to the lung, autoimmune response to lung-specific antigens need to be evaluated. Also, the portion of our At-Risk population that was sputum ACPA positive is likely higher than the portion that will go on to develop classified RA. Longitudinal studies will be informative to understand what factors lead to progression from local mucosal to systemic autoimmunity and ultimately classified RA. We also did not test for antibody cross-reactivity in this study. While future studies are needed in this area, we believe our current findings cannot all be explained by cross-reactivity, because some individuals had only 1 positive sputum antibody to a single cit antigen, and some individuals had sputum antibodies to cit antigens in the absence of non-cit antibody positivity. Finally, serum ACPA fine specificities differ between different RA populations(47), and it may be that similar findings could occur in the sputum, supporting the importance of additional sputum studies in other At-Risk populations.
In conclusion, we identified several sputum autoantibodies of particular interest in subjects At-Risk for future RA including sputum ACPAs with early cit specificity and those with strong associations with NETs in the sputum. These findings further support the hypothesis that the lung likely plays an important role in the development and evolution of RA-related autoimmunity.
Supplementary Material
Acknowledgments
Grants: This work was supported by the National Institutes of Health [grant numbers AR066712, AI101981, HD057022, AR063676 and AI103023], the Colorado Clinical and Translational Science Award UL1 TR001082-04, and the Intramural Research Program [NIAMS; project ZIAAR041199]. Rheumatology Research Foundation [Within our Reach and Scientist Development Award]; and the Walter S. and Lucienne Driskill Foundation. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Disclosures: Jeremy Sokolove is an employee of AbbVie pharmaceuticals.
References
- 1.Gyorgy B, Toth E, Tarcsa E, Falus A, Buzas EI. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol. 2006;38(10):1662–77. doi: 10.1016/j.biocel.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Baka Z, Gyorgy B, Geher P, Buzas EI, Falus A, Nagy G. Citrullination under physiological and pathological conditions. Joint Bone Spine. 2012;79(5):431–6. doi: 10.1016/j.jbspin.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Chandra PE, Sokolove J, Hipp BG, Lindstrom TM, Elder JT, Reveille JD, et al. Novel multiplex technology for diagnostic characterization of rheumatoid arthritis. Arthritis Res Ther. 2011;13(3):R102. doi: 10.1186/ar3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konig MF, Giles JT, Nigrovic PA, Andrade F. Antibodies to native and citrullinated RA33 (hnRNP A2/B1) challenge citrullination as the inciting principle underlying loss of tolerance in rheumatoid arthritis. Ann Rheum Dis. 2016;75(11):2022–8. doi: 10.1136/annrheumdis-2015-208529. [DOI] [PubMed] [Google Scholar]
- 5.de Pablo P, Dietrich T, Chapple IL, Milward M, Chowdhury M, Charles PJ, et al. The autoantibody repertoire in periodontitis: a role in the induction of autoimmunity to citrullinated proteins in rheumatoid arthritis? Ann Rheum Dis. 2014;73(3):580–6. doi: 10.1136/annrheumdis-2012-202701. [DOI] [PubMed] [Google Scholar]
- 6.Quirke AM, Perry E, Cartwright A, Kelly C, De Soyza A, Eggleton P, et al. Bronchiectasis is a Model for Chronic Bacterial Infection Inducing Autoimmunity in Rheumatoid Arthritis. Arthritis Rheumatol. 2015;67(9):2335–42. doi: 10.1002/art.39226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida M, Tsuji M, Kurosaka D, Kurosaka D, Yasuda J, Ito Y, et al. Autoimmunity to citrullinated type II collagen in rheumatoid arthritis. Mod Rheumatol. 2006;16(5):276–81. doi: 10.1007/s10165-006-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmona-Rivera C, Carlucci PM, Moore E, Lingampalli N, Uchtenhagen H, James E, et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Science Immunol. 2017;2(10) doi: 10.1126/sciimmunol.aag3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hueber W, Kidd BA, Tomooka BH, Lee BJ, Bruce B, Fries JF, et al. Antigen microarray profiling of autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2005;52(9):2645–55. doi: 10.1002/art.21269. [DOI] [PubMed] [Google Scholar]
- 10.Demoruelle MK, Parish MC, Derber LA, Kolfenbach JR, Hughes-Austin JM, Weisman MH, et al. Performance of anti-cyclic citrullinated Peptide assays differs in subjects at increased risk of rheumatoid arthritis and subjects with established disease. Arthritis Rheum. 2013;65(9):2243–52. doi: 10.1002/art.38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majka DS, Deane KD, Parrish LA, Lazar AA, Baron AE, Walker CW, et al. Duration of preclinical rheumatoid arthritis-related autoantibody positivity increases in subjects with older age at time of disease diagnosis. Ann Rheum Dis. 2008;67(6):801–7. doi: 10.1136/ard.2007.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 13.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7(5):e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brink M, Hansson M, Mathsson L, Jakobsson PJ, Holmdahl R, Hallmans G, et al. Multiplex analyses of antibodies against citrullinated peptides in individuals prior to development of rheumatoid arthritis. Arthritis Rheum. 2013;65(4):899–910. doi: 10.1002/art.37835. [DOI] [PubMed] [Google Scholar]
- 15.Finckh A, Escher M, Liang MH, Bansback N. Preventive Treatments for Rheumatoid Arthritis: Issues Regarding Patient Preferences. Curr Rheumatol Rep. 2016;18(8):51. doi: 10.1007/s11926-016-0598-4. [DOI] [PubMed] [Google Scholar]
- 16.Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum. 2012;64(6):1756–61. doi: 10.1002/art.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willis VC, Demoruelle MK, Derber LA, Chartier-Logan CJ, Parish MC, Pedraza IF, et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum. 2013;65(10):2545–54. doi: 10.1002/art.38066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynisdottir G, Karimi R, Joshua V, Olsen H, Hensvold AH, Harju A, et al. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheumatol. 2014;66(1):31–9. doi: 10.1002/art.38201. [DOI] [PubMed] [Google Scholar]
- 19.Demoruelle MK, Harrall KK, Ho L, Purmalek MM, Seto NL, Rothfuss HM, et al. Anti-citrullinated protein antibodies are associated with neutrophil extracellular traps in the sputum in relatives of rheumatoid arthritis patients. Arthritis Rheumatol. 2017 doi: 10.1002/art.40066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corsiero E, Bombardieri M, Carlotti E, Pratesi F, Robinson W, Migliorini P, et al. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann Rheum Dis. 2016;75(10):1866–75. doi: 10.1136/annrheumdis-2015-208356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolfenbach JR, Deane KD, Derber LA, O’Donnell C, Weisman MH, Buckner JH, et al. A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (RA) using first-degree relatives of probands with RA. Arthritis Rheum. 2009;61(12):1735–42. doi: 10.1002/art.24833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 24.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 25.in ‘t Veen JC, de Gouw HW, Smits HH, Sont JK, Hiemstra PS, Sterk PJ, et al. Repeatability of cellular and soluble markers of inflammation in induced sputum from patients with asthma. Eur Respir J. 1996;9(12):2441–7. doi: 10.1183/09031936.96.09122441. [DOI] [PubMed] [Google Scholar]
- 26.Gershman NH, Wong HH, Liu JT, Mahlmeister MJ, Fahy JV. Comparison of two methods of collecting induced sputum in asthmatic subjects. Eur Respir J. 1996;9(12):2448–53. doi: 10.1183/09031936.96.09122448. [DOI] [PubMed] [Google Scholar]
- 27.Robinson WH, DiGennaro C, Hueber W, Haab BB, Kamachi M, Dean EJ, et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med. 2002;8(3):295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 28.Monach PA, Hueber W, Kessler B, Tomooka BH, BenBarak M, Simmons BP, et al. A broad screen for targets of immune complexes decorating arthritic joints highlights deposition of nucleosomes in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106(37):15867–72. doi: 10.1073/pnas.0908032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22(2):146–53. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosseinzadeh A, Thompson PR, Segal BH, Urban CF. Nicotine induces neutrophil extracellular traps. J Leukoc Biol. 2016;100(5):1105–12. doi: 10.1189/jlb.3AB0815-379RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Luria A, Rhodes C, Raghu H, Lingampalli N, Sharpe O, et al. Nicotine drives neutrophil extracellular traps formation and accelerates collagen-induced arthritis. Rheumatology (Oxford) 2017;56(4):644–53. doi: 10.1093/rheumatology/kew449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aleyd E, Al M, Tuk CW, van der Laken CJ, van Egmond M. IgA Complexes in Plasma and Synovial Fluid of Patients with Rheumatoid Arthritis Induce Neutrophil Extracellular Traps via FcalphaRI. J Immunol. 2016;197(12):4552–9. doi: 10.4049/jimmunol.1502353. [DOI] [PubMed] [Google Scholar]
- 33.Limper AH, Roman J. Fibronectin. A versatile matrix protein with roles in thoracic development, repair and infection. Chest. 1992;101(6):1663–73. doi: 10.1378/chest.101.6.1663. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita CM, Fessler MB, Vasanthamohan L, Lac J, Madenspacher J, McCaig L, et al. Apolipoprotein E-deficient mice are susceptible to the development of acute lung injury. Respiration. 2014;87(5):416–27. doi: 10.1159/000358438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haidaris PJ. Induction of fibrinogen biosynthesis and secretion from cultured pulmonary epithelial cells. Blood. 1997;89(3):873–82. [PubMed] [Google Scholar]
- 36.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116(12):3183–94. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sohn DH, Rhodes C, Onuma K, Zhao X, Sharpe O, Gazitt T, et al. Local Joint inflammation and histone citrullination in a murine model of the transition from preclinical autoimmunity to inflammatory arthritis. Arthritis Rheumatol. 2015;67(11):2877–87. doi: 10.1002/art.39283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Woude D, Rantapaa-Dahlqvist S, Ioan-Facsinay A, Onnekink C, Schwarte CM, Verpoort KN, et al. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Ann Rheum Dis. 2010;69(8):1554–61. doi: 10.1136/ard.2009.124537. [DOI] [PubMed] [Google Scholar]
- 39.Papadaki G, Kambas K, Choulaki C, Vlachou K, Drakos E, Bertsias G, et al. Neutrophil extracellular traps exacerbate Th1-mediated autoimmune responses in rheumatoid arthritis by promoting DC maturation. Eur J Immunol. 2016;46(11):2542–54. doi: 10.1002/eji.201646542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith CK, Vivekanandan-Giri A, Tang C, Knight JS, Mathew A, Padilla RL, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 2014;66(9):2532–44. doi: 10.1002/art.38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spengler J, Lugonja B, Ytterberg AJ, Zubarev RA, Creese AJ, Pearson MJ, et al. Release of Active Peptidyl Arginine Deiminases by Neutrophils Can Explain Production of Extracellular Citrullinated Autoantigens in Rheumatoid Arthritis Synovial Fluid. Arthritis Rheumatol. 2015;67(12):3135–45. doi: 10.1002/art.39313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amara K, Steen J, Murray F, Morbach H, Fernandez-Rodriguez BM, Joshua V, et al. Monoclonal IgG antibodies generated from joint-derived B cells of RA patients have a strong bias toward citrullinated autoantigen recognition. J Exp Med. 2013;210(3):445–55. doi: 10.1084/jem.20121486. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Ioan-Facsinay A, el-Bannoudi H, Scherer HU, van der Woude D, Menard HA, Lora M, et al. Anti-cyclic citrullinated peptide antibodies are a collection of anti-citrullinated protein antibodies and contain overlapping and non-overlapping reactivities. Ann Rheum Dis. 2011;70(1):188–93. doi: 10.1136/ard.2010.131102. [DOI] [PubMed] [Google Scholar]
- 44.Masson-Bessiere C, Sebbag M, Girbal-Neuhauser E, Nogueira L, Vincent C, Senshu T, et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001;166(6):4177–84. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- 45.Li S, Yu Y, Yue Y, Liao H, Xie W, Thai J, et al. Autoantibodies From Single Circulating Plasmablasts React With Citrullinated Antigens and Porphyromonas gingivalis in Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(3):614–26. doi: 10.1002/art.39455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ytterberg AJ, Joshua V, Reynisdottir G, Tarasova NK, Rutishauser D, Ossipova E, et al. Shared immunological targets in the lungs and joints of patients with rheumatoid arthritis: identification and validation. Ann Rheum Dis. 2015;74(9):1772–7. doi: 10.1136/annrheumdis-2013-204912. [DOI] [PubMed] [Google Scholar]
- 47.Too CL, Murad S, Hansson M, Alm LM, Dhaliwal JS, Holmdahl R, et al. Differences in the Spectrum of Anti-Citrullinated Protein Antibody Fine Specificities Between Malaysian and Swedish Patients With Rheumatoid Arthritis: Implications for Disease Pathogenesis. Arthritis Rheumatol. 2017;69(1):58–69. doi: 10.1002/art.39827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.