Abstract
The particle size distribution, protein profile, pasting and dough rheological properties of meal from forty-two Indian durum wheat accessions were evaluated. Meal from accessions with higher grain hardness index (GHI) showed a high proportion of large size particles with higher protein content and lower paste viscosities. Elastic and viscous modulii (G′ and G″) of dough were negatively correlated with paste viscosities, which was associated with the presence/absence of LMW-GS and HMW-GS. Wheat accessions with allelic combinations of (13 + 16) with 97 + 91 kDa polypeptides (PPs) had higher G′ and G″. The accession with 35 kDa PP showed higher while those with 35 and 62 kDa PPs showed lower paste viscosity. Among all accessions, 25 accessions possess 7 + 8 (97 and 88 kDa) type HMW-GS allelic combination. Durum accessions with diverse GHI, particle size distribution, protein profile, paste and dough rheology indicates their variation in milling and processing behaviour.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3036-y) contains supplementary material, which is available to authorized users.
Keywords: Wheat, Pasting, Rheology, Particle size, SDS-PAGE
Introduction
Durum wheat (Triticum durum) is the most important part of diet of the Mediterranean people. The production of durum in India is standing second largest after bread wheat and the annual Indian durum wheat production is nearly 2.5 million tons per year. Morris (2002) reported that hardness of grain, an important factor in establishing the end use of wheat. Gazza et al. (2011) reported the effect of puroindolines on breadmaking quality in both common and durum wheat. Proteins and starch are the main constituents of durum wheat flour, the suitability and end use of durum wheat are greatly influenced by the composition, quantity, endosperm storage proteins and starches (Du Cros 1987). Various studies showed that quality of proteins and gluten quantity, formation of polymeric network of proteins and gelatinization of starches determines the end quality of durum products (Novaro et al. 1993). Dick and Youngs (1988) demonstrated that not only biochemical but the physical characteristics such as kernel size, kernel weight, grain hardness and vitreousness degree also affect the physicochemical, textural and sensory attributes of durum wheat directly or indirectly (Troccoli et al. 2000). Dynamic oscillatory measurement involving small deformation is an important approach and is being preferred for studying the structural and fundamental properties of wheat flour dough (Song and Zheng 2007). The Indian durum wheat improvement programme achieved the goal of the development of new durum accessions, which are dwarf in nature; contain a high degree of field resistance to rust and Karnal bunt. These varieties were developed according to the diverse Indian agro climatic zones and are having improved grain quality, high yielding and having increased adaptability and gaining popularity in Indian farmers. Durum wheat has shown the narrower adaptation, yield fluctuations over varying environments as compared to common wheat (Saini and Gautam 1990) and resulted in a challenge for durum wheat producers to chase the demands of very high quality standards imposed by millers, bakers and consumers of the international market. Sasaki et al. (2008) determined viscoelastic properties of flours, starch and gluten-starch mixture with varying amylose content. The objective of present study was to evaluate various durum wheat accessions for grain hardness, pasting, mixographic and rheological properties along with gliadins and glutenins analysis to understand the improvement in present day wheat for diverse uses.
Materials and methods
Materials
Durum wheat accessions namely, EC445268, EC445094, EC444996, EC445030, EC445308, EC445182, EC445070, EC445377, EC445203, IC335732, IC335735, EC445177, EC534549, EC277348, IC252912, EC576895, EC519488, EC276668, EC374955, EC577687, IC335829, IC75209, IC543401, IC335620, IC539641, EC445018, IC252906, IC549340, EC574400, EC577473, EC299141, EC277127, EC577467, EC575770, IC532026, IC576640, DWR1006, IC444777, IC75208, EC296359, IC416334 and UAS415 were procured from NBPGR, New Delhi. These germplasm lines included 16 indigenous and 24 exotic lines from Mexico, United States of America, Syria, Israel and Wales. The indigenous lines comprised of germplasm collections, breeding material and old cultivars from UAS, Dharwad; IIWBR, Karnal; PAU, Ludhiana and IARI, Pune.
Methods
Grain characteristics
Various grain characteristics were determined as described earlier by Kaur et al. (2015).
Wheat milling
Wheat grains were milled into meal by using Newport Scientific super mill and pass though a 60-mesh sieve for further analyses.
Flour characteristics
Protein content of meal was determined using AOAC methods (1990).
Particle size distribution
Particle size analyses of meal were determined by using a Microtrac S3500 Turbotrac Particle size analyzer (Microtrac Inc. USA).
SDS-PAGE analysis
Gliadins and glutenins of meal were extracted from different Indian durum wheat accessions as described earlier by Kaur et al. (2015).
Pasting properties
Pasting properties of meal from different durum wheat accessions were determined using a rheometer (MCR-301, Anton Paar, Austria) as described earlier (Kaur et al. 2014). Parameters evaluated were pasting temperature (PT), final viscosity (FV), peak viscosity (PV), setback viscosity (SBV) and breakdown viscosity (BDV).
Mixographic characteristics
Dough mixing properties were analysed using Mixograph as described earlier (Kaur et al. 2014). Various mixographic parameters evaluated were weakening slope (WS), mixograph peak time (MPT), mixograph peak width (MPW) and mixograph peak value (MPV).
Dynamic rheometry of dough
Dynamic rheology of dough was performed using a RheoStress 6000 (Haake, Karlsruhe, Germany) as described earlier by Kaur et al. (2013).
Statistical analysis
The data reported are an average of three replications. Pearson correlation (r) was carried out for determining the relationship between different variables using Minitab Release 14 Statistical Software (Soft College, PA, USA). PCA results were graphically represented using the same software.
Result and discussion
Grain characteristics
GD and TGW of meal milled from different durum wheat accessions ranged from 2.61 to 3.35 mm and 32.38 to 54.76 g, respectively (Table 1). The lowest and the highest value of TGW and GD were observed for IC576640 and EC445268. A strong positive correlation was observed between TGW and GD (r = 0.937, p ≤ 0.005). Earlier similar correlation between TGW and GD were reported for French durum wheat varieties (Raggiri et al. 2016). GHI, an indication of resistance to fracture of different durum wheat accessions ranged from 33 to 111. Grain hardness was reported to be an important parameter for defining grain quality and attributed to the distinctive genetic makeup of grains (Morris et al. 2011). IC335732, EC335735 and IC576640 showed lower GHI (33–35) and were softer in texture. Murray et al. (2017) termed durum wheat varieties with GHI between 19.4 and 40 as soft durum. While EC445094, EC405070 and EC25291 showed higher GHI (108–111). The two genes (Puroindoline a and b) resides on the 5D chromosome were absent in durum wheat accessions contributing to hard texture of grains (Li et al. 2014).
Table 1.
Physical properties of grains from different durum wheat accessions
| Sample | GHI | TGW (g) | GD (mm) |
|---|---|---|---|
| EC445268 | 102 ± 2.00e | 54.76 ± 2.26e | 3.35 ± 0.3e |
| EC445094 | 111 ± 3.53g | 34.14 ± 1.07b | 2.84 ± 0.1b |
| EC444996 | 96 ± 1.02d | 35.09 ± 1.45b | 2.85 ± 0.1b |
| EC445030 | 103 ± 1.00e | 32.40 ± 1.45ab | 2.79 ± 0.1b |
| EC445308 | 95 ± 2.00d | 38.12 ± 1.22b | 2.91 ± 0.01b |
| EC445182 | 95 ± 1.02d | 39.31 ± 1.66bc | 3.01 ± 0.01bc |
| EC445070 | 108 ± 2.00f | 33.91 ± 1.44ab | 2.77 ± 0.03ab |
| EC445377 | 95 ± 1.01d | 40.91 ± 1.69bc | 2.99 ± 0.05bc |
| EC445203 | 101 ± 1.00e | 32.31 ± 1.11ab | 2.77 ± 0.20ab |
| IC335732 | 34 ± 1.00a | 37.93 ± 1.68b | 2.85 ± 0.04b |
| IC335735 | 34 ± 2.02a | 37.76 ± 1.58b | 2.82 ± 0.02ab |
| EC445177 | 89 ± 2.01c | 38.56 ± 1.88b | 2.97 ± 0.02bc |
| EC534549 | 83 ± 2.01b | 35.83 ± 1.29b | 2.88 ± 0.02b |
| EC277348 | 92 ± 2.01c | 48.56 ± 2.16d | 3.19 ± 0.02cd |
| IC252912 | 108 ± 1.00f | 46.51 ± 2.29cd | 3.26 ± 0.04d |
| EC576895 | 93 ± 1.51cd | 30.66 ± 1.97a | 2.64 ± 0.10a |
| EC519488 | 85 ± 2.01b | 42.30 ± 2.07bc | 3.03 ± 0.03c |
| EC276668 | 98 ± 2.01d | 42.34 ± 2.06bc | 3.10 ± 0.02c |
| EC374955 | 99 ± 3.00d | 32.62 ± 1.79ab | 2.73 ± 0.03ab |
| EC577687 | 90 ± 2.01c | 33.13 ± 1.88ab | 2.85 ± 0.10b |
| IC335829 | 97 ± 1.01d | 39.84 ± 1.92bc | 3.05 ± 0.02c |
| IC75209 | 97 ± 2.00d | 44.09 ± 2.32c | 3.14 ± 0.02c |
| IC543401 | 94 ± 2.00cd | 39.11 ± 2.01bc | 3.07 ± 0.03c |
| IC335620 | 97 ± 1.54d | 44.28 ± 2.44c | 3.14 ± 0.09c |
| IC539641 | 84 ± 1.00bb | 42.76 ± 2.24bc | 3.01 ± 0.01bc |
| EC445018 | 103 ± 1.04e | 34.01 ± 1.00ab | 2.78 ± 0.10ab |
| IC252906 | 96 ± 1.00d | 41.41 ± 1.83bc | 3.15 ± 0.05c |
| IC549340 | 107 ± 2.01f | 37.18 ± 1.62b | 2.90 ± 0.02bc |
| EC574400 | 35 ± 0.14a | 40.10 ± 2.12bc | 2.97 ± 0.01bc |
| EC577473 | 90 ± 1.02c | 37.58 ± 1.90b | 2.98 ± 0.01bc |
| EC299141 | 91 ± 1.01c | 37.65 ± 1.73b | 3.00 ± 0.01bc |
| EC277127 | 98 ± 2.00d | 45.57 ± 2.14c | 3.15 ± 0.03c |
| EC577467 | 100 ± 1.00de | 41.60 ± 2.2bc | 3.03 ± 0.03bc |
| EC575770 | 105 ± 2.01e | 35.51 ± 1.48ab | 2.87 ± 0.02b |
| IC532026 | 100 ± 1.01de | 41.12 ± 2.3bc | 3.06 ± 0.02c |
| IC576640 | 33 ± 1.02a | 32.38 ± 1.78ab | 2.61 ± 0.01a |
| DWR1006 | 92 ± 2.00c | 38.81 ± 1.12b | 2.95 ± 0.04bc |
| IC444777 | 104 ± 2.00e | 43.93 ± 2.49c | 3.15 ± 0.01c |
| IC75208 | 94 ± 1.01cd | 36.79 ± 1.55ab | 2.96 ± 0.02bc |
| EC296359 | 99 ± 1.03d | 47.13 ± 2.25cd | 3.24 ± 0.02d |
| IC416334 | 92 ± 2.01c | 31.93 ± 1.35ab | 2.83 ± 0.03ab |
| UAS415 | 98 ± 2.00d | 38.87 ± 1.65b | 3.00 ± 0.07bc |
| LSD | 2.71 | 2.95 | 0.12 |
Data represented as mean value ± SD. Means with similar superscripts in a column do not differ significantly (p ≤ 0.05)
GHI grain hardness index, TGW thousand grain weight, GD grain diameter
Flour characteristics
Protein content
Meal milled from various Indian durum wheat accessions showed protein content between 8.38 and 13.89% (Table 2). The lowest and the highest value were observed for EC534549 and EC445018, respectively. Rharrabti et al. (2003) reported that the protein content ranged from 13.1 to 16.5%, for durum wheat accessions. Katyal et al. (2016) and Singh et al. (2011) reported the protein content in different Indian wheat accessions ranged from 8.89 to 12.77% and 8.26 to 12.85%. Most of the accessions in present study showed accumulation of average protein content ≥ 10% indicating their suitability for noodles and pasta production. Kaur et al. (2015) reported protein content of durum wheat accessions between 11.66 and 15.13%. The differences in the composition and protein characteristics led to the alteration in noodle-making properties of flours from durum wheat accessions (Novaro et al. 1993). Baasandroj et al. (2015) reported that an increase in protein content of flour with increase in GHI, consistent with present results.
Table 2.
Protein content and particle size distribution of meal from different durum wheat accessions
| Sample | Protein content (%) | Large size particles (> 105 µm) | Medium size particles (55–105 µm) | Small size particles (< 55 µm) |
|---|---|---|---|---|
| EC445268 | 12.06 ± 0.04j | 47.16 ± 2.03de | 13.04 ± 0.6cd | 39.8 ± 1.14c |
| EC445094 | 11.82 ± 0.10ij | 49.14 ± 2.16de | 12.00 ± 0.5c | 38.86 ± 1.26bc |
| EC444996 | 11.66 ± 0.30ij | 50.81 ± 2.16de | 12.17 ± 0.7c | 37.02 ± 1.29bc |
| EC445030 | 12.61 ± 0.20l | 54.67 ± 2.26ef | 10.49 ± 0.5ab | 34.84 ± 1.12b |
| EC445308 | 12.22 ± 0.10jk | 50.60 ± 2.09de | 12.17 ± 0.2c | 37.23 ± 1.32bc |
| EC445182 | 9.98 ± 0.01e | 52.13 ± 2.39e | 10.06 ± 0.02ab | 37.81 ± 1.26bc |
| EC445070 | 12.38 ± 0.30k | 48.13 ± 1.98de | 11.76 ± 0.2bc | 40.11 ± 1.59c |
| EC445377 | 8.86 ± 0.10c | 41.58 ± 1.87c | 10.92 ± 0.26b | 47.50 ± 1.68de |
| EC445203 | 9.42 ± 0.31d | 48.62 ± 1.99de | 11.24 ± 0.46b | 40.14 ± 1.59c |
| IC335732 | 10.86 ± 0.11gh | 34.67 ± 1.76ab | 10.90 ± 0.47b | 54.43 ± 2.19ef |
| IC335735 | 11.90 ± 0.05j | 31.48 ± 1.46a | 11.07 ± 0.59b | 57.45 ± 2.48f |
| EC445177 | 12.06 ± 0.02j | 48.96 ± 2.48de | 11.73 ± 0.59bc | 39.31 ± 1.74c |
| EC534549 | 8.38 ± 0.30b | 46.49 ± 2.39d | 9.65 ± 0.36a | 43.86 ± 2.09d |
| EC277348 | 10.38 ± 0.02fg | 47.22 ± 2.47d | 11.53 ± 0.49bc | 41.25 ± 2.18cd |
| IC252912 | 11.90 ± 0.02j | 50.20 ± 2.79de | 12.12 ± 0.75c | 37.68 ± 1.97bc |
| EC576895 | 10.70 ± 0.05g | 42.57 ± 1.94c | 11.90 ± 0.67bc | 45.53 ± 2.28de |
| EC519488 | 11.02 ± 0.02h | 38.67 ± 1.68bc | 11.75 ± 0.2bc | 49.58 ± 2.39e |
| EC276668 | 11.10 ± 0.01h | 42.25 ± 1.87c | 12.58 ± 0.3cd | 45.17 ± 2.19de |
| EC374955 | 12.77 ± 0.15l | 48.81 ± 1.76de | 10.83 ± 0.1b | 40.30 ± 2.29cd |
| EC577687 | 11.58 ± 0.4i | 49.14 ± 1.96de | 13.49 ± 0.5d | 37.37 ± 1.79bc |
| IC335829 | 7.98 ± 0.01a | 35.98 ± 1.48ab | 14.57 ± 0.74de | 49.45 ± 2.49e |
| IC75209 | 9.66 ± 0.2de | 48.68 ± 1.82de | 13.14 ± 0.68cd | 38.18 ± 1.47bc |
| IC543401 | 9.42 ± 0.10d | 31.28 ± 1.64a | 11.21 ± 0.29b | 57.71 ± 2.49f |
| IC335620 | 9.90 ± 0.05e | 47.43 ± 1.79d | 10.70 ± 0.19ab | 41.87 ± 2.18cd |
| IC539641 | 10.54 ± 0.20g | 50.03 ± 2.08de | 11.44 ± 0.33bc | 38.53 ± 1.99bc |
| EC445018 | 13.89 ± 0.10m | 52.68 ± 2.12e | 12.33 ± 0.42c | 34.99 ± 1.05b |
| IC252906 | 11.02 ± 0.01h | 50.47 ± 2.09de | 9.89 ± 0.17a | 39.64 ± 1.49c |
| IC549340 | 10.30 ± 0.01f | 58.07 ± 2.26f | 11.14 ± 0.36b | 30.79 ± 1.16ab |
| EC574400 | 8.62 ± 0.20bc | 36.57 ± 1.59b | 13.15 ± 0.86cd | 50.28 ± 2.07e |
| EC577473 | 8.94 ± 0.03c | 34.50 ± 1.49ab | 9.34 ± 0.26a | 56.16 ± 2.19f |
| EC299141 | 10.62 ± 0.30g | 46.15 ± 2.13d | 11.22 ± 0.4b | 42.63 ± 1.91cd |
| EC277127 | 12.30 ± 0.20k | 57.23 ± 2.48f | 14.89 ± 0.9e | 27.88 ± 1.24a |
| EC577467 | 10.06 ± 0.03ef | 45.41 ± 1.93cd | 12.79 ± 0.2cd | 41.80 ± 2.16cd |
| EC575770 | 10.46 ± 0.20fg | 35.27 ± 1.29ab | 11.24 ± 0.4b | 53.49 ± 2.39ef |
| IC532026 | 9.90 ± 0.01e | 41.98 ± 1.79c | 11.28 ± 0.2b | 46.74 ± 2.03de |
| IC576640 | 11.66 ± 0.02ij | 30.78 ± 1.48a | 13.56 ± 0.3d | 55.66 ± 2.38f |
| DWR1006 | 10.06 ± 0.02ef | 45.19 ± 2.16cd | 10.36 ± 0.3ab | 44.45 ± 1.72de |
| IC444777 | 11.58 ± 0.10i | 45.79 ± 1.72cd | 13.38 ± 0.3d | 40.83 ± 1.88cd |
| IC75208 | 10.54 ± 0.02g | 51.42 ± 2.11de | 9.70 ± 0.3a | 38.88 ± 1.27bc |
| EC296359 | 11.66 ± 0.03ij | 46.49 ± 1.49d | 9.32 ± 0.6a | 44.19 ± 1.46de |
| IC416334 | 8.94 ± 0.03c | 39.73 ± 1.29bc | 9.44 ± 0.4a | 50.83 ± 2.55e |
| UAS415 | 10.30 ± 0.02ef | 45.97 ± 1.84cd | 12.04 ± 0.04c | 41.99 ± 2.04cd |
| LSD | 0.24 | 3.16 | 0.74 | 3.05 |
Data represented as mean value ± SD. Means with similar superscripts in a column do not differ significantly (p ≤ 0.05)
Particle size distribution
The particle size analysis of meal milled from different durum wheat accessions showed bi-modular behavior. The proportions of particles of small, medium and large size ranged from 27.88 to 57.71, 9.32 to 14.89, and 30.78 to 58.07 μm, respectively (Table 2). The large size particles constitute the major proportions followed by small and medium size particles. Large size particles showed positive correlation (r = 0.402, p ≤ 0.005) while small size particles had negative correlation (r = −0.434, p ≤ 0.005) with protein content. Results indicated that accessions with higher GHI had higher protein content that milled into meal with lower proportion of small size and higher of large size particles. The results clearly reflected that durum wheat accessions with high GHI would give high recovery of coarse semolina, primarily used in the production of pasta. Particle size distribution greatly affected by GHI and contributed to the functionality and baking quality of flours (Galliard and Gallagher 1988). GHI showed negative correlation with small size particles (r = −0.576, p ≤ 0.005) and positive with large size particles (r = 0.600, p ≤ 0.005) ((Supplementary Table 1). Kaur et al. (2014) showed similar correlations of flours milled from different durum wheat varieties. Earlier studies have reflected that durum wheat endosperm is very brittle and contributed to the high level of virtuousness that consequently fragmented spontaneously during milling (Haraszi et al. 2016).
SDS-PAGE analysis of gliadins and glutenins
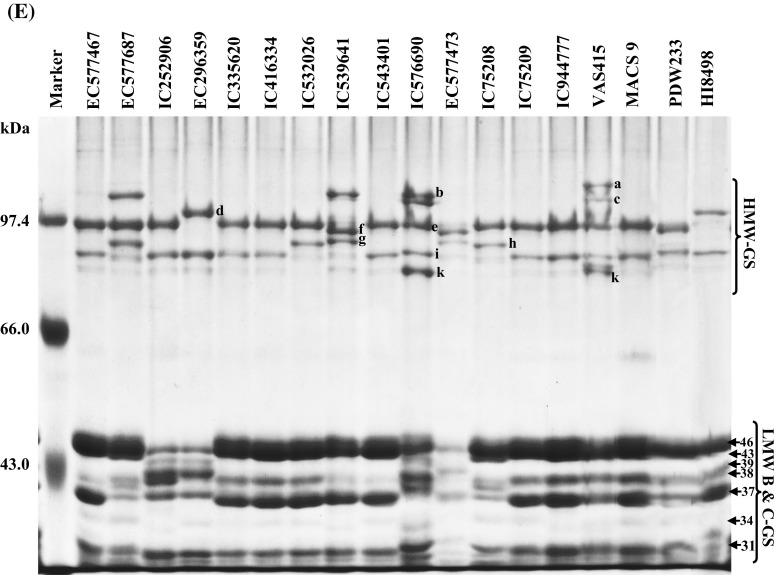
SDS-PAGE analysis revealed differential accumulation of different polypeptide (PPs) of gliadins and glutenins and storage of these PPs was varietal dependent. Gliadins showed the presence of 18 PPs with molecular weight ranged from 28 to 88 kDa (± 2 kDa) (Fig. 1a–c). MACS9, PDW233, and HI8498 were included in the analysis as standard with known PPs architecture, for the comparison of gliadins and glutenins in different accessions. The accumulation of PPs ranged from 43 to 88 kDa and varied significantly. The storage of 88 kDa PP was least or was absent in DWR1006, EC299141, EC335735, EC335829, EC519488, EC534549, EC574400, IC252906, EC296359, IC576690 and EC577473 (Fig. 1a, b). The PPs of 61 kDa was also least accumulated in EC445377 and EC574400 and IC75208, whereas, EC519488, EC534549 and EC575770 showed a doublet of PPs of 59 and 61 kDa instead of a 60 kDa PP (Fig. 1a). On the contrary, the accumulation of 60 kDa was higher in IC252906 and EC296359 (Fig. 1b). 43 kDa PP was observed only in EC299141. However, 39, 34, 32, 31, 30 and 28 kDa PPs were present in all durum accessions but accumulation of these PPs slightly varied. Higher accumulation of 37 kDa PP in EC519488, EC534549, EC575770, EC576895, EC577687, IC252906, EC296359 and IC75208 was distinguishable from other durum accessions. EC299141 and IC576690, EC577473 and IC75208 showed a different kind of banding pattern for small molecular weight gliadins, which were absent in other accessions. PPs ranged from 88 to 48 kDa were classified, as ω gliadins whereas, PPs ranged between 43 and 28 kDa were known as α-, β-, and γ gliadins. Major variations were observed in the banding pattern of ω gliadins. Earlier studies carried out by Aalami et al. (2007) and Edwards et al. (2007) demonstrated that γ gliadins play a key role in pasta and other products made from durum wheat. Boggini and Pogna (1989) earlier reported that Locus Gli-B1 of bread wheat encode three types of γ-gliadins that determine the strength of dough and gluten based on lower to higher ranking of γ-gliadins (γ-44 > γ-45 > γ-43.5) for durum wheat. These results thus revealed that the polymorphism in γ gliadins in many durum accessions might be associated with varied pasting properties. Glutenins in different durum was ranged from 31 to 113 kDa (± 2 kDa) while HMW-GS and LMW-GS were of 81–113 and 31–46 kDa, respectively. EC299141 and IC539641 showed the accumulation of 110 kDa HMW-GS PPs which was also depicted in IC335732, EC335735, EC335829, EC574400, EC577687 and VAS415 (Fig. 1d, e). HMW-GS of 113 kDa was exclusively depicted in EC335829, EC575770 and VAS415 as compared to other accessions, a rare type of HMW-GS of 113 and 110 kDa with 107 kDa was observed in EC335829 and IC576690, respectively. EC405070, EC445094, EC577687, IC532026 and IC75208 showed accumulation of 97 and 91 kDa HMW-GS. On the contrary, the accumulation of 95 and 92 kDa HMW-GS PPs was depicted in EC299141, EC445268, IC539641 and EC577473 (Supplementary Table 2). Two different types of HMW-GS allelic combinations were also observed in IC335732, EC335735 and EC335829, which showed 97 and 81 kDa PPs instead of 97 and 88 kDa PPs (Fig. 1d). DWR1006, EC335829, EC374955, EC445308, EC575770, EC576895 and EC577467 showed accumulation of 81 kDa HMW-GS PPs. EC374955, EC445308, EC575770, EC296359 and HI8498 showed the presence of 101 and 88 kDa PPs. The intensity of this type of HMW-GS allelic combination was also very low in durum accessions. Among durum wheat accessions, 24 accessions showed HMW-GS allelic combination of 97 kDa + 88 kDa (7 + 8). On the contrary, HI8498, EC374955, EC445308, EC575770 and EC296359 showed the presence of 101w + 88 kDa (6 + 8) HMW-GS combinations along with 113 and 81 kDa PPs. Whereas, five accessions (EC405070, EC445094, EC577687, IC532026 and IC752085) showed the HMW-GS allelic combination of 97 + 91 kDa (13 + 16). The HMW-GS allelic combination of 95 + 92 kDa (13 + 19) was depicted in EC299141, EC445268, IC539641 and EC577473. The HMW-GS allelic combination of 107 + 97 + 83 + 81 appeared to be 2 * (7 + 9) which was depicted in IC335732, EC335735 and EC574400, however the identity of 83 kDa PP, present in these accessions could not be ascertained. LMW-GS in wheat were encoded genes localized and at the Glu-3 loci, tightly associated with Gli-1 loci and affects the end use quality of wheat (Singh and Shepherd 1988; Singh et al. 1991). The genes on Gli-B1 locus, which is localized at the short arm of 1B, control the synthesis of γ-nul, γ-42, γ-44 or γ-45 gliadins, associated with good or poor pasta making qualities of durum. The Gli-B1 locus is firmly associated with Glu-B3 locus in durum wheat (Singh and Shepherd 1988, Nieto-Taladriz et al. 1997). The methodology for extraction of HMW-GS and LMW-GS was according to Singh and Shepherd (1991), whereas, the studies of Nieto-Taladriz et al. (1997) conceived different LMW-GS extraction methodology and resulted in slight variations in the electrophoretic mobility of LMW-GS polypeptide bands. Therefore, the molecular weight of LMW-glutenin-subunits were used for the discussion of the role of LMW-GS in dough pasting and rheological properties. Accumulation of LMW-GS was differential in different durum accessions and LMW-GS PPs ranged from 31 to 46 kDa (Fig. 1d, e). The storage of 46, 43, 39, 38, and 31 kDa PPs varied qualitatively as well as quantitatively and IC335732, EC335735, EC519488, EC534549, EC574400, EC575770, IC252906, EC296359 and EC577473 showed absence or lesser biosynthesis of 46, 43 and 38 kDa PPs (Fig. 1d, e). Whereas, EC299141, EC445094, IC539641, IC543401and EC577473 showed lesser accumulation or absence of 39 kDa PP. On the Contrary, IC335732, EC335735, EC574400, EC575770, EC577687, IC252906, IC576690 and IC75208 exhibited two PPs of 40 and 38.5 kDa as compared to 39 kDa PP, present in majority of accessions. IC335732, EC335735 possessed higher levels of 31 kDa PP which was absent in EC577473 (Fig. 1e). Low levels of polymorphism in LMW-GS at PPs levels may be associated with tight association Gli-1 loci, encodes different gliadins, and Glu-3 loci that encode LMW-GS, and inherited together, as depicted in bread wheat (Ram et al. 2011). Aalami et al. (2007) demonstrated that PDW 215, with HMW-GS 20, and MACS 1967 with 13 + 16 combination produced very good quality spaghetti. Edwards et al. (2007) also demonstrated the relationships between quantity and composition of polymeric protein and dough strength, for diverse durum wheat genotypes. Since durum is popular due to demand for a specific range of products, therefore, the breeding program should be focused on quality traits development in addition to disease resistance and yield.
Fig. 1.
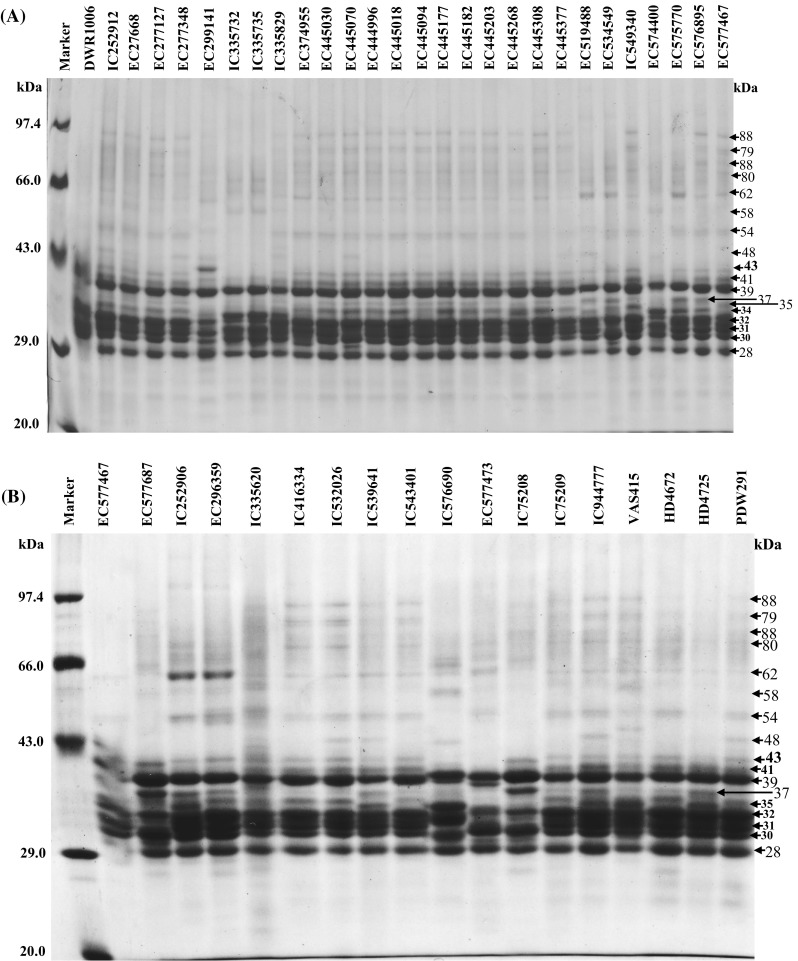
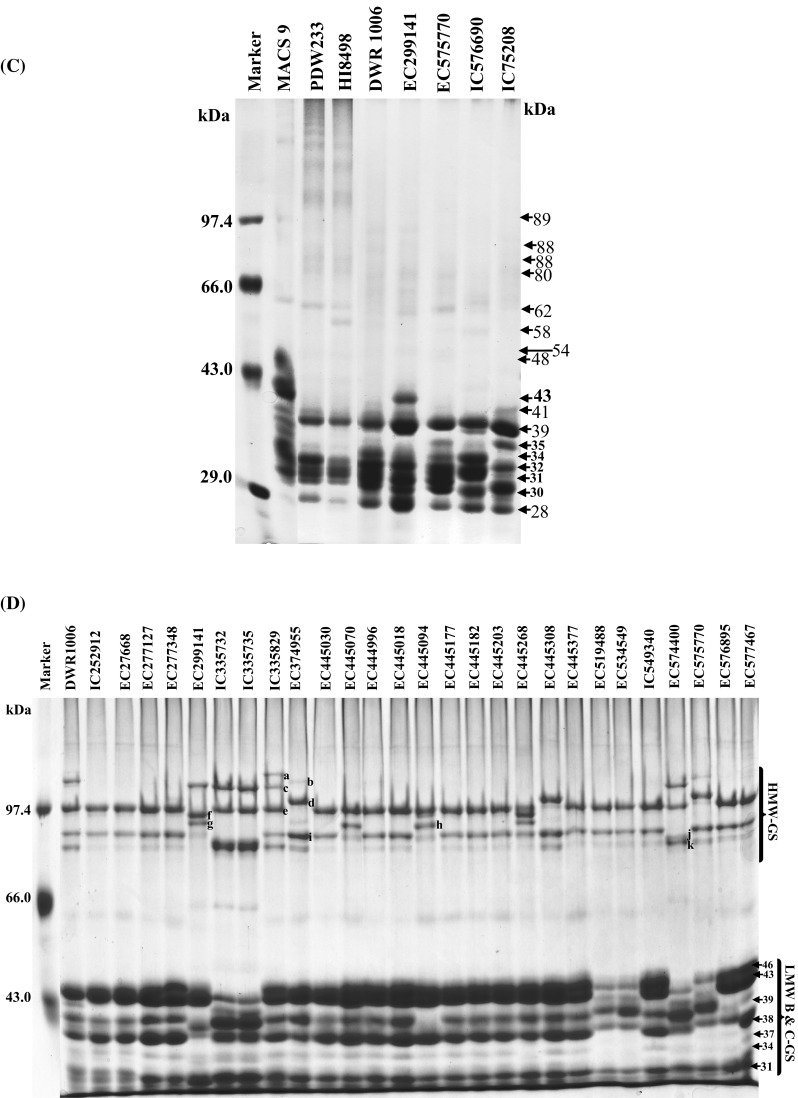
a SDS-PAGE analysis of gliadins of different durum wheat accessions. Gliadins were extracted from meal of durum wheat by 60% ethanol and subjected to SDS-PAGE analysis under non-reducing conditions by omitting β-mercaptoethanol from sample buffer. After electrophoresis, gels were fixed in 8% tri chloro acetic acid for 30 min at room temperature and 20 ml of 0.2% (w/v) coomassie brilliant blue R 250 dye, dissolved in absolute ethanol and passed through Whatman filter paper no. 1, was mixed in fixing solution. Gels were washed thoroughly with de-ionized water and scanned with HP G4010 flatbed scanner. The molecular weight analysis was done by using AlphaEase® FC v 6.0.0 gel analyzer software. b SDS-PAGE analysis of gliadins of different durum wheat accessions. The extraction and processing of gliadins was done as described in a. c Comparative SDS-PAGE analysis of gliadins between durum wheat and standard wheat. MACS9, PDW233, HI8498 were used as standards for which the gliadin architecture was well established. d SDS-PAGE analysis of different glutenins-subunits extracted from different durum wheat accessions. Reduced and alkylated glutenins were extracted from fine flour of durum wheat by glutenin extraction buffer (80 mM Tris HCl, pH 8.0, 50% propanol, 1% DL-Dithiothreitol, 0.4% 4-vinyle pyridine) thrice at 65 °C for 30 min each and subjected to SDS-PAGE analysis under reducing conditions. After electrophoresis, gels were stained in a staining solution containing 0.2% (w/v) commassie brilliant blue R 250 dye in 50% methanol for overnight and gels were destained in 50% methanol and washed thoroughly with de-ionized water. De-stained gels were scanned with HP G4010 flatbed scanner and the molecular weight analysis was done by using AlphaEase FC v 6.0.0 gel analyzer software. e SDS-PAGE analysis of different glutenins of different durum wheat accessions. Extraction, electrophoresis and gel documentation of durum glutenin were done as described in a. Molecular of different polypeptides are in kilo Dalton (kDa). HMW-GS molecular weights a = 113 kDa; b = 110 kDa; c = 107 kDa; d = 101 kDa; e = 97 kDa; f = 95 kDa; g = 92 kDa; h = 91; i = 88 kDa; j = 83 kDa; k = 81 kDa
Pasting properties
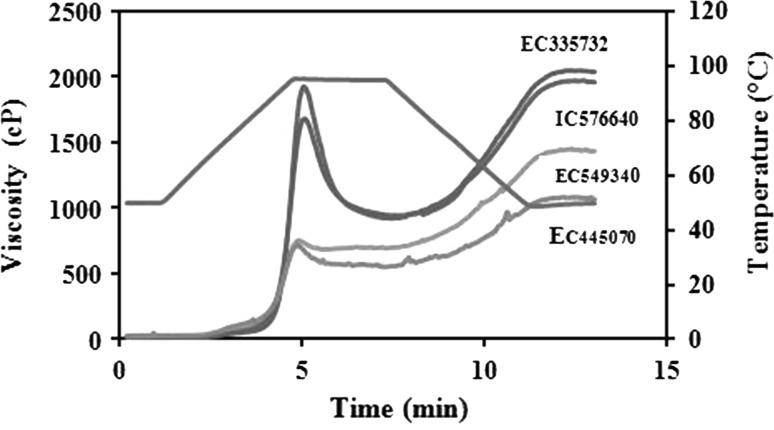
Pasting parameters (PV, BDV, SBV, FV and PT) of meal milled from different durum wheat accessions are shown in Table 3. PV and BDV of meal milled from different durum wheat accessions ranged from 666 to 1924cP and 7 to 968 cP, respectively. IC252906 showed the lowest whereas IC576640 showed the highest PV (Fig. 2). The highest value was observed for IC576640 while IC549340 showed the lowest value for BDV. PV had a strong negative correlation with proportion of large size particles (r = −0.582, p ≤ 0.005) and positive correlation with small size particles (r = 0.542, p ≤ 0.005). BDV had a strong negative correlation with large size particles (r = −0.594, p ≤ 0.005) but positive correlation with small size particles (r = 0.547, p ≤ 0.005). Both PV and BDV showed significant negative correlation with GHI (r = −0.835 and −0.722, respectively, p ≤ 0.005). These results reflected that accessions with lower grain hardness resulted into flour with higher pasting properties. Flour with high protein content showed lower BDV (Singh et al. 2011). The presence of 35 kDa polypeptide in IC576690, IC944777, HD4725, IC335732, EC335735, EC335829, EC574400, EC575770 and EC576895 showed higher PV. On the contrary, IC252906 possesses 35 kDa along with 62 kDa PP that might be associated with lower PV. Since the banding pattern of this accession was comparable to other accessions because of the presence of 62 kD. The 62 kDa PP fall in ω- gliadin category and have been associated with PV of dough. SBV and FV of meal from different durum wheat accessions ranged from 446 to 1090 cP and 1068 to 2036 cP. EC445070 showed the lowest while IC335732 had the highest SBV. EC445070 showed the lowest while IC335732 had the highest FV. SBV and FV had a strong negative correlation with large size particles (r = −0.579, p ≤ 0.005) and positive correlation with small size particles (r = 0.572, p ≤ 0.005). Both FV and SBV were also negatively correlated with protein content (r = −0.464 and −0.489, respectively, p ≤ 0.005). Both SBV and FV negatively correlated with GHI (r = −0.732 and −0.864, respectively, p ≤ 0.005). Results showed that the meal with lower GHI had more proportions of small size particles and had lower protein content that resulted in higher pasting properties. PT of meal from different durum wheat accessions ranged from 60.12 to 69.28 °C. EC445268 showed the lowest whereas IC335732 showed the highest PT and showed a strong negative correlation with large size particles (r = −0.302, p ≤ 0.005). PT negatively correlated with GHI (r = −0.346, p ≤ 0.05) indicating that hard accessions had more PT. Singh et al. (2016) showed similar values of pasting properties of various durum wheat accessions. These results indicated that the presence of higher protein delayed PT.
Table 3.
Pasting properties of meal and rheological properties of dough made from meal of different durum wheat accessions
| Sample | PV (cP) | FV (cP) | SBV (cP) | BDV (cP) | PT (◦C) | G′ (Pa) | G″ (Pa) | Tan δ |
|---|---|---|---|---|---|---|---|---|
| EC445268 | 719 ± 69b | 1225 ± 85bc | 596 ± 44d | 90.1 ± 28fg | 60.12 ± 0.1a | 27,490 ± 800h | 11,060 ± 186g | 0.40 ± 0.01j |
| EC445094 | 821 ± 73d | 1378 ± 88d | 630 ± 36de | 72.7 ± 21e | 62.61 ± 0.2c | 47,620 ± 1050o | 16,280 ± 198l | 0.34 ± 0.02e |
| EC444996 | 752 ± 67c | 1286 ± 89c | 566 ± 40c | 31.6 ± 18c | 63.95 ± 0.04e | 36,690 ± 925k | 12,300 ± 176h | 0.34 ± 0.03e |
| EC445030 | 768 ± 64c | 1245 ± 86bc | 571 ± 39c | 93.8 ± 17g | 65.44 ± 0.4gh | 29,220 ± 700h | 10,473 ± 168f | 0.36 ± 0.02f |
| EC445308 | 738 ± 59c | 1180 ± 76b | 538 ± 37b | 96.5 ± 20g | 63.47 ± 0.2d | 38,770 ± 840l | 13,700 ± 204i | 0.35 ± 0.02f |
| EC445182 | 947 ± 67e | 1503 ± 82f | 738 ± 16h | 181.8 ± 1jk | 64.47 ± 0.1f | 57,220 ± 910h | 17,410 ± 296m | 0.30 ± 0.01b |
| EC445070 | 716 ± 54b | 1068 ± 49a | 446 ± 9a | 94.2 ± 4g | 62.60 ± 0.2c | 50,770 ± 890p | 19,970 ± 253n | 0.39 ± 0.02i |
| EC445377 | 957 ± 76e | 1494 ± 86f | 742 ± 25h | 205.5 ± 15l | 62.61 ± 0.2c | 54,110 ± 905q | 17,140 ± 189m | 0.32 ± 0.01c |
| EC445203 | 895 ± 60de | 1443 ± 81ef | 697 ± 16f | 148.8 ± 5i | 63.50 ± 0.31d | 44,830 ± 730n | 14,120 ± 149j | 0.31 ± 0.01c |
| IC335732 | 1679 ± 82l | 2036 ± 93k | 1090 ± 21n | 733.4 ± 10r | 69.28 ± 0.1l | 15,560 ± 119c | 6415 ± 101b | 0.41 ± 0.02k |
| IC335735 | 1579 ± 85k | 1902 ± 89i | 994 ± 32l | 670.6 ± 28q | 64.96 ± 0.03g | 12,850 ± 108a | 5582 ± 98a | 0.43 ± 0.03l |
| EC445177 | 879 ± 71de | 1438 ± 59ef | 705 ± 10f | 146.5 ± 22hi | 62.12 ± 0.1c | 26,670 ± 220h | 10,068 ± 110f | 0.38 ± 0.01h |
| EC534549 | 1311 ± 85j | 1970 ± 93j | 1039 ± 20m | 379.8 ± 12o | 64.96 ± 0.01g | 25,790 ± 265g | 10,456 ± 113f | 0.41 ± 0.03k |
| EC277348 | 1108 ± 75h | 1592 ± 56g | 828 ± 5j | 344.1 ± 24n | 61.62 ± 0.2b | 36,750 ± 276k | 12,280 ± 123h | 0.33 ± 0.02d |
| IC252912 | 995 ± 59ef | 1417 ± 41e | 665 ± 6e | 242.6 ± 12lm | 62.11 ± 0.1c | 33,030 ± 264i | 12,480 ± 136h | 0.38 ± 0.04h |
| EC576895 | 955 ± 62e | 1358 ± 53d | 699 ± 1f | 296.3 ± 10m | 65.46 ± 0.2h | 14,980 ± 186b | 6152 ± 103b | 0.41 ± 0.01k |
| EC519488 | 803 ± 44d | 1294 ± 43c | 652 ± 0e | 161.5 ± 1j | 67.29 ± 0.2j | 24,330 ± 259f | 8952 ± 116e | 0.37 ± 0.02g |
| EC276668 | 840 ± 56d | 1287 ± 49bc | 643 ± 0de | 195.2 ± 7k | 66.43 ± 0.31i | 76,860 ± 781u | 23,880 ± 299o | 0.31 ± 0.01c |
| EC374955 | 858 ± 58d | 1322 ± 56cd | 556 ± 1b | 92 ± 1fg | 64.96 ± 0.02g | 20,830 ± 215de | 9002 ± 109e | 0.43 ± 0.05l |
| EC577687 | 806 ± 62d | 1292 ± 47c | 633 ± 6de | 147.3 ± 21hi | 66.44 ± 0.2i | 18,190 ± 198d | 7460 ± 94c | 0.41 ± 0.06k |
| IC335829 | 1254 ± 86i | 1794 ± 98h | 951 ± 27k | 411.4 ± 15p | 64.47 ± 1.16h | 18,370 ± 191d | 6879 ± 92b | 0.37 ± 0.04g |
| IC75209 | 959 ± 74e | 1520 ± 87fg | 615 ± 8de | 53.6 ± 5d | 64.01 ± 0.01e | 35,560 ± 256j | 11,510 ± 222g | 0.32 ± 0.03cd |
| IC543401 | 824 ± 73d | 1396 ± 68de | 668 ± 5e | 95.6 ± 10g | 67.38 ± 0.3j | 71,880 ± 691t | 20,170 ± 299n | 0.28 ± 0.02a |
| IC335620 | 714 ± 64b | 1295 ± 59c | 665 ± 6e | 83 ± 11f | 65.42 ± 0.4h | 49,810 ± 306p | 15,980 ± 182k | 0.32 ± 0.01cd |
| IC539641 | 637 ± 59a | 1214 ± 53bc | 658 ± 13e | 81.6 ± 19f | 66.92 ± 0.02j | 25,840 ± 192g | 10,558 ± 93f | 0.41 ± 0.05k |
| EC445018 | 729 ± 60b | 1153 ± 59b | 554 ± 10b | 129.7 ± 11gh | 67.39 ± 0.1j | 34,900 ± 265i | 11,266 ± 146g | 0.32 ± 0.01c |
| IC252906 | 666 ± 55a | 1255 ± 40bc | 607 ± 12d | 18.2 ± 3.01b | 60.17 ± 0.1a | 30,780 ± 249h | 12,490 ± 158h | 0.41 ± 0.06k |
| IC549340 | 761 ± 57c | 1439 ± 56ef | 685 ± 4e | 6.6 ± 3.01a | 62.11 ± 0.1c | 50,710 ± 482p | 15,180 ± 169k | 0.30 ± 0.02b |
| EC574400 | 1582 ± 76k | 1913 ± 89i | 1057 ± 28m | 725. 7 ± 15r | 67.79 ± 0.2k | 27,120 ± 211g | 10,293 ± 103f | 0.38 ± 0.05h |
| EC577473 | 1006 ± 71f | 1586 ± 63g | 803 ± 3i | 223 ± 5lm | 66.41 ± 0.4i | 43,040 ± 691n | 16,430 ± 166l | 0.38 ± 0.04h |
| EC299141 | 826 ± 51d | 1290 ± 39c | 606 ± 3d | 142.7 ± 9h | 65.48 ± 0.2h | 21,690 ± 235e | 8822 ± 101d | 0.41 ± 0.03k |
| EC277127 | 975 ± 57e | 1364 ± 45d | 664 ± 9e | 274.8 ± 3m | 66.37 ± 0.3i | 33,570 ± 283i | 12,740 ± 182h | 0.38 ± 0.06h |
| EC577467 | 702 ± 68b | 1236 ± 39bc | 600 ± 4d | 65.7 ± 25de | 66.46 ± 0.2i | 78,620 ± 796v | 26,140 ± 256p | 0.33 ± 0.05d |
| EC575770 | 830 ± 77d | 1416 ± 61e | 726 ± 11g | 139.7 ± 27h | 66.92 ± 0.02i | 43,650 ± 320n | 15,430 ± 179k | 0.35 ± 0.02f |
| IC532026 | 826 ± 63d | 1387 ± 36d | 711 ± 12fg | 150.1 ± 15i | 66.81 ± 0.01i | 44,740 ± 280n | 13,470 ± 142i | 0.30 ± 0.09b |
| IC576640 | 1924 ± 89m | 1956 ± 97ij | 1000 ± 26l | 968 ± 18s | 64.98 ± 0.01g | 12,870 ± 172a | 5425 ± 88j | 0.42 ± 0.04l |
| DWR1006 | 1088 ± 49g | 1597 ± 72g | 819 ± 8ij | 309.6 ± 15mn | 68.34 ± 0.2k | 41,430 ± 321m | 14,520 ± 152a | 0.35 ± 0.06f |
| IC444777 | 1059 ± 57g | 1614 ± 61g | 831 ± 3j | 276.4 ± 1m | 60.62 ± 0.1ab | 34,750 ± 268j | 11,990 ± 123g | 0.35 ± 0.08ef |
| IC75208 | 1082 ± 60g | 1532 ± 52fg | 804 ± 6i | 354 ± 2n | 68.71 ± 0.1k | 18,740 ± 192d | 7431 ± 94c | 0.40 ± 0.09j |
| EC296359 | 684 ± 40a | 1309 ± 42c | 680 ± 4e | 55.7 ± 6d | 66.39 ± 0.1i | 20,540 ± 216de | 8406 ± 108d | 0.41 ± 0.04k |
| IC416334 | 997 ± 50ef | 1504 ± 43f | 741 ± 20h | 233.3 ± 13lm | 62.07 ± 0.02c | 59,740 ± 325s | 17,920 ± 196m | 0.30 ± 0.10b |
| UAS415 | 1019 ± 54f | 1567 ± 64fg | 816 ± 13ij | 267.5 ± 3m | 63.03 ± 0.02d | 33,070 ± 203i | 11,170 ± 146g | 0.34 ± 0.20e |
| LSD | 105.43 | 108.49 | 30.14 | 23.47 | 0.41 | 814.85 | 269.56 | 0.005 |
Data represented as mean value ± SD. Means with similar superscripts in a column do not differ significantly (p ≤ 0.05)
PT pasting temperature, PV peak viscosity, BDV breakdown viscosity, FV final viscosity, SBV setback viscosity, G′ elastic modulus, G″ viscous modulus
Fig. 2.
Pasting profile of meal from different durum wheat accessions
Mixographic properties
Several mixographic parameters of meal obtained from different durum wheat accessions are shown in Table 4. MPT and MPW of dough made from meal ranged from 1.14 to 6.59 min and 15.34 to 60.58%, respectively. EC577473 showed the lowest while IC335732 showed the highest MPT. EC576895 and IC335735 showed lower while EC445203 showed the highest MPW. MPT was positively correlated with protein content (r = 0.352, p ≤ 0.05) indicated its contribution to dough development time. Baasandroj et al. (2015) reported that less protein content of flours resulted into longer peak time. Earlier similar values of MPT and MPW of flours milled from different Indian wheat accessions were reported by Kaur et al. (2015) and Singh et al. (2016). The mixograms of flours milled from different durum wheat accessions are shown in Supplementary Fig. 1. MPW was negatively correlated with PV and BDV (r = −0.282 and −0.340, respectively, p ≤ 0.05). This indicated that paste and dough consistency of flour was related to each other. LPV and LPW ranged from 13.08 to 44.46% and 12.56 to 56.79%, respectively. LPV was the highest for EC276668 and the lowest for EC577473. LPW was the highest for EC276668 and the lowest for EC374955. LPV was positively correlated with protein content showing that consistency of dough was related to flour protein content. RPV and RPW ranged from 24.05 to 44.98% and 11.25 to 44.76%, respectively. RPV was the highest for IC576640 and the lowest for IC335829 while RPW showed higher value for EC445203, EC445377 and the lowest value for IC335829. Oak et al. (2006) reported that the dough with more MPT and wider MPW resulted into stable and strong dough. Martinant et al. (1998) reported that RPW indicates the width of peak 1 min after MPT, exhibited dough tolerance during over mixing. RPW showed negative correlation with BDV (r = −0.305, p ≤ 0.05). WS reflected the breakdown rate and its sensitivity to mechanical treatment. WS of dough made from flour milled from different durum wheat accessions ranged from 0.9 to 19.26%Tq*min, the highest value for EC575770 and the Lowest for IC335732. WS was negatively correlated with PV and BDV. Lower dough strength indicated by mixograph parameters for HD4672 might be due to higher accumulation of 52, 46, 41 and 38 kDa PPs consistent with the earlier reports (Singh et al. 1991). It was thus evident from these studies that the HMW-GS and LMW-GS in durum played principal role in product quality improvement.
Table 4.
Mixographic properties of dough made from meal of different durum wheat accessions
| Sample | MPT (min) | MPV (%) | MPW (%) | LPV (%) | LPW (%) | RPV (%) | RPW (%) | WS (%/Tq × min) |
|---|---|---|---|---|---|---|---|---|
| EC445268 | 2.60 ± 0.2c | 36.67 ± 2bc | 17.39 ± 1ab | 33.68 ± 3ef | 22.44 ± 1cd | 33.38 ± 1.12bc | 18.21 ± 0.98bc | 9.72 ± 0.12g |
| EC445094 | 4.13 ± 0.1e | 41.16 ± 2.5cd | 32.27 ± 2.1cd | 37.98 ± 1fg | 31.49 ± 1.5f | 40.17 ± 2.16d | 21.47 ± 1.07c | 7.15 ± 0.3ef |
| EC444996 | 2.40 ± 0.4bc | 36.77 ± 1.8bc | 39.61 ± 1d | 29.48 ± 1.5de | 28.17 ± 1.1e | 32.86 ± 1.16bc | 29.20 ± 1.15e | 7.64 ± 0.6f |
| EC445030 | 3.90 ± 0.1e | 38.86 ± 1.95c | 22.19 ± 1.5b | 37.75 ± 2.5fg | 30.46 ± 1.3ef | 37.18 ± 1.46cd | 20.36 ± 1.26bc | 4.09 ± 0.06d |
| EC445308 | 4.02 ± 0.2e | 38.98 ± 2.2c | 45.55 ± 3.2e | 32.14 ± 1.9e | 37.72 ± 1.6gh | 33.76 ± 1.23bc | 23.91 ± 1.14cd | 6.85 ± 0.1ef |
| EC445182 | 2.43 ± 0.31bc | 34.92 ± 2.1bc | 43.67 ± 3de | 29.54 ± 1.4de | 37.71 ± 1.8gh | 32.82 ± 1.19bc | 35.45 ± 1.88f | 4.8 ± 0.1d |
| EC445070 | 4.39 ± 0.30f | 41.65 ± 2.6cd | 45.01 ± 3.3e | 40.25 ± 2.9g | 49.88 ± 2i | 39.43 ± 1.45d | 26.42 ± 1.23d | 7.76 ± 0.1f |
| EC445377 | 1.99 ± 0.30b | 37.76 ± 2bc | 57.74 ± 3.5fg | 27.21 ± 1.6d | 37.97 ± 1.85gh | 32.43 ± 1.25bc | 44.78 ± 2.59i | 6.18 ± 0e |
| EC445203 | 2.55 ± 0.40c | 42.66 ± 2.8cd | 60.58 ± 3.6g | 36.61 ± 2.1f | 53.52 ± 2.32j | 38.75 ± 1.45d | 44.76 ± 2.47i | 12.71 ± 1j |
| IC335732 | 6.59 ± 0.31i | 38.37 ± 2.3c | 18.33 ± 1.2ab | 37.77 ± 2.5fg | 19.88 ± 0.9c | 37.83 ± 1.35cd | 14.25 ± 0.89ab | 0.90 ± 0.2a |
| IC335735 | 6.45 ± 0.31i | 38.27 ± 2c | 15.63 ± 1a | 37.46 ± 1fg | 19.28 ± 0.8c | 36.51 ± 1.36cd | 12.41 ± 0.42a | 2.71 ± 0.2c |
| EC445177 | 3.28 ± 0.02d | 35.96 ± 1.7bc | 16.52 ± 1.4ab | 35.28 ± 0.2ef | 15.97 ± 0.7b | 34.51 ± 1.26bc | 16.46 ± 0.64b | 5.68 ± 0.1e |
| EC534549 | 1.63 ± 0.3ab | 37.74 ± 1.5bc | 50.62 ± 2.2ef | 21.31 ± 1.1c | 28.76 ± 0.75e | 31.55 ± 1.16bc | 34.37 ± 1.79f | 13.76 ± 0.1k |
| EC277348 | 1.83 ± 0.03b | 39.39 ± 1c | 52.44 ± 2.3f | 24.82 ± 1.6cd | 30.84 ± 0.95ef | 36.76 ± 1.38cd | 23.86 ± 1.45cd | 12.80 ± 0.7j |
| IC252912 | 1.79 ± 0.2b | 36.12 ± 2bc | 37.31 ± 2.3d | 28.93 ± 1.5de | 37.79 ± 1.35gh | 35.05 ± 1.49cd | 21.50 ± 1.39c | 8.00 ± 0.8f |
| EC576895 | 2.76 ± 0.2c | 32.22 ± 1b | 15.34 ± 1a | 28.87 ± 1.1de | 18.71 ± 0.45bc | 30.55 ± 1.22b | 14.07 ± 1.1ab | 6.11 ± 0.1e |
| EC519488 | 2.14 ± 0.1bc | 35.67 ± 2.2bc | 42.42 ± 2.6de | 30.19 ± 1.3de | 31.77 ± 1.38f | 31.79 ± 1.34bc | 16.55 ± 1.16b | 10.09 ± 0.6gh |
| EC276668 | 5.87 ± 0.1h | 46.65 ± 3d | 45.79 ± 2.9e | 44.46 ± 2.9h | 56.79 ± 2.45k | 42.44 ± 2.22de | 34.27 ± 1.98f | 4.12 ± 0.8d |
| EC374955 | 1.20 ± 0.2ab | 36.72 ± 2bc | 47.30 ± 2.7e | 10.46 ± 0.4a | 12.56 ± 0.36a | 35.35 ± 1.56c | 29.27 ± 1.13e | 10.61 ± 0.5h |
| EC577687 | 2.57 ± 0.03c | 37.09 ± 1bc | 17.16 ± 1ab | 33.33 ± 1e | 29.53 ± 1.46ef | 35.20 ± 1.47c | 15.91 ± 0.25b | 10.80 ± 0.22h |
| IC335829 | 1.74 ± 0.03b | 24.21 ± 1a | 20.59 ± 1.6ab | 14.62 ± 0.46b | 14.30 ± 0.6ab | 24.05 ± 1.04a | 11.25 ± 0.18a | 4.45 ± 0.01d |
| IC75209 | 2.98 ± 0.02cd | 38.57 ± 2.6c | 44.23 ± 3de | 33.33 ± 1.3e | 42.33 ± 2.15h | 33.55 ± 1.29bc | 23.22 ± 1.28cd | 7.10 ± 0.91ef |
| IC543401 | 3.91 ± 0.08e | 43.11 ± 3.5cd | 56.98 ± 3.4fg | 40.42 ± 2.5g | 53.73 ± 2.35j | 37.99 ± 1.45cd | 36.21 ± 1.57fg | 9.11 ± 1.92fg |
| IC335620 | 2.64 ± 0.3c | 39.41 ± 2.7c | 52.61 ± 2f | 34.98 ± 1.8ef | 44.91 ± 2.09hi | 35.84 ± 1.52c | 31.44 ± 1.43ef | 7.84 ± 1.21f |
| IC539641 | 1.45 ± 0.2ab | 38.27 ± 2.9c | 41.59 ± 2.2de | 17.03 ± 0.7bc | 19.88 ± 0.1c | 37.93 ± 1.64cd | 18.77 ± 0.79bc | 12.70 ± 1.62j |
| EC445018 | 5.23 ± 0.1gh | 41.65 ± 2cd | 23.75 ± 1b | 41.08 ± 28g | 23.01 ± 1d | 40.69 ± 2.11d | 21.64 ± 0.96c | 2.03 ± 0.1b |
| IC252906 | 1.47 ± 0.2ab | 40.51 ± 3c | 52.48 ± 3.46f | 18.26 ± 1.3bc | 22.69 ± 0.75cd | 35.06 ± 1.57c | 29.25 ± 1.04e | 15.68 ± 1.12l |
| IC549340 | 2.56 ± 0.41c | 41.38 ± 3.1cd | 56.86 ± 3.6fg | 34.94 ± 1ef | 41.61 ± 2.25h | 37.64 ± 1.69cd | 39.00 ± 2.26g | 7.29 ± 0.64ef |
| EC574400 | 1.22 ± 0.2ab | 32.08 ± 2b | 32.11 ± 2c | 16.07 ± 0.5b | 19.83 ± 0.36c | 29.44 ± 1.23b | 17.50 ± 0.73b | 8.10 ± 0.56f |
| EC577473 | 1.14 ± 0.1a | 40.61 ± 2.4c | 54.22 ± 3.2fg | 13.08 ± 0.4ab | 14.39 ± 0.3ab | 39.68 ± 1.45d | 38.00 ± 2.11g | 9.72 ± 0.17g |
| EC299141 | 2.21 ± 0.01c | 29.49 ± 1.4ab | 26.05 ± 1bc | 21.63 ± 1.2c | 19.28 ± 0.4c | 25.99 ± 1.11ab | 12.20 ± 0.8a | 11.06 ± 0.16h |
| EC277127 | 2.21 ± 0.01c | 42.34 ± 3cd | 53.73 ± 3f | 37.88 ± 1.7f | 51.38 ± 2.46ij | 40.09 ± 2.42d | 21.79 ± 1.71c | 11.38 ± 0.84h |
| EC577467 | 4.95 ± 0.04g | 35.02 ± 2bc | 50.04 ± 2ef | 32.69 ± 1.6e | 48.84 ± 1.49i | 32.24 ± 1.39bc | 42.53 ± 2.49h | 6.70 ± 0.01ef |
| EC575770 | 1.91 ± 0.08b | 44.31 ± 3.1cd | 58.85 ± 3.5g | 30.82 ± 1de | 40.52 ± 1.34h | 30.18 ± 1.19b | 16.53 ± 0.62b | 19.26 ± 1.08m |
| IC532026 | 3.06 ± 0.05d | 40.07 ± 2c | 54.79 ± 3fg | 35.03 ± 1.8ef | 50.89 ± 2.28ij | 35.03 ± 1.25c | 36.80 ± 1.36fg | 7.90 ± 0.26f |
| IC576640 | 4.22 ± 0.02ef | 45.74 ± 34d | 33.34 ± 2.5c | 42.06 ± 2.4g | 35.76 ± 1.64g | 44.98 ± 2.45e | 23.51 ± 1.25cd | 4.75 ± 0.89d |
| DWR1006 | 2.15 ± 0.04bc | 41.97 ± 2cd | 40.62 ± 2.6d | 32.04 ± 1e | 37.20 ± 1.76gh | 39.10 ± 2.08cd | 23.19 ± 1.36cd | 12.32 ± 0.58i |
| IC444777 | 2.39 ± 0.3bc | 37.77 ± 2.9bc | 38.41 ± 2.4d | 31.79 ± 1e | 35.59 ± 1.48g | 37.25 ± 1.97cd | 24.14 ± 1.47cd | 6.20 ± 1.06e |
| IC75208 | 3.26 ± 0.03d | 30.55 ± 1.9b | 25.93 ± 1bc | 21.35 ± 0.65c | 24.33 ± 1.19d | 27.65 ± 1.63ab | 14.58 ± 0.4ab | 8.42 ± 0.65f |
| EC296359 | 1.43 ± 0.2ab | 38.79 ± 2c | 39.47 ± 2.1d | 17.08 ± 0.5bc | 18.45 ± 1.09bc | 35.16 ± 1.78c | 17.68 ± 0.3b | 27.16 ± 0.98n |
| IC416334 | 3.02 ± 0.02d | 40.22 ± 3.2c | 55.14 ± 3.6fg | 36.99 ± 1.3f | 45.56 ± 2.17hi | 34.46 ± 1.69bc | 41.36 ± 2.19gh | 8.46 ± 1.08f |
| UAS415 | 3.14 ± 0.1d | 37.3 ± 2.6bc | 19.87 ± 1.1ab | 35.36 ± 1.6ef | 36.81 ± 1.55g | 36.36 ± 1.78cd | 15.37 ± 0.3ab | 9.31 ± 0.91fg |
| LSD | 0.32 | 3.75 | 3.94 | 2.62 | 2.42 | 2.51 | 2.24 | 1.16 |
Data represented as mean value ± SD. Means with similar superscripts in a column do not differ significantly (p ≤ 0.05)
MPT mixograph peak time, MPW mixograph peak width, LPV left peak value, LPW left peak width, RPV right peak value, RPW right peak width, WS weakening slope, MPV Mixograph peak value
Dynamic rheology of dough
G′, G″ and tan δ of dough made from meal of different durum wheat accessions were evaluated (Table 3). G′ and G″ ranged from 12,850 to 76,860 Pa and 5425 to 23,880 Pa, respectively. EC276668 showed the highest while IC335735 and IC576640 showed lower values of G′ and G″. Tanδ ranged from 0.31 to 0.43, the highest value was observed for IC335735. EC276668 showed the lowest tan δ value as compared to other accessions. G′ were greater than G″ for all accessions, indicated the more elastic behavior of dough (Singh et al. 2011). Ewart (1972) reported that gliadin to glutenin ratio affected the viscous and elastic properties of dough. Higher values of both modulii for meal milled from accessions with higher GHI was observed. Both G′ and G″ were strongly negatively correlated with PV, FV as well as SBV (Supplementary Table 1). Both modulii were positively correlated with mixographic parameters (Supplementary Table 1). RPW, the indicator of dough mixing tolerance was negatively correlated to tan δ. Tan δ showed a positive correlation with protein content (r = 0.307, p ≤ 0.05). G′ and G″ value demonstrated that the accessions having 113, 110 and 107 kDa (1, 2 and 2*) PPs along with 97 and 88 kDa (7 + 8) or 97 and 81 kDa (7 + 9) showed lower G′ and G″ and higher Tan δ. G′ and G″ indicated the elastic character of dough, therefore, it was likely that the accumulation of 113, 110 and 107 kDa PPs in EC299141, IC335732, EC335735, EC335829, EC574400, EC575770, EC577467, EC577687, IC539641, IC576690, and VAS415 might be associated with lower consistency of dough (Table 4). Whereas, studies carried out by Kaur et al. (2016) also revealed that PDW291, having 14 + 15 and type 2 HMW-GS allelic combination showed exceptionally higher G′ and G″ and also showed the best noodle making properties. The values of G′ and G″ were associated with the presence or absence of accumulation of LMW-GS and HMW-GS. The highest value of G′ and G″ might be associated with the presence of HMW-GS, which was not yet evaluated. In the present study, the accumulation of 97 + 91 kDa PPs (13 + 16) in EC445070, EC445094, IC532026 showed higher G′ and G″ and lower Tan δ, except EC577687, which possess 107 kDa PP along with 97 + 91 kDa PPs. The accessions (EC445070, EC445094 and IC532026) with higher G′ and G″ with allelic combinations of (13 + 16) with 97 + 91 kDa PPs showed best noodles/pasta making properties. Accession with higher G′ and G″ may result into higher cooked spaghetti firmness. A positive correlation of cooked spaghetti firmness with unextractable polymeric protein by Ohm et al. (2017) and of unextractable polymeric proteins with G′ and G″ by Singh et al. (2016) was observed. The storage of 107 kDa PP in EC577687 and lack of 40 and 38 kDa PPs in EC577687 and IC75208 may be responsible for lower G′ and G″ (Fig. 2 and Table 3).
Conclusion
The results reflected that durum wheat accessions with higher GHI and protein content on milling produced a large amount of coarse particles. Accessions with higher GHI will be suitable for milling into coarse semolina, as these will give higher recovery of semolina. Accessions (EC445070, EC445094 and IC532026) with allelic combinations of 13 + 16 and 97 + 91 kDa PPs showed higher G′ and G″ and will be more suitable for noodles/pasta making. Accessions (IC576690, IC944777, HD4725, IC335732, EC335735, EC335829, EC574400, EC575770 and EC576895) with 35 kDa PP showed higher while IC252906 with both 35 kDa and 62 kDa PP had lower paste viscosity and higher G′ and G″. Accession with higher G′ and G″ may result into higher cooked spaghetti firmness.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
NS acknowledges MOFPI, Govt. of India, for providing funds in the form of a research project. MK acknowledges UGC-BSR for providing financial assistance in the form of Fellowship. The authors are thankful to Dr. Arvind Kumar Ahlawat for measuring the grain hardness index of accessions.
Abbreviations
- GHI
Grain hardness index
- TGW
Thousand grain weight
- GD
Grain diameter
- PT
Pasting temperature
- PV
Peak viscosity
- BDV
Breakdown viscosity
- FV
Final viscosity
- SBV
Setback viscosity
- WS
Weakening slope
- LPV
Left peak value
- RPV
Right peak value
- LPW
Left peak width
- RPW
Right peak width
- MPW
Mixograph peak width
- MPT
Mixograph peak time
- G′
Elastic modulus
- G″
Viscous modulus
- LMW-GS
Low molecular weight glutenin subunits
- HMW-GS
High molecular weight glutenin subunits
- MPV
Mixograph peak value
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3036-y) contains supplementary material, which is available to authorized users.
References
- AACC International . Approved methods of American Association of Cereal Chemists. 10. St. Paul: AACC International Press; 2000. [Google Scholar]
- Aalami M, Rao UJS, Leelavathi K. Physicochemical and biochemical characteristics of Indian durum wheat varieties: relationship to semolina milling and spaghetti making quality. Food Chem. 2007;102:993–1005. doi: 10.1016/j.foodchem.2006.06.052. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 15. Washington: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Baasandroj T, Ohm JB, Manthey F, Simsek S. Effect of kernal size and mill type on protein, milling yield and baking quality of hard red spring wheat. Cereal Chem. 2015;92:81–87. doi: 10.1094/CCHEM-12-13-0259-R. [DOI] [Google Scholar]
- Boggini G, Pogna NE. The bread making quality and storage protein composition of Italian durum wheat. J Cereal Sci. 1989;9:131–138. doi: 10.1016/S0733-5210(89)80013-X. [DOI] [Google Scholar]
- Dick JW, Youngs VL. Evaluation of durum wheat, semolina, and pasta in the United States. In: Fabriani G, Lintas C, editors. Durum wheat: chemistry and technology. St. Paul: AACC; 1988. pp. 237–248. [Google Scholar]
- Du Cros DL. Glutenin proteins and gluten strength in durum wheat. J Cereal Sci. 1987;5:3–12. doi: 10.1016/S0733-5210(87)80003-6. [DOI] [Google Scholar]
- Edwards NM, Gianibelli MC, McCaig TN, Clarke JM, Ames NP, Larroque OR, Dexter JE. Relationships between dough strength, polymeric protein quantity and composition for diverse durum wheat genotypes. J Cereal Sci. 2007;45:140–149. doi: 10.1016/j.jcs.2006.07.012. [DOI] [Google Scholar]
- Ewart JAD. A modified hypothesis for the structure and rheology of glutenins. J Sci Food Agric. 1972;23:687–699. doi: 10.1002/jsfa.2740230604. [DOI] [PubMed] [Google Scholar]
- Galliard T, Gallagher DM. The effects of wheat bran particle size and storage period on bran flavor and baking quality of bran/flour blends. J Cereal Sci. 1988;8:147–154. doi: 10.1016/S0733-5210(88)80025-0. [DOI] [Google Scholar]
- Gazza L, Sgrulletta D, Cammerata A, Gazzelloni G, Perenzin M, Pogna NE. Pasta making and bread making quality of soft textured durum wheat lines. J Cereal Sci. 2011;54:481–487. doi: 10.1016/j.jcs.2011.09.003. [DOI] [Google Scholar]
- Haraszi R, Sissons M, Juhasz A, Kadkol G, Tamas L, Anderssen RS. Using rheological phenotype phases to predict rheological features of wheat hardness and milling potential of durum wheat. Cereal Chem. 2016;93:369–376. doi: 10.1094/CCHEM-12-15-0255-R. [DOI] [Google Scholar]
- Katyal M, Virdi AS, Kaur A, Singh N, Kaur S, Ahlawat AK, Singh AM. Diversity in quality traits amongst Indian wheat cultivars I: flour and protein characteristics. Food Chem. 2016;194:337–344. doi: 10.1016/j.foodchem.2015.07.125. [DOI] [PubMed] [Google Scholar]
- Kaur A, Singh N, Ahlawat AK, Kaur S, Singh AM, Chauhan H, Singh GP. Diversity in grain, flour, dough and gluten properties amongst Indian wheat cultivars varying in high molecular weight subunits (HMW-GS) Food Res Int. 2013;53:63–72. doi: 10.1016/j.foodres.2013.03.009. [DOI] [Google Scholar]
- Kaur A, Singh N, Kaur S, Ahlawat AK, Singh AM. Relationships of flour retention capacity, secondary structure and rheological properties with the cookie making characteristics of wheat cultivars. Food Chem. 2014;158:48–55. doi: 10.1016/j.foodchem.2014.02.096. [DOI] [PubMed] [Google Scholar]
- Kaur A, Singh N, Kaur S, Katyal M, Virdi AS, Kaur D, Ahlawat AK, Singh AM. Relationship of various flour properties with noodle making characteristics amongst durum wheat varieties. Food Chem. 2015;188:517–526. doi: 10.1016/j.foodchem.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Li Y, Mao X, Wang Q, Zhang J, Li X, Ma F, Sun F, Chang J, Chen M, Wang Y, Li K, Yang G, He G. Overexpression of puroindoline a gene in transgenic durum wheat (Triticum turgidum ssp. durum) leads to a medium-hard kernel texture. Mol Breed. 2014;33:545–554. doi: 10.1007/s11032-013-9971-4. [DOI] [Google Scholar]
- Martinant JP, Nicolas Y, Bouguennec A, Popineau Y, Saulnier L, Branlard G. Relationships between mixograph parameters and indices of wheat grain quality. J Cereal Sci. 1998;27:179–189. doi: 10.1006/jcrs.1997.0156. [DOI] [Google Scholar]
- Morris CF. Puroindolines: the molecular genetic basis of wheat grain hardness. Plant Mol Biol. 2002;48:633–647. doi: 10.1023/A:1014837431178. [DOI] [PubMed] [Google Scholar]
- Morris CF, Simeone MC, King GE, Lafi andra D. Transfer of soft kernel texture from Triticum aestivum to durum wheat Triticum turgidum ssp. durum. Crop Sci. 2011;51:114–122. doi: 10.2135/cropsci2010.05.0306. [DOI] [Google Scholar]
- Murray JC, Kiszonas AM, Morris CF. Influence of soft kernel texture on the flour, water absorption, rheology, and baking quality of durum wheat. Cereal Chem. 2017;94:215–222. doi: 10.1094/CCHEM-06-16-0163-R. [DOI] [Google Scholar]
- Nieto-Taladriz MT, Ruiz M, Martínez MC, Vázquez JF, Carrillo JM. Variation and classification of B low-molecular-weight glutenin subunit alleles in durum wheat. Theor Appl Genet. 1997;95:1155–1160. doi: 10.1007/s001220050676. [DOI] [Google Scholar]
- Novaro P, D’Egidio MG, Mariani BM, Nardi S. Combined effect of protein content and high-temperature drying systems on pasta cooking quality. Cereal Chem. 1993;70:716–719. [Google Scholar]
- Oak MD, Sissons M, Egan N, Tamhankar SA, Rao VS, Bhosale SB. Relationship between gluten strength and pasta firmness in Indian durum wheats. J Food Sci Technol. 2006;41:538–544. doi: 10.1111/j.1365-2621.2005.01103.x. [DOI] [Google Scholar]
- Ohm JB, Manthey F, Elias EM. Variation and correlation of protein molecular weight distribution and semolina quality parameters for durum genotypes grown in North Dakota. Cereal Chem. 2017;94:780–788. doi: 10.1094/CCHEM-07-16-0189-R. [DOI] [Google Scholar]
- Raggiri V, Barron C, Abecassis J, Lullien-Pellerin V. In-depth study of durum wheat grain tissue distribution at milling. Cereal Chem. 2016;93:219–225. doi: 10.1094/CCHEM-08-15-0177-R. [DOI] [Google Scholar]
- Ram S, Sharma S, Verma A, Tyagi BS, Peña RJ. Comparative analyses of LMW glutenin alleles in bread wheat using allele-specific PCR and SDS-PAGE. J Cereal Sci. 2011;54:488–493. doi: 10.1016/j.jcs.2011.09.004. [DOI] [Google Scholar]
- Rharrabti Y, Royo C, Villegas D, Aparicio N, del Moral LG. Durum wheat quality in Mediterranean environments: I. Quality expression under different zones, latitudes and water regimes across Spain. Field Crop Res. 2003;80:123–131. doi: 10.1016/S0378-4290(02)00176-4. [DOI] [Google Scholar]
- Saini DP, Gautam PL. Notes on G × E analysis in segregating populations of durum wheat. Indian J Genet. 1990;50:199–201. [Google Scholar]
- Sasaki T, Yasui T, Kohyama K. Influence of starch and gluten characteristics on rheological properties of wheat flour gels at small and large deformation. Cereal Chem. 2008;85:329–334. doi: 10.1094/CCHEM-85-3-0329. [DOI] [Google Scholar]
- Singh NK, Shepherd KW. Linkage mapping of the genes controlling endosperm proteins in wheat. 1. Genes on the short arms of group-1 chromosome. Theor Appl Genet. 1988;75:628–641. doi: 10.1007/BF00289132. [DOI] [Google Scholar]
- Singh NK, Shepherd KW, Cornish GB. A simplified SDS-PAGE procedure for separating LMW subunits of glutenin. J Cereal Sci. 1991;14:203–208. doi: 10.1016/S0733-5210(09)80039-8. [DOI] [Google Scholar]
- Singh S, Singh N, MacRitchie F. Relationship of polymeric proteins with pasting, gel dynamic- and dough empirical-rheology in different Indian wheat varieties. Food Hydrocoll. 2011;25:19–24. doi: 10.1016/j.foodhyd.2010.05.001. [DOI] [Google Scholar]
- Singh N, Kaur A, Katyal M, Singh AM, Ahlawat AK, Bhinder S. Diversity in quality traits amongst Indian wheat varieties II: paste, dough and muffin making properties. Food Chem. 2016;197:316–324. doi: 10.1016/j.foodchem.2015.10.035. [DOI] [PubMed] [Google Scholar]
- Song Y, Zheng Q. Dynamic rheological properties of wheat flour dough and proteins. Trends Food Sci Technol. 2007;18:132–138. doi: 10.1016/j.tifs.2006.11.003. [DOI] [Google Scholar]
- Troccoli A, Borreli GM, De Vita P, Fares C, Di Fonzo N. Durum wheat quality: a multidisciplinary concept. J Cereal Sci. 2000;32:99–113. doi: 10.1006/jcrs.2000.0322. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.