Summary
Most well-characterized enhancers are deeply conserved. In contrast, genome-wide comparative studies of steady state systems showed that only a small fraction of active enhancers are conserved. To better understand conservation of enhancer activity we used a comparative genomics approach that integrates temporal expression and epigenetic profiles in an innate immune system. We found that gene expression programs diverge among mildly induced genes while being highly conserved for strongly induced genes. The fraction of conserved enhancers varies greatly across gene expression programs, with induced genes and early response genes in particular, being regulated by a higher fraction of conserved enhancers. Clustering of conserved accessible DNA sequence within enhancers resulted in over 80 sequence motifs including motifs for known factors as well as many with unknown function. We further show that the number of instances of these motifs is a strong predictor of the responsiveness of a gene to pathogen detection.
eTOC blurb
A comparison of the transcriptome and chromatin landscape between mouse and human innate immune cells reveals higher conservation of regulatory elements that control specific gene expression programs. These conserved elements contain a large set of constrained sequence motifs, which can be used as features to successfully predict gene induction in stimulated mouse and human innate immune cells.
Introduction
Enhancers act over long chromosomal distances to control gene expression in a cell type-specific fashion (Ong and Corces, 2011). Recent advances in genomic methods have revealed hundreds of thousands of enhancers defined by biochemical signatures that include p300 binding, H3K27ac and H3K4me1 modifications (Heintzman et al., 2007; Rada-Iglesias et al., 2011; Visel et al., 2009). These studies have shown that the vast majority of regulatory elements are species-specific. Furthermore, gain or loss of species-specific enhancers across phylogeny is not concomitant with gain or loss of genomic sequence. Instead, the majority of species-specific enhancers are composed of ancestral sequences that gain enhancer activity in a species-specific manner (Ballester et al., 2014; Kunarso et al., 2010; Mikkelsen et al., 2010; Odom et al., 2007; Schmidt et al., 2010; Villar et al., 2015).
Rapid turnover of species-specific enhancers stands in stark contrast to the highly conserved nature of well-known enhancers that play essential roles in development (Chew et al., 2005; Crocker and Erives, 2008; Lettice et al., 2003), metabolism (Claussnitzer et al., 2015) and viral defense (Panne et al., 2007). Comparative sequence analysis revealed millions of conserved non-coding elements in the human genome that are likely to act as functional enhancers in-vivo (Pennacchio et al., 2006). Given the general expectation that most functional elements are under purifying selection, there is currently a disconnect between enhancers that are defined by biochemical activity and those defined by evolutionary conservation.
Several arguments have been proposed to reconcile this apparent contradiction between the high turnover rate of biochemical signatures of enhancers observed in comparative studies and the high conservation of a handful of well-characterized examples. One proposed explanation is that typical enhancer elements are redundant, with shadow enhancers that can compensate for the loss of another enhancer (Dunipace et al., 2011; He et al., 2011; Perry et al., 2010). However, redundant enhancers show no relaxation of sequence constraint compared to non-redundant enhancers (Cannavò et al., 2016). Another proposal is that genetic drift may sometimes yield new transcription factor binding sites, eventually leading to novel regulatory elements that make old ones redundant (Ludwig et al., 2000). Accordingly, individual binding sites within enhancers may be shuffled over time and even be replaced by sites occurring on different enhancers. Although both arguments would explain the reduced selective pressure on typical enhancers, they do not explain the apparent strong purifying selection of functionally important enhancers.
An alternative explanation is that most of the biochemically defined enhancers might not be critical in controlling conserved gene regulatory programs. Instead, conserved gene regulatory programs are controlled by a small subset of conserved enhancers. Here we revisited the question of enhancer conservation by studying the transcriptional regulation of genes that respond to Lipopolysaccharide (LPS). LPS is a cell wall component of gram negative bacteria, that is detected by the TLR4-MD-2 complex (Park et al., 2009). This is a well-defined inducible response in both human and mouse dendritic cells (Amit et al., 2009; Garber et al., 2012; Parnas et al., 2015), which involves hundreds of genes and, in its early stages offers a virtually synchronous response that is mostly transcriptionally controlled (Rabani et al., 2011). Focusing on LPS-responsive genes reduces many confounding factors such as the role of post-transcriptional regulation that make steady state analysis more complex. We focused on the evolutionary profile of enhancers that are associated with both species-specific and shared LPS-responsive genes. Our results reconcile the biochemical and conservation-based definitions of enhancers and demonstrate the importance of evolutionary selection of enhancers in controlling conserved transcriptional programs.
Results
Transcriptional dynamics of human and mouse DCs in response to LPS
We generated dendritic cells (DCs) from the bone marrow of two C57BL/6 mice and from human peripheral blood mononuclear cells (PBMCs) from two donors. We stimulated each set of DCs with LPS and collected cells at 0, 1, 2, 4, and 6 hours post-stimulation. We measured genome-wide gene expression by RNA sequencing (RNA-Seq), chromatin accessibility by ATAC-Seq (Buenrostro et al., 2013) and enhancer activity by chromatin immunoprecipitation of H3K27ac followed by sequencing (ChIP-Seq).
To compare human and mouse response to LPS we focused on genes that could be mapped unambiguously between human and mouse (one-to-one homologs). Immature mouse and human DCs have similar transcriptional profiles with 72% (6,370) of all one-to-one homologous genes detected in at least one species being expressed in both. Among the 3,642 genes that are LPS-responsive in at least one species only 740 have similar expression kinetics (Figure 1A, STAR Methods). However, induced genes with similar patterns showed greater induction levels (3.7-fold higher on average, Figure S1A), and were enriched in effectors (cytokines and chemokines p < 10−5, hypergeometric test) and transcription factors (TFs, p < 0.0001, hypergeometric test) compared to genes induced in only one species. Overall, the bulk of the differences between mouse and human DCs involve small fold changes and genes that are not critical to the LPS response. There are, however, interesting exceptions of highly induced genes that are species-specific. A well-known example, Nitric Oxide Synthase 2 (NOS2), has an important role in the mouse immune response to microbes but is not induced by LPS in human innate immune cells (Bogdan, 2001; Mestas and Hughes, 2004). Conversely, we find that the T-Cell effector Indoleamine 2,3-dioxygenase (IDO1) gene is highly induced in the human DCs (Mellor and Munn, 2004), but is not induced in mouse DCs.
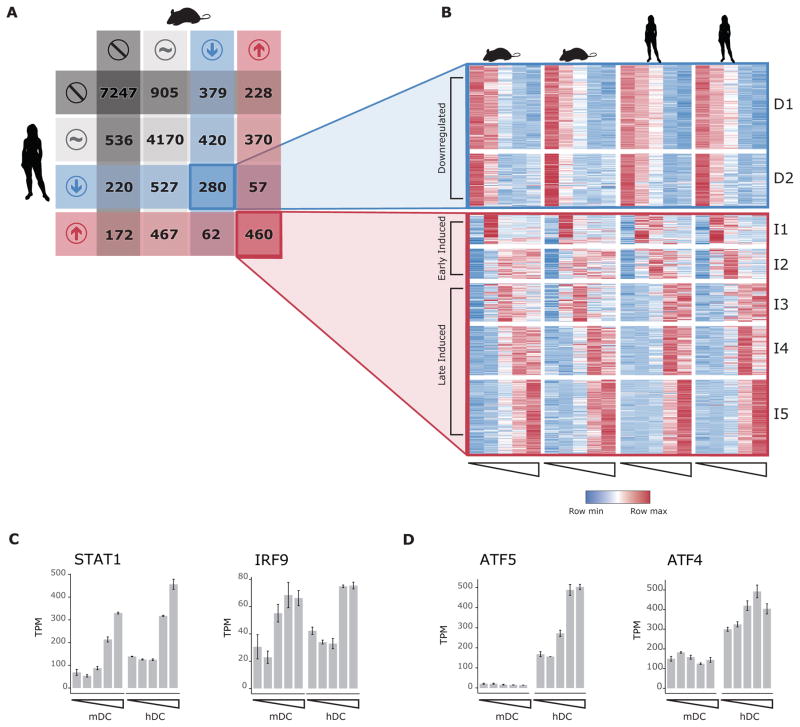
Figure 1. Highly induced LPS-responsive genes have similar expression kinetics in human and mouse dendritic cells.
A) Classification of 16,500 homolog genes in mouse and human as not expressed (dark grey), expressed without significant change after LPS stimulation (light grey), downregulated (blue) or induced (red) B) Heatmap showing normalized expression values for genes with shared response to LPS across five timepoints (Unstimulated, 1h, 2h, 4h and 6h post-LPS) in DCs derived from two different C57BL/6 mouse (left) and two human donors (right). Genes were grouped by spectral clustering into two clusters of shared downregulated genes (D1 and D2, top), and five clusters of shared induced genes (I1-I5, bottom). Induced gene clusters can be classified as early (I1 and I2) or late (I3, I4 and I5). C) Average normalized expression (TPM) for two shared late induced transcription factors (TFs), Stat1 and Irf9. D) Average normalized expression (TPM) for ATF family TFs with species-specific response.
We next clustered the genes that were responsive in both human and mouse DCs (Figure 1B, STAR Methods). We observed three broad shared expression trends: genes that were downregulated in both species (clusters D1 and D2), genes that were induced within 1h after LPS stimulation (early induced genes, clusters I1 and I2), and genes that were induced at least 2h after LPS stimulation (clusters I3, I4 and I5). These different clusters showed broad similar expression trends while also reflecting subtle differences in species-specific timing of peak expression. Shared early induced genes were enriched for cytokines and TFs (adjusted p < 10−5, hypergeometric test). Cluster I1 specifically, was 5.4-fold enriched in TFs (p < 10−7, hypergeometric test), including immediate-early genes such as JUN and FOSB. Shared late induced genes included the TFs STAT1 and IRF9 (Figure 1C), which are involved in autocrine signals from IFNβ and TNFα resulting from LPS detection (Toshchakov et al., 2002).
Although most species-specific genes were induced at relatively low levels, these differences may result from either changes in cis-regulatory elements or from differences in TF expression. We first focused on differences in TF expression. Overall, 530 TFs were expressed in at least one species, of which most (70%) were expressed in both species (Figure S1B), and most TFs detected only in one species had significant lower expression (Figure S1C, p < 10−15 Wilcoxon rank-sum test). Further, most TFs that respond to LPS have well conserved kinetics (STAR Methods, Figure S1D) and although we find specific TFs having diverging expression patterns, in most cases other members of the same family (defined by TF Class, Wingender et al., 2013) show similar kinetics. For only 15 TFs we found no evidence of compensatory changes, most of these cases involved TFs with a low peak expression or induction (Figure S1E). These results suggest that TF expression is conserved between mouse and human DCs. Two interesting exceptions are the AP1 factors ATF5 and ATF4, which are highly expressed and induced only in human DCs (Figure 1D). These two TFs respond to a variety of other stress stimuli, such as amino acid starvation, heat shock and oxidative stress (Harding et al., 2003; Wang et al., 2007a; Watatani et al., 2007), suggesting a human-specific role for cellular stress response in DC response to LPS. We next turned to cis-regulatory elements to further determine the source of changes in expression profiles.
The epigenetic landscape of regulatory elements in human and mouse DC response to LPS
To define the regulatory landscape of mouse and human DCs we followed a two-step process. First, we mapped candidate enhancer regions using ChIP of histone marks that are typical of transcriptionally active regions (Heintzman et al., 2007; Rada-Iglesias et al., 2011; Shlyueva et al., 2014). We then used ATAC-Seq signal to identify accessible regions within our H3K27ac-defined regions (Buenrostro et al., 2013) (STAR Methods, Figure 2A).
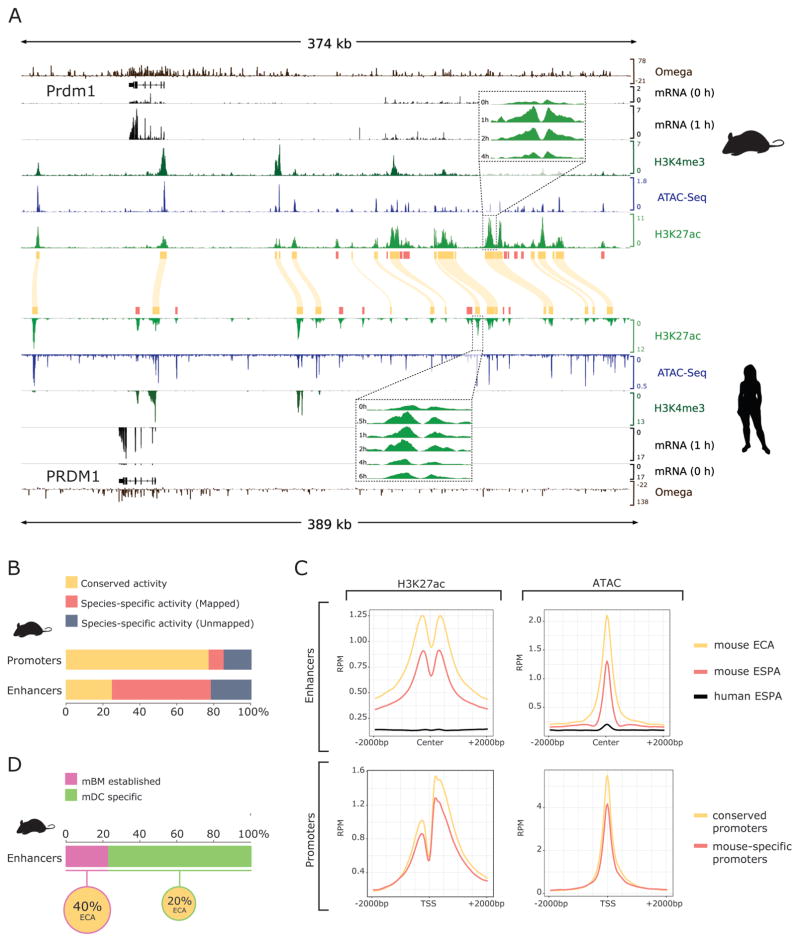
Figure 2. Rapid turnover of enhancer elements.
A) Integrative Genome Viewer diagram of the PRDM1 regulatory region in both mouse (top) and human (bottom) displaying the data used in this study. Tracks display from top to bottom: sequence conservation as estimated by SiPhy (Omega), RefSeq gene annotations, RNA-Seq coverage for unstimulated and one hour post-LPS, overlaid H3K4Me3, ATAC and H3K27ac coverage. Human data in reverse orientation, yellow boxes and curved lines indicate conserved H3K27ac peaks (regulatory regions with conserved activity: promoters or ECAs). Inlets show individual tracks for H3K27ac time course after LPS stimulation. Red boxes indicate H3K27ac peaks with species-specific activity. B) Proportion of regulatory regions with conserved activity: conserved promoters or ECAs, mouse-specific with clear human homologous sequence (mapped promoters or ESPA) and mouse-specific with no clear homologous sequence in human (unmapped promoters or ESPA) C) Average signal for mouse H3K27ac (left) and ATAC-Seq (right) signal over regulatory elements. Enhancer (top) H3K27ac or ATAC-Seq signal is centered in open regions, defined by ATAC-Seq peaks. Promoter (bottom) H3K27ac or ATAC-Seq signal is centered in the TSS. Data is shown for conserved enhancers and promoters (yellow), mouse-specific enhancers and promoters (red) and all other mouse genome coordinates for mapped human-specific enhancers and promoters (black). RPM = reads per million mapped reads D) Fraction of mouse enhancers that are active (pre-established) in bone marrow (mBM) cells and enhancers that are mDC specific, and fraction of mBM pre-established or mDC specific enhancers that are conserved (ECA).
As in previous studies (Cheng et al., 2014; Vierstra et al., 2014; Villar et al., 2015), we defined Enhancers with Conserved Activity (ECAs) as enhancers whose sequence could be uniquely mapped across species and which also had H3K27ac signal in both species. We defined Enhancers with SPecies-specific Activity (ESPAs) to include both species-specific sequences with H3K27ac signal and homologous sequences with species-specific H3K27ac signal. Consistent with previous studies (Villar et al., 2015), for the majority of the enhancers and promoters found in one species it was possible to unambiguously identify homologous sequence in the other species (Figure 2A,B, S2A and STAR Methods). However, as observed in other systems (Mikkelsen et al., 2010; Schmidt et al., 2010), conservation of H3K27ac signal paints a different picture: While 77% of mouse DC promoters mapped to human sequence with H3K27ac signal, for mouse DC enhancers this fraction is only 25% (Figure 2B, S2A). Among transposase-accessible regions within mouse enhancers, only 19% of homologous regions are transposase-accessible in human (Figure S2B, S2C). However, among enhancer sequences with conserved H3K27ac signal, 59% also had conserved accessibility in both species. This shows that accessible regions within enhancers and hence TF binding is maintained across evolutionary time whenever the activity of the larger region is also conserved. Overall, the fraction of ECAs (25%) observed in DC enhancers was similar to the one observed between mouse and human liver enhancers (Villar et al., 2015). Thus, in spite of the strong positive selection acting on innate immune cells, the regulatory landscape has not diverged much further than in liver, likely owing to the critical nature of this response for the organism’s survival. Since TF expression is well conserved while cis-regulatory elements have drastically diverged, it appears that most differences in LPS-responsive expression between human and mouse are the result of cis-regulatory changes rather than differences in trans-regulators.
We observed a stronger H3K27ac and ATAC signal in enhancers and promoters that are active in both species, compared to species-specific regions (Figure 2C, S2D). This observation could result from a threshold bias to define conserved active loci, with one species having a lower signal that fails to meet the enrichment threshold. However, the H3K27ac signal on the homologous regions of ESPAs was indistinguishable from background (black lines, Figure 2C, S2D). Thus, our classification of an active regulatory region as species-specific is not influenced by differing signal intensity.
Enhancers that are active in progenitor cells are more conserved but are not involved in the response to LPS
Mouse DCs are derived from bone marrow (mBM), whereas human DCs are derived from monocytes. We therefore hypothesized that observed differences in enhancer activity in these cells could be the result of prior activity in progenitor cells. To identify such enhancers we relied on H3K27ac ChIP-Seq data from mBMs (Yue et al., 2014) and generated similar data for human monocytes. Although the fraction of pre-established active enhancers is different in mouse (23% in bone marrow) and human (55% in monocytes), enhancers that are pre-established are more conserved than those that are DC-specific (Figure 2D, S2E). Consequently, pre-established active enhancers are not likely to explain the differences we observed in the transcriptional response to LPS in human and mouse DCs.
The higher degree of conservation among enhancers that are active in progenitors may indicate that they belong to a family of ubiquitous enhancers that have been shown to be more conserved in evolution (Cheng et al., 2014). Consistent with this, nearly half (40%) of the enhancers that are pre-established in mouse bone marrow are also active in liver. Further, we found that pre-established enhancers constitute 39% of all enhancers for genes with rapid downregulation in both species (Cluster D2, Figure 1B), compared to 23% for all genes. This indicates that ubiquitous enhancers, albeit being more highly conserved than cell type specific enhancers, are not involved in response to stimulus, and are not likely to play an important role in the regulation of LPS response.
Regulation of early LPS-induced genes is both complex and conserved
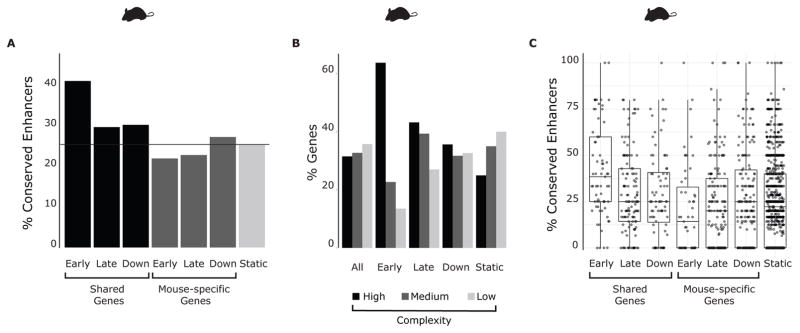
Previous comparative analyses have shown that conserved enhancers are associated with genes involved in specific biological processes (Ballester et al., 2014; Kunarso et al., 2010; Mikkelsen et al., 2010; Schmidt et al., 2010). While there is a slight increase in the fraction of ECAs among shared induced genes compared to enhancers of non-induced or species-specific induced genes, the largest increase (40%, almost double than for non-induced genes) is found on enhancers associated with shared early induced genes (p < 10−12, Fisher exact test) (Figure 3A, S3A). This shows that selection does not act uniformly across all enhancers but rather, that it depends on the particular transcriptional program in which the enhancers function.
Figure 3. Genes with shared transcriptional response to LPS have complex regulatory loci and a higher conservation of enhancer activity.
A) Fraction of ECAs that are associated to genes that are early induced, late induced or downregulated upon stimulation with LPS in mouse DCs. The black horizontal line shows the average enhancer conservation for all genes B) Fraction of genes in temporal clusters that are associated to high-, medium- or low-complexity enhancer loci. C) Fraction of ECAs in high complexity genes that have shared or species-specific response. The response patterns are: early induced, late induced, downregulated or unchanged.
Visual inspection of highly induced genes after LPS stimulation such as NFKBIZ, IL6 and PRDM1 (Figure 2A), suggested that these genes were associated with a high number of enhancers and with super enhancers (Whyte et al., 2013). Such regulatory complexity was previously observed in genes that have a cell type specific regulation during lineage commitment (González et al., 2015). Interestingly, genes with high regulatory complexity (having four or more enhancers) were highly enriched in LPS-responsive genes and particularly, in early induced genes (Figure 3B, S3B). Consistent with our initial observation, genes in the top regulatory complexity tier reached higher maximal expression after induction (Figure S3C). Enhancers that regulate highly induced early genes were also more likely to be conserved. Indeed, on average 2/5 of the enhancers are conserved for shared early response genes with complex regulatory loci, compared to only 1/5 for species-specific early response genes that also have complex regulatory loci (Figure 3C, S3D). In general, genes with shared temporal patterns constitute the core of LPS response, and accordingly, their regulation is under strong purifying selection.
Conserved lexicon within accessible regions
Chromatin accessibility is widely considered critical for transcription factor binding (John et al., 2011; Wang et al., 2012), and we confirmed the strong preference of TF binding on accessible regions using our previous transcription factor occupancy maps (Garber et al., 2012) (Figure S4A). As such, DNA accessible regions hold key information related to regulatory activity. Therefore, we next sought to establish the degree to which DNA accessible regions within ECAs are under purifying selection. To this end, we estimated the substitution rate of DNA accessible regions at 10-base resolution (Garber et al., 2009), using a multiple sequence alignment that included 41 mammalian genomes and 2 vertebrate genomes (STAR Methods). Comparison of the substitution rate between DNA accessible regions within ECAs and ESPAs showed a marked reduction in substitution rate (p-value < 10−15, KS-Test, Figure 4A, S4B). Therefore, ECAs are not only preserved in their activity but there are clear marks of purifying selection in the chromatin accessible sequence within, which is most amenable to TF binding.
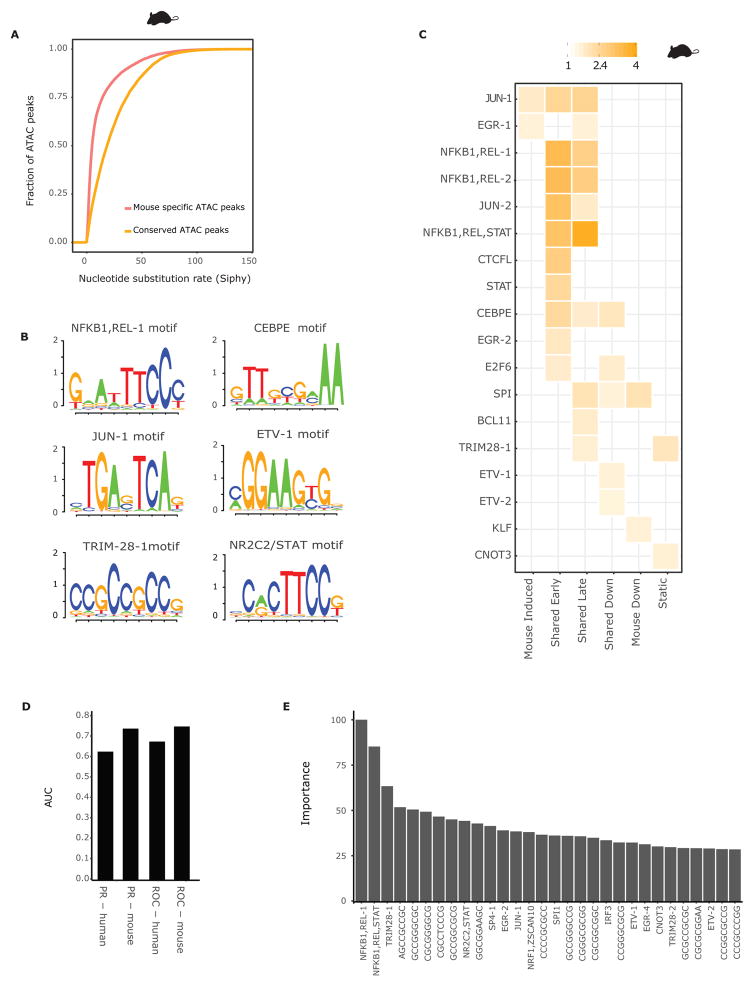
Figure 4. Enhancers with conserved activity contain a conserved lexicon.
A) Distribution of SiPhy omega log-odds scores for 200bp regions around the summits of ATAC-seq peaks that have conserved signal (yellow) and species-specific signal (red) in mouse DCs. B) Examples of sequence logos of the clusters of kmers obtained after clustering the sequences in ATAC regions with conserved signal that have a log-odds score greater than 30. C) Enrichment heatmap showing the observed over expected values for each motif in ATAC-seq peaks with conserved signal associated to the gene groups defined in Figure 1. D) AUC of the PR and ROC curves of a random forest model, predicting whether a gene will be induced or maintain constant expression following LPS stimulation. The features were the number of instances of each cPWM across all regulatory regions of a gene. E) Feature importance of the classifier, defined as the difference in mean accuracy across all trees between the model and the model after permuting the feature. The importance values were then scaled to span the range of 0 to 100. The 30 features with the highest importance values are shown.
To identify sequence elements at the core of ECA function, we clustered conserved 10-mers within ECAs (STAR Methods). Clustering resulted in 66 distinct conserved sequence motifs which we represent by conserved position weight matrices (cPWMs). 31 cPWMs have a clear match to a known transcription factor motif and include all major regulators of TLR4 signaling (STAT, AP1, NFKB, ETV, Figure 4B, Table S2). In addition, we identified 35 cPWMs with no clear similarity to any reported motif in public databases (STAR Methods).
Analysis of both known and unidentified cPWMs showed enrichment for genes with specific temporal expression patterns and, in particular, genes with shared response (Figure 4C, S4C). Importantly, the enrichment of motifs on induced genes was consistent with the expression kinetics of TFs that have affinity for these motifs and recapitulated previous reports (Garber et al., 2012; Medzhitov and Horng, 2009).
To measure the contribution of this conserved lexicon to gene regulation we next trained a random forest classifier to predict if a gene would be strongly induced (> 4-fold) or maintain constant expression following LPS stimulation (STAR Methods). The classifier performed well, achieving a mean area under the curve (AUC) value of 0.75 of the receiver operating characteristic curve (ROC) and a mean AUC value of 0.74 for the precision recall (PR) curve in 10-fold cross-validation (Figure 4D). This confirms the ability of cPWMs to predict gene induction, but also suggests that cPWM instances alone are not sufficient predictors.
Importantly, when we applied the model we trained in mouse to predict expression induction in human, it performed with similar accuracy and precision, achieving an AUROC of 0.68 and an AUC value of 0.63 for the PR curve (Figure 4D). Motifs of the key regulators such as NFKB, AP1, STAT and EGR along with several novel GC rich motifs are amongst the top classifying features (Figure 4E).
Enhanceosomes in conserved innate immune responses
Enhancers are thought to function in two broadly different mechanisms (Arnosti and Kulkarni, 2005). In enhanceosomes, TFs act cooperatively and their binding results in an on/off signal, where loss of even one TF binding site profoundly disrupts the function of the enhanceosome. Billboards on the other hand, are modular enhancers where the binding of each TF is not necessary for enhancer activity but rather has an additive or synergistic effect.
The prototypical enhanceosome is the IFNβ proximal enhancer, which requires the assembly of 6 TFs to induce IFNβ expression (Thanos and Maniatis, 1995). Mutations that disrupt a single binding site disrupt the enhancer and are highly deleterious. Consistent with this, the IFNβ enhanceosome sequence is more highly constrained than the protein coding sequence of IFNβ, the gene it regulates (Figure S5). Since the effect of mutations on enhanceosomes can be highly penetrant, we sought to identify and catalog enhancers that have characteristics typical of enhanceosomes and that may help prioritize non-coding mutations associated with immune disease.
We scanned for candidate enhanceosome regions in chromatin accessible regions within ECAs that were 1) Bound by at least six TFs, based on our previous binding maps of 14 TFs and 2) Had a large portion (> 30%) of their sequence conserved. Our scan identified 80 chromatin accessible regions (Figure 5 for example & Table S3) that resemble enhanceosomes, such as the IFNβ proximal enhancer (Figure S5). Consistent with their innate immune specific function, genes associated with these conserved, highly bound regions tend to have similar temporal induction in both human and mouse (p < 0.01 Fisher’s exact test) and are highly enriched in IRF1, RELA (also known as p65) and RUNX1 binding (p < 10−10, Fisher’s exact test). The high evolutionary sequence constraint that we required to define enhanceosome candidates translates to low variation across the human population. Indeed, human regulatory regions with similar evolutionary constraint are depleted of Single Nucleotide Polymorphisms (SNPs) having an average of only 25 SNPs compared to an average of 400 (and a minimum of 369) in similarly sized genomic regions.
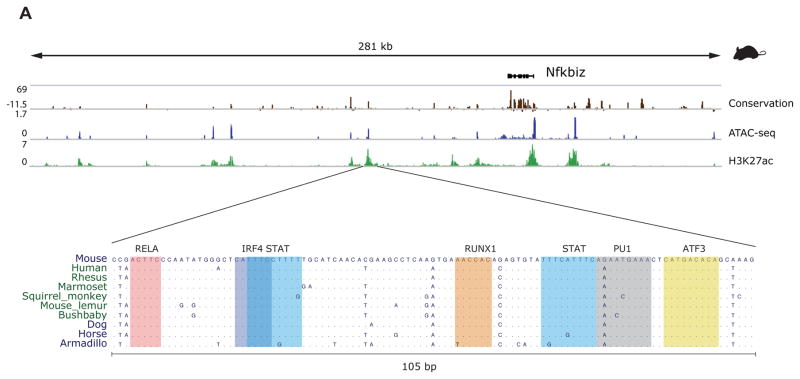
Figure 5. Candidate enhanceosome regions are highly conserved and bound by multiple TFs.
A) Example of an enhanceosome-like regulatory element in the NFKBIZ locus in mouse (top panel) showing the multiple sequence alignment of the conserved DNA accessible region.
Regulatory regions with conserved activity and temporal patterns regulate highly induced genes with shared kinetics
Response to LPS affects both the acetylation and chromatin accessibility of thousands of enhancers (Figure 6A, S6A–C). Although the chromatin state of most enhancers (72%) is unaffected by LPS, enhancers that show temporal kinetics tend to associate with genes having similar transcriptional kinetics. Indeed, regions whose DNA accessibility increases upon LPS stimulation are associated with induced genes (1.6-fold enrichment) while regions that close over time are associated with downregulated genes (2-fold enrichment, Figure 6B). We further observed a clear enrichment of cPWMs, including NFKB, STAT and AP1 motifs, on DNA accessible regions that show increased ATAC signal after LPS stimulation. On the other hand, cPWMs associated with ETV and STAT transcription factor families are enriched in accessible DNA regions that become less accessible in response to LPS. Enrichment of ETV and STAT motifs on regions that lose availability is consistent with their reported repressive function (Icardi et al., 2012; Mavrothalassitis and Ghysdael, 2000) (Figure 6C). It is interesting that STAT motifs are enriched in both down and upregulated elements. These motifs may recruit different members of the STAT family or attract complexes involving different TFs that modulate the STAT TF function. Our previously generated mouse binding data for STAT1 and STAT2 shows that these proteins bind mostly to regions that become increasingly accessible upon LPS stimulation. This suggests that motifs in regions whose DNA availability decreases after LPS stimulation are likely bound by different STAT TFs or other factors that can bind this motif.
Figure 6. Regulatory regions with conserved activity and conserved kinetics regulate genes with shared induction kinetics.
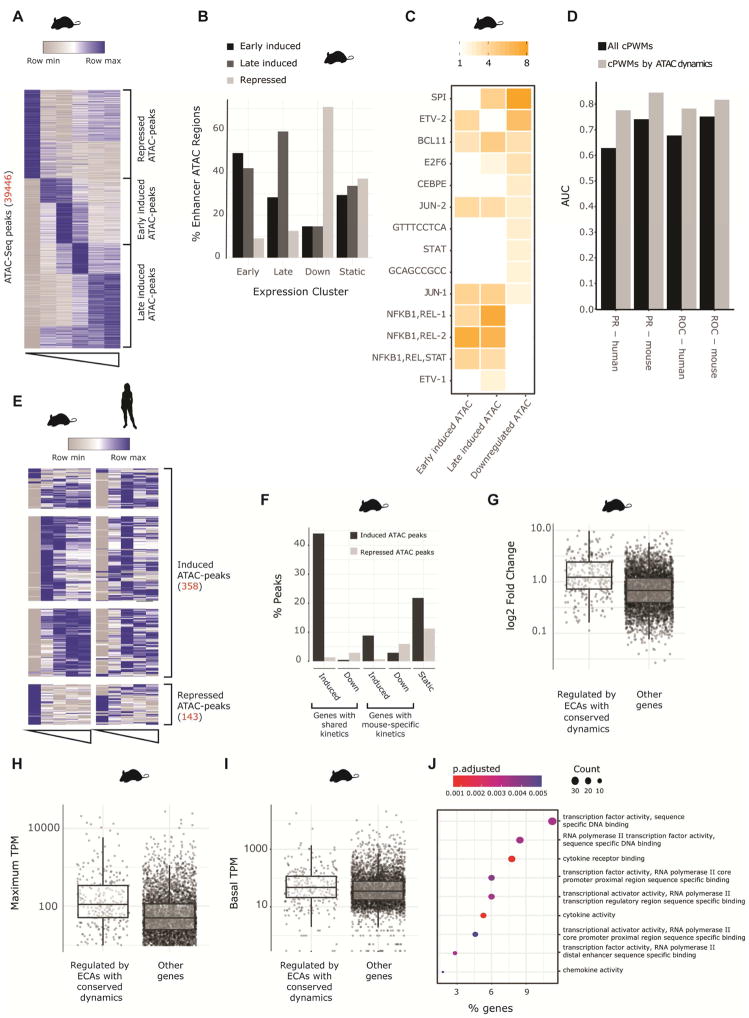
A) Heatmap showing k-means clustering of temporal patterns of mean signal per bp for ATAC-Seq peaks (at enhancer or promoter regions) with dynamic response to LPS in mouse DCs (Unstimulated, 30 minutes, 1 hours, 2 hours, 4 hours and 6 hours). Regions were classified as repressed, early induced or late induced. B) Fraction of early induced, late induced, downregulated or non-changing genes that are associated to dynamic ATAC peaks. C) Enrichment of cPWMs in ATAC peaks that are under purifying selection (Fig 4A) clustered into temporal groups. D) AUC of the PR and ROC curves of a random forest model, predicting whether a gene will be induced or maintain constant expression following LPS stimulation. The features for each model were the number of instances of each cPWM across all regulatory regions of a gene (black bars), or all instances separated by the temporal pattern of the regulatory element (grey bars) E) Heatmap showing the temporal patterns of ATAC-seq peaks with conserved signal that are dynamic in both mouse and human. F) Enrichment of ATAC-seq peaks with conserved signal associated to genes that are induced in both mouse and human DCs, induced only in mouse DCs, downregulated in both mouse and human DCs, downregulated only in mouse DCs and not responsive to LPS in mouse DCs. G-I) The maximum absolute fold change, maximum tpm and baseline tpm of genes that are associated with ATAC-seq peaks with conserved signal that have same temporal response in both mouse and human versus all other genes J) Gene ontology analysis of genes associated with regulatory regions with conserved LPS response kinetics.
To further determine the importance of cPWMs in regulating the LPS response, we proceeded to build a random forest classifier as above, but this time we associated each cPWM with three features per gene: the number of cPWMs in regulatory regions with increased, diminished or unchanged DNA accessibility upon LPS stimulation. This dramatically improved the model performance which now showed an average AUROC of 0.82 in mouse in a 10-fold cross-validation and an AUROC of 0.78 when applied to human (Figure 6D). This highlights the importance of the chromatin context and helps explain the weaker performance of a model that was trained on sequence alone.
Given that regions with LPS-responsive chromatin dynamics were important when evaluating sequence features, we next investigated the conservation of DNA accessibility dynamics. Interestingly, although regions with LPS-induced DNA accessibility are present in both human and mouse (28% and 30%, respectively), very few are LPS-responsive in both. By simultaneously clustering ATAC-Seq peaks from ECAs that had significant LPS-induced signal changes in at least one species (Figure S6D), we found that only 500 such regions (13%) are responsive in both mouse and human DCs (Figure 6E).
These 500 regions are associated with 325 genes, of which 57% have similar expression kinetics in human and mouse, while only 21% of all the expressed genes have similar expression patterns in both species (p < 10−20, Fisher-exact test, Figure 6F). Genes associated with these regions have much higher induction levels and reached higher maximal expression than other genes with no difference in baseline expression (Figure 6G–I, 6G: p < 2.2e−16 Wilcox-rank test, 6H: p < 2.2e−16 Wilcox-rank test, 6I: not significant Fisher-exact test). They include cytokines (e.g IL1B, IL6) and key transcription factors (e.g. REL, NFKB1, BCL2, NFKBIZ) (Figure 6J, p-adjusted < 0.004). Regions with conserved dynamics are enriched near genes with similar temporal dynamics and have maintained enhancer activity since the rodent/primate divergence. This suggests that they are crucial elements regulating this set of genes.
Transposable elements are enriched in cis-regulatory regions of LPS-induced genes
Most cis-regulatory elements are composed of ancestral sequence (Cheng et al., 2014; Villar et al., 2015) (Figure 2B). Therefore turnover of ancestral activity rather than sequence seems to be the major force reshaping regulatory regions. Sequence changes can still be an important source of difference between the human and mouse response. Since lineage specific transposable elements (TEs) have been shown to significantly modify transcriptional networks (Lowe and Haussler, 2012; Wang et al., 2007b), we next sought to determine whether TEs have contributed to regulatory sequence involved in the LPS response. We identified 25 families of TEs in mouse and 15 in human that are enriched in regulatory regions (enhancers or promoters) of induced genes (Figure 7A). These enriched TE families fall into two categories: those that were actively mobile prior to the human-mouse divergence, and newer elements that have only been active in either the mouse or human lineage. The majority belong to one of the ancestral TE families of Mammalian-wide interspersed repeats (MIRs), with MIR3 elements being the most enriched (Figure 7B) and having the largest number of elements within regulatory regions. MIR elements are some of the oldest (Smit and Riggs, 1995) and most conserved families of mobile elements (Jjingo et al., 2014), and have been reported to contribute to the regulation of cell type specific expression (Jjingo et al., 2014). Our data further suggests that MIRs, and MIR3 in particular, have been co-opted into regulation of innate immune responses prior to the euarchontoglires ancestor. As one might expect for important regulatory sequences, we observed that MIRs have been under clear purifying selection (Figure S7A).
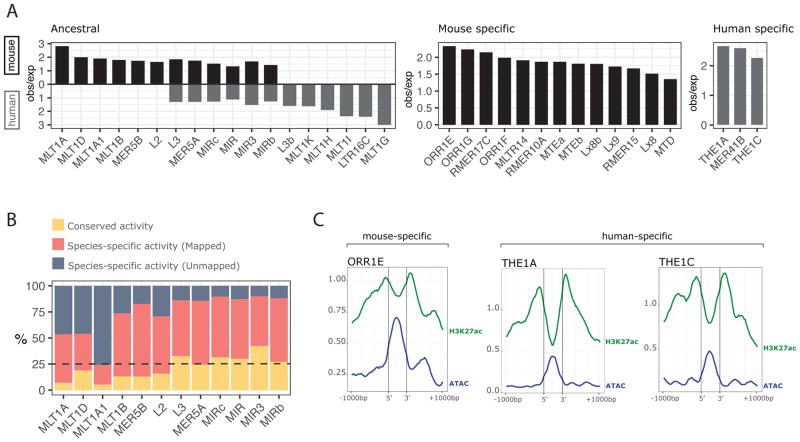
Figure 7. Mobile elements of ancestral and recent origin have reshaped response to environmental stimulus.
A) Families of transposable elements (TEs) enriched in regulatory regions of induced genes in mouse and human. Observed over expected (obs/exp) values are shown for each TE only when the enrichment is significant in that species (p value < 0.004, permutation test; p adjusted < 0.05). Panels show families of TEs that have instances in the mouse and human genomes (Ancestral, Left), only in mouse (Mouse specific, Center), or only in human (Human specific, Right). B) Conservation rate of the enhancer regions that overlap each ancestral TE. C) Average aggregation signal of H3K27ac and ATAC-Seq over TE instances that overlap regulatory elements. Region is centered in each TE instance, delimited by the vertical bars, and the 2kb surrounding region is shown.
Lineage specific TEs enriched in DC regulatory regions include mainly endogenous retroviral Long Terminal Repeat (LTR) elements. We found that elements from these families (ORR1E in mouse and THE1A and THE1C in human) tend to be positioned at the most accessible regions within enhancers, possibly indicating a role in creating or facilitating opening of chromatin that is more favorable to transcription factor binding and more likely to function as a regulatory element (Figure 7C).
Discussion
Massive parallel sequencing has revealed hundreds of thousands of active non-coding regions, most of which are classified by their chromatin signatures as enhancers or long noncoding RNAs (lncRNAs). Comparative analyses of enhancers and lncRNAs have shown that although the majority are encoded by ancestral sequence, their activity is generally species-specific (Chen et al., 2016; Cheng et al., 2014; Kutter et al., 2012; Necsulea et al., 2014; Ponjavic et al., 2007; Ulitsky, 2016; Vierstra et al., 2014; Villar et al., 2015; Washietl et al., 2014). Here we showed that a higher fraction of enhancers that regulate specific pathways tend to be conserved over longer evolutionary time.
As opposed to previous studies, we used a dynamic system and focused on temporal expression patterns rather than steady state expression. In this system, changes in mRNA levels in early time points are mostly the result of transcription rather than post-transcriptional processes (Rabani et al., 2011); this helps isolating and measuring the contribution of cis-regulatory elements to expression changes. Temporal analysis also allowed us to study different regulatory programs individually rather than analyzing all regulatory programs together or by broad functional classes (Figure 1B). As a result, we were able to find that regulatory element conservation is not homogeneous across all enhancers, but rather that it differs across programs. We find that regulatory elements associated with shared early-induced genes are conserved at twice the rate than those associated with other expressed genes. Not only regulatory element activity is conserved, but also the underlying sequence is under purifying selection. This allowed us to use comparative sequence analysis to identify a large set of constrained sequence motifs within active enhancers. Functional validation of these enhancers as well as the novel motifs we found will be critical, but this study provides a clear path towards the goal of functionally characterizing a well-defined set of regulatory regions involved in well-understood cellular processes.
It is interesting that, besides enhancers associated with shared induced genes, the other set of enhancers preserved since the euarchontoglires ancestor are ubiquitous or active in progenitor cells but are not associated with genes induced by TLR4 signaling. Instead, these enhancers tend to lose active marks following LPS stimulation. This is consistent with previous observations that basic cellular processes are passively downregulated upon induction of a large transcriptional program (Cheng et al., 2009; Garber et al., 2012), perhaps due to a shift of limited resources towards the response to immune challenge.
The greater conservation of enhancers associated with early induced genes is surprising, with conserved enhancers accounting for 40% of all enhancers associated with these genes. This arises an interesting question: why are the regulatory elements of early-induced genes under stronger selection? It is reasonable to argue that this initial wave of transcription triggers a program that, although necessary for immune defense, is deleterious to the individual when misregulated. Tight control of the initiation of the program may be critical to avoid unwanted harm. It is also interesting that in our previous analysis of mouse DC enhancers we observed a low degree of sequence constraint of most enhancers, and concluded that early-induced genes were regulated by a highly redundant regulatory architecture that functioned by recruiting many different TFs in a nonspecific fashion. Our comparison with human DCs paints a more nuanced picture. Early induced genes are regulated by a mix of highly constrained enhancers that have been preserved over hundreds of millions of years and newly evolved species-specific enhancers. The ECAs have clear signatures of undergoing purifying selection and may be necessary for induction. Nonetheless, the majority of enhancers is species-specific and may play redundant, subtler roles or have no impact on gene expression. Further functional studies will be needed to determine how different enhancers function and how they interact to produce reproducible, precise patterns of expression.
Our study sheds some light on the long-standing question of how selection acts on gene expression (Gilad et al., 2006). Although our study was not designed to answer this, we find two very clear modes of selection. On one hand, highly induced genes tend to have shared induction and are regulated by conserved regulatory elements. These observations are consistent with strong stabilizing selection. On the other hand, there is great divergence among genes with mild induction, which is consistent with neutral selection (Gilad et al., 2006). We reason that, while mutations that disrupt the level and timing of highly induced genes may have strong deleterious effect, for genes that are mildly induced, changes are tolerated.
Our comparative map provides a unique resource for future studies of in-vitro derived DCs. It provides a reference map of the genomic elements that can be mapped and translated from a mouse model to human biology. Further, recent reports on underlying differences in the cell types obtained in mouse and human DC in-vitro cultures (Helft et al., 2015) highlights the need to compare these two systems at the molecular level. In this work, we focused on understanding both the similarities and differences between the two. Given the overall similarity in TF expression, this system offers a deep platform to understand the impact of cis-regulatory changes on expression.
STAR Methods
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited Data | ||
| List of 147 data-sets used in this study | This paper | Table S4 |
| Human 10-mers substitution rates | This paper | http://garberlab.umassmed.edu/data/conservation/hg19/omega/ |
| Mouse 10-mers substitution rates | This paper | http://garberlab.umassmed.edu/data/conservation/mm10/mm10.omega |
| Software or Algorithms | ||
| gkm-SVM | (Ghandi et al., 2016) | v1.3 |
| Spectral clustering | This paper | https://github.com/nimezhu/ClsViz |
| Trimmomatic | (Bolger et al., 2014) | V0.32 |
| Bowtie2 | (Langmead and Salzberg, 2012) | v2.2.23 |
| Samtools | (Li et al., 2009) | v0.1.19 |
| DESeq2 | (Love et al., 2014) | v1.10.1 |
| Bedtools | (Quinlan and Hall, 2010) | V2.25.0 |
| MACS2 | (Zhang et al., 2008) | V2 |
| IGVtools | (Robinson et al., 2011) | V2.3.31 |
| RSEM | (Li and Dewey, 2011) | v1.2.28 |
| SiPhy | (Garber et al., 2009) | https://github.com/garber-lab/siphy |
| Antibodies & Reagents | ||
| H3K27ac | Diagenode | C15410196 |
| H3K4me3 | EMD Millipore | 05-745R |
| Ovation Human FFPE RNA-Seq Library System | NuGen | 0340 |
| Ovation mouse System RNA-Seq Library | NuGen | 0348 |
| RNeasy mini plus kit | Qiagen | 74134 |
| Nextra TDE-1 transposase, | Illumina | FC-121-1030 |
| Covaris tru-ChIP Chromatin Shearing and Reagent Kit | Covaris | 520154 |
| Agencourt AMPure XP | Beckman Coulter | A63880 |
| GMCSF | Miltenyi | 130-095-735 |
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Manuel Garber (Manuel.Garber@umassmed.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human Subjects
Anonymous, healthy donor leukopaks (New York Biologics, Southampton, NY), were used in accordance with UMMS-IRB protocol ID #H00004971
Mice
All mice were housed in specific pathogen-free condition in accordance with the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School. C57BL6 female mice were euthanized at 6–8 weeks of age to harvest bone marrow.
METHOD DETAILS
Cell culture
All cells were maintained at 37° C in 5% CO2 humidi fied incubators.
Human monocyte derived dendritic cells
Human dendritic cells were derived from peripheral blood mononuclear cells (PBMCs) isolated from de-identified, healthy donor leukopaks (New York Biologics, Southampton, NY), in accordance with UMMS-IRB protocol ID #H00004971. Mononuclear leukocytes were isolated by gradient centrifugation on Histopaque-1077 (Sigma-Aldrich, St. Louis, MO). CD14+ mononuclear cells were enriched via positive selection using anti-CD14 antibody MicroBead conjugates (Miltenyi, San Diego, CA), according to the manufacturer’s protocol. CD14+ cells were then plated at a density of 1 to 2 x 106 cells/ml in RPMI-1640 supplemented with 5% heat inactivated human AB+ serum (Omega Scientific, Tarzana, CA), 20 mM L-glutamine (ThermoFisher, Waltham, MA), 25 mM HEPES pH 7.2 (Sigma-Aldrich), 1 mM sodium pyruvate (ThermoFisher), and 1 x MEM non-essential amino acids (ThermoFisher). Differentiation of the CD14+ monocytes into dendritic cells (human DCs) was promoted by addition of recombinant human GM-CSF and human IL-4; cytokines were produced from HEK293 cells stably transduced with pAIP-hGMCSF-co or pAIP-hIL4-co, respectively, as previously described (Reinhard et al., 2014), with each cytokine supernatant added at a dilution of 1:100.
Mouse bone marrow derived dendritic cells
Mouse dendritic cells were derived from bone marrow harvested from 6–8 week old female C57BL6 mice. Bone marrow was then dissociated into single cells and filtered through 70um cell strainer. The cells were then incubated with red blood cell lysis buffer for 5 minutes. To differentiate bone marrow to dendritic cells, bone marrow cells were plated at 200,000 cells/mL in non-tissue culture treated plates. These cells were supplemented with media on day 2 and day 7. On day 5 cells were harvested and resuspended in fresh media. On day 8 all the floating cells were collected as mouse bone marrow derived dendritic cells. The media used for culturing and differentiating contains RPMI (Gibco) supplemented with 10% heat inactivated FBS (Gibco), β-mercaptoethanol (50uM, Gibco), MEM non-essential amino acids (1X, Gibco), sodium pyruvate (1mM, Gibco), and GM-CSF (20 ng/ml; Miltenyi).
Library preparation and Sequencing
RNAseq
Total RNA was isolated from frozen dendritic cell pellets using the RNeasy mini plus kit (QIAGEN). The RNAs were additionally treated with RNase-free DNase I for 15 minutes at room temperature to eliminate most genomic DNA. RNA-Seq libraries were prepared from 70 ng of starting RNA using the Ovation Human FFPE RNA-Seq Library System (NuGEN) or Ovation mouse RNA-Seq Library System (NuGEN), according to the manufacturer’s protocol. Fragmentation of the cDNA was achieved by sonication using the M220 sonicator (Covaris) with the following conditions: sonication time = 350 seconds; temp = 20°C; peak power = 50; duty factor = 20; cycles/burst = 200. The quality of the isolated RNA, as well as of the final libraries, was assessed using the 2100 Bioanalyzer (Agilent) and Qubit (Invitrogen). The libraries were pooled according to donor in equimolar ratios and denatured. Pooled libraries were sequenced for 2 x 100 cycles to obtain paired end reads, using a HiSeq 2000 (Illumina) for human DCs and 2 x 75 cycles using Nextseq 500 for mouse DCs.
ATAC-Seq
For each time point, 5 x 105 scraped DC’s were collected by centrifugation 500 x g for 5 min. and lysed for ATAC-seq following the protocol described in (Buenrostro et al., 2015). Each sample was tagmented using 12.5 ul Nextera TDE-1 transposase (Illumina) for 30 minutes at 37, then quenched by addition of 5 volumes DNA Binding Buffer (Zymo Research) and cleaned using Zymo Research DNA Clean and Concentrator-5 columns according to the supplied protocol. Tagmented DNA was PCR-amplified using indexed primers as described in (Buenrostro et al., 2015), using total cycle numbers for enrichment as determined empirically by qPCR to minimize PCR duplicates. The resulting libraries were purified twice by Zymo Research DNA Clean and Concentrator-5 columns using a ratio of 5:1 DNA Binding Buffer:Sample, and quantified by Qubit HS-DNA Assay (Thermo Fisher Scientific) and Bioanalyzer High-Sensitivity DNA (Agilent Technologies). Final ATAC-seq libraries were pooled (equimolar) and sequenced on an Illumina Nextseq 500.
ChIP-Seq
Harvest and Formaldehyde crosslinking
For each timepoint and donor, 5–7 x 106 unstimulated or LPS-stimulated hDCs were harvested by scraping in medium and centrifugation at 500 x g for 5 minutes. Each cell pellet was washed once with 2 mL PBS and gentle flicking of the tube, followed by centrifugation at 500 x g for 5 min. Cells were uniformly resuspended in 1 mL 1X Fixing Buffer A from the Covaris tru-ChIP Chromatin Shearing and Reagent Kit and fixed by adding 1 mL 2% methanol-free formaldehyde (Thermo Fisher Scientific) diluted in 1X Fixing Buffer A (1% formaldehyde final, 2.5–3.5x106 cells/mL) and rotated end-over end for 5 min. at room temperature. Fixation was quenched by adding 240 mL Quenching Buffer E (Covaris tru-ChIP kit) and rotating for an additional 5 min. Purified BSA was then added to 0.5% w/v final to prevent cell adherence to the tube, and crosslinked cells were harvested by centrifugation, 500 x g for 5 min. at 4°C. Crosslinked cells were washed twice in 2 mL ice-cold PBS + 0.5% BSA with centrifugation as above, and aliquoted evenly into 3 fresh 1.5 mL tubes during the second wash. Cells were finally pelleted by centrifugation at 16,000 x g, flash-frozen as dry pellets in liquid nitrogen, and stored at −80°C.
Lysis, Shearing, and Quantification
Individual crosslinked cell pellets (1.5–2 x 106 cells each) were lysed according to the Covaris tru-ChIP Chromatin Shearing and Reagent Kit instructions. Following lysis, nuclei were resuspended in 130 mL ice-cold Shearing Buffer D3 and transferred to 1.5 mL BioRupter Pico Microtubes (Diagenode) on ice. Chromatin was sheared to uniform fragment lengths (150–400 bp) by sonication at 4°C in a BioRupter Pico (Diagenode) set to 6 cycles of 30s ON and 30s OFF. Sheared chromatin was diluted in 10 volumes of ChRIPA buffer (1X PBS, 1 mM EDTA pH 8.0, 0.5 mM EGTA pH 8.0, 0.5% sodium deoxycholate, 1% Igepal CA-630, 0.1% SDS, 1X Roche cOmplete Protease Inhibitor Cocktail) and insoluble material was removed by centrifugation >15,000 x g for 10 minutes. Lysate was pre-cleared against 60 mL Dynabeads Protein A (Thermo Fisher Scientific) per 106 cells for 2h at 4°C with end-over-end rotation followed by two rounds of magnetic bead removal and transfer to fresh tubes. 2% of pre-cleared lysate was removed for DNA quantification and the remaining lysate was either flash-frozen in liquid nitrogen and stored at −80°C, or stored overnight at 4°C for use in immunoprecipitation. For quantification, 2% pre-cleared lysate was treated with 10 mg RNase A (Thermo Fisher Scientific) for 30 min. at 37°C, followed by addition of 100 mg Proteinase K (New England Biolabs) and crosslink reversal overnight at 65°C. DNA was purified using DNA Clean and Concentrator-5 columns (Zymo Research). Average sheared DNA fragment sizes were determined by agarose gel and chromatin yield was estimated by Qubit HS-DNA Assay. 50–100 ng purified DNA was saved as Input.
Chromatin Immunoprecipitation
Antibodies used for ChIP were rabbit anti-H3K27ac (Diagenode C15410196) and rabbit anti-H3K4me3 (EMD Millipore 05-745R). 1 mg antibody was added to 0.5 mg (anti-H3K27ac) or 1 mg (anti-H3K4me3) pre-cleared crosslinked lysate and incubated overnight with continuous mixing at 4°C. IgG/chromatin complexes were captured for 1h at room temperature on 25 mL Dynabeads Protein A that were pre-blocked for at least 1h with Blocking Buffer (1X PBS, 0.5% BSA, 0.5% Tween-20). Complexed beads were washed 5 times with ice-cold ChRIPA Buffer, twice with room temperature RIPA-500 Buffer (10 mM Tris pH 8.0, 500 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS), twice with ice-cold LiCl Wash Buffer (10 mM Tris pH 8.0, 250 mM LiCl, 1 mM EDTA, 0.5% Igepal CA-630, 0.5% sodium deoxycholate), and twice with ice-cold TE buffer. Each chromatin sample was eluted from beads using 50 ul Direct Elution Buffer (10 mM Tris pH 8.0, 5 mM EDTA, 300 mM NaCl, 0.5% SDS) and supplemented with 20 mg RNase A, incubating for 30 min. at 37°C. 20 mg glycogen was added to each bead/eluate suspension, and crosslinks were reversed by addition of of 50 mg Proteinase K and incubation at 37°C for an additional 2h, followed by overnight at 65°C. Dynabeads were removed by magnet capture, and the supernatant was mixed thoroughly with 2.3 volumes of Agencourt AMPure XP (Beckman Coulter) bead suspension and incubated for 10 minutes at room temperature prior to bead capture and washing. Purified DNA was eluted in 10 mM Tris pH 8.0.
Library Preparation and Sequencing
Sequencing libraries were prepared from half of each ChIP sample and 50 ng Input DNA using the Ovation Ultralow System V2 kit (NuGEN) according to supplier’s instructions, with the total numbers of enrichment PCR cycles determined empirically for each sample by qPCR to minimize PCR duplication rates. Barcoded libraries were quantified using Qubit HS-DNA Assay, qualified using Agilent Bioanalyzer High-Sensitivity DNA, and pooled for sequencing on Illumina Nextseq 500.
QUANTIFICATION AND STATISTICAL ANALYSIS
Alignment and processing of reads
RNA-Seq
Trimmomatic-0.32 (Bolger et al., 2014) was used to remove 5’ or 3’ stretches of bases having an average quality of less than 20 in a window size of 10. Only reads longer than 36 bases were kept for further analysis. Reads were then aligned to human or mouse ribosomal RNA using Bowtie2 v2.2.3 (Langmead and Salzberg, 2012) with parameters -p 2 -N 1 --no-unal. All reads mapped to rRNA were discarded from further analysis. RSEM v1.2.28 (Li and Dewey, 2011) was used to estimate gene expression in Transcripts per Million (TPM), with parameters -p 4 --bowtie-e 70 --bowtie-chunkmbs 100 --strand-specific. RSEM is configured to use Bowtie v0.12.9. Quantification was run against the transcriptome (RefSeq v69 downloaded from UCSC Table Browser (Pruitt et al., 2012). Genes with more than 10 TPM in any time point were considered expressed, and genes that did not achieve this threshold were removed from further analysis. Moderate batch effects were observed between samples from different mice and between the two human donors. We used the log transformed TPM normalized expression values as input to ComBat (package sva version 3.18.0) (Johnson et al., 2007; Leek et al., 2012) with default parameters and a model that specified different donors or mice as batches. Corrected TPM values were transformed back to read counts using the expected size of each transcript informed by RSEM. We only considered genes with at least 10 TPMs in at least one replicate at any time point.
ATAC-Seq
Paired-end reads were trimmed to remove adapter sequence using Cutadapt version 1.3, and then aligned with Bowtie2, version 2.1.0, parameter –X 2000. Reference genome hg19 was used for human samples and mm10 for mouse samples. The alignments were then filtered using Samtools (Li et al., 2009), version 0.0.19, to remove (i) PCR duplicates, as identified by Picard’s MarkDuplicates, and (ii) aligned reads with mapping quality below 4. While the reads were aligned as paired-end to optimize the alignment accuracy, the alignments were then further processed as if they were aligned single-end sequence data, so that each aligned read corresponded to a Tn5 cut-site.
Peak Calling
Each aligned read was first trimmed to the 9-bases at the 5’-end, the region where the Tn5 transposase cuts the DNA, and then extended 10-bases upstream and down, for smoothing. Peaks were called using these adjusted 29-base aligned reads with MACS2 (Zhang et al., 2008)], parameters --bw 29 --tsize 29 and --qvalue 0.0001. For visualization, the adjusted aligned reads were converted to tdf files using IGVTools, version 2.3.31 (Robinson et al., 2011) (IGVtools count –w 5).
Quality Control
Following the standard practice (Buenrostro et al., 2015), for each sample, we examined the fragment length distribution, as well as a comparison of the aggregate nucleosome signal to the aggregate nucleosome-free signal over transcription start sites for those genes found to be expressed for at least one time point in our RNA-Seq time series. Signal-to-noise ratios were computed for the peaks as f/(1 –f) where f is the fraction of reads overlapping peaks.
ChIP-Seq
Along with in house generated data we also analyzed publicly available data for mouse bone-marrow progenitors generated by the Encode consortium (Accession: GSM1000108). Paired-end reads were trimmed to remove sequencing adapters and leading and trailing bases with quality scores less than 5. Reads that were longer than 36 bases after trimming were kept for further analysis. The reads were then aligned to human reference genome hg19 or mouse genome mm10 using Bowtie2 with options -k 1 --un-conc to filter out reads that map to multiple locations in the genome and that align un-concordantly. Duplicated reads were filtered out using picard-tools-1.131 MarkDuplicates function. Peaks were then called using MACS2 with --bw=230 --tsize=75 and --qvalue 0.0001. Alignment files were also converted to tdf format using IGVtools count function using -w 5 --pairs options for visualizing. H3K27ac ChIP-Seq peaks were filtered to retain only the peaks that are two-fold enriched over input.
Gene classification and clustering
Homologs
All our analysis were restricted to genes that had homologous pair between human and mouse defined in the Homologene release 68 (NCBI Resource Coordinators, 2016), resulting in a list of 16,500 one to one homologous gene pairs.
Gene Classification
The expressed gene list was filtered to include only genes with homologs as defined by the previous step. We used the batch corrected (see above) counts per gene to identify differentially expressed genes by at least 2 fold between unstimulated cells (time 0) and any time point following LPS stimulation whose change in expression was significant (p-adjusted < 0.05) according to the package DESeq2 (v1.10.1) (Love et al., 2014) in R (v3.3.1). Due to the large transcriptional changes observed in this system, we turned off the fold change shrinkage in DESeq2 with betaPrior=FALSE and we added a pseudocount of 32 to all timepoints to avoid spurious large fold change estimates from lowly abundant genes. Genes were then classified based on their response to LPS stimulation in each species (induced, downregulated or non-responsive).
Clustering expression patterns
For genes expressed in both species and presenting similar response following LPS stimulation (induced in both species or downregulated in both), we applied a spectral clustering approach (von Luxburg, 2007) to identify genes with conserved expression patterns in mouse and human. Briefly, let {g1,g2,g3,…,gn} represent the set of response genes, and let EMi and EHi,1 ≤ i ≤ n, represent the expression time courses in TPM for gene gi in mouse and human respectively. Further, let ρM = [ρMij], 1< i,j< n represent the Pearson correlation coefficient matrix, where ρMij is the coefficient of correlation of EMi with EMj. The human correlation coefficient matrix, ρH is defined similarly. We define similarity matrices [SMij] and [SHij], for mouse and human respectively, where sMij = exp(−(sin(cos−1(ρMij)/2)2), and sHij = exp(−(sin(cos−1(ρHij)/2)2). Then the matrix W = [ wij ] = [SMij SHij] defines a similarity matrix for {g1, g2, … , gn} and can be viewed as an adjacency matrix for a weighted graph, where each gene represents a node in the graph. We associate to W its graph Laplacian L = D − W, where D is the diagonal degree matrix with entries dii = Σj=1nwij. L is positive, semi-definite and therefore has n real non-negative eigenvalues, λi, 1≤ i ≤ n, which we list in descending order, λ1 ≥ λ2≥ … ≥ λn. We select k, the number of clusters, to be the smallest positive integer such that (λ1 + λ2 + … + λk)/tr(L) > 0.95, where tr(L) is the trace of L. We then construct a matrix with columns set to the first k eigenvalues of L and apply k-means clustering to the rows of this matrix to cluster the genes into k distinct clusters. The python script used for spectral clustering is available on https://github.com/nimezhu/ClsViz.
We analyzed enrichments for specific Gene Ontology categories using clusterProfiler (Yu et al., 2012).
Transcription Factor network
We sought to first determined the extent to which the TF network in response to LPS is conserved between human and mouse DCs. To systematically explore core changes in the regulatory network, we compared the overall trends of the 258 transcription factors that responded to LPS-stimulation in at least one of the two species (Figure S1D). We calculated the Pearson correlation between the expression patterns across all timepoints for TFs with response to LPS per species. The resulting distance matrix was hierarchically clustered and displayed as a heat map. We chose the number of groups in each clustering by visual inspection of the dendrogram and selection of a threshold. Membership in each cluster was then compared across species to identify the corresponding groups.
Transcription Factor Network Overview
There are 3 large co-regulated groups of transcription factors with no major changes between the species, and a fourth cluster in mouse composed of only 8 TFs (Table S1) with very small changes in expression in mouse (< 2 fold), that are scattered across all three human clusters. The largest cluster in mouse contained 115 genes that were downregulated following LPS treatment. Further, 73% of the factors that were also expressed in human remained in the same cluster and showed a similar transcriptional downregulation pattern in human (Figure S1D, top right). Similarly, the vast majority (77%) of induced transcription factors were induced in both species, with 17 factors (19%) having different induction timing in each species (Table S1). The largest of the induced clusters (pink cluster, Figure S1D), contained mostly TFs with conserved kinetics (66% in mouse and 57% in human, Figure S1D, bottom right). This group included members of the NFKB, IRF, and STAT families (Figure 1C). The smaller cluster of induced transcription factors also contained important rapidly upregulated TFs (blue cluster, Figure S1D, middle right), including members of the FOS and JUN families, as well as MAFF, PRDM1, and EGR3, all of which show a conserved pattern in the human response. 17 mouse-specific and 12 human-specific TFs were induced by LPS. Interestingly, to the best of our knowledge, none of the species-specific factors have been studied in the context of innate immune signaling. Two mouse-specific TFs, ID1 and SIX1, are highly induced in mouse, although not detectable in human. Similarly, MSC is highly induced in human DCs but has no detectable expression in mouse DCs. Outliers such as these however, are rare, and most TFs with different responses in mouse and human DCs have moderate induction compared to genes with conserved response.
Substitution rate scan
We used SiPhy (Garber et al., 2009) to compute the substitution rate (ω) for every 10-mer in the mouse and human genomes. For human we used the vertebrate multiple sequence alignment available from the UCSC genome browser for the hg19 assembly. We removed the vertebrates danRer6, petMar1, oryLat2, gasAcu1, fr2, tetNig2 which left us with the following phylogeny: (((((((((((((((((hg19:0.006653,panTro2:0.006688):0.002482,gorGor1:0.008783):0.009697,p onAbe2:0.018183):0.040003,rheMac2:0.008812):0.002489,papHam1:0.008723):0.045139,calJac 1:0.066437):0.057049,tarSyr1:0.137822):0.010992,(micMur1:0.092888,otoGar1:0.1295):0.03 5423):0.015348,tupBel1:0.186424):0.004886,(((((mm9:0.084505,rn4:0.091627):0.197835,dip Ord1:0.211666):0.022945,cavPor3:0.225634):0.010077,speTri1:0.148511):0.025643,(oryCun2 :0.114421,ochPri2:0.201003):0.101624):0.015291):0.020683,(((vicPac1:0.107267,(turTru1: 0.064676,bosTau4:0.123573):0.025145):0.040411,((equCab2:0.109311,(felCat3:0.098636,can Fam2:0.102486):0.049838):0.006202,(myoLuc1:0.14262,pteVam1:0.113246):0.033792):0.00445 6):0.011576,(eriEur1:0.221758,sorAra1:0.269694):0.056557):0.021228):0.023628,(((loxAfr 3:0.082165,proCap1:0.155353):0.026774,echTel1:0.246266):0.049887,(dasNov2:0.116609,cho Hof1:0.096318):0.053052):0.006229):0.399651,macEug1:0.133617):0.002474,monDom5:0.15092 1):0.199105,ornAna1:0.461732):0.116917,((galGal3:0.164668,taeGut1:0.172833):0.200238,a noCar1:0.48763):0.10284):0.186338,xenTro2:0.834181):0.324842
Spanning 8.44 substitutions per site. We excluded 10-mers that after removing species with no alignable sequence due to either alignment gaps or missing sequence had a total branch length of less than 0.75. Data is available from http://garberlab.umassmed.edu/data/conservation/hg19/omega/
For mouse we used the vertebrate multiple sequence alignment available from the UCSC genome browser for the mm10 assembly. We removed petMar1, gadMor1, oryLat2, gasAcu1, oreNil2, fr3, tetNig2, latCha1, xenTro3, chrPic1, anoCar2, melUnd1, taeGut1, melGal1, ornAna1, macEug2, sarHar1 vertebrate assemblies which left us with the following phylogeny: (((((((((((mm10:0.0861604,rn5:0.0923189):0.20235,dipOrd1:0.210872):0.0258938,(hetGla2: 0.0916296,cavPor3:0.136929):0.0994423):0.00913482,speTri2:0.145406):0.0275377,(oryCun2 :0.10975,ochPri2:0.200956):0.102105):0.0142197,(((((((((hg19:0.00672748,panTro4:0.0069 0586):0.00329132,gorGor3:0.00918574):0.00952813,ponAbe2:0.019182):0.00354391,nomLeu2:0 .0218123):0.0117068,(rheMac3:0.00815625,papHam1:0.00799922):0.0289552):0.0208613,(calJ ac3:0.0342486,saiBol1:0.0333278):0.0358206):0.0593959,tarSyr1:0.137561):0.0111487,(mic Mur1:0.0919295,otoGar3:0.127188):0.0351183):0.0153325,tupBel1:0.188903):0.0042042):0.0 215023,((susScr3:0.121671,(vicPac1:0.10979,(turTru2:0.0635601,(oviAri1:0.0392014,bosTa u7:0.0315737):0.0939007):0.0204197):0.00365643):0.0444426,((((felCat5:0.0897916,(canFa m3:0.0888559,ailMel1:0.0767967):0.0218058):0.050101,equCab2:0.109329):0.00604713,(myoL uc2:0.137323,pteVam1:0.113957):0.0339856):0.00384687,(eriEur1:0.227177,sorAra1:0.27056 4):0.0629454):0.00322051):0.0291201):0.0231348,((((loxAfr3:0.0788116,proCap1:0.160315) :0.00818092,echTel1:0.266806):0.00328658,triMan1:0.068537):0.0736006,(dasNov3:0.112113 ,choHof1:0.0974595):0.0536232):0.00734155):0.246266,monDom5:0.35412299999999997):0.212 5305,galGal4:0.5622546999999999):0.6482475,danRer7:0.871611):0.49907
Spanning 8.21 substitutions per site. We excluded 10-mers that after removing species with no alignable sequence due to either alignment gaps or missing sequence had a total branch length of less than 0.5. Data is available from http://garberlab.umassmed.edu/data/conservation/mm10/mm10.omega
The models used were downloaded directly from UCSC and correspond to the alignments used.
Enhancer and promoter definition and conservation analysis
Enhancers and promoters were defined by H3K27ac peaks. We then merged all peaks from each time point located within 200bp from each other. Our maps consist of 28,142 and 29,273 H3K27ac regions (signal peaks) in mouse and human, respectively. We calculated the distance from each peak to the nearest transcription start site (TSS) of the highest expressed isoform for each gene using bedtools closest -D ref -t all (Quinlan and Hall, 2010). We classified all H3K27ac peaks that had a distance smaller than 500 bp to the nearest TSS as promoters, and the remaining peaks were considered enhancers. Enhancers were assigned to the nearest gene based on the same TSS distances as above. Unlike promoters, which were associated to the gene with the overlapping TSS independent of expression, enhancers were only associated to the closest expressed gene within 300 kb (Garber et al., 2012; González et al., 2015). This assignment of enhancers to nearby genes will misassign enhancers that either interact with more than one gene or interact with no adjacent genes. However, the majority of enhancers have been reported to interact with the neighboring gene (González et al., 2015). Overall, 2/3 of the peaks were annotated as enhancers in each species, consistent with previous studies (Villar et al., 2015). We filtered ATAC peaks to include only peaks that overlapped with a H3K27ac region. We classified ATAC peaks as enhancers or promoters based on the H3K27ac peak definition, and maintained the association to genes defined for H3K27ac peaks. To determine the conservation of mouse enhancer and promoters in human, peaks were mapped to the human genome corresponding locations using liftOver -minMatch=0.1 -multiple (Hinrichs et al., 2006). We filtered out peaks that mapped to more than 3 locations and used the remaining peak locations to intersect with the human enhancer and promoter coordinates to determine if that region was also active in the human dendritic cells. To generate aggregation plots of the H3K27ac and ATAC-Seq signal, we used the center position of ATAC peaks for enhancers and the TSS for the genes associated to the peaks as coordinates for input to ngs.plot (Shen et al., 2014). The coverage was calculated for a 4kb region surrounding the center position (−L 2000). We selected the regions corresponding to each group of interest from the output matrix and calculated the mean signal per group.
ATAC and H3K27ac dynamics
The mean signal across each ATAC-seq or H3K27ac peak was calculated by averaging the number of reads per base pair. The average signal across the libraries are normalized to the depth of each library using DESeq2 (v1.10.1) in R (v3.3.1). ATAC-seq or H3K27ac peaks were considered dynamic in response to LPS if they have greater than two fold-change in their mean signal compared to unstimulated state. The dynamic ATAC-seq or H3K27ac peaks identified are clustered using k-means algorithm to identify groups of ATAC-seq H3K27ac peaks that are induced or repressed following LPS stimulation.
Motif analysis
Motif analysis was done on 200 bp regions around the summits of the ATAC-seq peaks. The log-odds substitution rate for each 10 base-pair window across the summits of ECAs and ESPAs ATAC-seq peaks was calculated using SiPhy (Garber et al., 2009). The value of log-odd substitution score at the top ten percentile of a given peak was assigned as the conservation score for each peak. The kmers that intersected the ATAC-seq summits and which had log-odds score greater than 30 were considered for building cPWMs. To get a background set, we shuffled these 200bp ATAC-seq peaks within the enclosing H3K27ac peaks and considered all the kmers with log-odds score greater than 30. To identify kmers that distinguish the conserved ATAC peaks from background, we used the string kernel built-in gkm-svm R package (Ghandi et al., 2016) with 5 fold cross validation which resulted in 4500 unique kmers as features for conserved ATAC peaks. These kmers were clustered into 66 PWMs using k-medoids clustering algorithm with Euclidean distance, within the clara function in the cluster package in R (Blashfield, 1991). The cPWMs were then matched to the known motifs from CIS-BP database (Weirauch et al., 2014) using Tomtom (Gupta et al., 2007). Multiple motifs matched to the same TF are identified by numbers. For example JUN-1 and JUN-2. To find the cPWMs enriched in temporal gene groups or temporal ATAC peaks we used the Fisher exact test and all cPWMs with p value < 0.05 were considered enriched.
All cPWMs identified are available from http://garberlab.umassmed.edu/publications/conserved_lexicon_Dec_2017/cPWMs.motifcPWMs.motif
Transposable element analysis
We used the transposable element annotation by RepeatMasker (Smit et al., 2004) to identify TE instances in each genome that overlapped at least 10% with the regulatory regions (enhancers and promoters) associated to induced genes. As a background, we shuffled these cis-regulatory regions in the genome inside boundaries defined by the regulatory regions associated to expressed genes with no response to LPS, expanded by 10kb in each direction. We then identified the number of instances for each TE family that overlapped at least 10% with these shuffled peaks. We performed this shuffling process 1000 times and compared the initial counts obtained for each TE family to this null distribution. We computed a p-value for this permutation and corrected it using the Benjamini Hochberg method. All TE families with adjusted p-value under 0.05 were considered to be overrepresented in the regulatory regions of induced genes. For each instance of these elements in induced genes, we identified the corresponding region in the other species' genome through liftOver as described above. We then evaluated if the region that can be identified in the other genome also overlaps a H3K27ac peak, classifying it as an ECA. H3K27ac and ATAC-Seq signal aggregation plots were generated as described above, with the TE start and end genomic coordinates as the target region, flanked by 1kb on each side.
Predictive model of gene induction from cPWM instances
Feature selection
For the selected set of 66 cPWMs, all instances were detected across all ATAC peaks (promoters and enhancers) using fimo (Grant et al., 2011), with a q-value threshold of 1e-4. We tested the models using two representations of the cPWMs as features: 1. All cPWM instances together - For each gene and each cPWM, we counted the number of instances across all regulatory elements of the gene. 2. All cPWM instances, separated by ATAC temporal pattern - each cPWM was separated to three features - the number of instances in LPS-induced regions (based on ATAC-seq data), number of instances in repressed regions and number of instances in unchanging regions.
Gene filtering
To build an informative model and to reduce noise from lowly expressed genes, we focused on highly expressed genes by taking only genes that were in the top 30% of expressed genes in at least one time point. Furthermore, to clearly distinguish induced from non-induced genes, we classified genes with a log2 fold change > 2 as induced, and genes with a log2 fold change between −0.3 and 0.3 as not induced, and discarded all the rest. Next, to create a balanced set of induced and non-induced genes, we downsampled the number of non-induced genes. This resulted in a total of 676 genes (338 induced and 338 non-induced) in mouse and 748 genes in human.
Model evaluation
All model training and evaluations was done in R, using the caret (v6.0.77) (Kuhn et al.) and randomForest (v4.6.12) (Liaw et al., 2002) packages. For each feature set, we evaluated the accuracy of the model on the mouse data with 10-fold cross validation. For each one of the training data in the cross validation, hyperparameters tuning was performed using 10-fold inner cross validation with the “train” command, using the following parameters: tuneLength = 20, metric = “ROC”. To evaluate how well the model predicts induction on the human data, we trained a model on the full mouse data (again using 10-fold cross validation for hyperparameters selection) and applied the selected model on the human data.
Feature Importance
Importance measurement for each feature was computed with the “varImp” command, defined as the difference in mean accuracy across all trees between the model and the model after permuting the feature. The importance values were then scaled to span the range of 0 to 100.
DATA AND SOFTWARE AVAILABILITY
All samples generated for this work were submitted to NCBI as part of the Genomics of Gene Regulation Project, under accession number PRJNA356880. A list of samples used is specified on Table S4.
Supplementary Material
Highlights.
Conservation of enhancer activity differs across gene expression programs
Shared early induced genes in response to LPS have complex, conserved regulatory loci
Conserved DNA accessible regions hold specific constrained sequence motifs
Motif lexicon can successfully predict gene induction in both species
Acknowledgments
We want to thank Mitch Guttman, Jenny Chen, Ido Amit, Zhiping Weng, Scott Wolfe and members of the Garber Lab for valuable discussions and comments on the manuscript. We thank Idan Gabdank for help managing our data submission and to Sigrid Knemeyer for assistance with figures. This project was supported by the NHGRI U01 HG007910 (M.G., J.L., N.Y.), NIDA DP1DA034990 (J.L.), NIAID RO1AI111809 (M.G., J.L) and NCATS UL1 TR001453-02 (M.G.).
Footnotes
Author Contributions
P.V. and E.D designed and performed the data analysis. S.A. and N.Y. designed and implemented the induced gene classifier. P.V. performed the mouse DC experiments and constructed the high-throughput sequencing libraries. B.T. developed the ATAC-Seq processing pipeline and advised in data analysis. S.M. performed the human DC experiments. A.N. constructed the high-throughput sequencing libraries for human DCs. A.K. helped implement the data processing pipelines and managed sample metadata. X.Z. designed and implemented the gene expression spectral clustering algorithm. W.D. and E.D. performed the TE analysis. P.M. Supervised, developed protocols and planned all high-throughput sequencing experiments. M.G., J.L., and N.Y. conceived the project, advised on the analysis and data collection and supervised the research. E.D., P.V. and M.G. wrote the paper with input from all authors.
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amit I, Garber M, Chevrier N, Leite AP, Donner Y, Eisenhaure T, Guttman M, Grenier JK, Li W, Zuk O, et al. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science. 2009;326:257–263. doi: 10.1126/science.1179050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnosti DN, Kulkarni MM. Transcriptional enhancers: Intelligent enhanceosomes or flexible billboards? J Cell Biochem. 2005;94:890–898. doi: 10.1002/jcb.20352. [DOI] [PubMed] [Google Scholar]
- Ballester B, Medina-Rivera A, Schmidt D, Gonzàlez-Porta M, Carlucci M, Chen X, Chessman K, Faure AJ, Funnell APW, Goncalves A, et al. Multi-species, multi-transcription factor binding highlights conserved control of tissue-specific biological pathways. Elife. 2014;3:e02626. doi: 10.7554/eLife.02626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blashfield RK. In: Finding groups in data-an introduction to cluster-analysis. Kaufman L, Rousseeuw PJ, editors. 1991. [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013 doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Wu B, Chang HY, Greenleaf WJ. ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide. Curr Protoc Mol Biol. 2015;109:21.29.1–9. doi: 10.1002/0471142727.mb2129s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannavò E, Khoueiry P, Garfield DA, Geeleher P, Zichner T, Gustafson EH, Ciglar L, Korbel JO, Furlong EEM. Shadow Enhancers Are Pervasive Features of Developmental Regulatory Networks. Curr Biol. 2016;26:38–51. doi: 10.1016/j.cub.2015.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Shishkin AA, Zhu X, Kadri S, Maza I, Guttman M, Hanna JH, Regev A, Garber M. Evolutionary analysis across mammals reveals distinct classes of long non-coding RNAs. Genome Biol. 2016;17:19. doi: 10.1186/s13059-016-0880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Wu W, Kumar SA, Yu D, Deng W, Tripic T, King DC, Chen KB, Zhang Y, Drautz D, et al. Erythroid GATA1 function revealed by genome-wide analysis of transcription factor occupancy, histone modifications, and mRNA expression. Genome Res. 2009;19:2172–2184. doi: 10.1101/gr.098921.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Ma Z, Kim BH, Wu W, Cayting P, Boyle AP, Sundaram V, Xing X, Dogan N, Li J, et al. Principles of regulatory information conservation between mouse and human. Nature. 2014;515:371–375. doi: 10.1038/nature13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS, Li P, Ang YS, Lim B, Robson P, et al. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol. 2005;25:6031–6046. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussnitzer M, Dankel SN, Kim KH, Quon G, Meuleman W, Haugen C, Glunk V, Sousa IS, Beaudry JL, Puviindran V, et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. N Engl J Med. 2015;373:895–907. doi: 10.1056/NEJMoa1502214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker J, Erives A. A closer look at the eve stripe 2 enhancers of Drosophila and Themira. PLoS Genet. 2008;4:e1000276. doi: 10.1371/journal.pgen.1000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunipace L, Ozdemir A, Stathopoulos A. Complex interactions between cis-regulatory modules in native conformation are critical for Drosophila snail expression. Development. 2011;138:4075–4084. doi: 10.1242/dev.069146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber M, Guttman M, Clamp M, Zody MC, Friedman N, Xie X. Identifying novel constrained elements by exploiting biased substitution patterns. Bioinformatics. 2009;25:i54–i62. doi: 10.1093/bioinformatics/btp190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber M, Yosef N, Goren A, Raychowdhury R, Thielke A, Guttman M, Robinson J, Minie B, Chevrier N, Itzhaki Z, et al. A high-throughput chromatin immunoprecipitation approach reveals principles of dynamic gene regulation in mammals. Mol Cell. 2012;47:810–822. doi: 10.1016/j.molcel.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandi M, Mohammad-Noori M, Ghareghani N, Lee D, Garraway L, Beer MA. gkmSVM: an R package for gapped-kmer SVM. Bioinformatics. 2016;32:2205–2207. doi: 10.1093/bioinformatics/btw203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad Y, Oshlack A, Rifkin SA. Natural selection on gene expression. Trends Genet. 2006;22:456–461. doi: 10.1016/j.tig.2006.06.002. [DOI] [PubMed] [Google Scholar]
- González AJ, Setty M, Leslie CS. Early enhancer establishment and regulatory locus complexity shape transcriptional programs in hematopoietic differentiation. Nature Publishing Group. 2015;47:1249–1259. doi: 10.1038/ng.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant CE, Bailey TL, Noble WS. FIMO: scanning for occurrences of a given motif. Bioinformatics. 2011;27:1017–1018. doi: 10.1093/bioinformatics/btr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Stamatoyannopoulos JA, Bailey TL, Noble WS. Quantifying similarity between motifs. Genome Biol. 2007;8:R24. doi: 10.1186/gb-2007-8-2-r24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- He Q, Bardet AF, Patton B, Purvis J, Johnston J, Paulson A, Gogol M, Stark A, Zeitlinger J. High conservation of transcription factor binding and evidence for combinatorial regulation across six Drosophila species. Nat Genet. 2011;43:414–420. doi: 10.1038/ng.808. [DOI] [PubMed] [Google Scholar]
- Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- Helft J, Böttcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, Goubau D, Reis e Sousa C. GM-CSF Mouse Bone Marrow Cultures Comprise a Heterogeneous Population of CD11c+MHCII+ Macrophages and Dendritic Cells. Immunity. 2015;42:1197–1211. doi: 10.1016/j.immuni.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Hinrichs AS, Karolchik D, Baertsch R, Barber GP, Bejerano G, Clawson H, Diekhans M, Furey TS, Harte RA, Hsu F, et al. The UCSC Genome Browser Database: update 2006. Nucleic Acids Res. 2006;34:D590–D598. doi: 10.1093/nar/gkj144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icardi L, Mori R, Gesellchen V, Eyckerman S, De Cauwer L, Verhelst J, Vercauteren K, Saelens X, Meuleman P, Leroux-Roels G, et al. The Sin3a repressor complex is a master regulator of STAT transcriptional activity. Proc Natl Acad Sci U S A. 2012;109:12058–12063. doi: 10.1073/pnas.1206458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jjingo D, Conley AB, Wang J, Mariño-Ramírez L, Lunyak VV, Jordan IK. Mammalian-wide interspersed repeat (MIR)-derived enhancers and the regulation of human gene expression. Mob DNA. 2014;5:14. doi: 10.1186/1759-8753-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S, Sabo PJ, Thurman RE, Sung MH, Biddie SC, Johnson TA, Hager GL, Stamatoyannopoulos JA. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet. 2011;43:264–268. doi: 10.1038/ng.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- Kuhn M, Wing J, Weston S, Williams A, Keefer C, Engelhardt A. Others caret: Classification and regression training, 2011. R Package Version 4 [Google Scholar]
- Kunarso G, Chia NY, Jeyakani J, Hwang C, Lu X, Chan YS, Ng HH, Bourque G. Transposable elements have rewired the core regulatory network of human embryonic stem cells. Nat Genet. 2010;42:631–634. doi: 10.1038/ng.600. [DOI] [PubMed] [Google Scholar]
- Kutter C, Watt S, Stefflova K, Wilson MD, Goncalves A, Ponting CP, Odom DT, Marques AC. Rapid turnover of long noncoding RNAs and the evolution of gene expression. PLoS Genet. 2012;8:e1002841. doi: 10.1371/journal.pgen.1002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettice LA, Heaney SJH, Purdie LA, Li L, de Beer P, Oostra BA, Goode D, Elgar G, Hill RE, de Graaff E. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet. 2003;12:1725–1735. doi: 10.1093/hmg/ddg180. [DOI] [PubMed] [Google Scholar]
- Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw A, Wiener M, et al. Classification and regression by randomForest. R News. 2002;2:18–22. [Google Scholar]
- Love M, Anders S, Huber W. Differential analysis of count data--the DESeq2 package. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe CB, Haussler D. 29 mammalian genomes reveal novel exaptations of mobile elements for likely regulatory functions in the human genome. PLoS One. 2012;7:e43128. doi: 10.1371/journal.pone.0043128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig MZ, Bergman C, Patel NH, Kreitman M. Evidence for stabilizing selection in a eukaryotic enhancer element. Nature. 2000;403:564–567. doi: 10.1038/35000615. [DOI] [PubMed] [Google Scholar]
- von Luxburg U. A tutorial on spectral clustering. Stat Comput. 2007;17:395–416. [Google Scholar]
- Mavrothalassitis G, Ghysdael J. Proteins of the ETS family with transcriptional repressor activity. Oncogene. 2000;19:6524–6532. doi: 10.1038/sj.onc.1204045. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Xu Z, Zhang X, Wang L, Gimble JM, Lander ES, Rosen ED. Comparative epigenomic analysis of murine and human adipogenesis. Cell. 2010;143:156–169. doi: 10.1016/j.cell.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2016;44:D7–D19. doi: 10.1093/nar/gkv1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necsulea A, Soumillon M, Warnefors M, Liechti A, Daish T, Zeller U, Baker JC, Grützner F, Kaessmann H. The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature. 2014 doi: 10.1038/nature12943. [DOI] [PubMed] [Google Scholar]
- Odom DT, Dowell RD, Jacobsen ES, Gordon W, Danford TW, MacIsaac KD, Rolfe PA, Conboy CM, Gifford DK, Fraenkel E. Tissue-specific transcriptional regulation has diverged significantly between human and mouse. Nat Genet. 2007;39:730–732. doi: 10.1038/ng2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CT, Corces VG. Enhancer function: new insights into the regulation of tissue-specific gene expression. Nat Rev Genet. 2011;12:283–293. doi: 10.1038/nrg2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panne D, Maniatis T, Harrison SC. An Atomic Model of the Interferon-β Enhanceosome. Cell. 2007;129:1111–1123. doi: 10.1016/j.cell.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas O, Jovanovic M, Eisenhaure TM, Herbst RH, Dixit A, Ye CJ, Przybylski D, Platt RJ, Tirosh I, Sanjana NE, et al. A Genome-wide CRISPR Screen in Primary Immune Cells to Dissect Regulatory Networks. Cell. 2015;162:675–686. doi: 10.1016/j.cell.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchio LA, Ahituv N, Moses AM, Prabhakar S, Nobrega MA, Shoukry M, Minovitsky S, Dubchak I, Holt A, Lewis KD, et al. In vivo enhancer analysis of human conserved non-coding sequences. Nature. 2006;444:499–502. doi: 10.1038/nature05295. [DOI] [PubMed] [Google Scholar]
- Perry MW, Boettiger AN, Bothma JP, Levine M. Shadow enhancers foster robustness of Drosophila gastrulation. Curr Biol. 2010;20:1562–1567. doi: 10.1016/j.cub.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponjavic J, Ponting CP, Lunter G. Functionality or transcriptional noise? Evidence for selection within long noncoding RNAs. Genome Res. 2007;17:556–565. doi: 10.1101/gr.6036807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt KD, Tatusova T, Brown GR, Maglott DR. NCBI Reference Sequences (RefSeq): current status, new features and genome annotation policy. Nucleic Acids Res. 2012;40:D130–D135. doi: 10.1093/nar/gkr1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabani M, Levin JZ, Fan L, Adiconis X, Raychowdhury R, Garber M, Gnirke A, Nusbaum C, Hacohen N, Friedman N, et al. Metabolic labeling of RNA uncovers principles of RNA production and degradation dynamics in mammalian cells. Nat Biotechnol. 2011;29:436–442. doi: 10.1038/nbt.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard C, Bottinelli D, Kim B, Luban J. Vpx rescue of HIV-1 from the antiviral state in mature dendritic cells is independent of the intracellular deoxynucleotide concentration. Retrovirology. 2014;11:12. doi: 10.1186/1742-4690-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Wilson MD, Ballester B, Schwalie PC, Brown GD, Marshall A, Kutter C, Watt S, Martinez-Jimenez CP, Mackay S, et al. Five-vertebrate ChIP-seq reveals the evolutionary dynamics of transcription factor binding. Science. 2010;328:1036–1040. doi: 10.1126/science.1186176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Shao N, Liu X, Nestler E. ngs.plot: Quick mining and visualization of next-generation sequencing data by integrating genomic databases. BMC Genomics. 2014;15:284. doi: 10.1186/1471-2164-15-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlyueva D, Stampfel G, Stark A. Transcriptional enhancers: from properties to genome-wide predictions. Nat Rev Genet. 2014;15:272–286. doi: 10.1038/nrg3682. [DOI] [PubMed] [Google Scholar]
- Smit AF, Riggs AD. MIRs are classic, tRNA-derived SINEs that amplified before the mammalian radiation. Nucleic Acids Res. 1995;23:98–102. doi: 10.1093/nar/23.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit A, Hubley R, Green P. RepeatMasker Open-3.0. Seattle (WA): Institute for Systems Biology; 2004. [Google Scholar]
- Thanos D, Maniatis T. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell. 1995;83:1091–1100. doi: 10.1016/0092-8674(95)90136-1. [DOI] [PubMed] [Google Scholar]
- Toshchakov V, Jones BW, Perera PY, Thomas K, Cody MJ, Zhang S, Williams BRG, Major J, Hamilton TA, Fenton MJ, et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat Immunol. 2002;3:392–398. doi: 10.1038/ni774. [DOI] [PubMed] [Google Scholar]
- Ulitsky I. Evolution to the rescue: using comparative genomics to understand long non-coding RNAs. Nat Rev Genet. 2016;17:601–614. doi: 10.1038/nrg.2016.85. [DOI] [PubMed] [Google Scholar]
- Vierstra J, Rynes E, Sandstrom R, Zhang M, Canfield T, Hansen RS, Stehling-Sun S, Sabo PJ, Byron R, Humbert R, et al. Mouse regulatory DNA landscapes reveal global principles of cis-regulatory evolution. Science. 2014 doi: 10.1126/science.1246426. 1246426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar D, Berthelot C, Aldridge S, Rayner TF, Lukk M, Pignatelli M, Park TJ, Deaville R, Erichsen JT, Jasinska AJ, et al. Enhancer evolution across 20 mammalian species. Cell. 2015;160:554–566. doi: 10.1016/j.cell.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visel A, Blow MJ, Li Z, Zhang T, Akiyama JA, Holt A, Plajzer-Frick I, Shoukry M, Wright C, Chen F, et al. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature. 2009;457:854–858. doi: 10.1038/nature07730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lin G, Zhang Z. ATF5 promotes cell survival through transcriptional activation of Hsp27 in H9c2 cells. Cell Biol Int. 2007a;31:1309–1315. doi: 10.1016/j.cellbi.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhuang J, Iyer S, Lin X, Whitfield TW, Greven MC, Pierce BG, Dong X, Kundaje A, Cheng Y, et al. Sequence features and chromatin structure around the genomic regions bound by 119 human transcription factors. Genome Res. 2012;22:1798–1812. doi: 10.1101/gr.139105.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zeng J, Lowe CB, Sellers RG, Salama SR, Yang M, Burgess SM, Brachmann RK, Haussler D. Species-specific endogenous retroviruses shape the transcriptional network of the human tumor suppressor protein p53. Proceedings of the National Academy of Sciences. 2007b;104:18613–18618. doi: 10.1073/pnas.0703637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washietl S, Kellis M, Garber M. Evolutionary dynamics and tissue specificity of human long noncoding RNAs in six mammals. Genome Res. 2014 doi: 10.1101/gr.165035.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watatani Y, Kimura N, Shimizu YI, Akiyama I, Tonaki D, Hirose H, Takahashi S, Takahashi Y. Amino acid limitation induces expression of ATF5 mRNA at the post-transcriptional level. Life Sci. 2007;80:879–885. doi: 10.1016/j.lfs.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Weirauch MT, Yang A, Albu M, Cote AG, Montenegro-Montero A, Drewe P, Najafabadi HS, Lambert SA, Mann I, Cook K, et al. Determination and inference of eukaryotic transcription factor sequence specificity. Cell. 2014;158:1431–1443. doi: 10.1016/j.cell.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender E, Schoeps T, Dönitz J. TFClass: an expandable hierarchical classification of human transcription factors. Nucleic Acids Res. 2013;41:D165–D170. doi: 10.1093/nar/gks1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue F, Cheng Y, Breschi A, Vierstra J, Wu W, Ryba T, Sandstrom R, Ma Z, Davis C, Pope BD, et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515:355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.