Abstract
The effect of shelf storage under ambient conditions of cut apple dices on degradation of bioactive compounds such ascorbic acid, total phenols, antioxidant activity (% DPPH inhibition) and PPO activity were investigated. The results indicated that antioxidant activity declined significantly over 80 min storage of diced apples at ambient temperature. Similar trend was observed for ascorbic acid, total phenols and PPO activity. Ascorbic acid, total phenols and antioxidant activity degradation followed first-order kinetics where the rate constant (k) was found to be in range for all the thirteen cultivars, though initial ascorbic acid and phenol content varied in different apple cultivars. The reaction rate constant (k) for first order degradation ranged from 1.16 to 1.97, 0.89 to 1.29 and 0.37 to 1.54 for antioxidant activity, total phenols and ascorbic acid, respectively. This explains that antioxidant activity degrades at higher rate than total phenols and ascorbic acid, which also corroborates that antioxidant activity is affected by both total phenols and ascorbic acid content. In general, total antioxidant activity for apple dices kept for 80 min under ambient conditions exhibited lower values as compared to control.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3041-1) contains supplementary material, which is available to authorized users.
Keywords: Degradation kinetics, Antioxidant activity, Total phenols, Polyphenol oxidase activity, Ascorbic acid
Introduction
Based on size of production, apple is one of the main fruit crop in the world. It is a frequently consumed fruit and constitutes one of the main sources of polyphenols in diet (Boyer and Liu 2004). Polyphenols present in apples are important because of their health promoting antioxidant properties (Sluis et al. 2002) and contribution to the sensory quality of the fresh and processed fruits. Antioxidant activity of apple polyphenols is among the highest in fruits and vegetables commonly consumed (Lee et al. 2003).
Fresh-cut apples form an important and rapidly developing segment of food market, because of its convenience and fresh-like quality (Moreira et al. 2015). Cut apples have a very low shelf life ranging from minutes to hours manifested by change of color and loss of nutritive value. Due to tendency towards fast ripening and texture breakdown, apple is difficult to keep well for longer period of time (Wijewardane and Guleria 2013). Fresh apples contain more than 80/100 g water within a rigid cell wall structure, which is not only responsible for their crisp and crunchy texture, but also for their fast deterioration (Potter and Hotchkiss 1995). The primary deterioration reaction is enzymatic browning. The degree of browning depends on various factors such as presence of oxygen, reducing substances, metallic ions, pH, temperature, and the activity of different oxidizing enzymes, especially polyphenol oxidases (PPO) (Nicolas et al. 2007). This group of enzymes catalyzes two different reactions in the presence of molecular oxygen: the hydroxylation of monophenols to o-diphenols (monophenolase activity) followed by oxidation of o-diphenols to o-quinones (diphenolase activity).
Ascorbic acid is a major constituent in apples that contributes towards its antioxidant quality. Fang et al. (2017) found great variability in ascorbic acid content of apple germplasm. Various researches have related the decreasing proportion of ascorbic acid with browning development in apples (Sapers et al. 2001; Nicolas et al. 2007). Ascorbic acid is the best known chemical agent for reducing the browning reaction (Sapers et al. 2001). It is a labile vitamin that loses activity due to a number of factors, including pH, moisture content, oxygen, temperature and metal ion catalysis (Uddin et al. 2001). Ascorbic acid prevents oxidation of phenols by itself being a substrate for oxidation and converts to dehydro-ascorbic acid thereby inhibiting or delaying browning. Kinetic models are often used for an objective, fast and economic assessment of food quality. Kinetic modelling may also be employed to predict the influence of processing on critical quality parameters. Kinetic models have been developed to evaluate degradation of quality parameters including colour, browning, ascorbic acid during thermal processing, high pressure processing, sonication, ozonation and pulsed electric field processing (Polydera et al. 2005; Tiwari et al. 2008).
In this research relation between degradation of ascorbic acid and polyphenols with browning of apples dices has been studied. The paper aims to suggest kinetics of enzymatic browning with respect to concentration of phenols and ascorbic acid in apple dices.
Materials and methods
Raw material
Thirteen commercially grown cultivars of apple, namely, ‘Royal Delicious’, ‘Red Chief’, ‘Gale Gala’, ‘Scarlet Gala’, ‘Starkrimson’, ‘Vance Delicious’, ‘Well Spur’, ‘Silver Spur’, ‘Top Red’, ‘Super Chief’, ‘Scarlet Spur I’, ‘Royal Gala’ and ‘Oregon Spur II’ were procured from Regional Research Station, YSPUH&F, Seobagh, Kullu, Himachal Pradesh at optimum maturity. The apples were harvested at commercial maturity, having starch pattern index (SPI) of 3.0. After harvesting and sorting, the fruits were transported to New Delhi and stored at 2 °C with 80–90% relative humidity until further studied. TSS of apple cultivars ranged from 11 to 13 °B (‘Gale Gala’ 16 °B), titrable acidity ranged from 0.27 to 0.64% and firmness ranged from 10.32 to 12.64 N. All chemicals (2,2-diphenyl-1-picrylhydrazyl (DPPH); Folin-Ciocalteu reagent, metaphosphoric acid, 2,6 dichlorophenol indophenol) were of analytical grade and procured from Sigma Aldrich. Catechol was procured from SRL Chemicals.
Sample preparation
Ten apples from each of the thirteen cultivars were randomly selected for the study. The apples were peeled and the flesh was cut into cubes/dices of uniform size (1 cm3). The sample was analyzed for various parameters at zero time and then after every 10 min up to 80 min.
Antioxidant activity
Antioxidant activity was analyzed by DPPH assay (Mensor et al. 2001). DPPH is a stable free radical, it changes color to yellow on scavenging and this property is employed for antioxidant activity analysis. Methanolic extract of 1 g sample in 20 ml methanol were prepared by keeping them overnight. The samples were centrifuged at 10,000 rpm for 20 min at 4 °C. One ml of extract solution was mixed with 3.9 ml of DPPH and kept in dark for 30 min. Absorbance of reaction mixture was recorded using UV–VIS Spectrophotometer (Spectra Max M2, Molecuar Devices, USA) at 517 nm. Percent change in OD from blank sample was noted as antioxidant activity (% DPPH inhibition).
where AB = OD for blank; AS = OD for sample.
Total phenolic content
Methanolic extracts as prepared above were used for the analysis. Total phenolic content of the apple pulp samples was measured using a modified colorimetric Folin–Ciocalteu (FC) method (Singleton and Rossi 1965). FC develops blue color complex in presence of alkali (Na2CO3) and phenols. The absorbance was read at 765 nm using UV–VIS spectrophotometer (Spectra Max M2, Molecuar Devices, USA) and total phenols were expressed as mg GAE/100 g dry weight.
Polyphenol oxidase (PPO) activity
Polyphenol oxidase enzyme activity was analyzed by method of Sadasivam and Manickam (1997) with some modifications. Enzyme extract was prepared in the Phosphate buffer pH-6. For the extraction 0.5 g fruit was extracted in 1.5 ml Phosphate buffer and centrifuged at 12,000 rpm for 20 min. Supernatant was taken and kept at 4 °C until used as enzyme source. For the estimation, 3 ml of Catechol solution was taken in the test tube with 0.1 ml of enzyme extract. OD (495 nm) was recorded at zero time and after 3 min at 25 °C. The enzyme activity was expressed as ∆A495 by 0.001 ml−1 min−1.
Ascorbic acid
Ascorbic acid was determined by titration method using dye 2,6 dicholorophenol indophenol as described by AOAC (2000). Sample is weighed to 1 g and extracted in 3% metaphosphoric acid. Volume of extract is made up to 10 ml and titrated against dye till pink end point is obtained. Ascorbic acid (100 µg/ml) was used as standard.
where V1 = volume of dye consumed in standard titration; V2 = volume of dye consumed in sample titration.
All the analysis were done in triplicates and averaged.
Statistical analysis
All the analysis were done in triplicates and averaged. Statistical ranks were obtained using AGRES data entry module for AGRES statistical software, version 3.01. Based on factorial ANOVA, one factor analysis was done on individual variety. The statistical ranks (based on significant difference) obtained for each parameter are marked as superscripts over corresponding values. The correlation coefficient (R2) is a statistical measure of how close the data are to the fitted regression line which is calculated using zero and first order equations. Sum of squared residuals (SSR) is the sum of the squares of residuals (deviations predicted from actual empirical values of data). A small RSS indicates a tight fit of the model to the data. These predictors were used to describe the goodness of fit of the model.
Kinetics study
In the present work, zero order reaction equation (Eq. 1) was used to fit the experimental data followed by the Page model—first order equation (Eq. 2). The parameters of the model were calculated by non-linear regression (p < 0.05). The correlation coefficient (R2) and the sum of squared residuals, SSR (Eq. 3), were considered to evaluate the goodness of fit.
| 1 |
| 2 |
| 3 |
where X0 and Xt are observed values of parameter at time zero and time t respectively; Xexpt are expected values of parameter at time t; k is the rate constant and n is the expected rate of reaction.
Results and discussion
Antioxidant activity kinetics
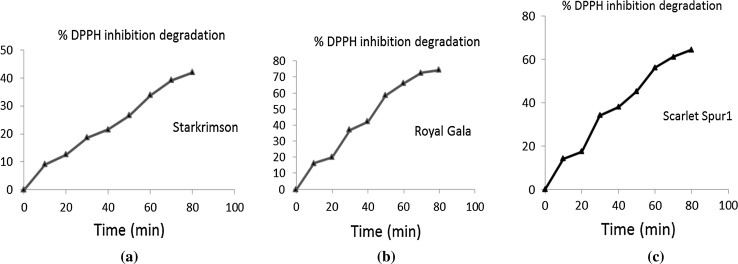
Antioxidant activity of apples was recorded as per cent DPPH inhibition and ranged between 7.84 and 21.68% in freshly cut apples with highest recorded value for the cultivar ‘Scarlet Spur I’ and least for the cultivar ‘Royal Gala’ (Table 1). A wide variation of antioxidant activity is observed in cultivars which might be attributed to genetic variability. Cultivation location (Wang et al. 2002; Hakkinen and Torronen 2003) and practices (Carbonaro et al. 2002), ripening stage (Raffo et al. 2002), harvested condition and seasons (Wu et al. 2004) also affect the antioxidant capacity of apples. Over a time of 80 min, antioxidant activity (% DPPH inhibition) declined by 41.99% in cultivar ‘Starkimson’ (most stable) and 74.3% in cultivar ‘Royal Gala’ (least stable). First order model equation (Eq. 2) was used to fit decline in antioxidant activity of apples after cutting. The sum of squared residuals (SSRs) was calculated to analyze the goodness of fit as shown in Table 1. The values indicate a good fit of first order reaction for decline of antioxidant activity of apples after cutting. Degradation curves for cultivars ‘Starkrimson’, ‘Royal gala’ and ‘Scarlet spur’ are shown in Fig. 1. A variety of factors have been shown to affect the antioxidant capacity of fruits including thermal processing (Dewanto et al. 2002; Quitão-Teixeira et al. 2008), unit operations such as slicing, peeling and storage regime (Piga et al. 2003). Lo Scalzo et al. (2004) have also reported that reduction of total antioxidant capacity of orange juice based on the radical scavenging of free radicals (hydroxyl and 1,1-diphenyl-2- picrylhydrazyl radicals) was attributed to the degradation of vitamin C during processing.
Table 1.
Parameters for degradation kinetics for first order
| S.no | Cultivar | Antioxidant activity degradation | Total phenol degradation | PPO activity degradation | Ascorbic acid degradation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial antioxidant activity (% DPPH inhibition) | K | SSR | R2 | Initial total phenols (mg GAE/100) | K | SSR | R2 | Initial PPO activity (ΔOD/min/ml) | K | SSR | R2 | Initial Ascorbic acid content (mg/100) | K | SSR | R2 | ||
| 1 | Starkrimson | 20.45a | 1.97 | 0.044 | 0.98 | 132.4a | 1.11 | 0.036 | 0.97 | 0.095hi | 1.48 | 0.230 | 0.93 | 5.88bc | 1.11 | 0.012 | 0.92 |
| 2 | Royal Gala | 7.84lm | 1.19 | 0.029 | 0.96 | 56.11ghi | 1.29 | 0.028 | 0.92 | 0.032cd | 1.13 | 0.042 | 0.84 | 6.62a | 1.54 | 0.021 | 0.86 |
| 3 | Scarlet Spur I | 21.68a | 1.19 | 0.036 | 0.95 | 65.85fg | 0.89 | 0.041 | 0.93 | 0.096hi | 1.1 | 0.027 | 0.89 | 5.23c | 1.01 | 0.036 | 0.93 |
| 4 | Vance Delicious | 18.03d | 1.54 | 0.028 | 0.94 | 90.96def | 1.13 | 0.042 | 0.96 | 0.057ef | 1.18 | 0.058 | 0.97 | 5.24c | 1.62 | 0.018 | 0.84 |
| 5 | Red Chief | 20.22cd | 1.32 | 0.016 | 0.91 | 134.2a | 1.16 | 0.038 | 0.88 | 0.090hi | 1.22 | 0.230 | 0.93 | 3.14ghi | 1.57 | 0.024 | 0.76 |
| 6 | Well Spur I | 20.69ab | 1.16 | 0.045 | 0.94 | 98.55de | 1.08 | 0.028 | 0.83 | 0.055ef | 1.11 | 0.053 | 0.96 | 4.14efg | 0.65 | 0.016 | 0.79 |
| 7 | Scarlet Gala | 20.91ab | 1.27 | 0.083 | 0.89 | 44.48ijk | 1.21 | 0.037 | 0.92 | 0.015a | 0.89 | 0.145 | 0.89 | 2.61ij | 0.75 | 0.032 | 0.82 |
| 8 | Top Red | 21.40a | 1.48 | 0.024 | 0.92 | 46.47ij | 1.18 | 0.049 | 0.89 | 0.072gh | 1.65 | 0.122 | 0.92 | 6.16ab | 0.78 | 0.014 | 0.83 |
| 9 | Royal Delicious | 14.68gh | 1.16 | 0.016 | 0.91 | 83.05fgh | 0.97 | 0.036 | 0.91 | 0.033cd | 1.36 | 0.413 | 0.87 | 3.22gh | 0.37 | 0.061 | 0.94 |
| 10 | Super Chief | 20.21abc | 1.19 | 0.014 | 0.96 | 42.82jkl | 1.11 | 0.021 | 0.93 | 0.097hi | 1.27 | 0.045 | 0.88 | 1.76jk | 0.77 | 0.042 | 0.91 |
| 11 | Silver Spur | 10.80jkl | 1.28 | 0.027 | 0.92 | 111.88cd | 1.12 | 0.027 | 0.95 | 0.016a | 1.30 | 0.026 | 0.92 | 3.68fg | 0.53 | 0.041 | 0.88 |
| 12 | Gale Gala | 15.11fg | 1.53 | 0.058 | 0.97 | 42.70jkl | 1.19 | 0.047 | 0.82 | 0.040cde | 1.41 | 0.132 | 0.94 | 5.04cd | 0.71 | 0.018 | 0.82 |
| 13 | Oregon Spur II | 17.51de | 1.18 | 0.024 | 0.96 | 86.53fg | 1.04 | 0.043 | 0.95 | 0.025bc | 1.60 | 0.234 | 0.93 | 4.25ef | 1.08 | 0.016 | 0.85 |
K values are estimated from the values of parameter using Eq. 2
Average of three determinations of a parameter were obtained for each evaluation
Superscripts denote significant different values for a particular parameter at 5% level of significance
Fig. 1.
Degradation curves (% degradation) of antioxidant activity (expessed as % DPPH inhibition) for cultivars a ‘Starkrimson’, b ‘Royal Gala’ and c ‘Scarlet spur I’
Total phenol degradation kinetics
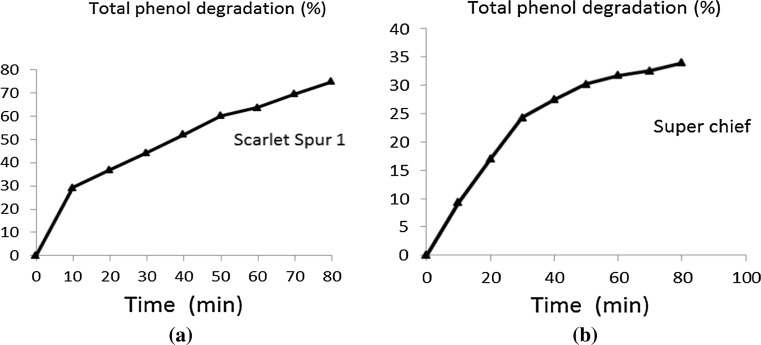
Total phenol content of different apple varieties ranged from 42.7 mg GAE/100 g in cultivar ‘Super Chief’ to 134.2 mg GAE/100 g in cultivar ‘Red Chief’ (Table 1). Total phenol content declined in all thirteen apple varieties with maximum stability demonstrated by ‘Super Chief’ (33.15% degradation in 80 min) and least by ‘Scarlet Spur I’ with 80 min degradation of 74.46% in total phenol content (Fig. 2). During fruit processing, cell structure is disrupted and the fruits become more prone to enzymatic oxidation which could be one of the main reasons for the loss in phenolic compounds (Patras et al. 2011). In our study, degradation of total phenols in cut apples followed first order reaction (Eq. 2) as evident by the SSRs for actual versus expected degradation values from zero time and R2 values for first order reaction (Table 1). When the logarithm of the total phenols was plotted against time, a straight line resulted, indicating pseudo first-order reaction kinetics for phenol degradation. The high R2 values of the range of 0.82–0.97 for all the thirteen cultivars indicate a good data fit to first order kinetic model. K value for the cultivars ranged from 0.89 to 1.21 depending upon the cultivar. It was observed that k value was higher for cultivar with high initial total phenol content. Michalczyk et al. (2009) found that the polyphenolic and anthocyanin contents in bilberries were increasingly reduced with long exposure time and high temperatures. Mrad et al. (2012) reasoned that damage to the membrane ultrastructure caused by cutting pears in parallel-epipedic pieces, permits rapid oxidation of phenolics compounds leading to degradation. They also found that total phenol degradation in pears during drying follows zero order reaction.
Fig. 2.
Degradation curves of total phenols for cultivars a ‘Scarlet Spur I’ and b ‘Super Chief’
Polyphenol oxidase (PPO) degradation kinetics
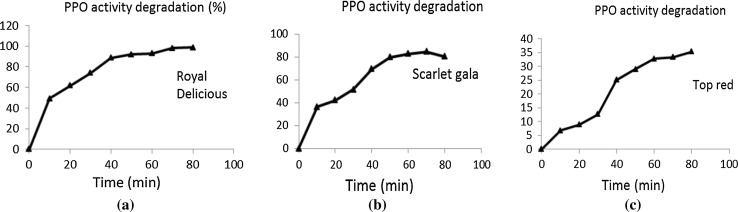
PPO activity degraded in apples after cutting simultaneous to browning development in all the cultivars at different rates. It was observed that PPO got increasingly inactivated with increasing shelf storage of apple dices at ambient conditions. Maximum PPO activity in fresh cut apples was found in cultivar ‘Royal Delicious’ (0.097 ∆OD/min/ml) and minimum in cultivar ‘Scarlet Gala’ (0.015 ∆OD/min/ml) (Table 1). Over a time of 80 min of shelf storage of diced apples, cultivar ‘Top Red’ demonstrated least PPO activity degradation of 35.31% whereas PPO of cultivar ‘Royal Delicious’ degraded fastest by 98.71% (Fig. 3). First order kinetic model (Eq. 2) of degradation of PPO activity at ambient conditions was found to be fit (Table 1).This indicates that PPO activity at ambient conditions degraded on its own with time in absence of compounding effect of any external factor. This may be due to progressive loss of substrates (polyphenols) in shelf stored apple dices happening simultaneous to browning. PPO inactivation has shown different kinetic study curves at the different range of temperature by various researchers. In the range of 40–80 °C, the PPO inactivation kinetics was clearly found to follow biphasic or nth-order reaction curves owing to the presence of isoenzyme fractions with different thermal stabilities (Yoruk and Marshall 2003). In contrast, Buckow et al. (2009) had observed that apple PPO inactivation kinetics followed a 2.2 order kinetics at the temperature ranging from 20 to 80 °C. In our study, inactivation is not induced by temperature but prevails at its own rate in apples after dicing.
Fig. 3.
Degradation curves of PPO activity for cultivars a ‘Royal Delicious’, b ‘Scarlet Gala’ and c ‘Top Red’
Ascorbic acid degradation kinetics
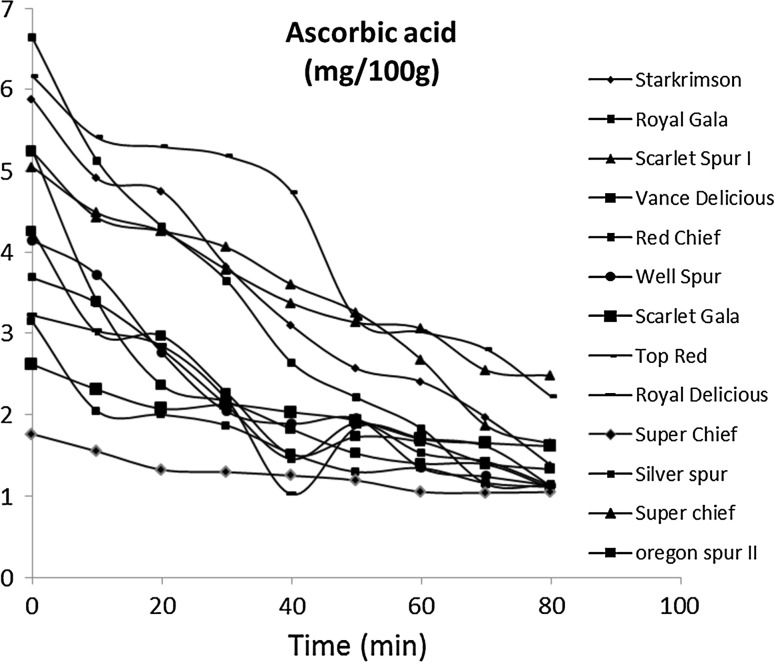
The average vitamin C content of fresh apples varied from 6.62 mg/100 g in cultivar ‘Royal Gala’ and 1.76 mg/100 g in cultivar ‘Royal Delicious’ (Table 1). Ascorbic acid content decreased in all the varieties of apple at different rates during shelf storage of 80 min (Fig. 4). Ascorbic acid in cultivar ‘Royal Gala’ was found to be least stable (82.71% degradation) and that of cultivar ‘Scarlet Gala’ (38.40% degradation) was found to be most stable during the 80 min shelf storage of cut apples dices. Considering as reference the ascorbic acid content at the beginning of air drying process, degradation curves were obtained for each cultivar. The declining ascorbic acid content of thirteen apple cultivars during shelf storage is shown in Fig. 4. This decline in ascorbic acid after cutting apples can be attributed to oxidation reactions converting ascorbic acid to dehydro-ascorbic acid. Equation (1) was fitted to experimental data by non-linear regression and the quality of the adjustment was evaluated through the statistical parameters R2 and SSR. Experimental data was analyzed for kinetics with different orders and first order reaction was found to be most fit owing to R2 values (Table 1). The decline in ascorbic acid was related to initial ascorbic acid content of apple and followed first order degradation kinetics in cut apple dices. Similar results were obtained by Abushita et al. (2000) when analyzing the content of this nutrient in tomatoes during post-harvest storage. This result is in agreement with the works of Erenturk et al. (2005) and Goula and Adamopoulos (2006), which had also observed a first order decay for ascorbic acid degradation. Ascorbic acid content was found to follow first-order degradation with R2 > 0.91 in strawberry jam during storage (Patras et al. 2011). Koutchma et al. (2009) reported that UV induced ascorbic acid degradation followed zero-order kinetics at concentrations between 341 and 660 mg/l. However, the experiments in that study were conducted using apple juice, and only 20–40% decline in ascorbic acid was achieved. In present study diced apples were left on shelf at ambient conditions and ascorbic acid degradation of 60–80% has been recorded in most of the apple cultivars. When k > 1 the reaction rate increases with time and the degradation curve assumes a sigmoidal shape. On the other hand, if k < 1 the reaction rate decreases with time and degradation rate higher than the exponential is observed at the process beginning (Cunha et al. 1998). Manso et al. (2001) obtained good results describing vitamin C degradation in orange juice and non-enzymatic browning kinetics by the Weibull model.
Fig. 4.
Change in ascorbic acid content of apple dices over 80 min shelf storage
Antioxidant interactions
As evident from above results, as browning proceeds antioxidant activity declines considerably during shelf storage of cut apples. This declining trend also repeats with total phenols and ascorbic acid. Correlation between antioxidant activity (% DPPH inhibition) and total phenols as well as antioxidant activity and ascorbic acid of different apple cultivars was significant ranging from 0.65 to 0.98 (antioxidant activity and total phenols) and 0.67–0.91 (antioxidant activity and ascorbic acid), thus highlighting the role of phenols and ascorbic acid in contributing towards antioxidant activity of apples. No such significant correlation was observed between total phenols and ascorbic acid or their rate of degradations. Patras et al. (2011) also found that total phenolic content was significantly correlated with total antioxidant activity (r = 0.84, p = 0.045) at 4 °C and (r = 0.98, p = 0.003).The reaction rate constants (k) for first order degradation ranged from 1.16 to 1.97, 0.89 to 1.29 and 0.37 to 1.54 for antioxidant activity, total phenols and ascorbic acid degradation kinetics respectively. This may determine that antioxidant activity degrades at higher rate than total phenols and ascorbic acid, which also corroborates that antioxidant activity, is affected cumulatively by both total phenols and ascorbic acid content amongst other factors.
Conclusion
In this study, degradation kinetics of antioxidant activity, total phenols, PPO activity and ascorbic acid of thirteen apple cultivars (after cutting) were investigated. The first-order kinetic model was found to be the best fit for the antioxidant activity, total phenols, PPO activity and ascorbic acid degradation. Total phenol content and antioxidant activity were highly correlated (R2 = 0.82). Similarly, a good correlation was also observed between total antioxidant activity and ascorbic acid content (R2 = 0.80). No such significant correlation was observed between total phenols and ascorbic acid or their rate of degradations. Degradation in antioxidant activity occurs in cut apples simultaneous to browning due to oxidation of polyphenols and ascorbic acid. Thus, the effects of cutting and storing of apples prior to serving is not only on color or aesthetic appeal but also on the nutritional properties of apples and hence, should be considered.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
Funding was provided by Indian Agricultural Research Institute.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3041-1) contains supplementary material, which is available to authorized users.
References
- Abushita AA, Daood HG, Biacs PA. Change in carotenoids and antioxidant vitamins in tomato as a function of varietal and technological factors. J Agric Food Chem. 2000;48:2075–2081. doi: 10.1021/jf990715p. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis of the association of official analytical chemists, official method 967.21. Washington DC: AOAC International; 2000. [Google Scholar]
- Boyer J, Liu RH. Review: apple phytochemicals and their health benefits. Nutr J. 2004;3:1–15. doi: 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckow R, Weiss U, Knorr D. Inactivation kinetics of apple polyphenol oxidase in different pressure-temperature domains. Inn Food Sci Emerg Technol. 2009;10:441–448. doi: 10.1016/j.ifset.2009.05.005. [DOI] [Google Scholar]
- Carbonaro M, Mattera M, Nicoli S, Bergamo P, Cappelloni M. Modulation of antioxidant compounds in organic vs. conventional fruit (peach, Prunuspersica L., and pear, Pyruscommunis L.) J Agric Food Chem. 2002;50:5458–5462. doi: 10.1021/jf0202584. [DOI] [PubMed] [Google Scholar]
- Cunha LM, Oliveira FAR, Oliveira JC. Optimal experimental design for estimating the kinetic parameters of processes described by the Weibull probability distribution function. J Food Eng. 1998;37:175–191. doi: 10.1016/S0260-8774(98)00085-5. [DOI] [Google Scholar]
- Dewanto V, Xianzhong W, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Erenturk S, Gulaboglu MS, Gultekin S. The effects of cutting and drying medium on the vitamin C content of rosehip during drying. J Food Eng. 2005;68(4):513–518. doi: 10.1016/j.jfoodeng.2004.07.012. [DOI] [Google Scholar]
- Fang T, Zhen Q, Liao L, Owiti A, Zhao L, Korban SS, Han Y. Variation of ascorbic acid concentration in fruits of cultivated and wild apples. Food Chem. 2017;225:132–137. doi: 10.1016/j.foodchem.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Goula AM, Adamopoulos KG. Retention of ascorbic acid during drying of tomato halves and tomato pulp. Dry Technol. 2006;24(1):57–64. doi: 10.1080/07373930500538709. [DOI] [Google Scholar]
- Hakkinen SH, Torronen AR. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species, influence of cultivar, cultivation site and technique. Food Res Int. 2003;33:517–524. doi: 10.1016/S0963-9969(00)00086-7. [DOI] [Google Scholar]
- Koutchma T, Forney LJ, Moraru CI. UV processing effects on quality of foods. In: Koutchma T, Forney LJ, Moraru CI, editors. Ultraviolet light in food technology—principles and applications. 1. Boca Raton: CRC Press; 2009. pp. 103–123. [Google Scholar]
- Lee KW, Kim YJ, Kim DO, Lee HJ, Lee CY. Major phenolics in apple and their contribution to the total antioxidant capacity. J Agric Food Chem. 2003;51:6516–6520. doi: 10.1021/jf034475w. [DOI] [PubMed] [Google Scholar]
- Lo Scalzo R, Iannoccari T, Summa C, Morelli R, Rapisarda P. Effect of thermal treatments on antioxidant and antiradical activity of blood orange juice. Food Chem. 2004;85:41–47. doi: 10.1016/j.foodchem.2003.05.005. [DOI] [Google Scholar]
- Manso MC, Oliveira FAR, Oliveira JC, Frias JM. Modeling ascorbic acid thermal degradation and browning in orange juice under aerobic conditions. Int J Food Sci Technol. 2001;36:303–312. doi: 10.1046/j.1365-2621.2001.t01-1-00460.x. [DOI] [Google Scholar]
- Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS, Leitao SG. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- Michalczyk M, Macura R, Matuszak I. The effect of air-drying, freeze drying and storage on the quality and antioxidant activity of some selected berries. J Food Process Preserv. 2009;33:11–21. doi: 10.1111/j.1745-4549.2008.00232.x. [DOI] [Google Scholar]
- Moreira MR, Cassani L, Belloso OM, Fortuny RS. Effects of polysaccharide-based edible coatings enriched with dietary fiber on quality attributes of fresh-cut apples. J Food Sci Technol. 2015;52(12):7795–7805. doi: 10.1007/s13197-015-1907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrad ND, Boudhrioua N, Kechaou N, Courtois F, Bonazzi C. Influence of air drying temperature on kinetics, physicochemical properties, total phenolic content and ascorbic acid of pears. Food Bioprod Process. 2012;90:433–441. doi: 10.1016/j.fbp.2011.11.009. [DOI] [Google Scholar]
- Nicolás JML, Núñez-Delicado E, Sánchez-Ferrer A, Carmona FG. Kinetic model of apple juice enzymatic browning in the presence of cyclodextrins, the use of maltosyl-b-cyclodextrin as secondary antioxidant. Food Chem. 2007;101:1164–1171. doi: 10.1016/j.foodchem.2006.03.018. [DOI] [Google Scholar]
- Patras A, Brunton NP, Tiwari BK, Butler F. Stability and degradation kinetics of bioactive compounds and colour in strawberry jam during storage. Food Bioprocess Technol. 2011;4:1245–1252. doi: 10.1007/s11947-009-0226-7. [DOI] [Google Scholar]
- Piga A, Caro DA, Agabbio M. Changes in ascorbic acid, polyphenol content and antioxidant activity in minimally processed cactus pear fruit. LWT Food Sci Technol. 2003;36:257–262. doi: 10.1016/S0023-6438(02)00227-X. [DOI] [Google Scholar]
- Polydera AC, Stoforos NG, Taoukis PS. Quality degradation kinetics of pasteurised and high pressure processed fresh Navel orange juice, nutritional parameters and shelf life. Innov Food Sci Emerg Technol. 2005;6:1–9. doi: 10.1016/j.ifset.2004.10.004. [DOI] [Google Scholar]
- Potter NN, Hotchkiss JH (1996 ed.) Food science. Constituents of foods: properties and significance, 5th edn. Chapman & Hall, New York, c1995, pp 43–44
- Quitão-Teixeira LJ, Aguiló-Aguayo I, Ramos AM, Martín-Belloso O. Inactivation of oxidative enzymes by high intensity pulsed electric field for retention of color in carrot juice. Food Bioprocess Technol. 2008;1:364–373. doi: 10.1007/s11947-007-0018-x. [DOI] [Google Scholar]
- Raffo A, Leonardi C, Fogliano V, Ambrosino P, Salucci M, Gennaro L. Nutritional value of cherry tomatoes (Lycopersicon esculentum Cv. Naomi F1) harvested at different ripening stages. J Agric Food Chem. 2002;50:6550–6556. doi: 10.1021/jf020315t. [DOI] [PubMed] [Google Scholar]
- Sadasivam S, Manickam A. Biochemical methods. 2. New Delhi: New Age International Pvt. Ltd. Publishers; 1997. pp. 110–111. [Google Scholar]
- Sapers GM, Hicks KB, Miller RL. Food additives-revised and expanded. In: Branen AL, Davidson PM, Salminen S, Thorngate JH, editors. Antibrowning agents. 2. New York: Marcel Dekker. Inc; 2001. pp. 543–561. [Google Scholar]
- Singleton VL, Rossi JA. Colourimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–153. [Google Scholar]
- Sluis VAA, Dekker M, Skrede G, Jongen WMF. Activity and concentration of polyphenolic antioxidants in apple juice-effect of existing production methods. J Agric Food Chem. 2002;50:7211–7219. doi: 10.1021/jf020115h. [DOI] [PubMed] [Google Scholar]
- Tiwari BK, Muthukumarappan K, O’Donnell CP, Cullen PJ. Effects of sonication on the kinetics of orange juice quality parameters. J Agric Food Chem. 2008;56(7):2423–2428. doi: 10.1021/jf073503y. [DOI] [PubMed] [Google Scholar]
- Uddin MS, Hawlader MNA, Zhou L. Kinetics of ascorbic acid degradation in dried kiwifruits during storage. Dry Technol. 2001;19:437–446. doi: 10.1081/DRT-100102916. [DOI] [Google Scholar]
- Wang SY, Zheng W, Galletta GJ. Cultural system affects fruit quality and antioxidant capacity in strawberries. J Agric Food Chem. 2002;50:6534–6542. doi: 10.1021/jf020614i. [DOI] [PubMed] [Google Scholar]
- Wijewardane RMNA, Guleria SPS. Effect of pre-cooling, fruit coating and packaging on postharvest quality of apple. J Food Sci Technol. 2013;50(2):325–331. doi: 10.1007/s13197-011-0322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gu L, Holden J, Haytowitz DB, Gebhardt SE, Beecher G. Development of a database for total antioxidant capacity in foods, a preliminary study. J Agric Food Chem. 2004;17:407–422. [Google Scholar]
- Yoruk R, Marshall MR. Physicochemical properties and function of plant polyphenol oxidase, a review. J Food Biochem. 2003;27:361–422. doi: 10.1111/j.1745-4514.2003.tb00289.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.