Abstract
Organic acids (OAs) are small non-volatile molecules with widespread usage in processed foods, feeds and instant beverages. The prime aim of this study was to explore major OAs in local citrus fruits (Citrus limetta, Citrus aurantifolia, Citrus nobilis, Citrus karna, Citrus medica, Citrus ichangensis and Citrus aurantium) and assessment of their bioactivities. A RP–HPLC–DAD method was developed using buffer free solvent system for rapid detection and quantification of major OAs from citrus fruits and derived products. Method validation studies showed good linear calibration curve (0.985–0.998) for all OAs. The values of %RSD ranged between 0.0001–1.129 and 0.142–1.941 for interday and intraday variability respectively. The limit of detection and limit of quantification values for different OAs were ranged between 1.5–12 and 5–40 µg mL−1. The juice of above mentioned citrus fruit cultivars were assessed for OAs, total phenolics, free radical scavenging antioxidants and their antimicrobial potential against selected bacterial and fungal strains. The results showed variable contents of phenolics [0.28 ± 0.001–1.17 ± 0.014 mg (GAE) mL−1] and antioxidant compounds (1.26 ± 0.009–2.84 ± 0.006 mg of trolox equivalents mL−1) in all juice samples besides significant antifungal activity against C. albicans and A. niger strains. However, in case of antibacterial activity, only C. aurantifolia showed inhibitory effects against selected strains. It was found that citrus fruits have immense potential for their utilization as economic source of natural OAs and development of value added products, beverages and bio-preservatives.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3045-x) contains supplementary material, which is available to authorized users.
Keywords: Citrus fruits, Organic acids, RP–HPLC, Phenolics, Antioxidants, Antimicrobial activity
Introduction
Organic acids are naturally occurring compounds which are abundantly present in different fruits, plants and vegetables. Particularly citrus fruits are renowned source of natural OAs. The widespread usage of OAs as food decontaminants has been practiced from very early times (Mani-Lopez et al. 2012). These are non-volatile compounds used in variable quantities in various foods and beverages with undeniable contribution in maintaining taste and quality of the product (Scherer et al. 2012; Narayan et al. 2015). Citric acid is the most frequently used OA accompanied by ascorbic, malic, oxalic, succinic and tartaric acid.
Apart from their contribution in enhancing organoleptic properties these compounds have extensive usage in food and beverage processing as pH adjusting agents, flavourings and accidulants (Smulders and Greer 1998; Ali et al. 2015; Narayan et al. 2015). Citrus fruits also contain wide range of health beneficial phytochemicals (Sdiri et al. 2012). These phytochemicals are well known for their antioxidant and antimicrobial properties (Mani-Lopez et al. 2012; Rostamzad et al. 2011). Earlier Rostamzad et al. (2011) have reported enhanced combinatorial antioxidant activity of citric and ascorbic acid. Furthermore, it was found that dietary intake of free radical scavenging phenolic antioxidants may play important role in prevention of various metabolic disorders (Pandey and Rizvi 2009). Hence organic acids obtained from citrus fruits might act as promising antioxidants and has potential to replace artificial antioxidants used in various products.
The qualitative and quantitative analysis of OAs is performed by LC/MS (Ibanez and Bauer 2014), or capillary electrophoresis (Li et al. 2015) and or HPLC using UV–visible detector, photodiode array detector (PDA) and electrochemical detector (Cunha et al. 2002; Kotani et al. 2004). Amongst HPLC is the routine analysis technique. Majority of available analytical HPLC methods have long run time while use of buffered solvents for separation of organic acids is quiet common (Tyagi et al. 2014). But extensive use of buffers might have deteriorating effect on column life. Hence easy and fast monitoring of these bioactives is highly required.
As per our knowledge Citrus limetta, Citrus aurantifolia, Citrus aurantium, Citrus karna, Citrus medica, Citrus ichangensis and Citrus nobilis are locally available fruit varieties which grow widely in forests and waste lands. Though some of these fruits are consumed fresh as well as in form of pickles from very early times but still they failed to receive much attention.
Therefore, prime aim of present investigation is to explore major bioactive compounds from these underutilized local citrus fruits along with their qualitative analysis with the help of new analytical HPLC method. Further aim of the study is to investigate the therapeutic potential of citrus fruit juices and assessment of active constituents for the development of value added food products.
Materials and methods
Chemicals and reagents
HPLC grade solvents, analytical standards and Trifluoroacetic acid were purchased from Merck Specialties Pvt. Ltd. (Mumbai, India).
Collection of material and sample preparation
Different citrus fruits viz. C. aurantifolia, C. nobilis, C. ichangensis, C. karna, C. medica, C. limetta and C. aurantium were collected from adjoining localities of CSIR-IHBT, Palampur during 2015-16. The fruits peel was removed and juice was squeezed out manually and filtered with Millipore nylon membrane syringe filters (0.45 µm). The filtered samples were stored at 4 °C till further analysis.
Method development and validation
A rapid RP–HPLC–DAD method was developed and validated using Waters HPLC system equipped with 2707 autosampler, 2998 PDA detector and temperature control module to adjust column oven temperature. For separation of compounds, Synergi MAX-RP C12 column with 4 µm particle size and 150 × 3.00 mm dimensions was used. Stock solutions of different standards (oxalic acid, tartaric acid, ascorbic acid, succinic acid, citric acid and malic acid) mg mL−1 were prepared with HPLC grade solvents. An isocratic solvent system containing 0.03% TFA in water was optimized at the flow rate of 1.2 mL min−1. The temperature of column was optimized at 30° C and injection volume kept 10 µL. The chromatographic spectrum was recorded at 200–700 nm via 3D wavelength program and final data was collected at 220 nm for all standards and samples.
Linearity
Varying concentrations of all standards were prepared from respective stock solutions to prepare linear calibration curves. The calibration curve was plotted against concentration verses peak area solely for each and every standard.
Sensitivity
Limit of detection (LOD) and limit of quantification (LOQ) value of all the standards were calculated by signal to noise S/N ratio of three and ten respectively.
Precision
Repeatability studies of the developed method was performed by analyzing aliquot of standard mixture at different time intervals, while reproducibility study was performed by analysing aliquot mixtures at three consecutive days in duplicates.
Accuracy
Accuracy was checked by measuring experimental values to the true values of recovery. Recovery percentage was calculated using formulae:
Triplicate analyses for each standard were performed.
Specificity
Peak specificity is the monitoring of interference of peaks with one another. The method was validated for specificity by observing retention time of all standards individually as well as in mixture.
Robustness
Robustness shows the flexibility of the methods with minor change in chromatographic conditions. Robustness of the method was tested by slightly varying temperature, pH, and flow rate of developed method.
Determination of pH, ºbrix and colour value
The hydronium ion concentration of citrus fruit juices was determined by pH meter (Labindia). TSS content of all the samples was determined by using hand refractometer (Erma Tokyo Hand Refractometer). Colour value (L*, a*, b* and ΔE) analysis was done using Chroma-Meter (CR-406, Konica Minolta). It is used to measure surface colour by reflectance of light illumination.
Total carbohydrate analysis
Total carbohydrate content of different juice samples was performed by modified anthrone—sulphuric acid method (Morris 1948). Aliquots (100 µL) from each sample were taken in 10 mL volumetric flasks and total volume was made up to 4 mL by addition of anthrone reagent. After mixing the reaction mixture the flasks were heated for 10 min in a hot water bath at temperature (60–70 °C). The final absorbance was measured at 630 nm in Hitachi 150–20 spectrophotometer after colour stabilization. The results of total carbohydrate contents were expressed as milligram glucose, sucrose and fructose equivalents mL−1 of juice. Linear calibration curves of glucose, sucrose and fructose were prepared at different concentrations (20, 40, 60, 80 and 100 µg mL−1) for quantification of total carbohydrate content in different juice samples.
Total polyphenolic content
Total polyphenolic content in citrus fruit juices was determined by Folin’s ciocalteau method (Swain and Hill 1959). 100 µL aliquot of different juices were taken in 25 mL volumetric flasks in triplicates. 1 mL of 1 N Folin’s ciocalteau reagent was added followed by 1 mL 35% Na2CO3 solution. Final volume was made 25 mL with distilled water. The reaction mixture was incubated for 30 min at room temperature. Absorbance was recorded at 730 nm using Hitachi UV–Visible spectrophotometer. Total polyphenolic content of all samples were calculated as gallic acid equivalent using calibration curve of gallic acid prepared at different concentrations.
Evaluation of antioxidant activity
Free radical scavenging activity of citrus fruits juices was evaluated using 2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay of Brand-Williams (Brand-Williams et al. 1995). Aliquots 100 µL of all juice samples were diluted with equal volume of 70% methanol and poured into test tube containing 2.8 mL of 0.1 mM solution of DPPH prepared in 70% methanol. Reaction mixture was shaken and incubated in dark for 30 min at room temperature. Final absorbance was measured at 517 nm wavelength and 70% methanolic solution was used as blank.
Antimicrobial activity
Test bacteria and fungus
The antimicrobial activity of citrus fruit juices and standard compounds citric and ascorbic acid were evaluated against four bacterial strains i.e. Bacillus subtilis (MTCC 721), Staphylococcus aureus (MTCC 3160) and Escherichia coli (MTCC 43), Klebsiella pneumonia (MTCC 109) and two fungal strains i.e. Aspergillus niger (MTCC 404) and Candida albicans (MTCC 3017). The cultures were procured from CSIR-Institute of Microbial Technology, MTCC Chandigarh (India).
Well diffusion method
A well diffusion method was used to evaluate the antimicrobial activity of citrus fruit juices, following procedure as discussed in Rana et al. (2016). Nutrient broth was used for the growth of bacterial culture. 50 µL inoculum of each selected bacterium was uniformly spreaded on Muller Hinton agar plate with the help of glass spreader. The wells (5 mm diameter) were bored into the inoculated petriplates with the help of sterile borer. Three different concentrations 50, 75 and 100 µL of each citrus fruit juice sample and 1.5, 2, 2.5 mg of citric acid and ascorbic acid in 50 µL aliquots were added to the wells. Antibiotic, streptomycin (5 mg mL−1) was used as positive control. The plates were incubated at 37 °C for 24 h and zone of inhibition was observed. For fungal strains, potato dextrose agar (PDA) plates were prepared and 50 µL inoculums of each selected fungus were uniformly spreaded on plates. 10, 20 and 50 µL concentration for fruit juices sample and 0.5, 1.0, 1.5 mg for ascorbic and citric acid were made in 50µL was poured into the wells. Nystatin (5 mg mL−1) was used as standard for antifungal activity. The plates were incubated at 27 °C for 24–48 h and fungal growth was observed.
Statistical analysis
All values recorded in triplicates from all experiments were given as mean ± standard deviation. Significance difference between the values was calculated by analysis of variance (ANOVA) using Statistica 7.0 software. “p” value difference calculated by Duncan test which was less than 0.05.
Results and discussion
Organic acids (OAs) are low molecular weight naturally occurring molecules which are present in different fruits and vegetables (Joslyn 1970) and have strong impact on the sensory properties and keeping quality of foods and beverages. Knowingly and unknowingly usage of OAs is practiced from very early times as preservatives in different foods and beverages. They also exhibit antioxidant properties acting as free radical scavengers. Ascorbic acid is a well-known antioxidant used widely in various processed foods. In present study efforts are on to determine their content in different fruits and further investigation of their alternative roles. Hence, quantitative estimation of selective OAs was performed in different citrus fruits by newly developed and validated RP–HPLC method followed by their comparative bioactivity studies.
Method development and validation
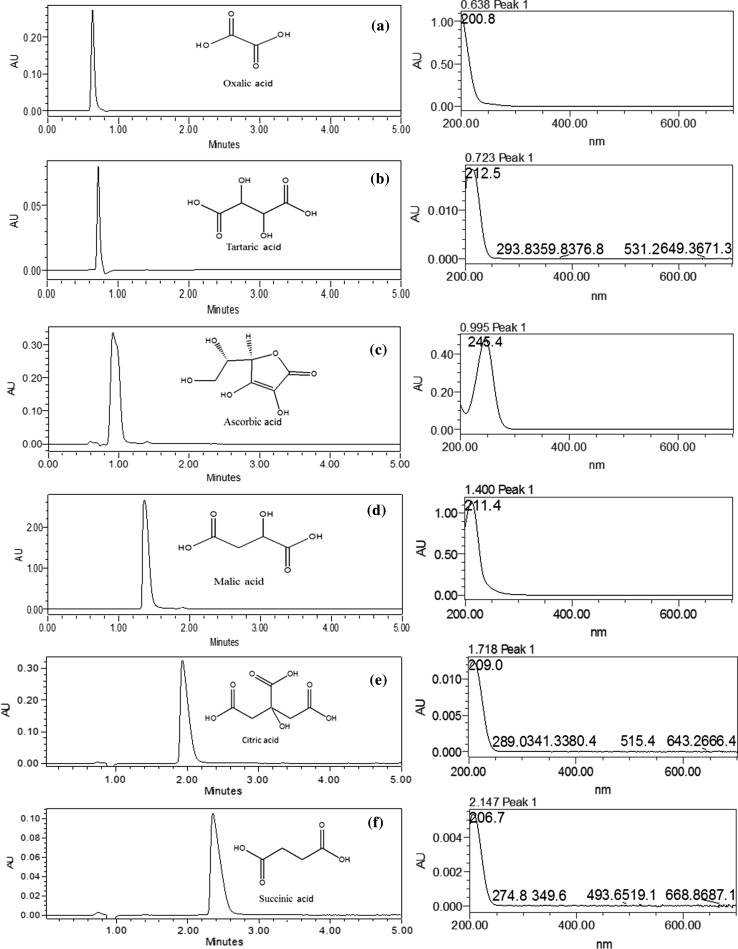
A new RP–HPLC–DAD method was developed and validated for the quantification of major OAs. Developed method showed separation of all the constituents within 5 min, which is very fast compared to previously reported methods (Hees et al. 1999; Kelebek et al. 2009). Use of buffers is completely avoided to protect the column. For separation of OAs, a new C12 silica stationary phase column was used which gave better peak resolution and sensitivity. Extensive studies were performed to optimize the solvent system and solvent flow rate for separation of all the constituents. Finally, H2O with 0.03% TFA was selected as solvent of choice for separation of all the organic acids at flow rate of 1.2 mL min−1. Absorbance spectra of all OAs was recorded at 200 to 700 nm and final data was retrieved at 220 nm for all compounds (Fig. 1). The elution order of OAs was oxalic–tartaric–ascorbic–malic–citric and succinic acid. Figure 1 showed the separation of all six organic acids within 5 min.
Fig. 1.
RP–HPLC chromatogram of individual standards; a oxalic acid, b tartaric acid, c ascorbic acid, d malic acid, e citric acid, f succinic acid with their UV–Vis spectra
The developed method was further validated by determining linearity, precision, sensitivity, accuracy, robustness and specificity studies. Linear standard curves were prepared for all standards with six working concentrations (n = 6). The concentrations used to attain linear standard curve, ranged 18–500 µg mL−1. Linearity values between 0.985 and 0.998 attained for all OAs. Regression coefficients of all OAs standards are greater than 0.984 which means there is higher correlation between peak area and concentration (Table 1). Precision analysis was performed by measuring inter day and intraday variation of standards. Interday (n = 3) and intraday variability (n = 3) for each constituent was determined by calculating %RSD. The values of %RSD ranged between 0.0001–1.129 and 0.142–1.941 for interday and intraday variability, respectively (Table 1). The higher %RSD was recorded for tartaric acid (1.129), whereas lowest in malic acid (0.0001) during interday analysis. The results of intraday variability showed highest %RSD for succinic acid (1.941), and lowest for malic acid (0.142) (Table 1). Sensitivity of the developed method was checked by calculating LOD and LOQ values. Limit of detection (LOD) and limit of quantification (LOQ) values for different organic acids were ranged between 1.5 and 12 µg mL−1and 5 - 40 µg mL−1(Table 1). Succinic acid showed lowest LOD (1.5 µg mL−1) and LOQ (5.0 µg mL−1) values. However, ascorbic and malic acid showed higher values for LOD (12.0 µg mL−1) and LOQ (40 µg mL−1). Method validation results showed good repeatability and reproducibility for all organic acids. Accuracy was determined by calculating % recovery for all the standards. Peak spiking was done by adding known amount of standards to previously analyzed known concentrations (w/v). Maximum recovery was obtained for succinic acid (117%) followed by citric acid (113%), malic acid (100%) and ascorbic acid (109%). Minimum recovery was shown by tartaric and oxalic acid i.e. 95 and 98% respectively (Table 1). For specificity studies injection of individual standard was injected and then mixtures of all standards were analyzed. Peaks obtained from individual compounds were spiked with the chromatogram of OAs mixture and results positively showed similar retention time. No interference of peaks was observed. This shows that developed method stands well within varying chromatographic conditions. Developed method was also tested for its robustness. Temperature range of the column was varied from 27 to 32 °C and no such noticeable change in retention time of peaks was observed. Similarly, slight variation of pH of solvent system does not affect peak separation. Flow rate of the solvent was also varied from 1 mL to 1.5 mL min−1. The method remained unaffected with slight changes which can be neglected. It is clearly indicated that the developed method is accurate and precise for the quantification of organic acids in different samples.
Table 1.
Repeatability and reproducibility of developed method and retention time, linearity, limit of detection (LOD) and limit of quantification (LOQ), % recovery of developed method
| Standards | Interday variability | RSD (%) | Intraday variability | RSD % | Retention time | Linearity range (mg mL−1) | Regression equation | r2 | LOD (µg mL−1) | LOQ (µg mL−1) | % Recovery |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxalic acid | 0.173 | 0.333 | 0.141 | 0.709 | 0.63 | 0.018–0.5 | y = 0.176x − 0.022 | 0.997 | 2.0 | 6.0 | 98 |
| Tartaric acid | 0.256 | 1.129 | 0.208 | 0.481 | 0.72 | 0.018–0.5 | y = 0.039x + 0.003 | 0.997 | 6.0 | 20.0 | 95 |
| Ascorbic acid | 0.267 | 0.216 | 0.210 | 0.476 | 0.99 | 0.018–0.5 | y = 5.766x − 0.018 | 0.998 | 12.0 | 40.0 | 109 |
| Malic acid | 0.730 | 0.000 | 0.702 | 0.142 | 1.40 | 0.018–0.5 | y = 30.37x + 0.021 | 0.998 | 12.0 | 40.0 | 100 |
| Citric acid | 0.242 | 0.239 | 0.178 | 0.858 | 1.71 | 0.018–0.5 | y = 0.501x − 0.014 | 0.985 | 3.0 | 10.0 | 113 |
| Succinic acid | 0.121 | 0.476 | 0.120 | 1.941 | 2.14 | 0.050–0.3 | y = 0.119x − 0.001 | 0.998 | 1.5 | 5.0 | 117 |
RSD Relative standard deviation, LOD limit of detection, LOQ limit of quantification
Estimation of major organic acids in different citrus fruit juices
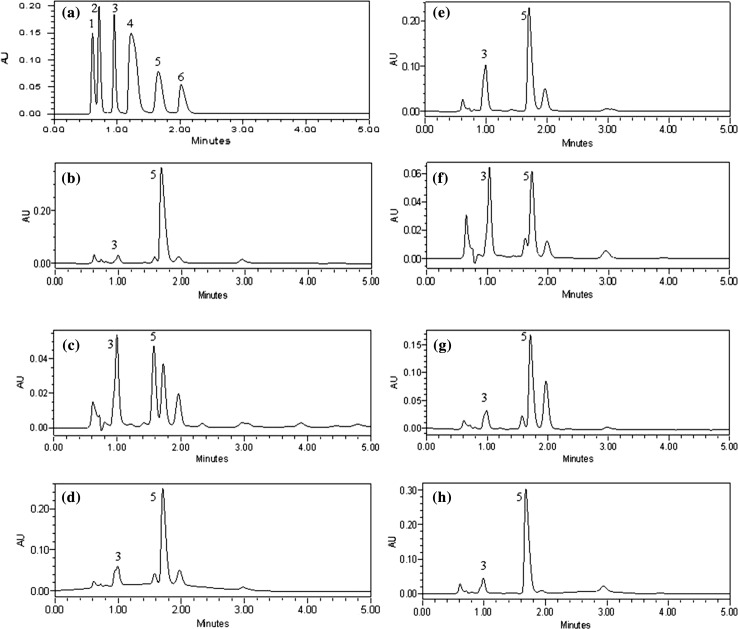
Quantification of major OAs from seven citrus fruit juices was done using newly developed RP–HPLC method. Organic acids quantification was performed by using linear equations of standards after comparing retention time and absorbance of samples peak with respective standards. Further confirmation was curtained by adding known amount of specific standard into the sample (peak spiking). Figure 2 shows RP–HPLC chromatogram of major organic acids present in different citrus fruit juices. Among all OAs, citric acid and ascorbic acid were found to be predominant in all samples. Citric acid content in different fruit juice samples ranged 3.40–35.36 mg mL−1. C. aurantium juice showed highest content of citric acid (35.36 mg mL−1) compared to other samples. However, lowest content of citric acid recorded in C. nobilis juice (3.40 mg mL−1). Ascorbic acid content in different samples recorded between 0.083 and 0.54 mg mL−1. C. nobilis showed highest ascorbic acid content (0.54 mg mL−1) while lowest amount (0.083 mg mL−1) was observed in C. karna juice sample. In earlier study, Scherer et al. (2012) reported 0.29 mg mL−1 ascorbic acid and 67.97 mg mL−1 citric acid in C. aurantifolia juice which is higher compared to present study. The variability in results might be attributed to the environmental factors, analytical technique and juice processing conditions. Results of organic acid analysis for all samples are shown in Table 3.
Fig. 2.
RP–HPLC chromatogram of a standard mixture and Citrus juices: b C. aurantium c C. nobilis, d C. medica, e C. ichangensis, f C. limetta, g C. karna, h C. aurantifolia. Peaks are labeled as: (1) Oxalic acid, (2) Tartaric acid, (3) Ascorbic acid, (4) Malic acid, (5) Citric acid and (6) Succinic acid
Table 3.
Total citric acid, ascorbic acid, phenolic content and antioxidant activity of juice samples
| Samples | TAAC (mg mL−1) | TCAC (mg mL−1) | TPC (mg GAE mL−1) | TEAC (mg mL−1) |
|---|---|---|---|---|
| C aurantifolia | 0.18c | 30.40b | 0.33 ± 0.003e | 1.70 ± 0.012b |
| C aurantium | 0.13d | 35.36a | 0.58 ± 0.008d | 1.96 ± 0.013b |
| C nobilis | 0.54a | 03.40e | 0.72 ± 0.007b | 2.37 ± 0.023a |
| C ichangensis | 0.20c | 21.79c | 0.68 ± 0.021c | 2.84 ± 0.006a |
| C karna | 0.08e | 15.80d | 0.75 ± 0.021b | 1.26 ± 0.010c |
| C medica | 0.11d | 22.67c | 0.28 ± 0.001f | 1.26 ± 0.009c |
| C limetta | 0.49b | 05.00e | 1.17 ± 0.014a | 2.82 ± 0.001a |
TAAC Total ascorbic acid content, TCAC total citric acid content, TPC total phenolic content, TEAC Trolox equivalent antioxidant activity, GAE Gallic acid equivalent
Within column numbers followed by different letters are significantly different. p values ≤ 0.05 by Duncan test
pH, ºbrix, colour and total carbohydrate content of citrus fruit juices
The pH values for all citrus fruit juices indicated their acidic nature. The pH values of the samples were ranged from 2.45 to 4.07 (Table 2). Maximum value of pH among all citrus fruits was recorded for C. limetta i.e. 4.07 while minimum value recorded for C. medica i.e. 2.45. In a previous study, Riaz et al. (2015) reported 4.2 pH for C. nobilis juice likewise our observation. °Brix represented the percentage of soluble solids in sample solution. The highest value of °Brix was recorded in C. nobilis juice (13°) whereas, lowest in C. ichangenesis i.e. 6°. Soluble solids mainly include sugars in juices and herein justified with high carbohydrate content in C. nobilis. The °Brix values for all the citrus samples ranged from 6 to 13° Brix (Table 2). The total carbohydrate content of citrus fruit juices were measured by anthrone sulphuric acid method (Morris 1948). Results of total carbohydrate analysis were expressed as glucose, fructose and sucrose equivalent. C. nobilis juice possessed highest carbohydrate content as glucose (537 mg mL−1), fructose (125 mg mL−1) and sucrose equivalent (140 mg mL−1), respectively. The lowest carbohydrate content was showed by C. medica juice (82.5 mg mL−1) as glucose equivalent whereas, C. aurantifolia (17.87 mg mL−1) and C. aurantium (2.85 mg mL−1) showed lowest values as sucrose and fructose equivalent, respectively (Table 2). Colour values of all the citrus juice samples were determined by calculating L*, a*, b* and ∆E value, where L value defines lightness factor of sample ranging between 0 and 100. a* Value defines colour from green to red region of light and b* value defines blue to yellow region of light spectra. Positive values for L* were observed for all samples. Maximum value of lightness was recorded in case of C. limetta (42.28) and minimum values for C. ichangensis and C. aurantium i.e. 38.25. All the citrus samples showed negative a* values ranging between − 0.44 and − 3.19. The b* value for all the fruit juices were positive and ranged from 2.48 to 9.34. ∆E value represent the color difference between L*, a*, b* values. The results showed highest ∆E value (9.34) for C. limetta juice whereas; C. ichangensis possesses lowest ∆E (5.28) (Table 2).
Table 2.
Total carbohydrate content, pH value, colour index and °brix of different citrus fruit juices
| GECC (mg mL−1) | SECC (mg mL−1) | FECC (mg mL−1) | pH | L* | a* | b* | ∆E | °Brix | |
|---|---|---|---|---|---|---|---|---|---|
| C. nobilis | 537 ± 0.70a | 140 ± 0.70a | 125.1 ± 0.77a | 4.02 | 40.44a | − 0.44c | 9.34a | 8.67a | 13 |
| C. ichangensis | 84.0 ± 0.35e | 27.5 ± 0.77d | 4.4 ± 0.28d | 2.70 | 38.25b | − 2.35a | 4.27b | 5.28b | 6.0 |
| C. medica | 82.5 ± 0.42e | 27.12 ± 0.08d | 4.8 ± 0.56d | 2.45 | 41.62a | − 2.84a | 3.27c | 8.69a | 7.0 |
| C. limetta | 155.5 ± 0.42c | 45.37 ± 0.19c | 16 ± 0.63c | 4.07 | 42.28a | − 3.12b | 4.65b | 9.34a | 7.5 |
| C. karna | 179.5 ± 1.76b | 51.37 ± 0.89b | 22.8 ± 0.56b | 2.74 | 38.50b | − 2.14a | 2.48d | 5.67b | 9.0 |
| C. aurantium | 89.5 ± 0.35d | 28.87 ± 0.61d | 2.85 ± 0.03e | 3.43 | 38.25b | − 2.33a | 2.68d | 5.40b | 7.5 |
| C. aurantifolia | 45.5 ± 0.98f | 17.87 ± 0.61e | 15.42 ± 29c | 2.56 | 41.70a | − 3.19b | 4.64b | 8.09a | 8.0 |
L* Lightness, a* red/green coordinate, b* yellow/blue coordinate, ∆E total colour difference
GECC Glucose equivalent carbohydrate content, SECC sucrose equivalent carbohydrate content, FECC fructose equivalent carbohydrate content
Within column numbers followed by different letters are significantly different. p values ≤ 0.05 by Duncan test
Determination of total polyphenolic content
Polyphenols are also called as polyhydroxyphenols because structure contains at least one aromatic ring and one or many hydroxyl groups. They are derived from shikimate pathway and are plants secondary metabolites. Polyphenols are bioactive molecules and have potential to scavenge electrophiles so their assessment and quantification is valuable. Total polyphenolic content (TPC) in citrus fruit juices was evaluated by Folin’s ciocalteau method. All samples showed presence of substantial amount of phenolics. Polyphenolic content for all samples were determined from regression equation prepared by using gallic acid as standard. Calibration curve was plotted at different concentrations to obtain regression equation (y = 0.005x + 0.035, R2 = 0.998). Total polyphenolic content ranged between 0.28 ± 0.001 to 1.17 ± 0.014 mg as gallic acid equivalent (GAE) mL−1 of juice. Varying content of phenolics was recorded in different varieties of citrus fruit juices. C. limetta showed highest phenolic content (1.17 ± 0.014 mg mL−1) while, lowest for C. medica (0.28 ± 0.001 mg GAE mL−1). Previously Guimaraes et al. (2010) reported 11.7 mg (GAE) polyphenols gram−1 of lemon extract as dry weight basis. Whereas our findings are similar to Ersus and Cam (2007) who have reported 56.9 ± 2.4 mg GAE 100 mL−1 polyphenols in C. aurantium juice. In our study, 0.51 ± 0.008 mg GAE mL−1 polyphenols in C. aurantium juice are recorded. Results of total polyphenolic content of different citrus fruit juices are given in Table 3, Fig. S3.
DPPH free radical scavenging activity
Free radicals are highly reactive molecules and can be counted as one of the main factors responsible for many diseases like tumors, inflammation, dementia etc. Antioxidant molecules are capable of stabilizing these free radicals by donating electron/proton. Polyphenols are also associated to impart free radical scavenging activity so antioxidant activity of citrus fruits was checked. Antioxidant activity of seven citrus fruit varieties were evaluated against stable DPPH free radical. In general, when DPPH free radical react with antioxidant compound it get reduced to become stable and reaction was monitored by change in colour from violet to yellow, measured spectrophotometrically at 517 nm. Antioxidant potential of samples against DPPH free radicals was determined and measured using trolox as reference standard. All the citrus samples showed significant antioxidant capacity against DPPH free radical. The intensity of discoloration defined extent of different samples to act as antioxidants. The free radical scavenging activity of citrus fruit juices was recorded 1.26 ± 0.009–2.84 ± 0.006 mg of Trolox equivalents mL−1. Higher the trolox equivalent amount of sample higher is the antioxidant activity. With this context the highest antioxidant activity was showed by C. ichangensis juice (2.84 ± 0.006 mg TEAC mL−1) while, lowest antioxidant capacity was recorded for C. medica (1.26 ± 0.009 mg TEAC mL−1). Barreca et al. (2011) have reported 10.8 µM trolox equivalents DPPH free radical scavenging activity for C. aurantium. In present study C. aurantium showed 1.96 mg TEAC mL−1. The results of antioxidant activity for citrus fruits juice are presented in Table 3, Fig. S3.
Antimicrobial activity
Antibacterial activity of Citrus fruit juice
Agar well diffusion method was used to access antimicrobial activity of citrus fruit juices against four pathogenic bacterial strains i.e. Escherichia coli, Bacillus subtilis, Staphylococcus aureus and Klebisella pneumonie. The zone of inhibition against four pathogenic bacteria was measured in mm. The results of antibacterial activity of fresh citrus juices are presented in Table S1. The antimicrobial activity of citrus fruit juices was recorded at three different concentrations. C. limetta showed highest (19 mm) inhibitory zone against S. aureus however, it was unable to show inhibitory effects against other bacterial strains. C. aurantifolia was found to possess good inhibitory effects against all the selected pathogens with highest zone of inhibition against S. aureus at similar concentration.. Hayes and Markovic (2002) studied the antimicrobial activity of lemon and reported significant inhibitory effects against S. aureus, K. pneumonie and E. coli. In present study, C. aurantium showed inhibitory effects against two pathogens i.e. E. coli and K. pneumonia. C. karna juice also inhibit all bacterial strains and showed almost similar inhibitory effects (5.0 ± 0.00) for all pathogens. While C. medica and C. nobilis juice does not inhibit any strain at all concentrations. C. ichangenesis were found to possess inhibitory activity against E. coli at all concentrations with highest activity at 100 µL. Thus in order to establish the role of citric acid as well as ascorbic acid as major OAs against antibacterial property of juices, the antibacterial activity of respective standards was also evaluated (Table S1). The results showed commendable antibacterial activities against all the pathogenic bacteria at concentration of 2.5 mg. The results also reveal that citric acid was more active against S. aureus (17.33 ± 0.70) at 2.5 mg followed by K. pneumonia (15.00 ± 0.00) at 2.5 mg, whereas ascorbic acid showed high inhibitory zone for K. pneumonia (13.00 ± 0.00) at 2.5 mg. Earlier Shokri (2011) reported that higher antibacterial activity of citric acid may be due to inhibitory mechanisms like alteration in pH of microbes as they are pH sensitive or varying the permeability of cell membranes. Several studies showed that citric acid and its salts inhibit the growth of the most common bacterial pathogens such as Arcobacter spp. Campylobacter spp. lactobacilli, E. coli and L. monocytogenes (Blaszyk and Holley 1998). The high antibacterial activity of citrus fruit juices with elevated content of citric and ascorbic acid clearly established their role in growth inhibition of selected bacterial strains.
Antifungal activity of Citrus fruit juice
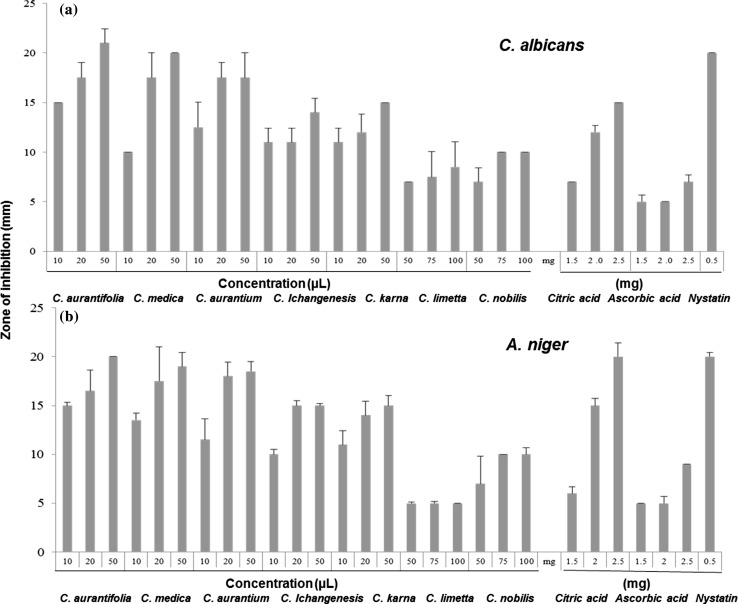
The antifungal potential of citrus fruit juices was assessed against two highly devastating fungus strains i.e. C. albicans and A. niger which are primarily responsible for spoilage of food and beverages. All the citrus fruit juices exhibited good antifungal activity against these two fungal strains. The results of the study are presented in Fig. 3. These results revealed that only 50 µL juices of C. aurantifolia, C. medica, C. aurantium, C. ichangensis and C. karna showed optimum inhibitory effects against both the fungal strains whereas, among all samples C. aurantifolia juice showed highest antifungal activity against C. albicnas (21.0 ± 1.41) and A. niger (20.0 ± 0.00) at 50 µL concentration. In a recent study, Oiekh et al. (2015) also reported significant antifungal activity of C. aurantifolia against C. albicans with 24 mm inhibition zone. The results of antifungal activity for A. niger ranged between 5 to 20 mm. Antifungal activity of standard citric and ascorbic acid was also assessed to check whether these OAs being major constituents play role in imparting antifungal activity of citrus fruit juices. Citric acid showed maximum zone of inhibition against both the fungal strains C. albicans (15 ± 0.00) and A. niger (20 ± 0.00) at concentration of 2.5 mg mL−1 whereas, ascorbic acid showed lower inhibitory activity against studied fungal strains (Fig. 3). Our results are in accordance with earlier studies that reports role of citric acids in imparting antifungal properties (Shokri 2011). From our results it is also concluded that citric acid has more antifungal potential as compared to ascorbic acid. These findings clearly showed that citrus fruit juices can be used as antifungal agents for controlling A. niger and C. albicans growth as indicated by zone of inhibition.
Fig. 3.
Antifungal activity of Citrus fruit juice against a C. albicans and b A. niger and their comparison with citric acid, ascorbic acid and nystatin
Conclusion
This study was performed with the aim to explore local citrus fruits as sustainable source of natural OAs for their utilization in diversified value added products. Thus, a new analytical RP–HPLC–DAD method with 5 min run time was developed and validated for investigation of major OAs in different citrus fruits, fruit juices, foods and beverages containing OAs. This method implies rapid and precise analysis of major OAs without using any buffer which might be helpful to extend column life. During the investigation both citric acid (3.40 to 35.36 mg mL−1) and ascorbic acid (0.083 to 0.54 mg mL−1) were recorded as major OAs in local cultivars of citrus fruits. The comparative analysis of total carbohydrates, phenolics and free radical scavenging antioxidants was also performed along with the assessment of their antimicrobial potential. These results report abundant amount of phenolics (0.28 ± 0.001–1.17 ± 0.014 mg GAE mL−1) and antioxidants (1.26 ± 0.009–2.84 ± 0.006 mg TEAC mL−1) in all juices. Promising antimicrobial results were recorded against A. niger and C. Albicans strains which evidently highlights the potential of citrus fruits for developing new antifungal bio-preservative formulations. The inhibitory activities of citric and ascorbic acid positively relate their role in citrus fruit juice as potential antimicrobials. The samples having higher content of these OAs simultaneously showed higher antimicrobial activity. Hence this study reports a rapid analytical method for screening and quantification of major organic acids from wide range of samples. The therapeutic evaluation studies of citrus fruits have clearly showed that due to incurrence of elevated content of natural OAs like citric and ascorbic acid their lies huge perspective for their exploration in food, beverage, pharma and cosmetic industries.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are very grateful to Director CSIR-IHBT Palampur Dr. Sanjay Kumar for his invaluable guidance and inspiration. Authors are also thankful to Council of Scientific and Industrial Research (CSIR) New Delhi India for financial support under MLP-0070 and DST, Science and Engineering Research Board (SERB) under grant no. YSS/2015/000097.
Compliance with ethical standards
Conflict of interests
Authors declare that they have no conflict of interest including any financial and personal with other people or organizations.
Human and animal subjects
This article does not contain any studies with human or animal subjects.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3045-x) contains supplementary material, which is available to authorized users.
References
- Ali HM, El-Gizawy AM, El-Bassiouny REI, Saleh MA. Browning inhibition mechanisms by cysteine, ascorbic acid and citric acid, and identifying PPO-catechol-cysteine reaction products. J Food Sci Technol. 2015;52(6):3651–3659. doi: 10.1007/s13197-014-1437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreca D, Bellocco E, Caristi C, Leuzzi U, Gattuso G. Distribution of C- and O-glycosyl flavonoids, (3-hydroxy-3-methylglutaryl) glycosyl flavanones and furocoumarins in Citrus aurantium L. juice. Food Chem. 2011;124:576–582. doi: 10.1016/j.foodchem.2010.06.076. [DOI] [Google Scholar]
- Blaszyk M, Holley RA. Interaction of monolaurin, eugenol and sodium citrate on growth of common meat spoilage and pathogenic organisms. Int J Food Microbiol. 1998;39:175–183. doi: 10.1016/S0168-1605(97)00134-7. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Barset C. Use of free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Cunha SC, Fernandes JO, Ferreira IM. HPLC/UV determination of organic acids in fruit juices and nectars. Eur Food Res Technol. 2002;1:67–71. doi: 10.1007/s002170100412. [DOI] [Google Scholar]
- Ersus S, Cam M. Determination of organic acids, total phenolic content, and antioxidant capacity of sour Citrus aurantium fruits. Chem Nat Compd. 2007;43:607–609. doi: 10.1007/s10600-007-0203-1. [DOI] [Google Scholar]
- Guimaraes R, Barros L, Barreira JCM, Sousa MJ, Carvalho AM, Ferreira ICFR. Targeting excessive free radicals with peels and juices of citrus fruits: Grapefruit, Lemon, Lime and Orange. Food Chem Toxicol. 2010;48:99–106. doi: 10.1016/j.fct.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Markovic B. Toxicity of Australian essential oil Backhousia citriodora (Lemon myrtle). Part I. Antimicrobial activity and in vitro cytotoxicity. Food Chem Toxicol. 2002;40:535–543. doi: 10.1016/S0278-6915(01)00103-X. [DOI] [PubMed] [Google Scholar]
- Hees PAW, Dahlen J, Lundstrom US, Boren H, Allard B. Determination of low molecular weight organic acids in soil solution by HPLC. Talanta. 1999;48:173–179. doi: 10.1016/S0039-9140(98)00236-7. [DOI] [PubMed] [Google Scholar]
- Ibanez AB, Bauer S. Analytical method for the determination of organic acids in dilute acid pretreated biomass hydrolysate by liquid chromatography-time-of-flight mass spectrometry. Biotechnol Biofuels. 2014;7:145. doi: 10.1186/s13068-014-0145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslyn MA. Methods of food analysis: Physical, chemical and instrumental methods of analysis. New York: Academic Press; 1970. [Google Scholar]
- Kelebek H, Selli S, Canbas A, Cabaroglu T. HPLC determination of organic acids, sugars, phenolic compositions and antioxidant capacity of orange juice and orange wine made from a Turkish cv. Kozan. Microchem J. 2009;91:187–192. doi: 10.1016/j.microc.2008.10.008. [DOI] [Google Scholar]
- Kotani A, Miyaguchi Y, Tomita E, Takamura K, Kusu F. Determination of organic acids by high-performance liquid chromatography with electrochemical detection during wine brewing. J Agric Food Chem. 2004;52:1440–1444. doi: 10.1021/jf0306486. [DOI] [PubMed] [Google Scholar]
- Li B, Yongku L, Wang X, Wang F, Wang X, Wang Y, Meng X. Simultaneous separation and determination of organic acids in blueberry juices by capillary electrophoresis-electrospray ionization mass spectrometry. J Food Sci Technol. 2015;52(8):5228–5235. doi: 10.1007/s13197-014-1611-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani-Lopez E, Garcia HS, Lopez-Malo A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res Int. 2012;45:713–721. doi: 10.1016/j.foodres.2011.04.043. [DOI] [Google Scholar]
- Morris DL. Quantitative determination of carbohydrates with Drywood’s anthrone reagent. Science. 1948;107:254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- Narayan R, Mendiratta SK, Mane BG. Effects of citric acid, cucumis powder and pressure cooking on quality attributes of goat meat curry. J Food Sci Technol. 2015;52(3):1772–1777. doi: 10.1007/s13197-013-1023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oiekh EI, Omoregie ES, Oviasogie FE, Oriakhi K. Phytochemical, antimicrobial, and antioxidant activities of different citrus juices concentrates. Food Sci Nutr. 2015;4(1):103–108. doi: 10.1002/fsn3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana A, Sharma E, Rawat K, Sharma R, Verma S, Padwad Y, Gulati A. Screening and purification of catechins from underutilized tea plant parts and their bioactivity studies. J Food Sci Technol. 2016;53(11):4023–4032. doi: 10.1007/s13197-016-2406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz M, Zamir T, Rashid N, Jamil N, Masood Z, Jabeen U, Mandokhel F, Behlil F, Mengal F, Khan M. Quality assessment in different stages of maturity of fruits, mandarins kinnow and feutrell’s early collected from the fruit market of Quetta city at in relation to their benefits for human health. Am Euras J Toxicol Sci. 2015;7(3):203–208. [Google Scholar]
- Rostamzad H, Shabanpour B, Kashaninejad M, Shabani A. Antioxidative activity of citric and ascorbic acids and their preventive effect on lipid oxidation in frozen persian sturgeon fillets. Latin Am Appl Res. 2011;41:135–140. [Google Scholar]
- Scherer R, Rybka ACP, Ballus CA, Meinhart AD, Filho JT, Godoy HT. Validation of HPLC method for simultaneous determination of main organic acids in fruits and juices. Food Chem. 2012;135:150–154. doi: 10.1016/j.foodchem.2012.03.111. [DOI] [Google Scholar]
- Sdiri S, Bermejo A, Aleza P, Navarro P, Salvador A. Phenolic composition, organic acids, sugars vitamin C and antioxidant activity in the juice of two new triploid late-season mandarins. Food Res Int. 2012;49:462–468. doi: 10.1016/j.foodres.2012.07.040. [DOI] [Google Scholar]
- Shokri H. Evaluation of inhibitory effects of citric and tartaric acids and their combination on the growth of Trichophyton mentagrophytes, Aspergillus fumigatus, Candida albicans and Malassezia furfur. Comp Clin Path. 2011;20:543–545. doi: 10.1007/s00580-011-1195-6. [DOI] [Google Scholar]
- Smulders FJM, Greer GG. Integrating microbial decontamination with organic acids in HACCP programmes for muscle foods: prospects and controversies. Int J Food Microbiol. 1998;44:149–169. doi: 10.1016/S0168-1605(98)00123-8. [DOI] [PubMed] [Google Scholar]
- Swain T, Hill E. The phenolic constituents of Prunus domestica. I. The quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Tyagi G, Jangir DK, Singh P, Mehrotra R, Ganeshan R, Gopal ESR. Rapid determination of main constituents of packed juices by reverse phase-high performance liquid chromatography: an insight into commercial fruit drinks. J Food Sci Technol. 2014;51(3):476–484. doi: 10.1007/s13197-011-0502-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.