Abstract
To determine the effect of maturity stage on the food attributes of hihatsumodoki (Piper retrofractum Vahl) fresh fruit, the flavor characteristics and antioxidant capacities were investigated at green (GM), orange (OM), and red maturity (RM) stages. Total organic acids, total free amino acids (FAA), and piperine decreased with increasing fruit maturation, reaching minima at the RM stage. Conversely, total sugars and the FAA that contribute to both umami and sweetness were the highest RM stage. Principal component analysis revealed that the volatile composition of the fruit at the GM stage was clearly different from that at the other stages. The DPPH radical scavenging activity and total phenolic content, as measures of antioxidant capacity, decreased with increasing fruit maturation from GM to RM, which was consistent with the changes in piperine content. Therefore, the maturity stage has a significant influence on the flavor and antioxidant characteristics of hihatsumodoki fresh fruit.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3040-2) contains supplementary material, which is available to authorized users.
Keywords: Piper retrofractum, Maturity stage, Flavor, Piperine, Antioxidant capacity
Introduction
Javanese long pepper (Piper retrofractum Vahl), known as hihatsumodoki in Japan, is native to Southeast Asia and mostly cultivated in Indonesia and Thailand. In Japan, the fresh and/or dried fruit of the pepper are traditionally used not only as a seasoning with a unique pungent taste and aroma, but also for various therapeutic purposes. In fact, the pepper fruit contains medicinal ingredients, such as the main pungent alkaloid piperine and other phenolic compounds (Luyen et al. 2014), which show anti-obesity (Kim et al. 2011), hepatoprotective (Matsuda et al. 2009), and antioxidative effects (Chonpathompikunlert et al. 2010).
The response of common fruits and vegetables to processing can be affected by many factors, such as cultivar, maturity stage, food-processing, post-cutting treatment, and storage conditions (Gil et al. 2006). In most fruits, the maturity stage is an important factor that influences the composition of the flavor compounds that contribute to the taste and aroma of the fruit. Furthermore, the maturity stage has a direct effect on the bioactive compounds in fruits. For example, the total antioxidant capacity in persimmon fruit changes with the content of phenolic compounds, such as flavonoids, tannins, and phenolic acids, during maturation (Sanchísa et al. 2015).
With maturation, the color of hihatsumodoki fresh fruit changes from green (GM) to orange (OM), and finally to red (RM). The fruit is unsuitable for eating or processing until it ripens to the GM stage, and the flavor characteristics of the fruit change throughout maturation. However, the production of various processed products does not, in general, use fruit at a specific maturity stage because there is no information available related to the effect of maturity stage on food attributes. Accordingly, it is of great interest to determine the food qualities, such as the antioxidative capacity, in hihatsumodoki fresh fruit at the three edible maturity stages (GM, OM, and RM), to determine the appropriate fruit properties for various processed products.
The aim of this work was to evaluate the influence of maturity stage (GM, OM, and RM) on several flavor quality attributes, such as the composition of sugars, amino acids, and organic acids, and the content of piperine and volatile components. To determine the importance of the phytochemical and antioxidant properties of the pepper, we evaluated the functionality of the fruit at each maturity stage by analysing the total phenolic content (TPC) and antioxidant capacity using 1,1-diphenyl-2-picrylhydrazine (DPPH) free radical scavenging activity assays. This is the first report on the flavor characteristics, in relation to taste and aroma, and bioactive functionality, including antioxidant capacity, of P. retrofractum Vahl fresh fruit at different maturity stages.
Materials and methods
Hihatsumodoki fruit samples
The fresh hihatsumodoki (P. retrofractum Vahl) fruit used in this study was cultivated at the Subtropical Field Science Center, University of the Ryukyus, Okinawa, Japan (latitude 26°24′N, longitude 127°75′E). A total of 20 clusters of the pepper fruit were harvested randomly from different parts of several trees of the same species in August 2015 at three edible maturity stages, as determined by the surface color based on Hunter Lab scale. This scale uses L* (luminosity, white–black), a* (green–red), and b* (yellow–blue) color parameters. The fruit were sorted into three maturity stages based on the external color: green maturity (GM; L* = 29.80 ± 2.19, a* = 6.35 ± 1.81, b* = 16.06 ± 2.79), orange maturity (OM; L* = 32.22 ± 2.37, a* = 10.91 ± 3.33, b* = 19.98 ± 2.48), and red maturity (RM; L* = 33.44 ± 3.69, a* = 20.63 ± 4.24, b* = 24.32 ± 3.90). The fruit were frozen and stored at –30 °C in a freezer until they were analyzed, unless otherwise indicated.
Chemicals
Standard sugars (sucrose, glucose, and fructose) and organic acids (malic, succinic, and citric acids) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Chemicals used as standards to identify the amino acid components were obtained from Sigma-Aldrich (St. Louis, MO, USA). Chemicals used as standards to identify the volatile components were obtained from Tokyo Chemical Industry (Tokyo, Japan) and Sigma-Aldrich. Gallic acid and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were obtained from Wako Pure Chemical Industries. 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) was purchased from Calbiochem (San Diego, CA, USA). Folin–Ciocalteu reagent was purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). All other reagents were of analytical grade.
Sugar composition analysis
Sugars (sucrose, glucose, and fructose) were extracted from the fruit according to the method of Sakamoto et al. (2012) with slight modifications. Freeze-dried samples of whole fruit with the peduncles removed were ground into powder. Then, a 0.5 g aliquot was put into 10 mL of purified water. The mixture was shaken for 1 h at 80 °C, and then centrifuged (CR 20 GIII, Hitachi, Japan) at 1800×g for 20 min. The extraction was repeated two times and the supernatant was collected. Equal volumes of the supernatant and acetonitrile were mixed well then centrifuged at 16,000×g for 5 min. The sugars in the supernatant were analyzed using high-performance liquid chromatography (HPLC) on a Unison UK-Amino column (250 × 3.0 mm i.d., Imtact Corp., Kyoto, Japan) with a low-temperature evaporative light-scattering detector (ELSD-LT; Shimadzu Corp., Kyoto, Japan) using a mobile phase consisting of water and acetonitrile (20:80, v/v). The flow rate and oven temperature were 0.5 mL/min and 45 °C, respectively. All measurements reported here are the means calculated from at least three independent experiments. The concentration of each sugar was calibrated using the water content of each sample and expressed as mg/100 g-fresh weight (FW).
Amino acid composition analysis
The free amino acid (FAA) composition of the pepper fruit was determined using an HPLC system with a Nexera SIL-30AC autosampler (Shimadzu Corp., Kyoto, Japan) for automated pretreatment (Herbert et al. 2000). Briefly, the extract was prepared using the procedure described in “Sugar composition analysis” section. Equal volumes of the extraction solution (500 μL) and ethanol (500 μL) were well mixed and then 800 μL of a 0.1 N HCl aqueous solution was added. After filtration, the mixture was analyzed using HPLC. The FAA were automatically derivatized with o-phthalaldehyde (OPA) within the autosampler to obtain fluorescent substance. After the derivatization reaction, the OPA-derivatized amino acids were separated using an ultra-high-speed YMC-Triart C18 column (75 mm × 3.0 mm i.d., YMC Co., Ltd., Kyoto, Japan) using a gradient solvent program consisting of various proportions (from 89:11 to 0:100, v/v) of 20 mmol/L phosphate potassium buffer (pH 6.9) and a 45:40:15 (v/v) acetonitrile/methanol/water solution at a flow rate of 0.8 mL/min. The OPA-derivatized amino acids (λx = 490 nm, λm = 520 nm) were detected with a RF-20Axs fluorescence detector (Shimadzu Corp., Kyoto, Japan). The concentration of each FAA was calibrated using the water content of each sample and expressed as mg/100 g-FW.
Organic acid composition analysis
The organic acid composition (particularly malic, succinic, and citric acids) of the pepper fruit was determined using HPLC with electroconductivity detection (Ji et al. 2009). Briefly, the fruit were sliced and then an approximately 5 g sample was homogenized with 20 mL of purified water using a homogenizer (Ultra Turrax T25 basic, Labortechnik, Wasserburg, Germany). The mixture was centrifuged at 25,000×g at 4 °C for 30 min. The supernatant was collected and filtered through Sep-Pak C18 and a 0.45 μm cellulose acetate membrane filter, and then diluted before injection. A Shim-pack SCR-102H column (300 mm × 8 mm i.d., Shimadzu Corp., Kyoto, Japan) connected to a guard column (50 mm × 6 mm i.d.) was used. Two Shimadzu LC-10 AD pumps were used for the mobile phase containing 5 mmol/L p-toluenesulfonic acid and the post-column detection reagent containing 5 mmol/L p-toluenesulfonic acid, 100 μmol/L ethylenediaminetetraacetic acid disodium salt, and 20 mmol/L Bis–Tris buffer in isocratic mode at a flow rate of 0.7 mL/min. The mobile phase and post-column detection solvent were mixed in a post-column reactor in a 1:1 ratio before detection with a Shimadzu CDD-6A conductivity detector. The column, guard column, and post-column reactor were maintained at a constant temperature of 40 °C using a Shimadzu CTO-10 AC oven, and the injection volume was 10 μL. The concentrations of malic, succinic, and citric acids were expressed as mg/100 g-FW. All assays were performed in triplicate.
Piperine analysis
The piperine content in the pepper fruit was determined using HPLC with UV/Vis detection (Wu et al. 2004). Briefly, the fresh fruit were sliced and then an approximately 3 g sample was homogenized with 10 mL of methanol using a homogenizer. The obtained suspension was centrifuged at 25,000×g at 25 °C for 30 min. The supernatant was collected, filtered, and then diluted before injection. Separation of piperine was achieved using a YMC Triart PFP (150 mm × 4.6 mm) packed column (YMC Co., Ltd., Kyoto, Japan) with a CTO-20 AC column oven set at 25 °C. Up to 2 μL of sample was injected and eluted with an isocratic gradient of 0.1% formic acid aqueous solution and acetonitrile (60:40, v/v) with a flow rate of 1.5 mL/min. The wavelength employed for fluorescence detection was 340 nm. Quantification was performed using external calibration curve of piperine standard, and the concentration was expressed as mg/g-FW.
Volatile aroma component analysis
The volatile aroma components in the pepper fruit samples were analyzed using headspace gas chromatography-flame ionization detection/mass spectrometry (HSGC-FID/MS) (Takahashi et al. 2016). Briefly, 1 g of sliced fruit was placed into a 20 mL headspace vial, which was immediately sealed with an aluminium crimp cap. The HSGC-FID analysis was performed using a 7890A gas chromatograph (GC) equipped with a G1888 headspace auto-sampler (Agilent, Santa Clara, CA, USA). The extraction conditions of the sampler were as follows: oven temperature, 60 °C; loop temperature, 170 °C; transfer line temperature, 210 °C; and sample equilibration time, 20 min. The extracted volatile compounds were injected using a split ratio of 1:10 and then separated on a fused silica capillary column (DB-Wax, 60 m × 0.25 mm i.d., 0.25 μm film thickness, Agilent). The oven temperature was initially set to 40 °C for 5 min, then increased to 200 °C at a rate of 5 °C/min, and then held isothermally at 200 °C for 3 min. The peak area response of the volatile components was monitored to evaluate their relative amounts (%) without using correction factors.
Mass spectral analysis of the volatile components was performed using the same GC instrument (with the same GC conditions) coupled to a 5975C mass spectrophotometer (Agilent). For mass spectrophotometry (MS) detection, both the electron impact ion source and interface temperatures were 230 °C, and the ionization energy was 70 eV. The mass acquisition scan range and rate were (m/z) 29–450 amu and 1.77 scans/s, respectively. The volatile components were identified by comparison of the linear retention indices (RIs) and mass spectra fragmentation patterns with MS data for the corresponding compounds obtained from the National Institute of Standards and Technology (NIST) MS Library, Version 2008, and peak enrichment upon co-injection with authentic standards. The linear RIs of the volatile components were determined relative to the retention times of a series of n-alkanes (C7–C28). All assays were performed in triplicate.
Total phenolic content analysis
The TPC of freeze-dried piper samples was examined using the Folin–Ciocalteu method (Takahashi et al. 2016). Briefly, the fruit were freeze-dried and then approximately 0.5 g of dried fruit was incubated with 10 mL of 80% ethanol for 90 min at room temperature. The obtained suspension was centrifuged at 25,000×g at 25 °C for 30 min, and the supernatant was used for the TPC assays. Various concentrations of diluted sample (20 μL), distilled water (60 μL), and Folin–Ciocalteu reagent (15 μL, previously diluted twofold with distilled water) were transferred to a 96-well microplate (Nunc, Roskilde, Denmark) and mixed well. The microplate was immediately placed in a microplate reader (PowerWave™ XS2, BioTek, Winooski, VT, USA), agitated, and then allowed to stand for 15 min until stable absorption values were obtained. The absorbance was then measured at 750 nm. The TPC was calculated from a linear gallic acid calibration curve and expressed as milligrams of gallic acid equivalents (GAE)/g-FW. All assays were performed in triplicate.
DPPH radical scavenging activity analysis
The antioxidant capacities of the pepper fruit samples were evaluated in terms of their DPPH radical scavenging activities (Takahashi et al. 2016). Briefly, the diluted pepper fruit samples were prepared as described in “Total phenolic content analysis” section for TPC analysis, and various concentrations of diluted sample (50 μL) and 0.1 mM DPPH methanol solution (150 μL) were mixed in a Nunc 96-well microplate. After vigorous shaking, the microplate was immediately placed in a PowerWave™ XS2 microplate reader and left to stand for 30 min until stable absorption values were obtained. The reduction in DPPH radicals by the sample was examined by measuring the absorption at 517 nm. The DPPH radical scavenging activity was calculated from a linear Trolox calibration curve and expressed as µmol Trolox equivalents (TE)/g-FW. All assays were performed in triplicate.
Statistical analysis
The differentiation of volatile components in hihatsumodoki fresh fruit at three edible maturity stages was evaluated using principal component analysis (PCA) with Microsoft Office Excel 2007 (Microsoft Corp., Redmond, WA, USA). The results are expressed as mean ± S.D. The data were analyzed using one-way ANOVA, and differences among the means of groups were analyzed using Fisher’s least significant difference post hoc test. The differences between means were considered significant at p < 0.05.
Results and discussion
Effects of maturity on sugar and organic acid compositions
The sugar and organic acid compositions of hihatsumodoki fresh fruit at the three edible maturity stages are shown in Table 1. The maturity stage had a significant influence on the sugar and organic acid composition in the fruit. The total sugar content in each fruit significant increased (p < 0.05) with maturation, and the fruit at the RM stage (489.85 mg/100 g-FW) were observed to have 2.4- and 1.7-fold higher amounts of total sugars than the fruit at the GM (202.56 mg/100 g-FW) and OM stages (284.98 mg/100 g-FW), respectively. The sugar in each fruit mainly consisted of glucose and fructose, and the ratio of glucose to fructose (G/F) decreased with fruit maturation; that is, the mean amount of fructose increased relative to glucose during the fruit ripening process, with fructose being the dominant sugar in the fruit at the RM stage. Furthermore, this trend is in agreement with the general trend reported in numerous studies of increasing levels of fructose compared with glucose at the advanced stages of fruit maturity for a variety of fruits, including apple, medlar, strawberry, and grape (Zhao et al. 2015).
Table 1.
Sugars and organic acids in P. retrofractum fresh fruit at different maturity stages
| Analysis | GM | OM | RM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sugars (mg/100 g-FW) | |||||||||
| Glucose | 95.49 | ± | 1.55 c | 119.46 | ± | 0.38 b | 134.32 | ± | 1.84 a |
| Fructose | 107.07 | ± | 5.40 c | 165.52 | ± | 6.27 b | 355.53 | ± | 11.53 a |
| Sucrose | N.D. | N.D. | N.D. | ||||||
| Total sugars | 202.56 | ± | 6.94 c | 284.98 | ± | 5.92 b | 489.85 | ± | 13.33 a |
| Glucose/fructose (G/F) | 0.89 | ± | 0.03 a | 0.72 | ± | 0.03 b | 0.38 | ± | 0.01c |
| Organic acid (mg/100 g-FW) | |||||||||
| Malic acid | 8.71 | ± | 0.20 a | 5.43 | ± | 0.34 b | 4.86 | ± | 0.11 b |
| Succinic acid | 0.04 | ± | 0.01 b | 0.05 | ± | 0.01 b | 0.08 | ± | 0.01 a |
| Citric acid | 0.58 | ± | 0.03 a | 0.44 | ± | 0.01 b | 0.64 | ± | 0.02 a |
| Total acids | 9.33 | ± | 0.22 a | 5.92 | ± | 0.35 b | 5.58 | ± | 0.10 b |
Values are expressed as mean ± S.D. (n = 3). Different letters indicate values that are significantly different by the Fisher’s least significant difference post hoc test (p < 0.05)
N.D. not detected
The composition and concentration of organic acids are important factors that determine consumer perceptions of both sweetness and sourness in numerous fruits, including spices (Fawole and Opara 2013). In this study, the fruit at the GM, OM, and RM stages had a total acid content of 9.33, 5.29, and 5.58 mg/100 g-FW, respectively; the most abundant organic acid was malic acid (93.8, 91.7, and 87.1%, respectively), followed by citric acid and succinic acid at each maturity stage (Table 1). The malic acid content was significantly decreased with fruit maturation, whereas the succinic and citric acid contents remained constant. The evolution of total organic acids in this study supports the general phenomenon of organic acids accumulating during fruit growth and being used as respiratory substrates in mature fruit (Fawole and Opara 2013).
Effects of maturity on amino acid composition
The taste characteristics of each FAA are greatly influenced by their concentration, pH, and other coexisting substances, such as inhibitors and enhancers, which may blur the boundaries for taste classification (Murata 2002). In several foods, FAA can be grouped as flavor enhancing (desirable) or flavor detracting (undesirable) for the Japanese palate (Woods et al. 2008). Flavor-enhancing amino acids are typically sweet (e.g. glycine, alanine, serine, and threonine), umami and/or sour (e.g. glutamic acid, aspartate, and glutamate). In contrast, flavor-detracting amino acids are bitter (e.g. arginine, histidine, lysine, phenylalanine, tyrosine, methionine, tryptophan, leucine, isoleucine, and valine). FAA with no detectable flavor (tasteless) are asparagine, cysteine, and γ-aminobutanoic acid (Woods et al. 2008).
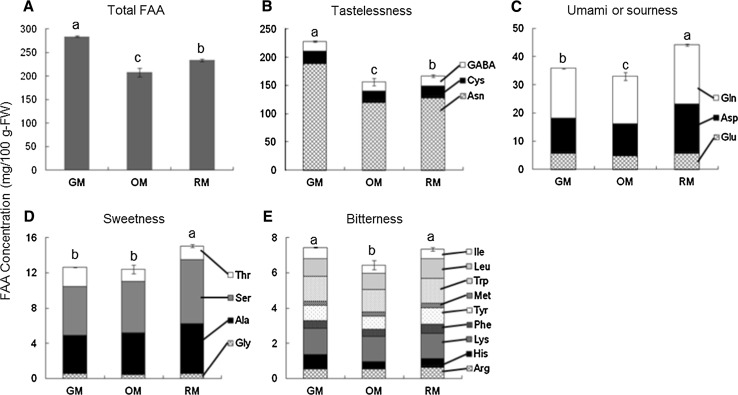
In this study, the total FAA content decreased during fruit maturation with 284.1, 208.3, and 233.9 mg/100 g-FW at the GM, OM, and RM stages, respectively (Fig. 1A). This decrease was attributed mainly to a decrease in the total tasteless FAA, including the most abundant FAA, asparagine (Fig. 1B). In view of the taste classifications, umami and/or sour were the dominant flavor throughout fruit maturation, followed by sweetness and bitterness. With fruit growth, the contents of glutamic acid, aspartate, and glutamate, as umami and/or sour tastes in hihatsumodoki fruit, initially decreased and then increased, with the total content of umami and/or sour FAA reaching a maximum (44.2 mg/100 g-FW) at the RM stage (Fig. 1C). The total content of sweet FAA at the RM stage was also significantly increased compared with those at the GM and OM stages (Fig. 1D), whereas there was no significant difference in the total bitter FAA between the GM and RM stages (Fig. 1E). These results indicate that hihatsumodoki fruit has more flavor-enhancing FAA than flavor-detracting FAA in all maturity stages, with increased flavor-enhancement at the RM stage compared with the GM and OM stages. Thus, the FAA content and the composition of the umami and/or sourness and sweetness indices might play an important role in determining acceptance of the fruit at the three maturity stages by consumers.
Fig. 1.
Free amino acid (FAA) composition in P. retrofractum fresh fruit at different maturity stages. Each graph shows a FAA taste classification: A total FAA, B tastelessness, C umami or sourness, D sweetness, and E bitterness. The values are expressed as mean ± S.D. (n = 3). Different letters indicate values that are significantly different by the Fisher’s least significant difference post hoc test (p < 0.05)
Effects of maturity on piperine
Piperine is responsible for the pungency of hihatsumodoki. The piperine content in the fresh fruit at the three edible maturity stages is shown in Fig. 2. The maturity stage had a significant influence on the piperine content, with a significantly higher (p < 0.05) piperine content in the fruit at the GM stage (23.59 mg/g-FW) than at the OM (14.24 mg/g-FW) and RM stages (12.97 mg/g-FW). The quantities of piperine obtained in P. retrofractum in this study were higher than those previously reported for the fresh fruit of various Piper species harvested in India, such as P. nigrum (7.22 mg/g-FW), P. retrofractum (5.53 mg/g-FW), P. longum (2.15 mg/g-FW), P. hymenophyllum (0.31 mg/g-FW), P. umbellatum (2.10 μg/g-FW), and P. attenuatum (1.65 μg/g-FW) (Chandra et al. 2015). This could be attributed to differences in the cultivar and sources of the materials, as well as regional differences (Wang et al. 2016). These results indicate that hihatsumodoki fresh fruit in Japan is one of the most abundant sources of piperine among Piper species, and the piperine content is clearly dependent on maturity stage. For certain applications, it might be important to ensure that hihatsumodoki fruit is harvested at the GM stage, when the piperine content is highest.
Fig. 2.
Piperine concentration in P. retrofractum fresh fruit at different maturity stages. The values are expressed as mean ± S.D. (n = 3). Different letters indicate values that are significantly different by the Fisher’s least significant difference post hoc test (p < 0.05)
Effects of maturity on volatile compounds
Thirty-four volatile compounds, including aldehydes, alcohols, hydrocarbons, ketones, ethers, and an acid, were identified in hihatsumodoki fresh fruit at the three edible maturity stages (Table 2). a strongly influence on the total volatile compound content in the fruit, with the total identified volatile compounds decreasing from a total area (× 105) of 1547.42 (GM) to 1109.28 (OM), and then to 1068.67 (RM). The total areas of 28 compounds identified in the fruit at the GM stage had clearly decreased at the RM stage, whereas only 6 constituent compounds increased during fruit maturation. The aldehyde 3-methyl butanal, which imparts a malty/green note and was reported as one of the most potent odorants in black pepper (Jagella and Grosch 1999), was the most predominant volatile aroma compound at any maturity stage in this work. The levels of 3-methyl butanal increased from 247.91 (GM) to 268.51 (OM), and then to 323.13 (RM) in total area.
Table 2.
Volatile compounds in P. retrofractum fresh fruit at different maturity stages
| # | RIa | Compound | Total area (× 105) | Odor descriptorb | Identificationc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | OM | RM | |||||||||||
| Aldehydes | |||||||||||||
| 1 | 892 | 3-Methyl-1-butanal | 247.91 | ± | 6.09 | 268.51 | ± | 5.33 | 323.13 | ± | 1.01 | Maltyd, greene | RI, MS |
| 2 | 1075 | Hexanal | 10.85 | ± | 3.91 | 4.25 | ± | 1.59 | 2.05 | ± | 0.60 | Green, grassf | RI, MS, PC |
| 3 | 1214 | 2-Hexenal | 15.84 | ± | 12.05 | 4.02 | ± | 1.63 | 1.77 | ± | 0.83 | Fragrant, sweetf, fruity, greene | RI, MS, PC |
| Sub-total | 274.60 | 276.78 | 326.95 | ||||||||||
| Alcohols | |||||||||||||
| 4 | 909 | Ethanol | 6.08 | ± | 0.88 | 2.75 | ± | 0.25 | 3.27 | ± | 0.70 | Alcoholg | RI, MS |
| 5 | 1544 | Linalool | 169.53 | ± | 8.64 | 97.00 | ± | 2.93 | 94.59 | ± | 2.88 | Fresh, floral, clean, sweet, lemong | RI, MS, PC |
| Sub-total | 175.60 | 99.75 | 97.87 | ||||||||||
| Hydrocarbons | |||||||||||||
| 6 | 1015 | α-Pinene | 62.87 | ± | 5.47 | 32.45 | ± | 8.00 | 28.75 | ± | 3.09 | Fruity, pineyh | RI, MS, PC |
| 7 | 1060 | Camphene | 30.82 | ± | 1.04 | 15.73 | ± | 1.49 | 16.34 | ± | 1.79 | Camphoraceous, mild-oilyi | RI, MS, PC |
| 8 | 1096 | β-Pinene | 43.75 | ± | 7.43 | 24.00 | ± | 4.73 | 21.46 | ± | 2.11 | Woody, pineyh | RI, MS, PC |
| 9 | 1151 | β-Myrcene | 14.21 | ± | 2.44 | 7.58 | ± | 0.72 | 7.39 | ± | 0.95 | Mild, sweeti | RI, MS, PC |
| 10 | 1188 | α-Limonene | 29.18 | ± | 5.54 | 15.58 | ± | 2.55 | 13.62 | ± | 1.99 | Fresh, citrus, lemonj | RI, MS, PC |
| 11 | 1224 | β-Ocimene | 50.61 | ± | 0.29 | 28.11 | ± | 3.60 | 22.75 | ± | 3.24 | Green, herbali, fruityh | RI, MS |
| 12 | 1242 | α-Ocimene | 95.40 | ± | 1.18 | 48.98 | ± | 4.32 | 41.25 | ± | 3.79 | Fruity, wet clothh | RI, MS |
| 13 | 1256 | Terpinolene | 3.46 | ± | 1.60 | 1.86 | ± | 0.26 | 2.15 | ± | 0.28 | Pineyi, lemonj | RI, MS, PC |
| 14 | 1289 | Tridecane | 9.09 | ± | 1.38 | 15.27 | ± | 3.98 | 17.76 | ± | 1.28 | – | RI, MS, PC |
| 15 | 1494 | n-Pentadecane | 26.00 | ± | 4.60 | 48.44 | ± | 8.84 | 52.83 | ± | 3.03 | – | RI, MS, PC |
| 16 | 1501 | α-Copaene | 9.75 | ± | 1.26 | 5.02 | ± | 0.41 | 4.58 | ± | 0.45 | Sweet, floralk | RI, MS |
| 17 | 1614 | β-Caryophyllene | 235.27 | ± | 4.28 | 126.18 | ± | 5.76 | 100.18 | ± | 6.52 | Woody, spicyj | RI, MS, PC |
| 18 | 1666 | trans-β-Farnesene | 12.72 | ± | 5.18 | 13.22 | ± | 3.86 | 10.70 | ± | 1.53 | Citrus, greenk | RI, MS |
| 19 | 1689 | α-Caryophyllene | 155.69 | ± | 3.79 | 87.90 | ± | 2.85 | 72.66 | ± | 5.36 | Woody, spicyj | RI, MS, PC |
| 20 | 1696 | α-Cadinene | 13.85 | ± | 0.99 | 25.66 | ± | 1.36 | 26.63 | ± | 2.54 | Woodyl | RI, MS |
| 21 | 1704 | β-Bisabolene | 6.67 | ± | 0.56 | 4.10 | ± | 0.67 | 4.10 | ± | 0.78 | Woody, leafm | RI, MS, PC |
| 22 | 1709 | 8-Heptadecene | 8.59 | ± | 0.80 | 4.45 | ± | 1.66 | 3.16 | ± | 0.93 | Earthy, mossn | RI, MS |
| 23 | 1719 | Epizonarene | 8.69 | ± | 0.39 | 17.16 | ± | 1.43 | 17.35 | ± | 0.74 | – | RI, MS |
| 24 | 1729 | γ-Muurolene | 155.75 | ± | 1.98 | 132.74 | ± | 7.91 | 108.33 | ± | 2.66 | Woodyo | RI, MS |
| 25 | 1745 | α-Selinene | 21.19 | ± | 1.69 | 12.47 | ± | 1.73 | 11.22 | ± | 1.56 | Woodyj | RI, MS |
| 26 | 1772 | (+)-δ-Cadinene | 11.49 | ± | 0.87 | 6.94 | ± | 1.08 | 7.16 | ± | 1.68 | Spicy, woodyj | RI, MS |
| 27 | 1778 | γ-Cadinene | 7.40 | ± | 0.58 | 4.40 | ± | 0.63 | 4.58 | ± | 0.92 | Fragrantp | RI, MS |
| 28 | 1786 | α-Panasinsene | 19.45 | ± | 2.04 | 11.26 | ± | 1.57 | 9.77 | ± | 0.84 | – | RI, MS |
| Sub-total | 1031.89 | 689.52 | 604.70 | ||||||||||
| Acid | |||||||||||||
| 29 | 1451 | Acetic acid | 15.27 | ± | 0.52 | 9.60 | ± | 1.48 | 7.65 | ± | 1.70 | Sour, vinegarj | RI, MS, PC |
| Sub-total | 15.27 | 9.60 | 7.65 | ||||||||||
| Ketones | |||||||||||||
| 30 | 1389 | 2-Nonanone | 14.22 | ± | 7.98 | 7.04 | ± | 1.12 | 8.56 | ± | 1.73 | Mintq | RI, MS |
| 31 | 1526 | Camphor | 8.93 | ± | 7.21 | 17.13 | ± | 6.14 | 14.50 | ± | 1.50 | Camphorr | RI, MS, PC |
| 32 | 1588 | Calarene | 6.00 | ± | 0.67 | 3.59 | ± | 0.43 | 3.36 | ± | 0.19 | Greenl | RI, MS |
| Sub-total | 29.15 | 27.77 | 26.42 | ||||||||||
| Ethers | |||||||||||||
| 33 | 817 | Methyl acetate | 6.24 | ± | 1.37 | 2.23 | ± | 0.22 | 1.72 | ± | 0.10 | Fruitys | RI, MS |
| 34 | 1861 | Methyl 3-phenylpropanoate | 14.67 | ± | 1.73 | 3.63 | ± | 0.72 | 3.36 | ± | 0.15 | – | RI, MS |
| Sub-total | 20.91 | 5.86 | 5.08 | ||||||||||
| Total identified/detected | 1547.42 | 1109.28 | 1068.67 | ||||||||||
Values are expressed as mean ± S.D. (n = 3)
aRetention indices relative to n-alkanes on a polar DB-Wax column
bOdor descriptors of volatile compounds identified in the literature
cIdentification based on retention index (RI), NIST MS library (MS), and authentic standards analyzed by mass spectrometry (PC)
dKumazawa and Masuda (2002)
eWang et al. (2008)
fQin et al. (2013)
gJirovetz et al. (2005)
hJordán et al. (2003)
iAlvarez et al. (2011)
jCheong et al. (2012)
kUsami et al. (2013)
lCosta et al. (2008)
mMacleod and De Troconis (1982)
nSelli et al. (2006)
oSant’Anna et al. (2007)
pMacLeod and Pieris (1982)
qGalvão et al. (2011)
rVera et al. (2014)
sSu and Chien (2010)
Notably, 23 of the constituent volatile compounds in the fruit were terpene hydrocarbons, including 10 monoterpene compounds (32.88% of total volatile compounds at the GM stage) and 13 sesquiterpene compounds (42.90%). Some of these terpene compounds are key aroma components in various pepper fruits, imparting mostly pleasant and herbaceous characteristics (Jagella and Grosch 1999). The most abundant monoterpene was linalool (10.96% of total volatile compounds at the GM stage), followed by α-ocimene (6.16%), α-pinene (4.06%), β-ocimene (3.27%), camphene (1.99%), and α-limonene (1.89%), which contribute potent sweet, fresh, citrus, and fruity odors (Alvarez et al. 2011; Jirovetz et al. 2005; Jordán et al. 2003). In addition, β-caryophyllene, γ-muurolene, and α-caryophyllene were the principal sesquiterpene hydrocarbons in the fruit (15.20, 10.07, and 10.06% of total volatile compounds at the GM stage, respectively), which might provide woody, herbal, and floral odors (Cheong et al. 2012; Sant’Anna et al. 2007; Su and Chien 2010). Each mono- and sesquiterpene, except camphor, continuously decreased as the fruit matured, with the total detected mono- and sesquiterpene compounds decreasing by 24.59% and 35.62%, respectively, from the GM stage. In addition to 3-methyl-1-butanal and terpene compounds, other odorants detected in the fruit at the GM, OM, and RM stages included hexanal and 2-hexenal (aldehydes), ethanol (alcohol), acetic acid (acid), 2-nonanone (ketone), and methyl acetate (ether). However, with the exception of acetic acid, these compounds have not been observed in other Piper species, such as P. nigrum, P. longum, and P. guneense (Jirovetz et al. 2002; Liu et al. 2007). These compounds exhibited continuously decreasing trends with fruit maturation, similar to the trend observed for the total terpene content.
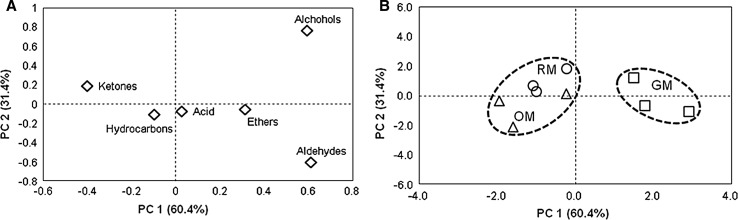
Differences in the composition and content of the isolated volatile aroma components were also examined using PCA. Approximately 91.8% of the total variance was accounted for by the first two identified principal components (Fig. 3). The first factor (PC 1) was responsible for 60.4% of the total variation, whereas the second factor (PC 2) explained only 31.4% of the total variation, indicating that the maximum possible variation in the fruit maturity indices was explained by PC 1 (Fig. 3a). Accordingly, compositional discrimination of the volatile compounds categorized by 6 functional groups (aldehydes, alcohols, hydrocarbons, acids, ketones, and ethers) could be achieved for hihatsumodoki fresh fruit at the GM, OM, and RM stages. Clear separation of each type of functional group in the volatile compounds was realized. The groups most positively correlated with PC 1 are aldehydes and alcohols, including the oxygenated monoterpene linalool, followed by ethers and acids, whereas ketones and hydrocarbons, which mostly comprise terpene hydrocarbons, were negatively associated with PC 1 (Fig. 3a). Positive scores in PC 1 corresponded to the GM stage, whereas the OM and RM stages had negative scores in PC 1 (Fig. 3b). Interestingly, the fruit at the GM stage was clearly separated from those the OM and RM stages, which were overlapped. As the fruit advanced in maturity, there was a shift from right to left along PC 1, with an increase in fruit color formation and odors of both hydrocarbons and ketones (Fig. 3a, b). These discrimination results for the functional groups composition agree with the volatile aroma components of the fruit presented in Table 2, revealing the distinctive of the fruit in each maturity stage. Accordingly, the different relative levels of volatile aroma components in hihatsumodoki fresh fruit might explain the distinctive aroma of hihatsumodoki when compared with other Piper species, and might also lead to different aroma properties at the GM, OM, and RM stages.
Fig. 3.
PCA plots of volatile compounds of P. retrofractum fresh fruit: a distribution of volatile compounds categorized by six functional groups and b discrimination of different maturity stages based on the contents of six functional groups in the volatile compounds
Effects of maturity on DPPH radical scavenging activity and TPC
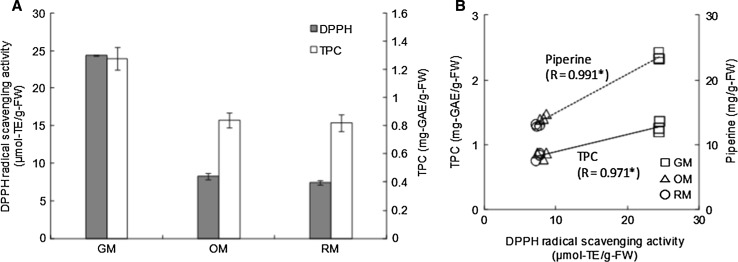
The DPPH radical scavenging activity and TPC were determined in hihatsumodoki fresh fruit at the three maturity stages (Fig. 4). The DPPH radical scavenging activity in the fruit at the GM stage (24.37 µmol-TE/g-FW) was about 3.0-fold and 3.3-fold higher than that at the OM (8.23 µmol-TE/g-FW) and RM stages (7.40 µmol-TE/g-FW), respectively (Fig. 4a). In addition, TPC and DPPH radical scavenging activity in the fruit displayed similar trends, decreasing from the GM to RM stages. This result is in accordance with previously reported results (Wang et al. 2016), and the DPPH radical scavenging activity in the pepper fruit at the GM, OM, and RM stages showed a significant positive correlation with TPC (R = 0.971, p < 0.05) (Fig. 4b). These results are in good agreement with previously reported findings on the phytoconstituents obtained from different Piper species (Chandra et al. 2015). Notably, many studies have revealed that piperamides, especially piperine, in different Piper species possess remarkable biological activities, including good antioxidant properties (Luyen et al. 2014). Interestingly, the DPPH radical scavenging activity showed significant positive correlation with piperine content in the fresh fruit at different maturity stages in our study (R = 0.991, p < 0.05) (Fig. 4b). This result indicates that phenolic compounds and piperine might be responsible for most of the antioxidant capacity in hihatsumodoki fresh fruit. However, further studies are needed to identify the unknown antioxidant substances and the major phenolic compounds present in hihatsumodoki fresh fruit at the different maturity stages.
Fig. 4.
a DPPH radical scavenging activity and total phenolic content (TPC). b Correlation coefficient (R) between TPC and DPPH radical scavenging activity, and between TPC and piperine content in P. retrofractum fresh fruit at different maturity stages. The values are expressed as mean ± S.D. (n = 3). Different letters indicate values that are significantly different by the Fisher’s least significant difference post hoc test (p < 0.05)
Conclusion
The maturity stage had a strong influence on the flavor characteristics and antioxidant capacity of the fruit. The concentrations of sugars, including glucose and fructose, in the fruit were the highest at the RM stage, whereas the total acid contents in the fruit were lowest at the RM stage. At the GM stage, the fruit had the highest content of total FAA, while the content of FAA that contribute umami and/or sourness and sweetness was highest at the RM stage. Moreover, the fruit at the GM stage had a significantly higher piperine content than the fruit at the OM and RM stages, which might influence the pungency of the fruit. Using PCA, the volatile aroma composition in the fruit at the GM stage was clearly separated from the other stages. These changes in the volatile composition are likely to influence the aroma notes. The antioxidant capability of the fruit, measured as the DPPH radical scavenging activity and TPC, decreased with maturation from the GM to RM stage, and these changes were correlated with changes in piperine content. Consequently, the variability in flavor and antioxidant capacities found in this study reveals appropriate harvesting periods for hihatsumodoki fresh fruit with various characteristics. This information can provide a basis for selecting among the three maturity stages of the fruit for various applications by considering the qualities of taste, aroma, and biological activity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (No. 15K00788) from the Japan Society for the Promotion of Science.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3040-2) contains supplementary material, which is available to authorized users.
References
- Alvarez RQ, Passaro CC, Lara OG, Julian L. Relationship between chromatographic profiling by HS-SPME and sensory quality of mandarin juices: effect of squeeze technology. Procedia Food Sci. 2011;1:1396–1403. doi: 10.1016/j.profoo.2011.09.207. [DOI] [Google Scholar]
- Chandra P, Pandeya R, Srivastva M, Rameshkumar KB, Kumar B. Quantitative determination of chemical constituents of Piper spp.using UPLC-ESI-MS/MS. Ind Crops Prod. 2015;76:967–976. doi: 10.1016/j.indcrop.2015.08.010. [DOI] [Google Scholar]
- Cheong MW, Liu SQ, Zhou W, Curran P, Yu B. Chemical composition and sensory profile of pomelo (Citrus grandis (L.) Osbeck) juice. Food Chem. 2012;135:2505–2513. doi: 10.1016/j.foodchem.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Chonpathompikunlert P, Wattanathorn J, Muchimapura S. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer’s disease. Food Chem Toxicol. 2010;48:798–802. doi: 10.1016/j.fct.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Costa R, d’Acampora Zellner B, Crupi ML, De Fina MR, Valentino MR, Dugo P, Dugo G, Mondello L. GC-MS, GC-O and enantio-GC investigation of the essential oil of Tarchonanthus camphoratus L. Flavour Frag J. 2008;23:40–48. doi: 10.1002/ffj.1854. [DOI] [Google Scholar]
- Fawole OA, Opara UL. Changes in physical properties, chemical and elemental composition and antioxidant capacity of pomegranate (cv. Ruby) fruit at five maturity stages. Sci Hortic. 2013;150:37–46. doi: 10.1016/j.scienta.2012.10.026. [DOI] [Google Scholar]
- Galvão MDS, Narain N, dos Santos MDSP, Nunes ML. Volatile compounds and descriptive odor attributes in umbu (Spondias tuberosa) fruits during maturation. Food Res Int. 2011;44:1919–1926. doi: 10.1016/j.foodres.2011.01.020. [DOI] [Google Scholar]
- Gil MI, Aguayo E, Kader AA. Quality changes and nutrient retention in fresh-cut versus whole fruits during storage. J Agric Food Chem. 2006;54:4284–4296. doi: 10.1021/jf060303y. [DOI] [PubMed] [Google Scholar]
- Herbert P, Barros P, Ratola N, Alves A. HPLC determination of amino acids in musts and port wine using OPA/FMOC derivatives. J Food Sci. 2000;65:1130–1133. doi: 10.1111/j.1365-2621.2000.tb10251.x. [DOI] [Google Scholar]
- Jagella T, Grosch W. Flavour and off-flavour compounds of black and white pepper (Piper nigrum L.) I. Evaluation of potent odorants of black pepper by dilution and concentration techniques. Eur Food Res Technol. 1999;209:16–21. doi: 10.1007/s002170050449. [DOI] [Google Scholar]
- Ji FD, Ji BP, Li B, Lu F. Effect of fermentation on nitrate, nitrite and organic acid contents in traditional pickled Chinese cabbage. J Food Process Preserv. 2009;33:175–186. doi: 10.1111/j.1745-4549.2008.00291.x. [DOI] [Google Scholar]
- Jirovetz L, Buchbauer G, Ngassoum MB, Geissler M. Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction-gas chromatography, solid-phase microextraction-gas chromatography-mass spectrometry and olfactometry. J Chromatogr A. 2002;976:265–275. doi: 10.1016/S0021-9673(02)00376-X. [DOI] [PubMed] [Google Scholar]
- Jirovetz L, Buchbauer G, Stoyanova A, Balinova A, Guangjiun Z, Xihan M. Solid phase microextraction/gas chromatographic and olfactory analysis of the scent and fixative properties of the essential oil of Rosa damascena L. from China. Flavour Fragr J. 2005;20:7–12. doi: 10.1002/ffj.1375. [DOI] [Google Scholar]
- Jordán MJ, Margaría CA, Shaw PE, Goodner KL. Volatile components and aroma active compounds in aqueous essence and fresh pink guava fruit puree (Psidium guajava L.) by GC-MS and multidimensional GC/GC-O. J Agric Food Chem. 2003;51:1421–1426. doi: 10.1021/jf020765l. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Lee MS, Jo K, Hwang JK. Piperidine alkaloids from Piper retrofractum Vahl. protect against high-fat diet-induced obesity by regulating lipid metabolism and activating AMP-activated protein kinase. Biochem Biophys Res Commun. 2011;411:219–225. doi: 10.1016/j.bbrc.2011.06.153. [DOI] [PubMed] [Google Scholar]
- Kumazawa K, Masuda H. Identification of potent odorants in different green tea varieties using flavor dilution technique. J Agric Food Chem. 2002;50:5660–5663. doi: 10.1021/jf020498j. [DOI] [PubMed] [Google Scholar]
- Liu L, Song G, Hu Y. GC-MS analysis of the essential oils of Piper nigrum L. and Piper longum L. Chromatographia. 2007;66:785–790. doi: 10.1365/s10337-007-0408-2. [DOI] [Google Scholar]
- Luyen BTT, Tai BH, Thao NR, Yang SY, Cuong NM. A new phenylpropanoid and an alkylglycoside from Piper retrofractum leaves with their antioxidant and α-glucosidase inhibitory activity. Bioorg Med Chem Lett. 2014;24:4120–4124. doi: 10.1016/j.bmcl.2014.07.057. [DOI] [PubMed] [Google Scholar]
- Macleod AJ, De Troconis NG. Volatile flavour components of Guava. Phytochemistry. 1982;21:1339–1342. doi: 10.1016/0031-9422(82)80138-6. [DOI] [Google Scholar]
- Macleod AJ, Pieris NM. Volatile flavour components of mangosteen, Garcinia mangostana. Phytochemistry. 1982;21:117–119. doi: 10.1016/0031-9422(82)80025-3. [DOI] [Google Scholar]
- Matsuda H, Ninomiya K, Morikawa T, Daisuke Y, Yamaguchi I, Yoshikawa M. Hepatoprotective amide constituents from the fruit of Piper chaba: structural requirements, mode of action, and new amides. Bioorgan Med Chem. 2009;17:7313–7323. doi: 10.1016/j.bmc.2009.08.050. [DOI] [PubMed] [Google Scholar]
- Murata Y. Studies on a novel bitter amino acid Pulcherrimine in the green sea urchin gonads. Bull Fish Res Agen. 2002;3:31–61. [Google Scholar]
- Qin Z, Pang X, Chen D, Cheng H, Hu X, Wu J. Evaluation of chinese tea by the electronic nose and gas chromatography-mass spectrometry: correlation with sensory properties and classification according to grade level. Food Res Int. 2013;53:864–874. doi: 10.1016/j.foodres.2013.02.005. [DOI] [Google Scholar]
- Sakamoto T, Hasunuma T, Hori Y, Yamada R, Kondo A. Direct ethanol production from hemicellulosic materials of rice straw by use of an engineered yeast strain codisplaying three types of hemicellulolytic enzymes on the surface of xylose-utilizing Saccharomyces cerevisiae cells. J Biotechnol. 2012;158:203–210. doi: 10.1016/j.jbiotec.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Sanchísa E, Mateosb M, Pérez-Gago MB. Effect of maturity stage at processing and antioxidant treatments on the physico-chemical, sensory and nutritional quality of fresh-cut ‘Rojo Brillante’ persimmon. Postharvest Biol Technol. 2015;105:34–44. doi: 10.1016/j.postharvbio.2015.03.010. [DOI] [Google Scholar]
- Sant’Anna BMP, Fontes SP, Pinto AC, Rezende CM. Characterization of woody odorant contributors in copaiba oil (Copaifera multijuga Hayne) J Brazil Chem Soc. 2007;18:984–989. doi: 10.1590/S0103-50532007000500016. [DOI] [Google Scholar]
- Selli S, Rannou C, Prost C, Robin J, Serot T. Characterization of aroma-active compounds in rainbow trout (Oncorhynchus mykiss) eliciting an off-odor. J Agric Food Chem. 2006;54:9496–9502. doi: 10.1021/jf0619582. [DOI] [PubMed] [Google Scholar]
- Su MS, Chien PJ. Aroma impact components of rabbiteye blueberry (Vaccinium ashei) vinegars. Food Chem. 2010;119:923–928. doi: 10.1016/j.foodchem.2009.07.053. [DOI] [Google Scholar]
- Takahashi M, Ishmael M, Asikin Y, Hirose N, Mizu M, Shikanai T, Tamaki H, Wada K. Composition, taste, aroma, and antioxidant activity of solidified non-centrifugal brown sugars prepared from whole stalk and separated pith of sugarcane (Saccharum officinarum L.) J Food Sci. 2016;81:2647–2655. doi: 10.1111/1750-3841.13531. [DOI] [PubMed] [Google Scholar]
- Usami A, Ono T, Marumoto S, Miyazawa M. Comparison of volatile compounds with characteristic odor in flowers and leaves of nojigiku (Chrysanthemum japonense) J Oleo Sci. 2013;62:631–636. doi: 10.5650/jos.62.631. [DOI] [PubMed] [Google Scholar]
- Vera V, Canellas E, Nerín C. Migration of odorous compounds from adhesives used in market samples of food packaging materials by chromatography olfactometry and mass spectrometry (GC-O-MS) Food Chem. 2014;145:237–244. doi: 10.1016/j.foodchem.2013.06.087. [DOI] [PubMed] [Google Scholar]
- Wang LF, Lee YJ, Chung JO, Baik JH, So S, Park SK. Discrimination of teas with different degrees of fermentation by SPME-GC analysis of the characteristic volatile flavour compounds. Food Chem. 2008;109:196–206. doi: 10.1016/j.foodchem.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Wang B, Huang Q, Venkitasamy C, Chai H, Gao H, Cheng N, Cao W, Lv X, Pan Z. Changes in phenolic compounds and their antioxidant capacities in jujube (Ziziphus jujuba Miller) during three edible maturity stages. LWT Food Sci Technol. 2016;66:56–62. doi: 10.1016/j.lwt.2015.10.005. [DOI] [Google Scholar]
- Woods CMC, James PJ, Moss GA, Wright J, Siikavuopio S. A comparison of the effect of urchin size and diet on gonad yield and quality in the sea urchin Evechinus chloroticus Valenciennes. Aqua Int. 2008;16:49–68. doi: 10.1007/s10499-007-9124-z. [DOI] [Google Scholar]
- Wu S, Sun C, Pei S, Lu Y, Pan Y. Preparative isolation and purification of amides from the fruits of Piper longum L. by upright counter-current chromatography and reversed-phase liquid chromatography. J Chromatogr A. 2004;1040:193–204. doi: 10.1016/j.chroma.2004.03.056. [DOI] [PubMed] [Google Scholar]
- Zhao J, Li H, Xi W, An W, Niu L, Cao Y, Wang H, Wang Y, Yin Y. Changes in sugars and organic acids in wolfberry (Lycium barbarum L.) fruit during development and maturation. Food Chem. 2015;173:718–724. doi: 10.1016/j.foodchem.2014.10.082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.