Abstract
Mayonnaise is one of the most commonly used sauces all over the world but it is vulnerable to oxidation because of its high oil content. Using natural antioxidants instead of synthetic ones is a popular and promising topic in the food industry. The aim of this study was to increase the oxidative stability of mayonnaise using cold-pressed black cumin oil (BCO), which has high antioxidant activity due to its phenolic content. Four different mayonnaise formulations were used: Mayo-Control, Mayo-5% BCO, Mayo-10% BCO, and Mayo-20% BCO, which refer to a 0 (control), 5, 10, and 20% BCO replacement of total sunflower oil content, respectively. Thymoquinone content of the mayonnaises including BCO increased with the increasing BCO ratios. At the end of the storage for 4 weeks at 20 °C, peroxide values of Mayo-Control, Mayo-5% BCO, Mayo-10% BCO, and Mayo-20% BCO samples were recorded as 36.07 ± 1.51, 26.76 ± 0.67, 25.60 ± 0.57, and 17.66 ± 1.93 meq O2/kg oil, respectively. The conjugated diene and triene values of the mayonnaises prepared by adding BCO were lower than those of the control group during storage. Overall acceptability of Mayo-5% BCO in sensory analysis was higher than that of Mayo-Control. Using BCO in mayonnaise improved its oxidative stability and flavor.
Keywords: Black cumin oil, Thymoquinone, Oxidation, Sensory
Introduction
Lipid oxidation has negative effects on food quality and human health. The oxidative changes of lipids lead to some changes in flavor, color, and odor and decrease the concentration of bioactive compounds, which reduces the nutritional value of foods. Therefore some precautions must be taken to minimize oxidation and improve the oxidative stability of lipid products (Raghavan 2007; Shahidi and Zhong 2010). Natural bioactive compounds have been frequently applied in the food industry for quality and safety preservation of the food products (Luther et al. 2007).
Mayonnaise is one of the oldest and most widely used sauces in the world. Traditional mayonnaise is a mixture of oil (70–80%), egg yolk, vinegar and spices. Due to its high content of oil, mayonnaise is susceptible to spoilage and more prone to auto-oxidation (Depree and Savage 2001).
Nowadays, consumers are interested in natural foods and natural preservatives for healthier lifestyles. Therefore, the food industry has been searching for new and unique spice flavorings for ethnic and cross-cultural cuisines (Raghavan 2007). Black cumin (Nigella sativa L.) seeds and its crude or essential oils are widely used in functional foods, nutraceuticals and pharmaceutical products (Hassanien et al. 2015) because of their antioxidant properties (Lutterodt et al. 2010) and health benefits (El-Abhar et al. 2003).
In comparison to the traditional extraction method, cold-pressed extraction is an alternative for seed oil production since it involves no heat treatment or solvent. A refining process is not applied to cold pressed black cumin oil (BCO) therefore it contains a higher level of natural antioxidants and thymoquinone (major bioactive and phenolic component of N. sativa L.) (Lutterodt et al. 2010; Ramadan 2013).
Several studies have been published on black cumin oil recently but no data was found in the literature about the antioxidative activity of BCO in mayonnaise. The purpose of the present study was to investigate the oxidative stability and sensory quality of mayonnaise incorporated with BCO.
Materials and methods
Material
BCO was kindly donated by Oneva (Istanbul, Turkey). Refined sunflower oil (SFO), vinegar, and pasteurized egg yolk were purchased from a local market in Ankara. FAME standards (C8–C24), tocopherol standards (alpha, beta, gamma, and delta), and thymoquinone were obtained from Sigma-Aldrich (Taufkirchen, Germany). All chemicals were of analytical grade.
Fatty acid composition
Fatty acid methyl esters (FAME) of BCO and SFO were prepared according to IUPAC (1987). The compositions of FAMEs were determined using a GC (Shimadzu, Kyoto, Japan) equipped with an FID detector and a DB 23 capillary column (30 m, 0.25 mm i.d. 0.25 µm film thickness) (Agilent J&W, USA). Injector and detector temperatures were 230 and 240 °C, respectively. Initial oven temperature was 40 °C for 5 min, then the oven temperature was programmed to rise from 40 to 190 °C at 10 °C/min and kept at 190 °C for 10 min. Helium was the carrier gas at a linear flow of 1.0 mL/min. 1 µL sample was injected to the GC by splitting (1:100). Identification of FAMEs was performed by comparison of their retention times with those of the reference standards and the fatty acid composition (percentage by weight) was calculated from their corresponding integration data.
Tocopherol content
Tocopherol analyses were performed according to AOCS Official Method Ce 8-89 (2005) using a normal-phase HPLC. A Shimadzu SCL-10A HPLC system equipped with Lichrosorb SI 60-5 column (250 × 4.6 mm id, particle size S-5 µm) (Hichrom, Reading, UK) was used for the analyses. The sample 1 g of oil was dissolved in 9 ml of hexane and injected into the system; isocratic elution was used with the mixture of n-hexane/iso-propanol (99/1) at a flow rate of 1 ml/min. The column temperature was maintained at 25 °C and total run time was 20 min. A photodiode array detector was used to detect tocopherol homologues at 295 nm. Quantification of tocopherols was carried out using external standards of α, β, γ, and δ-tocopherol.
Preparation of mayonnaise
The recipe was based on the following formulation: oil content (80%), pasteurized egg yolk (10%), cider vinegar (7%), sugar (2%), and salt (1%). Four different mayonnaise (Mayo) formulations were prepared with 0 (control), 5, 10, and 20% of BCO replacement of the total SFO content, which were indicated as Mayo-Control, Mayo-5% BCO, Mayo-10% BCO, and Mayo-20% BCO, respectively. Initially, egg yolk and vinegar were mixed for 1 min using a standard hand mixer (220 v- 50 Hz, Philips, Holland). Then salt and sugar were added to the mixture and mixed for 1 min. The oil (sunflower oil or the oil blends) was then incorporated into the mixture slowly and mixed gently for 10 min. Mayonnaise samples were stored at 4 °C until analyses.
Color measurement
The color of the oils was determined using a Lovibond tintometer (PFXi-195/3 spectrocolorimeter, Amesbury, UK). The results were presented as L*, a*, and b* values of the Hunter color system.
The color intensity of the various mayonnaises was measured using a chroma meter (Konica Minolta CR-400, Tokyo, Japan) and expressed as L*, a*, and b* values against a known white background. L* represents lightness with values varying from 0 (black) to 100 (white), a* represents red (+ a) or green (− a), and b* represents yellow (+ b) or blue (− b).
Sensory evaluation
For sensory evaluation, 10 trained panelists scored the mayonnaises for their appearance, color, odor, taste, texture and overall acceptability using a nine-point hedonic scale; 1 = dislike extremely, 9 = like extremely. All mayonnaise samples were evaluated in triplicate and weighed in transparent plastic cups of 5 g each. The samples, coded with three-digit numbers, were served in a completely random order. Water and crispy bread were provided for panelists to rinse and clean their mouths between each sample, and coffee was provided for the odor scoring. Tests were conducted in a well-ventilated and well-lit room.
Thymoquinone content in BCO and the mayonnaises
The thymoquinone content of BCO and the oils extracted from the mayonnaises was determined according to the method described by Murkovic et al. (2004). 2.5 g of oil was weighed in a centrifuge tube and mixed with a 2.5 mL mixture of methanol/water (80/20, v/v) then centrifuged at 9000 rpm for 10 min. The upper (methanolic) phase was removed from the tube and filtered through by a 0.45 µm pore size membrane filter. The extract was injected into a Shimadzu SCL-10A HPLC system equipped with Inertsil (250 × 4.6 mm2, ODS-3, 5 µm particle size, Tokyo, Japan) column. The mobile phase consisted of (A) water (pH adjusted to 3.1 with acetic acid) and (B) methanol (90:10, v/v) at flow rate of 1 mL/min. The gradient elution conditions were as follows: starting with from 10 to 30% B at 10 min, 30% B at 30 min, 40% B at 40 min, 50% B at 45 min until the end of the run. The phenolic compounds were detected at 280 and 320 nm by a photodiode array detector. Oven temperature was set at 35 °C and injection volume was 50 μL.
Storage of mayonnaise
Mayonnaise samples were stored in 50 mL plastic flasks and kept in an incubator at 20 °C for 28 days. The samples were taken every 7 days for oxidative stability analyses.
Oxidation experiments in mayonnaise
Oils were extracted from the mayonnaise as described by Altunkaya et al. (2013) with some modifications. Mayonnaise samples were left to freeze at − 18 °C overnight. After freezing, the samples were thawed at room temperature in darkness, and then centrifuged for 15 min at 9000 rpm. The extracted oil was stored in brown bottles at − 18 °C before analyses.
The peroxide value (PV) and ultraviolet (UV) absorption characteristics (K232 and K270 extinction coefficients) were determined according to AOCS Official Method Cd 8-53 and Ch 5-91, respectively.
Statistical analyses
All experiments were performed in triplicate. Analyses of variance was carried out using SAS Statistical software for Windows (ver. 21) and LSD comparisons were used to detect any significant differences (p < 0.05) between variables. Linear regression and analysis of covariance with 95% CI were used.
Results and discussions
Fatty acid composition and tocopherol content of SFO and BCO
The fatty acid compositions of the oils are shown in Table 1. The main fatty acids of SFO and BCO were linoleic, oleic, palmitic, and stearic acids. The differences in oleic and palmitic acid contents of the oils were remarkable. The ratio of oleic acid and palmitic acid in SFO were 34.82 and 6.07% while their ratios in BCO were 23.98 and 12.07%, respectively. The results in the present study were similar to previous studies published by Kiralan et al. (2017) and Ramadan and Wahdan (2012).
Table 1.
Fatty acid composition (%) and tocopherol content (μg/g) of SFO and BCO
| Fatty acid | SFO | BCO |
|---|---|---|
| C14:00 | 0.06 ± 0.00 | 0.13 ± 0.00 |
| C16:00 | 6.07 ± 0.04 | 12.07 ± 0.10 |
| C16:01 | 0.11 ± 0.00 | 0.25 ± 0.06 |
| C17:00 | 0.03 ± 0.00 | 0.06 ± 0.01 |
| C17:01 | 0.02 ± 0.00 | 0.03 ± 0.00 |
| C18:00 | 3.03 ± 0.02 | 3.06 ± 0.01 |
| C18:01 | 34.82 ± 0.06 | 23.98 ± 0.07 |
| C18:02 | 55.40 ± 0.04 | 57.33 ± 0.12 |
| C18:03 | 0.16 ± 0.02 | 0.49 ± 0.01 |
| C20:00 | 0.19 ± 0.01 | 0.14 ± 0.01 |
| C20:01 | 0.11 ± 0.01 | 0.23 ± 0.01 |
| C20:02 | – | 2.22 ± 0.10 |
| C24:00 | – | 0.2 ± 0.03 |
| α-tocopherol (μg/g) | 563.12 ± 5.86 | 9.12 ± 0.35 |
| γ-tocopherol (μg/g) | 36.35 ± 0.97 | 156.26 ± 2.91 |
| Total tocopherol (μg/g) | 599.47 | 165.39 |
Values given are averages of three replicates ± standard deviations
Vitamin E is a collective term and represents tocopherols and tocotrienols which are also known as “tocochromanols”. The tocopherols and the tocotrienols are characterized by a 6-chromanol ring structure and they are methylated at varying degrees at the 5, 7, and 8 positions. The plants store tocochromanols in several parts like oily seeds and fruits to prevent lipid oxidation by enhancing the induction period of the lipids (Ahsan et al. 2015).
The qualitative and quantitative tocopherol compositions of SFO and BCO are given in Table 1. According to the results, SFO is rich in α-tocopherol whereas BCO is rich in γ-tocopherol. β and δ-tocopherols were not detected in both oils. Similar results were obtained in recent studies. Ag-Seleci et al. (2015) and Choe (2013) could not detect β and δ-tocopherols in black cumin oil obtained by soxhlet extraction and in sunflower oil, respectively. Rudzińska et al. (2016) found BCO is rich in γ-tocopherol. Kiralan et al. (2017) stated that the amount of β and δ-tocopherols in BCO were very low. The tocopherol content of black cumin oil was lower than that reported by Ramadan and Wahdan (2012) while higher than that reported by Kiralan et al. (2014).
Color measurement
The results of color measurement are presented in Table 2. It can be clearly seen that SFO and BCO had completely different color characteristics. The higher lightness belonged to SFO while the higher greenness and yellowness values were related to BCO. Additionally, it is important to notice the huge difference in b* values between these oils.
Table 2.
Colour values of the oils and the mayonnaises
| Colour | Oils | Mayonnaises | ||||
|---|---|---|---|---|---|---|
| SFO | BCO | Mayo-Control | Mayo-5% BCO | Mayo-10% BCO | Mayo-20% BCO | |
| L* | 99.27 ± 0.09A | 87.88 ± 0.44B | 61.09 ± 0.09a | 57.28 ± 2.18b | 58.35 ± 2.22ab | 56.78 ± 0.93b |
| a* | − 3.21 ± 0.02A | − 11.06 ± 0.16B | − 1.79 ± 0.08a | − 1.60 ± 0.46a | − 1.70 ± 0.53a | − 1.54 ± 0.04a |
| b* | 9.93 ± 0.09B | 80.04 ± 0.52A | 22.42 ± 0.94ab | 20.18 ± 0.98b | 23.10 ± 1.45a | 22.68 ± 1.06a |
Values given are averages of three replicates ± standard deviations
a–bDifferent uppercase letters indicate significant differences between the mayonnaises in row (p < 0.05)
A–BDifferent uppercase letters indicate significant differences between the oils in row (p < 0.05)
For the mayonnaises, Mayo-Control had the highest lightness while Mayo-20% BCO had the lowest. This result probably arose from the higher lightness level of SFO than BCO. There was no statistical difference between a* values of the mayonnaises. Mayo-10% BCO and Mayo-20% BCO had the highest yellowness values relative to the increasing levels of BCO substitution.
Sensory evaluation
Table 3 presents the results of sensory analysis. As seen in the table, Mayo-Control and Mayo-5% BCO samples had significantly higher appearance and color values. The lightness of the oils supported this result. For all samples, there were no statistical differences in odor, taste, and texture scores. The higher concentration of BCO in Mayo-10% BCO and Mayo-20% BCO samples resulted in lower overall acceptability scores because of the higher phenolic content which contributes to bitterness and astringency. Mayo-5% BCO had the highest score in overall acceptability.
Table 3.
Sensory evaluation results of the mayonnaises
| Level of BCO | Appearance | Colour | Odour | Taste | Texture | Overall acceptability |
|---|---|---|---|---|---|---|
| Mayo-control | 7.6 ± 0.1a | 7.5 ± 0.1a | 7.0 ± 0.2a | 7.0 ± 0.3a | 7.2 ± 0.4a | 7.0 ± 0.2ab |
| Mayo-5% BCO | 7.7 ± 0.2a | 7.4 ± 0.2a | 6.9 ± 0.2a | 7.3 ± 0.2a | 7.3 ± 0.3a | 7.3 ± 0.1a |
| Mayo-10% BCO | 7.2 ± 0.1b | 6.8 ± 0.3b | 6.9 ± 0.2a | 6.8 ± 0.6a | 7.4 ± 0.3a | 7.0 ± 0.3ab |
| Mayo-20% BCO | 7.0 ± 0.1b | 6.5 ± 0.2b | 6.8 ± 0.1a | 6.7 ± 0.6a | 7.0 ± 0.3a | 6.6 ± 0.2b |
Values given are averages of three replicates ± standard deviations
a–bDifferent uppercase letters indicate significant differences between the mayonnaises (p < 0.05)
Thymoquinone content in BCO and the mayonnaises
Thymoquinone is the main bioactive and phenolic compound of BCO (Gray et al. 2016). Phenols are known as primary antioxidants which behave like hydrogen donors and radical scavengers. They delay the initiation step or interrupt the propagation step of lipid oxidation (Kiokias et al. 2008). Therefore, phenolic compounds prevent formation of aldehydes, ketones, alcohols, and hydrocarbons which cause a rancid taste and odor in foods containing lipids (Shahidi and Ambigaipalan 2015).
Thymoquinone distributions of BCO and the mayonnaises were determined on day 0. Thymoquinone contents decreased according to the descending amount of BCO in the mayonnaise samples. Its content (µg/g oil) in the oils extracted from mayonnaises ranged as follows: Mayo-20% BCO (230.03 ± 24.69) > Mayo-10% BCO (104.99 ± 1.43) > Mayo-5% BCO (27.87 ± 3.16). It was determined that Mayo-Control had no thymoquinone content (data not shown). The thymoquinone concentration of BCO (1185.70 ± 64.82 µg/g oil) was lower than those stated by Lutterodt et al. (2010). They reported that the thymoquinone content of six different BCO ranged from 3480 to 8730 µg/g oil. This difference is possibly because of the origin and growing conditions of the black cumin seed, extraction method, and storage of the oil.
Oxidation stability results
Lipid oxidation involves three stages: initiation, propagation, and termination (McClements and Decker 2000). PV is carried out to measure initial and intermediary oxidation products (hydroperoxides). Conjugated dienes (CD) present formation of intermediary products (hydroperoxides and conjugated dienes) while conjugated trienes (CT) represent formation of end products (alcohols, aldehydes, hydrocarbons, and ketones) (McClements and Decker 2000).
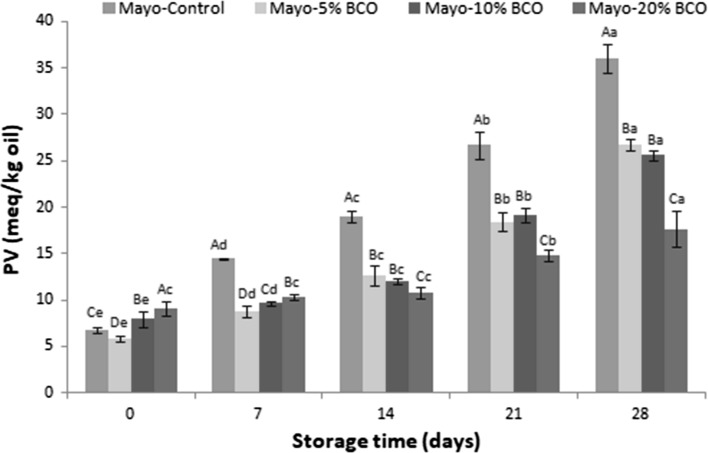
The PV values of the mayonnaises are shown in Fig. 1. After preparing the mayonnaises (day 0), the highest PV value was obtained in Mayo-20% BCO, followed by Mayo-10% BCO because BCO had initially a higher PV than SFO. The PV values of BCO and SFO were 36.87 ± 0.90 and 1.65 ± 0.29 meq/kg oil, respectively (data not shown). Kreps et al. (2014) indicated that the existences of transition metal and light in crude oils can be the reasons for high PV values. BCO-incorporated mayonnaises had lower PV values than the Mayo-Control between 7th and 28th days of storage. According to the results, only Mayo-20% BCO had a lag phase where its PV values did not increase in the first 14 days of storage. After 7 days of storage, the PV values of the mayonnaises decreased as follows: Mayo-Control > Mayo-5% BCO > Mayo-10% BCO > Mayo-20% BCO. Additionally, after 7 days of the storage, there was no significant difference between the PV results of Mayo-5% BCO and Mayo-10% BCO (p < 0.05). Adding 5, 10, and 20% BCO to the oil phase of mayonnaise inhibited the rate of lipid hydroperoxide formation in the mayonnaises by 25.82, 29.05, and 51.04%, respectively, compared to the Mayo-Control at the end of 28 days. Similar findings were also reported in the literature. Li et al. (2014) and Raikos et al. (2015) who added purple corn extract and beetroot to mayonnaise, respectively. They indicated that the oxidative stabilities of mayonnaises were enhanced by using these ingredients. According to these studies, phenolic compounds in these materials may act like antioxidants and prevent or delay lipid oxidation in mayonnaise.
Fig. 1.
Changes in the peroxide value of the mayonnaises during storage. Values expressed in meq/kg oil (mean ± SD, n = 3). A–D Different uppercase letters represent significant differences between the mayonnaises (p < 0.05). a–e Different uppercase letters represent significant differences between the storage times of each mayonnaises (p < 0.05)
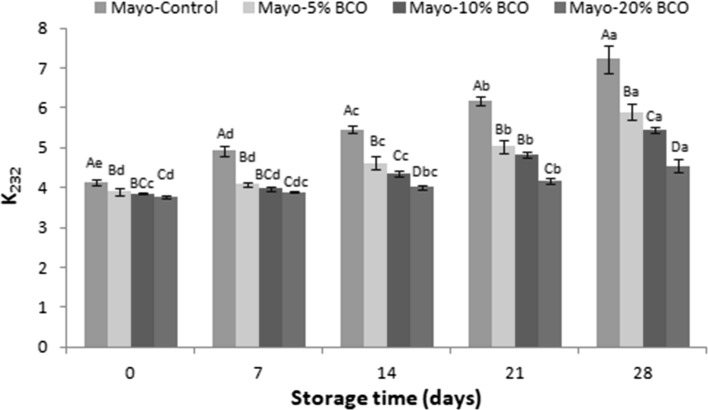
All mayonnaises which included BCO showed lower CD than the control group during the storage period (p < 0.05) (Fig. 2). The CD values of the mayonnaises ranged as follows: Mayo-Control > Mayo-5% BCO > Mayo-10% BCO > Mayo-20% BCO, throughout the storage. The CD values of BCO and SFO were 3.40 ± 0.08 and 3.93 ± 0.01, respectively (data not shown). The high BCO concentration was related to high phenolic content, which resulted in low CD values in the mayonnaises. Altunkaya et al. (2013) indicated that enrichment of mayonnaise with grape seed extract caused lower CD than mayonnaise without the extract.
Fig. 2.
Changes in conjugated diene of the mayonnaises during storage. Values given are the mean of three replicates and error bars show the variations of three determinations in terms of standard deviation. A–D Different uppercase letters indicate significant differences between the mayonnaises (p < 0.05). a–e Different uppercase letters indicate significant differences between the storage times of each mayonnaises (p < 0.05)
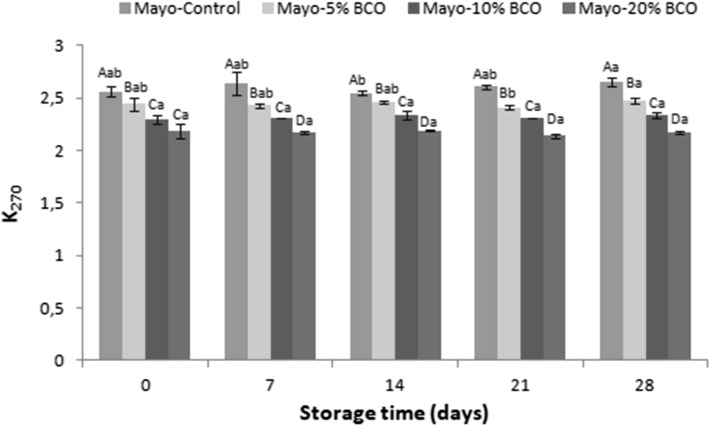
The CT results of the mayonnaises are shown in Fig. 3. The CT values of BCO and SFO were 2.17 ± 1.27 and 2.52 ± 0.01, respectively (data not shown). The CT values for Mayo-Control, Mayo-5% BCO, Mayo-10% BCO, and Mayo-20% BCO corresponded to 2.56, 2.44, 2.29, and 2.19, respectively, at the beginning of the storage. These values changed to 2.65, 2.47, 2.34, and 2.17 in the same order at the end of the storage. According to the results, little changes in the CT values of the samples were observed. Probably, these changes arose from replacement of double bound positions of lipids containing dienes or polyenes during oxidation (Chirinos et al. 2015). The highest CT value was obtained from Mayo-Control while the lowest belonged to Mayo-20% BCO during storage. CD and CT results prove the effectiveness of BCO. Similar results were revealed by Ramadan and Wahdan (2012). They improved the thermal stability of high linoleic-corn oil by adding BCO. The CT values of BCO-enriched blends were lower than those of the control during storage.
Fig. 3.
Changes in conjugated triene of the mayonnaises during storage. Values given are the mean of three replicates and error bars show the variations of three determinations in terms of standard deviation. A–D Different uppercase letters show significant differences between the mayonnaises (p < 0.05). a–b Different uppercase letters show significant differences between the storage times of each mayonnaises (p < 0.05)
Conclusion
BCO-enriched mayonnaises showed better oxidative stability as compared to mayonnaise prepared with SFO. The effect of BCO on oxidative stability may be related to phenolic compounds, mainly thymoquinone. The mayonnaise formulation having the lowest BCO also had a higher sensorial acceptability than the other formulations. As a natural antioxidant source, using BCO in mayonnaise formulations could be useful in reducing oxidation rate and thus enhancing shelf life and flavor. It is recommended that further investigations should be performed to determine the effect of BCO on the textural properties of mayonnaise.
Funding
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Ag-Seleci D, Gümüş ZP, Yavuz M, Şeleci M, Bongartz R, Stahl F, Çoşkunol H, Timur S, Scheper T. A case study on in vitro investigations of the potent biological activities of wheat germ and black cumin seed oil. Turk J Chem. 2015;39:801–812. doi: 10.3906/kim-1502-72. [DOI] [Google Scholar]
- Ahsan H, Ahad A, Siddiqui WA. A review of characterization of tocotrienols from plant oils and foods. J Chem Biol. 2015;8:45–59. doi: 10.1007/s12154-014-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altunkaya A, Hedegaard RV, Harholt J, Brimer L, Gökmen V, Skibsted LH. Oxidative stability and chemical safety of mayonnaise enriched with grape seed extract. Food Funct. 2013;4:1647–1653. doi: 10.1039/c3fo60204d. [DOI] [PubMed] [Google Scholar]
- AOCS . Official methods and recommended practices of the American Oil Chemists’ Society. 5. Champaign: AOCS Press; 2005. [Google Scholar]
- Chirinos R, Pedreschi R, Cedano I, Campos D. Antioxidants from Mashua (Tropaeolum tuberosum) control lipid oxidation in Sacha Inchi (Plukenetia volubilis L.) oil and raw ground pork meat. J Food Process Preserv. 2015;39:2612–2619. doi: 10.1111/jfpp.12511. [DOI] [Google Scholar]
- Choe E. Interaction of light and temperature on tocopherols during oxidation of sunflower oil. J Am Oil Chem Soc. 2013;90:1851–1857. doi: 10.1007/s11746-013-2330-0. [DOI] [Google Scholar]
- Depree JA, Savage GP. Physical and flavour stability of mayonnaise. Trends Food Sci Tech. 2001;12:157–163. doi: 10.1016/S0924-2244(01)00079-6. [DOI] [Google Scholar]
- El-Abhar HS, Abdallah DM, Saleh S. Gastroprotective activity of Nigella sativa oil and its constituent, thymoquinone, against gastric mucosal injury induced by ischaemia/reperfusion in rats. J Ethnopharmacol. 2003;84:251–258. doi: 10.1016/S0378-8741(02)00324-0. [DOI] [PubMed] [Google Scholar]
- Gray JP, Burgos DZ, Yuan T, Seeram N, Rebar R, Follmer R, Heart EA. Thymoquinone, a bioactive component of Nigella sativa, normalizes insulin secretion from pancreatic-cells under glucose overload via regulation of malonyl-CoA. Am J Physiol Endocrinol Metab. 2016;310:E394–E404. doi: 10.1152/ajpendo.00250.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanien MF, Assiri AM, Alzohairy AM, Oraby HF. Health-promoting value and food applications of black cumin essential oil: an overview. J Food Sci Tech. 2015;52:6136–6142. doi: 10.1007/s13197-015-1785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUPAC . Standard methods for analysis of oils, fats and derivatives. 7. Boston: Blackwell Scientific Publications; 1987. [Google Scholar]
- Kiokias S, Varzakas T, Oreopoulou V. In vitro activity of vitamins, flavonoids, and natural phenolic antioxidants against the oxidative deterioration of oil-based systems. Crit Rev Food Sci Nutr. 2008;48:78–93. doi: 10.1080/10408390601079975. [DOI] [PubMed] [Google Scholar]
- Kiralan M, Özkan G, Bayrak A, Ramadan MF. Physicochemical properties and stability of black cumin (Nigella sativa) seed oil as affected by different extraction methods. Ind Crops Prod. 2014;57:52–58. doi: 10.1016/j.indcrop.2014.03.026. [DOI] [Google Scholar]
- Kiralan M, Ulaş M, Özaydin A, Özdemir N, Özkan G, Bayrak A, Ramadan MF. Blends of cold pressed black cumin oil and sunflower oil with improved stability: a study based on changes in the levels of volatiles, tocopherols and thymoquinone during accelerated oxidation conditions. J Food Biochem. 2017;41:1–10. doi: 10.1111/jfbc.12272. [DOI] [Google Scholar]
- Kreps F, Vrbiková L, Schmidt Š. Influence of industrial physical refining on tocopherol, chlorophyll and beta-carotene content in sunflower and rapeseed oil. Eur J Lipid Sci Technol. 2014;116:1572–1582. doi: 10.1002/ejlt.201300460. [DOI] [Google Scholar]
- Li CY, Kim HW, Li H, Lee DC, Rhee HI. Antioxidative effect of purple corn extracts during storage of mayonnaise. Food Chem. 2014;152:592–596. doi: 10.1016/j.foodchem.2013.11.152. [DOI] [PubMed] [Google Scholar]
- Luther M, Parry J, Moore J, Meng J, Zhang Y, Cheng Z, Yu LL. Inhibitory effect of Chardonnay and black raspberry seed extracts on lipid oxidation in fish oil and their radical scavenging and antimicrobial properties. Food Chem. 2007;104:1065–1073. doi: 10.1016/j.foodchem.2007.01.034. [DOI] [Google Scholar]
- Lutterodt H, Luther M, Slavin M, Yin JJ, Parry J, Gao JM, Yu LL. Fatty acid profile, thymoquinone content, oxidative stability, and antioxidant properties of cold-pressed black cumin seed oils. LWT Food Sci Technol. 2010;43:1409–1413. doi: 10.1016/j.lwt.2010.04.009. [DOI] [Google Scholar]
- McClements DJ, Decker EA. Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. J Food Sci. 2000;65:1270–1282. doi: 10.1111/j.1365-2621.2000.tb10596.x. [DOI] [Google Scholar]
- Murkovic M, Lechner S, Pietzka A, Bratacos M, Katzogiannos E. Analysis of minor components in olive oil. J Biochem Biophys Methods. 2004;61:155–160. doi: 10.1016/j.jbbm.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Raghavan S. Handbook of spices, seasonings, and flavorings. 2. Boca Raton: CRC Press; 2007. [Google Scholar]
- Raikos V, Neacsu M, Morrice P, Duthie G. Anti- and pro-oxidative effect of fresh and freeze-dried vegetables during storage of mayonnaise. J Food Sci Technol. 2015;52:7914–7923. doi: 10.1007/s13197-015-1897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan MF. Healthy blends of high linoleic sunflower oil with selected cold pressed oils: functionality, stability and antioxidative characteristics. Ind Crops Prod. 2013;43:65–72. doi: 10.1016/j.indcrop.2012.07.013. [DOI] [Google Scholar]
- Ramadan MF, Wahdan KMM. Blending of corn oil with black cumin (Nigella sativa) and coriander (Coriandrum sativum) seed oils: impact on functionality, stability and radical scavenging activity. Food Chem. 2012;132:873–879. doi: 10.1016/j.foodchem.2011.11.054. [DOI] [Google Scholar]
- Rudzińska M, Hassanein MMM, Abdel-Razek AG, Ratusz K, Siger A. Blends of rapeseed oil with black cumin and rice bran oils for increasing the oxidative stability. J Food Sci Technol. 2016;53:1055–1062. doi: 10.1007/s13197-015-2140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects—a review. J Funct Foods. 2015;18:820–897. doi: 10.1016/j.jff.2015.06.018. [DOI] [Google Scholar]
- Shahidi F, Zhong Y. Lipid oxidation and improving the oxidative stability. Chem Soc Rev. 2010;39:4067–4079. doi: 10.1039/b922183m. [DOI] [PubMed] [Google Scholar]