Abstract
World’s vegetable oil demand is increasing day by day and oil seed supply is limited to a dozen oil seed crops on commercial scale. Efforts were made to explore the potential of water melon a traditionally grown native crop of Indian arid zone having oil content over 30% and seed yield potential of 500–600 kg per hectare under rainfed conditions. An analysis was carried out to explore the suitability of watermelon [Citrullus lanatus (Thunb.)] oil for human consumption on the basis of fatty acid (FA) composition in selected genotypes. Total oil content ranged between 10.0 and 31.0%. Eleven FA were identified in seed oil. Linoleic, stearic, palmitic and oleic acid were found as major FA while myristic, heptadecanoic, arachidic, 9-hexadecenoic and 14-eicosenoic acid was present in traces. Linoleic acid single polyunsaturated FA contributor found in the range of 43.95% (WM-44) to 55.29% (WM-18). Saturated FA content ranged between 32.24 and 37.61%. Significant genetic variation was observed for mono-unsaturated FA. Metabolic capacity to inter-conversion of FA and nutritive value of watermelon oil was described on the basis of ratio of FA group. Total phenolics, antioxidant activity, peroxide value and oxidizability were also estimated along with oxidative stability of oil. Multivariate analysis showed that, oil content has positive correlation with linoleic acid. The Euclidean based UPGMA clustering revealed that genotypes WM-18 is most suitable for trait specific breeding program for high linoleic acid (n–6), desaturation ratio and oleic desaturation ratio with higher oil content and lowest palmitic acid.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3074-5) contains supplementary material, which is available to authorized users.
Keywords: Fatty acids, Antioxidant activity, Edible oil, Correlation
Introduction
Vegetable oils account for 80% of the world’s natural oils and fat supply with increasing importance in nutrition and industries owing to their dietary energy, antioxidant, bio-fuels and raw materials potentials for oleochemicals, lubricants, pharmaceuticals and cosmetics (Fasina and Colley 2008). Only 12 of the 5,00,000 known plant species are currently commercially exploited to produce vegetable oils in order to meet the world’s increasing demand (Mabaleha et al. 2007). In developing countries like India, more than 50% of domestic vegetable oil demand is being met through imports (14.0 million tons costing over Rs. 10,000 million USD) besides fourth largest oilseed producing country in the world after USA, China and Brazil. Thus, there is urgent need to identify native crops having potential to produce oil in substantial quantity for commercial exploitation.
Watermelon [Citrullus lanatus (Thunb.) Matsumura & Nakai] cultivated in warmer parts all over the world (Robinson and Decker-Walters 1997; Jeffrey 2001) for fresh consumption of the juicy and sweet flesh of mature fruit. But throughout sub-Saharan Africa and arid regions of India, it is being grown in mixed cropping under resource constraint situations of rainfed agriculture to minimize the risk of crop failure under aberrant weather situations for its highly priced seeds. The seeds of watermelon are rich source of protein 25–37% and oil 37.8–45.4% (Ziyada and Elhussien 2008). The watermelon seed contains about 28% crude fat and 23% crude protein; the corresponding values for the kernel are about 49 and 40%, respectively (Das et al. 2002). Watermelon seed oils, often characterized by high-linoleic acid content (> 60%) and is used for oil production at the subsistence level in different parts of the world such as West Africa and the Middle East (Ziyada and Elhussien 2008; Baboli and Kordi 2010; Jarret and Levy 2012). Seeds of watermelon are rich in natural antioxidant and phytochemicals such as flavonoids, vitamin C, thiamine, riboflavin and polyphenolic compounds.
In recent past, watermelon seeds demand is increasing day by day due to people’s health concerns and changing food habits. Further, such crops support livelihood in the hostile situations where commercial crop diversification is not feasible. Therefore, concept of seed purpose watermelon in Indian Thar Desert has been advocated by Mahla and Choudhary (2013) following the rigorous evaluation of watermelon germplasm under rainfed condition. The genotypes provided seed yield of 700–800 kg per hectare with 28–35% oil content (Mahla et al. 2014). There are meager reports on extent of genotypic variability in cultivated genotypes of seed purpose watermelon in India for fatty acid and other quality parameters for edible use. Hence, present study has been conducted to explore the genetic variation in selected genotypes on the basis of oil, fatty acids and their oxidative stability. This would help introduce novelty for developing new cultivars from non-established oilseed crops that too from the plant part hitherto been considered wastes, for innovative end uses. Therefore, an attempt was made to analyze fatty acid profile and other quality parameters of watermelon seed oil for its commercial exploitation.
Materials and methods
One hundred and fifty germplasm accessions including exotic egusi types (50), local landraces (30) and elite breeding lines (70) were initially evaluated at Central Arid Zone Research Institute, Regional Research Station, Jaisalmer for seed yield and further, oil content was estimated at National Research Centre on Seed Spices, Tabiji, Ajmer, Rajasthan India. Subsequently, selected fifteen genotypes (10 for higher oil content and five with low oil content) were analyzed for fatty acid profile and oxidizing stability of oil to evaluate its suitability for edible purpose (Supplementary Table 1).
Oil extraction and FAME analysis
All reagents and fatty acid standards used in the analysis were of analytical grade. Oil content was measured using accelerated solvent extraction system (Dionex India Pvt. Ltd.) which accelerates the traditional extraction process by using solvent at elevated temperatures and pressures. Oil was obtained after evaporating the solvent in rotary evaporator. Thirty gram seed powder was utilized for oil extraction and n-hexene was used as solvent.
Fatty Acid Methyl Esters (FAME) were prepared according to AOCS Method CE 1–62. Diluted FAME were separated on an Agilent Series GC–MS (Agilent, USA; GC-7820 A, MS-5975) equipped with an HP5 (Universal column) (30 m × 0.325 mm × 0.25 µm); Agilent J & W GC column with an auto sampler. A sample of 1 µl was used in split mode (20:1) with an auto sampler. Helium was used as the carrier gas at a flow rate of 1.0 ml/min. The column temperature was programmed from 50 to 280 °C with equilibrium time of 3 min, held for 30 min. Injector temperatures were set at 250 °C. The fatty acids were identified by a comparison of their retention indices and their identification was confirmed by computer matching of their mass spectral fragmentation patterns of compounds in the NIST-MS library and published mass spectra with the help of Chemstation software (Agilent Technologies, USA).
Phenol, flavonoid, antioxidant activity and scavenging capacity
Total phenol concentrations were determined using a Folin–Ciocalteu assay, as described by Amin et al. (2006). Total flavonoid concentration was determined by using previously reported method by Chang et al. (2002). The antioxidant activity of oil extract was evaluated on the basis of its activity in scavenging the stable DPPH radical using the method described by Shimada et al. (1992). Oil extract was diluted in methanol to give at least 5 different concentrations. An aliquot (1, 1.5, 2, 2.5 ml) of the oil extract of each concentration was mixed with 1 ml of 1 M DPPH solution. The mixture was then homogenized and left to stand for 30 min in the dark. The absorbance was measured at 517 nm against a blank of methanol using a spectrophotometer. DPPH solution plus methanol was used as control and Butyl hydroxyl toluene (BHT) was used as a standard reference synthetic antioxidant with R2 value ranged from 0.95 to 0.99. Results were expressed as µg Butyl hydroxyl toluene (BHT) Equivalent/ml oil.
Results were expressed as a mean standard deviation from three replicate measurements. The percent scavenging effect was calculated as follows:
Oxidative stability and fatty acid ratio
Peroxide value of the watermelon seed oil extract was determined as per standard methods (AOAC 2005). The ratio between polyunsaturated fatty acids (PUFA) and saturated fatty acids (SFA), known as polyene index (PI), was measured according to Mendez et al. (1996). The oxidizability is the primary factor explaining differences in induction time (McCormick et al. 2007), is defined as follows:
Elongation ratio (ER), and desaturation ratio (DR) was based on the formula suggested by Velasco et al. (1998) while oleic desaturation ratio (ODR) were calculated as per Pleines and Friedt (1988) with modifications.
Ratio of MUFA/SFA (M/S), PUFA/SFA(P/S), PUFA/MUFA (P/M) and (PUFA + MUFA)/SFA (M + P/S) ratio have been worked out to analyze the potential of individual genotype for nutritional importance (Badr et al. 2014).
Statistical analysis
Data were analyzed in completely randomized design (CRD) and correlation coefficients for fatty acid were calculated as suggested by Panse and Sukhatme (1978). Experiment was conducted in triplicate. Pair wise fatty acid diversity for fifteen genotypes compared by a Euclidean distance matrix and dendrogram were prepared using XLSTAT software version 2015. The same software was used to perform principal component analysis (PCA). Cluster analysis was performed based on the genetic distance matrices generated by the Euclidean distance method to reveal the patterns of genetic relationships among genotypes.
Results and discussion
Total oil was estimated from the seeds of 150 germplasm lines of seed purpose watermelon through extraction process. Further, the fatty acid and other chemical characteristics were analyzed on ten foremost oil containing genotypes and five having least oil content. Analysis of variance as per completely randomized design showed the significant differences among the watermelon genotypes for total oil, FAME and all characteristics (Supplementary Table 2). It clearly shows that variability exists among the genotypes for total oil content, component fatty acids and their characteristics except TUFA.
Total oil and fatty acids
Hexane-extracted oil contents from watermelon seeds varied from 10.0 to 31.0% (g 100 g−1 dried seed weight basis) with a mean of 20.5% among the genotype tested. The highest (31.0%) oil yield was exhibited by watermelon genotype WM-23, whereas the lowest (10.0%) by genotype WM-51 (Table 1) showing significant variation (p < 0.05) among the genotypes which might be linked to their differing genetic makeup. The oil contents in the analyzed watermelon genotypes were in close agreement with the previous reports of Das et al. (2002); Raziq et al. (2012); Oluba et al. (2008); Jarret and Levy (2012). Although oil recovery was lower in comparison to reports of Baboli and Kordi (2010); Edidiong and Ubong (2013) and Jacob et al. (2015). Such variation in oil yield from different regions might be attributed to the genetic, seed quality or varied agroclimatic conditions. The levels of seed oil contents (28.3–35.7%) were found to exceed those of three other conventional oilseed crops including cotton seed (15.0–24.0%), soybean (17.0–21.0%) and olive (20.0–25.0%) (Pritchard 1991).
Table 1.
Total oil content and fatty acid composition of watermelon genotypes
| Genotypea | Oil ± SD | 14:0 ± SD | 16:0 ± SD | 16:1 ± SD | 17:0 ± SD | 18:0 ± SD | 18:1 ± SD | 18:2 ± SD | 20:0 ± SD | 20:1 ± SD | 22:0 ± SD | 24:0 ± SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WM-23 | 31.0 ± 1.7 | 0.09 ± 0.01 | 16.6 ± 1.10 | 0.24 ± 0.01 | 0.16 ± 0.02 | 18.7 ± 1.39 | 13.2 ± 1.27 | 48.8 ± 2.21 | 1.19 ± 0.12 | 0.25 ± 0.03 | 0.16 ± 0.01 | 0.16 ± 0.02 |
| WM-17 | 30.2 ± 1.9 | 0.09 ± 0.00 | 18.8 ± 3.07 | 0.27 ± 0.00 | 0.18 ± 0.00 | 17.2 ± 1.39 | 10.6 ± 0.94 | 51.2 ± 2.00 | 1.01 ± 0.14 | 0.35 ± 0.03 | 0.17 ± 0.02 | 0.17 ± 0.01 |
| WM-18 | 29.4 ± 1.8 | 0.10 ± 0.00 | 14.6 ± 0.75 | 0.24 ± 0.01 | 0.21 ± 0.01 | 17.2 ± 0.97 | 10.5 ± 0.27 | 55.3 ± 0.99 | 1.02 ± 0.13 | 0.35 ± 0.07 | 0.17 ± 0.02 | 0.29 ± 0.01 |
| WM-20 | 28.3 ± 1.0 | 0.08 ± 0.01 | 19.5 ± 1.33 | 0.26 ± 0.00 | 0.15 ± 0.00 | 17.2 ± 1.39 | 12.5 ± 0.93 | 48.4 ± 3.19 | 0.81 ± 0.06 | 0.31 ± 0.04 | 0.11 ± 0.03 | 0.12 ± 0.02 |
| WM-65 | 27.6 ± 1.8 | 0.10 ± 0.01 | 16.3 ± 1.11 | 0.57 ± 0.01 | 0.20 ± 0.01 | 19.3 ± 1.23 | 15.1 ± 1.37 | 45.4 ± 1.78 | 1.24 ± 0.14 | 0.35 ± 0.03 | 0.21 ± 0.03 | 0.19 ± 0.01 |
| WM-73 | 27.1 ± 0.9 | 0.08 ± 0.00 | 17.0 ± 1.04 | 0.30 ± 0.01 | 0.16 ± 0.03 | 18.2 ± 1.10 | 18.3 ± 0.65 | 44.0 ± 5.46 | 0.96 ± 0.10 | 0.23 ± 0.02 | 0.13 ± 0.18 | 0.18 ± 0.02 |
| WM-21 | 26.5 ± 1.2 | 0.00 ± 0.00 | 16.9 ± 0.92 | 0.26 ± 0.02 | 0.15 ± 0.01 | 14.1 ± 1.28 | 15.5 ± 1.29 | 51.7 ± 2.61 | 0.82 ± 0.06 | 0.27 ± 0.03 | 0.12 ± 0.02 | 0.15 ± 0.02 |
| WM-55 | 26.4 ± 1.4 | 0.00 ± 0.00 | 16.0 ± 1.57 | 0.25 ± 0.01 | 0.18 ± 0.01 | 18.5 ± 1.01 | 12.7 ± 1.4 | 49.5 ± 2.59 | 1.20 ± 0.15 | 0.24 ± 0.03 | 0.21 ± 0.01 | 0.25 ± 0.03 |
| WM-105 | 26.3 ± 2.3 | 0.09 ± 0.00 | 16.1 ± 0.25 | 0.25 ± 0.01 | 0.16 ± 0.01 | 16.6 ± 0.90 | 13.5 ± 1.44 | 51.0 ± 4.42 | 1.02 ± 0.10 | 0.32 ± 0.03 | 0.17 ± 0.04 | 0.23 ± 0.02 |
| WM-19 | 25.9 ± 1.6 | 0.08 ± 0.01 | 16.9 ± 1.10 | 0.37 ± 0.01 | 0.16 ± 0.00 | 17.2 ± 1.26 | 13.4 ± 1.57 | 49.9 ± 3.61 | 0.88 ± 0.12 | 0.31 ± 0.03 | 0.13 ± 0.02 | 0.20 ± 0.02 |
| WM-98 | 13.9 ± 1.0 | 0.10 ± 0.00 | 16.8 ± 1.10 | 0.28 ± 0.01 | 0.18 ± 0.00 | 18.2 ± 1.35 | 16.5 ± 0.76 | 45.6 ± 1.90 | 1.16 ± 0.16 | 0.26 ± 0.03 | 0.19 ± 0.01 | 0.17 ± 0.02 |
| WM-51 | 10.0 ± 0.7 | 0.08 ± 0.01 | 18.2 ± 1.01 | 0.26 ± 0.01 | 0.13 ± 0.01 | 17.2 ± 0.97 | 14.0 ± 0.82 | 48.3 ± 3.84 | 0.75 ± 0.13 | 0.20 ± 0.01 | 0.09 ± 0.01 | 0.10 ± 0.02 |
| WM-44 | 12.0 ± 0.8 | 0.12 ± 0.01 | 16.9 ± 1.25 | 0.29 ± 0.01 | 0.16 ± 0.01 | 17.1 ± 0.82 | 19.5 ± 2.51 | 43.9 ± 1.15 | 1.17 ± 0.17 | 0.24 ± 0.06 | 0.24 ± 0.01 | 0.26 ± 0.03 |
| WM-124 | 12.3 ± 1.7 | 0.12 ± 0.00 | 16.1 ± 0.93 | 0.32 ± 0.01 | 0.17 ± 0.01 | 18.1 ± 1.40 | 14.2 ± 1.73 | 47.6 ± 2.84 | 1.49 ± 0.19 | 0.36 ± 0.05 | 0.27 ± 0.03 | 0.27 ± 0.02 |
| WM-150 | 12.4 ± 1.0 | 0.00 ± 0.00 | 15.9 ± 0.89 | 0.22 ± 0.06 | 0.17 ± 0.00 | 17.2 ± 0.84 | 14.3 ± 1.88 | 49.8 ± 2.61 | 1.04 ± 0.11 | 0.29 ± 0.05 | 0.17 ± 0.02 | 0.34 ± 0.07 |
aWM watermelon, SD standard deviation, 14:0: myristic acid, 16:0: palmitic acid; 16:1: hexadecenoic acid; 17:0: margaric acid; 18:0: stearic acid; 18:1: oleic acid; 18:2: linoleic acid; 20:0: arachidic acid; 20:1: 11,14-eicosadienoic acid; 22:0: docosanoic acid and 24:0: tetracosanoic acid
The indigenous collection with the mean oil content of 19.6% lagged behind exotic collections (21.8%) and advanced lines from crosses (20.8%). The advanced breeding lines involving the exotic parent EC 677165 had more oil content compared to lines derived from crosses among indigenous parents. High oil content in exotic types might be due to their thin coat as Jarret and Levy (2012) reported negative correlation of oil content to the proportionate contribution of hull in the seed. Indian collections have thick coat, contributing to about 30–35% of total seed weight as against 10–15% in thin coated exotic collections (unpublished data). Consequently, all the high oil containing lines (10) were derived from a cross involving one exotic parent with most of the lines (6) represented by a single cross IC-449393 × EC-677165 (WM-17, WM-18, WM-19, WM-20, WM-21 and WM-73) providing an added advantage to derive information on the possibility of generating diversity through hybridization program.
FAME analysis revealed the presence of eleven fatty acids in watermelon seed oil. Linoleic acid (18:2 n–6), stearic acid (18:0), palmitic acid (16:0) and oleic acid (18:1 n–9) were the pre- dominant fatty acids present in watermelon seed oil (Table 1). Linoleic acid, stearic acid, palmitic acid and oleic acid were reported as the major fatty acids in watermelon by Das et al. (2002); Sabahelkhier et al. (2011); Raziq et al. (2012); Jarret and Levy (2012); Edidiong and Ubong (2013) and Jacob et al. (2015); however in variable ratios. Other fatty acids viz, myristic acid (14:0), 9-hexadecenoic acid (16:1 n–7), heptadecanoic acid (17:0), Arachidic (20:0), 11-eicosadienoic acid (20:1 n–9), behenic acid (22:0) and tetracosanoic acid were also present in watermelon seed oil.
Perusal of the data (Table 2) revealed a significant variation in SFA content varying from 32.24% (WM-21) to 37.99% (WM-20) with an average availability of 35.97%. The level of unsaturated fatty acid have been reported to about 70% or more (Oyolu and Mcfarlane 1982; Baboli and Kordi 2010; Sabahelkhier et al. 2011; Jarret and Levy 2012). Among SFA, stearic acid contributed maximum in the range of 14.1% (WM-21) to 19.3% (WM-65) followed by palmitic acid (14.6% in WM-18 to 19.5% in WM-20) (Table 1). Myristic acid (14:0) and heptadecanoic were observed below 1% in all watermelon genotypes, while arachidic (20:0) varied from 0.75 to 1.49%. Amount with some variation and type of SFA in present study are in lieu with the results presented by previously workers Das et al. (2002); Kamel et al. (1985); Baboli and Kordi (2010); Raziq et al. (2012). Being high in stearic acid, watermelon oil is healthier to other fats rich in saturated fatty acids. It also lacks medium chain saturated fatty acids that are implicated in the induction of arteriosclerosis and hence better alternative to palm kernel and coconut oil (Katan et al. 1995).
Table 2.
Fatty acid groups and fatty acid ratios for chain elongation and desideration in watermelon genotypes
| Genotypea | SFA ± SD | MUFA ± SD | PUFA ± SD | TUFA ± SD | ER ± SD | DR ± SD | ODR ± SD |
|---|---|---|---|---|---|---|---|
| WM-23 | 37.07 ± 2.63 | 13.72 ± 1.30 | 48.79 ± 2.21 | 62.51 ± 3.51 | 0.004 ± 0.0 | 0.784 ± 0.01 | 0.787 ± 0.01 |
| WM-17 | 37.61 ± 1.68 | 11.17 ± 0.91 | 51.20 ± 2.00 | 62.37 ± 1.09 | 0.006 ± 0.0 | 0.824 ± 0.02 | 0.829 ± 0.02 |
| WM-18 | 33.56 ± 1.75 | 11.06 ± 0.66 | 55.29 ± 3.84 | 66.36 ± 3.55 | 0.005 ± 0.0 | 0.836 ± 0.01 | 0.841 ± 0.01 |
| WM-20 | 37.99 ± 0.02 | 13.04 ± 0.90 | 48.39 ± 3.19 | 61.43 ± 2.29 | 0.005 ± 0.0 | 0.790 ± 0.02 | 0.795 ± 0.02 |
| WM-65 | 37.57 ± 2.44 | 16.06 ± 1.34 | 45.42 ± 1.78 | 61.48 ± 3.11 | 0.006 ± 0.0 | 0.746 ± 0.01 | 0.750 ± 0.01 |
| WM-73 | 36.68 ± 0.14 | 18.85 ± 0.68 | 44.04 ± 5.46 | 62.89 ± 4.78 | 0.004 ± 0.0 | 0.701 ± 0.03 | 0.704 ± 0.03 |
| WM-21 | 32.24 ± 2.17 | 16.04 ± 1.29 | 51.69 ± 2.61 | 67.74 ± 3.89 | 0.004 ± 0.0 | 0.766 ± 0.01 | 0.769 ± 0.01 |
| WM-55 | 36.38 ± 0.46 | 13.25 ± 1.12 | 49.51 ± 2.59 | 62.76 ± 3.70 | 0.004 ± 0.0 | 0.792 ± 0.01 | 0.795 ± 0.01 |
| WM-105 | 34.32 ± 0.61 | 14.11 ± 1.46 | 50.98 ± 4.42 | 65.09 ± 2.95 | 0.005 ± 0.0 | 0.785 ± 0.03 | 0.789 ± 0.03 |
| WM-19 | 35.50 ± 2.45 | 14.11 ± 1.53 | 49.89 ± 3.61 | 64.00 ± 5.14 | 0.005 ± 0.0 | 0.784 ± 0.01 | 0.788 ± 0.01 |
| WM-98 | 36.85 ± 0.37 | 17.03 ± 0.78 | 45.60 ± 1.90 | 62.63 ± 1.14 | 0.004 ± 0.0 | 0.729 ± 0.02 | 0.732 ± 0.02 |
| WM-51 | 36.57 ± 1.82 | 14.48 ± 0.83 | 48.26 ± 0.99 | 62.73 ± 1.81 | 0.003 ± 0.0 | 0.772 ± 0.02 | 0.774 ± 0.02 |
| WM-44 | 35.88 ± 0.55 | 20.04 ± 2.63 | 43.95 ± 1.15 | 63.99 ± 3.75 | 0.004 ± 0.0 | 0.690 ± 0.02 | 0.693 ± 0.02 |
| WM-124 | 36.55 ± 0.32 | 14.91 ± 1.76 | 47.64 ± 2.84 | 62.54 ± 4.60 | 0.006 ± 0.0 | 0.766 ± 0.01 | 0.771 ± 0.01 |
| WM-150 | 34.76 ± 0.02 | 14.79 ± 1.77 | 49.81 ± 2.61 | 64.60 ± 4.38 | 0.005 ± 0.0 | 0.775 ± 0.01 | 0.778 ± 0.01 |
aWM watermelon, SFA saturated fatty acids, MUFA mono-unsaturated fatty acids, PUFA poly-unsaturated fatty acids, TUFA total unsaturated fatty acids, ER elongation ratio (ER), DR desaturation ratio (DR), ODR oleic desaturation ratio(ODR), SD standard deviation
The MUFA content ranged from 11.06% in genotype WM-18 to 20.04% (WM-44). Among MUFA, oleic acid, a member of n-9 group was dominant fatty acid, ranging from a minimum of 10.5% (WM-18) to a maximum of 19.5% (WM-44), while other MUFA below 1% (Table 1). Level of MUFA in present study was similar to reports by Oluba et al. (2008) and Raziq et al. (2012). The high content of monounsaturated fatty acids (MUFAs) especially oleic acid (18:1) is associated with decreased total cholesterol and low-density lipoprotein cholesterol (Dennys et al. 2006), thus low incidence of coronary heart disease (CHD).
Similarly, PUFA were the dominant fatty acids in watermelon seed oil and linoleic acid was the only representatives of this group. However, it was most abundant among all the fatty acids ranging from 43.95% (WM-44) to 55.29% (WM-18) with an average of 48.70% (Table 2). TUFA varied from 61.43% (WM-20)–67.74% (WM-21) with an average of 63.54%. It is reported that linoleic acid is one of the most significant PUFA in human diet due to its ability of preventing heart and vascular diseases. Some fatty acids myristic (14:0), palmitoleic (16:1), arachidic (C20:0) ecosadienoic acid (20:1 n–9), (22:0) and (24:0) are present in our study as minor fatty acids, but these fatty acids were not reported earlier and might be included in other fatty acids due to their minute quantity. However, the availability of linolenic, myristic and lauric acids in traces was also reported by Mabaleha et al. (2007). Presence of these fatty acids might be due to genetic, seed quality or seed production environment and the efficacy of the analytical system applied.
Phenol, flavonoid contents, antioxidant activity and scavenging capacity
All the watermelon genotypes showed good amount of phenolic and flavonoid content in seed oil (Table 3). Total phenolic content was ranging from a minimum of 28.2 µg GA E/ml (WM-44) to a maximum of 44.4 µg GA E/ml (WM-65) with an average of 35.72 µg GA E/ml while flavanoid content was observed minimum in WM-150 (29.7 µg QE Eq/ml) and maximum in WM-17 (136.1 µg QE Eq/ml). Whereas total antioxidant activity was maximum in WM-17 (144 µg BHT Eq/ml) and minimum in WM-44 (109.1 µg BHT Eq/ml) with an average of 130.20 µg BHT Eq/ml. Free radical scavenging capacity was exhibited maximum by the genotype WM-17 (25.4%) while minimum in WM-98 (12.5%). Results indicated that watermelon seed oil possesses significant antioxidant activity. Presence of phenolic and flavonoid contents positively contributes to antioxidant activity (Table 3). Thus the antioxidant activity may be attributed to vitamin E in oil or positive correlation with phenolics (Mahatma et al. 2016). Misuna et al. (2008) observed that the antioxidant capacity was closely related to the content of phenolic compounds in peanut. A strong correlation (r2 value 0.966) was observed between phenolics with antioxidant capacity in legumes (Marathe et al. 2011).
Table 3.
Fatty acid ratios for nutritive value and oxidizing satiability attributes in oil of watermelon genotypes
| Genotype | M/S ± SD | P/S (PI) ± SD | P/M ± SD | M + P/S ± SD | PH1 ± SD | FC2 ± SD | AO3 ± SD | SC4 ± SD | PV5 ± SD | OZY6 ± SD |
|---|---|---|---|---|---|---|---|---|---|---|
| WM-23 | 0.366 ± 0.01 | 1.305 ± 0.03 | 3.567 ± 0.18 | 1.671 ± 0.03 | 32.9 ± 1.3 | 71.6 ± 8.1 | 133.7 ± 6.3 | 18.6 ± 1.5 | 8.3 ± 0.4 | 0.491 ± 0.02 |
| WM-17 | 0.298 ± 0.04 | 1.362 ± 0.04 | 4.613 ± 0.56 | 1.660 ± 0.05 | 35.5 ± 1.5 | 136.1 ± 6.4 | 144.0 ± 8.0 | 25.4 ± 1.1 | 8.2 ± 0.6 | 0.514 ± 0.02 |
| WM-18 | 0.316 ± 0.02 | 1.426 ± 0.18 | 4.515 ± 0.54 | 1.742 ± 0.20 | 41.1 ± 2.2 | 113.7 ± 6.7 | 137.4 ± 8.4 | 25.2 ± 1.1 | 9.4 ± 0.5 | 0.555 ± 0.01 |
| WM-20 | 0.343 ± 0.02 | 1.274 ± 0.09 | 3.734 ± 0.50 | 1.617 ± 0.06 | 29.2 ± 1.4 | 118.4 ± 7.4 | 143.7 ± 6.3 | 17.5 ± 1.7 | 8.4 ± 0.3 | 0.486 ± 0.03 |
| WM-65 | 0.430 ± 0.06 | 1.214 ± 0.13 | 2.836 ± 0.13 | 1.645 ± 0.20 | 44.4 ± 1.9 | 93.0 ± 4.5 | 137.3 ± 7.3 | 18.5 ± 1.5 | 8.5 ± 0.3 | 0.457 ± 0.02 |
| WM-73 | 0.516 ± 0.02 | 1.201 ± 0.01 | 2.334 ± 0.37 | 1.717 ± 0.14 | 38.7 ± 1.9 | 74.1 ± 5.2 | 140.4 ± 7.6 | 21.2 ± 2.3 | 8.1 ± 0.5 | 0.444 ± 0.06 |
| WM-21 | 0.497 ± 0.01 | 1.605 ± 0.03 | 3.227 ± 0.10 | 2.102 ± 0.02 | 37.3 ± 1.7 | 94.9 ± 5.4 | 133.4 ± 9.4 | 20.1 ± 1.8 | 9.4 ± 0.6 | 0.520 ± 0.3 |
| WM-55 | 0.364 ± 0.03 | 1.361 ± 0.05 | 3.743 ± 0.12 | 1.725 ± 0.08 | 39.5 ± 2.0 | 69.6 ± 2.8 | 126.1 ± 6.1 | 18.6 ± 2.1 | 9.4 ± 0.3 | 0.498 ± 0.03 |
| WM-105 | 0.412 ± 0.05 | 1.484 ± 0.10 | 3.661 ± 0.70 | 1.896 ± 0.05 | 42.4 ± 1.9 | 63.5 ± 3.3 | 132.6 ± 8.6 | 20.2 ± 2.2 | 8.8 ± 0.4 | 0.513 ± 0.04 |
| WM-19 | 0.401 ± 0.07 | 1.414 ± 0.20 | 3.545 ± 0.13 | 1.815 ± 0.27 | 35.5 ± 1.7 | 80.9 ± 5.3 | 138.7 ± 6.3 | 19.8 ± 1.6 | 8.5 ± 0.4 | 0.502 ± 0.04 |
| WM-98 | 0.467 ± 0.02 | 1.238 ± 0.06 | 2.658 ± 0.23 | 1.705 ± 0.05 | 28.3 ± 1.5 | 67.4 ± 6.8 | 117.1 ± 3.0 | 12.5 ± 1.9 | 8.7 ± 0.4 | 0.459 ± 0.02 |
| WM-51 | 0.397 ± 0.04 | 1.515 ± 0.10 | 3.826 ± 0.15 | 1.913 ± 0.15 | 33.6 ± 1.4 | 133.3 ± 7.2 | 121.3 ± 2.8 | 14.5 ± 1.7 | 9.3 ± 0.5 | 0.485 ± 0.04 |
| WM-44 | 0.549 ± 0.07 | 1.225 ± 0.01 | 2.251 ± 0.23 | 1.774 ± 0.08 | 28.2 ± 2.0 | 50.9 ± 3.9 | 109.1 ± 6.4 | 16.8 ± 1.4 | 8.9 ± 0.5 | 0.443 ± 0.01 |
| WM-124 | 0.408 ± 0.05 | 1.304 ± 0.09 | 3.211 ± 0.20 | 1.712 ± 0.14 | 29.9 ± 2.5 | 54.5 ± 3.5 | 118.0 ± 3.5 | 13.8 ± 1.6 | 9.6 ± 0.5 | 0.479 ± 0.03 |
| WM-150 | 0.426 ± 0.05 | 1.433 ± 0.08 | 3.385 ± 0.23 | 1.858 ± 0.13 | 39.3 ± 1.2 | 29.7 ± 4.3 | 120.2 ± 5.2 | 16.8 ± 1.2 | 9.4 ± 0.4 | 0.501 ± 0.03 |
aWM watermelon, M mono-unsaturated fatty acids, S saturated fatty acids, P poly-unsaturated fatty acids, (1) PH expressed as Phenol µg GA Eq/ml, (2) flavanoid content (FC) expressed as µg QE Eq/ml, (3) anti-oxidant activity (AO) µg BHT Eq/ml, (4) free radical scavenging capacity (SC) expressed as (%), (5) peroxide value (PV) expressed as mequiv. O2 Kg−1oil and (6) oxidizability (OZY)
Peroxide value (PV), polyene index and oxidizability
PV was exhibited highest by the oil of WM-124 (9.6 mequiv. O2 Kg−1) and minimum by WM-73 (8.1 mequiv. O2 Kg−1 oil). The level of PV reported was in conformity with Oluba et al. (2008) and Sabahelkhier et al. (2011). Much lower PV viz. 3.24 and 2.80 m equiv. Kg−1 oil was reported by Baboli and Kordi (2010) and Egbuonu et al. (2015), respectively. A high peroxide value indicated oil with a high susceptibility to auto-oxidation due to the presence of moisture or trace elements and high degree of unsaturation (Adebisi and Olagunju 2011). Thus, the low peroxide value of the watermelon seed oil suggested its high degree of stability and non-susceptibility to oxidative rancidity. Very small amounts of secondary oxidation products were reported in Citrullus oil by Kamel et al. (1985) and Das et al. (2002) and good storability lasting up to 6 months by Teotia and Ramakrishna (1984). PV value of all watermelon genotypes falls well within the Codex standards for vegetable oils. These measurements indicate the amount of primary oxidation products and free fatty acid released by hydrolysis in watermelon seed oil was lower than sunflower seed oil and a little higher than soybean oil. The peroxide value of seed oil was lower than 10 mequiv. O2 Kg−1 oil required for standard oils (Ngassapa and Othman 2001) and could be suitable for human consumption.
Polyene Index (PI) was recorded highest in WM-21 (1.605) and minimum in WM-73 (1.201). The ratio between polyunsaturated fatty acids (PUFA) and saturated fatty acids (SFA) known as polyene index (PI), is usually taken as a measure of the extent of polyunsaturation of an oil and obviously, of its tendency to undergo autoxidation (Mendez et al. 1996). Oxidizability was observed maximum in WM-18 (0.555) and minimum in WM-44 (0.443). The coefficients for oleic (C18:1) and linoleic (C18:2) fatty esters are proportional to the relative rates of oxidation of these compounds. The polyunsaturated content (oxidizability) has the largest impact on oxidation of food oil (McCormick et al. 2007). Therefore, the better stability of oil extracted from genotype WM-44 compared to WM-18 (Table 3). The watermelon oil had higher oxidative stability (Egbuonu et al. 2015) which is higher than sunflower and less than soybean (Baboli and Kordi 2010).
Fatty acid ratio
To describe the biochemical capacities of metabolic pathway and suitability for human diet of watermelon oil, various FA ratios were calculated. Genotypes WM-51 showed minimum ER (0.003) while maximum (0.006) by the genotypes WM-17, WM-65 and WM-124. The lower ER estimates the relative weight of the elongation pathway from oleic acid to longer chain fatty acids i.e. C20, C22 fatty acids. Value of DR was however, maximum in WM-18 (0.836) and minimum in WM-44 (0.690) with an average of 0.769. DR is an estimate of relative weight of the desaturation pathway from oleic acid to PUFA i.e. LA which is more desirable for n–6 content. ODR value was observed maximum in WM-18 (0.841) genotype and minimum level was observed in WM-44 (0.693). ODR is the indicator of the efficiency of desaturation systems from C18:1 to C18:2 (Table 3). Highest linoleic acid might be due to higher DR and ODR value. These ratios are responsible for desaturation in fatty acids and conversion of oleic to linoleic acid. A high PUFA is due to a higher amount of its only one member (linoleic acid) and lower amount of MUFA is due to a lower amount of its main contributor oleic acid. Oxidizability is directly linked with higher amount of PUFA or linoleic acid.
To describe the potential of watermelon oil for human consumption various important FA ratios viz. M/S, P/S and (M + P)/S have been calculated and presented in Table 3. M/S ratio pointed minimum in WM-17 (0.298) and maximum in WM-44 (0.549). P/S, varied from 1.201 to 1.605 in WM-73 and WM-21, respectively. A wide range of P/M ratio from 2.251 (WM-44)–4.613 (WM-17) with an average of 3.406 was observed. Similarly, (M + P)/S ratio was found maximum in WM-21 and minimum in WM-20 (2.102 and 1.617, respectively). The higher ratios of P/S and (M + P)/S in WM-21 may be due to lower SFA and higher MUFA and PUFA content. A low M/S ratio and high (P + M)/S ratios are prerequisites for keeping plasma and liver cholesterol low (Chang and Huang 1998). Oils and fats with higher value of P/S index more than one are considered to have good nutritional value. Several studies indicated that higher value of P/S index means a smaller deposition of lipids in the body (Lawton et al. 2000). The diets high in a polyunsaturated-to-saturated fatty acid (P/S) ratio ameliorated insulin action in streptozotocin induced diabetic rats fed a high-fat diet, but a high P/S ratio diet enhances oxidative stress because PUFA are highly susceptible to lipid per-oxidation (Kang et al. 2005).
Correlation analysis
Oil content was positively correlated with linoleic acid (PUFA), while significantly negative associated with oleic acid and MUFA (Supplementary Table 4). Palmitic acid showed significant positive correlation with SFA, while negative correlation with 17:0, 24:0 and TUFA. Stearic acid exhibited a positive correlation with arachidic acid (20:0) and SFA, while it showed negative correlation with TUFA at higher level of significance. Being only major contributor oleic acid was positively correlated to MUFA and its desaturation to linoleic acid led to negative correlation with PUFA. Similarly, linoleic acid (PUFA) was positively associated with TUFA and negatively correlated with SFA and MUFA at higher level of significance. Progressive conversion led to positive correlation of 20:0 with 22:0 and 22:0 with 24:0. SFA exhibited a high significant negative correlation with TUFA (Supplementary Table 4). Two of the important fatty acids, oleic and linoleic, were negatively correlated indicating competitive efficiency of desaturation of oleic to linoleic acid. While, Oyolu and Mcfarlane (1982) reported positive correlation among palmitic and oleic acid and negative among palmitic and linoleic acid. However, no such correlations were observed by Jarret and Levy (2012). The variation among progenies of same cross further indicated possibility for manipulation of these ratios in desired direction.
Principal component analysis (PCA)
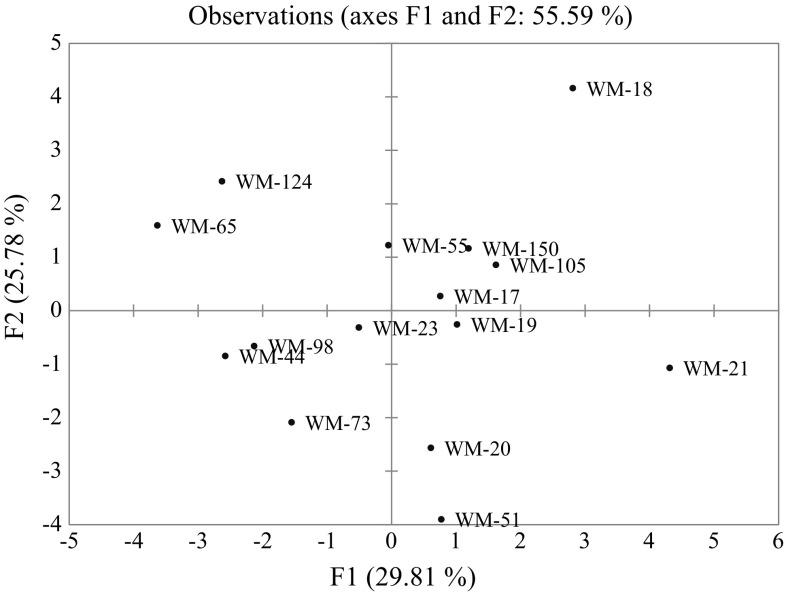
PCA was conducted using all the quality parameters studied in order to derive important components for graphical presentation of groups. First five components with Eigen values > 1 contributed 89.32% of variance for oil, FAME and fatty acid group in watermelon seed oil. A variation of 29.81% was explained by the first component with linoleic acid (PUFA), TUFA and oil main contributors, whereas negative contribution was observed for stearic acid, (14:0), (20:0), (22:0), oleic acid, MUFA and SFA (Supplementary Table 3). For PC2, the variability of 25.78% was observed and (17:0), (20:0), (20:1 n–9), (22:0) (24:0) and stearic acid contributed more positively, while palmitic acid contributed negatively. PC3, accounted for 19.77% variability with oleic acid, MUFA and TUFA positive contributors, while oil content and SFA negatively contributed (Fig. 1).
Fig. 1.
PCA plot of watermelon genotypes based on oil, FAME profile and fatty acid group
Cluster analysis
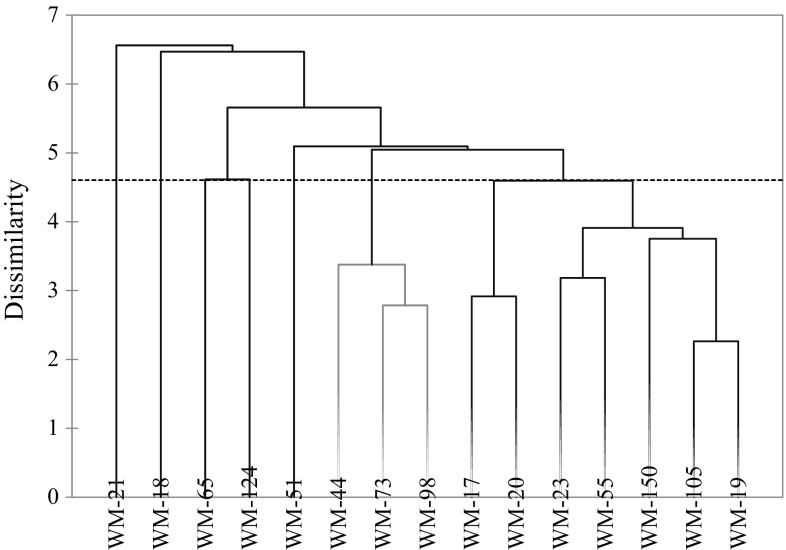
Clusters analysis was done on the basis of UPGMA. All studied genotypes were divided on 50% Euclidean distances into 7 clusters for total oil and FAME and their characteristics (Fig. 2). Cluster-I consisted of 7 genotypes (WM-23, WM-17, WM-20, WM-55, WM-105, WM-19, WM-150), whereas cluster-II and III had one genotypes each, WM-18 and WM-65, respectively. Cluster-IV constructed with three genotypes viz. WM-73, WM-44 and WM-98. Cluster V, VI, VII were represented by one genotype each, respectively, WM-21, WM-51 and WM-124.
Fig. 2.
Dendrogram produced by UPGMA clustering based on oil and FAME profile
Genotypes present in cluster-I exhibited a low variation in fatty acid value and their characteristics except oil content. Oil content exhibited higher side values in this cluster except WM-50. Genotypes belong to this cluster showed higher side antioxidant activity as well as free radical scavenging activity. Genotype (WM-18) present in cluster-II exhibited higher linoleic (PUFA), DR, ODR and oxydizibility with higher oil content. This group also exhibited lowest palmitic acid, oleic acid and MUFA. The only member of cluster-III was characterized with highest level of stearic acid as well as higher SFA and PH. A high SFA content is directly linked with higher value of its main contributor stearic acid. Cluster–IV, exhibited higher oleic acid, MUFA and M/S with lower linoleic acid, PUFA, DR, ODR, PI, P/M, PH, AO and OZY. These all characters were interrelated with each other. A higher oleic acid directly contributes to MUFA and higher ratio of M/S. Lower DR and ODR ratios might be responsible for lower level of linoleic acid and this fatty acid was the main contributor to PUFA. A lower PI and P/M might be due to lower PUFA. A lower antioxidant activity is directly linked with amount of lower flavanoides. These cluster posses near about just opposite characters as of cluster-II.
Cluster–V having one genotype exhibited higher TUFA, PI and (M + P)/S with lower value of stearic acid, SFA, and absence of 14:0. A higher level of TUFA is mainly due to higher side value of two major contributors, oleic and linoleic acid. A higher TUFA and lower SFA is directly responsible for higher PI value and (M + P)/S ratios. A lower SFA is due to less value of its main contributor stearic acid. Cluster-VI (WM-51) exhibited lower values for long chain fatty acids (20:0, 20:1, 22:0) with lower ER. Cluster-VII (WM-124) have a higher level of 20:0, 20:1 n–6 and 22:0, ER and PV. Higher level of 20:0, 20:1 n–6 and 22:0 might be due to higher ER, which is responsible for chain elongation in fatty acids.
Conclusion
In present study, genotype WM-18 (cluster-II) was most suitable for further selection in breeding program for improvement of desirable characters of edible oil i.e. high linoleic acid (n–6) as well as PUFA, DR, ODR with higher oil content. This group also exhibited the lowest palmitic acid which is less desirable in edible oil. Genotype, WM-21 (cluster-V) may be selected for higher oleic acid, TUFA, PI and (M + P)/S with lower value of stearic acid. Further, with the use of breeding techniques genotypes WM-18 (higher linoleic acid and lower palmitic acid and higher oil), WM-21 (higher TUFA and lower stearic acid), WM-17 (higher FC and AO) may be used for developing a good quality edible oil. Interestingly, all high oil containing genotypes were derived from a single cross involving IC-449393 (indigenous) and EC-677165 (exotic parent), proving importance of exotic collections for oil content and quality improvement.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to Directors, ICAR—Central Arid Zone Research Institute, Jodhpur and ICAR—National Research Centre on Seed Spices, Tabiji, Ajmer for providing necessary facilities for conducting the research work.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3074-5) contains supplementary material, which is available to authorized users.
References
- Adebisi GA, Olagunju EO. Nutritional potential of the seed of fluted pumpkin (Telfairia occidentalis) J New Trends Sci Technol Appl. 2011;1:7–18. [Google Scholar]
- Amin I, Norazaidah Y, Hainida KIE. Antioxidant activity and phenolic content of raw and blanched Amaranthus species. Food Chem. 2006;94:47–52. doi: 10.1016/j.foodchem.2004.10.048. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 18. Washington, DC: Association of Official Analytical Chemists; 2005. [Google Scholar]
- Baboli ZM, Kordi AAS. Characteristics and composition of watermelon seed oil and solvent extraction parameters effects. J Am Oil Chem Soc. 2010;87:667–671. doi: 10.1007/s11746-010-1546-5. [DOI] [Google Scholar]
- Badr N, Arzoo S, Bakeet ZAN. Characteristics and fatty acid composition of commonly consumed cooking oil marketed locally in Riyadh City. Int J Biosci. 2014;4:227–238. [Google Scholar]
- Chang NW, Huang PC. Effects of the ratio of polyunsaturated and monounsaturated fatty acid to saturated fatty acid on rat plasma and liver lipid concentrations. Lipids. 1998;33:481–487. doi: 10.1007/s11745-998-0231-9. [DOI] [PubMed] [Google Scholar]
- Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Das M, Das SK, Suthar SH. Composition of seed and characteristics of oil from karingada [Citrullus lanatus (Thumb) Mansf] Int J Food Sci Tech. 2002;37:893–896. doi: 10.1046/j.1365-2621.2002.00638.x. [DOI] [Google Scholar]
- Dennys ECC, Andre GVC, Maria do CGP, Sergio Matta DS, Marco TCS, Neuza MBC. Lipid profile of rats fed high-fat diets based on flaxseed, peanut, trout or chicken skin. Nutrition. 2006;22:197–205. doi: 10.1016/j.nut.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Edidiong AE, Ubong ME. Chemical analysis of Citrullus lanatus seed oil obtained from Southern Nigeria. Elix Org Chem. 2013;54:12700–12703. [Google Scholar]
- Egbuonu ACC, Aguguesi RG, Samuel R, Ojunkwu O, Onyenmeri F, Uzuegbu U. Some physicochemical properties of the petroleum ether extracted water melon (Citrullus lanatus) seed oil. Asian J Sci Res. 2015;8:519–525. doi: 10.3923/ajsr.2015.519.525. [DOI] [Google Scholar]
- Fasina OO, Colley Z. Viscosity and specific heat of vegetable oils as a function of temperature: 35 C to 180 C. Int J Food Prop. 2008;11:738–746. doi: 10.1080/10942910701586273. [DOI] [Google Scholar]
- Jacob AG, Etong DI, Tijjani A. Proximate, mineral and anti-nutritional compositions of melon (Citrullus lanatus) seeds. Br J Res. 2015;2:142–151. [Google Scholar]
- Jarret RL, Levy IJ. Oil and fatty acid contents in seed of Citrullus lanatus Schrad. J Agric Food Chem. 2012;60:5199–5204. doi: 10.1021/jf300046f. [DOI] [PubMed] [Google Scholar]
- Jeffrey C. Cucurbitaceae. In: Hanelt P, editor. Mansfeld’s encyclopedia of agricultural and horticultural crops. Berlin: Springer; 2001. [Google Scholar]
- Kamel BS, Dawson H, Kakuda Y. Characteristics and compositions of melon and grape seed oils and cakes. J Am Oil Chem Soc. 1985;62:881–883. doi: 10.1007/BF02541750. [DOI] [Google Scholar]
- Kang MJ, Shin MS, Park JN, Lee SS. The effects of polyunsaturated: saturated fatty acids ratios and peroxidisability index values of dietary fats on serum lipid profiles and hepatic enzyme activities in rats. Br J Nutr. 2005;94:526–532. doi: 10.1079/BJN20051523. [DOI] [PubMed] [Google Scholar]
- Katan MB, Zock PL, Mensink RP. Trans fatty acids and their effects on lipoproteins in humans. Annu Rev Nutr. 1995;15:473–493. doi: 10.1146/annurev.nu.15.070195.002353. [DOI] [PubMed] [Google Scholar]
- Lawton CL, Delargry HJ, Brockman J, Simith RC, Blundell JE. The degree of saturation of fatty acids influences in post ingestive satiety. Br J Nutr. 2000;83:473–482. [PubMed] [Google Scholar]
- Mabaleha MB, Mitei YC, Yeboah SO. A comparative study of the properties of selected melon seed oils as potential candidates for development into commercial edible vegetable oils. J Am Oil Chem Soc. 2007;84:31–36. doi: 10.1007/s11746-006-1003-7. [DOI] [Google Scholar]
- Mahatma MK, Thawait LK, Bishi SK, Khatediya N, Rathnakumar AL, Lalwani HB, Misra JB. Nutritional composition and antioxidant activity of Spanish and Virginia groundnuts (Arachis hypogaea L.): a comparative study. J Food Sci Technol. 2016;53:2279–2286. doi: 10.1007/s13197-016-2187-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahla HR, Choudhary BR. Genetic diversity in seed purpose watermelon (Citrullus lanatus) genotypes under rainfed situations of Thar Desert. Ind J Agric Sci. 2013;83:300–303. [Google Scholar]
- Mahla HR, Singh JP, Roy MM. Seed purpose watermelon in arid zone. Jodhpur: Central Arid Zone Research Institute; 2014. [Google Scholar]
- Marathe SA, Rajalakshmi V, Jamdar SN, Sharma A. Comparative study on antioxidant activity of different varieties of commonly consumed legumes in India. Food Chem Toxicol. 2011;49:2005–2012. doi: 10.1016/j.fct.2011.04.039. [DOI] [PubMed] [Google Scholar]
- McCormick RL, Ratcliff M, Moens L, Lawrence R. Several factors affecting the stability of biodiesel in standard accelerated tests. Fuel Process Tech. 2007;88:651–657. doi: 10.1016/j.fuproc.2007.01.006. [DOI] [Google Scholar]
- Mendez E, Sanhueza J, Speisky H, Valenzuela A. Validation of the rancimat test for the assessment of the relative stability of fish oils. J Am Oil Chem Soc. 1996;73:1033–1037. doi: 10.1007/BF02523412. [DOI] [Google Scholar]
- Misuna S, Swatsitang P, Jogloy S. Fatty acids content and antioxidant capacity of peanut. KKU Sci J. 2008;36:64–74. [Google Scholar]
- Ngassapa FN, Othman OC. Physicochemical characteristics of some locally manufactured edible vegetable oils marketed in Dar es Salaam, Tanzania. J Sci. 2001;27:49–58. [Google Scholar]
- Oluba OM, Ogunlowo YR, Ojieh GC, Adebisi KE, Eidangbe GO, Isiosio IO. Physicochemical properties and fatty acid composition of Citrullus lanatus (egusi melon) seed oil. J Biol Sci. 2008;8:814–817. doi: 10.3923/jbs.2008.814.817. [DOI] [Google Scholar]
- Oyulu C, Macfarlane N. A study of the oil and soluble protein components of five Egusi (Colocynthis citrullus L.) cutivars. Trop Sci. 1982;24:93–98. [Google Scholar]
- Panse VC, Sukhatme PV. Statistical methods for agricultural workers. III Rev. New Delhi: ICAR; 1978. [Google Scholar]
- Pleines S, Friedt W. Breeding for improved C:18 fatty acid composition in rapeseed (Brassica napus L.) Fat Sci Technol. 1988;90:167–171. [Google Scholar]
- Pritchard JLR. Analysis and properties of oilseeds. In: Rossell JB, Pritchard JLR, editors. Analysis of oilseeds, fats and fatty foods. New York: Elsevier; 1991. pp. 39–102. [Google Scholar]
- Raziq S, Anwar F, Mahmood Z, Shahid SA, Nadeem R. Characterization of seed oils from different varieties of watermelon [Citrullus lanatus (Thunb.)] from Pakistan. Grasasy Aceites. 2012;63:365–372. doi: 10.3989/gya.022212. [DOI] [Google Scholar]
- Robinson RW, Decker-Walters DS. Cucurbits. Oxon: CAB International Wallingford; 1997. [Google Scholar]
- Sabahelkhier MK, Ishag KEA, Sabir Ali AK. Fatty acid profile, ash composition and oil characteristics of seeds of watermelon grown in Sudan. Br J Sci. 2011;1:76–80. [Google Scholar]
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthin on autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–948. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- Teotia MS, Ramakrishna P. Chemistry and technology of melon seeds. J Food Sci Technol. 1984;21:332–340. [Google Scholar]
- Velasco L, Goffman FD, Becker HC. Variability for the fatty acid composition of the seed oil in germplasm collection of the genus Brassica. Genet Res Crop Evol. 1998;45:371–382. doi: 10.1023/A:1008628624867. [DOI] [Google Scholar]
- Ziyada AK, Elhussien SA. Physical and chemical characteristics of Citrullus lanatus var Colocynthoide seed oil. J Phys Sci. 2008;19:69–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.