Abstract
Purpose
T2 MRI oximetry can non-invasively determine oxygen saturation (Y) but requires empirical MR calibration models to convert the measured blood transverse relaxation (T2b) into Y. The accuracy of existing T2b models in the presence of blood disorders such as sickle cell disease (SCD) remains unknown.
Methods
A Carr Purcell Meiboom Gill T2 preparation sequence was used to make 83 whole blood measurements from 11 subjects with SCD in order to derive an ex-vivo hemoglobin S (HbS) T2b model. Forearm venous blood gas, sagittal sinus T2 (T2 Relaxation Under Spin Tagging) and total brain blood flow (phase contrast MRI) were measured in 37 healthy controls and 33 SCD subjects (age 24.6 ± 10.2 years). Cerebral oxygen saturation, extraction fraction and metabolic rate estimates were calculated using three separate T2b models. Cerebral and forearm oxygen extraction fraction were compared.
Results
Ex-vivo, SCD blood had greater saturation dependent relaxivity than control blood, with a weak dependence on HbS and no dependence on hematocrit. In-vivo, the HbS T2b model predicted Yv values with lowest coefficient of variation (compared to existing T2b models) and the strongest correlation with peripheral venous oximetry (r2=.29). The HbS T2b model predicted systematically higher Yv measurements in SCD patients (73 ± 5 and 61 ± 6, p<0.0001) which was mirrored by peripheral venous measurements (75 ± 20 and 45 ± 20, p<0.0001).
Conclusion
Cerebral and peripheral oxygen extraction are decreased in SCD patients, suggesting either blood flow is increased beyond metabolic demands or the presence of physiological arterial-venous shunting.
Keywords: Sickle Cell Disease, Oxygen Extraction, T2 Relaxation Under Spin Tagging, Cerebral Metabolic Rate of Oxygen, Oxygenation, Hyperemia
Introduction
Cerebral MRI oximetry is a promising technology because it offers a noninvasive metric of venous oxygen saturation and oxygen extraction fraction (OEF)(1). When paired with a MRI based cerebral blood flow (CBF) technique such as arterial spin labeling(2) or phase contrast(3), MRI oximetry allows for calculation of cerebral metabolic rate of oxygen (CMRO2) quickly and easily within a single MRI examination(4).
Several techniques for MRI oximetry exist, all of which utilize the fact that hemoglobin undergoes a change in magnetic susceptibility when transitioning from the oxyghemoglobin to deoxyhemoglobin configuration(5). Of these, T2 Relaxation Under Spin Tagging (TRUST)(1) is the most widely reported MRI oximetry technique because it is easily performed, reproducible, rapid and well tolerated (6). TRUST uses arterial spin labeling to magnetically tag and isolate blood in the sagittal sinus and a Carr Purcell Meiboom Gill (CPMG)(7) T2 preparation pulse train to apply T2 weighting to blood, allowing for the determination of the blood transverse relaxation (T2b). Predetermined MR signal models relating T2b and oxygen saturation (Y) are then used to convert the measured T2b into venous oxygen saturation (Yv) in vivo. Due to the ease and efficacy of TRUST MRI, there are several recent reports of Yv, OEF and CMRO2 in a number of pathologic and physiologic states and conditions including vascular aging, drug addiction, multiple sclerosis and sickle cell disease(8–11).
Although TRUST has excellent intersubject, interscan and intersession reproducibility(6), the accuracy of MR oximetry in pathologic blood conditions, such as sickle cell disease, remains an open question. The most commonly used T2b model for oximetry calibration was derived from bovine blood (which has smaller cells that do not exhibit red cell aggregation) and is defined over a range of hematocrit not applicable for anemic patients(12,13).
In order to strengthen the reliability of TRUST and T2 based MRI oximetry in patients with sickle cell disease, this work sought to determine the impact of sickle hemoglobin (HbS) on ex vivo T2b model calibrations and derived Yv, OEF and CMRO2 estimates in healthy controls and patients with sickle cell disease.
Methods
All studies were performed at Children’s Hospital Los Angeles and approved by the Committee on Clinical Investigation (CCI# 11-00083). Informed consent or assent was obtained from all study participants. The study was performed in two separate segments: ex vivo T2b signal model determination and in vivo brain imaging. Participants were separated into two study populations: healthy control subjects (CTL) and subjects with sickle cell disease (SCD). We restricted the population to adolescents and young adults without previous overt strokes or known cerebrovascular disease. Other exclusion criteria were the following: 1) pregnancy; 2) occurrence of acute chest or pain crisis hospitalization within one month; and 3) additional conditions such as epilepsy or traumatic brain injury.
TRUST imaging
All imaging was performed on a Philips Achieva 3T MR system with an eight channel, receive only head coil.
The in vivo, TRUST pulse sequence used in this study is thoroughly explained in prior publications(12,13). Briefly, a transverse, single slice TRUST sequence was used to measure T2b in the sagittal sinus. The TRUST sequence consisted of a pulsed arterial spin labeling tag, a CPMG T2 preparation module, 1022ms delay and an echo planar readout (Figure 1). Pairwise subtraction of images with and without tagging were used to render a difference image that isolated venous blood (Figure 2). The interecho spacing (tau) of the CPMG module was 10ms. Phase cycled, MLEV16 composite pulses were used to limit off resonance effects (90x° 180y° 90x°). The number of inversion pulses were varied across four image acquisitions (0, 4 8 16 pulses) to acquire difference images with effective echo times of 0ms, 40ms, 80ms and 160ms. A 1022ms delay after T2 preparation was used to allow tagged blood to reach the sagittal sinus where images were acquired with a single-shot echo planar readout, TR/TE, 1978/3.77 ms.
Figure 1.
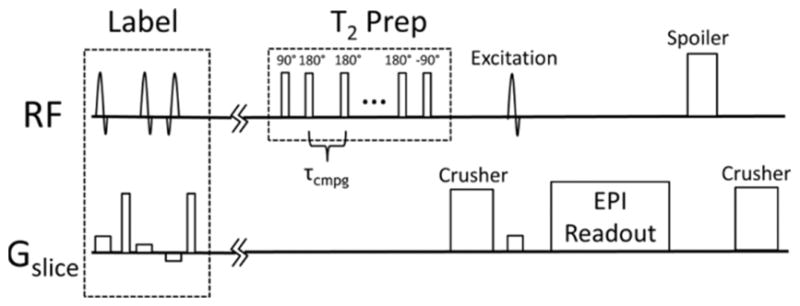
A) T2 Relaxation Under Spin Tagging (TRUST) pulse sequence that was used to measure T2b within the sagittal sinus.
Figure 2.
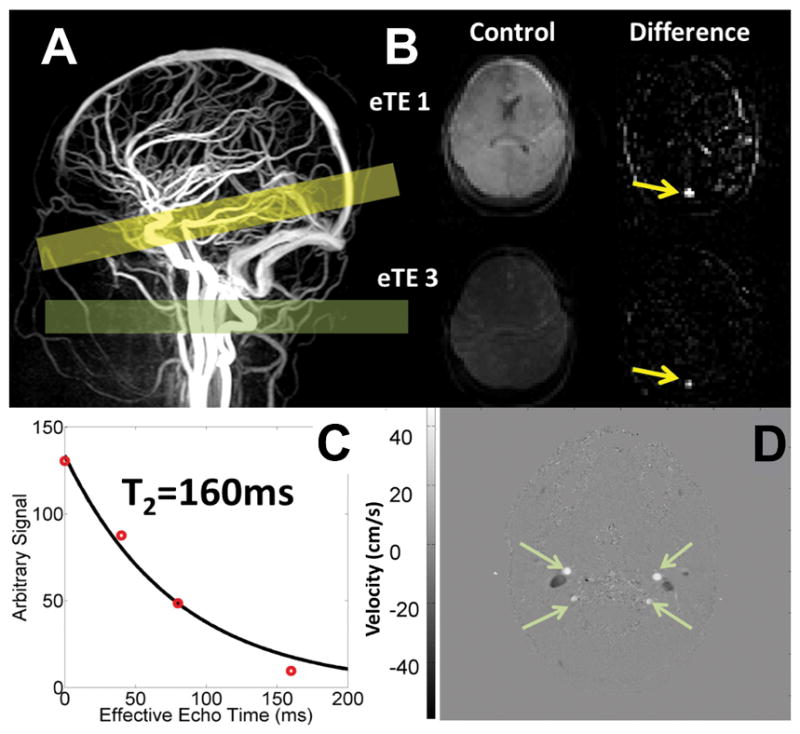
A) Head and neck angiogram and the localization of the TRUST imaging plane (yellow bar) and the Phase Contrast imaging plane (green bar). B) Representative TRUST images showing the anatomical, control images the first and third effective echo time and the resulting difference image upon subtraction of the control and labeled TRUST images. Yellow arrows correspond to the isolated blood signal in the sagittal sinus of the difference images. C) Mono-exponential fitting of all four difference images is then performed to estimate T2b that is then converted to a blood oxygenation value using empirically derived blood calibrations. Phase contrast (D), phase based velocity image of the four major cerebral feeding vessels of the neck demonstrate how velocity and global CBF can be quantified.
The difference signal in the sagittal sinus was averaged and the four measured eTE were fit to the monoexponential decay equation:
| [1] |
where ΔSignal0 is constant across echo times and T1b is the blood longitudinal relaxation. A fixed T1b was used in patients with SCD(14) and hematocrit corrected T1b was used in the CTL subjects(14,15).
Blood Experiments
The TRUST sequence was modified for the ex vivo blood measurements to reduce the field of view and disable the ASL tagging because there was no blood flow. Additionally, a three-shot acquisition was used to reduce boundary susceptibility artifacts. A head-to-head comparison with and without ASL and EPI modifications demonstrated no statistical difference in T2 estimation (−1.2% ± 5.1%). The remaining CPMG T2 preparation sequence parameters included a repetition time of 1978ms, 110cm FOV, 5mm slice thickness and in plane resolution of 1.7mm. These modifications did not alter the fundamental measured T2b(13).
Approximately 30ml of blood was acquired from the antecubital vein into heparinized tubes. A small aliquot was immediately (<10 minutes) used for determination of peripheral venous oxygen saturation (Alere Inc., EPOC Blood Analysis System, Walthan, MA). Complete blood count and hemoglobin electrophoresis was also performed. Remaining blood was placed at 4° C until analysis. All studies were performed within 12 hours of phlebotomy.
The remaining blood was warmed and reoxygenated by ambient exposure to room air during ex vivo experimentation. Oxygen saturation, hemoglobin, methemoglobin and carboxyhemolgobin were measured using a bench top CO-oximeter (Radiometer, OSM3, Copenhagen, Denmark). The blood was intentionally left at the native hematocrit level, as to disturb the blood samples as little as possible, increasing reliability of T2b estimates but lowering the measurable hematocrit range.
A previously described, custom blood phantom chamber was used to image the blood samples within the MRI scanner(13). The blood phantom chamber was sealed to the atmosphere and temperature controlled to 37°C.
All blood images were acquired in the sagittal orientation. Once the blood reached 37˚C, it was gently agitated by aspiration with a syringe system and then sealed from the atmosphere. The blood oxygen saturation was then measured on the table top CO-oximeter and MR imaging was initiated. Next, a series of three TRUST images were performed: first τ=10ms, next τ=20ms and then another τ=10ms. The blood sample was gently agitated prior to each image acquisition, in order to reduce blood sedimentation and aggregation (13). Temperature and blood oxygen saturation were measured following each imaging series. Blood was then removed from the imaging phantom and placed inside a temperature-controlled gas chamber and deoxygenated using 37° C, humidified gas and a membrane oxygenator (Living System Instrumentation, Burlington, Vermont, USA). The gas mixture used for deoxygenation was 95% N2 and 5% CO2 in order to maintain a physiologic pH. The blood was deoxygenated by 10–30% and then was placed back into the blood phantom chamber for imaging. The blood was again gently agitated, temperature and blood oxygenation were measured and the imaging series was repeated. TRUST imaging and deoxygenation were repeated until the blood reached an oxygen saturation of approximately 30%. The blood was then safely discarded.
Linear regression was used to determine and correct for reoxygenation and aggregation mediated T2 drift(13).
Following completion of the ex vivo T2b measurements, T2b was derived by applying monoexponential fitting to the blood pool signal and the values were fit to a bilinear relationship of hematocrit (Hct) and oxygen saturation (Y) as follows:
| [2] |
where A1, A2, A3, and A4 are empirically determined coefficients(13).
In Vivo Imaging
Phase Contrast
In addition to TRUST, whole brain cerebral blood flow was measured using phase contrast MRI(3). Cerebral blood flow data has been previously reported in these patients(13,16). In short, a single, two dimensional phase contrast slice was positioned approximately 1–5 cms superior to the carotid bifurcation and optimally orthogonal to both carotid and vertebral arteries (Figure 2). Image parameters included 260x260mm FOV, TE=7.5ms, 5mm slice thickness, 150 cm/s velocity encoding gradient (VENC), 10 signal averages. Vessel boundaries were semi-automatically segmented from the complex difference images using Canny edge detection and mapped to the phase difference image for flow calculation. The coefficient of variation of these measurements was 4.6% ± 3.5% over a 30 minute interval(16).
Phlebotomy for complete blood count, venous blood gas and hemoglobin electrophoresis were performed on all subjects. Fingertip pulse oximetry was measured continuously throughout the MRI examination.
Calculated Parameters
In vivo TRUST Yv values from SCD patients were subsequently calculated using three different T2b models, 1) the original bovine calibration(12), 2) the calibration previously reported using the blood of healthy subjects(13), and 3) the newly derived HbS specific, SCD calibration. Linear regressions of oxygen saturation, oxygen extraction and cerebral metabolic rate of oxygen versus oxygen content were performed to examine the influence of T2b model on physiologic oxygenation predictions. Regression coefficients were compared using a z-score test of equality(17).
Several physiological parameters were derived. Cerebral metabolic rate of oxygen (CMRO2) was defined as:
| [3] |
where CaO2 and CvO2 are the arterial and venous oxygen contents respectively:
| [4] |
| [5] |
where pO2 is the vessel specific partial pressure of oxygen and Ya is the arterial oxygen saturation. Pulse oximetry and phlebotomy were used to obtain Ya and hemoglobin, respectively, for oxygen content quantification. Since arterial and venous blood gasses were not obtained, the partial pressure of oxygen was assumed to be 100 torr in the systemic circulation and 40 torr in the venous circulation.
Therefore, CMRO2 can be rewritten as
| [6] |
where OEF is the oxygen extraction fraction defined as:
| [7] |
Results
The blood of 11 patients with SCD (five HbSS, two HbSS receiving transfusion therapy, one HbSC, two HbSβ+, one HbSβ0) was separately studied for a total of 83 HbS T2b measurements over a range of physiologically relevant oxygen saturation values (18%–99%) and hematocrit levels (24%–40%). The white blood cell count was 10.8±7.3 cells/dL, reticulocyte count 7.4±4.9%, hemoglobin F% 6.3±9.9% and hemoglobin S% 66.4 ±22.1% across the blood samples from subjects with SCD.
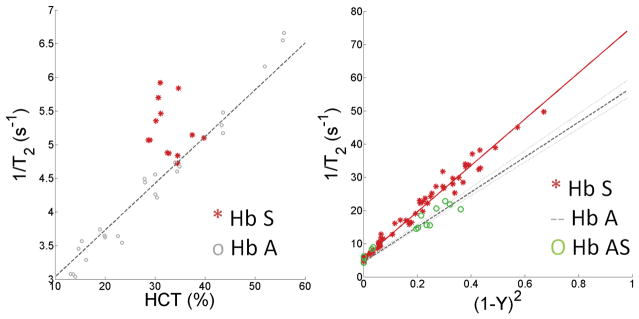
Neither the bovine nor the HbA T2b model from equation [2], adequately fit the observed T2b relationship for sickle cell blood (Figure 3). Hematocrit had no influence on the measured T2b for both fully saturated (>95% saturation) (Figure 3) and desaturated HbS blood, (r2 = 0.00090, p = 0.79). Though the inverse of T2b was highly dependent on (1-Y)2, the slope of this relationship was steeper than that observed with the previously derived HbA T2b model (Figure 3). Given these findings, we defined the following data driven, hematocrit independent, linear HbS T2b model:
| [8] |
where A was 70.0 and B was 5.75 when a tau=10ms was used and 93.1 and 7.16 when tau=20ms. This relationship demonstrated an r2=0.98, p<0.0001. The residual of this relationship was weakly dependent on HbS percentage r2=0.04, p=0.03, but was not included in the model because HbS% is not routinely known when patients with SCD undergo brain MRI. Additionally, a total of 18 measurements where made using the blood of two subjects with sickle cell trait, HbS % 29.9 ±3.3. The T2b measurements were statistically identical to the HbA T2b model (Figure 3).
Figure 3.
Left) Ex vivo relationship between hematocrit and 1/T2b0 for fully saturated blood from sickle cell disease patients (filled circles) and normal controls (open circles, previously reported (13)). HbS sickle blood exhibits greater relaxivities than non-sickle blood and does not exhibit an obvious hematocrit dependence (r2=0.00090, p=0.79). Right) Despite lower hematocrit levels, blood from SCD patients (filled circles) exhibits a steeper slope with (1-Y)2 compared to healthy HbA blood, (r2=0.981, p<0.0001). In contrast, blood from subjects with sickle cell traits (open green circles) exhibits relaxation behavior similar to healthy HbA blood. For reference, the HbA model fit for a hematocrit of 35% is displayed by the dashed line, with 25% to 75% confidence intervals shown in gray.
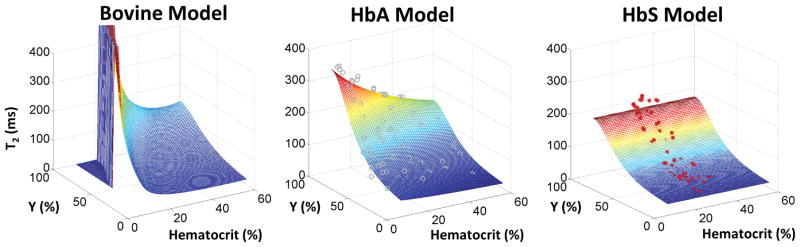
The three dimensional relationships between T2b, hematocrit and oxygen saturation produced by each T2b model can be seen in Figure 4. The differences in T2b model formulation translated into model specific predictions of Yv, OEF and CMRO2 when using TRUST MRI in vivo.
Figure 4.
Three distinct, empirically derived T2b signal models are presented. Left) The most widely used T2b model was derived using bovine blood over hematocrit values of 35%–55% and is highly nonlinear at low hematocrit. Middle) The HbA model (13) was calibrated across hematocrit values of 10%–55% using blood of healthy subjects and demonstrates a bilinear relationship between 1/T2b, hematocrit, and (1-Y)2. The gray circles represent individual measurements. Right) The ex vivo T2b model derived in this work is fairly distinct from both aforementioned T2b models, demonstrating no hematocrit dependence. The red asterisks denote the individual ex vivo measurements used to derive the T2b model fits.
In vivo Brain Imaging
Brain MRI examinations were performed on 70 subjects in order to determine Yv, OEF and CMRO2 using TRUST MRI. Additional demographic information can be found in Table 1.
Table 1.
Demographic and Oximetry Data.
| Healthy Control Subjects | Subjects with Sickle Cell Anemia (SCD) | |
|---|---|---|
| N | 37 | 33 |
| Sex | 10 Male | 16 Male |
| Age (years) | 27.2 ± 10.6 | 21.8 ± 9.0* |
| Hematocrit (%) | 40 ± 4 | 28 ± 4* |
| Hemoglobin (g/dl) | 13.5 ± 1.3 | 9.7 ± 1.7* |
| Phase Contrast CBF (ml/100g/min) | 58.8 ± 9.4 | 96.0 ± 21.4* |
| T2 (ms) | 74.0 ± 12.7 | 96.9 ± 12.7 * |
| Bovine Model Yv (%) | 65 ± 6 | 60 ± 8* |
| HbA Model Yv (%) | 61 ± 6 | 64 ± 6 |
| HbA and HbS Model Yv (%) | 61 ± 6 | 73 ± 5* |
| Bovine Model OEF | 0.34 ± .05 | 0.38 ± .08 |
| HbA model OEF | 0.38 ± .06 | 0.34 ± .06* |
| HbA and HbS model OEF | 0.38 ± .06 | 0.24 ± .04* |
| Bovine Model CMRO2 (mlO2/100g/min) | 3.63 ±0.80 | 4.45 ± 0.85* |
| HbA model CMRO2 (mlO2/100g/min) | 3.99 ± 0.81 | 3.96 ± 0.60 |
| HbA and HbS model CMRO2 (mlO2/100g/min) | 3.99 ± 0.81 | 2.88 ± 0.52* |
| Peripheral Yv (%) | 45.0±19.9 | 74.9±20.5* |
| Peripheral OEF | 0.54±0.20 | 0.23±0.21* |
The average cerebral flow and oxygenation parameters measured by PC and TRUST MRI varied between populations and T2b models (Table 1). Using the bovine T2b model, Yv was lower and OEF trended higher in the SCD patients compared to controls. In contrast, the HbA T2b model yielded systematically higher Yv values for SCD patients and lower Yv values for controls. The difference in absolute oxygenation percentage using the bovine calibration compared to the HbA was −3±1% in CTL (p<0.0001) and 4±4% in SCD (p<0.0001). The newly derived HbS T2b model demonstrated even larger differences for SCD patients, with the absolute differences in Yv increasing to 13±6.4% in SCD (p<0.0001). Though the coefficients of variation were similar in controls across T2b models (bovine 9.2% and HbA 9.8%), the HbS demonstrated the lowest coefficient of variation in SCD subjects (bovine 13.3%, HbA 9.4%, HbS 6.8%).
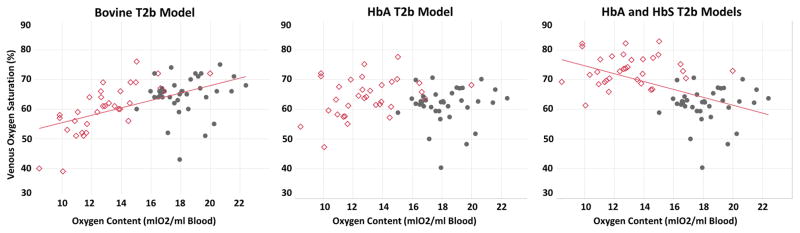
Insight into these model discrepancies can be gained from regression analysis with oxygen content. According to the bovine model, increasing anemia was associated with progressively lower Yv, as well as increased OEF and CMRO2 (Figure 5). However, the HbA and HbS calibrations exhibited completely different physiologic predictions. The HbA T2b model demonstrated no relationship between Yv and oxygen content. In contrast, the HbS T2b model predicted that Yv varies inversely with oxygen content. The linear coefficients (mean ± standard error) for the bovine (slope=1.2±0.2, intercept= 42.8±3.8, p<0.0001, r2=0.29), HbA (slope=−0.08±0.2, intercept= 64.8±3.7, p=0.7, r2=.002), and HbS (slope=−1.3±0.2, intercept= 87.5±3.5, p<0.0001, r2=.30) T2b models were all significantly different from one another (p<0.01).
Figure 5.
TRUST oximetry derived, in vivo venous oxygen saturation predictions in healthy subjects (gray circles) and subjects with sickle cell disease (red diamonds) with respect to oxygen content using three separate T2b models. Each model demonstrates a unique physiological relationship between oxygen content and cerebral venous oxygen saturation. The bovine T2b (left) predicts an increasing oxygen venous saturations with respect to oxygen content (r2=0.21, p<0.0001) whereas the newly derived HbS model (right) predicts a diametrical opposite relationship (r2=0.29, p<0.0001). The HbS model statistically outperformed the other models in SCD patients, producing the lowest coefficient of variation (bovine 13.3%, HbA 9.4%, HbS 6.8%).
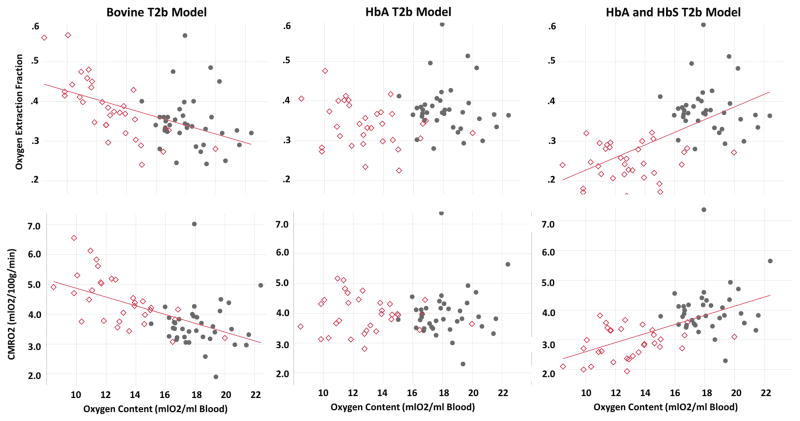
The systematic Yv differences across T2b models also gave rise to conflicting relationships between OEF, CMRO2 and oxygen content (Figure 6). OEF and CMRO2 were negatively correlated with oxygen content in the bovine model (Figure 6, left panels), were independent of oxygen content in the HbA model (Figure 6, center panels) and were positively correlated with oxygen content in the HbS model (Figure 6, right panels).
Figure 6.
Derived estimates of cerebral oxygen extraction fraction and cerebral metabolic rate of oxygen using TRUST measured venous oxygen saturation, peripheral pulse oximetry and global cerebral blood flow, equation [3–7], in healthy subjects and subjects with sickle cell disease (red diamonds) with respect to oxygen content using three separate T2b models. Similar to Figure 4, T2b model selection had a large effect on the derived oxygenation estimates, although the coefficient of variation of OEF was lowest when the HbS T2b model was used (bovine 21.1%, HbA 17.6% and HbS 16.7%).
To place the different cerebral oximetry predictions into physiological context, we also measured antecubital vein Yv and OEF using cooximetry of venous blood samples. The peripheral Yv measurements were higher in the SCD (74.9±20.5%) compared to the CTL group (45.0±19.9%) (Figure 7), most similar to cerebral Yv predictions using the HbS T2b model. Peripheral OEF estimates had the strongest correlation with cerebral OEF estimates made using the HbS model (p<0.0001, r2=0.30), were weakly correlated with the HbA model (p=0.009, r2=0.14) and were uncorrelated when the bovine model was used (p=0.7, r2=0.003). Upon multivariate analysis of cerebral Yv with peripheral Yv and oxygen content, oxygen content had an r2=0.41 and peripheral oxygenation was no longer correlated.
Figure 7.
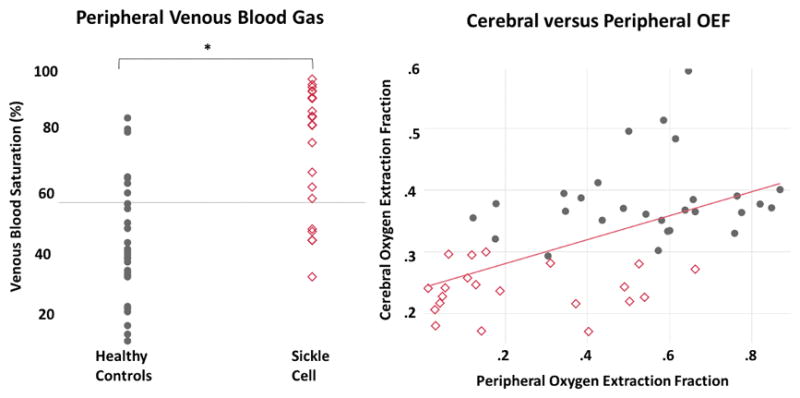
(Left) Peripheral venous blood gas saturation measurements were higher in the subjects with SCD compared to control, most similar to the cerebral Yv predictions made by the HbS T2b model. (Right) Peripheral venous blood had the strongest correlation coefficient (r2=0.30) to cerebral Yv TRUST estimates when the HbS model was used in the SCD subjects. This relationship was no longer significant once oxygen content was accounted for in multivariate analysis, suggesting anemia and/or hyperemia is driving similar physiology in both vascular beds.
Discussion
This work uses validated empirical techniques and the blood of subjects with sickle cell disease to derive a HbS T2b model that confers improved Yv predictions in SCD when to compared to existing T2b models. These Yv predictions and the resulting physiological conclusions are contradictory to recently published MR oximetry results in SCD(9,18,19). Given the documented limitations of the extrapolating the bovine T2b models outside its calibrated range(13), this work calls into question recent MR oximetry conclusions(9,18,19) and demonstrates that SCD patients exhibit decreased OEF proportional to their level of anemia.
To date, all existing MR oximetry Yv measurements in SCD subjects were derived using a bovine T2b model(12), which has several limitations for use in SCD(9,18,19). First, the bovine T2b model was originally defined over a hematocrit range from (35–55%) and extrapolates poorly to hematocrit ranges observed in anemic subjects(13). Next, bovine red blood cells are smaller than human red blood cells and do not aggregate or form rouleux(20). Shape of the exchange unit is a fundamental property in Luz Meiboom T2b models(5) and red cell aggregation has been shown to decrease the relaxivity of blood(21). These limitations were addressed by previous calibration experiments performed in whole human blood (HbA model) over a wide hematocrit range of 10%–55%(13). However, neither the bovine, nor the HbA model account for the pathologic, hematological or rheological properties unique to whole, sickle cell blood.
Sickle Model for T2b
When deoxygenated, hemoglobin S molecules undergo a double nucleation process, rapidly polymerizing into long rigid fibers within the red blood cell(22–24). These polymer fibers often deform and may lyse the red blood cell membrane. Though the kinetics of hemoglobin polymerization and cellular deformation have been well described(22–24), the implications of this phenomenon on magnetic transverse relaxation is unknown, prompting the ex vivo T2b calibration study.
Blood transverse relaxation rate was linear with (1-Y)2, similar to other Luz-Meiboom based models(25,26). Unlike the HbA T2b model, the HbS T2b model was independent of hematocrit. This may be due, in part, to the relatively small HCT range studied in our patients with SCD. We might have been able to demonstrate a T2b -hematocrit relationship by artificially manipulating the blood over a broader hematocrit range. However, sickle red blood cells are fragile and we aimed to minimize red cell mechanical stressor and hemolysis. Alternatively, the relaxation rate of sickle blood may be intrinsically less hematocrit sensitive. According to the two compartment exchange relationship(5), the slope of (1-Y)2 is governed by size, shape and permeability of the cellular environment. In normal blood, shape and permeability are relatively constant across subjects and oxygenation status(13). In SCD blood, red cell shape and permeability(27,28) may vary dramatically across subjects and oxygenation status, potentially dwarfing the hematocrit dependency. A similar hematocrit independence was previously observed in deoxygenated SCD blood T1 measurements(14). This is striking because T1 is even more hematocrit dependent than T2 measurements(15). Other factors, such as the presence of hemolysis(29) and iron inclusion bodies(30), could also add variability into the empirical relationships.
Surprisingly, the concentration of hemoglobin S contributed little to the observed relaxivity (r2=0.04), suggesting that the red blood cell shape, surface area and/or permeability abnormalities responsible for the transverse relaxivity changes are more enigmatic than a simple relationship with hemoglobin S concentration. The weak relationship between relaxation and hemoglobin S concentration suggests that the HbS T2b model is valid across sickle cell phenotypes. This is clinically fortuitous for MR oximetry, in that hemoglobin S concentration can be difficult to know precisely without hemoglobin electrophoresis testing. Further evidence that sickle hemoglobin is acting though an indirect mechanism, such as shape change and permeability, is that the sickle cell trait subjects follow the HbA T2b calibration even though they have 30–40% hemoglobin S concentration (Figure 3). In sickle cell trait, hemoglobin rarely polymerizes and the red blood cell are morphologically normal under most physiologic conditions because every cell contains a stable mixture of normal and sickle hemoglobin(23). Despite the complexity of the sickle cell whole blood relaxation, our HbS T2b model accounted for over 98% of the variability in the ex vivo measurements in the presence of broad range of HbS genotypes and sickle hemoglobin concentrations. Importantly, our calibration was also consistent with independent observations in six nontransfused homozygous sickle cell disease patients(31).
In Vivo TRUST and Using the Sickle Model
In light of the newly derived HbS T2b model, previous physiologic interpretations using the bovine T2b model should be reexamined.
Predictions of the Bovine Model
In our data, the bovine model predicted a direct relationship between Yv increased and oxygen content whereas OEF and CMRO2 varied inversely, replicating previous observations(9,18,19). Those authors concluded that cerebral autoregulation was impaired in patients with SCD, similar to patient with chronic renal disease(32) and critical carotid stenosis(33), resulting in elevated OEF(9). However, no previous, non-MR cerebral oxygenation study in patients with SCD using PET or nitrous oxide methods has observed elevated cerebral OEF as a pathophysiologic consequence(34,35). To the contrary, studies have denoted elevated peripheral Yv in anemic subjects(36), which is exacerbated during SCD pain crisis(37). Furthermore, the SCD patients in our study were healthy, well managed since birth, had normal MRA and were free from known stoke. Therefore, given the proven biases demonstrated in the bovine model for hematocrit <35%(13) (Figure 4), we argue that the increased OEF and CMRO2 in anemic subjects predicted by this model and reported previously may be spurious.
Predictions for the Hemoglobin S model
This work demonstrates several reasons why the presented HbS model is superior to existing T2b calibration models when used in patients with sickle cell disease. First, the HbS model statistically outperformed both the bovine and HbA T2b models. In vivo, intersubject variability in the SCD subjects, reflected by the coefficient of variability in Yv and OEF estimates, was lowest when the HbS was used. Second, while the predicted cerebral Yv and OEF using the HbS T2b model seem paradoxical, they correlated well with peripheral oxygen extraction (Figure 7). Although the cerebral vasculature is under distinct autoregulatory control from the systemic vasculature, Figure 7 suggests that both the systemic and cerebral oxygen delivery are increased relative to demand. Furthermore, the loss of correlation between peripheral and cerebral Yv following multivariate analysis with oxygen content, further suggests anemia and/or high flow are driving reduced oxygen extraction in both vascular beds. It is important to note that the peripheral oximetry measurements are robust to hemoglobin subtypes, changes in hemoglobin dissociation and corrected for dyshemoglobins (carboxyhemoglobin, methemoglobin). Third, there is a historical precedent for the observation of decreased oxygen extraction with anemia. Lower cerebral arterio-venous oxygen difference (35) and elevated systemic venous oxygenation have been described in SCD patients but the mechanism remains unclear(36). Furthermore, a highly defensible explanation for decreased oxygen extraction in anemic patients is outlined below.
Microvascular Arteriovenous Shunting
In this work, we observed that at higher levels of anemia and cerebral blood flow, there is the lowest levels of oxygen extraction and utilization (Figure 6). This mismatch in local tissue oxygen delivery and bulk, organ oxygen delivery is likely caused by microvascular arteriovenous shunting. Recent work using a pseudo continuous arterial spin labeling has demonstrated that cerebral vascular shunting may be present in high cerebral blood flow populations with reduced microvascular transit time such as sickle cell disease, other anemic syndromes and pediatric populations(38,39). Shunting pathophysiology has also been observed in several other vascular beds in SCD(40,41), which is exacerbated during hyperemic flow conditions common in crisis(42). Several analytical microvascular models have also shown that nonlinear matching between CBF and CMRO2 leads to reduced OEF during hyperemia due to oxygen diffusion limitations at the microvascular level(43–45), however this is an area of ongoing debate(46). Uncoupling between CBF and CMRO2 is also a well described phenomenon following the acute hyperemia during the BOLD response(47). Additionally, analytical and animal work has shown that variability in microvascular transit time leads to diminished OEF and apparent uncoupling of OEF and CMRO2 (44,48). SCD subjects are known to have microvascular damage that leads to highly heterogeneous microvascular bed(49) and shortened transit times(50), likely contributing to the observed decrease in global OEF.
At this time, it is unclear whether the decreased CMRO2 is primary or secondary to the diminished local tissue oxygen utilization. On one hand, decreased CMRO2 may be a protective mechanism staving off more catastrophic ischemia, similar to hibernation. On the other hand, microvascular arteriovenous shunting may be reducing the bioavailability of oxygen, driving down CMRO2.
Either way, the microvascular arteriovenous shunting hypothesis is provocative and understanding the interplay of macro and microvascular oxygen transport may impact treatment strategies in SCD. For instance, increasing oxygen carrying capacity, through transfusions, hydroxyurea or other pharmacological means not only increases oxygen bioavailability but may also jointly slow blood flow thereby improving oxygen exchange. However, future therapies in SCD must balance oxygen transport kinetics with the impact of increased blood viscosity, capillary transit time heterogeneity, hemoglobin polymerization and red cell sickling. Further work is necessary to characterize the interplay between cerebral blood flow, capillary transit heterogeneity and cerebral oxygenation in sickle cell disease subjects.
Limitations
The presented data should be considered within their limitations. The ex vivo calibration studies place greater mechanical stress on sickle red blood cells than is encountered in vivo. More importantly, the red cells remain deoxygenated for a longer period time. In vivo, the red blood cell releases oxygen, transits the remaining microvasculature and arrives in the imaging plane in a matter of seconds. Based on polymerization kinetics, intracellular polymerization has a rapid onset and relatively few red cells will undergo a shape change by the time blood is imaged in vivo using TRUST(24). On the other hand, ex vivo, deoxygenation and TRUST imaging can take many minutes, potentially amplifying the shape and permeability changes. Therefore, it is possible that the in vivo HbS T2b model has behavior intermediate to the ex vivo HbA and HbS models. However, both the HbA and HbS models predict quite different behavior than the bovine model and are better aligned with non-MRI based oximetry results in SCD(34,35).
This study would have benefited from direct jugular venous sampling for validation. However, the barriers to doing so are formidable and no previous cerebral MRI oximetry study has performed this validation. Given the preponderance of evidence to date, we conclude that the HbS T2b model should be provisionally used for MR oximetry in SCD subjects.
Conclusion
In conclusion, a de novo, ex vivo HbS T2b model of sickle cell blood differs dramatically from bovine and HbA T2b models, suggesting that morphologic and/or hematologic changes of sickle cell containing blood are critically important to T2b MRI oximetry estimation. TRUST oximetry shows that cerebral oxygen extraction and metabolism is lower in SCD subjects, suggesting hyperemic condition give rise to uncoupling in tissue oxygen delivery and oxygen utilization. Future studies are needed to confirm these conclusions and their implications on ischemic cerebrovascular disease.
Acknowledgments
This work was supported by the NHLBI of the NIH (1U01HL117718-01). Adam Bush is sponsored through a NHLBI minority supplement (1U01HL117718-01).
Contributor Information
Adam M Bush, Department of Radiology, Stanford University, Stanford, California USA.
Thomas D Coates, Division of Hematology/Oncology, Children’s Hospital Los Angeles, Los Angeles, California, USA.
John C Wood, Division of Cardiology, Children’s Hospital Los Angeles, Los Angeles, California, USA.
References
- 1.Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med. 2008;60(2):357–363. doi: 10.1002/mrm.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsop DC, USA BIDMCaHMSDoRBM, Detre JA, USA UoPDoNaRPP, Golay X, UK UIoNDoBRaRL, Günther M, Germany FMB, University Bremen Bremen G, Germany MGH, Hendrikse J, Netherlands UMCUDoRUT, Hernandez-Garcia L, University of Michigan FMRI Laboratory DoBEAAMU, Lu H, USA USMCAIRCDT, MacIntosh BJ, Canada UoTDoMBT, Canada SRIDoPST, Parkes LM, University of Manchester Centre for Imaging Science IoPH, Faculty of Medical and Human Sciences Manchester UK, Smits M, University Medical Centre Rotterdam Department of Radiology EMRTN, Osch MJP, Leiden University Medical Center C J Gorter Center for High Field MRI DoRLTN, Wang DJJ, USA UoCLADoNLAC, Wong EC, USA UoCSDDoRaPLJC, Zaharchuk G., USA SUDoRSC Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine. 2016;73(1):102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enzmann DR, Ross MR, Marks MP, Pelc NJ. Blood flow in major cerebral arteries measured by phase-contrast cine MR. AJNR Am J Neuroradiol. 1994;15(1):123–129. [PMC free article] [PubMed] [Google Scholar]
- 4.Xu F, Ge Y, Lu H. Noninvasive quantification of whole-brain cerebral metabolic rate of oxygen (CMRO2) by MRI. Magn Reson Med. 2009;62(1):141–148. doi: 10.1002/mrm.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright GA, Hu BS, Macovski A. 1991 I.I. Rabi Award. Estimating oxygen saturation of blood in vivo with MR imaging at 1. 5 T. J Magn Reson Imaging. 1991;1(3):275–283. doi: 10.1002/jmri.1880010303. [DOI] [PubMed] [Google Scholar]
- 6.Liu P, Xu F, Lu H. Test-retest reproducibility of a rapid method to measure brain oxygen metabolism. Magn Reson Med. 2013;69(3):675–681. doi: 10.1002/mrm.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meiboom S, Gill D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Review of Scientific Instruments. 1958;29(688) [Google Scholar]
- 8.Ge Y, Zhang Z, Lu H, Tang L, Jaggi H, Herbert J, Babb JS, Rusinek H, Grossman RI. Characterizing Brain Oxygen Metabolism in Patients with Multiple Sclerosis with T2-Relaxation-Under-Spin-Tagging MRI. 2012 doi: 10.1038/jcbfm.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan LC, Gindville MC, Scott AO, Juttukonda MR, Strother MK, Kassim AA, Chen SC, Lu H, Pruthi S, Shyr Y, Donahue MJ. Non-invasive imaging of oxygen extraction fraction in adults with sickle cell anaemia. Brain. 2016;139(Pt 3):738–750. doi: 10.1093/brain/awv397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng SL, Dumas JA, Park DC, Liu P, Filbey FM, McAdams CJ, Pinkham AE, Adinoff B, Zhang R, Lu H. Age-related increase of resting metabolic rate in the human brain. Neuroimage. 2014;98:176–183. doi: 10.1016/j.neuroimage.2014.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu P, Lu H, Filbey FM, Tamminga CA, Cao Y, Adinoff B. MRI assessment of cerebral oxygen metabolism in cocaine-addicted individuals: Hypoactivity and dose dependence. NMR Biomed. 2014;27(6):726–732. doi: 10.1002/nbm.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu H, Xu F, Grgac K, Liu P, Qin Q, van Zijl P. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn Reson Med. 2012;67(1):42–49. doi: 10.1002/mrm.22970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bush A, Borzage M, Detterich J, Kato RM, Meiselman HJ, Coates T, Wood JC. Empirical model of human blood transverse relaxation at 3 T improves MRI T2 oximetry. Magn Reson Med. 2017;77(6):2364–2371. doi: 10.1002/mrm.26311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaclavu L, van der Land V, Heijtel DF, van Osch MJ, Cnossen MH, Majoie CB, Bush A, Wood JC, Fijnvandraat KJ, Mutsaerts HJ, Nederveen AJ. In Vivo T1 of Blood Measurements in Children with Sickle Cell Disease Improve Cerebral Blood Flow Quantification from Arterial Spin-Labeling MRI. AJNR Am J Neuroradiol. 2016;37(9):1727–1732. doi: 10.3174/ajnr.A4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3. 0 Tesla. Magn Reson Med. 2004;52(3):679–682. doi: 10.1002/mrm.20178. [DOI] [PubMed] [Google Scholar]
- 16.Borzage MT, Bush AM, Choi S, Nederveen AJ, Vaclavu L, Coates TD, Wood JC. Predictors of cerebral blood flow in patients with and without anemia. J Appl Physiol (1985) 2016;120(8):976–981. doi: 10.1152/japplphysiol.00994.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paternoster R. USING THE CORRECT STATISTICAL TEST FOR THE EQUALITY OF REGRESSION COEFFICIENTS. Criminology. 1998;36(4):859–866. [Google Scholar]
- 18.Bush AM, Borzage M, Choi S, Coates T, Wood JC. Elevated Cerebral Metabolic Oxygen Consumption in Sickle Cell Disease. 2014 [Google Scholar]
- 19.Watchmaker JM, Juttukonda MR, Davis LT, Scott AO, Faraco CC, Gindville MC, Jordan LC, Cogswell PM, Jefferson AL, Kirshner HS, Donahue MJ. Hemodynamic mechanisms underlying elevated oxygen extraction fraction (OEF) in moyamoya and sickle cell anemia patients. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16682509. 271678x16682509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumler H, Neu B, Mitlohner R, Georgieva R, Meiselman HJ, Kiesewetter H. Electrophoretic and aggregation behavior of bovine, horse and human red blood cells in plasma and in polymer solutions. Biorheology. 2001;38(1):39–51. [PubMed] [Google Scholar]
- 21.Schuhmacher JH, Clorius JH, Semmler W, Hauser H, Matys ER, Maier-Borst W, Hull WE. NMR relaxation times T1 and T2 of water in plasma from patients with lung carcinoma: correlation of T2 with blood sedimentation rate. Magn Reson Med. 1987;5(6):537–547. doi: 10.1002/mrm.1910050604. [DOI] [PubMed] [Google Scholar]
- 22.Ferrone FA, Hofrichter J, Eaton WA. Kinetics of sickle hemoglobin polymerization. II. A double nucleation mechanism. J Mol Biol. 1985;183(4):611–631. doi: 10.1016/0022-2836(85)90175-5. [DOI] [PubMed] [Google Scholar]
- 23.Eaton WA, Hofrichter J. Hemoglobin S gelation and sickle cell disease. Blood. 1987;70(5):1245–1266. [PubMed] [Google Scholar]
- 24.Eaton WA, Hofrichter J. Sickle cell hemoglobin polymerization. Adv Protein Chem. 1990;40:63–279. doi: 10.1016/s0065-3233(08)60287-9. [DOI] [PubMed] [Google Scholar]
- 25.Golay X, Silvennoinen MJ, Zhou J, Clingman CS, Kauppinen RA, Pekar JJ, van Zijl PC. Measurement of tissue oxygen extraction ratios from venous blood T(2): increased precision and validation of principle. Magn Reson Med. 2001;46(2):282–291. doi: 10.1002/mrm.1189. [DOI] [PubMed] [Google Scholar]
- 26.Zhao JM, Clingman CS, Narvainen MJ, Kauppinen RA, van Zijl PC. Oxygenation and hematocrit dependence of transverse relaxation rates of blood at 3T. Magn Reson Med. 2007;58(3):592–597. doi: 10.1002/mrm.21342. [DOI] [PubMed] [Google Scholar]
- 27.Gibson JS, Ellory JC. Membrane transport in sickle cell disease. Blood Cells Mol Dis. 2002;28(3):303–314. doi: 10.1006/bcmd.2002.0515. [DOI] [PubMed] [Google Scholar]
- 28.Clark MR, Rossi ME. Permeability characteristics of deoxygenated sickle cells. Blood. 1990;76(10):2139–2145. [PubMed] [Google Scholar]
- 29.Grgac K, Li W, Huang A, Qin Q, van Zijl PC. Transverse water relaxation in whole blood and erythrocytes at 3T, 7T, 9.4T, 11.7T and 16. 4T; determination of intracellular hemoglobin and extracellular albumin relaxivities. Magn Reson Imaging. 2017;38:234–249. doi: 10.1016/j.mri.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinberg MH, Dreiling BJ. Clinical, hematologic and biosynthetic studies in sickle cell-betao-thalassemia: a comparison with sickle cell anemia. Am J Hematol. 1976;1(1):35–44. doi: 10.1002/ajh.2830010105. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Xu X, Liu P, Strouse J, Lu H, van Zijl P, Qin Q. Determination of Oxygenation Extraction Fraction for People with Sickle Cell Anemia using Calibration Model Specific to SCA Blood. Proceeding of the 25th Annual Meeting of the International Society of Magnetic Resonance in Medicine; Honolulu, Hawaii. 2017, April 22–27. [Google Scholar]
- 32.Hirakata H, Yao H, Osato S, Ibayashi S, Onoyama K, Otsuka M, Ichiya Y, Kuwabara Y, Masuda Y, Fujishima M. CBF and oxygen metabolism in hemodialysis patients: effects of anemia correction with recombinant human EPO. Am J Physiol. 1992;262(5 Pt 2):F737–743. doi: 10.1152/ajprenal.1992.262.5.F737. [DOI] [PubMed] [Google Scholar]
- 33.Derdeyn CP, Videen TO, Yundt KD, Fritsch SM, Carpenter DA, Grubb RL, Powers WJ. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain. 2002;125(Pt 3):595–607. doi: 10.1093/brain/awf047. [DOI] [PubMed] [Google Scholar]
- 34.Herold S, Brozovic M, Gibbs J, Lammertsma AA, Leenders KL, Carr D, Fleming JS, Jones T. Measurement of regional cerebral blood flow, blood volume and oxygen metabolism in patients with sickle cell disease using positron emission tomography. Stroke. 1986;17(4):692–698. doi: 10.1161/01.str.17.4.692. [DOI] [PubMed] [Google Scholar]
- 35.Heyman A, Patterson JL, Duke TW. CEREBRAL CIRCULATION AND METABOLISM IN SICKLE CELL AND OTHER CHRONIC ANEMIAS, WITH OBSERVATIONS ON THE EFFECTS OF OXYGEN INHALATION 1. J Clin Invest. 1952;31(9):824–828. doi: 10.1172/JCI102668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brannon ES, Merrill AJ, Warren JV, Stead EA. THE CARDIAC OUTPUT IN PATIENTS WITH CHRONIC ANEMIA AS MEASURED BY THE TECHNIQUE OF RIGHT ATRIAL CATHETERIZATION 1. J Clin Invest. 1945;24(3):332–336. doi: 10.1172/JCI101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manfredi F, Spoto AP, Saltzman HA, Sieker HO. Studies of peripheral circulation during sickle-cell crisis. Circulation. 1960;22:602–607. doi: 10.1161/01.cir.22.4.602. [DOI] [PubMed] [Google Scholar]
- 38.Wu WC, St Lawrence KS, Licht DJ, Wang DJ. Quantification issues in arterial spin labeling perfusion magnetic resonance imaging. Top Magn Reson Imaging. 2010;21(2):65–73. doi: 10.1097/RMR.0b013e31821e570a. [DOI] [PubMed] [Google Scholar]
- 39.Bush A, Coates T, Wood J. Pseudo Continous Arterial Spin Labeling Quantification Considerations in Hyperemic Cerebral Blood Flow. Society of Magnetic Resonance in Medicine; Honolulu, Hawaii. 2017 April 22–27; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dowling MM, USA USMCDoPaNNDT, Quinn CT, USA CCsHMCDoHCO, Ramaciotti C, USA USMCDoPDT, Kanter J, USA MUoSCDoPCS, Osunkwo I, USA CHSTLCICN, Inusa B, UK GsaSTNTHL, Iyer R, USA UoMMCJM, Kwiatkowski JL, USA UoPPSoMDoHPP, Johnson C, USA CCsHCSFWT, Rhodes M, USA UoMMCPHOJM, Owen W, USA CsHotKsDCsCaBDCNV, Strouse JJ, USA TJHUSoMBM, Panepinto JA, Medical College of Wisconsin/Children’s Hospital of Wisconsin Department of Pediatrics HOMWU, Neumayr L, USA UBCsHDoHOOC, Sarnaik S, USA WSUDoPDM, Plumb PA, USA USMCDoNNDT, Dlamini N, UK ECsHDoPNL, Kirkham F, UK UIoCHDNL, Hynan LS., USA USMCDoCSBaPDT Increased prevalence of potential right-to-left shunting in children with sickle cell anaemia and stroke. British Journal of Haematology. 2016 doi: 10.1111/bjh.14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varat MA, Adolph RJ, Fowler NO. Cardiovascular effects of anemia. Am Heart J. 1972;83(3):415–426. doi: 10.1016/0002-8703(72)90445-0. [DOI] [PubMed] [Google Scholar]
- 42.Nahavandi M, Millis RM, Tavakkoli F, Wyche MQ, Perlin E, Winter WP, Castro O. Arterialization of peripheral venous blood in sickle cell disease. J Natl Med Assoc. 2002;94(5):320–326. [PMC free article] [PubMed] [Google Scholar]
- 43.Jespersen SN, Østergaard L. The roles of cerebral blood flow, apillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab. 2012;32:264–277. doi: 10.1038/jcbfm.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angleys H, Ostergaard L, Jespersen SN. The effects of capillary transit time heterogeneity (CTH) on brain oxygenation. J Cereb Blood Flow Metab. 2015;35(5):806–817. doi: 10.1038/jcbfm.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buxton RB, Frank LR. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab. 1997;17(1):64–72. doi: 10.1097/00004647-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Mintun MA, Lundstrom BN, Snyder AZ, Vlassenko AG, Shulman GL, Raichle ME. Blood flow and oxygen delivery to human brain during functional activity: theoretical modeling and experimental data. Proc Natl Acad Sci U S A. 2001;98(12):6859–6864. doi: 10.1073/pnas.111164398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostergaard L, Chesler DA, Weisskoff RM, Sorensen AG, Rosen BR. Modeling cerebral blood flow and flow heterogeneity from magnetic resonance residue data. J Cereb Blood Flow Metab. 1999;19(6):690–699. doi: 10.1097/00004647-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 49.Cheung AT, Chen PC, Larkin EC, Duong PL, Ramanujam S, Tablin F, Wun T. Microvascular abnormalities in sickle cell disease: a computer-assisted intravital microscopy study. Blood. 2002;99(11):3999–4005. doi: 10.1182/blood.v99.11.3999. [DOI] [PubMed] [Google Scholar]
- 50.Juttukonda MR, Jordan LC, Gindville MC, Davis LT, Watchmaker JM, Pruthi S, Donahue MJ. Cerebral hemodynamics and pseudo-continuous arterial spin labeling considerations in adults with sickle cell anemia. NMR Biomed. 2017;30(2) doi: 10.1002/nbm.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]