Abstract
In this pilot-scale study supercritical carbon dioxide (SCCO2) extraction technique was used for decaffeination of black tea. Pressure (250, 375, 500 bar), extraction time (60, 180, 300 min), temperature (55, 62.5, 70 °C), CO2 flow rate (1, 2, 3 L/min) and modifier quantity (0, 2.5, 5 mol%) were selected as extraction parameters. Three-level and five-factor response surface methodology experimental design with a Box–Behnken type was employed to generate 46 different processing conditions. 100% of caffeine from black tea was removed under two different extraction conditions; one of which was consist of 375 bar pressure, 62.5 °C temperature, 300 min extraction time, 2 L/min CO2 flow rate and 5 mol% modifier concentration and the other was composed of same temperature, pressure and extraction time conditions with 3 L/min CO2 flow rate and 2.5 mol% modifier concentration. Results showed that extraction time, pressure, CO2 flow rate and modifier quantity had great impact on decaffeination yield.
Keywords: Supercritical carbon dioxide, Decaffeination, Black tea, Extraction
Introduction
Tea is one of the most widely consumed beverages in the world after water. Depending on the manufacturing processes more than 300 different kinds of tea are produced from the leaves of Camellia sinensis and about 78% of the tea production worldwide is black tea (Sang et al. 2011). Tea leaves contain between 2 and 5% caffeine depending on the variety. In addition to tea (Camellia sinensis), coffee (Coffea spp.), cocoa (Theobroma cacao), maté (Ilex paraguariensis), cola nuts (Cola vera) and guarana (Paullinia cupana) are the other well known natural sources of caffeine and also it is mostly taken to body by the consumption of coffee, tea, chocolate candy, soft drinks and commercial drugs (Sereshti and Samadi 2014). Caffeine (1,3,5-trimethylxanthine), an alkaloid is generally used as psychoactive substance and acts as a central nervous system stimulator. Low to moderate doses (50–300 mg/day) elevates alertness, decreases fatigue, promotes mood, reduces depressive symptoms, and decreases the risk of suicide (Penolazzi et al. 2012). However, higher levels of intake considerably trigger negative effects such as anxiety, restlessness, insomnia and tachychardia that are being observed primarily in caffeine-sensitive individuals. Additionally, higher dosages of caffeine show toxic effect on women taking an oral contraceptive, pregnant women, young children, and those with liver disease. Pregnant women should not consume foods and drinks containing too much caffeine. Because fetus can not metabolise the purine alkoloid and so accumulated high levels caffeine twofold endanger developing fetus (Penolazzi et al. 2012; Joshi et al. 2013). Because of these negative effects, health conscious consumers who do not want to digest caffeine provoke expanding new market for decaffeinated products, especially tea and coffee (Vuong and Roach 2014). The decaffeination process is usually done on black tea which makes it extremely sensitive to a change in its flavor. Valuable components of tea should be protected at maximum level after decaffeination. For that reason it is important to determine the most appropriate extraction method in order to protect quality and safety of decaffeinated tea (Hung et al. 2012). Various extraction methods such as solvent extraction, hot water extraction, ultrasound-assisted extraction, microwave-assisted extraction, accelerated solvent extraction and supercritical fluid extraction have been used to remove caffeine from tea, coffee, or other natural sources of caffeine. Conventional extraction methods like solvent extraction are very time consuming and require relatively large quantities of solvents to remove caffeine from tea or other caffeine-containing products. During extraction, not only caffeine, but also great amount of other valuable tea components like polyphenols are removed with solvents. In addition to these disadvantages, conventional methods generally cause the destruction of valuable substances due to the long extraction time and high application temperature (Sahena et al. 2009; Vuong and Roach 2014).
As an advanced separation and extraction technology, supercritical carbon dioxide (SCCO2) extraction has obvious advantages such as dissolving ability and good mass transfer performance. Flexibility of operation conditions, adjustable range parameters, the opportunity to work at low temperature, easy post-treatment are some other operational advantages of this method. It is also clean, safe, nonflammable, nontoxic and environment-friendly. Additionally, its energy consumption is lower than traditional solvent extraction methods (Tang et al. 2010; Bimakr et al. 2012). Even though supercritical fluids have been used to isolate natural products since the end of the 1970s, only a few of them such as carbon dioxide, methane, ethane, propane, ethanol, methanol and acetone are widely used in industry. With its advantages like having a higher diffusion coefficient and lower viscosity, rapid penetration ability, high selectivity, recyclability and safety SCCO2 has taken the place of organic solvents and proven its usefulness in food industry (Saldaña et al. 2002). Supercritical state is achieved when the temperature and the pressure of substance is raised over its critical value. Supercritical fluid has characteristics of both gases and liquids. The most important properties of supercritical fluid are its density, viscosity, diffusivity, heat capacity and thermal conductivity. Higher density of supercritical fluid positively affects solubilisation of compounds and low viscosity increases penetration rate into solids. Because of its availability, pressurized carbon dioxide is generally used as a supercritical solvent. The critical state of liquid CO2 is at a temperature of only 31.1 °C and pressure of 7.38 MPa and the corresponding critical density is 0.466 g/mL (Sahena et al. 2009). Furthermore, use of CO2 protects product quality and it is ideal for heat-sensitive molecules. At the end of the process, carbon dioxide and the extract are easily separated by pressure reduction of the extractor (Zhou et al. 2012). Some lab-scale studies regarding decaffeination of green tea (Park et al. 2007a, b), and of low quality black tea (Bahar et al. 2013) and also caffeine extraction from tea stalks and fibers (Guru and Icen 2004) by using supercritical carbon dioxide extraction have been reported. However, no study on decaffeination of black tea using pilot-scale supercritical carbon dioxide extraction has been found in the literature.
Materials and methods
Reagents and materials
Turkish black tea with particle size between 600 and 780 µm supplied from Caykur Cumhuriyet Tea Factory (Caykur, Rize, Turkey) was used as raw material. The tea samples were put into aluminum foil packages and kept in a cool (less than 22 °C) environment with humidity below 70% at the Caykur Ataturk Tea Research Institute Pilot Plant until they were used in the experiments. Extraction experiments were performed using a pilot-scale SCCO2 extractor (Applied Separations, Model No: 9843, USA) with two vessels each having 10 L volume. As solvent carbon dioxide (CO2, SFE grade) contained in a dip tube cylinder (35 kg CO2/tube) and used in the extraction processes was purchased from the KOGS Company (Trabzon, Turkey). In addition, as modifier food grade ethanol was purchased from Antalya Alkol (Antalya, Turkey). In order to use in High Performance Liquid Cromatography (HPLC) analysis 99% caffeine standard (Sigma Aldrich, China) and 99.9% methanol (Merck, Germany) were purchased. Depending on the purity of the caffeine standard used in this study, the correction factor was calculated to graph a calibration curve. The weight of the caffeine standard (mg) required for stock solution was determined by multiplying with 100/99.
Dynamic extraction using SCCO2
Pilot-scale SCCO2 extraction system shown in Fig. 1 was used in this study. At the beginning of each experiment, 500 g black tea with particle size of 600–780 µm and 2.16% caffeine content was soaked with 300 mL water as pretreatment and then put into the extractor vessel. As shown in Fig. 1, liquid CO2 from the dip tube (1) filled into the storage tank (3) that cooled by recirculating cooling bath (2) and then liquid CO2 passed through a cooler (4) at 0–4 °C to the high-pressure pump (5) and vessel (8) respectively. Thus, the pressure among the storage tank, the high-pressure pump and the vessel was balanced. Liquid CO2 was heated to desired experimental supercritical conditions using CO2 heater (6), then it was compressed to the desired working pressure using a high-pressure pump (Haskel, CA 91502, USA) (5). The pressurized and heated CO2 bypassing the separator-1, passed through the micro-metering valve (10), to the separator-2 (11). A small fraction of the liquid CO2 was intermittently discharged using the separator-2 collector (12) and the separator-2 drain (13). The remainder of the liquid CO2 was condensed using the CO2 condenser (14), introduced into the system and provided with circulation. For the experiments in which modifier was used, food grade ethanol was introduced into the system (mol% EtOH) using the modifier pump (7). In this way, dynamic extraction was performed in each of the planned 46 experiments. All parameters were entered and controlled via ASI-Vision DL application (National Instruments, Labview, USA) on the device-integrated computer (Tyco, ELO Touchscreen, USA). After each SCCO2 extraction process, the decaffeinated tea was dried at 70 °C in the oven (Nuve, KD 700, Turkey) until the final moisture content reached 4.0%.
Fig. 1.
Schematic flow diagram of pilot-scale SCCO2 extraction system
High-performance liquid chromatography analysis
A HPLC method (Anon 2005) was applied to determine the amounts of caffeine in both the unprocessed and decaffeinated tea samples. The HPLC system which consisted of degasser (Thermo, Spectra SYSTEM SCM 1000, Italy), gradient pump (Thermo, Spectra SYSTEM P4000, Italy), autosampler (Thermo, Spectra SYSTEM AS3000, Italy) and as a diode array detector PDA (Spectra SYSTEM UV6000LP, Thermo, Italy) was equipped with HPLC column (Phenomenex, Luna C-18 5 µm 4.6 × 150 mm, USA). System was connected to data control interface software (Termo, ChromQuest 4.0, USA). The deionized water used in caffeine analysis was obtained using a reverse osmosis purification system (Barnstead, Nanopure Diamond, England). The operating conditions of HPLC for caffeine analysis were as follows: the volume of the injection loop was 20 μL, the temperature was set to 38 °C, gradient chromatography was run at 1.0 mL/min, detection wave length was 280 nm and mobile phase was composed of 30% methanol/ultra pure water (v/v). Quantity calculations were made according to the linear calibration curves of caffeine standard.
Design of experiments (DoE) and RSM analysis
In this study, a three-level five-factor RSM experimental design with a Box–Behnken type was employed to determine extraction conditions for the removal of caffeine from black tea by using SCCO2 extraction technique. This design generated 46 treatments for the experiments. Pressure (250, 375, 500 bar; X1), temperature (55, 62.5, 70 °C; X2), extraction time (60, 180, 300 min; X3), flow rate (1, 2, 3 L/min, X4) and modifier concentration (0, 2.5, 5 mol% EtOH; X5) were the independent variables. Besides extraction yield (%) of caffeine was considered as response. The levels of the independent variables were determined based on the findings of previous lab-scale studies and the data of preliminary experiments made for this study.
Minitab 17.0 (Minitab Inc., USA) was used to test the most suitable model for the experimental design. Predicted R2 ranges between 0 and 100%. Larger value of predicted R2 indicates model of greater predictive ability. It is also known as Coefficient of Determination. R2 values of linear, linear + square, linear + interactions and full quadratic polinomial model were identified and it was seen that R2 value of full quadratic polinomial model was the largest among other models. Because larger value of R2 indicates model of greater predictive ability, full quadratic polinomial model was chosen. A quadratic polynomial equation was formed for determining regression coefficients with statistical significance of model terms and for fitting the mathematical models to the experimental data. ANOVA was performed for predicting Y variable which represented caffeine extraction yield (%). The generalized polynomial model proposed for predicting the response variable as a function of independent variables is given as
where Y is the predicted response; β0 is the intercept point; β1, β2, β3, β4 and β5 are the regression coefficients for linear effect terms; β11, β22, β33, β44 and β55 are the quadratic effects; and β12, β13, β14, β15, β23, β24, β25, β34, β35 and β45 are the interaction effects. In this model, X1, X2, X3, X4 and X5 are the independent variables. Minitab 17.0 (Minitab Inc., USA) was used as software to perform the regression analysis. By holding three independent variables constant, the effect of the other two independent variables on caffeine extraction yield was shown with response surface plots.
Results and discussion
Variation of caffeine extraction yield
Uncoded and coded values of the independent variables together with response variable were shown in Table 1. As the results of 46 experiments generated by Box–Behnken design, caffeine content of black tea samples were reduced from 2.16 to 0%. Namely, extraction yield of caffeine (%) varied between 16.2 and 100% depending on the experimental conditions. Two of these experiments produced 100% extraction yield under two different conditions one of which was consist of 375 bar pressure, 62.5 °C temperature, 300 min extraction time, 2 L/min CO2 flow rate and 5 mol% modifier concentration and the other was composed of 375 bar pressure, 62.5 °C temperature, 300 min extraction time, 3 L/min CO2 flow rate and 2.5 mol% modifier concentration. Minimum extraction yield of caffeine (16.2%) was obtained using 60 min extraction time, 1 L/min CO2 flow rate and 2.5 mol% modifier concentration under the same pressure (375 bar) and temperature (62.5 °C).
Table 1.
Coded and uncoded levels of independent variables for Box–Behnken design and response variable
| Coded level | Uncoded level | Response variable | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | Pressure (bar) | Temperature (°C) | Extraction time (min) | Flow rate (L/min) | Modifier concentration EtOH (mol%) | Extraction yield (%) | |
| 1 | 0 | 0 | 1 | 0 | − 1 | 375 | 62.5 | 300 | 2 | 0 | 84.7 |
| 2 | − 1 | 0 | 0 | 0 | 1 | 250 | 62.5 | 180 | 2 | 5 | 70.8 |
| 3 | 0 | 0 | 1 | 0 | 1 | 375 | 62.5 | 300 | 2 | 5 | 100.0 |
| 4 | 1 | 0 | 0 | 0 | − 1 | 500 | 62.5 | 180 | 2 | 0 | 87.0 |
| 5 | 1 | − 1 | 0 | 0 | 0 | 500 | 55 | 180 | 2 | 2.5 | 88.4 |
| 6 | 0 | 1 | 0 | 0 | − 1 | 375 | 70 | 180 | 2 | 0 | 80.6 |
| 7 | − 1 | 1 | 0 | 0 | 0 | 250 | 70 | 180 | 2 | 2.5 | 72.2 |
| 8 | 1 | 0 | − 1 | 0 | 0 | 500 | 62.5 | 60 | 2 | 2.5 | 38.4 |
| 9 | 0 | 1 | 0 | 1 | 0 | 375 | 70 | 180 | 3 | 2.5 | 93.5 |
| 10 | 0 | 0 | − 1 | 0 | − 1 | 375 | 62.5 | 60 | 2 | 0 | 56.5 |
| 11 | 0 | − 1 | − 1 | 0 | 0 | 375 | 55 | 60 | 2 | 2.5 | 53.7 |
| 12 | − 1 | − 1 | 0 | 0 | 0 | 250 | 55 | 180 | 2 | 2.5 | 58.8 |
| 13 | 0 | − 1 | 1 | 0 | 0 | 375 | 55 | 300 | 2 | 2.5 | 95.8 |
| 14 | 0 | 0 | 0 | 0 | 0 | 375 | 62.5 | 180 | 2 | 2.5 | 89.8 |
| 15 | 0 | 0 | 0 | 1 | − 1 | 375 | 62.5 | 180 | 3 | 0 | 94.9 |
| 16 | 1 | 1 | 0 | 0 | 0 | 500 | 70 | 180 | 2 | 2.5 | 91.2 |
| 17 | − 1 | 0 | 0 | 0 | − 1 | 250 | 62.5 | 180 | 2 | 0 | 52.3 |
| 18 | 0 | 0 | 0 | 1 | 1 | 375 | 62.5 | 180 | 3 | 5 | 98.6 |
| 19 | 1 | 0 | 0 | 1 | 0 | 500 | 62.5 | 180 | 3 | 2.5 | 98.6 |
| 20 | 0 | − 1 | 0 | − 1 | 0 | 375 | 55 | 180 | 1 | 2.5 | 52.8 |
| 21 | 0 | 0 | 0 | 0 | 0 | 375 | 62.5 | 180 | 2 | 2. 5 | 77.3 |
| 22 | 0 | 0 | 0 | 0 | 0 | 375 | 62.5 | 180 | 2 | 2.5 | 81.0 |
| 23 | 0 | 1 | 1 | 0 | 0 | 375 | 70 | 300 | 2 | 2.5 | 98.1 |
| 24 | 0 | − 1 | 0 | 0 | 1 | 375 | 55 | 180 | 2 | 5 | 95.4 |
| 25 | 0 | 0 | 1 | 1 | 0 | 375 | 62.5 | 300 | 3 | 2.5 | 100.0 |
| 26 | 0 | 0 | 0 | 0 | 0 | 375 | 62.5 | 180 | 2 | 2.5 | 82.4 |
| 27 | 0 | 0 | 0 | 0 | 0 | 375 | 62.5 | 180 | 2 | 2.5 | 83.8 |
| 28 | 0 | − 1 | 0 | 0 | − 1 | 375 | 55 | 180 | 2 | 0 | 49.5 |
| 29 | − 1 | 0 | 0 | 1 | 0 | 250 | 62.5 | 180 | 3 | 2.5 | 85.6 |
| 30 | 1 | 0 | 0 | 0 | 1 | 500 | 62.5 | 180 | 2 | 5 | 82.4 |
| 31 | 0 | 0 | 0 | − 1 | − 1 | 375 | 62.5 | 180 | 1 | 0 | 38.4 |
| 32 | 1 | 0 | 1 | 0 | 0 | 500 | 62.5 | 300 | 2 | 2.5 | 97.2 |
| 33 | 1 | 0 | 0 | − 1 | 0 | 500 | 62.5 | 180 | 1 | 2.5 | 55.6 |
| 34 | 0 | 0 | − 1 | 0 | 1 | 375 | 62.5 | 60 | 2 | 5 | 47.2 |
| 35 | − 1 | 0 | 0 | − 1 | 0 | 250 | 62.5 | 180 | 1 | 2.5 | 45.4 |
| 36 | 0 | 1 | − 1 | 0 | 0 | 375 | 70 | 60 | 2 | 2.5 | 39.4 |
| 37 | 0 | 1 | 0 | 0 | 1 | 375 | 70 | 180 | 2 | 5 | 97.2 |
| 38 | − 1 | 0 | 1 | 0 | 0 | 250 | 62.5 | 300 | 2 | 2.5 | 89.4 |
| 39 | 0 | 0 | 1 | − 1 | 0 | 375 | 62.5 | 300 | 1 | 2.5 | 72.2 |
| 40 | − 1 | 0 | − 1 | 0 | 0 | 250 | 62.5 | 60 | 2 | 2.5 | 28.7 |
| 41 | 0 | 0 | 0 | − 1 | 1 | 375 | 62.5 | 180 | 1 | 5 | 50.9 |
| 42 | 0 | − 1 | 0 | 1 | 0 | 375 | 55 | 180 | 3 | 2.5 | 90.7 |
| 43 | 0 | 0 | − 1 | 1 | 0 | 375 | 62.5 | 60 | 3 | 2.5 | 53.7 |
| 44 | 0 | 0 | 0 | 0 | 0 | 375 | 62.5 | 180 | 2 | 2.5 | 81.0 |
| 45 | 0 | 0 | − 1 | − 1 | 0 | 375 | 62.5 | 60 | 1 | 2.5 | 16.2 |
| 46 | 0 | 1 | 0 | − 1 | 0 | 375 | 70 | 180 | 1 | 2.5 | 55.1 |
Table 2 provides the regression coefficients obtained by fitting experimental data to response surface quadratic polynomial model for caffeine extraction yield (%). The statistical significance of regression equation was checked by ANOVA. The p value of the model was 0.000 which indicates the model fitness was significant. Furthermore, non-significancy of the lack of fit value (0.058) indicated that the model equation was adequate for predicting the extraction yield of caffeine under any combination of values of the variables. The R2 value for extraction yield (%) of caffeine was determined as 0.932 in accordance with full quadratic polynomial model. While linear and quadratic effects were significant (p < 0.05), 2-way interactions were not (p > 0.05). In addition it was seen in Table 2 that X1, X3, X4, X5, X1X1, X3X3 and X4X4 were significant (p < 0.05) model terms on extraction yield of caffeine.
Table 2.
Regression coefficients and ANOVA for the extraction yield of caffeine (%)
| Terms | Coefficients |
|---|---|
| Intercept | 82.55 |
| X1 (pressure) | 8.47* |
| X2 (temperature) | 2.64 |
| X3 (extraction time) | 25.22* |
| X4 (CO2 flow rate) | 20.56* |
| X5 (modifier concentration) | 6.16* |
| X1X1 | − 5.63* |
| X2X2 | 0.24 |
| X3X3 | − 11.53* |
| X4X4 | − 8.95* |
| X5X5 | − 1.93 |
| R2 | 0.9321 |
*Significant at p < 0.05
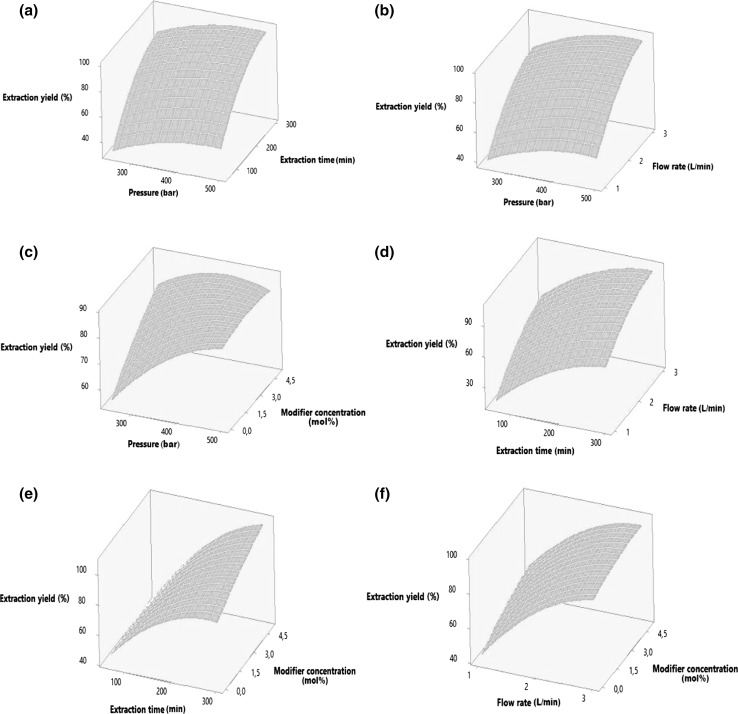
2-way interactions of the independent variables were represented as X1X2, X1X3, X1X4, X1X5, X2X3, X2X4, X2X5, X3X4, X3X5, X4X5. Interaction effects of some of these variables on the extraction yield were graphically shown with the three-dimensional (3D) response surface plots. Through these 3D plots, it is easy and convenient to understand the interactions between two variables and locate their optimum ranges. Figure 2 shows the effects of pressure and extraction time (a), pressure and flow rate (b), pressure and modifier concentration (c), extraction time and flow rate (d), extraction time and modifier concentration (e) and flow rate and modifier concentration (f) on the extraction yield of caffeine (%) respectively.
Fig. 2.
Response surface plots showing the effects of a pressure and extraction time (constants: temperature 62.5 °C, flow rate 2 L/min and modifier concentration 2.5 mol%), b pressure and flow rate (constants: temperature 62.5 °C, time 180 min and modifier concentration 2.5 mol%), c pressure and modifier concentration (constants: temperature 62.5 °C, time 180 min and flow rate 2 L/min), d extraction time and flow rate (constants: pressure 375 bar, temperature 62.5 °C and modifier concentration 2.5 mol%), e extraction time and modifier concentration (constants: pressure 375 bar, temperature 62.5 °C and flow rate 2 L/min), f flow rate and modifier concentration (constants: pressure 375 bar, temperature 62.5 °C and time 180 min) on the extraction yield of caffeine (%)
Effect of pressure
Figure 2a–c show the effect of pressure and extraction time, pressure and flow rate and pressure and modifier concentration, respectively. Caffeine extraction yield improved with increasing pressure, however the change in temperature did not show any significant effect at 500 bar; on the other hand at 250 bar, increasing temperature showed upwards effect on extraction yield of caffeine. As shown in Fig. 2a, b, response surface of pressure-extraction time and pressure-flow rate have similar effects on extraction yield of caffeine. As pressure increased, the extraction yield of caffeine showed slight increase. The increase in extraction time and flow rate each positively affected the extraction yield of caffeine. When the pressure-modifier concentration interaction shown in Fig. 2c was examined, the extraction yield of caffeine increased remarkably as the modifier concentration increased at 250 bar, whereas the increase in modifier concentration at 500 bar did not show any significant effect. Despite modifier was not used, extraction yield of caffeine considerably increased when the pressure increased from 250 to 375 bar. However, increased pressure from 375 to 500 bar has led to an increase in the extraction yield of caffeine at a lower rate. Table 2 indicated that both linear and quadratic effects of pressure on extraction yield of caffeine were significant (p < 0.05). Bahar et al. (2013) expressed that caffeine extraction increased while pressure was increasing up to about 300 bar. After that point increased pressure had an adverse effect on caffeine extraction yield. Icen and Guru (2009) observed that caffeine extraction yield increased considerably with increasing pressure up to 250 bar and then did not vary significantly at higher pressures. It has also been reported that pressure had a direct effect on SCCO2 extraction. As the pressure increases in the supercritical medium, higher densities are achieved. The increase in the density of the medium also increases the solubility of the solute (Casas et al. 2005; Maran et al. 2015). The results of these lab-scale studies mentioned above showed similarity to the findings of this research.
Effect of temperature
Caffeine extraction yield increased when the temperature increased at 250 bar pressure, whereas the temperature increase at 500 bar pressure had no significant effect on caffeine extraction yield. Although the extraction time progressed and flow rate increased, the change in the extraction yield of caffeine was very small, depending on the increase in extraction temperature. The temperature-modifier interaction showed a different effect on caffeine extraction yield than other interactions. As the modifier concentration increased, the extraction yield increased up to 62.5 °C, later the extraction efficiency decreased due to the increased temperature. Song et al. (2010) reported that when the pressure was constant, increasing temperature caused decreasing ethanol density and that reduced solvation power of ethanol. Bahar et al. (2013) expressed that the effect of temperature on caffeine extraction was not significant while Icen and Guru (2009) stated that the caffeine yield increased with increasing temperature up to 60 °C and then decreased for further increase in extraction temperature. Sun et al. (2010) reported that caffeine extraction yield of green tea decreased significantly (p < 0.05) at extraction temperatures ranging from 40 to 60 and 80 °C when the pressure was held at 300 bar. Caffeine extraction at these temperatures were determined as 27.09, 23.14 and 19.94 mg/g, respectively.
Effect of extraction time
Linear and quadratic effects of extraction time on extraction yield were found significant (p < 0.05) as shown in Table 2. Extraction time-flow rate interaction and extraction time-modified concentration interaction on caffeine extraction yield were shown in Fig. 2d, e. The increase of extraction time and the flow rate showed a linear effect on the extraction yield, while the increase of extraction time and modifier concentration showed a logarithmic effect on the extraction yield. Tello et al. (2011) reported that the extraction efficiency of caffeine was 35% in 120 min extraction time and 59% in 300 min extraction time (at 100 °C and 300 bar). Icen and Guru (2009) studied the effect of extraction time on caffeine extraction yield in tea stalk from 1 to 10 h. Researchers stated that the extraction efficiency increased exponentially up to 7 h and did not show any significant change after that point (constants: 55 °C, 10 g/min CO2 flow rate, 200 bar and 0.387 mm particle size). Park et al. (2007a) reported that at the beginning of the extraction period, the extraction rate of caffeine was higher than that in the later period of extraction. More than 75.7, 93.9 and 97.8% of caffeine were extracted after 40, 80, and 120 min extraction times, respectively. Researchers stated that due to the internal mass transfer resistance, more time and CO2 were required to remove the remained soluble solutes towards to the end of extraction time.
Effect of flow rate
Both linear and quadratic effects of flow rate on extraction yield of caffeine were found significant (p < 0.05) as shown in Table 2. Figure 2f shows flow rate-modifier concentration interaction on extraction yield of caffeine. As seen in this figure, the increasing modifier concentration at low flow rate of CO2 showed more impact on caffeine extraction yield than the increasing modifier concentration at high flow rate. Icen and Guru (2009) investigated the effect of carbon dioxide amount on the caffeine yield while extraction time of 7 h, temperature of 55 °C, pressure of 200 bar and mean particle size of 0.25 mm were kept constant. It was expressed as the caffeine extraction yield increased exponentially with increasing carbon dioxide flow rate up to 11 g/min and did not vary significantly at 12 g/min in this study. Kim et al. (2007) studied with the range of 5.04–28.08 kg CO2/kg green tea as flow rate of CO2 for decaffeination of green tea. They reported that depending on time, the amount of caffeine extracted increased with increasing the CO2 flow rate. This situation was expressed as, a large amount of SCCO2 at a higher flow rate brought out larger amount of caffeine during the same extraction time (constants: 323 K, 40 MPa, 20.8% water content). Tello et al. (2011) stated that the increase in the amount of CO2 would raise the extraction yield of caffeine, which could be achieved by increasing the CO2 flow rate.
Effect of modifier concentration
As shown in Table 2, the effects of modifier concentration on the extraction yield were found linearly significant (p < 0.05). The pressure-modifier concentration interaction was shown in Fig. 2c and explained under the subtitle of effect of pressure whilst the temperature-modifier concentration interaction was interpreted under the subtitle of effect of temperature. Similarly, extraction time-modifier concentration and flow rate-modifier concentration interactions were graphically depicted in Fig. 2e, f and also clarified under the subtitles of effect of extraction time and effect of flow rate respectively. Supercritical CO2 is a non-polar or low-polar solvent, therefore SCCO2 is generally utilized in the extraction of solutes possessing little or no polarity. Due to the high polarity of caffeine, it is desirable to add a polar solvent to SCCO2 as a modifier in order to increase the polarity of SCCO2, so that SCCO2 can be used to extract the caffeine from the green and black tea (Park et al. 2007b; MacHmudah et al. 2012; Joshi et al. 2013). As a polar solvent, ethanol is a well-known modifier in food industry. It is also easily removed from final product by evaporation at room temperature (Berna et al. 2001). The solubility of caffeine increases with the quantity of ethanol added to the supercritical solvent. Practically its solubility doubles when the ethanol concentration in supercritical mixture increases from 5 to 10%. The increase of caffeine solubility with the amount of ethanol added to the solvent is coherent with the proportional increase in the number of hydroxyl groups available for hydrogen bonding between ethanol and caffeine. The solubility of mixture which contained 10% ethanol was found to be about 8 times higher than those in pure supercritical CO2. In other words the modifier effect of 10% ethanol mixture was 8:1 which represents the ratio between the solubility of caffeine in the presence of the modifier and that in the pure CO2 at the same conditions of temperature and pressure. (Kopcak and Mohamed 2005). Icen and Guru (2010) expressed that according to the total flow rate when the mixture of 5% ethanol and 95% CO2 was used, caffeine that can be leached within 6–7 h, can be extracted in 2 h. Bahar et al. (2013) stated that ethanol concentration had significant effect (p < 0.05) on caffeine extraction. Park et al. (2007b) found caffeine extraction yields as 28.7, 35 and 40.3 mg/g dry tea with increasing modifier concentrations of 2.3, 4.6 and 7 g EtOH/100 g CO2, respectively. These findings are consistent with the results of this research.
Conclusion
This pilot scale study revealed that removal of 100% of caffeine from black tea could be achieved at a low temperature of 62.5 °C using SCCO2 extraction technique. Due to the fact that SCCO2 was used as solvent, there was no need for an additional process after extraction to remove solvent from decaffeinated tea. It was important to attain high extraction efficiency (100%) at a low processing temperature (62.5 °C) in a short extraction time (300 min) in terms of applicability of the results of this study in industrial scale.
In conclusion, the parameters obtained in this pilot-scale study could be used to optimize the processing conditions of decaffeinated black tea in industrial scale, taking into account of regulations, legislations, working conditions, operator preferences and desired final product quality.
Acknowledgements
This study was funded by The Scientific and Technological Research Council of Turkey (TUBITAK) (Project No. 112G074-2013).
References
- Anon (2005) ISO 14502-2: Determination of substances characteristic of green and black tea—part 2: content of catechins in green tea—method using high-performance liquid chromatography
- Bahar B, Pelvan E, Hasbay I, Alasalvar C. Decaffeinated black tea: process optimization and phenolic profiles. J Supercrit Fluids. 2013;82:116–121. doi: 10.1016/j.supflu.2013.07.002. [DOI] [Google Scholar]
- Berna A, Cháfer A, Montón J, Subirats S. High-pressure solubility data of system ethanol (1) + catechin (2) + CO2 (3) J Supercrit Fluids. 2001;20:157–162. doi: 10.1016/S0896-8446(01)00063-8. [DOI] [Google Scholar]
- Bimakr M, Rahman RA, Ganjloo A, et al. Optimization of supercritical carbon dioxide extraction of bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves by using response surface methodology. Food Bioprocess Technol. 2012;5:912–920. doi: 10.1007/s11947-010-0504-4. [DOI] [Google Scholar]
- Casas L, Mantell C, Rodríguez M, et al. Effect of the pre-treatment of the samples on the natural substances extraction from Helianthus annuus L. using supercritical carbon dioxide. Talanta. 2005;67:175–181. doi: 10.1016/j.talanta.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Guru M, Icen H. Obtaining of caffeine from Turkish tea fiber and stalk wastes. Bioresour Technol. 2004;94:17–19. doi: 10.1016/j.biortech.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Hung TN, Gumerov F, Gabitov F, et al. Improvement of the water brewing of Vietnamese green tea by pretreatment with supercritical carbon dioxide. J Supercrit Fluids. 2012;62:73–78. doi: 10.1016/j.supflu.2011.10.017. [DOI] [Google Scholar]
- Icen H, Guru M. Extraction of caffeine from tea stalk and fiber wastes using supercritical carbon dioxide. J Supercrit Fluids. 2009;50:225–228. doi: 10.1016/j.supflu.2009.06.014. [DOI] [Google Scholar]
- Icen H, Guru M. Effect of ethanol content on supercritical carbon dioxide extraction of caffeine from tea stalk and fiber wastes. J Supercrit Fluids. 2010;55:156–160. doi: 10.1016/j.supflu.2010.07.009. [DOI] [Google Scholar]
- Joshi R, Babu GDK, Gulati A. Effect of decaffeination conditions on quality parameters of Kangra orthodox black tea. Food Res Int. 2013;53:693–703. doi: 10.1016/j.foodres.2012.12.050. [DOI] [Google Scholar]
- Kim W, Kim J, Oh S. Supercritical carbon dioxide extraction of caffeine from Korean green tea. Sep Sci Technol. 2007;42:3229–3242. doi: 10.1080/01496390701513008. [DOI] [Google Scholar]
- Kopcak U, Mohamed RS. Caffeine solubility in supercritical carbon dioxide/co-solvent mixtures. J Supercrit Fluids. 2005;34:209–214. doi: 10.1016/j.supflu.2004.11.016. [DOI] [Google Scholar]
- MacHmudah S, Martin A, Sasaki M, Goto M. Mathematical modeling for simultaneous extraction and fractionation process of coffee beans with supercritical CO2 and water. J Supercrit Fluids. 2012;66:111–119. doi: 10.1016/j.supflu.2011.11.011. [DOI] [Google Scholar]
- Maran JP, Manikandan S, Priya B, Gurumoorthi P. Box–Behnken design based multi-response analysis and optimization of supercritical carbon dioxide extraction of bioactive flavonoid compounds from tea (Camellia sinensis L.) leaves. J Food Sci Technol. 2015;52:92–104. doi: 10.1007/s13197-013-0985-z. [DOI] [Google Scholar]
- Park HS, Choi H-K, Lee SJ, et al. Effect of mass transfer on the removal of caffeine from green tea by supercritical carbon dioxide. J Supercrit Fluids. 2007;42:205–211. doi: 10.1016/j.supflu.2007.03.002. [DOI] [Google Scholar]
- Park HS, Lee HJ, Shin MH, et al. Effects of cosolvents on the decaffeination of green tea by supercritical carbon dioxide. Food Chem. 2007;105:1011–1017. doi: 10.1016/j.foodchem.2007.04.064. [DOI] [Google Scholar]
- Penolazzi B, Natale V, Leone L, Russo PM. Individual differences affecting caffeine intake. Analysis of consumption behaviours for different times of day and caffeine sources. Appetite. 2012;58:971–977. doi: 10.1016/j.appet.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Sahena F, Zaidul ISM, Jinap S, et al. Application of supercritical CO2 in lipid extraction: a review. J Food Eng. 2009;95:240–253. doi: 10.1016/j.jfoodeng.2009.06.026. [DOI] [Google Scholar]
- Saldaña MDA, Zetzl C, Mohamed RS, Brunner G. Extraction of methylxanthines from guaraná seeds, maté leaves, and cocoa beans using supercritical carbon dioxide and ethanol. J Agric Food Chem. 2002;50:4820–4826. doi: 10.1021/jf020128v. [DOI] [PubMed] [Google Scholar]
- Sang S, Lambert JD, Ho C-T, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacol Res. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Sereshti H, Samadi S. A rapid and simple determination of caffeine in teas, coffees and eight beverages. Food Chem. 2014;158:8–13. doi: 10.1016/j.foodchem.2014.02.095. [DOI] [PubMed] [Google Scholar]
- Song Q, Zhu J, Wan J, Cao X. Measurement and modeling of epigallocatechin gallate solubility in supercritical carbon dioxide fluid with ethanol cosolvent. J Chem Eng Data. 2010;55:3946–3951. doi: 10.1021/je901025f. [DOI] [Google Scholar]
- Sun Q, Hua S, Ye J, et al. Decaffeination of green tea by supercritical carbon dioxide. J Med Plants Res. 2010;4:1161–1168. [Google Scholar]
- Tang WQ, Li DC, Lv YX, Jiang JG. Extraction and removal of caffeine from green tea by ultrasonic-enhanced supercritical fluid. J Food Sci. 2010 doi: 10.1111/j.1750-3841.2010.01604.x. [DOI] [PubMed] [Google Scholar]
- Tello J, Viguera M, Calvo L. Extraction of caffeine from robusta coffee (Coffea canephora var. Robusta) husks using supercritical carbon dioxide. J Supercrit Fluids. 2011;59:53–60. doi: 10.1016/j.supflu.2011.07.018. [DOI] [Google Scholar]
- Vuong QV, Roach PD. Caffeine in green tea: its removal and isolation. Sep Purif Rev. 2014;43:155–174. doi: 10.1080/15422119.2013.771127. [DOI] [Google Scholar]
- Zhou YS, Gu CM, Gu H. Supercritical CO2 extraction of tee seed oil from camellia seeds and composition analysis of tee seed oil extracts. Adv Mater Res. 2012;538–541:2372–2376. doi: 10.4028/www.scientific.net/AMR.538-541.2372. [DOI] [Google Scholar]