Abstract
Cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome (CANVAS) is a rare disorder with an unknown etiology. We present a British family with presumed autosomal dominant CANVAS with incomplete penetrance and variable expressivity. Exome sequencing identified a rare missense variant in the ELF2 gene at chr4:g.140058846 C > T, c.10G > A, p.A4T which segregated in all affected patients. By using transduced BE (2)-M17 cells, we found that the mutated ELF2 (mt-ELF2) gene increased ATXN2 and reduced ELOVL5 gene expression, the causal genes of type 2 and type 38 spinocerebellar ataxias. Both, western blot and confocal microscopy confirmed an increase of ataxin-2 in BE(2)-M17 cells transduced with lentivirus expressing mt-ELF2 (CEE-mt-ELF2), which was not observed in cells transduced with lentivirus expressing wt-ELF2 (CEE-wt-ELF2). Moreover, we observed a significant decrease in the number and size of lipid droplets in the CEE-mt-ELF2-transduced BE (2)-M17 cells, but not in the CEE-wt-ELF2-transduced BE (2)-M17. Furthermore, changes in the expression of ELOVL5 could be related with the reduction of lipid droplets in BE (2)-M17 cells. This work supports that ELF2 gene regulates the expression of ATXN2 and ELOVL5 genes, and defines new molecular links in the pathophysiology of cerebellar ataxias.
Keywords: cerebellar ataxia, vestibular hypofunction, neuropathy, whole-exome sequencing, ETS domain
Introduction
The triad of cerebellar ataxia, bilateral vestibulopathy, and peripheral neuropathy occurs between 9 and 32% of patients with bilateral vestibular failure (Bronstein et al., 1991; Zingler et al., 2007). It is a rare disorder termed Cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome (CANVAS; [MIM: 614575]). A review reported 51 patients seen over a 10-year period (Szmulewicz et al., 2015), in agreement with our own estimates of seeing 6–8 new cases per year.
Cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome is a late-onset, slowly progressive multi-system ataxia likely secondary to a neurodegenerative ganglionopathy. The combination of cerebellar ataxia and vestibular impairment produces a characteristic oculomotor sign of impaired (“broken up”) visually enhanced vestibulo-ocular reflex (VVOR) (Migliaccio et al., 2004). Phenotypic heterogeneity in CANVAS patients is recognized (Szmulewicz et al., 2014b). Although most cases are sporadic, the finding of six affected siblings’ pairs (Szmulewicz et al., 2014a) suggests a familial recessive disorder or a dominant inheritance with incomplete penetrance; however, the genes involved have not been elucidated.
Case Presentation
We describe a non-consanguineous family with three CANVAS patients from England (Figure 1A). Genetic testing excluded Friedreich ataxia and SCA 1, 2, 3, 6, 7, and 38 as potential diagnoses. All patients provided written informed consent for their participation for publication and the study protocol was approved by the institutional review board. Family members in the fourth generation were examined and remained asymptomatic; however, symptom onset is typically delayed and usually over 60 years of age.
FIGURE 1.
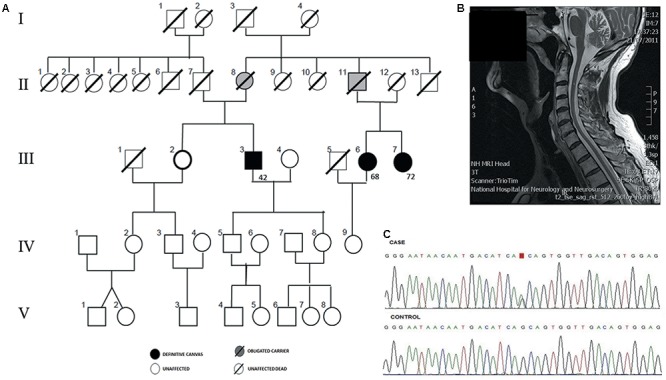
Genetic diagnosis of familial Cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome (CANVAS). (A) Pedigree of an autosomal dominant CANVAS family with three affected cases with the age of onset. (B) Sagittal MRI showing cerebellar atrophy in patient III:3. (C) Chromatogram of reverse chain of the variant chr4:g.140058846 G > A from an affected individual (III.3) is compared to the sequence from a familial control (III.2).
Patient III:3 (proband), was a 78 year old gentleman with 20 years of progressive loss of sensation distally in upper and lower limbs and a gradual deterioration in his balance. He developed oscillopsia in 2005 and in 2014 he noticed mild slurred speech and incoordination followed by development of a prominent dry cough, difficulty with micturition and erectile dysfunction. Examination revealed dysarthria, ataxic gait, and a positive Romberg test. Eye movement examination revealed downbeat nystagmus on lateral gaze. Smooth pursuit was broken horizontally and vertically. Saccades were moderately hypometric. The doll’s head-eye maneuver was abnormally jerky, with numerous “catch-up” saccades [abnormal VVOR; (Figure 2)]. Horizontal and vertical head impulse tests (HITs) were positive bilaterally. The rest of the cranial nerve examination was normal. Limb examination revealed normal tone and power throughout with no spasticity or extrapyramidal features. Reflexes were symmetrically present in the upper limbs, however, in the lower limbs, ankle jerks were absent and plantar were mute. There was a distal loss to light touch and pinprick sensation in all limbs, vibration sense was absent to the sternum with proprioceptive loss to ankles bilaterally. There was moderate bilateral upper and lower limb dysmetria. Romberg’s test was positive. Normal blood tests included negative anti-neuronal, anti-GAD, coeliac antibodies, anti-treponemal, paraneoplastic antibodies normal B1, B12, glucose, thyroid function, Mg, and vitamin E. Bithermal caloric and rotational electronystagmography confirmed bilateral absence of vestibular function. Nerve conduction study (NCS) revealed an axonal sensory neuronopathy. Sural nerve and muscle biopsy were normal. Autonomic function tests were normal. MRI brain showed cerebellar atrophy particularly involving the vermis (Figure 1B). The patient was diagnosed with CANVAS. His father (II:7) died of presumed stroke in his 60’s and his mother remained well until she died at the age of 96. Although the clinical record did not report any known neurological condition, II:8 was considered to be an obligated carrier. On further exploring the family history, it was discovered that III:6 and III:7 (maternal cousins of proband) had similar symptoms hence were also assessed. Of note, their father (II:11) had a balance disorder of unknown etiology therefore may have been affected.
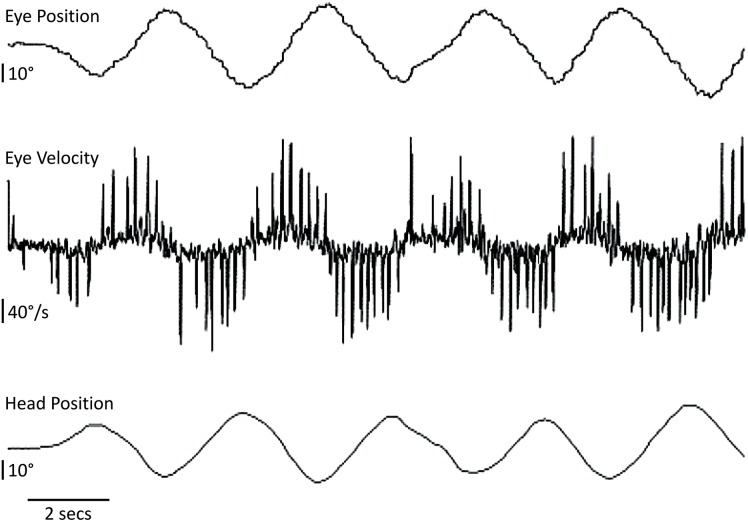
FIGURE 2.
Head and eye horizontal movements in the CANVAS proband. The patient fixates a visual target on the wall while the examiner manually oscillates his head from behind in a quasi-sinusoidal fashion [visually assisted vestibulo-ocular reflex or visually enhanced vestibulo-ocular reflex (VVOR)]. The compensatory eye movement elicited is severely broken-up or cog-wheeled due to the presence of multiple eye saccades (best seen as ‘spikes’ in the eye velocity trace). Upward deflections correspond to rightward head or eye movements.
Patient III:6, was a 78 year old lady with a 10 years history of slowly progressive imbalance, distal numbness, and dysesthesia. Over the last year she described dysphagia and occasional cough. Eye movement examination revealed an abnormal VVOR with HIT showing catch up saccades to the left. Pursuit movements were moderately broken up but in keeping with age. There was distal loss to pinprick in upper and lower limbs. Ankle reflexes were absent. She had an ataxic gait and Romberg’s was mildly positive. Bithermal caloric testing and rotational test (velocity steps and sinusoidal oscillation), showed significant bilateral reduction of vestibular function. Video-HIT showed consistent abnormal catch up saccades bilaterally. EMG/NCS confirmed a sensory neuronopathy. Autonomic function tests were normal. MRI brain revealed an incidental frontal cavernoma and mild global atrophy. This was in keeping with a diagnosis of incomplete (‘forme fruste’) CANVAS phenotype.
Patient III:7, was a 74 years old lady with a 2 years history of imbalance, especially in the dark, followed by distal neuropathic symptoms and severe coughing ‘fits.’ She denied any facial numbness or paresthesiae, speech or swallowing disturbance. Examination revealed a weak downbeat nystagmus in lateral gaze. Pursuit was broken in all directions and saccades were mildly hypometric. She had an abnormal VVOR and bilateral positive HIT. Reflexes were diminished throughout and ankle jerks were absent. There was distal sensory loss to light touch and pinprick in upper and lower limbs, proprioceptive impairment to wrists and ankles. Finger-nose testing was mildly impaired in upper limbs. She had a broad based ataxic gait and Romberg’s was positive. Investigations including cerebellar screening, blood tests, and genetic tests were normal. Autonomic function tests were normal. Bilateral vestibular hypofunction was confirmed on caloric and rotational test. EMG/NCS confirmed axonal sensory neuronopathy with absent sensory nerve action potentials. MRI brain showed fissural prominence within the superior cerebellar vermis. A cervical spine MRI showed a slender lower cervical/upper thoracic cord with flattening of the posterior surface and faint signal change dorsally, compatible with dorsal root ganglionopathy. These features represent a typical CANVAS phenotype.
The fourth subject (III: 2) was a 73 years old lady without any neurological symptoms and a normal neurological examination.
Whole-Exome Sequencing
We sequenced the exomes of four individuals in the family (III:3, III:6, III:7, and III:2) (Figure 1A). Exons capture, library preparation and sequencing were performed as we previously described, in a SOLiD 5500xl platform using the reference sequence GRChr37/hg19 (Martin-Sierra et al., 2016). Only variants were considered. Single nucleotide variants (SNVs) with coverage >30X and minor allele frequency (MAF) <0.001 were retrieved using a combined filtering strategy (Requena et al., 2017). Variants found in the non-affected sibling (III:2) (Figure 1C), were discarded and 3622 variants were retained for further analyses. ANNOVAR software was used to annotate and filter SNVs. Finally, 30 heterozygous SNVs remained after filtering by exome data from the Exome Aggregation Consortium, 1000 Genomes databases and in-house controls. Twenty-seven SNVs had been previously annotated and three of them were novel variants. We also used LOD scores derived from WES-common SNVs to reduce the list of candidate variants, as previously described (Gazal et al., 2016), and 10 candidate variants remained (Supplementary Tables S1, S2). The selected candidate variant, a missense heterozygous variant in the coding regions of ELF2 [NM_201999.2], that segregated with the phenotype was validated by Sanger sequencing. The candidate variant has been submitted to ClinVar database1.
We searched for rare variants in the ELF2 gene in exome sequencing datasets from two additional British CANVAS families, and we also performed Sanger sequencing of the ELF2 gene in these two families and a third one from Spain. So, a total of eight additional unrelated individuals with CANVAS were sequenced, however, none of them carried the variant or other rare variants in the coding regions of ELF2.
The rare variant leads to a change in the exon 2 of the transcript sequence (p.A4T). The predicted effect on protein function is probably damaging, since the beginning of the coding sequence is highly conserved across species and matches with the protein N-terminal elf transcription factor domain, encoded from 4th residue to 108th residue (Supplementary Figures S1, S2). At protein level, the elf-2 amino acids sequence has a 67 and 57% of positive homology matches with elf-1 and ets-1, respectively. The known ETS-binding domain has 87% homology among the three transcription factors (TFs), and the amino acid (p.A4) is conserved in the sequence of ETS-1, ELF-1, and ELF-2 (Supplementary Figure S3). A PAVIVE motif on N-terminal elf transcription factor domain, a relevant recognition motif in elf family, is conserved between elf-1 and elf-2 amino acids sequences.
BE(2)-M17 Cell Culture
Human neuroblastoma BE(2)-M17 cell line (ATCC® CRL-2267TM) was cultured and RT-PCR was used to confirm that the ELF2, ATXN2, and ELOV5L genes are constitutively expressed in BE(2)-M17 neuroblastoma cell line (Supplementary Figures S4A,C).
Lentiviral Vector Constructs Production and Neuroblastoma Transduction
The cDNA encoding for human ELF2 gene and the ELF2 gene with the variant described was cloned in the bicistronic lentiviral vector (LV) pHRSINcppt_CMVeGFP_ELF1α-TetR (also named CEET, available in our laboratory) using standard molecular biology techniques [PacI/MreI (Sse232I)] to obtain the lentiviral plasmids CEE-wt-ELF2 and CEE-mt-ELF2, respectively. Both LV expressed eGFP in addition to the wt-ELF2 or the mt-ELF2. LVs production was performed as previously described (Frecha et al., 2008). All the LVs used were titrated based on the percentage of eGFP expressing cells as previously described (Benabdellah et al., 2014).
The transduction efficiency was 95%. The number of LV integrated per cell was estimated by qRT-PCR as previously described (Cobo et al., 2013). Transduction was measured at 3, 7, 10, and 25 days). No significant differences were found between both transduced cell lines. Moreover, the transduction remained stable over time after day 3 (Supplementary Figure S4B).
Cell Viability and Proliferation Assays
Cell viability and proliferation assays were performed in BE(2)-M17 cells to investigate the effect of the ELF2 variant. For both cell viability and proliferation assays, there was no difference between the cells. (Supplementary Figure S5). These results suggest that overexpression of wt-ELF2 or mt-ELF2 gene did not have any influence on the proliferation or survival of BE(2)-M17 cells and overexpression of ATXN2 did not modify the morphology.
Functional Assays: qRT-PCR, Western Blot, Immunocytochemistry, and Confocal Microscopy
We also investigated the effect of mutant ELF2 on ATNX2 and ELOVL5 expression levels, since these genes are a direct target of ELF2, according to Curated Transcription Factor Targets Dataset (TRANSFAC), and both have been associated with SCA2 and SCA38 (Scoles et al., 2012; Di Gregorio et al., 2014; Hoxha et al., 2017).
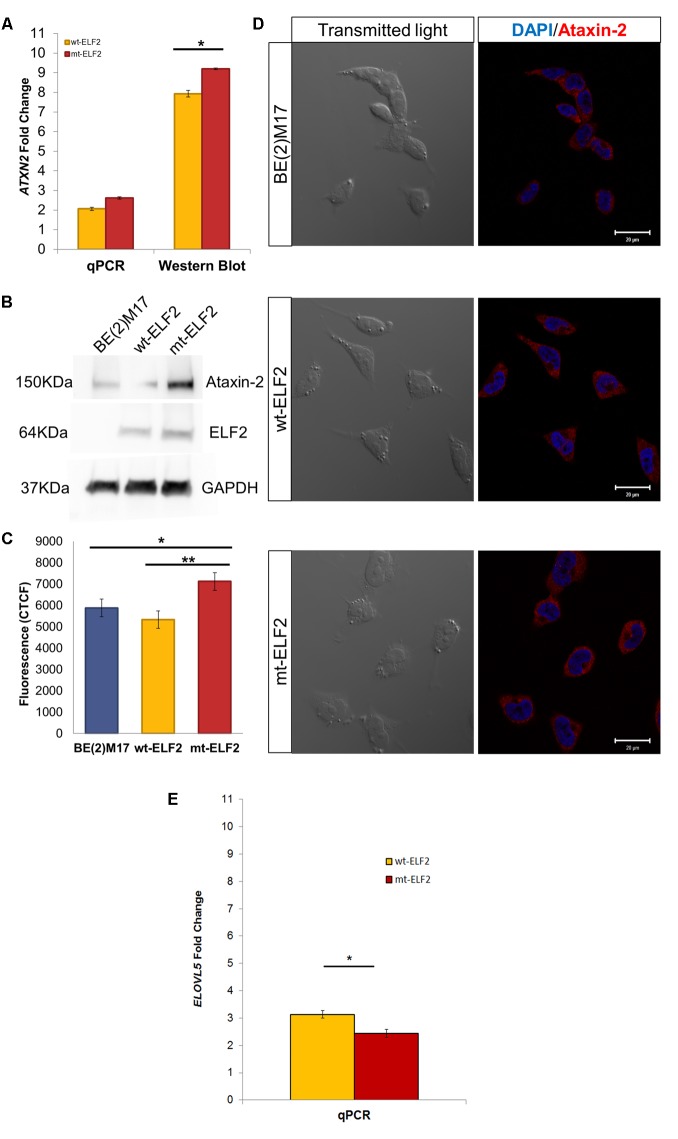
We confirmed that ELF2, ATXN2, and ELOVL5 genes were constitutively expressed in BE(2)-M17 cells by RT-PCR. We then evaluated ELF2, ATXN2, and ELOVL5 gene expression in CEE-wt-ELF2- and CEE-mt-ELF2-transduced BE(2)-M17 cells by qPCR and Western blot and found a significant increase in both ELF2 (p = 0.03) and ATNX2 (p = 0.002) expression at mRNA levels in the cells transduced with the CEE-mt-ELF2, but not in cells transduced with the CEE-wt-ELF2 (Figure 3A). In contrast, ELOVL5 was significantly decreased (p = 0.003) in cells transduced with the CEE-mt-ELF2, but not in cells transduced with the CEE-wt-ELF2 (Figure 3E). The ATXN2 increase was confirmed at protein levels in the CEE-mt-ELF2-transduced BE(2)-M17 cells, when they were compared to the wild type cell line (p = 0.019, Figures 3A,B).
FIGURE 3.
ATXN2 expression in BE(2)M17, wt-ELF2 and mt-ELF2 transduced cells. (A) ATXN2 qPCR and ataxin-2 Western blot show statistical differences between wt-ELF2 and mt-ELF2 transduced cells, both in qPCR and Western blot. (B) Representative western blot of BE(2)M17 exhibiting an increased content of ATXN2 in mt-ELF2 transduced cells. ATXN2 (#611378, 1:1000), Elf2 (#HPA006057-100UL, 1:1000), GAPDH (#AB2302, 1:3000 and secondary antibodies #HAF007, 1:6000, #HAF008, 1:3000, #A9046-1ML, 1:10000. (C) CTCF emitted by BE(2)M17 cells labeled with anti-ataxin-2 antibody in non-transduced, wt-ELF2 transduced and mt-ELF2 cells. (D) Representative immunocytochemistry image of ataxin-2 in non-transduced BE(2)M17, wt-ELF2, and mt-ELF2 transduced cells showing an increased staining in mt-ELF2 cell-line. ∗p < 0.02, ∗∗p < 0.002. Primary antibodies anti-ataxin-2 (1:250) and anti-ELF2 (1:500) and visualized with Alexa-555-conjugated goat anti-mouse #A-21422, 1:500 and Alexa-633-conjugated goat anti-rabbit #A-21071, 1:500, respectively. (E) ELOVL5 qPCR show statistical differences between wt-ELF2 and mt-ELF2 transduced cells. ∗p < 0.003.
Confocal microscopy imaging illustrated an overexpressed cytoplasmic distribution of ataxin-2 in CEE-mt-ELF2-transduced BE(2)M17 cells. We quantified the fluorescence intensity levels (Figure 3D). CEE-mt-ELF2 cell line was the most intensely labeled, followed by those cells that were not transduced and finally wt-ELF2 cells. Significant differences were found among non-transduced cells compared to mt-ELF2 (p = 0.03) and between wt-ELF2 as compared to mt-ELF2 (p = 0.003, Figure 3C). In addition, the immunocytochemistry showed that the transduction and mutation did not change elf2 location.
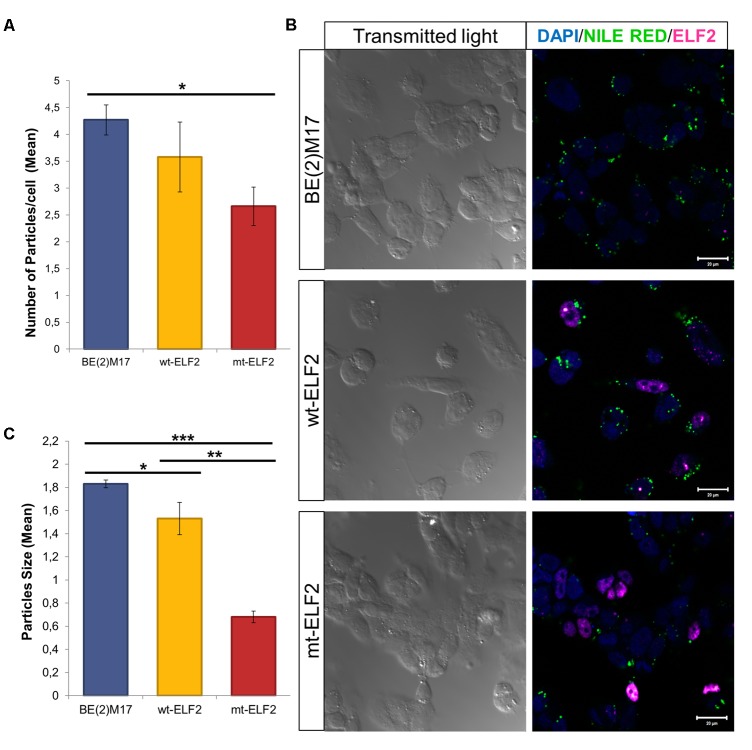
On comparing non-transduced BE(2)M17 cells with CEE-mt-ELF2 BE(2)M17-transduced cells, significant differences in the number of lipid droplets were observed with reduced lipid droplets present in the mutant cell line (p = 0.02, Figures 4A,B). In addition, we observed that lipid droplets were smaller in CEE-mt-ELF2 transduced BE(2)M17 cells (0.68 ± 0.05) when compared with CEE-wt-ELF2 BE(2)M17 transduced cells (1.53 ± 0.14, p = 1.54 × 10-8) and non-transduced BE(2)M17 cells (1.83 ± 0.03, p = 1.55 × 10-48, Figure 4C).
FIGURE 4.
Changes in Lipid droplets in transduced BE(2)M17 cell-lines. (A) Number of lipid droplets particles per cell in each cell-line (∗p = 0.02). (B) Representative immunocytochemistry image of Lipid droplets stained with Nile Red in non-transduced BE(2)M17, wt-ELF2 and mt-ELF2 transduced cells showing a decrease number and size of the droplets in mt-ELF2 cell-line. For lipid droplets experiments, cells were stained with Nile red to measure the number and size of lipid droplets. After Nile red staining, cells were fixed and staining with anti-ELF2 (1:500) and visualized with Alexa-633-conjugated goat anti-rabbit (1:500). (C) Mean of particles size in every cell-line. BE(2)M17 non-transduced cells vs. wt-ELF2 cells (∗p = 0.03); wt-ELF2 vs. mt-ELF2 transduced cells (∗∗p = 1.54 × 10-8); BE(2)M17 vs. mt-ELF2 (∗∗∗p = 1.55× 10-48).
Background
The ETS gene family is a group of TFs divided in 12 subfamilies. The ETS subfamily includes ETS1 and ETS2; the ELF subfamily includes ELF1, ELF2, and ELF4 (MEF) genes and the ELG subfamily consist of GABPα. All ETS TFs are defined by a highly conserved DNA binding domain, the ETS domain with a core GGA(A/T) DNA sequence (Sharrocks, 2001). Previous electrophoretic mobility shift assays (EMSAs) have demonstrated that ETS1, ELF2, and GABPα interact with the ETS domain within the 5′-UTR in the ATXN2 gene in HEK293 and SH-SY5Y nuclear lysates. HEK293 cells overexpressing ETS1 showed an increase in the expression of ATXN2 gene (Scoles et al., 2012). These findings suggested that the ETS domain in ATXN2 may be regulated by other TFs of the ETS gene family such as ELF-2. In the present study, we identified a novel missense variant in the ELF2 gene (E74-like factor 2; NERF), which segregates the complete phenotype and we present functional data showing the effect of mutated ELF2 (mt-ELF2) gene on ATXN2 and ELOVL5 two genes previously associated with spinocerebellar ataxia 2 and 38 (SCA2 and SCA38). No similar phenotype has been linked to ELF2 mutations at the time of submission (see section “Concluding Remarks”).
Discussion
Cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome is a rare syndrome, with less than 500 cases described worldwide (Szmulewicz et al., 2015), and familial cases have been described rarely (Szmulewicz et al., 2014a). We report a family with three CANVAS patients segregating a novel variant in ELF2 gene. Several lines of evidence support a pathogenic role for the ELF2 variant in this family. Firstly, multiple bioinformatics tools ranked this variant at the top of the candidate list; secondly, this novel variant was not found in the gnomAD and, perhaps more conclusively, the mt-ELF2 in a neuroblastoma cell line was able to modify the gene expression of two genes associated with ataxia in two ways. Firstly, by upregulating the expression and translation of ATXN2 (the gene involved in SCA2) and secondly, by decreasing the expression and translation of ELOVL5, (associated with SCA 38). Sequencing data were re-evaluated in our familial dataset in both genes, but no abnormal CAG repeat expansion in ATXN2 or pathogenic variants in ELOVL5 gene such as c.214C>G or c.689G>Tl were found in the patients.
ELF2 is a TF associated with RUNX1 and both interact in the regulation of gene expression (Wang et al., 1993). We have observed that ELF2 acts as a repressor of ATXN2 gene expression in neuroblastoma cells and that mt-ELF2 will not be likely to regulate its expression. Although our mutation is not within the ETS-binding domain, it is not possible to exclude the interaction of ELF2 and other TFs, such as RUNX1.
ELOVL5 is a target gene for ELF2 according to the TRANSFAC (Wingender et al., 2000; Wingender, 2008) and this gene is considered the causal gene of SCA 38 (Di Gregorio et al., 2014). Our results also confirm that mt-ELF2 also modifies the expression of ELOVL5. This gene is involved in the long-chain fatty acids elongation cycle, and it is highly expressed in Purkinje cells. Furthermore, the ELOVL5-/- mice develop ataxia and motor impairment during the balance beam test (Hoxha et al., 2017). Several neurological diseases, particularly hereditary spastic paraplegias (Dick et al., 2010; Tesson et al., 2012; Boukhris et al., 2013; Martin et al., 2013) display alterations of lipid metabolism. Increases in lipid droplets play a crucial role in the nervous system and have been associated with in vitro models of neurodegenerative disorders such as Huntington’s and Parkinson’s diseases (Martinez-Vicente et al., 2010; Thiam et al., 2013; Welte, 2015), emphasizing the importance of lipid homeostasis in brain membranes.
Although the expression of ELF2 gene in the human cerebellum is low according to the Allen Brain Atlas2 (Hawrylycz et al., 2012), and the same variant was not observed in other CANVAS patients, this may be attributed to the genetic heterogeneity commonly found in hereditary ataxias.
Furthermore, we have found strong evidence that the position chr4:g.140058846 C > T in the ELF2 gene is highly conserved in an evolutionary sense, therefore the variant is likely pathogenic and possibly interferes with protein function. Functional assays indicate a regulatory role of the ELF2 variant in vitro for two SCA genes, since we have shown that the expression of mt-ELF2, but not wt-ELF2, increases ATXN2 gene expression and ataxin-2 translation and decreases ELOVL5 gene expression in BE(2)-M17 cells.
Concluding Remarks
We describe a rare variant in ELF2 gene in this family with CANVAS syndrome and demonstrate its functional effects in ATXN2 and ELOV5 genes in BE(2)-M17 transduced cells. The interaction between ELF2, ATXN2, and ELOVL5 genes found suggests that the regulation of expression in these genes could potentially be a shared mechanism in hereditary ataxias.
Ethics Statement
This study was carried out in accordance with the recommendations of Imperial College Research Ethics Committee with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Imperial College Research Ethics Committee.
Author Contributions
HA, TR, LF, MC, AG-M, FM, JL-E, and AB substantially contributed to the conception and design of the work. AB and HA examined the patients. TR, LF, and MC carried out the lab experiments. AG-M and TR performed the bioinformatic analyses of NGS data. All authors analyzed and interpreted the data for the work, drafted the work, revised it critically for important intellectual content, finally approved the version to be published, and all agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer GH and handling Editor declared their shared affiliation.
Acknowledgments
We acknowledge Julia Jaldo and Carmen Martin-Sierra for their assistance with the cell culture. We thank the assistance of Raquel Marrero Díaz and Sara Moreno Sanjuán of the Microscopy Unit and all the staff of the Genomic Unit at Genyo. We also thank Dr. Diego Kaski and Mr. David Buckwell for their help in producing (Figure 2). We particularly appreciate the patient’s and their family member’s help and enthusiasm with this study.
Funding. This work was supported by an MRC grant MR/J004685/1 (AB), financial support to the Otology and Neurotology Group (JL-E), and by the Fondo de Investigaciones Sanitarias ISCIII (Spain), Fondo Europeo de Desarrollo Regional (FEDER) from the EU through research grants PI15/02015, TerCel: RD12/0019/0006, and by the CICE and CS de la Junta de Andalucía FEDER/Fondo de Cohesion Europeo (FSE) de Andalucía through research grants PI-57069 (FM), and PI-0407/2012 and PI-0318/2014 (MC).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2018.00085/full#supplementary-material
References
- Benabdellah K., Gutierrez-Guerrero A., Cobo M., Munoz P., Martin F. (2014). A chimeric HS4-SAR insulator (IS2) that prevents silencing and enhances expression of lentiviral vectors in pluripotent stem cells. PLoS One 9:e84268. 10.1371/journal.pone.0084268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhris A., Schule R., Loureiro J. L., Lourenco C. M., Mundwiller E., Gonzalez M. A., et al. (2013). Alteration of ganglioside biosynthesis responsible for complex hereditary spastic paraplegia. Am. J. Hum. Genet. 93 118–123. 10.1016/j.ajhg.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein A. M., Mossman S., Luxon L. M. (1991). The neck-eye reflex in patients with reduced vestibular and optokinetic function. Brain 114(Pt 1A), 1–11. [PubMed] [Google Scholar]
- Cobo M., Anderson P., Benabdellah K., Toscano M. G., Munoz P., Garcia-Perez A., et al. (2013). Mesenchymal stem cells expressing vasoactive intestinal peptide ameliorate symptoms in a model of chronic multiple sclerosis. Cell Transplant. 22 839–854. 10.3727/096368912X657404 [DOI] [PubMed] [Google Scholar]
- Di Gregorio E., Borroni B., Giorgio E., Lacerenza D., Ferrero M., Lo Buono N., et al. (2014). ELOVL5 mutations cause spinocerebellar ataxia 38. Am. J. Hum. Genet. 95 209–217. 10.1016/j.ajhg.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick K. J., Eckhardt M., Paisan-Ruiz C., Alshehhi A. A., Proukakis C., Sibtain N. A., et al. (2010). Mutation of FA2H underlies a complicated form of hereditary spastic paraplegia (SPG35). Hum. Mutat. 31 E1251–E1260. 10.1002/humu.21205 [DOI] [PubMed] [Google Scholar]
- Frecha C., Toscano M. G., Costa C., Saez-Lara M. J., Cosset F. L., Verhoeyen E., et al. (2008). Improved lentiviral vectors for Wiskott-Aldrich syndrome gene therapy mimic endogenous expression profiles throughout haematopoiesis. Gene Ther. 15 930–941. 10.1038/gt.2008.20 [DOI] [PubMed] [Google Scholar]
- Gazal S., Gosset S., Verdura E., Bergametti F., Guey S., Babron M. C., et al. (2016). Can whole-exome sequencing data be used for linkage analysis? Eur. J. Hum. Genet. 24 581–586. 10.1038/ejhg.2015.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylycz M. J., Lein E. S., Guillozet-Bongaarts A. L., Shen E. H., Ng L., Miller J. A., et al. (2012). An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489 391–399. 10.1038/nature11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxha E., Gabriele R. M. C., Balbo I., Masante L., Zambelli V., Mitro N., et al. (2017). Elovl5 knock-out mice as a model of spinocerebellar ataxia 38. Front. Cell. Neurosci. 95 209–217. 10.3389/fncel.2017.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E., Schule R., Smets K., Rastetter A., Boukhris A., Loureiro J. L., et al. (2013). Loss of function of glucocerebrosidase GBA2 is responsible for motor neuron defects in hereditary spastic paraplegia. Am. J. Hum. Genet. 92 238–244. 10.1016/j.ajhg.2012.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vicente M., Talloczy Z., Wong E., Tang G., Koga H., Kaushik S., et al. (2010). Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nat. Neurosci. 13 567–576. 10.1038/nn.2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Sierra C., Requena T., Frejo L., Price S. D., Gallego-Martinez A., Batuecas-Caletrio A., et al. (2016). A novel missense variant in PRKCB segregates low-frequency hearing loss in an autosomal dominant family with Meniere’s disease. Hum. Mol. Genet. 25 3407–3415. 10.1093/hmg/ddw183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A. A., Halmagyi G. M., Mcgarvie L. A., Cremer P. D. (2004). Cerebellar ataxia with bilateral vestibulopathy: description of a syndrome and its characteristic clinical sign. Brain 127 280–293. 10.1093/brain/awh030 [DOI] [PubMed] [Google Scholar]
- Requena T., Gallego-Martinez A., Lopez-Escamez J. A. (2017). A pipeline combining multiple strategies for prioritizing heterozygous variants for the identification of candidate genes in exome datasets. Hum. Genomics 11:11. 10.1186/s40246-017-0107-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoles D. R., Pflieger L. T., Thai K. K., Hansen S. T., Dansithong W., Pulst S. M. (2012). ETS1 regulates the expression of ATXN2. Hum. Mol. Genet. 21 5048–5065. 10.1093/hmg/dds349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrocks A. D. (2001). The ETS-domain transcription factor family. Nat. Rev. Mol. Cell Biol. 2 827–837. 10.1038/35099076 [DOI] [PubMed] [Google Scholar]
- Szmulewicz D. J., Mclean C. A., Macdougall H. G., Roberts L., Storey E., Halmagyi G. M. (2014a). CANVAS an update: clinical presentation, investigation and management. J. Vestib. Res. 24 465–474. 10.3233/VES-140536 [DOI] [PubMed] [Google Scholar]
- Szmulewicz D. J., Mclean C. A., Rodriguez M. L., Chancellor A. M., Mossman S., Lamont D., et al. (2014b). Dorsal root ganglionopathy is responsible for the sensory impairment in CANVAS. Neurology 82 1410–1415. 10.1212/WNL.0000000000000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmulewicz D. J., Seiderer L., Halmagyi G. M., Storey E., Roberts L. (2015). Neurophysiological evidence for generalized sensory neuronopathy in cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome. Muscle Nerve 51 600–603. 10.1002/mus.24422 [DOI] [PubMed] [Google Scholar]
- Tesson C., Nawara M., Salih M. A., Rossignol R., Zaki M. S., Al Balwi M., et al. (2012). Alteration of fatty-acid-metabolizing enzymes affects mitochondrial form and function in hereditary spastic paraplegia. Am. J. Hum. Genet. 91 1051–1064. 10.1016/j.ajhg.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiam A. R., Antonny B., Wang J., Delacotte J., Wilfling F., Walther T. C., et al. (2013). COPI buds 60-nm lipid droplets from reconstituted water-phospholipid-triacylglyceride interfaces, suggesting a tension clamp function. Proc. Natl. Acad. Sci. U.S.A. 110 13244–13249. 10.1073/pnas.1307685110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Y., Petryniak B., Thompson C. B., Kaelin W. G., Leiden J. M. (1993). Regulation of the Ets-related transcription factor Elf-1 by binding to the retinoblastoma protein. Science 260 1330–1335. 10.1126/science.8493578 [DOI] [PubMed] [Google Scholar]
- Welte M. A. (2015). Expanding roles for lipid droplets. Curr. Biol. 25 R470–R481. 10.1016/j.cub.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender E. (2008). The TRANSFAC project as an example of framework technology that supports the analysis of genomic regulation. Brief. Bioinform. 9 326–332. 10.1093/bib/bbn016 [DOI] [PubMed] [Google Scholar]
- Wingender E., Chen X., Hehl R., Karas H., Liebich I., Matys V., et al. (2000). TRANSFAC: an integrated system for gene expression regulation. Nucleic Acids Res. 28 316–319. 10.1093/nar/28.1.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingler V. C., Cnyrim C., Jahn K., Weintz E., Fernbacher J., Frenzel C., et al. (2007). Causative factors and epidemiology of bilateral vestibulopathy in 255 patients. Ann. Neurol. 61 524–532. 10.1002/ana.21105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.