Abstract
Saffron quality is commonly determined by three parameters: color, aroma, and taste. Several factors including harvesting and post-harvesting conditions, affect these parameters. In this study, the effect of storage time on saffron quality was evaluated. At first, the relative concentration of the saffron secondary metabolites in freshly dried and 2 years stored saffron samples prepared with ISO 3632 and UA-DLLME methods and then measured using UV–Vis and GC-FID techniques. In order to find saffron storage time biomarkers, the obtained data were subjected to several data analysis steps including data preprocessing, principal component analysis (PCA), partial least square discriminant analysis (PLS-DA) and variable selection methods. Based on the obtained main biomarkers and proposed molecule mechanism, it can be concluded that during the storage periods, the intensity of saffron color reduces, while its aroma increases, reflecting a negative correlation between them. Freshly dried samples have a higher level of the crocins as coloring agents, β-isophorone, 4-hydroxy-3,5,5-trimethylcyclohex-2-enone and picrocrocin, while the stored samples were more abundant by safranal as the main saffron aroma agent.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3046-9) contains supplementary material, which is available to authorized users.
Keywords: Saffron storage biomarkers, Metabolic fingerprinting, Volatile profile, Multivariate data analysis, Molecular mechanism
Introduction
The genus Crocus L. (Iridaceae) contains more than 100 species that are mainly distributed in the Mediterranean Europe and Western Asia (Carta et al. 2016; Peruzzi 2016). The most commonly cultivated species of the genus, Crocus sativus L., known as saffron, is a triploid (2n = 3x = 24) plant and not able to produce viable seeds (Rashed-Mohassel 2007). It is propagated from underground corms reproducing vegetatively from the main (parental) corms (Acar et al. 2015). The outstanding feature of the lilac flowers of the plant is the branched tri-partite stigmas which are red and becomes more intensified in color when dried (Reza Gohari et al. 2013). Saffron is mainly used as a spice for coloring and flavoring purposes in food and cosmetic industries. In addition, it has a variety of therapeutic properties in traditional and modern medicine (Abdullaev 2004; Alavizadeh and Hosseinzadeh 2014; Melnyk et al. 2010).
The three main parameters used to define the saffron quality are color (expressed as crocins), taste (expressed as picrocrocin) and aroma (expressed as safranal). Crocins are glycosyl esters of crocetin (8,8′-diapo-Ψ,Ψ′-carotenedioic acid) with glucose, gentiobiose, neapolitanose or triglucose sugar moieties (Sánchez et al. 2008). These hydrophilic sugar moieties make crocins water-soluble to create special orange-yellow color in the water. Picrocrocin (4-(-d-glucopyranosyl)-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde) is the main component which is responsible for saffron bitter taste (Sánchez et al. 2008). It has been proposed that picrocrocin is the main precursor of safranal (Carmona et al. 2006). Safranal (2,6,6-trimethylcyclohexane-1,3-dien-1-carboxaldehyde) is the most abundant volatile component and the most influential sensorial compound in the saffron aroma (Bononi et al. 2015).
Harvesting and post-harvesting conditions including climate, type of soil and fertilizer, planting time, the age of farm, age and size of the corm, irrigation system, drying system, and storage conditions affect the saffron quality (Ordoudi and Tsimidou 2004; Raina et al. 1996). Storage time is one of the major factors affecting the quality of saffron either through the formation of new materials or degradation of the other components. It is known that fresh saffron does not contain safranal. During the first 2 years of storage, its content increases and then start decreasing (Maggi et al. 2010). It has been proven that during the storage period many other saffron volatile components are transforming. Furthermore, picrocrocin loses its sugar moieties and converts to 4-hydroxy-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde (HTCC) and safranal. It is likely the reason why fresh saffron samples are bitterer than the old ones. Regarding the coloring agent, it was suggested that crocins are degraded and shift to volatiles compounds passing some internal mechanisms (Carmona et al. 2006).
Because of saffron nutritional and medicinal characteristics importance, the proper control of saffron quality is necessary. International Standard Organisation (ISO) developed some methods to assess the saffron quality and detect adulteration (ISO 3632 2014). According to the suggested UV–Vis spectrophotometric method, the quality of saffron is determined using its three main characteristics: color, taste, and aroma. Color and taste are well defined in this method. However, due to safranal low solubility in water and some spectral interference at the same wavelength, its detection, as the main compound in volatile components, is not efficient. Gas chromatography (GC) coupled with different detectors is the best technique for characterization of saffron volatile components (Bononi et al. 2015). However, the obtained GC chromatograms often consist of different problems such as high level of the baseline and peak overlapping. These problems mainly occur due to the complexity of the saffron volatile matrix, and cannot be fully eliminated by sample preparation methods. Therefore, many different chemometric methods have been introduced and developed to overcome these problems and to extract the important information from the obtained data (Aliakbarzadeh et al. 2016).
The aim of the present investigation is to assess how the storage time might affect the main quality parameters of saffron especially its important volatile components. For this purpose, saffron volatile components are extracted and preconcentrated with the UAE-DLLME method and analyze with gas chromatography. Moreover, color and taste parameters evaluated using the UV–Vis method recommended by ISO 3632. The obtained GC-FID chromatograms and UV–Vis spectra were subjected to appropriate chemometric preprocessing and modeling methods to classify them based on their storage period and to find the potential biomarkers of saffron storage time.
Materials and methods
Samples and chemicals
A total of 49 saffron samples were collected at two different times of storage from different regions of Khorasan province (north-east of Iran). Among these samples, 21 samples were harvested in autumn 2013 and stored for 2 years, and 28 samples were harvested in autumn 2015. All collected samples were dried at room temperature and stored at 4 °C in the absence of light until analysis. All the chemicals including methanol, acetonitrile, n-dodecane, chloroform and sodium chloride were purchased from Merck Chemicals (Darmstadt, Germany).
Instrumentation
The GC analyses were performed using an Agilent Technologies 7890 gas chromatograph (Santa Clara, CA, USA) equipped with a flame ionization detector (FID). The injection port (split/splitless) was set at 230 °C with the split ratio of 1:20. Separation of the extracted compounds was carried out on a HP-5 capillary column (30 m × 0.25 mm × 0.25 μm). Helium (99.999%) was used as carrier gas with a constant flow rate of 1.0 mL min−1 during the analysis. The column temperature was initially kept for 1 min at 80 °C, then increased to 140 °C with a rate of 15 °C min−1 and finally increased to 260 °C with a rate of 80 °C min−1 and kept for 33.5 min. The detector temperature was 270 °C.
For identification of saffron volatile components, a GC–MS system (Agilent Technologies, Santa Clara CA, USA) including a GC model 6890 with a HP5-MS capillary column (30 m × 0.32 mm × 0.25 μm) attached to a mass spectrometer was applied. The operatory conditions of GC–MS were similar to GC-FID conditions. The scanned mass range and ionizing energy was 20–500 m/z and 70 eV, respectively. A UV–Vis spectrophotometer (UV 2601, Beijing, China) was used for measurement of absorbance in the range of 200–700 nm according to the ISO 3632 method.
UV–Vis methodology
The main saffron components including picrocrocin, safranal, and crocin were determined using the proposed method by ISO 3632-2 (ISO 3632 2014). Briefly, 50 mL of distilled water was added to 50 mg of saffron powder (1%, w/v) and stirred for 1 h. Then, the solution was placed in a dark place for 24 h and then was filtered rapidly using a filter paper to obtain a clear solution. Afterward, 1 mL of the obtained solution was transferred to a 25 mL volumetric flask and made up with distilled water (0.004%, w/v). Finally, the absorbance of this solution was measured by UV–Vis spectrophotometry.
UA-DLLME/GC-FID procedure
The ultrasound-assisted extraction coupled with dispersive liquid–liquid microextraction (UA–DLLME) method was used for extraction of saffron volatile components (Sereshti et al. 2014). Briefly, 50 mg of the powdered saffron was placed in a 10 mL conical glass test tube and 1 mL of a mixture of MeOH/ACN (38:62, v/v%) containing 10 mg L−1 of dodecane (internal standard) was added to it. The mixture was then exposed to ultrasonic waves for 22 min at ambient temperature. Then, the mixture was centrifuged at 3000 rpm for 3 min to separate the solid particles from the solution. Next, 26 µL of chloroform was added to 0.5 mL of the supernatant and the mixture was sonicated for 2 min. Afterward, the mixture was injected into 2 mL of NaCl solution (7%, w/v) and a cloudy solution appeared in the conical test tube. The chloroform phase was sedimented in the bottom of the test tube by centrifugation at 3500 rpm for 3 min. Finally, 0.8 μL of the sediment phase was injected to GC-FID.
Statistical analysis
The raw GC chromatograms were arranged in a (n × p) data matrix, X, with n rows containing all 49 saffron samples and p variables containing 1401 elution time points. Before multivariate analysis, the row chromatograms were subjected to different pre-processing methods to remove baseline and noise contribution and retention times shifts (RTs) (Aliakbarzadeh et al. 2016; De La Mata-Espinosa et al. 2011). First, the chromatograms were subjected to Asymmetric Least Squares (AsLS) for baseline correction. Any possible shifts in the location of the chromatographic peaks should be removed before statistical analysis (Liland 2011). Therefore, correlation optimization warping (COW), a robust peak alignment method, was employed to correct the peak shifts between different chromatograms. Finally, saffron chromatograms were normalized toward the maximum value of the internal standard signal (dodecane, RT: 6.6–6.8 min) to compensate the effect of non-sample-related variations which occur during sample preparation or data acquisition. All chromatograms were then mean-centered and Pareto-scaled to remove the direction of the overall variance and increasing the importance of minor metabolites without increasing the noise levels. According to our prior knowledge, the volatile components of saffron elute between 4 and 9 min. Thus, it was considered as the saffron chromatographic fingerprint (Aliakbarzadeh et al. 2016). Similar to GC-FID chromatograms, the UV–Vis spectra were arranged in a matrix, with n rows containing all 49 saffron samples and p variables containing 509 wavelength points. The UV–Vis data were mean-centered and Pareto scaled before data analysis.
The pre-processed data were divided into training and test sets. The training sets, contain 31 GC chromatograms and 34 UV–Vis spectra. Eighteen chromatograms and 15 spectra were included in external test sets. In order to explore the data structure and to discriminate among the saffron samples based on their storage time, the training sets were modeled using PCA and PLS-DA methods. The VIP and rPLS variable selection methods were applied on the PLS models to investigate the most important variables. Finally, the prediction abilities of the obtained models were evaluated using test sets. Data analysis was performed with MATLAB (version 8.3, R2014a), Classification toolbox (version 4.0) (Ballabio and Consonni 2013) and PCA toolbox (version 1.0) (Ballabio 2015).
Result and discussion
Data pre-processing
The GC-FID chromatograms of all the saffron samples, before pre-processing steps, are illustrated in Fig. S1a (supplementary data file) and the zoomed out view of saffron fingerprint area (4–9 min) is shown in Fig. S1b. The chromatographic fingerprint is related to main secondary saffron volatile metabolites. As it is clear from these figures, saffron chromatograms contained many compounds. They also contained some chromatographic artifacts such as baseline and noise contribution and retention time shifts. Therefore, as explained in “Statistical analysis” section, the chromatograms were subjected to different pre-processing steps including baseline correction, peak alignment, and normalization against the maximum value of the internal standard peak. Figure S1c and S1d show the pre-processed full chromatograms and fingerprint segments, respectively. The relative standard deviations (RSD%) of the pre-processed and normalized fingerprint segments, calculated based on total peak area for each sample were in the range of 1.62–7.21 (n = 3). Finally, the chromatograms were mean-centered and Pareto-scaled before further analysis.
Principal component analysis (PCA)
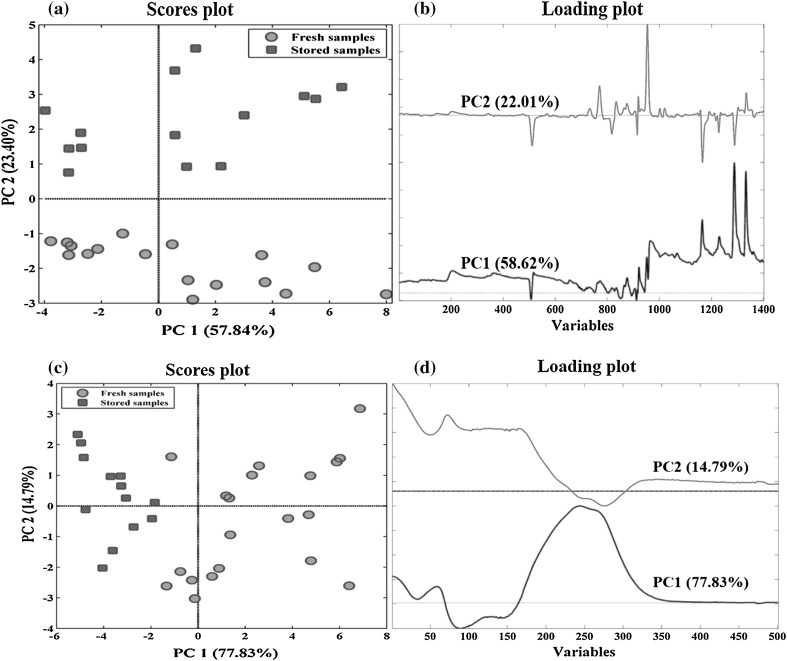
The pre-processed dataset was analyzed using PCA as an unsupervised method to evaluate similarities and dissimilarities among the samples. The PCA technique can be used to reduce the dimensionality of a multi-dimensional dataset. This method provides an overview of hidden information in the dataset (Vallejo et al. 2008). Figure 1a, b depicts the score and loadings plots of PCA for GC chromatograms. The first two principal components (PCs) (PC1: 58.62% and PC2: 22.01%) showed 80% of the total variance. PC1 did not offer any useful information about the storage condition of the saffron samples. The most important information about the effect of storage time on the saffron volatile components were obtained by PC2. As shown in Fig. 1a, the freshly dried samples collected in autumn 2015 (green points) and the old samples collected in autumn 2013 (red points) contained quite different volatile composition and therefore, are fairly separated in score plot.
Fig. 1.
PCA plots of the training data set for GC-FID a scatterplot and b the loadings plots and for UV–Vis data, c scatterplot and d the loadings plots for the first two PCs
Inspecting the raw chromatograms and loadings plots (Fig. 1b) revealed that old samples that had positive score values on PC2, compared to freshly dried samples, contained a much higher level of safranal (RT ≈ 4.61, data points ≈ 955) as the main ingredients of the saffron aroma profile. They also had higher content of 2-isopropylidene-3-methylhexa-3,5-dienal (RT ≈ 4.06, data points ≈ 730), α-isophorone (RT ≈ 4.16, data points ≈ 770), 2-hydroxy-isophorone (RT ≈ 4.31, data points ≈ 835), 1-acetyl-4-methylbicyclo[3.1.0]hexan-3-one (RT ≈ 4.38,data points ≈ 865), 2,6,6-trimethyl-1,4-cyclohexanedione (dihydrooxophorone) (RT ≈ 4.42, data point ≈ 875), eucarvone (RT ≈ 4.68, data point ≈ 1000), 3,5,5-trimethyl-2-hydroxy-1,4-cyclohexadione-2-ene (RT ≈ 4.73, data point ≈ 1020) and safranal isomer (RT ≈ 5.84, data point ≈ 1335). On the other hand, the volatile components of freshly dried saffron samples that had negative score values on second PC, contained approximately high level of β-isophorone (RT ≈ 3.57, data points ≈ 510), ketoisophorone (RT ≈ 4.27, data points ≈ 820), 4-hydroxy-3,5,5-trimethylcyclohex-2-enone (RT ≈ 5.12, data points ≈ 1165), α-ionol (RT ≈ 5.5, data point ≈ 1217), 2,6,6-trimethyl-4-hydroxy-1-cyclohexene-1-carboxaldehyde (HTCC) (RT ≈ 5.55, data point ≈ 1228) and 2-hydroxy-3-isopropyl-6-methyl-2-cyclohexen-1-one (RT ≈ 5.7, data points ≈ 1290), in comparison with the old samples.
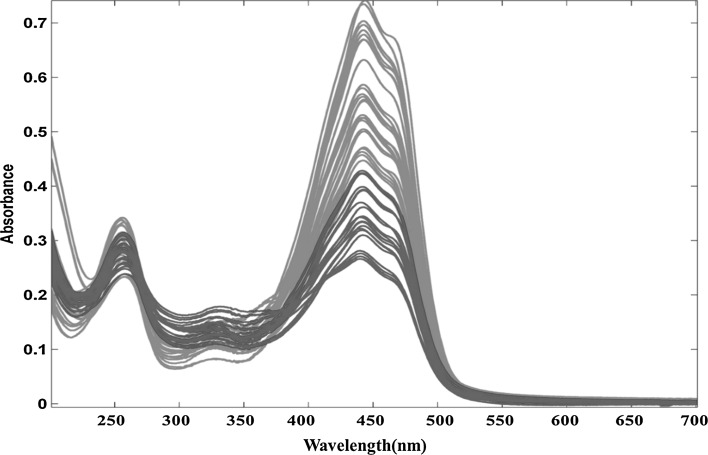
Figure 2 shows the raw UV–Vis spectra of all samples obtained according to ISO 3632-2 method. There are three peaks in saffron’s UV–Vis spectra; picrocrocin (Kmax = 257 nm), safranal (Kmax = 330 nm) and crocins (Kmax = 440 nm). Green and red colors represent freshly dried and 2-year stored samples, respectively. The scores and loadings plot of PCA for UV–Vis spectra are depicted in Fig. 1c, d. 92.62% of the total variance was explained by just two first PCs (PC1: 77.83% and PC2: 14.79%). In PC1 saffron samples with two different storage times were properly separated. The loadings plot shows the differences in three parameters in UV–Vis spectra for two storage times.
Fig. 2.
Raw UV–Vis spectra obtained for all analysed samples. The results marked in green and red color correspond to the freshly dried and 2-year stored samples of saffron, respectively
It can be concluded from Figs. 1c, d and 2 that amount of safranal (330 nm) was higher in the samples stored for 2 years. The loading plot of the GC data (Fig. 1b) confirmed this result. In contrast, there was a considerable decrease in the content of crocins (440 nm) during the storage. According to the obtained results, it seems that aroma profile of saffron especially safranal was negatively correlated with crocins content. This finding shows that while the color of saffron decreased during the storage time, its aroma increased.
Partial least squares-discriminant analysis (PLS-DA)
In order to discriminate between freshly dried and stored saffron samples and finding the most important saffron biomarkers, partial least squares discriminant analysis (PLS-DA), as a well-known supervised classification method (Ballabio and Consonni 2013), was used to classify saffron UV–Vis and GC fingerprints based on samples storage time. For choosing an optimum number of PLS latent variables (LVs), the error rate (ER) values were obtained using Venetian blinds cross-validation (VB-CV). Two latent variables (LVs) were used for developing the model in both UV and GC cases, based on which the minimum values of the error rate and also non-assigned samples were achieved.
The qualities of the models were evaluated by general classification and cross-validation parameters including sensitivity, specificity, accuracy, error rate (ER), and non-error rate (NER). The sensitivity of a class shows the ability of the model to correctly assign samples belonging to that class. Specificity describes the model ability to reject the samples of all the other classes. The rate of correctly assigned samples defines as accuracy. The non-error rate of each class is an average of its specificity and sensitivity and finally, its ER is calculated to equal to ER = 1 − NER. All these values are range from 0.00 to 1.00 and non-assigned samples are not included in their calculations (Ballabio and Consonni 2013). All of these values along with explained variance were summarized in Table 1.
Table 1.
Classification figures of merit for PLS-DA models
| Variable selection | Explained variance (X) | No. variables | LVsa | NERb | ERc | Accuracy | Old saffron | Freshly dried saffron | Not assigned | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Specificity | Sensitivity | Specificity | Sensitivity | |||||||||
| GC-FID | ||||||||||||
| Global PLS-DA | 79% | 1401 | 2 | Fitting | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | – |
| CVd | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | – | ||||
| Test set | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.20 | ||||
| rPLS | 96% | 27 | 2 | Fitting | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | – |
| CV | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | – | ||||
| Test set | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | – | ||||
| VIP | 77% | 139 | 2 | Fitting | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.03 |
| CV | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.03 | ||||
| Test set | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.60 | ||||
| UV–Vis | ||||||||||||
| Global PLS-DA | 92% | 501 | 2 | Fitting | 0.97 | 0.03 | 0.97 | 0.95 | 1.00 | 1.00 | 0.95 | – |
| CV | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.03 | ||||
| Test set | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.33 | ||||
| rPLS | 99% | 11 | 2 | Fitting | 0.97 | 0.03 | 0.97 | 0.95 | 1.00 | 1.00 | 0.95 | – |
| CV | 0.97 | 0.03 | 0.97 | 0.95 | 1.00 | 1.00 | 0.95 | – | ||||
| Test set | 0.93 | 0.07 | 0.93 | 0.86 | 1.00 | 1.00 | 0.86 | – | ||||
| VIP | 99% | 143 | 2 | Fitting | 0.97 | 0.03 | 0.97 | 0.95 | 1.00 | 1.00 | 0.95 | – |
| CV | 0.97 | 0.03 | 0.97 | 0.95 | 1.00 | 0.95 | 1.00 | – | ||||
| Test set | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | – | ||||
aLatent variables, bnon error rate, cerror rate
Based on the obtained results (Table 1), 20% of the samples of the test set were not assigned to any groups in the model build based on GC-FID data. However, the other model parameters were in acceptable range. By fitting a PLS-DA model on the UV–Vis spectra, 92% of variance explained for all 501 variables. The sensitivities were found to be equal to 1.00 and 0.95 for stored and freshly dried saffron, respectively. The resulting NER of the test set classification was equal to 97%. However, the rate of the not assigned samples in the external validation was also high in this case (33% of the test set samples were not assigned to any group).
Variable selection and important biomarkers
Variable selection is usually used for improving the model performance and prediction ability. Removal of noisy and unreliable variables will typically improve the prediction ability and reduce the model overfitting and complexity (Andersen and Bro 2010). Furthermore, the most important compounds that cause differences between samples can be detected by variable selection methods.
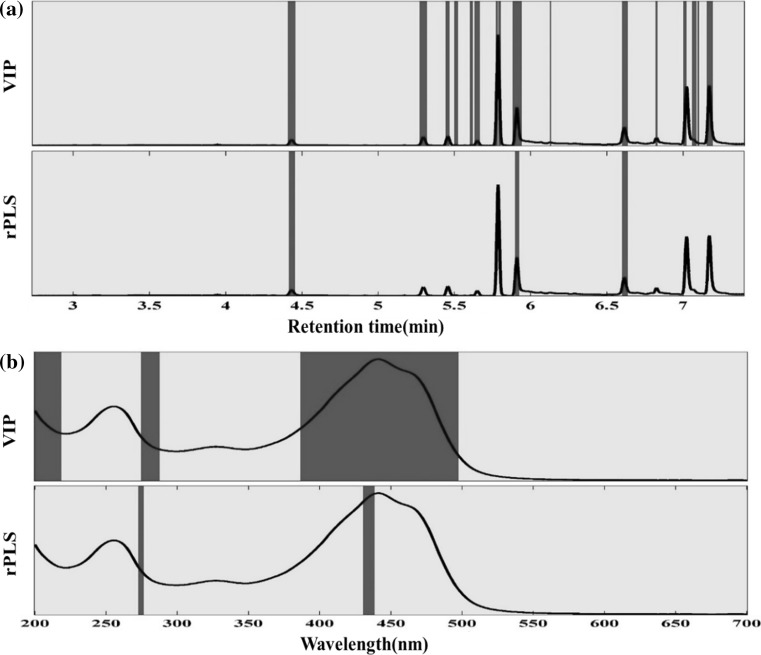
In the present study, two known variable selection methods including variable importance projection (VIP) and recursive partial least squares (rPLS) were applied to detect the discriminative variables for both GC and UV–Vis dataset. New PLS-DA models were built based on the variables chosen by these methods. The selected variables by two methods for GC and UV–Vis data are depicted in Fig. 3.
Fig. 3.
Selected variables with two different variable selection methods. a For GC-FID data. b For UV–Vis data
The VIP is one of the most popular variable selection methods in which a VIP score is calculated for each variable based on the accumulated PLS loadings weights of a variable in all the selected LVs (Andersen and Bro 2010). Applying the VIP method for GC data, decreased number of variables from 1401 to 139 important variables. A new PLS-DA model was built based on this new variable set. The model figures of merit including sensitivity, specificity, and accuracy for internal and external validation were all in the acceptable range. However, 60% of the test set samples were not assigned to any class (Table 1). In addition, the VIP model chooses internal standard as an important variable. Therefore, the obtained results from this method for GC data cannot be much relied. On the other hand, applying VIP method on the UV–Vis data results in the acceptable range of the PLS-DA model figures of merit in all cases.
The rPLS is one of the novel variable selection method (Rinnan et al. 2014). The method iteratively reweights the variables using the PLS regression coefficients to increase and decrease the weight of important and unimportant variables, respectively. The rPLS model reduced the number of variables to 27 from 1401 initial variables for GC data and 11 from 501 initial variables for UV–Vis data. By building the model using two LVs for both GC and UV–Vis data, the minimum value of the ER and the minimum number of unassigned samples were achieved. All the models’ figures of merit including the percent of the non-assigned samples were in an acceptable range (Table 1).
Based on the rPLS weights for GC data, the most important discriminator between two saffron classes in volatile components was β-isophorone (RT ≈ 3.57, data points ≈ 510), safranal (RT ≈ 5.84, data point ≈ 1335) and 4-hydroxy-3,5,5-trimethylcyclohex-2-enone (RT ≈ 5.12, data points ≈ 1165,). In VIP method, in addition to this three compound, several other compounds were considered to be important that one of them was internal standard. For UV–Vis dataset, the VIP method had chosen three important discriminators between two classes included crocins peak (color agent) and parts of before and after picrocrocin peak (taste agent), whereas rPLS had chosen only two important discriminators included crocins and picrocrocin peak. Both of this two variable selection method didn’t choose safranal (aroma agent) as an important variable due to the inability of UV–Vis method to detect volatile components.
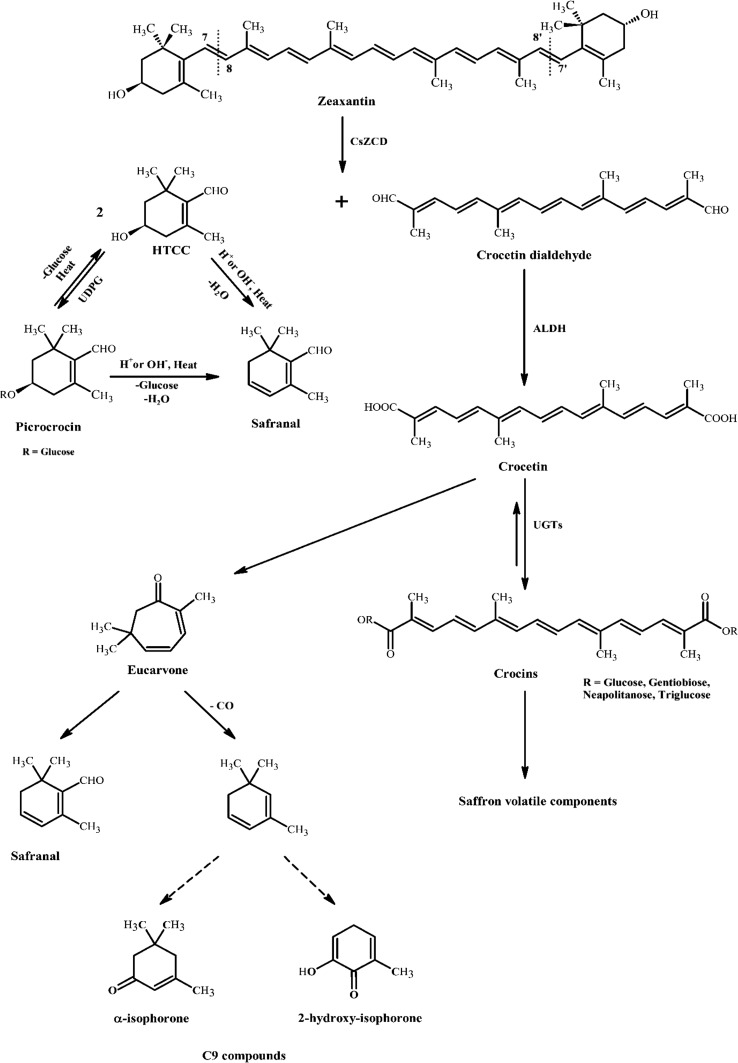
Molecular mechanisms
Figure 4 shows the main pathways involved in the production of the main saffron components (Carmona et al. 2006; Maggi et al. 2010). As can be seen, Crocus sativus zeaxanthin cleavage dioxygenase (CsZCD) is cleaved sequentially at C7 = C8 and C7′ = C8′ double bonds to form crocetin dialdehyde and hydroxyl-β-cyclocitral (HTCC) molecule. HTCC follows two paths: (1) releasing H+ or OH− and heat to form safranal directly; (2) under the action of UDPG-glucosyltransferase is converted to picrocrocin, the compound responsible for saffron bitterness (Frusciante et al. 2014). Picrocrocin can lose its glucosyl moieties and be converted to HTCC again or transformed by thermal treatment or alkaline-acid hydrolysis into safranal (Carmona et al. 2006).
Fig. 4.
Proposed mechanism for the generation and conversion of saffron important components
Crocetin dialdehyde is converted to crocetin, a dicarboxylic acid molecule, by aldehyde oxidoreductase enzymes (ALDH) (Frusciante et al. 2014). Crocetin is an intermediate molecule for the formation of saffron color and volatile agents and plays a key role in saffron molecular conversion pathways. Carmona and Zalancain proposed a pathway to convert crocetin to safranal and other volatile compounds in their work (Carmona et al. 2006). These pathways that are consistent with our results are shown also in a part of Fig. 4. In old samples, higher amounts of eucarvone are present. According to this pathway, eucarvone loses one CO molecule and forms C9 compounds like α -isophorone and 2-hydroxy-isophorone with a series of internal changes. The content of both of this compounds is higher in old samples. In another part of this pathway, eucarvone can be converted to safranal directly. α-Isophorone and β-isophorone are isomers. It is interesting that the amount of α-isophorone is higher in old samples and β-isophorone in freshly dried samples.
HTCC and isophorone that act as sources of safranal formation during storage time, would disappear during a 1–4 years storage period (Carmona et al. 2006; Maggi et al. 2010). In addition to formation of eucarvone, crocetin might follow other pathway to produce saffron volatiles, using UDPG-glucosyltransferase (UGTs), crocetin takes sugar moieties and converts to crocins. In given structure for crocins, R can be glucose, gentiobiose, neapolitanose or triglucose moieties. α-crocin with 2-glucosyl moieties is main crocin that is responsible for saffron color (Alavizadeh and Hosseinzadeh 2014). It seems conversion of crocetin to crocins are reversible. Reducing the content of color and in parallel increasing the content of safranal support this supposition. During the storage time, unknown enzymes or heat or light degrade crocins and cut their moieties. There are some proposed mechanisms for crocins degradation and convert them to volatile molecules (Carmona et al. 2006). It seems some intermediate molecules like TDOI (4,5,6,7-tetrahydro-7,7-dimethyl-5-oxo-3H-isobenzofuranone) play role in these proposed mechanisms. However, it was not detected in the volatile components.
Conclusion
Chemometric analysis of gas chromatographic and spectroscopic profiles obtained from freshly dried and 2-year stored saffron samples showed that the storage time has a great influence on the saffron chemical profile and quality. Moreover, it was proven that by inspecting only five saffron secondary metabolites (i.e., safranal, crocins, β-isophorone, 4-hydroxy-3,5,5-trimethylcyclohex-2-enone, and picrocrocin), the freshly dried and stored samples can be fairly distinguished. The freshly dried samples contained more crocins, β-isophorone, 4-hydroxy-3,5,5-trimethylcyclohex-2-enone and picrocrocin, while the level of safranal, the main aroma ingredient of saffron, was higher in old samples. Furthermore, it was found that the concentration of other volatile metabolites such as α-isophorone, 2-hydroxy-isophorone and eucarvone were higher in the stored saffron samples compared to the fresh ones. On the other hand, the freshly dried samples contained higher amounts of ketoisophorone and HTCC. Although these components were not considered as the saffron biomarkers, the obtained data led to a better understanding of phytochemical changes of saffron ingredients upon storage time.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3046-9) contains supplementary material, which is available to authorized users.
References
- Abdullaev FI. Antitumor effect of saffron (Crocus sativus L.): overview and perspectives. Acta Hortic. 2004;650:491–499. doi: 10.17660/ActaHortic.2004.650.60. [DOI] [Google Scholar]
- Acar B, Sadikoglu H, Doymaz I. Freeze-drying kinetics and diffusion modeling of saffron (Crocus sativus L.) J Food Process Preserv. 2015;39:142–149. doi: 10.1111/jfpp.12214. [DOI] [Google Scholar]
- Alavizadeh SH, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Aliakbarzadeh G, Sereshti H, Parastar H. Pattern recognition analysis of chromatographic fingerprints of Crocus sativus L. secondary metabolites towards source identification and quality control. Anal Bioanal Chem. 2016;408:3295–3307. doi: 10.1007/s00216-016-9400-8. [DOI] [PubMed] [Google Scholar]
- Andersen CM, Bro R. Variable selection in regression—a tutorial. J Chemom. 2010;24:728–737. doi: 10.1002/cem.1360. [DOI] [Google Scholar]
- Ballabio D. A MATLAB toolbox for principal component analysis and unsupervised exploration of data structure. Chemom Intell Lab Syst. 2015;149:1–9. doi: 10.1016/j.chemolab.2015.10.003. [DOI] [Google Scholar]
- Ballabio D, Consonni V. Classification tools in chemistry. Part 1: linear models. PLS-DA. Anal Methods. 2013;5:3790–3798. doi: 10.1039/c3ay40582f. [DOI] [Google Scholar]
- Bononi M, Milella P, Tateo F. Gas chromatography of safranal as preferable method for the commercial grading of saffron (Crocus sativus L.) Food Chem. 2015;176:17–21. doi: 10.1016/j.foodchem.2014.12.047. [DOI] [PubMed] [Google Scholar]
- Carmona M, Zalacain A, Salinas MR, Alonso GL. Generation of saffron volatiles by thermal carotenoid degradation. J Agric Food Chem. 2006;54:6825–6834. doi: 10.1021/jf0612326. [DOI] [PubMed] [Google Scholar]
- Carta A, Campigli S, Peruzzi L, Bedini G. The avoidance of self-interference in the Tuscan endemic spring geophyte Crocus etruscus Parl. (Iridaceae) Plant Biosyst. 2016;150:1358–1363. doi: 10.1080/11263504.2015.1118164. [DOI] [Google Scholar]
- De La Mata-Espinosa P, Bosque-Sendra JM, Bro R, Cuadros-Rodríguez L. Discriminating olive and non-olive oils using HPLC-CAD and chemometrics. Anal Bioanal Chem. 2011;399:2083–2092. doi: 10.1007/s00216-010-4366-4. [DOI] [PubMed] [Google Scholar]
- Frusciante S, Diretto G, Bruno M, Ferrante P, Pietrella M, Prado-Cabrero A, Giuliano G. Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proc Natl Acad Sci USA. 2014;111:12246–12251. doi: 10.1073/pnas.1404629111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO 3632–2 Technical Specification . Saffron (Crocus sativus L.), Part 2 (test methods) Geneva, Switzerland: International Organization for Standardization; 2014. [Google Scholar]
- Liland KH. Multivariate methods in metabolomics from pre-processing to dimension reduction and statistical analysis. Trends Anal Chem. 2011;30:827–841. doi: 10.1016/j.trac.2011.02.007. [DOI] [Google Scholar]
- Maggi L, Carmona M, Zalacain A, Kanakis CD, Anastasaki E, Tarantilis PA, Alonso GL. Changes in saffron volatile profile according to its storage time. Food Res Int. 2010;43:1329–1334. doi: 10.1016/j.foodres.2010.03.025. [DOI] [Google Scholar]
- Melnyk JP, Wang S, Marcone MF. Chemical and biological properties of the world’s most expensive spice: saffron. Food Res Int. 2010;43:1981–1989. doi: 10.1016/j.foodres.2010.07.033. [DOI] [Google Scholar]
- Ordoudi SA, Tsimidou MZ. Saffron quality: effect of agricultural practices, processing and storage. In: Dris R, Jain SM, editors. Production practices and quality assessment of food crops. Dordrecht: Springer; 2004. pp. 209–260. [Google Scholar]
- Peruzzi L. Crocus heuffelianus (Iridaceae), a new record for the Italian flora. Phytotaxa. 2016;261:291–294. doi: 10.11646/phytotaxa.261.3.10. [DOI] [Google Scholar]
- Raina BL, Agarwal SG, Bhatia AK, Gaur GS. Changes in pigments and volatiles of saffron (Crocus sativus L.) during processing and storage. J Sci Food Agric. 1996;71:27–32. doi: 10.1002/(SICI)1097-0010(199605)71:1<27::AID-JSFA542>3.0.CO;2-U. [DOI] [Google Scholar]
- Rashed-Mohassel MH. Saffron from wild to the field. Acta Hortic. 2007;739:187–193. doi: 10.17660/ActaHortic.2007.739.23. [DOI] [Google Scholar]
- Reza Gohari A, Saeidnia S, Kourepaz Mahmoodabadi M. An overview on saffron, phytochemicals, and medicinal properties. Pharmacogn Rev. 2013;7:61–66. doi: 10.4103/0973-7847.112850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinnan Å, Andersson M, Ridder C, Engelsen SB. Recursive weighted partial least squares (rPLS): an efficient variable selection method using PLS. J Chemom. 2014;28:439–447. doi: 10.1002/cem.2582. [DOI] [Google Scholar]
- Sánchez AM, Carmona M, Prodanov M, Alonso GL. Effect of centrifugal ultrafiltration on the composition of aqueous extracts of saffron spice (Crocus sativus L.) J Agric Food Chem. 2008;56:7293–7301. doi: 10.1021/jf801105x. [DOI] [PubMed] [Google Scholar]
- Sereshti H, Heidari R, Samadi S. Determination of volatile components of saffron by optimised ultrasound-assisted extraction in tandem with dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry. Food Chem. 2014;143:499–505. doi: 10.1016/j.foodchem.2013.08.024. [DOI] [PubMed] [Google Scholar]
- Vallejo M, Angulo S, García-Martínez D, García A, Barbas C. New perspective of diabetes response to an antioxidant treatment through metabolic fingerprinting of urine by capillary electrophoresis. J Chromatogr A. 2008;1187:267–274. doi: 10.1016/j.chroma.2008.02.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.