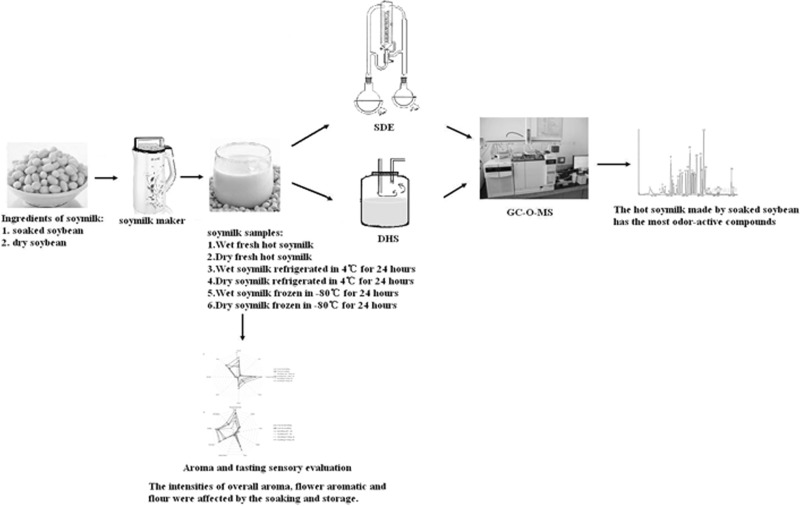
Abstract
Abstract
The influence of different processing and storage conditions on the aroma and taste of soymilk were investigated. Volatile components in soymilk were made by soymilk machine, half soybeans produced by grinding soaked with water, the other half soybeans without soaking. Then the soymilk was stored in different conditions for 24 h. Dynamic headspace dilution analysis and aroma extract dilution analysis in conjunction and gas chromatography–olfactometry–mass spectrometry were used to identify major aroma-active compounds. Sixteen odor-active compounds were identified in dry milling soymilk, while 21 odor-active compounds for wet milling one (smell was also more intense), among them, (E)-2-decanal (fatty, green), (E)-2-nonenal (sweet, fruity), 2-acetyl-1-pyrroline (popcorn), having the highest FD factors in SDE extract, were regarded as the most important odorants in soymilk. Investigation of the volatile components was affected to a greater extent by hot soymilk compared with refrigerated or frozen storage. Sensory results showed that intensities of overall aroma, flower aromatic, flour were affected by the soaking and storage.
Graphical Abstract
Keywords: Aroma-active components, Soymilk, Soaked samples, Refrigerated storage, Frozen storage, Gas chromatography–olfactometry–mass spectrometry (GC–O–MS)
Introduction
Soymilk is a traditional Chinese food and its protein content is nearly equal to that of cow milk (Kim and Kim 1999; Orthoefer and Liu 1995). There are certain amounts of soybean saponin, isoflavone, soybean oligosaccharide in soymilk, which are special health factors. Soymilk can play a role of balancing nutrition, regulating endocrine and decomposing excess fat in our diet. Soymilk is lactose-free, which is very good for lactose-intolerant people (Sacks et al. 2006). Evidences show that soybean consumption may result in a reduction of low-density lipoprotein (so called ‘bad cholesterol’). Soybean is a superior vegetable food, which is rich in protein (very similar to animal protein), unsaturated fatty acids, dietary fiber, vitamins and minerals (Darling et al. 2009). Soymilk is popular in China, and people of all ages are fond of the special bean-flavor of soymilk very much (Lei and Boatright 2001). In recent years soymilk has became more and more popular, and people have made it at home by themselves with soymilk maker. However, different processing methods can affect the major aroma-active components.
Efforts have been made by many researchers for the analysis methods and technologies of soymilk flavor. Among these methods, dynamic headspace dilution analysis (DHDA) was applied. The objectives of this project were to characterize the major aroma-active components generated by different processing methods and to compare the changes of the major aroma-active components under the different storage conditions, to assess the impact of different processing methods on the overall soymilk aroma and taste. The determination of volatile compounds responsible for the beany off-flavor in soymilk was reported in some literatures (Lozano et al. 2007; Lv et al. 2011; Yuan and Chang 2007). Kumazawa and Yuan studied soymilk of different soybeans made by traditional methods, and identified the key aroma compounds including 2-isopropyl-3-methoxypyrazine, cis-4,5-epoxy-(E)-2-decenal, trans-4,5-epoxy-(E)-2-decenal, 3-hydroxy-4,5-dimethyl-2(5H)-furanone, 2-aminoacetophenone, hexanal, 1-hexanol, trans-2-nonenal, 1-octen-3-ol, and (E,E)-2,4-decadienal (Yuan and Chang 2007; Kumazawa and Nishimura 2011). Because Korean consumers prefer hot or warmed soymilk (~ 50–60 °C) to cold or room temperature soymilk, Kim and others researchers investigated refrigerated and thermal storage on the volatile profile of commercial aseptic soymilk (Kim et al. 2009; Lozano et al. 2007). The volatile profile changes caused by thermal storage or refrigerated storage may influence the aroma quality of aseptic soymilk. Endo et al. (2004) demonstrated that the beany flavor of soymilk can be reduced by blanching soaked and swollen soybeans for 30 s in boiling water. The study of Achouri et al. (2007) showed that soymilk flavor, which was known to play a critical role in overall product quality, was affected not only by the compositions but also by the storage conditions. Storage of soymilk at 4 °C especially resulted in a keep of its quality, and a decrease of the aroma intensity over time, indicating that storage under refrigerated condition was better for soymilk quality. Using dynamic headspace and capillary gas chromatography, Min et al. (2005) found that factors such as soybean variety and growing location could have significant effects on the volatile compounds in soymilk. Catalyzed by lipoxygenase, the linoleic acid and linolenic acid in soybean were converted into off-flavor such as hexanal, 2-hexenal and 3-hexenol etc. (Kobayashi et al. 1995). Fu and Xia (2011) investigated volatiles of home-made soymilk, found that soaked soybean contained less beany compounds and more aroma compounds; thus, the soaking was very important for the preparation of high quality soymilk. Although the above information is very useful, characterizations of the flavor profiles generated as a result of different processing procedures continues to be lacking in literature. And to our knowledge, up to now, little is known on either the general flavor compounds or the aroma-active components of the soymilk made by soymilk machine. Gas chromatography–olfactometry (GC–O), combined with aroma extract dilution analysis (AEDA), is very useful for the identification and ranking of the key aroma components in various foods (Grosch 1993; Wettasinghe et al. 2001). The objective of this study was to identify and characterize the aroma-active compounds of the soymilk made by soymilk machine under different processing and storage conditions, by using gas chromatography–olfactometry–mass spectrometry (GC–O–MS) technique, together with aroma dilution analysis such as dynamic headspace dilution analysis (DHDA) and aroma extract dilution analysis (AEDA).
Materials and methods
Materials
Soybean sample was from Mudanjiang, Heilongjiang Province. Soymilk maker HD2070 was made by Philips Co., Ltd. (Shanghai, China).
Reagents and chemicals
Chemicals n-alkanes (C7–C22) (chromatographic reagent) were from Sigma-Aldrich (Santa clara, CA, USA). Dichloromethane and anhydrous sodium sulfate were from Huihai Scientific Instruments Co., Ltd. (Beijing, China).
Preparation of aroma concentrate of soymilk
Soaking is very important for soymilk making. Whole soybeans were divided into two groups, one portion of soybeans were soaked in distilled water (75 g soybeans with 964 ml distilled water) for 16 h at ambient temperature, another group was put in distilled water only (75 g soybeans with 964 ml distilled water), with no need for soaking. Then the soybeans were put into the soymilk maker HD2070 for the preparation of soymilk. It was as the following: (1) one cup of wet soybean was taken (soaking for 8 h); (2) soaked soybeans were put into the machine, and a certain amount of water was put into also; (3) the machine was powered on and started to operate; (4) about 24 min later, the making of soymilk was done (it was made by automated program). After cooling and filtering, the soymilk was obtained. Both groups of soymilk were stored either in refrigerator (control, 4 °C) or in freezer (control, − 80 °C).
Dynamic headspace sampling (DHS)
DHS was carried out on an Agilent 7890 GC instrument and an olfactometry port (Sniffer 9000, Brechbühler, Switzerland) was also mended on. Aroma compounds from Tenax trap were thermally desorbed at 280 °C using a TDSA2 system (Gerstel, Germany) into a cryo-cooled (− 150 °C) CIS inlet (Gerstel). Injection was splitless (inlet heating rate of 12 °C/min to 260 °C).
Dynamic headspace dilution analysis (DHDA)
Soymilk (60 ml) was put into a purge-and-trap vessel (150 ml volume). After equilibrating 30 min at 50 °C (water-bath circulation), the sample was purged with a nitrogen stream at a flow-rate of 100 ml/min for 60, 12.5, 2.5 or 0.5 min, the FD factors were 1, 5, 25, 125 respectively. Volatile compounds of the sample headspace were captured by Tenax trap put into the vessel. The Tenax trap was then dried by gentle nitrogen purge for 20 min (TD controller, Gerstel, Mulheim Germany) to remove moisture for aroma analysis.
Simultaneous distillation and extraction of volatile components (SDE)
Volatile components of 500 ml soymilk were extracted for 3 h with a simultaneous distillation and extraction (SDE) apparatus (model 523010-0000, Kontes, NJ). Redistilled dichloromethane (50 ml) was used as solvent. After boiling (sample solution), SDE operation was done for 3 h. The solvent extract was dried with anhydrous sodium sulfate, then concentrated to ca. 1 ml by a Vigreux column, and further to 0.2 ml by gentle purging of nitrogen (99.995% purity), and stored at −18 °C for next step.
Aroma extract dilution analysis (AEDA)
A serial of 3n dilutions were made from the initial SDE extract in the ratio of 1:2 in diethyl ether. Each dilution was then subjected to GC–O–MS analysis. The highest dilution in which the compound was detectable is the flavor dilution (FD) factor of that compound (Chung and Cadwallader 1994). All FD results are shown in an aroma diagram. Compounds with higher FD value are considered to be more important.
Gas chromatograph–olfactometry–mass spectrometer (GC–O–MS) analysis
The analysis of volatiles was carried out on a GC–MS of 7890A coupled to a Triple Quad 7000B (both Agilent, Palo Alto, CA), combined with a Sniffer 9000 Olfactometer (Brechbühler, Switzerland). Separations in GC were performed on DB-5MS UI (30 m × 0.250 mm × 0.25 µm, J & W Scientific, Folsom, CA, USA) and HP-INNOWAX (30 m × 0.25 mm × 0.25 μm, Agilent Inc., USA). Ultra-high purity helium was used as carrier gas and the flow rate was 1.2 ml/min. The temperature of GC oven was programmed as the following: firstly it was 40 °C, keeping for 3 min; then ramped at 5 °C/min to 230 °C, holding for 3 min. The temperatures of the injector and the GC–MS interface were 250 and 280 °C respectively. Electron-impact mass spectra were generated at 70 eV, with m/z scan range from 35 to 550 amu and the ion source temperature was 230 °C. Compounds were identified according to NIST 05 mass spectra libraries installed in the GC–MS equipment. The sniffing (GC–O) was accomplished by three experienced panelists.
Compounds identification
The identification of aroma-active compounds was done on both DB-5 column and DB-Wax columns. The RI values and odor descriptions of aroma-active compounds should match with the mass spectra and retention indices of reference compounds (Rychlik et al. 1998; www.odor.org.uk). n-Alkanes (C7–C22) were analyzed under the same conditions to calculate LRIs:
which was described by Dool and Krazt (1963).
Results and discussion
Aroma-active compounds
A total of 37 aroma-active compounds from hot soymilk made by the soymilk machine HD2070 were identified by SDE–GC–O–MS and DHS–GC–O–MS. There was no significant difference between the way of making soymilk from the machine and traditional production process in overall aroma aspect. The soymilk which we bought from the market made by traditional way was very complex. Soybeans were homogenized with distilled water by a mixer after soaking in distilled water for 12 h. Another some of distilled water was then added, and the mixture was boiled for few minutes. The advantage of DHS is that the aroma of soymilk could be enriched and original flavor of soymilk could be shown completely. Twenty compounds were identified as major odorants (Table 1) by DHS–GC–O–MS. Among them, heptanal (fatty, oily), 2-acetyl-1-pyrroline (popcorn), 1-octen-3-ol (mushroom), acetic acid (sour), (E,E)-2,4-nonadienal (stink) were considered as aroma components as their FD-factors were more than 125 in hot wet milling soymilk. 2-Acetyl-1-pyrroline (popcorn), acetic acid (sour) were also key odorants, with FD factors ≥ 125 in hot wet milling soymilk refrigerated at 4 °C for 24 h. With FD-factors ≥ 125, 2-acetyl-1-pyrroline (popcorn), acetic acid (sour), (E,E)-2,4-nonadienal (stink) were judged as predominant in hot dry milling soymilk. And being FD-factor ≥ 125, (E,E)-2,4-nonadienal (stink) was also a key odorant in hot dry milling soymilk refrigerated in 4 °C for 24 h. Especially, there are no compounds of FD-factors ≥ 125 in soymilk refrigerated in − 80 °C for 24 h (both wet milling soymilk and dry milling soymilk), implied that freezing could reduce aroma considerably. Besides, in Table 1, there were still two fairly important odorants remaining unknown in the soymilk (only in wet milling soymilk refrigerated at 4 °C), No. 18 and No. 20. Compound No. 18 had a pine-like note, its RI value was 1549 on DB-Wax column, while the RI of compound No. 20 was 1741 on DB-Wax column, giving a flour-like smell, and further identification was needed. Subsequently, the compounds detected by DHDA such as eugenol (mint-like), 2-pentylfuran (green-like), 2-ethylfuran, ethyl acetate (fruity) were not detected by SDE.
Table 1.
Key aroma compounds in the HD2070 samples by DHS
| Nr | Compound name | Odor property | RI | FD factors (DHDA) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DB-Wax | DB-5 | Wet milling soymilk | Dry milling soymilk | |||||||
| A | B | C | A | B | C | |||||
| 1 | Ethyl acetatea | Fruity | 885 | <600 | N | N | N | 1 | 5 | 5 |
| 2 | 3-methyl-1-butanola | Malt | 1206 | 651 | 5 | N | N | 1 | 1 | 1 |
| 3 | 2-ethylfurana | Green | 1257 | 701 | 1 | N | N | 1 | N | 1 |
| 4 | Hexanala | Cut grass | 1081 | 802 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5 | Heptanala | Fatty, oily | 1183 | 902 | 125 | 25 | 1 | 25 | 1 | 25 |
| 6 | 2-acetyl-1-pyrrolineb | Popcorn | 1307 | 924 | 125 | 125 | 25 | 125 | 25 | 25 |
| 7 | Benzaldehydea | Almond | 1515 | 924 | 25 | 25 | 25 | 5 | 25 | 5 |
| 8 | 1-octen-3-ola | Mushroom | 1441 | 980 | 125 | 125 | 1 | 25 | 25 | 5 |
| 9 | 2-pentylfurana | Fruity/green | 1231 | 995 | 1 | 1 | 1 | 1 | 1 | 1 |
| 10 | 1,2-dichlorobenzenea | Rotten | 1027 | N | N | N | 5 | N | N | |
| 11 | Acetic acida | Sour | 1457 | 1043 | 125 | 125 | 5 | 125 | 1 | N |
| 12 | Acetophenonea | Soap | 1668 | 1099 | 1 | 1 | 25 | 1 | 1 | 5 |
| 13 | Nonanala | Floral | 1388 | 1107 | 25 | 25 | N | 1 | 1 | 5 |
| 14 | (E,Z)-2,6-nonadienalb | Cucumber | 1593 | 1156 | 1 | 1 | 1 | 1 | 1 | N |
| 15 | Eugenola | Mint | 1727 | 1358 | 25 | 25 | N | 25 | 25 | 5 |
| 16 | 1-penten-3-ola | Burnt | 1164 | 1 | 1 | N | 1 | N | N | |
| 17 | Octanala | Soap | 1287 | 25 | 5 | N | 25 | 1 | N | |
| 18 | Unknown | Pine-like | 1549 | 1 | N | N | 1 | 1 | 1 | |
| 19 | (E,E)-2,4-nonadienala | Stink | 1673 | 125 | 25 | 25 | 125 | 125 | N | |
| 20 | (E)-2-Undecenal | Flour | 1741 | N | 25 | N | N | N | N | |
aCompounds were tentatively identified by RI value, odor properties, and MS
bCompounds were tentatively identified by GCO, comparing their RIs and odor properties with referenced RIs and odor qualities
A, fresh hot soymilk; B, soymilk refrigerated in 4 °C for 24 h; C, soymilk frozen in − 80 °C for 24 h
Compared to other solvent extraction methods, SDE could protect the activity of the protein and keep the aroma of boiled soymilk perfectly. Twenty-one compounds were identified as odorants in soymilk by AEDA. Among them, hexanal, amyl alcohol, 2-acetyl-1-pyrroline, (E)-2-decanal and (E)-2-nonenal, having log3FD factors of ≥ 2 (Table 2), were identified as key aroma components. Compounds with log3FD factor of ≥ 3 had popcorn/fatty note, including 2-acetyl-1-pyrroline, (E)-2-decanal. Besides these, other compounds in Table 2 were also important due to their slightly lower intensities. For wet milling soymilk, aroma of certain compounds were very active as their log3FD factors were 6, such as (E)-2-decanal and benzaldehyde. For aroma-active compounds of log3FD factor ≥ 2, seven were detected in dry milling soymilk; while ten were in fresh hot wet milling soymilk, so it could be seen that key aroma-active compounds in the wet milling soymilk had higher FD factors, this result coincided with the conclusion of sensory characterization, therefore, the sample of hot wet milling soymilk had the best flavor. The study showed that the content of hexanal (cut grass), 2-pentylfuran (green), 1-hexen-3-one (pungent, plastic, water bottle) and (E)-2-decanal (fatty, green) had no significant change after refrigerated 24 h. The content of 2-acetyl-1-pyrroline (popcorn) also had no remarkable change, which was the key compound of sweet aroma of soymilk. Hexanal (cut grass) and 2-pentylfuran (green) were the key aroma-active compounds of soymilk, which had no significant change after refrigerated 24 h. They were composed the faint scent of soymilk. However, the content of alcohols and aldehydes had a significant reduction. The aroma-active compounds loss was particularly serious in the soymilk after being frozen. Although the cold storage and frozen storage made the soymilk flavor loss more seriously, the key aroma compounds in soymilk which was soaked for 16 h, had higher FD factors and more classes than those of soybeans without soaking.
Table 2.
Key aroma compounds in the HD2070 samples by SDE
| Nr | Compound name | Oder property | RI | FD factors (Log3FD) | |||
|---|---|---|---|---|---|---|---|
| DB-Wax | A | B | C | D | |||
| 1 | Unknown | Milky | 995 | 3 | <1 | N/A | N/A |
| 2 | 3-Methylfurana | Fatty | 1032 | 1 | 1 | N/A | N/A |
| 3 | Hexanala | Green, cut-grass | 1086 | 2 | 2 | 2 | 2 |
| 4 | 1-Hexen-3-oneb | Pungent, plastic, water bottle | 1125 | 1 | 1 | 1 | 1 |
| 5 | 1-Octen-3-oneb | Mushroom | 1180 | N/A | 2 | N/A | N/A |
| 6 | Amyl alcoholb | Cooked potato-like, wine | 1248 | 3 | 1 | 4 | 2 |
| 7 | Octanala | Green, orange peel | 1293 | <1 | <1 | <1 | N/A |
| 8 | 2-Acetyl-1-pyrrolinea | Popcorn | 1340 | 6 | 5 | 5 | 5 |
| 9 | Hexanola | Wine | 1356 | <1 | 2 | N/A | N/A |
| 10 | (Z)-2-Octenala | Hay, stale | 1448 | 1 | 1 | <1 | N/A |
| 11 | 2-Nonanonea | Milky | 1450 | <1 | N/A | N/A | N/A |
| 12 | 1-octen-3-ola | Mushroom | 1452 | 5 | <1 | <1 | N/A |
| 13 | (E)-2-Decanala | Fatty, green | 1458 | 6 | 6 | 5 | 5 |
| 14 | (Z)-2-Nonenal | Fruity,fatty | 1523 | 4 | N/A | N/A | N/A |
| 15 | (E)-2-Nonenala | Sweet, fruity | 1539 | 1 | 4 | 4 | <1 |
| 16 | (E,Z)-2,6-Nonadienala | Cucumber | 1593 | 3 | 2 | N/A | N/A |
| 17 | (Z)-2-Decenal | Carton | 1640 | 6 | N/A | N/A | 2 |
| 18 | Benzaldehydeb | Almond-like | 1698 | 6 | 1 | N/A | 1 |
| 19 | Unknown | Peppermint | 1732 | <1 | N/A | N/A | N/A |
| 20 | (E)-2-Undecenalb | Cilantro | 1756 | 1 | N/A | <1 | N/A |
| 21 | (E,E)-2,4-Decadienala | Fatty, fried | 1817 | 1 | 1 | 1 | <1 |
aCompounds were tentatively identified by RI value, odor properties, and MS
bCompounds were tentatively identified by GCO, comparing their RIs and odor properties with referenced RIs and odor qualities
A, fresh hot wet milling soymilk; B, fresh hot dry milling soymilk; C, wet milling refrigerated in 4 °C for 24 h; D, dry milling refrigerated in 4 °C for 24 h
1-Octen-3-ol, (Z)-2-nonenal, (Z)-2-decenal, benzaldehyde are auto-oxidation products of linoleic acid. The (Z,Z)-1,4-pentadiene structure of linoleic acid is oxidized by lipoxygenase through the addition of molecular oxygen to form hydroperoxides which degrade to small molecules having beany odor. In this study, the overall aroma intensity of fresh wet milling soymilk was stronger than that of fresh dry milling soymilk, and volatile compounds such as 1-octen-3-ol, (Z)-2-nonenal, (Z)-2-decenal, benzaldehyde in fresh wet milling soymilk were more than that of fresh dry milling soymilk. Man et al. (1991) investigated the effect of soaking on biologically active components of soybean. When soybean was soaked in distilled water at 23 °C for 4 h, its lipoxygenase activity (LA) increased from 13.90 to 23.56 unit/mg solid and remained the same for another 4 h. The possible reason for LA increase was the decrease in solid content of soybean during the 8 h soaking. From Table 2, it could be seen that, the FD factors of 1-octen-3-ol, (Z)-2-nonenal, (Z)-2-decenal, benzaldehyde in fresh wet milling soymilk were much higher than that of in fresh dry milling soymilk, because after soaking, the lipoxygenase activity (LA) increased, more oxidized products of linoleic acid were generated, the concentrations of compounds mentioned above were increased. Badenhop and Wilkens’s work (1969) showed that, it was very likely that some amount of 1-octen-3-ol was formed during soaking process by enzymatically-catalyzed bio-conversion from linoleic acid.
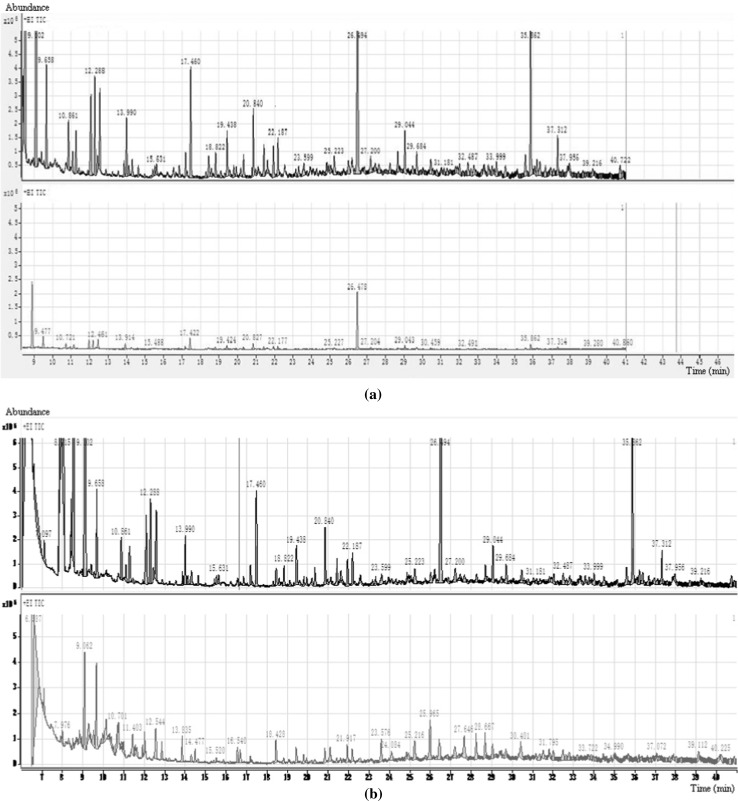
Fig. 1a shows that the technology of wet milling process is markedly better than that of dry milling. Sixteen odor-active compounds were identified in dry milling soymilk, while 21 odor-active compounds were identified in wet milling one (smell was also more intense). Among them, (E)-2-decanal (fatty, green), (E)-2-nonenal (sweet, fruity), and 2-acetyl-1-pyrroline (popcorn) were regarded as the most important odorants in soymilk, as they had the highest FD factors in SDE extract.
Fig. 1.
GC–MS TIC of different soymilks. a Up: TIC of wet milling soymilk; down: TIC of dry milling soymilk, b up: TIC of fresh wet milling soymilk; down: TIC of wet milling soymilk stored at 4 °C for overnight
The aroma components of fresh wet milling soymilk were also compared with that of the same one stored at 4 °C overnight. It was shown in Fig. 1b that although total number of volatile compounds was almost the same, from the peak height of TIC and intensity of sniffing, the odor loss of soymilk after a period of refrigeration was obviously serious.
Li et al. (2012) found large amount of benzaldehyde in soybean dregs, accounting for 11.8% of the total volatile substances. Benzaldehyde is known as an auto-oxidation product of linoleic acid, having a pleasant cheery, almond-like note, which is more for overall odor profile of soymilk. Moreover, hexanal is the main beany compound in soymilk, there was really a small amount of hexanal in the soymilk made by the soymilk machine. It was implied that, the more pleasant bean-note of the soymilk consisted of more benzaldehyde and less hexanal along with other odor-active compounds such as eugenol.
Conclusion
Compared to the odor-active compounds of soymilk made by soymilk machine under different processing and storage conditions, the hot soymilk made by soaked soybeans had the most odor-active compounds and the best taste. The aroma-active compounds were lost remarkably in the soymilk after frozen, so the low storage temperature should be avoided. It is helpful to understand the soymilk flavor formation, and the changes were observed that volatile compounds formed at different storage temperatures. Factors influenced the overall aroma profile of soymilk need to be further investigated, and this may provide information for the selection of soymilk maker heating methods and soymilk storage conditions.
References
- Achouri A, Boye JI, Zamani Y. Changes in soymilk quality as a function of composition and storage. J Food Qual. 2007;30:731–744. doi: 10.1111/j.1745-4557.2007.00153.x. [DOI] [Google Scholar]
- Badenhop AF, Wilkens WF. The formation of 1-octen-3-ol in soybeans during soaking. J Am Oil Chem Soc. 1969;46:179–182. doi: 10.1007/BF02635729. [DOI] [Google Scholar]
- Chung H, Cadwallader K. Aroma extract dilution analysis of blue crab claw meat volatiles. J Agric Food Chem. 1994;42:2867–2870. doi: 10.1021/jf00048a040. [DOI] [Google Scholar]
- Darling A, Millward D, Torgerson D, Hewitt C, Lanham-New S. Dietary protein and bone health: a systematic review and meta-analysis. Am J Clin Nutr. 2009;90(6):1674–1692. doi: 10.3945/ajcn.2009.27799. [DOI] [PubMed] [Google Scholar]
- Dool H, Kratz P. Generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J Chromatogr. 1963;2:463–470. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- Endo H, Ohno M, Tanji K, Shimada S, Kaneko K. Effect of heat treatment on the lipid peroxide content and aokusami (beany flavor) of soymilk. Food Sci Technol Res. 2004;10(3):328–333. doi: 10.3136/fstr.10.328. [DOI] [Google Scholar]
- Fu XJ, Xia SQ (2011) Effect of soaking on the quality of house-made soymilk. In: 2011 international conference on IEEE new technology of agricultural engineering (ICAE)
- Grosh W. Detection of potent odorants in food by aroma extract dilution analysis. Trends Food Sci Technol. 1993;41:68–73. doi: 10.1016/0924-2244(93)90187-F. [DOI] [Google Scholar]
- Kim Y, Kim C. Effects of extraction methods and heating times on physicochemical properties of soymilk. Korean Soybean Dig. 1999;16:40–55. [Google Scholar]
- Kim H, Cadwallader KR, Jeong EJ, Cha YJ. Effect of refrigerated and thermal storage on the volatile profile of commercial aseptic Korean soymilk. J Food Sci Nutr. 2009;14(1):76–85. [Google Scholar]
- Kobayashi A, Tsuda Y, Hirata N, Kubota K, Kitamura K. Aroma constituents of soybean [Glycine max (L.) Merrill.] milk lacking lipoxygenase isozymes. J Agric Food Chem. 1995;43:2449–2452. doi: 10.1021/jf00057a025. [DOI] [Google Scholar]
- Kumazawa K, Nishimura O. Studies on the key aroma compounds in soymilk made from three different soybean cultivars. J Agric Food Chem. 2011;59:12204–12209. doi: 10.1021/jf202942h. [DOI] [PubMed] [Google Scholar]
- Lei Q, Boatright W. Compounds constituting the odor of aqueous slurries of soy protein concentrate. J Food Sci. 2001;66:1306–1310. doi: 10.1111/j.1365-2621.2001.tb15206.x. [DOI] [Google Scholar]
- Li H, Peng J, Zhao G. Effect of different drying methods on aromatic composition of Okara as determined by headspace SPME–GC–MS. Food Sci. 2012;33:167–172. [Google Scholar]
- Lozano P, Drake M, Benitez D, Cadwallader K. Instrumental and sensory characterization of heat-induced odorants in aseptically packaged soy milk. J Agric Food Chem. 2007;55(8):3018–3026. doi: 10.1021/jf0631225. [DOI] [PubMed] [Google Scholar]
- Lv Y, Song H, Li X, Wu L, Guo S. Influence of blanching and grinding process with hot water on beany and non-beany flavor in soymilk. J Food Sci. 2011;76(1):20–25. doi: 10.1111/j.1750-3841.2010.01947.x. [DOI] [PubMed] [Google Scholar]
- Man YBC, Wei LS, Nelson AI, Yamashita N. Effects of soaking soybeans in dilute acids on biologically active components. J Am Oil Chem Soc. 1991;68:471–473. doi: 10.1007/BF02663815. [DOI] [Google Scholar]
- Min S, Yu Y, Yoo S, St. Martin S. Effect of soybean varieties and growing locations on the flavor of soymilk. J Food Sci. 2005;70:C1–C7. doi: 10.1111/j.1365-2621.2005.tb09009.x. [DOI] [Google Scholar]
- Orthoefer FT, Liu K. Soybeans for food uses. Food Mark Technol. 1995;9:4–8. [Google Scholar]
- Rychlik M, Schieberle P, Grosch W. Compilation of odor thresholds, odor qualities and retention indices of key food odorants. Garching: Deutche Forschungsanstalt fur Lebensmittelchemie; 1998. [Google Scholar]
- Sacks F, Lichtenstein A, van Horn L, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: a summary of a statement for professionals from the American Heart Association Nutrition Committee. Arterioscler Thromb Vasc Biol. 2006;26:1689–1692. doi: 10.1161/01.ATV.0000227471.00284.ef. [DOI] [PubMed] [Google Scholar]
- Wettasinghe M, Vasanthan T, Temelli F, Swallow K. Volatile flavor composition of cooked by-product blends of chicken, beef and pork: a quantitative GC–MS investigation. Food Res Int. 2001;34:149–158. doi: 10.1016/S0963-9969(00)00146-0. [DOI] [Google Scholar]
- Yuan S, Chang S. Selected odor compounds in soymilk as affected by chemical composition and lipoxygenases in five soybean materials. J Agric Food Chem. 2007;55:426–431. doi: 10.1021/jf062274x. [DOI] [PubMed] [Google Scholar]