Abstract
The effects of solid-state fermentation with Cordyceps militaris (L.) Fr. on the nutritional, physicochemical, and functional properties as well as angiotensin I converting enzyme (ACE) inhibitory activity of red bean (Phaseolus angularis [Willd.] W.F. Wight.) flour were determined. Fermentation increased the amount of small peptides but significantly decreased large peptides. Fermentation also increased proteins and essential amino acids (by 9.31 and 13.89%, respectively) and improved the in vitro protein digestibility (6.54%) of red beans. Moreover, fermentation increased the water holding capacity (from 2.36 to 2.59 mL/g), fat absorption capacity (from 84.65 to 114.55%), emulsion activity (from 10.96 to 52.77%), emulsion stability (from 5.43 to 53.82%), and foaming stability (from 11.95 to 20.68%). Fermented red bean flour achieved a lower least gelation concentration of 14% than that of the control (18%). In contrast to the non-fermented red bean, the fermented red bean showed ACE inhibitory activity, with IC50 value of 0.63 mg protein/mL. Overall, fermentation improved the nutritional, physicochemical, and functional properties as well as the biological activity of red bean flour. Thus, fermented red bean flour may serve as a novel nutritional and functional ingredient for applications in food design.
Keywords: Cordyceps militaris, Red bean, Solid-state fermentation, Functional properties, ACE inhibitory activity
Introduction
Red bean (Phaseolus angularis [Willd.] W.F. Wight.) is an important leguminous seed applied in healthy diets because of its nutritional value (Tiwari et al. 2011; Yu et al. 2011). Red beans are native to the northeastern China and are widely consumed as food in many countries (Tang and Sun 2011). Red beans are a health-promoting food and a nutritional source containing abundant carbohydrate fractions, proteins, amino acids, and minerals (Guillon and Champ 2002; Ma et al. 2009). However, many studies reported that the use of leguminous products is limited by the presence of anti-nutritional factors (e.g., phytates, lectins, protease inhibitors and cyanoglycosides) and inefficient digestibility of legume proteins with high molecular mass (Angulo-Bejarano et al. 2008; Reyes-Bastidas et al. 2010). Biotechnological processes, particularly fermentation, are an inexpensive method used to improve the nutritional, physicochemical, and functional properties as well as decrease the number of anti-nutritional factors of legume-based products (Angulo-Bejarano et al. 2008). Solid state fermentation (SSF) is a widely used technology for processing various types of legumes; this approach improves the nutritional and nutraceutical properties of legume-based products (Rochín-Medina et al. 2015; Xiao et al. 2015). Angulo-Bejarano et al. (2008) and Xiao et al. (2015) reported that chickpea processed by SSF with Rhizopus or Cordyceps militaris strains improved the nutritional properties, increased the amount of essential amino acids, and enhanced the in vitro protein digestibility of the product. During SSF, microbes produce many enzymes (e.g., proteases, amylases, esterases and celluases) which could hydrolyze the components such as protein, polysaccharide and lipid, and then contribute to the development of products with improved nutrition, flavor, and aroma (Ang et al. 2013; Chutmanop et al. 2008).
Other authors reported that SSF is a bioprocess used to generate bioactive peptides with low molecular masses (Pyo and Lee 2007; Xiao et al. 2015). SSF is also employed to produce legume-based foods with angiotensin I converting enzyme (ACE) inhibitory peptides for prevention and treatment of hypertension (Rochín-Medina et al. 2015). Fermented leguminous products, such as natto (Bacillus subtilis fermented soybean) and Monascus-fermented soybean, exhibit ACE inhibitory activity and are an important source of ACE inhibitory peptides (Juan et al. 2010; Pyo and Lee 2007). Therefore, SSF can be used to produce natural ACE inhibitors by using dietary bioprocessed legumes; such inhibitors are potentially safe and may thus be a preferred alternative for control of hypertension.
Cordyceps militaris (L.) Fr. is a food-grade fungus which is belonging to the class of Ascomycetes (Das et al. 2010). This organism contains many bioactive materials (e.g., cordycepin and cordycepic acid) that elicit antiarrhythmic, antitumor, and antivirus effects; this fungus was traditionally used as folk tonic and functional foods in many countries (Das et al. 2010). Previous studies evaluated the potential of using SSF with Rhizopus oligosporus, Aspergillus oryzae, or B. subtilis to improve the nutritional value and physicochemical properties of chickpea, common bean, and soybean (Reyes-Bastidas et al. 2010; Rochín-Medina et al. 2015). However, to our knowledge, there is no information regarding the effects of SSF with C. militaris on the nutritional, physicochemical, and functional properties of red beans. To develop functional foods or food formulations using C. militaris fermented red bean (FRB), scholars must elucidate the physicochemical and functional properties of the product. Therefore, this study aims to assess the effects of fermentation on protein composition; nutritional, physicochemical, and functional properties; and biological activity of red bean flour. Results could be used to determine potential applications of FRB in the development of novel food formulations and nutritious food products for health promotion.
Materials and methods
Materials, chemicals and microorganism
ACE, hippuryl-l-histidyl-l-leucine (HHL), hippuric acid (HA), sodium dodecyl sulfate (SDS), soybean oil, and solvents used for high-performance liquid chromatography (HPLC) analysis were purchased from the company Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and solvents were of analytical grade. Red beans were purchased from the local supermarket (Suguo supermarket, Nanjing, China). The fungus C. militaris SN-18 (Xiao et al. 2014; 2016) was cultivated and maintained in a potato dextrose agar (PDA) (Sigma, Aldrich) slanted and subcultured monthly. The culture was stored and activated for SSF by using the method detailed by Xiao et al. (2014). A suspension of spore (about 108–109 spores/mL) of the strain was prepared and used as the inoculum starter for red beans fermentation.
SSF of red beans
Red beans were fermented with C. militaris as described by Xiao et al. (2014). The red bean sample was washed and soaked in distilled water at 20 °C for 12 h. After decanting the water, the red beans were cracked mechanically in a commercial cracker (BE601AB, Midea, China) and sterilized in an autoclave at 121 °C for 25 min. After cooling, lactic acid (food grade, 0.5 mL per 100 g of wet red bean) was added to the sterile cracked seeds followed by inoculation of C. militaris spore suspension (2.5 mL of spore suspension for every 100 g wet red bean) and incubated at 25 °C for 7 days (C. militaris fermented red bean, FRB). In this study, the non-fermented red bean (RB, used as control) was prepared following the same procedure for processing FRB, except that red bean was not incubated with C. militaris spore suspension. Both FRB and RB were freeze-dried and powdered to fine flour (0.2 mm sieve) by using a mixer grinder. The flour was stored at 4 °C.
Proximate analysis
The proximate composition of FRB and RB, including crude protein, ash and crude fat were determined according to the methods of AOAC 14.108, 14.103 and 14.093, respectively (AOAC 1990). Total carbohydrate content was determined by subtracting the contents of crude protein, ash and crude fat from 100% of dry matter (Reyes-Moreno et al. 2004). The results were presented as grams per 100 g of dry basis (g/100 g d.b.).
Analysis of amino-acid composition
Amino-acid composition was determined in accordance with a published procedure (Angulo-Bejarano et al. 2008). A known amount of the sample (0.2 g each in triplicates) taken in sealed glass tubes was treated with 10 mL of 6 mol/L HCl and incubated in an oven operating at 110 °C for 24 h. After cooled to room temperature, hydrolysates were dried under vacuum and the dried hydrolysate was subsequently mixed with 5 mL 0.02 mol/L HCl. The mixture was then filtered with a syringe filter (0.22 µm PVDF membrane, Bedford, MA, USA). 20 µL of each sample was subjected to a fully automated amino acid analyzer HITACHI L-8900 (Hitachi Ltd., Japan). The results were expressed as milligram of amino acids per gram of dry basis (mg/g d.b.).
Physicochemical properties
Bulk density
Bulk density was evaluated by using the detail method of Wani et al. (2013). Briefly, a 10 mL graduated cylinder which previously weighed was employed to fill with red bean flour. The sample was then packed through gently tapping the graduated cylinder on a laboratory bench several times until there was no further diminution of the sample level after it was filled up to the 10 mL mark. Bulk density was calculated as the weight of the red bean flour per unit volume of sample (g/mL).
In vitro protein digestibility (IVPD)
IVPD was assessed according to the method of Yousif and El Tinay (2000). 200 mg of red bean flour sample was digested with 1.5 mg of pepsin (prepared in 15 mL of 0.1 mol/L HCl) for 3 h at 37 °C. Then 3.3 mL NaOH (0.5 mol/L) and 4 mg pancreatin in 7.5 mL phosphate buffer (0.2 mol/L, pH 8.0) were added. The mixture inoculated at 37 °C for 24 h. The reaction was then terminated by the addition of 10 mL of 10% trichloroacetic acid (10% TCA). The obtained mixture was centrifuged at 10,000g for 20 min. The supernatant was assayed for nitrogen using the micro-Kjeldahl method. IVPD rate was calculated in light of the following formula:
Protease activity
Protease activity was determined using the method described by Chutmanop et al. (2008) with slight modifications. 10 mL of deionized water was added to 1 g of the sample. The resulting slurry was agitated on a rotary shaker (180 rpm) for 1 h at 30 °C and centrifuged at 10,000g for 10 min at 4 °C. Then 1 mL of the diluted supernatant was mixed with 1 mL of solution containing 1% casein and incubated for 10 min at 40 °C on a gyratory shaker. The enzyme mixture was added with 2 mL of 0.4 mol/L TCA, stand for 30 min at room temperature, and centrifuged for 10 min (10,000g, 4 °C). One unit of protease activity was defined as the amount of enzyme that releases one μg of tyrosine per minute under the assay conditions. Result was expressed as U/g dry solid.
Functional properties
Fat absorption capacity (FAC) and water holding capacity (WHC)
FAC was determined using the procedure of Boye et al. (2010) with some modifications. 0.5 g of lyophilized samples were mixed with 5 mL of soybean oil in pre-weighed centrifuge tubes for 1 min. The tubes were then centrifuged for 30 min at 4000g. The supernatant was removed and the tubes were re-weighed. FAC was determined in light of the following equation:
WHC was evaluated in light of the detail method reported by Boye et al. (2010) and was expressed as the amount of water absorbed by 1 g of red bean flour.
Emulsion activity (EA) and emulsion stability (ES)
EA and ES were determined using the method of Kaur and Singh (2005) and Sridaran et al. (2012). Briefly, 3.5 g of red bean flour sample was homogenized with 50 mL distilled water for 30 s in a high-speed scattering machine (XHF-D, China). The mixture was homogenized again for 30 s after addition of 25 mL soybean oil. Then, other 25 mL soybean oil was added into the mixture and homogenized for 90 s. The obtained emulsion was divided evenly into two 50 mL centrifuge tubes and centrifuged for 5 min at 1100g. EA was then calculated based on the following equation:
The samples which prepared for evaluation of EA was used to determine the ES. They were heated at 85 °C for 15 min. After cooling, they were centrifuged for 5 min at 1100g. The ES was then calculated in light of the following equation:
Foaming capacity (FC) and foaming stability (FS)
FC and FS were determined using the method of Du et al. (2014) and Wani et al. (2015). Briefly, aqueous dispersions of the red bean flour samples (2%, w/v) were homogenized by a high-speed scattering machine (XHF-D, China) for 2 min at 10,000g. The volume was measured before and after whipping. FC was calculated as the percent increase in volume of the flour dispersion. In order to determine the FS, the foam was then allowed to stand for 1 h after whipping. Then, the volume of the samples was again measured. FC and FS were calculated using the formulas
where V1 is the volume of the suspension (mL) before whipping, V2 is the volume of the suspension (mL) after whipping, and V3 is the volume of the whipped suspension (mL) after it was allowed to stand for 60 min.
Least gelation concentration (LGC)
The LGCs of FRB and RB were determined using the method of Elkhalifa and Bernhardt (2010). Flour suspension of different concentrations (2, 4, 6, 8, 10, 12, 14, 16, 18, 20 and 25%) (w/v) were prepared by mixing the sample flour with 5 mL distilled water. Then, the test tubes which contain the suspensions were heated in boiling water bath for 1 h. Thereafter, the samples were rapidly cooled under cold water. The tubes were further cooling at 4 °C for 2 h. LGC was determined by visual observation whether any drop from the emulsion slipped out or not to the top of the inverted tubes.
SDS-polyacrylamide gel electrophoresis (PAGE)
SDS-PAGE analysis was conduced following the method of Xiao et al. (2015). 50 mg of flour sample was extracted in 1 mL of protein extraction buffer (pH 8.8, 0.05 mol/L Tris–HCl). The mixture was stirred on a rotary shaker at room temperature (25 °C) for 1 h. Following extraction, samples were centrifuged at 10,000 g for 10 min. The collected protein supernatant were mixed with sample buffer (0.5 mol/L Tris–HCl at pH 6.8, 2% SDS, 0.005% bromophenol blue, 20% glycerol and 10% β-mercaptoethanol) at a ratio of 1:1 and then heated at 100 °C for 5 min. Then, the sample was loaded on to a gel, and subsequently the gel was electrophoresized in a Miniprotein 3 unit (Bio-Rad Lab., Hercules, CA). 4% stacking and 10% separating gel was used to separate proteins of the sample. After electrophoresis, Coomassie brilliant blue R-250 was used to stain the protein bands. Then, the gel was photographed by Molecular Imager PharosFX Plus.
Reverse-phase HPLC (RP-HPLC)
RP-HPLC analysis was conducted to investigate changes in the peptide profile of the samples. FRB and RB were extracted with 40 folds of borate buffer (0.05 mol/L, pH 8.3). The mixture was stirred for 2 h at room temperature (25 °C) on a rotary shaker, and then centrifuged for 10 min at 10,000g. The collected supernatant was used as stock solution. The sample solutions were filtered with a syringe filter (0.45 µm polyvinylidene fluoride membrane, Bedford, MA, USA), and then 20 µL of the test solutions were injected into the HPLC system for analysis according to the detail method of Rui et al. (2015). The mobile phase consisted of water as solvent A (containing 0.1% trifluoroacetic acid) and acetonitrile as solvent B (containing 0.1% trifluoroacetic acid) with the following gradient: the time program started at 5% B and increased to 20% B in 25 min, then 20–30% B for over 5 min, 30–100% B for 15 min, 100–5% B in 5 min. The flow rate was 0.7 mL/min and the elution was detected at 280 nm using a DAD detector.
ACE inhibitory activity
Protein content in the sample solution (used for RP-HPLC analysis) was assayed by Lowry method with bovine serum albumin as standard. The solution was then diluted to different concentrations for analysis of ACE inhibitory activity. ACE inhibitory activity was determined by monitoring the liberation of HA from the substrate HHL through HPLC by using the method described by Xiao et al. (2015). Briefly, 200 µL of HHL solution (5 mM in 0.1 M borate buffer, containing 0.3 mol/L NaCl, pH 8.3) were incubated with 80 µL of sample solution at 37 °C for 5 min. The reactions were then initiated by adding 20 µl of ACE solution (0.1 U/mL) and incubated at 37 °C for 30 min. The reaction was terminated by adding 250 µL HCl (1.0 M). The samples were filtered, and released HA was determined by RP-HPLC on a C18 reverse-phase column (4.6 × 250 mm, 5 μm particle size, Agilent). The effluent was detected at 228 nm. The column was eluted with 50% (v/v) methanol in water with 0.1% trifluoroacetic acid (TFA) for 20 min at a flow rate of 0.8 mL/min. Absorbance of the hippuric acid peak was determined. ACE inhibitory activity (%) was calculated as follows:
where A was the absorbance of the test sample, B was the absorbance of the control, C was the absorbance of the blank.
Statistical analysis
All experiments were carried out independently in triplicate. The results were expressed as mean ± standard deviation (SD) of triplicate determinations. Statistical significance of the obtained results was analyzed by independent sample t test by using SPSS version 17.0.
Results and discussion
Proximate composition
The results of the proximate composition carried out on the RB and FRB are shown in Table 1. SSF slightly increased protein (by 9.31%) and ash content (by 5.57%) and decreased the carbohydrate content (by 3.23%) of red bean flour. In accordance with previous studies, SSF with R. oligosporus or C. militaris have also shown significant increase in protein and decrease in carbohydrate content of legumes (Reyes-Bastidas et al. 2010; Xiao et al. 2015). Sparringa and Owens (1999) found that fermentation for 72 h significantly increased protein content of soybean (by 21.70%), which reflected an increase in mold biomass. The reduction in carbohydrate content may be explained by its role as energy source of fungus for growth.
Table 1.
Proximate composition of C. militaris fermented and non-fermented red bean flours
| Parameter | Non-fermented red bean | C. militaris fermented red bean |
|---|---|---|
| Crude protein | 23.61 ± 0.50b | 25.81 ± 0.06a |
| Crude fat | 3.00 ± 0.08a | 2.92 ± 0.12a |
| Ash | 2.87 ± 0.01b | 3.03 ± 0.02a |
| Carbohydrates | 70.52 ± 0.42a | 68.24 ± 0.04b |
The results were expressed as grams per 100 g of dry basis (g/100 g d.b.)
Values are mean ± SD (n = 3). Different small letters in the same row denote statistical difference (p < 0.05)
Amino-acid composition
Amino-acid composition of FRB and RB is presented in Table 2. The major amino acid in both FRB and RB was glutamic acid. Previous studies reported glutamic acid as the major amino acid in fermented and non-fermented legumes (Song et al. 2008). Moreover, all essential amino acids in FRB were significantly higher than RB except for threonine (Table 2). The total amino acid and total essential amino acids of the red beans significantly enhanced 13.09 and 13.88%, respectively, during C. militaris fermentation. Many previous investigations have also demonstrated that fungi fermentation could also increase amino-acid levels. For instance, Reyes-Bastidas et al. (2010) reported that common beans by SSF with R. oligosporus significantly enhanced the Ile, Val, Thr, total aromatic (Phe + Tyr) and total sulfur (Met + Cys) contents, and a similar trend was found in the current study (Table 2). It was stated in literatures that the conversion of amino acids by the action of produced transaminases during fermentation could partly contribute to the increase in amino-acid levels (Angulo-Bejarano et al. 2008; Reyes-Bastidas et al. 2010). Thus, higher contents of total and essential amino-acid in FRB might be attributed to the synthesis or transamination occurring during SSF.
Table 2.
Amino-acid composition of C. militaris fermented and non-fermented red bean flours
| Amino acid composition | Non-fermented red bean | C. militaris fermented red bean |
|---|---|---|
| Non essential amino acids | ||
| Aspartic acid (Asp) | 11.63 ± 0.11a | 6.53 ± 0.08b |
| Serine (Ser) | 8.99 ± 0.04a | 8.56 ± 0.08b |
| Glutamic acid (Glu) | 32.40 ± 0.38b | 45.36 ± 0.31a |
| Glysine (Gly) | 6.16 ± 0.06a | 4.73 ± 0.05b |
| Alanine (Ala) | 8.52 ± 0.11b | 11.88 ± 0.16a |
| Arginine (Arg) | 12.23 ± 0.10b | 13.69 ± 0.20a |
| Proline (Pro) | 16.93 ± 0.06b | 18.13 ± 0.09a |
| Essential amino acids | ||
| Threonine (Thr) | 11.46 ± 0.07a | 7.50 ± 0.07b |
| Cysteine (Cys) | 9.20 ± 0.05b | 10.89 ± 0.03a |
| Valine (Val) | 1.26 ± 0.04b | 4.16 ± 0.13a |
| Methionine (Met) | 2.12 ± 0.05b | 4.05 ± 0.07a |
| Isoleusine (Ile) | 7.59 ± 0.07b | 9.13 ± 0.11a |
| Leucine (Leu) | 15.60 ± 0.17b | 18.57 ± 0.26a |
| Tyrosine (Tyr) | 4.76 ± 0.10b | 5.75 ± 0.08a |
| Phenylalanine (Phe) | 11.13 ± 0.10b | 12.71 ± 0.10a |
| Lysine (Lys) | 14.27 ± 0.06b | 15.77 ± 0.18a |
| Histidine (His) | 6.12 ± 0.34b | 6.57 ± 0.40a |
| Total essential amino acids | 83.52 ± 1.06b | 95.11 ± 1.43a |
| Total amino acids | 180.37 ± 0.83b | 203.98 ± 1.35a |
The results were presented as milligram of amino acids per gram of dry basis (mg/g d.b.)
Values are mean ± SD (n = 3). Different small letters in the same row denote statistical difference (p < 0.05)
Effect of fermentation on the protein molecular weight distribution and IVPD of red beans
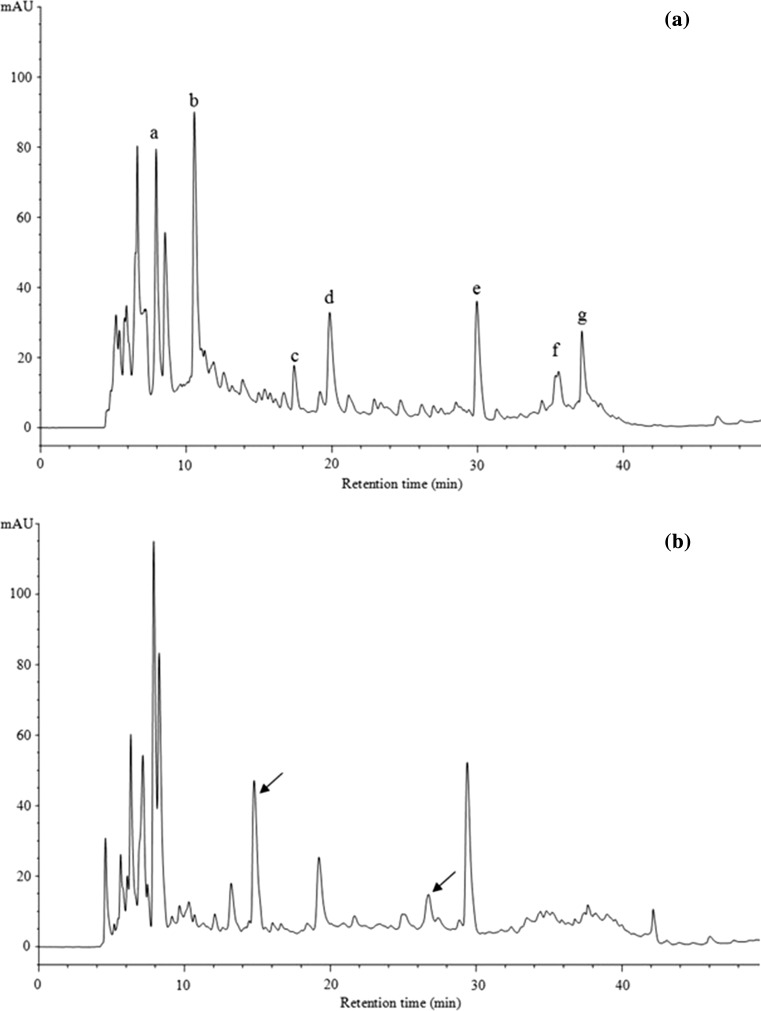
Changes in the protein profiles of red beans during fermentation were analyzed by SDS-PAGE, and the results are shown in Fig. 1. Notably, fermentation significantly affected the characteristics of proteins in red beans. The protein profiles of RB consisting of several major large-sized protein bands. It was found that ~ 33 and ~ 27 kDa bands were the most intense ones in RB (Fig. 1). Because of strong proteolysis during SSF, the large-sized and mid-to-large sized proteins of RB were hydrolyzed into smaller molecular masses, resulting in an increment in the amount of small-sized proteins or peptides (< 15 kDa). Some of the new peptides were also generated (arrows of FRB in Fig. 1). RP-HPLC further revealed the protein hydrolysis of the red beans during the fermentation process (Fig. 2). Notably, fermentation with C. militaris resulted in the hydrolysis of protein fractions, with several peaks that disappeared in size and new peaks that formed (arrows in Fig. 2b). For instance, the results suggested the complete hydrolysis of compounds from peaks b, c, f, and g and a reduction of compound from peak d. These results may correspond to the generation of smaller/more hydrophilic peptides. The RP-HPLC profiles indicated the intensive alterations of protein peaks during fermentation, which was previously reported by Aguirre et al. (2008) for soybean and by Rui et al. (2015) for navy bean.
Fig. 1.
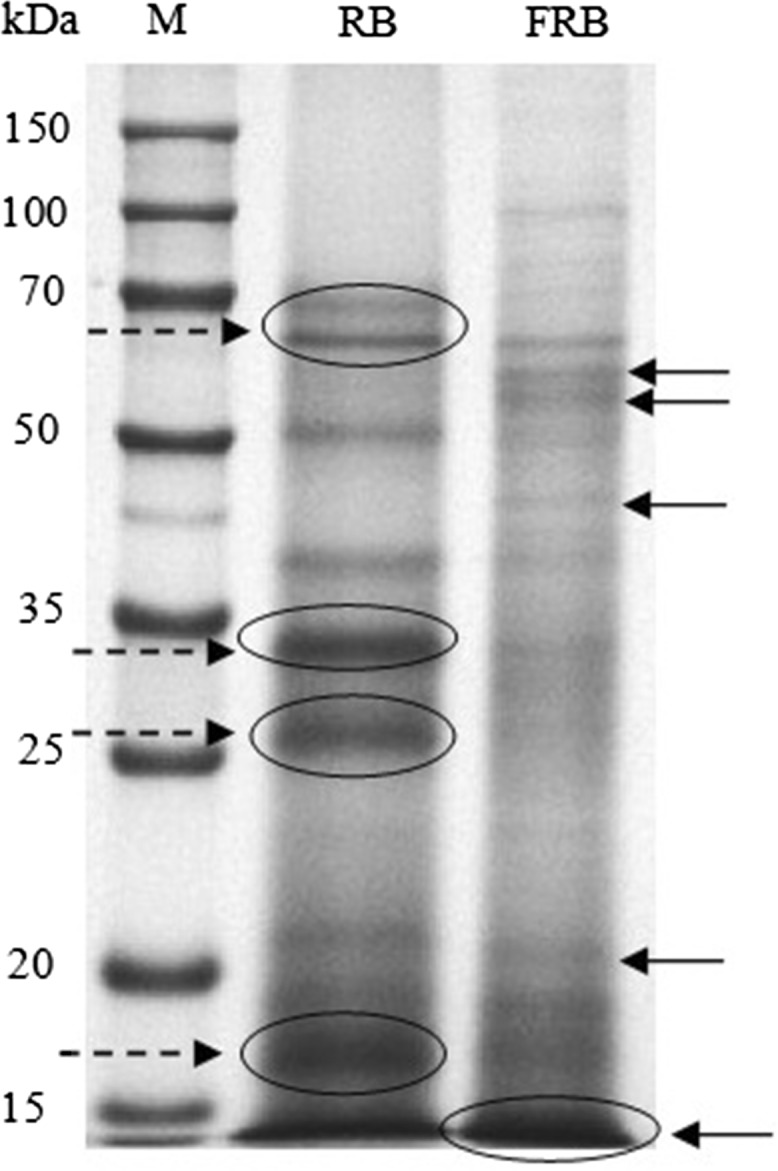
SDS-PAGE electrophoregram of C. militaris fermented and non-fermented red bean proteins. M, molecular weight marker; RB, non-fermented red bean; FRB, C. militaris fermented red bean
Fig. 2.
RP-HPLC patterns of soluble peptides present in non-fermented red bean (a) and C. militaris fermented red bean (b)
The hydrolysis of proteins to smaller peptides during fermentation has been reported in many previous published studies (Aguirre et al. 2008; Hong et al. 2004; Lim et al. 2010). For instance, Hong et al. (2004) and Xiao et al. (2015) demonstrated how A. oryzae fermented soybean or C. militaris fermented chickpea significantly decreased the amount of large peptides and increased those of small peptides; the release of small peptides in fermented products could be due to strong proteolysis during fermentation. Microorganisms are generally considered to possess a developed peptidase system, which could hydrolyze larger proteins/peptide to smaller peptide/amino acids. In this study, the protease activity from FRB was estimated to be 52.72 ± 2.84 U/g. Therefore, the results obtained could be explained by strong proteolysis occurring through the activation of protease produced by C. militaris.
In this study, the IVPD of RB was 78.29%, and SSF significantly improved (6.54%) the IVPD of red beans. The result was in accordance with those of Reyes-Bastidas et al. (2010) who reported that common beans by SSF with R. oligosporus significantly increased their IVPD. The increase in the IVPD of fermented products might be explained by the opening of proteins during SSF, facilitating easier access of the proteases (Angulo-Bejarano et al. 2008). It was reported by Ghumman et al. (2016a) that low IVPD might be due to the inhibition of protein hydrolysis by anti-nutritional factors (e.g., protease inhibitors and lectins) which presented in pulses. SSF could eliminate the anti-nutritional factors of pulses and thus improved their IVPD (Reyes-Bastidas et al. 2010). Furthermore, Rochín-Medina et al. (2015) also demonstrated that the improvement in IVPD could be associated with the elimination of anti-nutritional factors (e.g., hydrolysis of phytic acid) and protein denaturation during SSF, which making proteins more accessible and easier to enzyme action.
Physicochemical and functional properties of the FRB and RB flours
Bulk density
Cordyceps militaris fermentation significantly decreased the bulk density of red bean flour by about 19.72% (Table 3). Similar observations of lower bulk density after fermentation were reported by Elkhalifa et al. (2005) for sorghum flour and by Akubor and Chukwu (1999) for African oil bean seed flour. It has been reported in literatures that fermentation is a very useful and economic approach for processing low-bulk weaning foods (Akubor and Chukwu 1999). Therefore, the decrease in bulk density of fermented red bean flour would benefit the preparation of infant foods or weaning food formulations.
Table 3.
Physicochemical and functional properties of C. militaris fermented and non-fermented red bean flours
| Property | Non-fermented red bean | C. militaris fermented red bean |
|---|---|---|
| Bulk density (g/mL) | 0.71 ± 0.01a | 0.57 ± 0.01b |
| Fat absorption capacity (%) | 84.65 ± 3.09b | 114.55 ± 3.00a |
| Water holding capacity (mL/g) | 2.36 ± 0.03b | 2.59 ± 0.07a |
| Emulsion activity (%) | 10.96 ± 2.54b | 52.77 ± 2.19a |
| Emulsion stability (%) | 5.43 ± 0.89b | 53.82 ± 2.45a |
| Foaming capacity (%) | 16.98 ± 2.32a | 15.60 ± 3.36a |
| Foaming stability (%) | 11.95 ± 1.57b | 20.68 ± 4.47a |
Values are mean ± SD (n = 3). Different small letters in the same row denote statistical difference (p < 0.05)
FAC and WHC
Cordyceps militaris fermentation significantly increased the FAC of red bean flour (Table 3) by 29.91%. This result is consistent with that reported by Elkhalifa et al. (2005) for sorghum flour. Kaur and Singh (2005) have reported that the surface availability of hydrophobic amino acids in the sample could positively affect their binding of fat. Hence, enhancement of FAC in the fermented samples could be explained by the increase in the availability of these hydrophobic amino acids by unfolding the non-polar residues from the interior protein molecules. Shevkani et al. (2015b) have also reported that the higher FAC of the sample might be attributed to the greater availability of hydrophobic/non-polar amino acids which could react with hydrocarbon chains of the fats. FAC is attributed to the physical entrapment of fat; this notion is important because fat acts as a flavor retainer and enhances the mouth feel of food (Elkhalifa and Bernhardt 2010). High FAC of FRB implies that the flour would be very useful in food applications (e.g., doughnuts and pancakes), in which fat holding capacity is required (Elkhalifa et al. 2005).
The WHC of food formulations is an essential quality parameter for practical applications. Fermentation also significantly (p < 0.05) increased (by almost 9.75%) the WHC of red bean flour (Table 3). In literatures, WHCs of lentil, cowpea, sorghum, oil bean, and bengal gram were also reported to be increased by fermentation (Akubor and Chukwu 1999; Elkhalifa and Bernhardt 2010). Ghumman et al. (2016b) have stated that the increase in WHC may be due to the increase in number of polar protein groups and the increase in low molecular weight proteins during the bioprocess. Besides, Akubor and Chukwu (1999) reported that the breaking of peptide bonds as a result of proteolytic activity during fermentation may produced an increased number of polar groups and, thus, increase protein hydrophilicity. Therefore, high WHC of FRB may be attributed to the enhanced hydrophilic properties of the flour proteins and the superior water holding performance of polar amino acid side chains.
EA and ES
One of the most important functional properties of food ingredients is the emulsifying properties (i.e., EA and ES) (Shevkani et al. 2014). The EA generally associates with the ability and capacity of proteins to adsorb on the interface area of oil and water in an emulsion whereas ES depends on to the properties of this adsorbed layer (Shevkani et al. 2015a). As shown in Table 3, fermentation significantly increased (p < 0.05) the EA and ES of red bean flour. The EA and ES of FRB were 52.77 and 53.82%, respectively, which are significantly higher than that of RB. This may be due to the differences in the amount and composition of the proteins of FRB and RB induced by SSF. Shevkani et al. (2015a) stated that protein-hydrophobicity is an important factor which could determine the initial adsorption of proteins, and thus affect their EA and ES. Elkhalifa and Bernhardt (2010) reported that the bioprocess may induce the dissociation and partial opening of polypeptides, resulting in the exposure of hydrophobic amino acid residues, which consequently enhanced the adsorption and surface activity at the water and oil interface. As a result, a much greater protein surface area was rendered available, and EA was enhanced. In addition, Ghumman et al. (2016a) have reported that EA related with the surface charge/zeta potential of protein. SSF might enhance the surface charge/zeta potential of red bean protein, which might contribute to the higher EA as compared to non-fermented sample. Moreover, fermentation produced low molecular mass peptides which tend to easily migrate into the oil–water interface, and thus contribute to the improvement of EA (Lim et al. 2010). Therefore, fermentation may result in partial change of protein characteristics and improve the EA due to increased hydrophobicity of proteins, suggesting a positive relationship between EA and surface hydrophobicity. In addition, ES could also be improved by the absorption of more flexible peptide molecules which play an important role in its ES (Shevkani et al. 2015a). Thus, some of the hydrophilic and flexible proteins that formed during the SSF may have induced the increase in ES of the red bean flour.
FC and FS
FC and FS are important functional properties of food ingredients that determine their application in food systems, where aeration and overrun is an important consideration e.g., baked foods, whipped toppings and ice-cream mixes (Shevkani et al. 2015a). The FC of RB and FRB showed no significant difference, but fermentation significantly enhanced (by almost 8.73%) the FS relative to that of the RB (Table 3). FRB achieved a higher FS compared with that of RB, a result probably correlated to the high adsorption and surface activities of soluble proteins in the formed liquid phases (Kaur and Singh 2005). Elkhalifa and Bernhardt (2010) reported that conformational changes occurring during bioprocessing of the proteins may influence the FS of cereal flours. This notion may partially explain the higher FS of the FRB flour. Therefore, the increased FS might be attributed to the structural changes of protein during SSF, which tends to enhance the intermolecular interactions and thus enhance the formed viscoelastic film. FS is important property due to the usefulness of whipping agents depends on the agents’ ability to maintain the whipped form as long as possible (Kaur and Singh 2005).
LGC
LGC is generally considered as an important indicator of the gelation ability of food ingredients. A lower LGC of the sample signifies a better capacity to form gels. In the present study, the LGC for FRB was 14%, whereas that for RB was 18%. This finding suggests that the fermentation processes employed to produce the red bean flour significantly improved the flour’s gelling properties. Similarly, Elkhalifa et al. (2005) reported that fermentation significantly decreased the LGC of sorghum flour. Gelation involves the aggregation of denatured molecules. The differences in gelation among the flour samples may be attributed to difference in compositions, such as lipids, carbohydrates, and proteins, in the flours (Kaur and Singh 2005). Fermentation might have denatured the red bean proteins and carbohydrate and thus caused more aggregation than in the RB flour. These results suggested that FRB flour would be a suitable gel-forming or firming agent for food systems, such as snacks and pudding, which require thickening and gelling.
ACE inhibitory activity
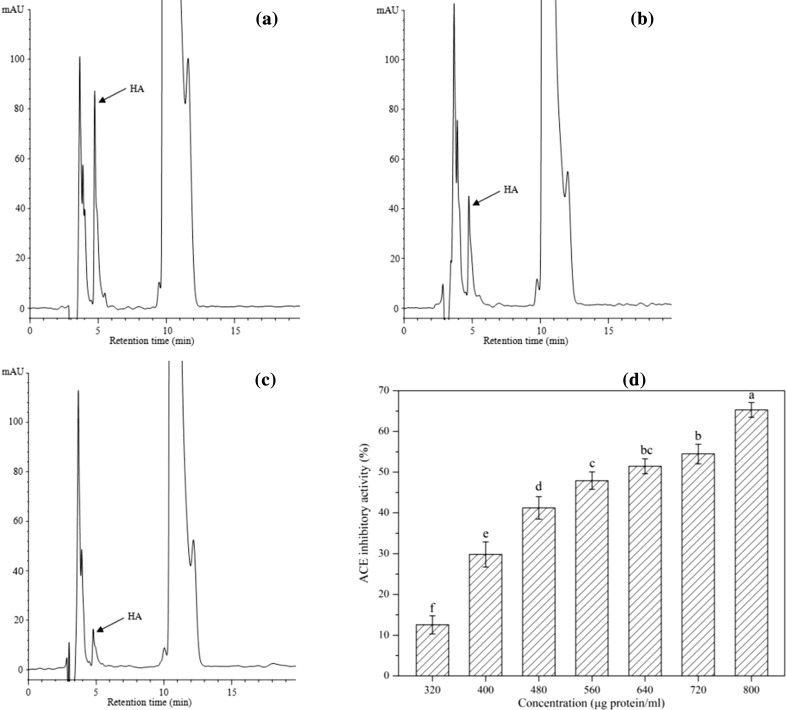
Inhibition of ACE by synthetic or natural inhibitors has been shown to prevent or treat blood pressure in in vivo clinical trials, and their consumption may play an important role in promoting cardiovascular health (Torino et al. 2013). In recent years, increasing attention has been paid to the natural sources of ACE inhibitory peptides which were considered to be green and safe (Rui et al. 2015). Early studies have demonstrated that ACE inhibitory peptides could be produced from the hydrolysates of legume proteins (Pyo and Lee 2007; Rui et al. 2015). The ACE inhibitory activity of the test sample was evaluated by measuring the content of HA released from HHL by the action of ACE. Lower content of liberated HA signifies a stronger ACE inhibitory activity of the test sample (Xiao et al. 2015). The amount of HA released in the presence of FRB (Fig. 3b) is significantly lower (p < 0.05) than that in the control (Fig. 3a). These results indicate that FRB showed ACE inhibitory activity. The dose-dependent ACE inhibitory activity of FRB is shown in Fig. 3d. The IC50 value (half maximal inhibitory concentration) was 0.63 mg protein/mL. The results imply that the C. militaris used in the present study contributed to the production of potential ACE inhibitors during red bean fermentation. However, the released HA for RB was in accordance with the result observed in Fig. 3a (data not shown), which indicated that RB did not exhibit ACE inhibitory activity.
Fig. 3.
ACE inhibitory activity of C. militaris fermented red bean. HPLC chromatogram of hippuric acid (HA) released without red bean protein extract (a), in the presence of C. militaris fermented red bean protein extract (b), and in the blank, which did not contain an ACE (c). Dose-dependent ACE inhibitory activities of the C. militaris fermented red bean protein extract (d). Values were presented as mean of triplicate analysis
Similar results were obtained by Rui et al. (2015) and Xiao et al. (2015), who reported that navy bean and chickpea showed no ACE inhibitory activity, whereas the corresponding fermented products exhibited ACE inhibitory activity. Accordingly, these findings revealed that ACE inhibitory peptides were liberated from the legume protein during microbial fermentation. Protein hydrolysis occurs throughout the fermentation process, and bioactive peptides with ACE inhibitory activity may be liberated (Torino et al. 2013). Small peptides are generally considered as the main substance that exhibits ACE inhibitory activity; the proteolysis of protein has been suggested as a primary cause leading to ACE inhibitory activity in fermented food (Pyo and Lee 2007). Therefore, the peptides formed from the hydrolytic action of proteases which produced by the starter microorganism afforded the FRB with ACE inhibitory activity. However, the ACE inhibitory effect was different for the variant materials and microorganisms employed in the study. For example, Pyo and Lee (2007), Torino et al. (2013), and Juan et al. (2010) demonstrated the ACE inhibitory activity of Monascus fermented soybean, Lactobacillus plantarum fermented lentil, and Bacillus spp. fermented black soybeans, with IC50 of 0.29, 0.20, and 1.81–2.35 mg/mL, respectively. The results suggest that FRB could be used as nutraceuticals or physiological functional food supplements for hypertensive therapy. Besides, further research is needed for the purification and identification of bioactive peptides in FRB, evaluation of the ACE inhibitory activity by in vivo studies, and determination of the peptides’ health-stimulating effects in the human body.
Conclusion
This study demonstrated that C. militaris fermentation can be employed to improve the nutritional, physicochemical, and functional properties as well as the biological activity of red bean flour. Fermentation caused remarkably beneficial changes in the protein composition of red bean. The increases in contents of protein, total amino acids, and essential amino acids, as well as the in vitro protein digestibility of red bean, were observed as a result of fermentation. SSF involved C. militaris, which degraded the red bean proteins and released some new small peptides. The FAC, WHC, EA, ES, and FS of the red bean flour were increased by SSF. In addition, fermentation significantly decreased the bulk density and LGC of red bean flour. Furthermore, FRB demonstrated ACE inhibitory activity for the generated low-molecular-weight peptides during SSF. Thus, FRB can be used as a new functional food ingredient in widely consumed fabricated foods, such as weaning foods and baked goods (bread and cookies). FRB can also be employed to help design some novel nutritious foods for people suffering from hypertension.
Acknowledgements
This work was co-financed by the National Natural Science Foundation of China (Nos. 31371807, 31201422) and the Jiangsu Provincial Postgraduate Innovation Project of China (No. KYLX15_0594). The authors also thank the Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control.
Abbreviations
- RB
Non-fermented red bean
- FRB
C. militaris-fermented red bean
- SSF
Solid state fermentation
- SDS-PAGE
Sodium dodecyl sulphate polyacrylamide gel electrophoresis
- IVPD
In vitro protein digestibility
- FAC
Fat absorption capacity
- FC
Foaming capacity
- EA
Emulsion activity
- ES
Emulsion stability
- FS
Foaming stability
- LGC
Least gelation concentration
- WHC
Water holding capacity
- ACE
Angiotensin I-converting enzyme
- HA
Hippuric acid
- HHL
Hippuryl-l-histidyl-l-leucine
Contributor Information
Yu Xiao, Email: yuxiao_89@163.com.
Mingsheng Dong, Phone: +86 25 84399090, Email: dongms@njau.edu.cn.
References
- Aguirre L, Garro MS, de Giori GS. Enzymatic hydrolysis of soybean protein using lactic acid bacteria. Food Chem. 2008;111:976–982. doi: 10.1016/j.foodchem.2008.05.018. [DOI] [Google Scholar]
- Akubor P, Chukwu J. Proximate composition and selected functional properties of fermented and unfermented African oil bean (Pentaclethra macrophylla) seed flour. Plant Food Hum Nutr. 1999;54:227–238. doi: 10.1023/A:1008100930856. [DOI] [PubMed] [Google Scholar]
- Ang SK, Shaza EM, Adibah Y, Suraini AA, Madihah MS. Production of cellulases and xylanase by Aspergillus fumigatus SK1 using untreated oil palm trunk through solid state fermentation. Process Biochem. 2013;48:1293–1302. doi: 10.1016/j.procbio.2013.06.019. [DOI] [Google Scholar]
- Angulo-Bejarano PI, Verdugo-Montoya NM, Cuevas-Rodríguez EO, Milán-Carrillo J, Mora-Escobedo R, Lopez-Valenzuela JA, Garzon-Tiznado JA, Reyes-Moreno C. Tempeh flour from chickpea (Cicer arietinum L.) nutritional and physicochemical properties. Food Chem. 2008;106:106–112. doi: 10.1016/j.foodchem.2007.05.049. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 15. Washington: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Boye J, Aksay S, Roufik S, Ribéreau S, Mondor M, Farnworth E, Rajamohamed S. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res Int. 2010;43:537–546. doi: 10.1016/j.foodres.2009.07.021. [DOI] [Google Scholar]
- Chutmanop J, Chuichulcherm S, Chisti Y, Srinophakun P. Protease production by Aspergillus oryzae in solid-state fermentation using agroindustrial substrates. J Chem Technol Biotechnol. 2008;83:1012–1018. doi: 10.1002/jctb.1907. [DOI] [Google Scholar]
- Das SK, Masuda M, Sakurai A, Sakakibara M. Medicinal uses of the mushroom Cordyceps militaris: current state and prospects. Fitoterapia. 2010;81:961–968. doi: 10.1016/j.fitote.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Du SK, Jiang H, Yu X, Jane JL. Physicochemical and functional properties of whole legume flour. LWT Food Sci Technol. 2014;55:308–313. doi: 10.1016/j.lwt.2013.06.001. [DOI] [Google Scholar]
- Elkhalifa AEO, Bernhardt R. Influence of grain germination on functional properties of sorghum flour. Food Chem. 2010;121:387–392. doi: 10.1016/j.foodchem.2009.12.041. [DOI] [Google Scholar]
- Elkhalifa AEO, Schiffler B, Bernhardt R. Effect of fermentation on the functional properties of sorghum flour. Food Chem. 2005;92:1–5. doi: 10.1016/j.foodchem.2004.05.058. [DOI] [Google Scholar]
- Ghumman A, Kaur A, Singh N. Functionality and digestibility of albumins and globulins from lentil and horse gram and their effect on starch rheology. Food Hydrocoll. 2016;61:843–850. doi: 10.1016/j.foodhyd.2016.07.013. [DOI] [Google Scholar]
- Ghumman A, Kaur A, Singh N. Impact of germination on flour, protein and starch characteristics of lentil (Lens culinari) and horsegram (Macrotyloma uniflorum L.) lines. LWT Food Sci Technol. 2016;65:137–144. doi: 10.1016/j.lwt.2015.07.075. [DOI] [Google Scholar]
- Guillon F, Champ MJ. Carbohydrate fractions of legumes: uses in human nutrition and potential for health. Br J Nutr. 2002;88:293–306. doi: 10.1079/BJN2002720. [DOI] [PubMed] [Google Scholar]
- Hong KJ, Lee CH, Kim SW. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J Med Food. 2004;7:430–435. doi: 10.1089/jmf.2004.7.430. [DOI] [PubMed] [Google Scholar]
- Juan MY, Wu CH, Chou CC. Fermentation with Bacillus spp. as a bioprocess to enhance anthocyanin content, the angiotensin converting enzyme inhibitory effect, and the reducing activity of black soybeans. Food Microbiol. 2010;27:918–923. doi: 10.1016/j.fm.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Kaur M, Singh N. Studies on functional, thermal and pasting properties of flours from different chickpea (Cicer arietinum L.) cultivars. Food Chem. 2005;91:403–411. doi: 10.1016/j.foodchem.2004.06.015. [DOI] [Google Scholar]
- Lim JY, Kim JJ, Lee DS, Kim GH, Shim JY, Lee I, Imm JY. Physicochemical characteristics and production of whole soymilk from Monascus fermented soybeans. Food Chem. 2010;120:255–260. doi: 10.1016/j.foodchem.2009.10.017. [DOI] [Google Scholar]
- Ma D, Wang H, Ng T. A peptide with potent antifungal and antiproliferative activities from Nepalese large red beans. Peptides. 2009;30:2089–2094. doi: 10.1016/j.peptides.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Pyo YH, Lee TC. The potential antioxidant capacity and angiotensin I-converting enzyme inhibitory activity of Monascus-fermented soybean extracts: evaluation of Monascus-fermented soybean extracts as multifunctional food additives. J Food Sci. 2007;72:S218–S223. doi: 10.1111/j.1750-3841.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Reyes-Bastidas M, Reyes-Fernandez EZ, Lopez-Cervantes J, Milan-Carrillo J, Loarca-Pina GF, Reyes-Moreno C. Physicochemical, nutritional and antioxidant properties of tempeh flour from common bean (Phaseolus vulgaris L.) Food Sci Technol Int. 2010;16:427–434. doi: 10.1177/1082013210367559. [DOI] [PubMed] [Google Scholar]
- Reyes-Moreno C, Cuevas-Rodríguez EO, Milán-Carrillo J, Cárdenas-Valenzuela OG, Barrón-Hoyos J. Solid state fermentation process for producing chickpea (Cicer arietinum L) tempeh flour. Physicochemical and nutritional characteristics of the product. J Sci Food Agric. 2004;84:271–278. doi: 10.1002/jsfa.1637. [DOI] [Google Scholar]
- Rochín-Medina JJ, Gutiérrez-Dorado R, Sánchez-Magaña LM, Milán-Carrillo J, Cuevas-Rodríguez EO, Mora-Rochín S, Valdez-Ortiz A, Reyes-Moreno C. Enhancement of nutritional properties, and antioxidant and antihypertensive potential of black common bean seeds by optimizing the solid-state bioconversion process. Int J Food Sci Nutr. 2015;66:498–504. doi: 10.3109/09637486.2015.1052377. [DOI] [PubMed] [Google Scholar]
- Rui X, Wen D, Li W, Chen X, Jiang M, Dong M. Enrichment of ACE inhibitory peptides in navy bean (Phaseolus vulgaris) using lactic acid bacteria. Food Funct. 2015;6:622–629. doi: 10.1039/C4FO00730A. [DOI] [PubMed] [Google Scholar]
- Shevkani K, Singh N, Rana JC, Kaur A. Relationship between physicochemical and functional properties of amaranth (Amaranthus hypochondriacus) protein isolates. Int J Food Sci Technol. 2014;49:541–550. doi: 10.1111/ijfs.12335. [DOI] [Google Scholar]
- Shevkani K, Singh N, Kaur A, Rana JC. Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocoll. 2015;43:679–689. doi: 10.1016/j.foodhyd.2014.07.024. [DOI] [Google Scholar]
- Shevkani K, Kaur A, Kumar S, Singh N. Cowpea protein isolates: functional properties and application in gluten-free rice muffins. LWT Food Sci Technol. 2015;63:927–933. doi: 10.1016/j.lwt.2015.04.058. [DOI] [Google Scholar]
- Song YS, Frías J, Martinez-Villaluenga C, Vidal-Valdeverde C, de Mejia EG. Immunoreactivity reduction of soybean meal by fermentation, effect on amino acid composition and antigenicity of commercial soy products. Food Chem. 2008;108:571–581. doi: 10.1016/j.foodchem.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Sparringa R, Owens J. Protein utilization during soybean tempe fermentation. J Agric Food Chem. 1999;47:4375–4378. doi: 10.1021/jf981279u. [DOI] [PubMed] [Google Scholar]
- Sridaran A, Karim AA, Bhat R. Pithecellobium jiringa legume flour for potential food applications: studies on their physico-chemical and functional properties. Food Chem. 2012;130:528–535. doi: 10.1016/j.foodchem.2011.07.062. [DOI] [Google Scholar]
- Tang CH, Sun X. Structure–physicochemical function relationships of 7S globulins (vicilins) from red bean (Phaseolus angularis) with different polypeptide constituents. Food Hydrocoll. 2011;25:536–544. doi: 10.1016/j.foodhyd.2010.08.009. [DOI] [Google Scholar]
- Tiwari BK, Gowen A, McKenna B, editors. Pulse foods: processing, quality and nutraceutical applications. London: Academic Press; 2011. [Google Scholar]
- Torino MI, Limón RI, Martínez-Villaluenga C, Mäkinen S, Pihlanto A, Vidal-Valverde C, Frias J. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem. 2013;136:1030–1037. doi: 10.1016/j.foodchem.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Wani IA, Sogi DS, Wani AA, Gill BS. Physico-chemical and functional properties of flours from Indian kidney bean (Phaseolus vulgaris L.) cultivars. LWT Food Sci Technol. 2013;53:278–284. doi: 10.1016/j.lwt.2013.02.006. [DOI] [Google Scholar]
- Wani IA, Wani AA, Gani A, Muzaffar S, Gul MK, Masoodi FA, Wani TA. Effect of gamma-irradiation on physico-chemical and functional properties of arrowhead (Sagittaria sagittifolia L.) tuber flour. Food Biosci. 2015;11:23–32. doi: 10.1016/j.fbio.2015.04.003. [DOI] [Google Scholar]
- Xiao Y, Xing G, Rui X, Li W, Chen X, Jiang M, Dong M. Enhancement of the antioxidant capacity of chickpeas by solid state fermentation with Cordyceps militaris SN-18. J Funct Food. 2014;10:210–222. doi: 10.1016/j.jff.2014.06.008. [DOI] [Google Scholar]
- Xiao Y, Xing G, Rui X, Li W, Chen X, Jiang M, Dong M. Effect of solid-state fermentation with Cordyceps militaris SN-18 on physicochemical and functional properties of chickpea (Cicer arietinum L.) flour. LWT Food Sci Technol. 2015;63:1317–1324. doi: 10.1016/j.lwt.2015.04.046. [DOI] [Google Scholar]
- Xiao Y, Huang L, Chen Y, Zhang S, Rui X, Dong M. Comparative study of the effects of fermented and non-fermented chickpea flour addition on quality and antioxidant properties of wheat bread. CyTA J Food. 2016;14:621–631. [Google Scholar]
- Yousif NE, El Tinay AH. Effect of fermentation on protein fractions and in vitro protein digestibility of maize. Food Chem. 2000;70:181–184. doi: 10.1016/S0308-8146(00)00069-8. [DOI] [PubMed] [Google Scholar]
- Yu T, Ahn HM, Shen T, et al. Anti-inflammatory activity of ethanol extract derived from Phaseolus angularis beans. J Ethnopharmacol. 2011;137:1197–1206. doi: 10.1016/j.jep.2011.07.048. [DOI] [PubMed] [Google Scholar]