Abstract
The Saccharomyces cerevisiae TEL1 gene is an ortholog of the human ATM (Ataxia telangiectasia mutated) gene. S. cerevisiae tel1 mutant (tel1∆) lacking Tel1p, share some of the cellular defects with ATM mutation that includes prevention of oxidative damage repair, premature aging and apoptosis. In the present study, we investigated the protective effects of quercetin on the sensitivity of yeast S. cerevisiae tel1∆ cells exposed to oxidative, apoptotic and DNA damaging stress and viability of tel1∆ cells during chronological aging. Quercetin improved the stress resistance of tel1∆ cells when challenged with oxidants such as hydrogen peroxide (H2O2), menadine bisulphite (MBS) and tertiary butyl hydroperoxide (t-BHP) by scavenging reactive oxygen species (ROS). Quercetin protected the tel1∆ cells from acetic acid-induced apoptotic cell death and sensitivity against hydroxyurea. We found that quercetin attenuated ROS accumulation and apoptotic markers in tel1∆ cells and therefore an increase in cell viability during chronological aging. Our results from the S. cerevisiae model, suggest that use of quercetin as a food supplement might alleviate oxidative stress mediated DNA damage, apoptosis and age related damaging effects in AT patients and also improve health beneficial effects in humans.
Keywords: Ataxia telangiectasia, ATM, TEL1, tel1∆, Oxidative stress, Apoptosis and aging
Introduction
Food derived polyphenols have antioxidant properties and play a significant role in the prevention of several chronic diseases such as cancer, cardiovascular disease, diabetes and neurodegenerative disorders. In addition, many polyphenols have been used as natural antioxidants in food industries to prolong shelf‐life of functional ingredient in foods and dietary supplements (Harwood et al. 2007). Among the polyphenols, quercetin is a major dietary flavonoid ubiquitously found in the human diet including onions, apples, grapes, berries, tea and red wine. It is one of the most active scavengers of reactive oxygen species (ROS) and reactive nitrogen species (RNS) both in vitro and in vivo conditions (Boots et al. 2008) with a wide range of biological properties including anti-inflammatory, anti-cancer, anti-neurodegenerative, anti-atherosclerotic, and antihypertensive properties (Li et al. 2016). Quercetin rich diet and supplements were reported to be very effective in disease prevention in rats (Ting et al. 2018). Quercetin being a potent antioxidant and having a variety of biological activities, World Health Organization (WHO) recommended quercetin to use as a food additive (JECFA 1977).
The S. cerevisiae TEL1 gene is an ortholog of human ATM (Ataxia telangiectasia mutated). Both Tel1 and ATM proteins that share amino acid identity and functional similarities are required for the phosphorylation of downstream proteins when cells are under various stress conditions including DNA damage. The mammalian serine/threonine protein kinase ATM signaling pathway plays a significant role in DNA damage sensitivity, cell cycle checkpoint deficiency, mitotic recombination, high cancer incidence and telomere length maintenance (Baldo et al. 2008). S. cerevisiae tel1 mutant (tel1∆) share some of the cellular defects with ATM mutation. Mutation or deficiency of ATM causes ataxia telangiectasia (AT) in humans. AT is a rare neurodegenerative disorder characterized by premature aging, oxidative damage, cerebellar ataxia with neuropathy, immunodeficiency, and predisposition to cancer. Moreover, ATM-deficient mice brains were reported to show significant alterations in the levels of antioxidant enzymes and thiol-containing compounds leading to accumulation of ROS levels, suggesting the absence of functional ATM results in oxidative stress, which may be an important cause of the degeneration of cerebellar neurons in AT (Kamsler et al. 2001). ATM-deficient cells were reported to be more sensitive to oxidative damage caused by hydrogen peroxide (Yi et al. 1990), t-butyl hydroperoxide (Shackelford et al. 2001), chromium IV, nitric oxide (NO) (Shackelford et al. 2004) and DNA damaging agents. Cells deficient in ATM were more susceptible than wild-type cells to apoptosis induced by various agents such as H2O2, bleomycin, C(2)-ceramide and ionizing radiation suggesting that AT disorder is associated with oxidative damage mediated apoptosis and that ATM might well act as a sensor for redox homeostasis in response to oxidative damage mediated apoptosis (Zhang et al. 2002). Accordingly, development of effective strategies for the treatment of AT is highly desirable. Many studies have shown the protective role of glucocorticoid analogues (betamethasone and dexamethasone) (Zannolli et al. 2012; Menotta et al. 2012) and antioxidant supplements (N-acetylcysteine, tempol, and 5-carboxy-1,1,3,3-tetramethylisoindolin-2-yloxyl) in AT associated neurological symptoms. However, no therapy is available at present for treating AT associated conditions. Therefore, there is an emerging need to develop drugs against AT associated disease conditions.
Over the past few years, there has been a surge of interest on the food derived antioxidant molecules for the development of potential therapeutics to improve the quality of life. Quercetin has been marketed in the United States primarily as a dietary supplement, with a recommended daily doses of 200–1200 mg. Whereas, food-grade quercetin of high purity (98.5% and higher) was suggested in the range of 10–125 mg/serving in a variety of foods (Harwood et al. 2007). Consequently, the use of quercetin as a therapeutic agent for the treatment of neurodegenerative diseases was suggested (Naidu et al. 2003). Pharmacological manipulation of ATM activity via iron chelation by antioxidants might have clinical efficacy in the treatment of AT associated neurodegenerative diseases (Edwin Shackelford et al. 2005). Many reports have been published using various gene-deletion mutants of the yeast S. cerevisiae to explain the molecular mechanism underlying several human diseases (Outeiro and Lindquist 2003; Ocampo et al. 2003). Therefore, the present study investigated the protective effects of quercetin on the sensitivity of yeast tel1∆ cells to oxidants (H2O2, MBS and t-BHP), acetic acid and hydroxyurea. Finally, we reported the protective effect of quercetin on the survival of yeast tel1∆ cells during chronological life span.
Materials and methods
Reagents
Yeast growth media components such as yeast extract, peptone, dextrose, yeast nitrogen base w/o ammonium sulphate (Cat. No. G090), complete synthetic mixture (CSM; Cat. No. G100), dimethyl sulfoxide (DMSO) and quercetin (Cat. No. RM6191) were purchased from Himedia, Mumbai, India. Other chemicals including 2′,7′-dichlorofluorescin diacetate (H2DCFDA; Cat. No. D6883), 4′,6-diamidino-2-phenylindole (DAPI; Cat. No. D9542), propidium iodide (PI; Cat. No. P4170), acridine orange (AO; Cat. No. A6014) and ethidium bromide (EtBr; Cat. No. E7637) were purchased from Sigma-Aldrich, USA.
Saccharomyces cerevisiae strains and growth conditions
S. cerevisiae wild type (BY4741) and tel1∆ strains were purchased from Fischer scientific, USA. For stress resistance assays, yeast cells were grown in YPD (1% yeast extract, 2% peptone and 2% dextrose, all in w/v) medium and incubated at 160 rpm at 30 °C. For aging studies, yeast cells were inoculated in synthetic complete medium (SDC) containing yeast nitrogen base (YNB), ammonium sulphate and complete synthetic mixture and incubated at 160 rpm at 30 °C (Fabrizio and Longo 2007).
Semi quantitative spot assays
Exponentially growing yeast cells (OD600 = 0.5–0.6) were pretreated with quercetin (200 μM) or equal volume of DMSO for 1 h under shaking at 160 rpm at 30 °C. Following incubation, yeast cultures (20 µL) were serially diluted to 10−4 dilution and 5 µL of each dilution was spotted on to the YPD supplemented with 2% agar containing different concentrations of hydrogen peroxide (H2O2) (2 and 3 mM) or menadine bisulphite (MBS) (0.2 mM) or tert-butyl hydroperoxide (t-BHP) (1 and 2 mM), or acetic acid (40 and 80 mM). Spotted plates were incubated at 30 °C for 48 h and photos were taken.
Oxidative stress resistance
Oxidative stress resistance assay was performed according to the method of Vilaca et al. (2012) with slight modifications. Briefly, exponentially growing yeast cultures were pretreated with quercetin (200 μM) or DMSO for 1 h and subsequently treated with 1 mM H2O2 or 15 mM MBS or 1 mM t-BHP for 1 h with shaking at 160 rpm at 30 °C. The cell suspensions were then serially diluted to reach 10−4 dilution in sterile distilled water and 100 µL of the same dilution are plated on to YPD plates. Colonies were counted after 48 h of incubation at 30 °C. Cell viability was calculated as the percentage of colony forming units.
Detection of intracellular reactive oxygen species
Intracellular oxidation level was examined using 2,7-dichlorofluorescein diacetate (H2DCFDA). Exponentially growing yeast cultures (OD600 = 0.5–0.6) were pretreated with quercetin (200 μM) or DMSO for 1 h and subsequently treated with 1 mM H2O2 or 15 mM MBS or 1 mM t-BHP at 30 °C with shaking at 160 rpm for 1 h. After treatment, cells were washed 3 times with phosphate buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 and 1.8 mM KH2PO4, pH 7.4) and incubated with H2DCFDA (at a final concentration of 10 µM) at 30 °C for 30 min. All the samples were washed again and resuspended in PBS. An aliquot of cell suspension was observed under fluorescence microscope using a blue filter. ROS generating cells appeared green in color (Doudican et al. 2005).
Acetic acid stress resistance
Acetic acid stress resistance assay was performed according to the method of Ludovico et al. (2001). Briefly, exponentially growing yeast cultures (OD600 = 0.5–0.6) were pretreated with quercetin (200 μM) or DMSO for 1 h. After incubation, cells were harvested and resuspended in YPD medium at pH 3.0 (set with HCl) containing 40 or 80 mM acetic acid under shaking at 160 rpm for 100 min at 30 °C. The cell suspensions were then serially diluted to reach 10−4 dilution in sterile distilled water and 100 µL of the same dilution are plated on to YPD plates. Colonies were counted after 48 h of incubation at 30 °C. Cell viability was calculated as the percentage of the colony forming units.
Analysis of apoptotic events in cells treated with acetic acid and chronologically aging cultures
DAPI and PI staining
Yeast cells were harvested, washed with PBS and fixed with 3.7% formaldehyde for 30 min. For nuclear staining yeast cells were washed with PBS and incubated with 2 μg/mL of DAPI for 10 min at room temperature in dark (Madeo et al. 1997). Then, cells were washed with PBS and observed under fluorescence microscope for chromatin condensation.
Cell membrane integrity was analyzed by propidium iodide (PI) staining procedure (Sousa et al. 2013). Briefly, cells were washed, suspended in 1 mL PBS and stained with 1.5 μg/mL of PI at room temperature for 10 min in dark. After staining, cells were washed with PBS, and the level of PI staining was analyzed by fluorescence microscope.
Acridine orange/ethidium bromide dual staining
Dual acridine orange/ethidium bromide (AO/EtBr) fluorescent staining was used to identify apoptosis-associated changes in cell membrane during the process of apoptosis (Malakar et al. 2008). Yeast cells were harvested and stained with 2 μL of AO/EtBr solution [AO in PBS (100 μg/mL) and EtBr in PBS (100 μg/mL) mixed 1:1, v/v], and examined under a fluorescence microscope. For quantitative assessment of AO/EtBr staining, about 150–250 yeast cells were counted per sample in each of three independent experiments performed in duplicate.
Hydroxyurea stress resistance
Exponentially growing yeast cultures (OD600 = 0.5–0.6) were pretreated with quercetin (200 μM) or DMSO for 1 h and subsequently treated with 100 or 200 mM hydroxyurea (HU) under shaking at 160 rpm for 1 h at 30 °C. The cell suspensions were then serially diluted to reach 10−4 dilution in sterile distilled water and 100 µL of the same dilution are plated on to YPD plates (Chabes et al. 2003). Colonies were counted after 48 h of incubation at 30 °C. Cell viability was calculated as the percentage of colony forming units.
Chronological life span assay
Chronological life span (CLS) experiments were carried out in SDC medium (0.18% yeast nitrogen base (YNB) without amino acids and ammonium sulfate, 0.5% ammonium sulfate, and 0.173% complete amino acid mix) with slight modifications (Fabrizio and Longo 2007licen). Briefly, cells were grown overnight, then subcultured into the flasks containing SDC medium (flask volume/medium volume ratio of 5:1) supplemented with quercetin (200 µM) and incubated with shaking at 160 rpm at 30 °C. The 3rd day cultures were considered as day 0 for CLS assay. To determine the number of viable cells, 20 μL of aliquots were removed, diluted 10,000 times in sterile water at different days (0, 3, 6, 9, 12, 16 and 20) and plated on to the YPD plates in duplicate. Plates were incubated at 30 °C for 2–3 days. Percent cell survival was assessed by counting colony forming units per milliliter (CFUs/mL). The day 0 CFUs were considered to be the 100% survival. At indicated time-points, serially diluted cultures were also spotted on to YPD plates alone and YPD plates containing H2O2 (2 mM) and acetic acid (40 mM).
Statistical analysis
The data analysis was done using the SPSS/16 software. Each experiment was performed thrice, and the data were presented as mean ± SD. Statistical analysis was carried out by a one way analysis of variance (ANOVA) test. The statistical probability of p < 0.05 was considered to be significant.
Results
Quercetin protects yeast tel1∆ cells from oxidant induced cell death
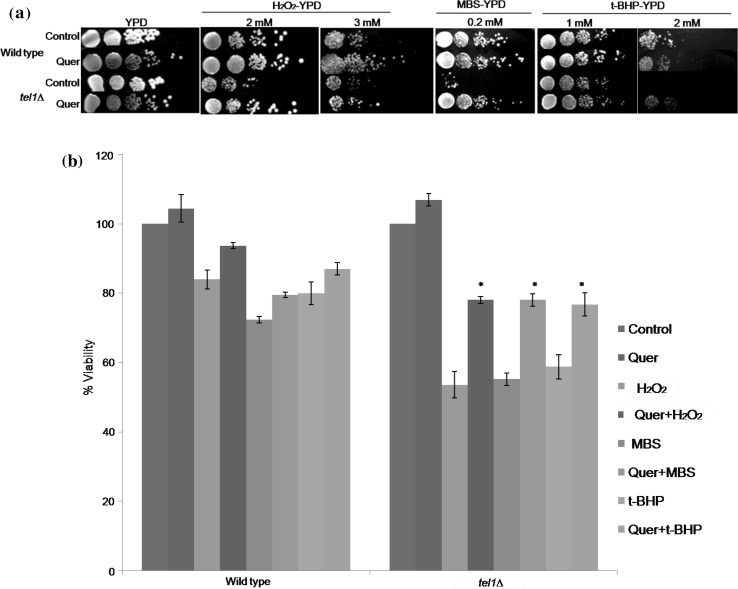
We investigated the protective effects of quercetin against oxidant induced cell death in yeast tel1 mutant. Yeast tel1∆ cells showed sensitivity to all the oxidants (H2O2, MBS and t-BHP) tested compared to DMSO treated control and wild type cells. However, pretreatment of yeast cells with quercetin, augmented the stress resistance of tel1∆ cells against oxidant induced toxicity and thereby increased viability (Fig. 1a).
Fig. 1.
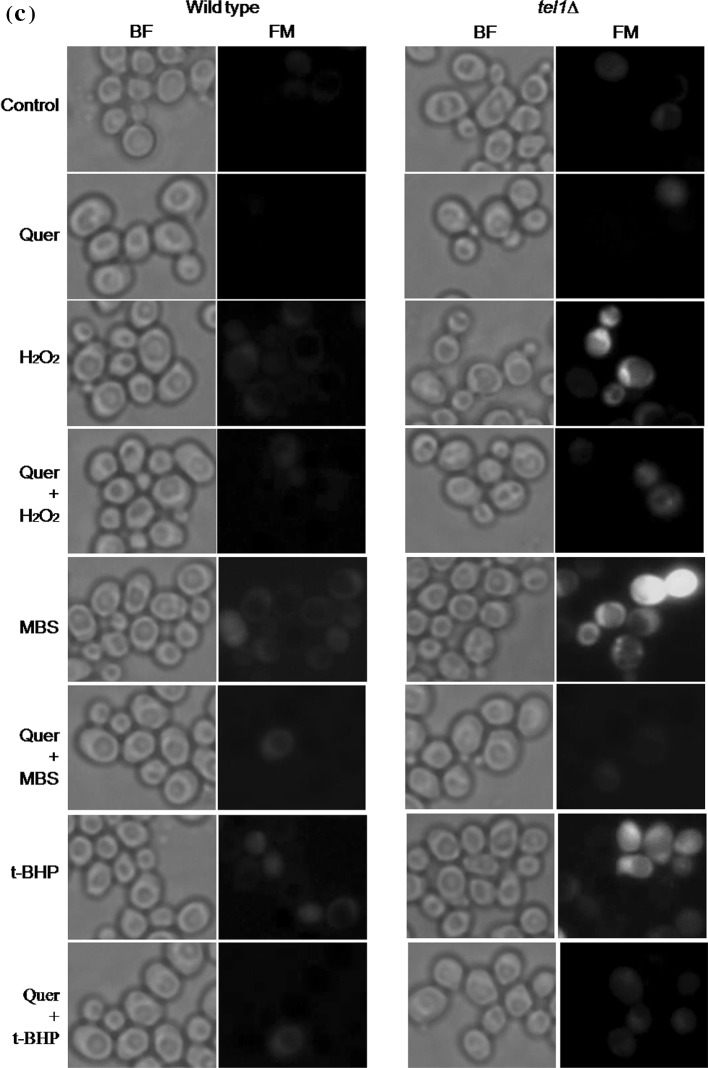
Quercetin protects yeast tel1 mutant cells exposed to different oxidants. a Spot assay. Exponentially growing wild type and tel1∆ cells were pretreated with 200 µM quercetin (Quer) or equal volume of DMSO (control) for 1 h. After incubation, cells were tenfold serially diluted and spotted on to YPD plates or YPD plates containing H2O2 (2 and 3 mM) or MBS (0.2 mM) or t-BHP (1 and 2 mM), and were incubated at 30 °C for 2–3 days. Representative images are shown from at least three independent experiments. b Colony forming unit assay. Viability of wild type and tel1∆ strains was measured after exposure of cells to different oxidants (H2O2-1 mM; MBS-0.2 mM; and tBHP-1 mM) for 1 h without (control) or with quercetin (200 µM). Values are mean ± SD of three independent experiments. *Significant increase in percent viability in quercetin treated tel1∆ cultures compared to tel1∆ cultures treated with H2O2, t-BHP and MBS alone, respectively (p < 0.05). c Detection of ROS using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) staining. Exponentially growing yeast cells were treated as described above, stained with H2DCFDA and observed under fluorescence microscope. Representative images are shown from at least three independent experiments. BF, bright field; FM, fluorescence microscopy
Further, we performed colony forming unit (CFU) assay to investigate the protective effects of quercetin on the survival of yeast tel1∆ cells exposed to different oxidants. Oxidant induced cell death is more pronounced in tel1∆ cells compared to wild type (Fig. 1b). Thus, results suggest the highest susceptibility of tel1∆ cells to tested oxidants. However, quercetin pretreatment increased the percentage viability of tel1∆ cells to 78.07, 78.06 and 76.79% for all the tested oxidants such as H2O2, MBS and t-BHP when compared to their respective DMSO treated controls (53.57, 55.20 and 58.77%). A significant increase in percentage viability of quercetin treated tel1 mutant was observed compared to wild type strain.
Further, we performed fluorescence microscopy to assess oxidant induced ROS accumulation in yeast cells. It is observed from the Fig. 1c that predominantly yeast tel1∆ cells with green fluorescence representing oxidant mediated ROS generation compared to wild type cells. However, quercetin pretreatment decreased the number of tel1∆ cells with green fluorescence. These results suggest that presence of quercetin lowered the number of ROS positive cells, thereby protecting yeast tel1∆ cells from oxidant mediated cell death.
Quercetin protected yeast tel1∆ cells from acetic acid induced apoptosis
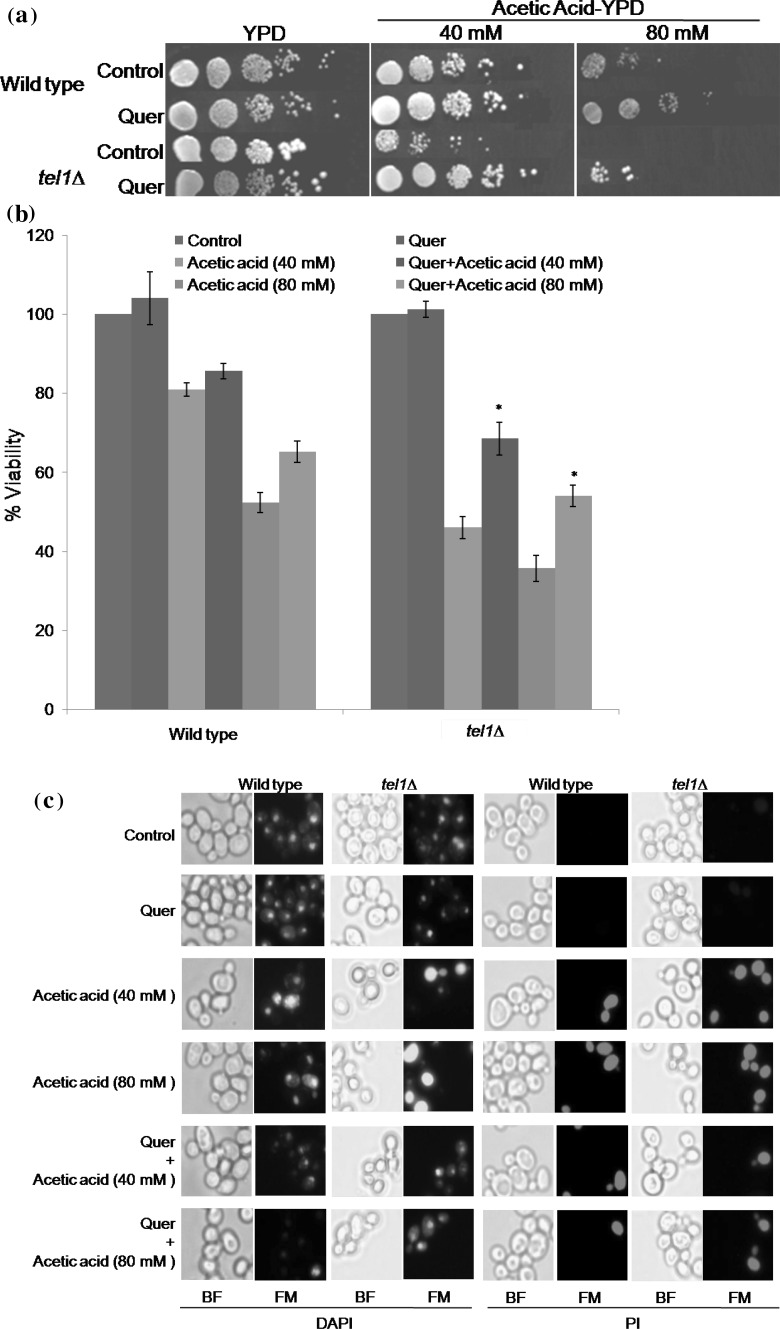
S. cerevisiae tel1∆ cells were found to be sensitive to acetic acid compared to wild type, while the quercetin pretreatment protected tel1∆ cells from acetic acid induced sensitivity when compared to its respective control (Fig. 2a). Furthermore, we performed CFU assay to calculate the percent viability of yeast tel1∆ cells exposed to acetic acid and protection offered by quercetin pretreatment. As shown in Fig. 2b, a significant difference was found in the sensitivity of tel1∆ cells to acetic acid induced cell death compared to respective control cells. In contrast, quercetin pretreatment significantly increased the percentage viability of tel1∆ cells to 68.48 and 54.01% at acetic acid concentrations 40 and 80 mM, respectively, when compared to control samples (46.02 and 35.68%). Similarly, the percentage increase in viability of wild type cells was found to be 80.55 and 60.17%, respectively at 40 and 80 mM concentrations of acetic acid compared to controls (72.21 and 52.32%). These results suggest that the anti-apoptotic potential of quercetin rescued the yeast tel1∆ cells from acetic acid induced apoptotic cell death.
Fig. 2.
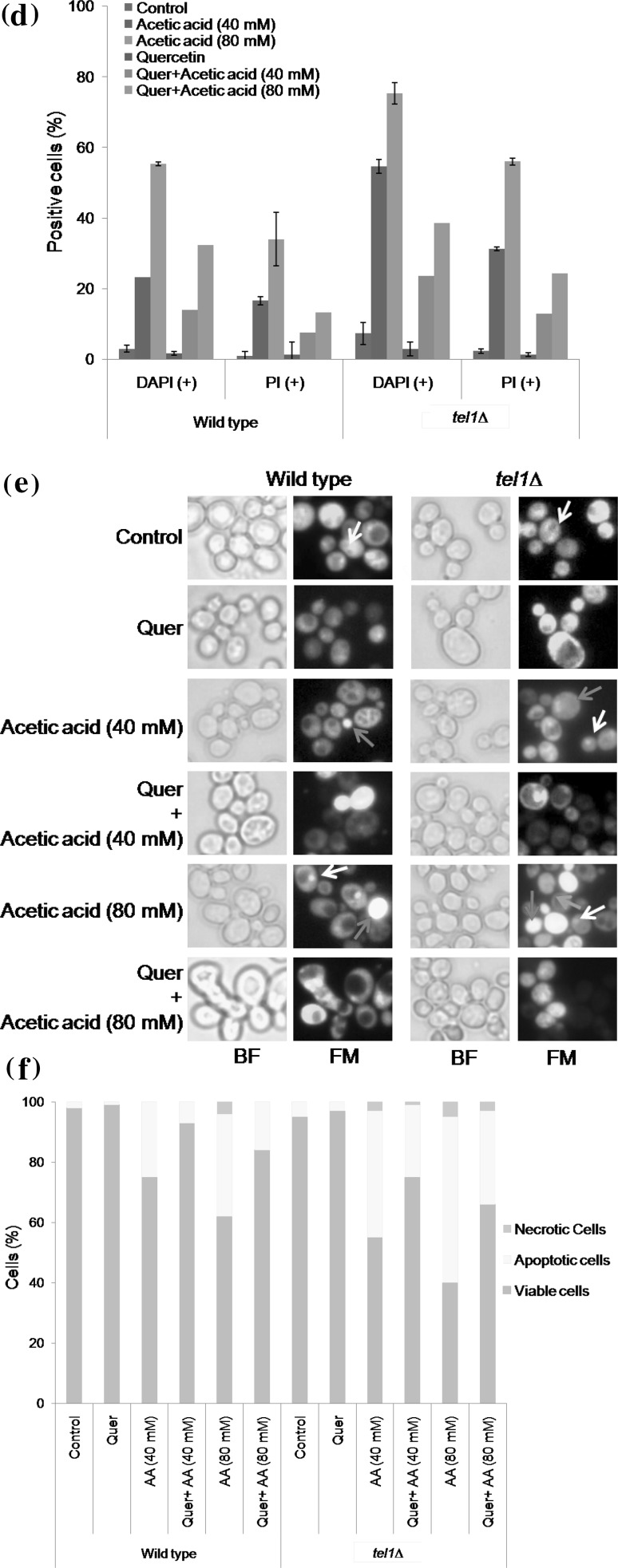
Quercetin protects yeast tel1 mutant cells from acetic acid stress. a Spot assay. Exponentially growing wild type and tel1∆ cells were pretreated with 200 µM quercetin (Quer) or equal volume of DMSO (control) for 1 h. After incubation, cells were tenfold serially diluted and spotted on to YPD plates or YPD plates containing different concentrations of acetic acid (40 and 80 mM). Spotted plates were incubated at 30 °C for 2–3 days. Representative images are shown from at least three independent experiments. b Colony forming unit assay. Viability of wild type and tel1∆ strains was measured after exposure of cells to acetic acid (40 and 80 mM) for 100 min without (control) or with quercetin (200 µM). Values are mean ± SD of three independent experiments (p < 0.05). * represents significant increase in tel1∆ percent viability of Quer + 40 mM acetic acid and Quer + 80 mM acetic acid treatment compared to acetic acid (40 and 80 mM) alone treatment, respectively. Analysis of apoptotic events by fluorescence microscopy c DAPI and PI staining. Exponentially growing yeast cells were treated as described above, stained with DAPI or PI and observed under fluorescence microscope. Representative images are shown from at least three independent experiments. d Percentage DAPI and PI positive cells. A total of 100 cells were examined for DAPI and PI positive cells. Data represent an average of three independent experiments with standard deviation. e Acridine orange/ethidium bromide staining. Exponentially growing yeast cells were treated as described above and stained with AO/EtBr solution. AO/EtBr staining cells shows 3 types of cells: (1) viable cells showed uniform green fluorescence (yellow arrow) with an organized structure and green cytoplasm (2) apoptotic cells exhibited chromatin condensation visible as bright green spot or fragments (white arrow) and yellow fluorescence with condensed or fragmented chromatin (red arrow) (3) necrotic cells stained in orange to red (blue arrow). Representative images are shown from at least three independent experiments. f Percentage of apoptotic cells. For quantitative assessment of AO/EtBr staining, about 150–250 yeast cells were counted per sample in each of three independent experiments. Data represent an average of three independent experiments is shown. BF, bright field; FM, fluorescence microscopy; AA, acetic acid (colour figure online)
Fluorescence microscopy was performed to investigate the acetic acid induced apoptotic markers such as chromatin fragmentation and plasma membrane integrity. As evident from the Fig. 2c, acetic acid (40 and 80 mM) treated tel1∆ cells showed a concentration dependent increase in chromatin condensation and decreased plasma membrane integrity represented by increased blue and red staining, respectively. In contrast, quercetin pretreatment led to decrease in chromatin condensation as well as increase in plasma membrane integrity (Fig. 2c). Exposure of tel1∆ cells to acetic acid increased the percentage of DAPI positive cells (54.66 and 75.33%) compared to wild type control cells (23.33 and 55.33%) at 40 and 80 mM concentrations, respectively (Fig. 2d). However, quercetin pretreatment reduced the chromatin fragmentation and thereby decreased percentage of DAPI positive tel1∆ cells to 23.66 and 38.6% at the concentrations tested (40 and 80 mM). Similarly, the percentage increase in loss of membrane integrity was more in tel1Δ than the wild type cells (Fig. 2d). However, quercetin pretreatment decreased the percentage of PI positive cells indicating the maintenance of membrane integrity in tel1Δ cells (24 and 31%, respectively, at the concentrations tested 40 and 80 mM).
The dual AO/EtBr dyes discriminate the live cells from the dead cells (Ferreira et al. 2011). Acridine orange (green dye) stains both live and dead cells, whereas ethidium bromide (orange dye) incorporates only in the apoptotic cell. Both wild type and tel1∆ control cells showed a uniform green color, indicating the normal physiology of live cells. Acetic acid treated cells showed a yellowish color indicating the signs of early apoptosis and orange-red represents late apoptotic cells, with more number of apoptotic cells in tel1∆ strain compared to wild type (Fig. 2e). However, quercetin pretreatment reduced the apoptotic markers in acetic acid treated tel1∆ cells compared to acetic acid alone treated cells. Exposure to acetic acid significantly increased the percentage of apoptotic tel1∆ cells (42 and 55% at 40 and 80 mM concentrations, respectively) compared to wild type (26% at 40 mM and 34% at 80 mM), while quercetin treatment significantly reduced the percentage of apoptotic tel1∆ cells. In addition to AO-positive cells (apoptotic cells), we also observed few necrotic cells in both tel1∆ and wild type cells treated with high concentration of acetic acid (80 mM).
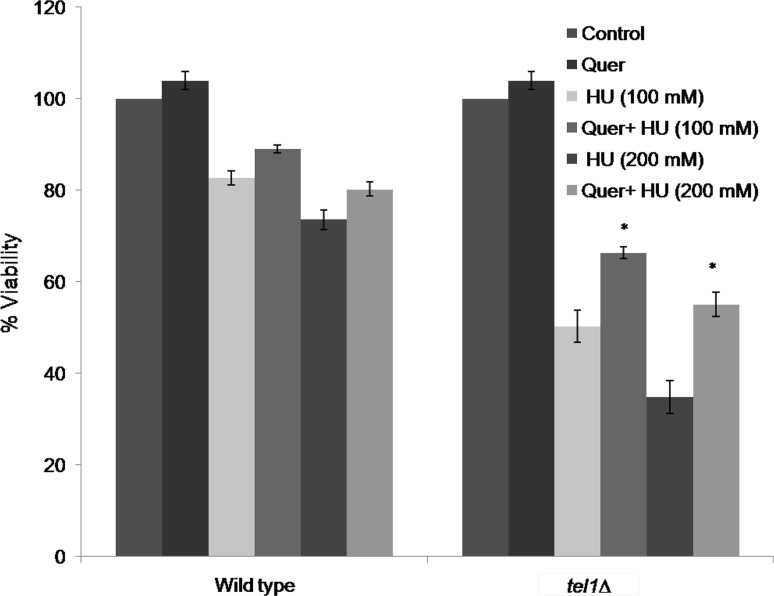
Quercetin protected yeast tel1∆ cells from hydroxyurea sensitivity
Hydroxyurea induces DNA damage via formation of hydrogen peroxide and nitric oxide. As shown in Fig. 3, tel1∆ cells were found to be sensitive to hydroxyurea compared to wild type strain. The percentage decrease in viability of tel1∆ cells was found to be 50.33 and 34.82%, respectively at 100 and 200 mM concentrations of hydroxyurea. In contrast, quercetin pretreatment increased the percentage viability of tel1∆ cells significantly, which was found to be 66.38 and 55.08% compared to their respective control samples (50.33 and 34.82%) at concentrations of hydroxyurea tested (100 and 200 mM).
Fig. 3.
Quercetin protects yeast tel1 mutant cells from hydroxyurea (HU) sensitivity. Viability of wild type and tel1∆ strains was measured after exposure of cells to HU (100 and 200 mM) for 1 h without (control) or with 200 µM Quer. Values are mean ± SD of three independent experiments. *Significant increase in tel1∆ percent viability of Quer + 100 mM HU and Quer + 200 mM HU treatment compared to HU (100 and 200 mM) treatment, respectively (p < 0.05)
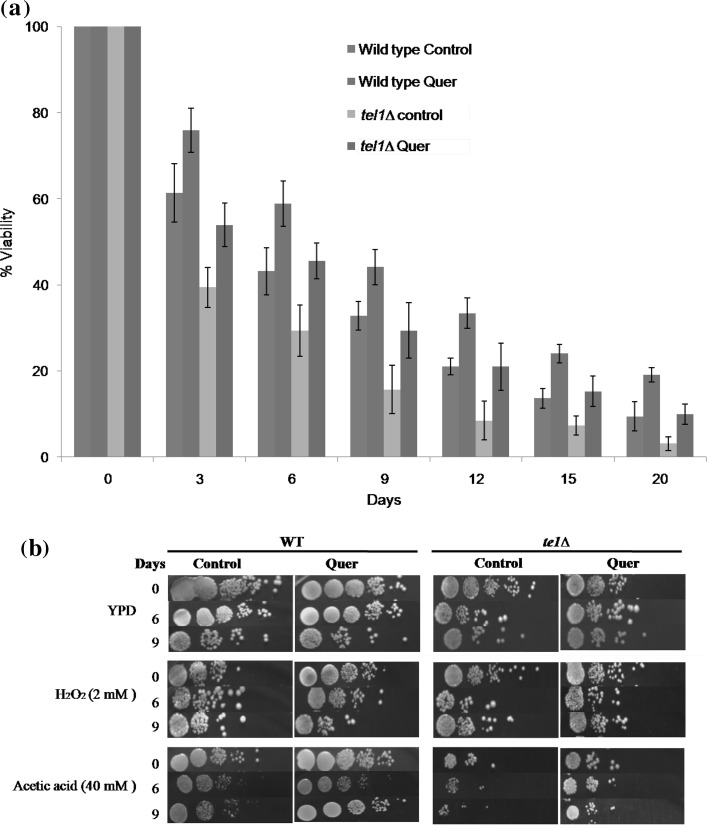
Effect of quercetin on chronological life span of yeast tel1∆ cells
The percentage viability of yeast tel1 mutant strain was decreased during chronological aging when compared to wild type. Whereas, presence of quercetin in the culture medium enhanced the percentage viability of tel1 mutant cells during CLS when compared to control cells (Fig. 4a). Interestingly, the increase in percent viability was found to be higher in quercetin treated in tel1Δ than wild type at day 3 and 6 when compared to respective controls. Further, we spotted the yeast cells from CLS assay (0, 6 and 9 days) on to YPD plate containing either H2O2 or acetic acid to assess oxidative and apoptotic stress resistance offered by quercetin treatment (Fig. 4b). Our results showed that quercetin increased the stress resistance of chronologically aged tel1Δ cells against H2O2 or acetic acid toxicity when compared to untreated controls.
Fig. 4.
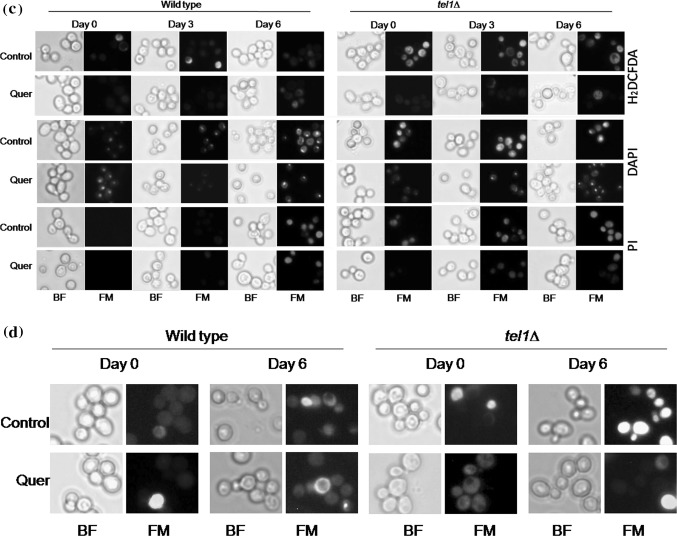
Quercetin extends the chronological life span of yeast cells. a Percent viability of wild type and tel1∆ yeast cells during chronological aging. Values are mean ± SD of three independent experiments. b Spot assay. Wild type and tel1∆ cultures from CLS assay were spotted on to YPD plates containing H2O2 (2 mM) and acetic acid (40 mM) and incubated at 30 °C for 2–3 days. Representative images are shown from at least 3 independent experiments. c Detection of ROS, nuclear condensation and plasma membrane integrity staining of Wild type and tel1∆ cells during chronological aging. ROS detection was studied by using a ROS sensing dye H2DCFDA (upper panel), the nuclear condensation was detected by using 4′,6-diamidino-2-phenylindole (DAPI) (middle panel) and plasma membrane integrity was detected by using propidium iodide (PI) (lower panel). Representative images are shown from at least three independent experiments. d AO/EtBr dual staining to detect apoptotic events. Yeast cells were stained with AO/EtBr staining solution as described above. Representative images are shown from at least three independent experiments
Figure 4c represents the fluorescence microscopic images of yeast cells stained with H2DCFDA or DAPI or PI at different time points (day 0, 3 and 6) during chronological aging. These results clearly show that increase in ROS accumulation, chromatin condensation and loss of plasma membrane integrity in control tel1Δ cells with age. In contrast, presence of quercetin in the medium reduced the age associated ROS accumulation and other apoptotic markersin tel1Δ cells. Furthermore, the apoptotic morphology of aging yeast cells were evaluated using AO/EtBr dual staining. As shown in Fig. 4d, age associated increase in apoptotic cells (indicated by yellow fluorescence cells) is more pronounced in control tel1∆ cells compared to wild type control. However, quercetin treatment led to reduction in apoptotic tel1∆ cells. These experimental results suggest that quercetin protected the apoptotic mediated cell death of tel1Δ cells and increased the percentage viability during CLS.
Discussion
Owing to the high degree of conservation of cellular activities from yeast to humans, several researchers have successfully used yeast S. cerevisiae as a eukaryotic model to study the molecular mechanisms underlying human diseases (Outeiro and Lindquist 2003; Ocampo et al. 2003). Many of the human diseases associated genes share homology with the yeast genes and their mutations in yeast show similar phenotypes under different stress conditions. The S. cerevisiae TEL1 is the ortholog of human ATM, plays an important role in maintaining the genome stability and coordinates the intricate array of cellular responses to DNA double-strand breaks. Mutation or inactivation of ATM gene leads to Ataxia Telangiectasia (AT) in human, whose hallmarks are increased oxidative damage, neuronal degeneration, immunodeficiency, genomic instability, premature aging and predisposition to cancer (Reichenbach et al. 2002; Guo et al. 2010). There is a great interest to understand ATM deficiency associated genome instability-oxidative stress connection and apoptosis (Poletto et al. 2017). These studies provide further insight into the phenotypes associated with genetic deficiencies of DNA damage responses and help in developing new therapeutic avenues for the management and treatment of AT (Reliene and Schiestl 2008). In the present study, we demonstrated the protective effects of quercetin, a natural antioxidant flavonoid on the sensitivity of yeast tel1∆ cells when challenged with oxidative, apoptotic, and DNA damaging agents. Furthermore, we investigated the anti-aging effect of quercetin on the CLS of tel1∆ cells. It has been reported that oxidants such as H2O2, MBS and t-BHP are known to cause oxidative stress in the cells mainly through decreasing the level of reduced glutathione and increasing the level of reactive oxygen species (Kwolek-Mirek and Zadrag-Tecza 2014). These compounds cause a number of negative changes in yeast cells, resulting in decreased cell viability. Our results showed that yeast tel1∆ cells are sensitive to oxidants (H2O2, MBS and t-BHP) compared to wild type (Fig. 1a, b), whereas quercetin treatment mitigated oxidant induced ROS accumulation (Fig. 1c), thereby increased stress resistance of tel1∆ cells. Within the flavonoid family, quercetin is the most potent scavenger of ROS and reactive nitrogen species (RNS) due to the presence of two antioxidant pharmacophores within the molecule that have the optimal configuration for free radical scavenging (Heijnen et al. 2001).
Further, we aimed at investigating the sensitivity of tel1∆ cells to acetic acid induced apoptosis. Acetic acid has been reported to induce apoptosis in S. cerevisiae (Ludovico et al. 2001). S. cerevisiae tel1 mutant showed high sensitivity to acetic acid represented by spot assay (Fig. 2a) as well as CFU assay (Fig. 2b) when compared to wild type strain, suggesting apoptosis mediated cell death of tel1∆. We employed different fluorescence dyes to visualize the apoptotic markers such as chromatic condensation and plasma membrane integrity and found that acetic acid induced apoptotic cell death of tel1∆ cells (Fig. 2c–f). Quercetin has also been shown to have anti-apoptotic properties across different model systems (Boots et al. 2008). Our results also showed that quercetin inhibits acetic acid induced characteristic alterations in the apoptotic morphology of tel1∆ cells (Fig. 2c, e) and prevented apoptosis mediated cell death. Apoptotic cell death has been reported in AT mutated cell lines, ATM gene knockout mice (ATM/-/-) and AT patients. From our yeast experimental results, we predict that quercetin might protect apoptotic mediated cell death in patients with ATM mutation.
Cells incubated with either high concentrations or prolonged treatment with low doses of hydroxyurea can result in cell death due to the DNA damage generated at arrested replication forks (Singh and Xu 2016). Recent studies in several model organisms have shown that oxidative stress and several other mechanisms may contribute to the majority of the cytotoxic effect of hydroxyurea (Marchetti et al. 2006; Nakayashiki and Mori 2013). Our results showed that tel1∆ cells were sensitive to HU compared to wild type and the sensitivity was rescued by quercetin pretreatment (Fig. 3), suggesting the antioxidant activity of quercetin might mitigate the oxidant mediated DNA damage by HU.
ATM gene mutation is also associated with age related neurodegeneration and predisposition to cancer. Therefore, we performed chronological lifespan assay for tel1 mutant along with wild type in presence or absence of quercetin. We observed a decrease in viability of tel1∆ cells during CLS and it was increased when treated with quercetin. The results from the present study indicated that quercetin could reduce accumulation ROS and apoptotic markers (chromatin condensation and plasma membrane integrity), thereby increasing the percentage viability of tel1∆ cells during chronological lifespan (Fig. 4a, c, d). Furthermore, our results showed that quercetin enhanced the stress resistance of aged cells against hydrogen peroxide and acetic acid as shown in the plate based assay (Fig. 4b).
There is no curative strategy for treating AT disease associated with ATM mutation. The current treatment has focused only on the management by slowing down the disease progression towards neurodegeneration and cancer. Short-term treatment with glucocorticoid analogues (betamethasone) improved in neurological symptoms associated with AT patients (Zannolli et al. 2012). Glucocorticoid therapy (dexamethasone) promotes alternative splicing in the ATM gene and possibly restored a residual ATM activity in AT cells (Menotta et al. 2012). It is proposed that decrease in antioxidant capacity, increase in oxidative stress and apoptosis contribute to the neurodegeneration in AT patients (Reichenbach et al. 2002). N-acetyl cysteine suppressed both oxidative DNA damage and DNA deletions in ATM deficient mice. Tempol, a stable nitroxide free radical and superoxide dismutase mimetic has shown to reduce ROS levels, protein oxidation and restored mitochondrial membrane potential in thymocytes implying that chemoprevention by tempol is associated with its antioxidant activity. Tempol treatment also reduced cell number in the thymus and decreased weight gain in ATM deficient mice. Another stable nitroxide free radical related compound, 5-carboxy-1,1,3,3-tetramethylisoindolin-2-yloxyl has shown to prolong the survival of ATM deficient mice (Menotta et al. 2012). In conclusion, we have shown that S. cerevisiae tel1 mutant was sensitive to oxidative, apoptotic and DNA damaging stress agents and the sensitivity was rescued by quercetin. It is also found that quercetin improved the oxidative and apoptotic stress résistance of tel1 mutant and thereby increased viability during chronological aging. Although consuming quercetin-rich food is beneficial, since the benefits are dose dependent, intake of quercetin as a food supplement may be much more effective in preventing AT and other diseases. Overall, our results suggest that quercetin may be used as an antioxidant therapeutic agent to slow or prevent the damaging effects in AT patients. Future studies are needed to examine the molecular mechanism underlying protective effects of quercetin against ATM-deficient mice and human cells.
Acknowledgements
The authors thankful to UGC-BSR (F NO 42-665/2013 (SR dated 25-03-2013)) for supporting this work and DBT-IPLS, DST-FIST for providing infrastructure. Phaniendra Alugoju thank to Pondicherry University and UGC-BSR Research fellowship F-7-370/2012 (BSR) for providing fellowship for the financial assistance.
Compliance with ethical standards
Conflict of interest
Authors declare that there is no conflict of interest.
References
- Baldo V, Testoni V, Lucchini G, Longhese MP. Dominant TEL1-hy mutations compensate for Mec1 lack of functions in the DNA damage response. Mol Cell Biol. 2008;28:358–375. doi: 10.1128/MCB.01214-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Chabes A, Georgieva B, Domkin V, Zhao X, Rothstein R, Thelander L. Survival of DNA damage in yeast directly depends on increased dNTP levels allowed by relaxed feedback inhibition of ribonucleotide reductase. Cell. 2003;112(3):391–401. doi: 10.1016/S0092-8674(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Doudican NA, Song B, Shadel GS, Doetsch PW. Oxidative DNA damage causes mitochondrial genomic instability in Saccharomyces cerevisiae. Mol Cell Biol. 2005;25(12):5196–5204. doi: 10.1128/MCB.25.12.5196-5204.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwin Shackelford R, Manuszak RP, Heard SC, Link CJ, Wang S. Pharmacological manipulation of ataxia-telangiectasia kinase activity as a treatment for Parkinson’s disease. Med Hypotheses. 2005;64:736–741. doi: 10.1016/j.mehy.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Longo VD. The chronological life span of Saccharomyces cerevisiae. Methods Mol Biol. 2007;371:89–95. doi: 10.1007/978-1-59745-361-5_8. [DOI] [PubMed] [Google Scholar]
- Ferreira TC, de Moraes LM, Campos EG. Cell density-dependent linoleic acid toxicity to Saccharomyces cerevisiae. FEMS Yeast Res. 2011;11(5):408–417. doi: 10.1111/j.1567-1364.2011.00729.x. [DOI] [PubMed] [Google Scholar]
- Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45(11):2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Heijnen CG, Haenen GR, Vekemans JA, Bast A. Peroxynitrite scavenging of flavonoids: structure activity relationship. Environ Toxicol Pharmacol. 2001;10(4):199–206. doi: 10.1016/S1382-6689(01)00083-7. [DOI] [PubMed] [Google Scholar]
- JECFA (1977) Evaluation of certain food additives. In: Proceedings of 21st JECFA session, April, 18–27, 1977, Geneva, Switzerland. WHO technical report series, no. 617. World Health Organization (WHO), Joint FAO/WHO Expert Committee on Food Additives (JECFA) & Food and Agriculture Organization of the United Nations, Geneva, Switzerland
- Kamsler A, Daily D, Hochman A, Stern N, Shiloh Y, Rotman G, Barzilai A. Increased oxidative stress in ataxia telangiectasia evidenced by alterations in redox state of brains from Atm-deficient mice. Cancer Res. 2001;61:1849–1854. [PubMed] [Google Scholar]
- Kwolek-Mirek M, Zadrag-Tecza R. Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast Res. 2014;14:1068–1079. doi: 10.1111/1567-1364.12202. [DOI] [PubMed] [Google Scholar]
- Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, Yin Y. Quercetin, inflammation and immunity. Nutrients. 2016;8:167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludovico P, Sousa MJ, Silva MT, Leao C, Corte-Real M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology. 2001;147:2409–2415. doi: 10.1099/00221287-147-9-2409. [DOI] [PubMed] [Google Scholar]
- Madeo F, Frohlich E, Frohlich KU. A yeast mutant showing diagnostic markers of early and late apoptosis. JCell Biol. 1997;139:729–734. doi: 10.1083/jcb.139.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakar D, Dey A, Basu A, Ghosh AK. Antiapoptotic role of S-adenosyl-l-methionine against hydrochloric acid induced cell death in Saccharomyces cerevisiae. Biochem Biophys Acta. 2008;1780(7–8):937–947. doi: 10.1016/j.bbagen.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Marchetti MA, Weinberger M, Murakami Y, Burhans WC, Huberman JA. Production of reactive oxygen species in response to replication stress and inappropriate mitosis in fission yeast. J Cell Sci. 2006;119(Pt 1):124–131. doi: 10.1242/jcs.02703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menotta M, Biagiotti S, Bianchi M, Chessa L, Magnani M. Dexamethasone partially rescues ataxia telangiectasia-mutated (ATM) deficiency in ataxia telangiectasia by promoting a shortened protein variant retaining kinase activity. J Biol Chem. 2012;287(49):41352–41363. doi: 10.1074/jbc.M112.344473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu PS, Singh A, Kulkarni SK. Quercetin, a bioflavonoid, attenuates haloperidol-induced orofacial dyskinesia. Neuropharmacology. 2003;44:1100–1106. doi: 10.1016/S0028-3908(03)00101-1. [DOI] [PubMed] [Google Scholar]
- Nakayashiki T, Mori H. Genome-wide screening with hydroxyurea reveals a link between nonessential ribosomal proteins and reactive oxygen species production. J Bacteriol. 2013;195(6):1226–1235. doi: 10.1128/JB.02145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocampo A, Liu J, Barrientos A. NAD+ salvage pathway proteins suppress proteotoxicity in yeast models of neurodegeneration by promoting the clearance of misfolded/oligomerized proteins. Hum Mol Genet. 2003;22(9):1699–1708. doi: 10.1093/hmg/ddt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science. 2003;302:1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletto M, Yang D, Fletcher SC, Vendrell I, Fischer R, Legrand AJ, Dianov GL. Modulation of proteostasis counteracts oxidative stress and affects DNA base excision repair capacity in ATM-deficient cells. Nucleic Acids Res. 2017;45(17):10042–10055. doi: 10.1093/nar/gkx635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach J, Schubert R, Schindler D, Muller K, Bohles H, Zielen S. Elevated oxidative stress in patients with ataxia telangiectasia. Antioxid Redox Signal. 2002;4:465–469. doi: 10.1089/15230860260196254. [DOI] [PubMed] [Google Scholar]
- Reliene R, Schiestl RH. Experimental antioxidant therapy in ataxia telangiectasia. Clin Med Oncol. 2008;2:431–436. doi: 10.4137/cmo.s535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford RE, Innes CL, Sieber SO, Heinloth AN, Leadon SA, Paules RS. The Ataxia telangiectasia gene product is required for oxidative stress-induced G1 and G2 checkpoint function in human fibroblasts. J Biol Chem. 2001;276:21951–21959. doi: 10.1074/jbc.M011303200. [DOI] [PubMed] [Google Scholar]
- Shackelford RE, Manuszak RP, Johnson CD, Hellrung DJ, Link CJ, Wang S. Iron chelators increase the resistance of Ataxia telangiectasia cells to oxidative stress. DNA Repair. 2004;3:1263–1272. doi: 10.1016/j.dnarep.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Singh A, Xu YJ. The cell killing mechanisms of hydroxyurea. Genes (Basel) 2016;7(11):99. doi: 10.3390/genes7110099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa M, Duarte AM, Fernandes TR, Chaves SR, Pacheco A, Leao C, Corte-Real M, Sousa MJ. Genome-wide identification of genes involved in the positive and negative regulation of acetic acid-induced programmed cell death in Saccharomyces cerevisiae. BMC Genom. 2013;14:838. doi: 10.1186/1471-2164-14-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting Y, Chang WT, Shiau DK, Chou PH, Wu MF, Hsu CL. Antiobesity efficacy of quercetin-rich supplement on diet-induced obese rats: effects on body composition, serum lipid profile, and gene expression. J Agric Food Chem. 2018;66(1):70–80. doi: 10.1021/acs.jafc.7b03551. [DOI] [PubMed] [Google Scholar]
- Vilaca R, Mendes V, Mendes MV, Carreto L, Amorim MA, de Freitas V, Moradas-Ferreira P, Mateus N, Costa V. Quercetin protects Saccharomyces cerevisiae against oxidative stress by inducing trehalose biosynthesis and the cell wall integrity pathway. PLoS ONE. 2012;7:e45494. doi: 10.1371/journal.pone.0045494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Rosin MP, Anderson CK. Response of fibroblast cultures from ataxia-telangiectasia patients to oxidative stress. Cancer Lett. 1990;54:43–50. doi: 10.1016/0304-3835(90)90089-G. [DOI] [PubMed] [Google Scholar]
- Zannolli R, Buoni S, Betti G, Salvucci S, Plebani A, Soresina A, Pietrogrande MC, Martino S, Leuzzi V, Finocchi A, Micheli R, Rossi LN, Brusco A, Misiani F, Fois A, Hayek J, Kelly C, Chessa L. A randomized trial of oral betamethasone to reduce ataxia symptoms in ataxia telangiectasia. Mov Disord. 2012;27(10):1312–1316. doi: 10.1002/mds.25126. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ma WY, Kaji A, Bode AM, Dong Z. Requirement of ATM in UVA-induced signaling and apoptosis. J Biol Chem. 2002;277:3124–3131. doi: 10.1074/jbc.M110245200. [DOI] [PubMed] [Google Scholar]