Abstract
Objective
In order to facilitate regionally-specific liver cancer prevention and control, this study estimates the fraction of hepatocellular carcinoma cases attributable to five major liver cancer risk factors by geographic region.
Methods
Prevalence estimates of major hepatocellular carcinoma risk factors, including chronic infection with hepatitis B and hepatitis C, alcohol drinking, tobacco smoking, obesity, and diabetes were extracted for each country from the literature, along with recent incidence and risk estimate data, to calculate regionally specific population attributable fractions.
Results
Overall, 44% of hepatocellular carcinoma cases worldwide were attributable to chronic hepatitis B infection, with the majority of cases occurring in Asia. Hepatitis C was responsible for 21% of cases. Lifestyle risk factors such as alcohol drinking and obesity were responsible for a larger percentage of cases in North America and Western, Central, and Eastern Europe. Additionally, strong gender disparities were observed when looking at lifestyle risk factors, particularly tobacco smoking, in Asia and Africa.
Conclusions
Prominent risk factors for hepatocellular carcinoma vary depending on region. Our findings provide useful data for developing regionally specific guidelines for liver cancer prevention and control worldwide.
Keywords: Liver neoplasms, Carcinoma, hepatocellular, Attributable fraction
Introduction
The etiology of liver cancer is geographically heterogeneous, and patterns of liver cancer mortality closely follow incidence patterns worldwide. Of the 782,451 new cases estimated worldwide in 2012, 83% were from low and middle income countries (Ferlay et al., 2013). Although lower rates are reported in high income countries of the world, mortality rates from liver cancer have already greatly increased in the United States (Bertuccio et al., 2017), and are projected to increase even more over the next twenty years (Rahib et al., 2014; Parkin et al., 2011).
Hepatocellular carcinoma (HCC) is the most common type of liver cancer. Major risk factors for HCC include chronic infection with hepatitis B virus (HBV) and hepatitis C virus (HCV), alcohol drinking, tobacco smoking, and aflatoxin exposure (American Cancer Society, 2016). Recently, evidence has emerged that obesity may also increase risk of liver cancer through chronic inflammation (Sun and Karin, 2012; World Cancer Research Fund/American Institute for Cancer Research, 2007). Furthermore, diabetes may increase the risk of liver cancer beyond the risk conferred by obesity alone (Miele et al., 2015; Bosetti et al., 2015).
Previously published studies on the major attributable risk factors of liver cancer use outdated data, and have often looked at a limited number of risk factors. Two studies estimated the worldwide risk of liver cancer due to infectious agents only (de Martel et al., 2012; Parkin, 2006), while another has estimated risk from alcohol intake only (Praud et al., 2016). Studies in France, the United Kingdom, the United States (USA), Japan, and China are country-specific and lack global and regional perspectives (IARC Working Group, 2007; Parkin et al., 2011; Inoue et al., 2012; Fan et al., 2013; Makarova-Rusher et al., 2016). The Global Burden of Disease Project has focused on mortality outcomes, estimating the years of life lost associated with liver cancer subtypes (Mortality and Causes of Death, 2016).
By combining worldwide prevalence of major HCC risk factors with estimates of gender and regionally-specific risk of cancer, we will describe the global patterns of HCC risk and provide preventive strategy by geographic region.
Methods
HCC Case Estimation
The 2012 estimated number of new cancer cases by country and gender are available for 27 of the major cancers in GLOBOCAN 2012 (Ferlay et al. 2013). Since only liver cancer cases were reported, the percentage of HCC cases by country and by geographic region was estimated using the International Agency for Research on Cancer’s age-standardized world incidence rates of microscopically verified cases by histological type (IARC 2014).
Incidence rates by histological type are reported by country. Country-wide data from large registries were prioritized, and if unavailable, then country-wide data was calculated from regional studies using averages weighted by verified study sample size. Studies where more than half of the cases were unspecified were not used. For countries missing histological data, the gender-specific average rate weighted by number of verified histological cases for the corresponding region was used to estimate the percentage of HCC cases. For regions missing histological data, the gender-specific rate weighted by the number of liver cancer cases for the continental region was used. Estimations of percent and number of HCC cases by country are presented in supplementary tables.
Risk Ratios (RRs)
To assess the RRs associated with individual risk factors and liver cancer, we searched publications on PubMed. The search words used with “liver cancer,” “HCC,” and “hepatocellular carcinoma” included: HBV, hepatitis B virus, HCV, hepatitis C virus, aflatoxin, alcohol, drinking, smoking, tobacco, obesity, diabetes, and meta-analysis. Separate RRs were abstracted when there was indication of variation by gender, geographical location, level of alcohol drinking, and/or HBV/HCV status.
Table 1 displays the RRs used in this study. Specifically, risk ratios used for HBV (22.5 CI: 19.5–26) and HCV (17.3 CI: 13.9–21.6) were the RRs from a meta-analysis using second generation measurement techniques (Donato et al., 1998). The risk associated with heavy drinking was reported by gender in a meta-analysis of 27 cohort studies and 63 case control studies (Chuang et al., 2015). A trend line was fitted to the gender-specific RRs, and the risks associated with 20g increases in daily alcohol intake were estimated. An RR of 1.43 (CI: 1.21–1.68) was used to estimate the risk associated with ever smoking on liver cancer risk (Lee et al., 2009). This estimate was adjusted for alcohol consumption. The gender-specific estimates of risk associated with an overweight BMI (>=25) and an obese BMI (>=30) were obtained from a meta-analysis of 26 prospective studies (Chen et al., 2012). An RR of 1.94 (CI: 1.58, 2.38) was used for diabetes and liver cancer (Wang et al., 2012). This estimate adjusted for BMI or obesity.
Table 1.
Summary of risk estimates used, by major risk factor
| Study | Study Design | Overall RR (95% CI) | Measure of Exposure | Heterogeneity by Region | Heterogeneity by Gender | Heterogeneity by Hepatitis | Other Adjustments | |
|---|---|---|---|---|---|---|---|---|
| HBV | ||||||||
| Donato, Boffeta & Puoti, 1998 | 32 studies, 21 case-control studies | 20.4 (18.0, 23.2) | HBsAg positivity vs HBsAg negative, in HCV-negative | 20.8 (17.8, 24.3) in Africa, China, & southeast Asia; 18.8 (11.8, 30.3) in Japan & Mediterranean | NA | 135 (79.7, 242) in anti-HCV/HCV RNA positive | 22.5 (19.5, 26) in 2nd generation HBsAg measurement | |
| This Study | 22.5 (19.5, 26) | |||||||
| HCV | ||||||||
| Donato, Boffeta & Puoti, 1998 | 32 studies, 21 case-control studies | 23.6 (20.0, 28.1) | Anti-HCV/HCV-RNA positivity vs negativity, in HBsAg negative | 11.5 (8.8, 15.0) in Africa, China, & southeast Asia; 31.2 (20.9, 47.4) in Japan & Mediterranean | NA | 135 (79.7, 242) in HBsAG positive | 17.3 (13.9, 21.6) in 2nd generation anti-HCV | |
| This Study | 17.3 (13.9, 21.6) | |||||||
| Alcohol | men | women | ||||||
| Chuang et al., 2015 | 27 cohort studies; 63 case control | 1.19 (1.12, 1.27) | 25g/day vs never | 1.28 (1.13, 1.44) in Asia, 1.39 (0.93, 1.85) in Europe, 1.20 (0.86, 1.54) in North America, ever vs never | 1.2 (1.1,1.3) | 1.3 (1.1,1.5) | 1.12 (0.82,1.52) | 2.06 (1.31, 2.81) in former vs never drinkers |
| 1.54 (1.36, 1.74) | 50g/day vs never | 1.5 (1.3,1.7) | 2.1 (1.2,3.6) | 1.74 (1.25,2.41) | ||||
| 2.14 (1.74, 2.62) | 75g/day vs never | 1.8 (1.5,2.3) | 4.7 (1.5,14.5) | 3.68 (2.15,6.29) | ||||
| 3.21 (2.34, 4.40) | 100g/day vs never | 2.3 (1.7,3.1) | 10.6 (3.66,30.7) | |||||
| This Study | Chuang et al. gender-specific estimates and former vs never estimate | |||||||
| Smoking | men | women | ||||||
| Lee et al., 2009 | 13 case-control; 7 cohort | 1.51 (1.37, 1.67) | Current smokers vs never tobacco smokers | 1. 56 (1.36, 1.79) in Asia | 1.61 (1.38, 1.89) | 1.86 (1.33, 2.60) | 1.38 (1.01, 1.88) adjusted for HBV, 1.55 (1.00, 2.38) adjusted for HCV | 1.43 (1.21, 1.68) adjusted for alcohol |
| This Study | 1.43 (1.21, 1.68) | |||||||
| Obesity | men | women | ||||||
| Chen et al., 2012 | 26 prospective studies | 1.48 (1.31, 1.67) | Overweight vs normal weight | 1.35 (1.18, 1.54) in Asia, 1.65 (1.36, 2.02) in non-Asian countries | 1.42 (1.22, 1.65) | 1.18 (1.08, 1.30) | 1.74 (1.35, 2.25) adjusted for HBV and/or HCV | 1.46 (1.25, 1.69) adjusted for alcohol |
| 1.83 (1.59, 2.11) | Obese vs normal weight | 1.91 (1.51, 2.41) | 1.55 (1.30, 1.85) | |||||
| This Study | Chen et al. gender-specific estimates | |||||||
| Diabetes | men | women | ||||||
| Wang et al., 2012 | 17 case-control; 32 cohort studies | 2.31 (1.87, 2.84) | diabetic vs not diabetic | 2.43 (1.94, 3.05) in North America, 2.51 (1.92, 3.27) in Europe, 2.00 (1.46, 2.75) in East Asia | 2.03 (1.58, 2.62) | 1.91 (1.22, 2.99) | 2.26 (1.62, 3.14) adjusted for hepatitis | 2.32 1.93, 2.79) adjusted for alcohol; 1.94 (1.58, 2.38) adjusted for BMI/obesity |
| This Study | 1.94 (1.58, 2.38) | |||||||
Risk Factor Prevalence Data
Prevalence data for major HCC risk factors was extracted from the literature and publicly accessible databanks. Specifically, HBV prevalence data was obtained from a study of 161 countries, estimating HBsAg seroprevalence worldwide from 1965–2012 (Schweitzer et al., 2015). Estimates were for both genders from 1990–2013. Estimates of HCV prevalence data came from a study of anti-HCV prevalence in 87 countries for both genders, estimated from 2000–2010 data (Gower et al., 2014). Alcohol exposure estimates by country were obtained from a study of 241 countries and territories, estimating the 2005 distribution of drinking in men and women ages 15 and older (Shield et al., 2013). Prevalence estimates were given for never drinkers, former drinkers, and in increments of 20 and 40g of alcohol per day. Prevalence of male and female current smokers and non-smokers ages 15 years and older from 1996 came from a study of 187 countries (Ng et al., 2014), while prevalence estimates of overweight and obese in men and women in 1996 were obtained from the WHO data repository (World Health Organization, 2017b, 2017a). Similarly, prevalence estimates of raised fasting blood glucose (>= 7.0 mmol/L or on medication) also came from the WHO (World Health Organization, 2017c).
Calculation of Attributable Cancer Cases
The fraction of new HCC cases attributable to HBV, HCV, tobacco smoking, and diabetes was calculated using Levin’s formula, given the prevalence of the risk factor and the associated relative risk:
The population attributable fraction (PAF) represents the proportion of cases of liver cancer that could be avoided had exposure to a major risk factor was removed. The population attributable cases (PAC), representing the number of cases that could have been avoided had exposure to the risk factor been removed, can be calculated by multiplying the number of HCC cases in each region by the PAF.
For alcohol consumption and BMI, estimates of prevalence and risk at different levels of exposure (e.g. the prevalence and risk associated with having a BMI of 25–30, 30+) were widely available. The PAF was then calculated as a summary measure of these categories to reflect varying risk as exposure level increases using the following formula:
The risk associated with each level of alcohol drinking or BMI was extracted from meta-analysis (Table 1).
95% confidence intervals were calculated using the 95% confidence intervals (CI) of the reported risk ratios and prevalence data, when available. Additionally, individuals may have more than one risk factor and these risk factors may interact with one another, making it possible that summing across categories gives a PAF exceeding 100%. Interactions for some risk factors are described in the results section.
Geographic divisions
The proportion of HCC cases attributable to each risk factor was estimated for each with available data. In order to look at trends on a regional level, global estimates were grouped into the 21 Global Burden of Disease (GBD) Regions (World Health Organization, 2002). The specific countries included in each grouping here are reported in the supplementary tables. Gender-specific PAFs on the regional level were estimated using the countries for which data was available, weighted by the number of HCC cases in each region.
Results
Worldwide, viral risk factors for liver cancer play a larger role compared to behavioral risk factors. Chronic infection with HBV contributes to 44% of all HCC cases worldwide, while 21% of HCC cases can be attributed to HCV (Table 2). Alcohol consumption was the strongest lifestyle risk factor, contributing to 26% of HCC cases, while smoking contributed to 13% and obesity to 9%.
Table 2.
Fraction of Cases Attributable to Major HCC Risk Factors by Geographic Region
| Region | HCC (N=) | HBV PAF | HCV PAF | Alcohol PAF | Smoking PAF | Obesity PAF | Diabetes PAF |
|---|---|---|---|---|---|---|---|
| (CI) | (CI) | (CI) | (CI) | (CI) | (CI) | ||
| Asia Pacific | 40,303 | 23% | 17% | 28% | 14% | 8% | 7% |
| (20%, 26%) | (5%, 32%) | (14%, 41%) | (7%, 21%) | (4%, 11%) | (5%, 9%) | ||
|
| |||||||
| Central Asia | 4,687 | 57% | 50% | 27% | 9% | 14% | 9% |
| (48%, 68%) | (33%, 67%) | (12%, 41%) | (4%, 14%) | (7%, 24%) | (6%, 12%) | ||
|
| |||||||
| East Asia | 310,366 | 54% | 18% | 27% | 14% | 7% | 7% |
| (50%, 59%) | (5%, 30%) | (13%, 40%) | (7%, 21%) | (4%, 10%) | (5%, 10%) | ||
|
| |||||||
| South Asia | 24,900 | 27% | 17% | 13% | 8% | 4% | 8% |
| (24%, 31%) | (7%, 25%) | (5%, 20%) | (4%, 13%) | (2%, 6%) | (5%, 11%) | ||
|
| |||||||
| Southeast Asia | 57,264 | 51% | 20% | 19% | 14% | 4% | 6% |
| (47%, 56%) | (12%, 39%) | (9%, 30%) | (7%, 21%) | (2%, 7%) | (4%, 8%) | ||
|
| |||||||
| Australasia | 1,457 | 16% | 22% | 28% | 10% | 21% | 6% |
| (14%, 18%) | (14%, 29%) | (14%, 41%) | (5%, 15%) | (12%, 31%) | (4%, 8%) | ||
|
| |||||||
| Central Europe | 5,198 | 29% | 20% | 35% | 12% | 19% | 6% |
| (24%, 35%) | (13%, 30%) | (17%, 51%) | (6%, 20%) | (10%, 29%) | (4%, 9%) | ||
|
| |||||||
| Eastern Europe | 5,475 | 39% | 39% | 39% | 13% | 18% | 6% |
| (34%, 45%) | (14%, 53%) | (18%, 56%) | (7%, 20%) | (9%, 27%) | (4%, 10%) | ||
|
| |||||||
| Western Europe | 32,766 | 12% | 15% | 30% | 12% | 20% | 5% |
| (10%, 15%) | (8%, 32%) | (15%, 44%) | (6%, 19%) | (11%, 30%) | (4%, 8%) | ||
|
| |||||||
| Oceania | 496 | 79% | 3% | 21% | 14% | 16% | 12% |
| (73%, 84%) | (3%, 16%) | (10%, 31%) | (6%, 22%) | (8%, 27%) | (7%, 17%) | ||
|
| |||||||
| Caribbean | 836 | 48% | NA | 28% | 7% | 13% | 8% |
| (34%, 63%) | (12%, 42%) | (3%, 12%) | (6%, 21%) | (5%, 12%) | |||
|
| |||||||
| Andean Latin America | 1,960 | 24% | 16% | 30% | 5% | 15% | 6% |
| (19%, 33%) | (5%, 26%) | (13%, 45%) | (2%, 9%) | (7%, 24%) | (4%, 9%) | ||
|
| |||||||
| Central Latin America | 7,231 | 9% | 19% | 26% | 6% | 17% | 8% |
| (6%, 11%) | (12%, 27%) | (11%, 41%) | (3%, 11%) | (9%, 27%) | (6%, 12%) | ||
|
| |||||||
| Southern Latin America | 1,648 | 14% | 20% | 30% | 11% | 20% | 8% |
| (12%, 17%) | (6%, 35%) | (14%, 44%) | (5%, 19%) | (10%, 30%) | (5%, 11%) | ||
|
| |||||||
| Tropical Latin America | 7,841 | 12% | 21% | 37% | 7% | 15% | 7% |
| (10%, 14%) | (13%, 26%) | (17%, 54%) | (3%, 13%) | (8%, 24%) | (4%, 10%) | ||
|
| |||||||
| North America | 25,898 | 6% | 17% | 32% | 9% | 24% | 6% |
| (4%, 8%) | (14%, 33%) | (15%, 47%) | (4%, 14%) | (13%, 34%) | (5%, 9%) | ||
|
| |||||||
| North Africa & Middle East | 22,147 | 28% | 56% | 21% | 9% | 19% | 12% |
| (24%, 32%) | (45%, 67%) | (8%, 35%) | (4%, 15%) | (10%, 29%) | (9%, 15%) | ||
|
| |||||||
| Central Sub-Saharan Africa | 3,778 | 60% | 41% | 27% | 4% | 5% | 5% |
| (54%, 65%) | (31%, 75%) | (11%, 41%) | (2%, 7%) | (2%, 8%) | (3%, 9%) | ||
|
| |||||||
| East Sub-Saharan Africa | 5,124 | 58% | 17% | 21% | 6% | 4% | 4% |
| (52%, 65%) | (9%, 46%) | (9%, 34%) | (2%, 10%) | (2%, 7%) | (3%, 7%) | ||
|
| |||||||
| Southern Sub-Saharan Africa | 2,449 | 62% | 22% | 23% | 9% | 13% | 8% |
| (56%, 69%) | (12%, 41%) | (10%, 37%) | (4%, 15%) | (6%, 20%) | (5%, 11%) | ||
|
| |||||||
| West Sub-Saharan Africa | 19,754 | 69% | 53% | 28% | 4% | 6% | 5% |
| (64%, 74%) | (31%, 73%) | (13%, 42%) | (2%, 8%) | (3%, 9%) | (3%, 8%) | ||
|
| |||||||
| WORLD | 581,579 | 44% | 21% | 26% | 13% | 9% | 7% |
| (40%, 48%) | (10%, 35%) | (12%, 39%) | (6%, 19%) | (5%, 13%) | (5%, 10%) | ||
Regions in italics are high income regions of the world
CI-confidence interval; PAF-population attributable fraction
Hepatitis B
Worldwide, more cases of HCC can be attributed to chronic infection with HBV than any other risk factor. 44% of the world total of liver cancer cases can be attributed to HBV (Table 2). The vast majority of cases attributable to chronic infection with HBV occurred in Asia, and in particular East Asia (166,977). PAFs were highest (>50%) in Oceania and Sub-Saharan Africa, as well as Central, East, and Southeast Asia.
Hepatitis C
About 122,132 cases of HCC (21% of all HCC cases) can be attributed to chronic HCV infection (Table 2). Similar to patterns observed with HBV, Central Asia, Central Sub-Saharan Africa, and West Sub-Saharan Africa had a high PAFs due to HCV (>30%), with high proportions of attributable cases also in North Africa and the Middle East and Eastern Europe. North Africa and the Middle East had the largest PAF due to HCV (56%), due to a large PAF (70.6%) for Egypt (Supplemental Table 3), where HCV is prevalent and is exacerbated by a possible interaction with schistosoma (Bedwani et al., 1996).
Interaction between HBV and HCV
Meta-analyses have reported conflicting results on the joint effects of HBV and HCV, reporting both subadditive (Cho et al., 2011) and superadditive effects (Donato et al., 1998; Shi et al., 2005). Cho et al. stratified studies and found an overall subadditive effect on risk of HCC in more recent studies (2000–2009), cohort studies, and studies conducted in areas where HBV and HCV infection were uncommon. If the effects are subadditive, then the sum of the PAFs reported here for HBV and HCV are an overestimate; however if the effects are superadditive, the sum is an underestimate.
Interaction between HBV and Aflatoxin
Aflatoxin is another risk factor for liver cancer thought to interact with HBV exposure. A review of available studies concluded that aflatoxin likely plays a causative role in 4.6–28.2% of all global HCC cases (25,000–155,000), with the majority of cases occurring in sub-Saharan Africa, Southeast Asia, and China (Liu et al., 2012). Exposure to aflatoxin in persons with chronic HBV infection is associated with a risk up to 30 times greater than in those exposed to aflatoxin alone (Groopman et al., 2008). Estimates for HBV infected individuals in regions where aflatoxin exposure is common, such as parts of Africa and Asia, are likely underestimates.
Alcohol
About 150,629 cases of HCC can be attributed to alcohol drinking, or 26% of the worldwide total (Table 2). Although infectious diseases had larger PAFs in Asia and Africa, PAFs for alcohol drinking were largest (>35%) in Central and Eastern Europe and Tropical Latin America. PAFs were larger for men in most regions of the world compared to women due to the higher prevalence of drinking among men (Table 3). However, in some regions where the prevalence was similar, including Southern and Andean Latin America, PAFs were higher in women due to the increased risk of HCC at lower levels of consumption.
Table 3.
Fraction of Cases Attributable to HCC Lifestyle Risk Factors by Geographic Region and Gender
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| GBDR | HCC | Alcohol PAF | Smoking PAF | Obesity PAF | Diabetes PAF | HCC | Alcohol PAF | Smoking PAF | Obesity PAF | Diabetes PAF |
| (N=) | (CI) | (CI) | (CI) | (CI) | (N=) | (CI) | (CI) | (CI) | (CI) | |
| Asia Pacific | 27,417 | 29% | 19% | 9% | 7% | 12,886 | 25% | 4% | 4% | 5% |
| (17%, 40%) | (10%, 28%) | (5%, 13%) | (5%, 9%) | (8%, 41%) | (2%, 8%) | (2%, 7%) | (4%, 7%) | |||
|
| ||||||||||
| Central Asia | 2,688 | 28% | 13% | 17% | 9% | 1,999 | 26% | 2% | 11% | 9% |
| (15%, 41%) | (6%, 21%) | (9%, 26%) | (6%, 13%) | (8%, 41%) | (1%, 4%) | (4%, 20%) | (6%, 12%) | |||
|
| ||||||||||
| East Asia | 230,372 | 28% | 19% | 7% | 8% | 79,995 | 23% | 2% | 4% | 6% |
| (15%, 41%) | (10%, 27%) | (4%, 10%) | (5%, 10%) | (7%, 36%) | (1%, 3%) | (2%, 7%) | (4%, 9%) | |||
|
| ||||||||||
| South Asia | 15,679 | 15% | 13% | 4% | 8% | 9,220 | 8% | 1% | 3% | 7% |
| (7%, 24%) | (6%, 20%) | (3%, 6%) | (6%, 11%) | (2%, 13%) | (1%, 2%) | (1%, 6%) | (5%, 10%) | |||
|
| ||||||||||
| Southeast Asia | 41,782 | 24% | 18% | 5% | 6% | 15,482 | 7% | 2% | 4% | 6% |
| (11%, 37%) | (9%, 27%) | (3%, 6%) | (4%, 8%) | (2%, 13%) | (1%, 4%) | (1%, 7%) | (4%, 9%) | |||
|
| ||||||||||
| Australasia | 1,033 | 27% | 10% | 25% | 6% | 424 | 28% | 9% | 13% | 5% |
| (16%, 39%) | (5%, 15%) | (14%, 36%) | (4%, 8%) | (8%, 46%) | (4%, 14%) | (6%, 21%) | (3%, 6%) | |||
|
| ||||||||||
| Central Europe | 3,293 | 36% | 14% | 23% | 6% | 1,904 | 34% | 9% | 13% | 6% |
| (21%, 50%) | (7%, 23%) | (12%, 33%) | (4%, 9%) | (11%, 54%) | (4%, 14%) | (6%, 22%) | (4%, 8%) | |||
|
| ||||||||||
| Eastern Europe | 3,134 | 39% | 19% | 20% | 6% | 2,341 | 38% | 6% | 15% | 7% |
| (23%, 55%) | (10%, 28%) | (11%, 29%) | (4%, 9%) | (12%, 59%) | (3%, 10%) | (7%, 24%) | (4%, 10%) | |||
|
| ||||||||||
| Western Europe | 23,137 | 30% | 13% | 23% | 6% | 9,629 | 31% | 10% | 12% | 4% |
| (18%, 42%) | (7%, 20%) | (13%, 34%) | (4%, 8%) | (9%, 50%) | (5%, 15%) | (6%, 20%) | (3%, 6%) | |||
|
| ||||||||||
| Oceania | 322 | 20% | 17% | 18% | 12% | 175 | 22% | 8% | 13% | 11% |
| (11%, 30%) | (8%, 26%) | (9%, 29%) | (7%, 17%) | (7%, 33%) | (3%, 14%) | (5%, 23%) | (7%, 16%) | |||
|
| ||||||||||
| Caribbean | 446 | 28% | 9% | 14% | 7% | 390 | 27% | 4% | 12% | 9% |
| (14%, 42%) | (4%, 15%) | (7%, 21%) | (4%, 11%) | (9%, 42%) | (2%, 8%) | (5%, 22%) | (5%, 13%) | |||
|
| ||||||||||
| Andean Latin America | 882 | 28% | 7% | 17% | 6% | 1,078 | 31% | 3% | 13% | 7% |
| (15%, 42%) | (3%, 13%) | (9%, 26%) | (3%, 9%) | (11%, 47%) | (1%, 5%) | (5%, 22%) | (4%, 10%) | |||
|
| ||||||||||
| Central Latin America | 3,523 | 29% | 10% | 20% | 8% | 3,708 | 24% | 3% | 14% | 9% |
| (15%, 44%) | (4%, 16%) | (11%, 30%) | (5%, 11%) | (7%, 39%) | (1%, 6%) | (6%, 24%) | (6%, 12%) | |||
|
| ||||||||||
| Southern Latin America | 912 | 27% | 12% | 23% | 8% | 736 | 34% | 10% | 15% | 8% |
| (16%, 38%) | (6%, 20%) | (13%, 35%) | (5%, 11%) | (11%, 51%) | (4%, 18%) | (7%, 25%) | (5%, 11%) | |||
|
| ||||||||||
| Tropical Latin America | 4,654 | 36% | 8% | 18% | 7% | 3,187 | 37% | 6% | 12% | 7% |
| (19%, 54%) | (4%, 15%) | (10%, 26%) | (4%, 9%) | (13%, 55%) | (2%, 11%) | (5%, 20%) | (5%, 10%) | |||
|
| ||||||||||
| North America | 19,132 | 32% | 9% | 26% | 7% | 6,767 | 31% | 8% | 15% | 5% |
| (18%, 46%) | (4%, 14%) | (15%, 38%) | (5%, 9%) | (10%, 48%) | (4%, 13%) | (8%, 25%) | (4%, 7%) | |||
|
| ||||||||||
| North Africa & Middle East | 15,076 | 27% | 13% | 20% | 11% | 7,071 | 8% | 1% | 16% | 13% |
| (10%, 45%) | (6%, 20%) | (11%, 30%) | (8%, 15%) | (3%, 13%) | (0%, 2%) | (8%, 27%) | (10%, 16%) | |||
|
| ||||||||||
| Central Sub-Saharan Africa | 2,292 | 26% | 6% | 4% | 5% | 1,486 | 28% | 1% | 5% | 5% |
| (12%, 41%) | (3%, 12%) | (3%, 6%) | (3%, 9%) | (10%, 41%) | (0%, 1%) | (2%, 9%) | (3%, 9%) | |||
|
| ||||||||||
| East Sub-Saharan Africa | 3,023 | 23% | 8% | 4% | 4% | 2,101 | 19% | 2% | 4% | 4% |
| (11%, 36%) | (4%, 14%) | (3%, 6%) | (3%, 7%) | (6%, 31%) | (1%, 3%) | (2%, 8%) | (3%, 7%) | |||
|
| ||||||||||
| Southern Sub-Saharan Africa | 1,452 | 27% | 13% | 11% | 7% | 996 | 16% | 3% | 15% | 9% |
| (14%, 40%) | (6%, 21%) | (6%, 17%) | (4%, 11%) | (4%, 31%) | (1%, 6%) | (7%, 25%) | (6%, 13%) | |||
|
| ||||||||||
| West Sub-Saharan Africa | 13,229 | 28% | 6% | 6% | 6% | 6,525 | 27% | 2% | 6% | 5% |
| (15%, 41%) | (2%, 10%) | (3%, 9%) | (3%, 8%) | (8%, 44%) | (1%, 3%) | (2%, 11%) | (3%, 8%) | |||
|
| ||||||||||
| WORLD | 413,479 | 27% | 17% | 10 | 7% | 168,100 | 22% | 3% | 7% | 6% |
| (15%, 41%) | (8%, 25%) | (6%, 14%) | (5%, 10%) | (7%, 35%) | (1%, 5%) | (3%, 11%) | (4%, 9%) | |||
Regions in italics are high income regions of the world
CI-confidence interval; PAF-population attributable fraction
Interaction between Alcohol and HBV/HCV
Table 1 presents results from a meta-analysis on the association between alcohol and HCC risk in those without hepatitis infection (Chuang et al., 2015). However, estimates came from only seven studies. If alcohol consumption accelerates progression to HCC in those with chronic viral hepatitis, then estimates for alcohol in HBV and HCV endemic regions are underestimates.
Tobacco Smoking
Overall, around 73,279 cases of liver cancer can be attributed to tobacco smoking, or 13% of the world total (Table 2). Men in all regions had higher attributable fractions compared to women due to a higher prevalence of smoking; however, this disparity was more pronounced in Asia and Africa. The largest number of cases was in East Asia, due to a large attributable fraction among men (19%), but not among women (2%) (Table 3). Central and Western Europe, Australasia, and Southern Latin America had high attributable fractions among women (>8%).
Obesity
Body fatness, encompassing those overweight and obese, is a risk factor for liver cancer. The World Cancer Research Fund has found that there is limited but suggestive evidence that body fatness is associated with liver cancer, and even a small increased risk of liver cancer could have wide-reaching future effects (World Cancer Research Fund/American Institute for Cancer Research, 2007).
Overall, 51,760 HCC cases worldwide in 2012 can be attributed to obesity, or 9% of the world total (Table 2). Australasia and North America had the highest attributable fractions of liver cancer cases due to a high prevalence of overweight and obese (>20%), while parts of Asia and Africa had the lowest attributable fractions (<5%) (Table 3). Although the worldwide prevalence of obesity was generally higher in women, the risk of HCC associated with obesity is higher in men, leading to higher PAFs in many geographic regions.
Interaction between Obesity and Alcohol
Obesity may interact with other lifestyle risk factors. Those who drink alcohol are more likely to be obese, and it may be difficult to assess the individual effect of each risk factor as they often co-occur. A meta-analysis of 26 prospective studies by Chen et al. found that the risk of liver cancer associated with obesity, adjusted for alcohol (RR: 1.46, 95% CI: 1.25, 1.69) was similar to the unadjusted estimate (Chen et al., 2012). Using the RR adjusted for alcohol consumption would slightly lower PAFs for obesity.
Diabetes
Diabetes is independently associated with liver cancer, conferring risk beyond obesity alone. About 7% of HCC cases worldwide can be attributed to diabetes, corresponding to approximately 40,711 cases worldwide (Table 2). Regions with the highest attributable fractions include Oceania, North Africa and the Middle East, as well as Central Asia. Estimates were similar among men and women.
The five countries with the largest number of HCC cases are: China, Japan, the USA, India, and Vietnam. China alone accounts for over half of the cases of HCC worldwide. More cases can be attributed to HBV than any other risk factor in China, India, and Vietnam (Table 4). Alcohol drinking and HCV were major risk factors in Japan and the USA, while obesity was also a main risk factor in the USA. Smoking was responsible for a high proportion of cases in men but not women in China, Japan, India, and Vietnam. Supplementary tables include additional country-specific estimates.
Table 4.
Fraction of Cases Attributable to Major HCC Risk Factors in Top 5 Countries
| Country | Population Attributable Fraction | Population Attributable Cases | ||
|---|---|---|---|---|
|
|
|
|||
| Men | Women | Men | Women | |
| 1. China | N=227,739 | N=78,770 | ||
| HBV | 53.8% (50.0%, 58.5%) | 164,813 (153,177, 179,421) | ||
| HCV | 17.5% (5.3%, 30.2%) | 53,593 (16,144, 92,466) | ||
| Alcohol | 27.8% (14.8%, 41.1%) | 23.2% (7.4%, 36.2%) | 63,368 (33,659, 93,569) | 18,247 (5,837, 28,529) |
| Smoking | 18.7% (9.8%, 27.4%) | 1.7% (0.8%, 2.9%) | 42,593 (22,226, 62,399) | 1,332 (591, 2,288) |
| Adiposity | 7.2% (4.1%, 10.4%) | 4.2% (1.8%, 7.4%) | 16,461 (9,423, 23,643) | 3,334 (1,446, 5,865) |
| Diabetes | 7.6% (5.4%, 10.3%) | 6.4% (4.4%, 8.7%) | 17,399 (12,350, 23,430) | 5,058 (3,468, 6,830) |
| 2. Japan | N=18,696 | N= 9,895 | ||
| HBV | 12.4% (10.7%, 14.6%) | 3,553 (3,069, 4,188) | ||
| HCV | 19.6% (6.5%, 32.2%) | 5,617 (1,858, 9,210) | ||
| Alcohol | 25.6% (14.8%, 36.3%) | 23.9% (6.9%, 39.6%) | 4,795 (2,767, 6,793) | 2,369 (680, 3,914) |
| Smoking | 18.2% (9.4%, 27.3%) | 5.2% (2.4%, 8.7%) | 3,411 (1,764, 5,096) | 512 (237, 860) |
| Adiposity | 9.1% (5.3%, 13.0%) | 3.9% (1.7%, 6.8%) | 1,703 (985, 2,427) | 387 (166, 675) |
| Diabetes | 7.2% (5.3%, 9.2%) | 4.7% (3.4%, 6.3%) | 1,338 (998, 470) | 470 (333, 619) |
| 3. United States | N=17,932 | N=6,291 | ||
| HBV | 5.3% (4.1%, 7.0%) | 1,282 (989, 1,698) | ||
| HCV | 17.5% (14.3%, 34.1%) | 4,235 (3,463, 8,270) | ||
| Alcohol | 32.0% (17.5%, 46.5%) | 30.7% (9.8%, 48.0%) | 5,730 (3,141, 8,342) | 1,934 (614, 3,022) |
| Smoking | 9.0% (4.5%, 14.1%) | 8.0% (3.8%, 12.6%) | 1,620 (799, 2,534) | 500 (241, 793) |
| Adiposity | 26.6% (15.2%, 37.8%) | 15.6% (7.7%, 24.8%) | 4,765 (2,733, 6,775) | 981 (485, 1,558) |
| Diabetes | 6.9% (5.1%, 9.3%) | 5.5% (4.0%, 7.3%) | 1,240 (912, 1,667) | 346 (250, 460) |
| 4. India | N=12,011 | N=7,094 | ||
| HBV | 23.6% (20.9%, 27.4%) | 4,516 (3,997, 5,230) | ||
| HCV | 11.5% (5.3%, 17.8%) | 2,204 (1,006, 3,394) | ||
| Alcohol | 18.8% (8.4%, 29.9%) | 10.0% (3.0%, 16.9%) | 2,259 (1,005, 3,590) | 713 (214, 1,200) |
| Smoking | 12.1% (5.9%, 18.9%) | 1.1% (0.5%, 2.1%) | 1,449 (705, 2,266) | 81 (34, 146) |
| Adiposity | 4.1% (2.5%, 5.7%) | 2.9% (1.2%, 5.4%) | 495 (295, 684) | 207 (85, 380) |
| Diabetes | 7.6% (5.3%, 10.3%) | 7.1% (4.9%, 9.6%) | 908 (641, 1,236) | 502 (349, 681) |
| 5. Vietnam | N=12,030 | N=3,708 | ||
| HBV | 69.8% (65.5%, 74.6%) | 10,988 (10,304, 11,736) | ||
| HCV | NA | NA | ||
| Alcohol | 23.9% (11.9%, 36.4%) | 4.3% (1.2%, 7.6%) | 2,880 (1,432, 4,382) | 158 (45, 282) |
| Smoking | 20.0% (10.5%, 29.3%) | 1.9% (0.8%, 3.4%) | 2,408 (1,258, 3,519) | 69 (29, 124) |
| Adiposity | 2.8% (1.8%, 3.5%) | 2.2% (0.9%, 4.0%) | 341 (222, 421) | 81 (32, 149) |
| Diabetes | 4.7% (3.0%, 6.6%) | 4.6% (3.0%, 6.3%) | 561 (362, 792) | 170 (112, 235) |
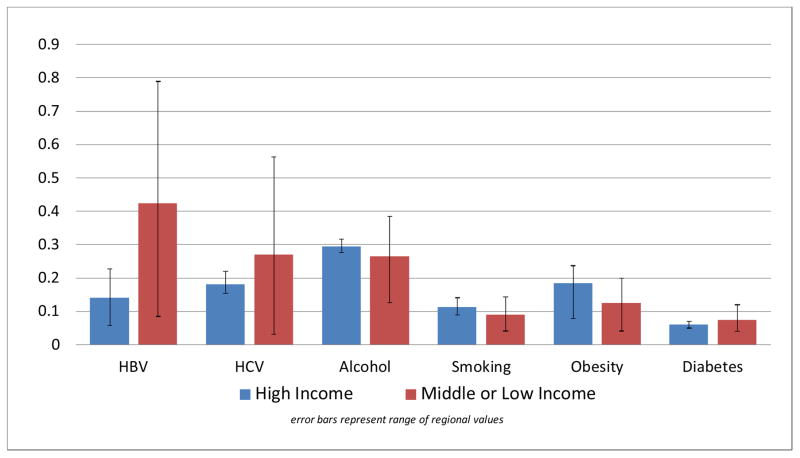
High income regions of the world (Asia Pacific, Australasia, Western Europe, and North America) had a distinct disease profile compared to other regions of the world (Figure 1). Other than diabetes, lifestyle risk factors were responsible for a larger percentage of HCC cases in high income regions compared to other regions of the world. On average the PAF for alcohol drinking in high income regions was 29.5% (vs. 26.5% in other regions), 11.2% for smoking (vs. 9.0%), and 18.4% for obesity (vs. 12.5%), while the average PAF for HBV (14.0%) and HCV (18.0%) was much smaller in high income regions compared to other regions of the world (42.3% and 27.0%, respectively) (Figure 1).
Figure 1.
Average Population Attributable Fraction for Major Hepatocellular Carcinoma Risk Factors by Regional Income Status
Discussion
This report provides an estimate of the contribution of known modifiable risk factors of HCC cases worldwide. The majority of HCC cases are attributed to HBV and HCV infections, with regions in Asia and Africa having the highest attributable risk fractions. These regions have the highest burden of HCC worldwide, and vaccination programs and treatment are extremely effective. Compounding the risk associated with hepatitis infection, aflatoxin exposure is also seen more commonly in Asia and Africa.
While infectious disease and aflatoxin contamination of cereals are responsible for the majority of HCC cases in Asia and Africa, a higher percentage of cases are due to tobacco smoking, alcohol drinking, and obesity in high income regions of the world. As control of viral agents improves, cases due to lifestyle risk factors are likely to grow in number. Furthermore, the prevalence of lifestyle risk factors is increasing in many middle or low income countries and will possibly play a larger role in the future global burden of liver cancer.
Results from this study were comparable to similar studies. Studies conducted in France (IARC Working Group, 2007), the United Kingdom (Parkin et al., 2011), Japan (Inoue et al., 2012), and China (Fan et al., 2013) found that the PAFs due to infectious diseases (HBV and HCV) in Europe were much lower (France: 64.4% United Kingdom: 41.6%) than the percent of cases due to infectious disease in Asia (Japan: 92.1% China: 70%). Similarly, we found that PAFs due to infectious agents were much higher in Asia and Africa than in western populations. Another study estimated 12.8% of liver cancers worldwide are attributable to alcohol (Praud et al., 2016), compared to our estimate of 25.9%. This lower PAF is due to using different RRs and alcohol consumption prevalence estimates; however these two estimates are not significantly heterogeneous.
One limitation of this study is the availability of data for estimation of continuous variables such as alcohol drinking, obesity, and tobacco smoking. PAFs for alcohol drinking and obesity were estimated using categorical prevalence and risk estimates, while these measures were not available for smoking. It is possible that reported estimates do not adequately reflect the population distribution of smoking within each region. Measurements for diabetes combined those with raised blood fasting blood glucose with those on medication, although there is evidence that risk of liver cancer can vary depending on the type of diabetes medication taken (Miele et al., 2015; Bosetti et al., 2015; Wang et al., 2012). Furthermore, the regions themselves are aggregate measures of country-level data. Supplementary tables include country-specific estimates of HCC cases attributable to major risk factors, reflecting variations in risk within broader regions.
This study gives researchers and policymakers an updated estimation of the percentage of incident cases of HCC that can be attributed to major viral and lifestyle risk factors. Our results may be informative for policies and programs on reducing the burden of worldwide.
Supplementary Material
Acknowledgments
Funding: The work by Aileen Baecker was partially funded by a training grant from the National Cancer Institute (Grant Number T32 CA009142).
We would like to thank the International Agency for Research on Cancer and the World Health Organization for access to data, and Dr. Donald Maxwell Parkin for his time, insight, and input during draft revisions. This research was partially supported by the National Institutes of Health [Grant Numbers T32 CA009142] and the Alper Research Funds for Environmental Genomics of the UCLA Jonsson Comprehensive Cancer Center.
Footnotes
Conflicts of interest: We have no conflicts of interests to declare.
References
- American Cancer Society. [Accessed August 8th];Liver cancer risk factors. 2016 http://www.cancer.org/cancer/livercancer/detailedguide/liver-cancer-risk-factors.
- Bedwani R, El-Khwsky F, El-Shazly M, Seif HA, Zaki A, Renganathan E, et al. Hepatitis viruses, schistosomal infection and liver cancer in Egypt. Int J Cancer. 1996;68:688–9. doi: 10.1002/1097-0215(19961127)68:5<688::aid-ijc2910680502>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Bosetti C, Franchi M, Nicotra F, Asciutto R, Merlino L, La Vecchia C, et al. Insulin and other antidiabetic drugs and hepatocellular carcinoma risk: a nested case-control study based on Italian healthcare utilization databases. Pharmacoepidemiol Drug Saf. 2015;24:771–8. doi: 10.1002/pds.3801. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang X, Wang J, Yan Z, Luo J. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. Eur J Cancer. 2012;48:2137–45. doi: 10.1016/j.ejca.2012.02.063. [DOI] [PubMed] [Google Scholar]
- Cho LY, Yang JJ, Ko KP, Park B, Shin A, Lim MK, et al. Coinfection of hepatitis B and C viruses and risk of hepatocellular carcinoma: systematic review and meta-analysis. Int J Cancer. 2011;128:176–84. doi: 10.1002/ijc.25321. [DOI] [PubMed] [Google Scholar]
- Chuang SC, Lee YC, Wu GJ, Straif K, Hashibe M. Alcohol consumption and liver cancer risk: a meta-analysis. Cancer Causes Control. 2015;26:1205–31. doi: 10.1007/s10552-015-0615-3. [DOI] [PubMed] [Google Scholar]
- de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–15. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998;75:347–54. doi: 10.1002/(sici)1097-0215(19980130)75:3<347::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Fan JH, Wang JB, Jiang Y, Xiang W, Liang H, Wei WQ, et al. Attributable causes of liver cancer mortality and incidence in china. Asian Pac J Cancer Prev. 2013;14:7251–6. doi: 10.7314/apjcp.2013.14.12.7251. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, Dishit R, Eser S, Mathers C, et al. [Accessed March 1, 2015];GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. 2013 [Internet] http://globocan.iarc.fr.
- Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Groopman JD, Kensler TW, Wild CP. Protective interventions to prevent aflatoxin-induced carcinogenesis in developing countries. Annu Rev Public Health. 2008;29:187–203. doi: 10.1146/annurev.publhealth.29.020907.090859. [DOI] [PubMed] [Google Scholar]
- IARC Working Group. IARC Working Group Reports. International Agency for Research on Cancer; 2007. Attributable Causes of Cancer in France in the year 2000; p. 48. [Google Scholar]
- Inoue M, Sawada N, Matsuda T, Iwasaki M, Sasazuki S, Shimazu T, et al. Attributable causes of cancer in Japan in 2005--systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann Oncol. 2012;23:1362–9. doi: 10.1093/annonc/mdr437. [DOI] [PubMed] [Google Scholar]
- Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. 2009;38:1497–511. doi: 10.1093/ije/dyp280. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chang CC, Marsh GM, Wu F. Population attributable risk of aflatoxin-related liver cancer: systematic review and meta-analysis. Eur J Cancer. 2012;48:2125–36. doi: 10.1016/j.ejca.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova-Rusher OV, Altekruse SF, McNeel TS, Ulahannan S, Duffy AG, Graubard BI, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122:1757–65. doi: 10.1002/cncr.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele L, Bosetti C, Turati F, Rapaccini G, Gasbarrini A, La Vecchia C, et al. Diabetes and Insulin Therapy, but Not Metformin, Are Related to Hepatocellular Cancer Risk. Gastroenterol Res Pract. 2015;2015:570356. doi: 10.1155/2015/570356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortality G.B.D. Collaborators Causes of Death. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311:183–92. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Boyd L, Walker LC. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105(Suppl 2):S77–81. doi: 10.1038/bjc.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praud D, Rota M, Rehm J, Shield K, Zatonski W, Hashibe M, et al. Cancer incidence and mortality attributable to alcohol consumption. Int J Cancer. 2016;138:1380–7. doi: 10.1002/ijc.29890. [DOI] [PubMed] [Google Scholar]
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–55. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhu L, Liu S, Xie WF. A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer. 2005;92:607–12. doi: 10.1038/sj.bjc.6602333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield KD, Rylett M, Gmel G, Gmel G, Kehoe-Chan TA, Rehm J. Global alcohol exposure estimates by country, territory and region for 2005--a contribution to the Comparative Risk Assessment for the 2010 Global Burden of Disease Study. Addiction. 2013;108:912–22. doi: 10.1111/add.12112. [DOI] [PubMed] [Google Scholar]
- Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56:704–13. doi: 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130:1639–48. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. 2007. [Google Scholar]
- World Health Organization. The World Health Report 2002: List of Member States by WHO Region and Mortality Stratum. 2002. [Google Scholar]
- World Health Organization. Obesity (body mass index ≥ 30), age-standardized (%) Estimates by country. 2017a. [Google Scholar]
- World Health Organization. Overweight (body mass index ≥ 25), age-standardized (%) Estimates by country. 2017b. [Google Scholar]
- World Health Organization. Raised fasting blood glucose (>= 7.0 mmol/L or on medication); Data by country. 2017c. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.